Abstract
OBJECTIVE
To investigate whether dietary phylloquinone and menaquinones intakes are related to risk of type 2 diabetes.
RESEARCH DESIGN AND METHODS
We used data from a prospective cohort study in 38,094 Dutch men and women, aged 20–70 years. Dietary phylloquinone and menaquinones intakes were assessed using a validated food frequency questionnaire. Diabetes case patients were ascertained mainly via self-report and verified against medical records.
RESULTS
During 10.3 years of follow-up, 918 incident cases of diabetes were documented. In a multivariate model adjusting for diabetes risk factors and dietary factors, phylloquinone intake tended to be associated (P = 0.08) with a reduced risk of type 2 diabetes with a hazard ratio (HR) of 0.81 (95% CI 0.66–0.99) for the highest versus the lowest quartile. For menaquinones intake, a linear, inverse association (P = 0.038) with risk of type 2 diabetes was observed with an HR of 0.93 (0.87–1.00) for each 10-μg increment in the multivariate model.
CONCLUSIONS
This study shows that both phylloquinone and menaquinones intakes may be associated with a reduced risk of type 2 diabetes.
Vitamin K is a fat-soluble vitamin occurring in two biologically active forms; vitamin K1 (phylloquinone) and vitamin K2 (menaquinones). Phylloquinone, the most common form, is present in green, leafy vegetables and certain vegetable oils (1), whereas menaquinones occur in animal products such as meat, eggs, and cheese (2). However, strong regional differences in the amount and forms of menaquinones intake exist (2). Vitamin K functions as a cofactor in the γ-carboxylation of certain glutamic acid (Gla) residues of vitamin K-dependent proteins for their activation (3). Vitamin K was known mainly as a cofactor to carboxylate clotting factors such as prothrombin (3). More recently, it became apparent that vitamin K also carboxylates other proteins such as osteocalcin, a regulator of bone mineral maturation (3).
A recent study showed that osteocalcin concentrations may also affect insulin sensitivity and type 2 diabetes by regulating the expression of insulin genes and β-cell proliferation markers (4). In mice, osteocalcin was shown to increase insulin secretion and insulin sensitivity and decrease the severity of type 2 diabetes (4). This animal study suggested a specific role for the uncarboxylated form of osteocalcin (4), contradicting a role for vitamin K. Subsequent human studies, however, observed relations between high total or carboxylated osteocalcin and improved insulin sensitivity (5,6). These latter studies suggest that vitamin K could reduce insulin resistance and risk of type 2 diabetes by carboxylating osteocalcin.
To date, no studies have investigated the relation between vitamin K intake and risk of type 2 diabetes and only a few have explored relations with insulin sensitivity. In rats, vitamin K deficiency delayed the insulin response and decreased plasma glucose (7). Similar results have been shown in small-scale human studies among young men with a low risk of diabetes (8). Recently, two larger studies investigated the relation between dietary phylloquinone and insulin sensitivity. An observational study showed that high phylloquinone intake was associated with improved insulin sensitivity and glycemic control (9). A randomized controlled trial showed improved insulin sensitivity after phylloquinone supplementation among men (10). Whether dietary phylloquinone or menaquinones intakes are associated with a reduced risk of type 2 diabetes is unknown. Therefore, we investigated whether dietary phylloquinone and menaquinones intakes are inversely associated with type 2 diabetes in a prospective cohort of Dutch men and women. Because previous studies suggested relations between vitamin K and inflammatory factors or blood lipid profile (11–14), we explored relations between phylloquinone and menaquinones intakes and high-sensitivity C-reactive protein (CRP), blood lipid profile, and A1C as a marker of diabetes risk.
RESEARCH DESIGN AND METHODS
The European Prospective Investigation into Cancer and Nutrition (EPIC)-NL consists of the two Dutch contributions to the EPIC study, the Prospect-EPIC and MORGEN-EPIC cohorts. These cohorts were set up simultaneously in 1993–1997 and merged into one Dutch EPIC cohort. The design and rationale of EPIC-NL are described elsewhere (15). The Prospect-EPIC study includes 17,357 women aged 49–70 years living in Utrecht and vicinity. The MORGEN-EPIC cohort consists of 22,654 adults aged 21–64 years selected from random samples of the Dutch population in three Dutch towns. All participants provided informed consent before study inclusion. The study complies with the Declaration of Helsinki and was approved by the institutional review board of the University Medical Center Utrecht (Prospect) and the Medical Ethical Committee of TNO Nutrition and Food Research (MORGEN). After exclusion of individuals with prevalent diabetes (n = 615), individuals with abnormal energy intake (kcal <600 or >5,000; n = 108), missing nutritional data (n = 213), and missing follow-up (n = 981), 38,094 participants were left for analysis.
