Abstract
OBJECTIVE
Compare GHb among people with diabetes who have and have not received periodontal care.
RESEARCH DESIGN AND METHODS
This cross-sectional study linked 5 years of electronic medical record and dental insurance data for dually insured patients with diabetes, ages 40–70 years (n = 5,103). We assessed the association between annual mean GHb (%) and periodontal care (a proxy for periodontitis) defined using claim codes. Among patients who received periodontal care, we assessed the association between GHb and periodontal treatment intensity. We determined associations using linear regression adjusted for potential confounders and tested for effect modification by age, sex, insulin use, diabetes severity, BMI, and smoking.
RESULTS
Mean GHb was 7.66%; 38% of participants received periodontal care during the 5 years. After multivariate adjustment, patients who received periodontal care had a GHb level 0.08 percentage points higher than patients who did not (P = 0.02). In stratified analyses, the association was present for women (0.18 percentage points higher GHb with periodontal care, P < 0.001) but not significant for men (0.008 percentage points lower, P = 0.86). In patients who received periodontal care, those with one, and with two or more, surgical treatments had GHb 0.25 (P = 0.04) and 0.36 (P = 0.002) percentage points lower, respectively, than patients without periodontal surgeries.
CONCLUSIONS
This population-based cross-sectional study showed small associations between periodontal care (a proxy for periodontitis) and higher GHb. Well-controlled longitudinal studies or clinical trials are needed to evaluate causality and temporal trends. Sub-analyses suggest that further investigation of this association among women, and by intensity of periodontal treatment, may be of interest.
An estimated 23.6 million Americans have diabetes, and the prevalence is increasing. Periodontal disease may have a systemic effect that could worsen glycemic control. Reports have linked periodontitis to higher plasma levels of C-reactive protein (1), interleukin-6 (2), and tumor necrosis factor-α (2); these factors have been associated with insulin resistance, potentially worsening glycemic control (3,4). Some investigators suggest this association may be due to confounding by shared causal factors such as an unhealthy diet or smoking (5,6).
Observational studies have assessed the association between periodontal disease and GHb in patients with type 2 diabetes. Most (7–13) but not all (14) studies suggest an association with higher GHb. These studies were typically small and were often performed in patient groups subject to selection biases. The impact of periodontal treatment on glycemic control is controversial, with two meta-analyses reporting conflicting results (15,16), reflecting the biases that may plague these small studies.
We sought to examine these associations in a large population-based cross-sectional study. Our primary hypothesis was that GHb levels would be higher in participants with claims for periodontal care (a proxy for periodontitis). A secondary hypothesis was that, in patients who received periodontal care, GHb levels would be lower in patients who received higher-intensity treatment.
RESEARCH DESIGN AND METHODS
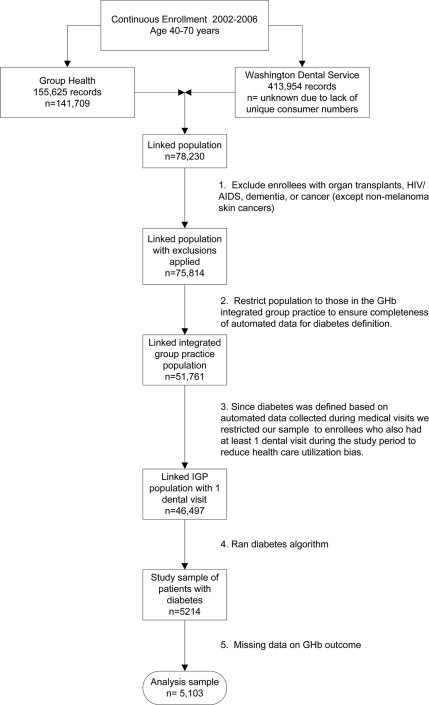
Data were extracted from automated dental and medical databases. Instead of the usual 1-year study period, we used 5 years, to ensure adequate time for participants to receive both dental and medical care. Figure 1 shows the inclusion and exclusion criteria. We used four identifiers to link medical and dental data for individuals aged 40–70 years who were continuously insured from 2002 to 2006 by both the Washington Dental Service (a dental insurer) and Group Health Cooperative (an integrated health care system) in Washington state. These procedures are described elsewhere (17).