Intakes of phylloquinone, menaquinones, and other nutrients
Daily nutritional intake was obtained from a food frequency questionnaire (FFQ) containing questions on the usual frequency of consumption of 79 main food items during the year preceding enrollment. This questionnaire allows the estimation of the mean daily consumption of 178 foods. The FFQ has been validated against 12 24-h dietary recalls (16). The 1996 Dutch food consumption table was used to calculate energy and nutrient intakes. This table does not contain information on vitamin K content of foods. Therefore, the concentrations of phylloquinone and menaquinones (menaquinones subtypes, menaquinones-4 [MK4] through menaquinones-10 [MK10]) in a series of Dutch foods were assessed at the Biochemistry Laboratory, Maastricht University (2). For some foods, published data by others were used to update the dietary database for vitamin K (2,17–19). In total, vitamin K contents of 260 foods were collected and tabulated to estimate phylloquinone and menaquinones intakes. We used data from our validation study to determine reliability of the FFQ to estimate vitamin K intake against 12 24-h recalls in 58 women and 63 men (16). We observed a low relative validity of phylloquinone and MK10 intakes with correlations of the FFQ against 24-h recalls of 0.24 and 0.23, respectively. Relative validity for intakes of menaquinones and MK4 to MK9 was reasonable to good with correlation ranging from 0.51 for MK7 to 0.73 for MK5. Intakes of nutrients were adjusted for energy intake by the regression residual method.
Diabetes
Occurrence of diabetes during follow-up was self-reported in two follow-up questionnaires with 3- to 5-year intervals. Participants were asked whether diabetes was diagnosed, in what year, and by whom and what treatment was received. In the Prospect study, incident cases of diabetes were detected via a urinary glucose strip test, sent out with the first follow-up questionnaire, for detection of glucosuria. Diagnoses of diabetes were also obtained from the Dutch Centre for Health Care Information, which holds a standardized computerized register of hospital discharge diagnoses. In this register, admission files have been entered continuously from all general and university hospitals in the Netherlands from 1990 onward. All diagnoses were coded according to the ICD-9-CM. Follow-up was complete on 1 January 2006. Potential cases identified by any of these methods were verified against participants' general practitioner or pharmacist information through mailed questionnaires. Diabetes was defined as being present when either of these confirmed the diagnosis. For 89% of participants with potential diabetes, verification information was available, and 72% were verified as having type 2 diabetes and were used for the analysis.
Other measurements
At baseline, participants filled out a general questionnaire containing questions on demographics, presence of chronic diseases, and risk factors for chronic diseases. Smoking was categorized into current, past, and never smoker and parental history of diabetes into none, one, or two parents. Systolic and diastolic blood pressure measurements were performed twice on the right arm with the participant in the supine position using a Boso Oscillomat (Bosch & Son, Jungingen, Germany) (Prospect) or on the left arm using a random zero sphygmomanometer (MORGEN), and the mean was taken. Hypertension was defined as being present based on diastolic blood pressure ≥90 mmHg, systolic blood pressure ≥140 mmHg, self-reported use of antihypertensive medication, or self-reported presence of hypertension. Physical activity was assessed using a questionnaire validated in an elderly population, and the Cambridge Physical Activity Score was calculated and used to categorize physical activity (20). Because we could not calculate a total physical activity score for 14% of all participants, we imputed missing scores by means of single linear regression modeling (SPSS MVA procedure). Waist circumference, height, and weight were measured and BMI was calculated (weight in kilograms divided by the square of height in meters). A 6.5% random sample (n = 2,604) of the baseline cohort was drawn for more detailed biochemical measurements. The baseline characteristics of this random sample were similar to the baseline characteristics of the entire cohort (15). Thus, the random sample is representative of the full baseline cohort and can therefore be used to explore relations between risk markers and other exposures. A1C was measured in erythrocytes using an immunoturbidimetric latex test, total cholesterol and triglycerides were measured using enzymatic methods, high-sensitivity CRP was measured with a turbidimetric method, and HDL cholesterol and LDL cholesterol were measured using a homogeneous assay with an enzymatic end point (15).
Data analysis
Baseline characteristics by quartiles of energy-adjusted dietary phylloquinone and menaquinones were inspected using analysis of variance for continuous variables and a χ2 test for categorical variables. We calculated person-years of follow-up for each participant from the date of return of the questionnaire to the date of type 2 diabetes, the date of death, or 1 January 2006. We used Cox regression to estimate hazard ratios (HRs) for type 2 diabetes for quartiles of either energy-adjusted phylloquinone or menaquinones intake and for each 50-μg increment of energy-adjusted phylloquinone intake and for each 10-μg increment of energy-adjusted menaquinones intake. These increments were based on an approximately half SD for phylloquinone (SD 98) and menaquinones (SD 17). We adjusted for type 2 diabetes risk factors and dietary factors using three models. The first model was adjusted for age, sex, and waist circumference. In a second model, we included smoking status (non/current/former), physical activity (four categories), education (three categories), hypertension, alcohol consumption, and total energy intake (both continuous). In the final multivariate model, we also adjusted for diet by including energy-adjusted intake of saturated, polyunsaturated, and monounsaturated fat, protein, fiber, calcium, vitamin C, and vitamin E in the model (all continuous). We checked whether interaction of sex, waist circumference, and BMI with phylloquinone and menaquinones intakes was present by including the interaction terms in the model. The presence of a nonlinear association of phylloquinone or menaquinones intake was explored by including the quadratic term of phylloquinone and menaquinones in the model with the linear term. The possibility of a nonlinear relation was further examined nonparametrically with restricted cubic splines (21). The likelihood ratio text was used for nonlinearity, comparing the model with only the linear term to the model with the linear and cubic spline terms. Associations between risk markers and phylloquinone and menaquinones intakes (both modeled continuously per 50 and 10 μg) were assessed by linear regression using the third multivariate model in the baseline random sample. In these analyses, we excluded those with CRP >10 mg/l (indicating active infection; n = 72) and A1C >6.5% (indicating the presence of diabetes; n = 60). Data analysis was performed using SPSS (version 15.0 for Windows) and SAS (version 9.1 for Windows).
RESULTS
Intake of phylloquinone was 200 ± 98 μg/day and intake of menaquinones was 31 ± 7 μg/day in our study population. Vegetables contributed 78% of phylloquinone intake, whereas cheese contributed 53%, milk products 19%, and meat 17% of menaquinones intake. Age- and energy-adjusted intake of protein was higher with higher phylloquinone and menaquinones intakes, whereas percent men and prevalence of physical inactivity were lower with higher intakes of phylloquinone and menaquinones (Table 1). With higher phylloquinone intake, we observed higher intakes of energy-adjusted fiber and vitamin C, whereas alcohol consumption was lower. Alcohol consumption and energy-adjusted intake of saturated fat and calcium were higher with higher menaquinones intake and prevalence of smoking was lower with higher menaquinone intake (Table 1).
Table 1.
Baseline characteristics according to quartiles of energy-adjusted phylloquinone and menaquinones intake of 38,094 Dutch adults
| Characteristics | Phylloquinone |
Menaquinones |
||||||
|---|---|---|---|---|---|---|---|---|
| Quartile 1 (low) | Quartile 2 | Quartile 3 | Quartile 4 (high) | Quartile 1 (low) | Quartile 2 | Quartile 3 | Quartile 4 (high) | |
| n | 9,523 | 9,524 | 9,524 | 9,523 | 9,486 | 9,557 | 9,557 | 9,494 |
| Phylloquinone (μg) | 95.7 ± 24.6 | 155.8 ± 15.1 | 213.1 ± 19.1 | 332.9 ± 82.5 | 187.1 ± 97.3 | 200.6 ± 96.8 | 205.3 ± 99.5 | 204.5 ± 98.6 |
| Menaquinones (μg) | 29.4 ± 14.5 | 30.3 ± 13.6 | 30.9 ± 13.3 | 31.5 ± 13.5 | 15.4 ± 3.7 | 24.5 ± 2.2 | 33.0 ± 2.8 | 49.1 ± 10.8 |
| Male sex | 3,204 (33.6) | 2,738 (28.7) | 2,191 (23.0) | 1,607 (16.9) | 3,509 (37.0) | 2,585 (27.0) | 2,033 (21.3) | 1,613 (17.0) |
| Age (years) | 46.3 ± 12.4 | 48.2 ± 11.8 | 49.8 ± 11.6 | 52.2 ± 10.9 | 44.9 ± 12.9 | 49.0 ± 11.9 | 50.5 ± 11.2 | 52.0 ± 10.2 |
| BMI (kg/m2) | 25.3 ± 3.9 | 25.4 ± 3.9 | 25.6 ± 3.9 | 26.1 ± 4.2 | 25.3 ± 3.9 | 25.7 ± 4.0 | 25.8 ± 4.0 | 25.8 ± 4.1 |
| Waist circumference (cm) | 84.7 ± 11.7 | 84.8 ± 11.4 | 85.0 ± 11.2 | 85.7 ± 11.3 | 84.9 ± 11.7 | 85.4 ± 11.4 | 85.2 ± 11.2 | 84.8 ± 11.4 |
| Current smoker | 3,167 (33.3) | 2,860 (30.1) | 2,754 (29.0) | 2,849 (30.0) | 3,376 (35.7) | 2,979 (31.3) | 2,707 (28.4) | 2,568 (27.1) |
| Physically inactive* | 1,079 (11.3) | 808 (8.5) | 785 (8.2) | 829 (8.7) | 1,169 (12.3) | 871 (9.1) | 750 (7.8) | 711 (7.5) |
| Higher education | 2,257 (23.7) | 2,030 (21.3) | 1,955 (20.5) | 1,588 (16.7) | 1,676 (17.7) | 1,770 (18.5) | 2,024 (21.2) | 2,360 (24.9) |
| Family history of diabetes | 1,570 (16.5) | 1,674 (17.6) | 1,759 (18.5) | 1,860 (19.5) | 1,482 (15.6) | 1,783 (18.7) | 1,851 (19.4) | 1,747 (18.4) |
| Systolic blood pressure (mmHg) | 124.3 ± 18.5 | 125.1 ± 18.1 | 126.7 ± 19.0 | 128.1 ± 19.5 | 123.6 ± 18.0 | 126.1 ± 18.6 | 127.0 ± 19.1 | 127.3 ± 19.3 |
| Diastolic blood pressure (mmHg) | 77.4 ± 10.8 | 77.6 ± 10.4 | 77.9 ± 10.6 | 78.2 ± 10.7 | 77.1 ± 10.4 | 77.9 ± 10.7 | 78.0 ± 10.7 | 78.0 ± 10.7 |
| Hypertension | 3,225 (33.9) | 3,307 (34.7) | 3,605 (37.9) | 3,908 (41.0) | 3,096 (32.6) | 3,592 (37.6) | 3,664 (38.3) | 3,693 (38.9) |
| Hyperlipidemia | 771 (8.1) | 787 (8.3) | 748 (7.9) | 828 (8.7) | 958 (10.1) | 836 (8.7) | 738 (7.7) | 602 (6.3) |
| Alcohol intake (g/day)† | 5.6 (16.7) | 5.7 (15.3) | 5.2 (14.3) | 4.1 (13.7) | 4.3 (15.7) | 5.2 (15.4) | 5.2 (14.1) | 5.7 (14.9) |
| Diet‡ | ||||||||
| Energy intake (kcal/day) | 2,060 ± 649 | 2,103 ± 624 | 2,071 ± 612 | 1,984 ± 590 | 2,087 ± 669 | 2,064 ± 618 | 2,043 ± 590 | 2,024 ± 601 |
| Saturated fat intake (g/day) | 31.9 ± 5.9 | 32.5 ± 5.8 | 32.8 ± 5.7 | 33.1 ± 6.1 | 29.4 ± 5.4 | 31.7 ± 5.2 | 33.2 ± 5.1 | 35.9 ± 5.8 |
| PUFA intake (g/day) | 14.5 ± 3.9 | 14.9 ± 3.7 | 15.1 ± 3.8 | 15.3 ± 4.1 | 15.5 ± 4.2 | 15.2 ± 3.8 | 14.9 ± 3.7 | 14.3 ± 3.7 |
| MUFA intake (g/day) | 29.2 ± 5.2 | 29.5 ± 5.0 | 29.6 ± 5.1 | 29.5 ± 5.3 | 29.0 ± 5.6 | 29.4 ± 5.1 | 29.5 ± 4.9 | 30.0 ± 5.0 |
| Protein intake (g/day) | 73.5 ± 11.3 | 75.0 ± 10.4 | 76.2 ± 10.5 | 78.2 ± 11.2 | 69.0 ± 10.3 | 74.1 ± 9.4 | 77.5 ± 9.5 | 82.3 ± 10.2 |
| Fiber intake (g/day) | 21.1 ± 4.6 | 22.9 ± 4.4 | 23.9 ± 4.4 | 25.5 ± 4.7 | 23.0 ± 5.3 | 23.4 ± 4.8 | 23.6 ± 4.5 | 23.4 ± 4.6 |
| Vitamin C intake (mg/day) | 94.2 ± 39.6 | 107.0 ± 42.9 | 113.6 ± 44.3 | 122.8 ± 49.2 | 101.5 ± 47.2 | 108.5 ± 44.3 | 113.0 ± 43.9 | 114.6 ± 44.8 |
| Vitamin E intake (mg/day) | 11.5 ± 3.2 | 12.0 ± 3.1 | 12.4 ± 3.1 | 13.0 ± 3.4 | 12.6 ± 3.5 | 12.4 ± 3.3 | 12.2 ± 3.1 | 11.7 ± 3.0 |
| Calcium intake (mg/day) | 1,003 ± 370 | 1,050 ± 347 | 1,079 ± 343 | 1,130 ± 349 | 804 ± 296 | 990 ± 285 | 1,127 ± 283 | 1,340 ± 320 |
Data are means ± SD or n (%) unless otherwise indicated. Ptrend < 0.001 except for waist circumference over menaquinones categories. MUFA, monounsaturated fat; PUFA, polyunsaturated fat.
*Inactive according to the Cambridge physical activity index.
†Median (interquartile range).
‡All nutrients are energy adjusted except energy intake.
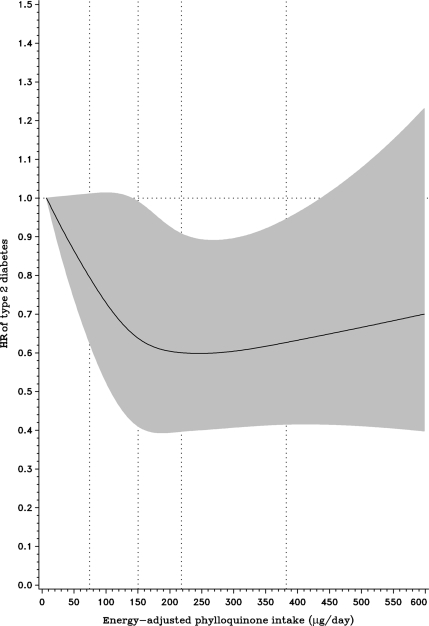
During a median follow-up of 10.3 years, we documented 918 verified cases of type 2 diabetes. In an age-, sex-, and waist-adjusted model, phylloquinone intake was not associated with risk of type 2 diabetes with an HR of 1.00 (95% CI 0.97–1.03) for each 50-μg increment (Table 2). However, with adjustment for diabetes risk factors and dietary factors, quartiles of phylloquinone intake tended to be associated (P = 0.08) with a reduced risk of type 2 diabetes with an HR of 0.81 (0.66–0.99) for the highest versus the lowest quartile. This association, however, remained nonsignificant when modeled linearly per 50 μg. This result was due to the presence of a nonlinear relation as indicated by a significant quadratic term (P = 0.016). Spline regression indeed showed evidence of a nonlinear relation (P = 0.053) between phylloquinone intake and type 2 diabetes with a linear risk reduction at lower levels, reaching a plateau at higher levels of intake with an HR of ∼0.65 (Fig. 1).
Table 2.
Energy-adjusted phylloquinone and menaquinones intake and risk of type 2 diabetes among 38.094 Dutch men and women
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | Ptrend value | Per 50 μg | |
|---|---|---|---|---|---|---|
| Phylloquinone | ||||||
| Intake (μg/day) | 100.1 | 155.7 | 211.4 | 308.1 | ||
| Age-, sex-, waist-adjusted | 1.0 | 0.89 (0.73–1.08) | 0.95 (0.79–1.14) | 0.89 (0.74–1.07) | 0.35 | 1.00 (0.97–1.03) P = 0.92 |
| Multivariate adjusted* | 1.0 | 0.89 (0.74–1.09) | 0.94 (0.78–1.14) | 0.88 (0.73–1.06) | 0.26 | 0.99 (0.96–1.02) P = 0.65 |
| Multivariate adjusted† | 1.0 | 0.87 (0.71–1.06) | 0.90 (0.74–1.09) | 0.81 (0.66–0.99) | 0.08 | 0.98 (0.95–1.02) P = 0.31 |
| Menaquinones | ||||||
| Intake (μg/day) | 16.0 | 24.5 | 32.9 | 46.1 | Per 10 μg | |
| Age-, sex-, waist-adjusted | 1.0 | 1.03 (0.85–1.25) | 0.95 (0.78–1.15) | 0.86 (0.71–1.05) | 0.07 | 0.95 (0.91–1.01) P = 0.060 |
| Multivariate adjusted* | 1.0 | 1.04 (0.86–1.26) | 0.97 (0.80–1.17) | 0.88 (0.73–1.08) | 0.13 | 0.96 (0.91–1.02) P = 0.12 |
| Multivariate adjusted† | 1.0 | 0.99 (0.82–1.21) | 0.89 (0.72–1.10) | 0.80 (0.62–1.02) | 0.04 | 0.93 (0.87–1.00) P = 0.038 |
Data are HRs (95% CI).
*Adjusted for age, sex, waist circumference, smoking status, physical activity, hypertension, education, alcohol consumption, and total energy intake.
†Adjusted for confounders in footnote * and energy-adjusted intake of saturated, polyunsaturated, and monounsaturated fat, protein, fiber, calcium, vitamin C, and vitamin E.
Figure 1.
Association between phylloquinone intake and risk of type 2 diabetes modeled continuously using splines; HR (——) with 95% CI in gray.
Menaquinones intake tended to be inversely associated (P = 0.060) with risk of type 2 diabetes with an HR of 0.95 (95% CI 0.91–1.01) for each 10-μg increment in an age-, sex-, and waist-adjusted model. In the final multivariate model, an inverse association (P = 0.038) was observed with an HR of 0.93 (0.87–1.00) for each 10-μg increment of menaquinones intake. These results were similar using quartiles of menaquinones intake. Spline regression showed a linear inverse association (P = 0.035) between menaquinones intake and type 2 diabetes without evidence for a nonlinear relation.
The interaction between sex and phylloquinone or menaquinones intakes was not significant, nor was the interaction with waist circumference or BMI. If waist circumference was replaced with BMI in the model, similar results were observed for menaquinones (HR 0.93 [95% CI 0.86–0.99]) and phylloquinone (0.98 [0.95–1.02]). Adjustment for parental history of type 2 diabetes did not change the results for phylloquinone (0.99 [0.95–1.02]) or menaquinones (0.94 [0.87–1.00]) intake nor did adjustment for vitamin D intake (menaquinones: 0.93 [0.87–1.00]; phylloquinone: 0.98 [0.95–1.02]).
In the baseline random sample (n = 1,604), used for the hypothesis-generating analysis, HDL cholesterol tended to be higher with higher menaquinones intake (ß 0.01 ± 0.007; P = 0.11). The HDL cholesterol-to-total cholesterol ratio was higher with higher menaquinones intake (ß 0.003 ± 0.001; P = 0.034). High-sensitivity CRP was inversely associated with menaquinones intake (ß −0.10 ± 0.04; P = 0.016). None of the other risk markers (A1C, total cholesterol, LDL cholesterol, or triglycerides) were associated with menaquinones intake. Phylloquinone intake was not associated with any of the risk markers (data not shown).
CONCLUSIONS
In this large cohort of 38,094 Dutch men and women, both dietary phylloquinone and menaquinones intakes were associated with a reduced risk of type 2 diabetes. This association was linear inverse for menaquinones, whereas a significant risk reduction for phylloquinone was observed at higher intakes, in particular. High dietary menaquinones intake was associated with lower CRP concentrations and an improved blood lipid profile.
The strengths of this study include its prospective design, long follow-up, large sample size, and verification of type 2 diabetes against medical records. However, certain limitations need to be addressed. The main limitation was the determination of dietary intakes of phylloquinone and menaquinones using an FFQ. Although the FFQ was not validated for phylloquinone and menaquinones at that time, we estimated relative validity of phylloquinone and menaquinones using the data of the validation study. Relative validity of our FFQ for phylloquinone intake was low, but for menaquinones intake it was reasonable with correlation coefficients well in line with those of many other nutrients estimated using FFQs (16). In addition, FFQs are valid tools to rank participants according to nutritional intake but are not designed to estimate absolute intakes. Therefore, we can only use our data to show the shape of associations, but exact amounts for a threshold cannot be determined.
Second, as in any observational study, our results could be influenced in part by differences in factors other than vitamin K intake. Phylloquinone and menaquinones intakes are associated with different, almost opposite, lifestyle behaviors. Vegetable intake contributes mostly to phylloquinone intake and is associated with a healthy lifestyle. Residual confounding in this case could therefore lead to an inverse association between phylloquinone intake and type 2 diabetes. In contrast, consumption of meat, milk products, and cheese contributes to menaquinones intake and is related to an unhealthier lifestyle and higher risk of type 2 diabetes. Residual confounding in this case would therefore attenuate our results for menaquinones intake. Although we simultaneously adjusted for several diabetes risk factors and these dietary factors in our analysis, residual confounding may be present.
Finally, relations between phylloquinone and menaquinones intakes and risk markers were explored cross-sectionally in a baseline random sample. These results should therefore be regarded as hypothesis-generating to explain the relation with type 2 diabetes and not as a causal relation.
To the best of our knowledge, this is the first study to investigate the relation between vitamin K intake and risk of type 2 diabetes. Two previous studies have investigated the relation between phylloquinone intake and insulin sensitivity or glycemic markers. An observational study showed an inverse relation of phylloquinone intake with 2-h glucose concentrations and insulin sensitivity among 2,719 women (9). A subsequent trial among nondiabetic men and women showed reduced progression of insulin resistance after 3 years of supplementation with 500 μg daily among men only (10). In this large population of Dutch men and women, phylloquinone intake was associated with a reduced risk of type 2 diabetes particularly at higher intakes, in line with these previous studies. For menaquinones intake, we observed a linear, inverse association with type 2 diabetes. No previous studies have studied the relation of menaquinones intake with insulin sensitivity or markers of glycemic status. The different shape of the associations of phylloquinone and menaquinones may be due to the lower ranges of intake of menaquinones compared with that of phylloquinone. Sensitivity of FFQs to differentiate at higher levels of intake may also be involved, because a plateau effect in the relation between plasma phylloquinone and phylloquinone intake from an FFQ has been reported previously (22).
On the other hand, the differences may also be explained by differences in distribution and metabolism of both forms. Because phylloquinone is transported with the triacylglycerol-rich fraction and menaquinones are transported both by triacylglycerol-rich lipoprotein and LDL, phylloquinone is more effectively cleared from the circulation by the liver for activation of clotting factors than menaquinones (23). An intervention study indeed suggested that MK7 more effectively carboxylated osteocalcin than phylloquinone (24). However, because both forms have the same function in carboxylation of proteins, both should have similar effects that could only differ in dosage. More research is, however, needed to assess whether differences are indeed present between both forms and at what dosage.
The underlying mechanism for the relation between vitamin K intake and type 2 diabetes is unknown, but several pathways may be involved. First, vitamin K could influence insulin sensitivity and risk of type 2 diabetes by carboxylating osteocalcin. Osteocalcin was suggested to function as a hormone in energy metabolism, regulating insulin sensitivity through an effect on adiponectin (4). However, only the uncarboxylated form seems to function hormonally in energy metabolism (4), making it unlikely to explain effects through carboxylation by vitamin K.
Osteocalcin also functions as a regulator of bone mineral maturation by binding calcium (3). Because both calcium and vitamin D insufficiency are consistently associated with an increased risk of type 2 diabetes (25), vitamin K could reduce insulin resistance and risk of type 2 diabetes through effects on calcium metabolism. However, adjustment for calcium intake and vitamin D in our study did not affect the associations. Finally, preliminary studies have shown that vitamin K influences other diabetes risk factors. Both in vitro (13) and observational (14) studies showed that vitamin K intake may decrease inflammation, which could also improve insulin sensitivity. Previous studies showed that menaquinones supplementation improved the blood lipid profile (12), whereas phylloquinone supplementation increased triglyceride and decreased HDL cholesterol concentrations after 6 weeks (11). These studies are confirmed by our exploratory results with risk markers and could explain the inverse relation between dietary menaquinones and type 2 diabetes.
In summary, the findings of this study show that both phylloquinone and menaquinones intakes may be associated with a reduced risk of type 2 diabetes. For phylloquinone intake, these risk reductions occurred at higher levels of intake, whereas for menaquinones a linear inverse association was observed.
Acknowledgments
The EPIC-NL study was funded by “Europe against Cancer” Programme of the European Commission, the Dutch Ministry of Public Health, Welfare and Sports (formerly Ministry of Welfare, Public Health and Culture), the Dutch Cancer Society, ZonMW, the Netherlands Organisation for Health Research and Development, and the World Cancer Research Fund (WCRF). J.W.J.B. is supported by a personal Dr. Dekker postdoctoral grant (2008T062) from the Netherlands Heart Foundation.
No potential conflicts of interest relevant to this article were reported.
J.W.J.B., D.E.G., and Y.T.S. designed the study. J.W.J.B., D.L.A., D.E.G., I.S., A.M.W.S., and Y.T.S. acquired data. J.W.J.B. analyzed data. J.W.J.B., D.L.A., D.E.G., I.S., A.M.W.S., and Y.T.S. interpreted data. J.W.J.B. wrote the manuscript. J.W.J.B., D.L.A., D.E.G., I.S., A.M.W.S., and Y.T.S. reviewed/edited the manuscript.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Bolton-Smith C, Price RJ, Fenton ST, Harrington DJ, Shearer MJ: Compilation of a provisional UK database for the phylloquinone (vitamin K1) content of foods. Br J Nutr 2000;83:389–399 [PubMed] [Google Scholar]
- 2. Schurgers LJ, Vermeer C: Determination of phylloquinone and menaquinones in food. Effect of food matrix on circulating vitamin K concentrations Haemostasis 2000;30:298–307 [DOI] [PubMed] [Google Scholar]
- 3. Cranenburg EC, Schurgers LJ, Vermeer C., Vitamin K: The coagulation vitamin that became omnipotent. Thromb Haemost 2007;98:120–125 [PubMed] [Google Scholar]
- 4. Ferron M, Hinoi E, Karsenty G, Ducy P: Osteocalcin differentially regulates β cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci USA 2008;105:5266–5270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pittas AG, Harris SS, Eliades M, Stark P, Dawson-Hughes B: Association between serum osteocalcin and markers of metabolic phenotype. J Clin Endocrinol Metab 2009;94:827–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shea MK, Gundberg CM, Meigs JB, Dallal GE, Saltzman E, Yoshida M, Jacques PF, Booth SL: γ-Carboxylation of osteocalcin and insulin resistance in older men and women. Am J Clin Nutr 2009;90:1230–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sakamoto N, Wakabayashi I, Sakamoto K: Low vitamin K intake effects on glucose tolerance in rats. Int J Vitam Nutr Res 1999;69:27–31 [DOI] [PubMed] [Google Scholar]
- 8. Sakamoto N, Nishiike T, Iguchi H, Sakamoto K: Possible effects of one week vitamin K (menaquinone-4) tablets intake on glucose tolerance in healthy young male volunteers with different descarboxy prothrombin levels. Clin Nutr 2000;19:259–263 [DOI] [PubMed] [Google Scholar]
- 9. Yoshida M, Booth SL, Meigs JB, Saltzman E, Jacques PF: Phylloquinone intake, insulin sensitivity, and glycemic status in men and women. Am J Clin Nutr 2008;88:210–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yoshida M, Jacques PF, Meigs JB, Saltzman E, Shea MK, Gundberg C, Dawson-Hughes B, Dallal G, Booth SL: Effect of vitamin K supplementation on insulin resistance in older men and women. Diabetes Care 2008;31:2092–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kristensen M, Kudsk J, Bügel S: Six weeks phylloquinone supplementation produces undesirable effects on blood lipids with no changes in inflammatory and fibrinolytic markers in postmenopausal women. Eur J Nutr 2008;47:375–379 [DOI] [PubMed] [Google Scholar]
- 12. Nagasawa Y, Fujii M, Kajimoto Y, Imai E, Hori M: Vitamin K2 and serum cholesterol in patients on continuous ambulatory peritoneal dialysis. Lancet 1998;351:724. [DOI] [PubMed] [Google Scholar]
- 13. Reddi K, Henderson B, Meghji S, Wilson M, Poole S, Hopper C, Harris M, Hodges SJ: Interleukin 6 production by lipopolysaccharide-stimulated human fibroblasts is potently inhibited by naphthoquinone (vitamin K) compounds. Cytokine 1995;7:287–290 [DOI] [PubMed] [Google Scholar]
- 14. Shea MK, Booth SL, Massaro JM, Jacques PF, D'Agostino RB, Sr, Dawson-Hughes B, Ordovas JM, O'Donnell CJ, Kathiresan S, Keaney JF, Jr, Vasan RS, Benjamin EJ: Vitamin K and vitamin D status: associations with inflammatory markers in the Framingham Offspring Study. Am J Epidemiol 2008;167:313–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Beulens JW, Monninkhof EM, Verschuren WM, van der Schouw YT, Smit J, Ocke MC, Jansen EH, van DS, Grobbee DE, Peeters PH, Bueno-de-Mesquita HB: Cohort profile: the EPIC-NL study. Int J Epidemiol 8 July 2009. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16. Ocke MC, Bueno-de-Mesquita HB, Pols MA, Smit HA, Van Staveren WA, Kromhout D: The Dutch EPIC food frequency questionnaire. II. Relative validity and reproducibility for nutrients. Int J Epidemiol 1997;26(Suppl. 1):S49–S58 [DOI] [PubMed] [Google Scholar]
- 17. Beulens JW, Bots ML, Atsma F, Bartelink ML, Prokop M, Geleijnse JM, Witteman JC, Grobbee DE, van der Schouw YT: High dietary menaquinone intake is associated with reduced coronary calcification. Atherosclerosis 2009;203:489–493 [DOI] [PubMed] [Google Scholar]
- 18. Booth SL, Sadowski JA, Weihrauch JL, Ferland G: Vitamin K-1 (phylloquinone) content of foods: a provisional table. J Food Comp Anal 1993;6:109–120 [Google Scholar]
- 19. Shearer MJ, Bach A, Kohlmeier M: Chemistry, nutritional sources, tissue distribution and metabolism of vitamin K with special reference to bone health. J Nutr 1996;126:1181S–1186S [DOI] [PubMed] [Google Scholar]
- 20. Wareham NJ, Jakes RW, Rennie KL, Schuit J, Mitchell J, Hennings S, Day NE: Validity and repeatability of a simple index derived from the short physical activity questionnaire used in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Public Health Nutr 2003;6:407–413 [DOI] [PubMed] [Google Scholar]
- 21. Durrleman S, Simon R: Flexible regression models with cubic splines. Stat Med 1989;8:551–561 [DOI] [PubMed] [Google Scholar]
- 22. McKeown NM, Jacques PF, Gundberg CM, Peterson JW, Tucker KL, Kiel DP, Wilson PW, Booth SL: Dietary and nondietary determinants of vitamin K biochemical measures in men and women. J Nutr 2002;132:1329–1334 [DOI] [PubMed] [Google Scholar]
- 23. Schurgers LJ, Vermeer C: Differential lipoprotein transport pathways of K-vitamins in healthy subjects. Biochim Biophys Acta 2002;1570:27–32 [DOI] [PubMed] [Google Scholar]
- 24. Schurgers LJ, Teunissen KJ, Hamulyák K, Knapen MH, Vik H, Vermeer C: Vitamin K-containing dietary supplements: comparison of synthetic vitamin K1 and natto-derived menaquinone-7. Blood 2007;109:3279–3283 [DOI] [PubMed] [Google Scholar]
- 25. Pittas AG, Lau J, Hu FB, Dawson-Hughes B: The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab 2007;92:2017–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]