Figure 1.
Flow diagram showing study inclusion and exclusion criteria.
Diabetes was defined as follows: two fasting glucose levels ≥126 mg/dl, two nonfasting glucose levels ≥200 mg/dl, or one of each within 12 months; GHb ≥7.0%; any filled prescription for insulin or oral diabetic agents; or one inpatient or two outpatient diabetes diagnoses. We excluded patients with gestational and secondary diabetes (18). The population with diabetes included both types 1 and type 2 diabetes; but given the age-specific prevalence, most probably have type 2 diabetes (18). Group Health's institutional review board approved all procedures.
Our exposure variable, periodontal care, was defined by identifying any dental claim submitted with at least one Current Dental Terminology (CDT) periodontal procedure code during the 5-year study period. The CDT codes taken as evidence of periodontal disease included periodontal maintenance (D4910), gingival (D4240, D4241) and apically (D4245) positioned flaps, osseous surgery (D4260, D4261), bone replacement graft (D4263, D4264), tissue regeneration with biologic materials (D4265), guided tissue regeneration (D4266, D4267), periodontal scaling/root planing (D4341, D4342, D4345), full mouth debridement (D4355), and localized antimicrobial delivery (D4381). Periodontal treatment intensity was defined by two variables: 1) the occurrence and frequency of periodontal surgeries (D4240, D4241, D4245, D4260, D4261, D4263, D4264, D4265, D4266, D4267) (0, 1, ≥ 2) and 2) an indicator (0/1) of whether the total number of nonsurgical periodontal claims (D4342, D4345, D4355, D4381, D4910) was above the median (7). These exposure variables were assigned once during the 5-year period.
Potential confounders, extracted from the medical databases, included age, sex, and medical insurance type in 2002. Smoking status, routinely collected at medical visits, was defined as nonsmoker if clinical staff recorded patients as nonsmokers on 90% or more of their visits during the 5-year period, continuous smokers if >90% of visits were labeled as smoking, and intermittent smokers for the remainder. BMI recorded during clinical visits was calculated using the median of the first three measurements during the 5-year period and categorized (≤24.9, 25–29.9, 30–39.9, and ≥40). To control for potential utilization biases, we included markers of medical care use (number of primary care/urgent visits [square root], number of specialty visits [square root], number of emergency visits [0, 1, ≥2], and the number of GHb tests performed), and surrogate markers of preventive health care–seeking behaviors (number of preventive [well-care] visits [square root] and number of retinal eye exams [square root]). We used RxRisk scores to control for chronic disease comorbidity at the beginning of the study (2002) (19). This score is based on an individual's age, sex, insurance status, and chronic condition profile measured by outpatient pharmacy dispensing. Using pharmacy records, we classified diabetes treatment intensity during the 5-year period as “no diabetes medications,” “oral hypoglycemic only,” and “any use of insulin.” We quantified diabetes severity using the 11-point Diabetes Complication Severity Index (0, 1, 1+) (20).
We used Pearson χ2 for categorical variables and ANOVA F tests for continuous variables to test differences in percentages and means of population characteristics by periodontal care status.
The outcome variable, mean GHb (%), was calculated for each study year. While the distribution of annual mean GHb (%) was right skewed, log transformation yielded similar results, and we elected to use the untransformed mean to ease interpretation. Because an individual can have up to five annual GHb means (2002–2006), we used generalized estimating equations with an independence working correlation structure to account for within-person correlation. Some covariates had extremely high values and were either categorized or square-root transformed to reduce their influence on the overall fit. Linear regression models with robust standard errors (regress command in STATA release 10) were used to model annual mean GHb as a function of single measures of periodontal care and periodontal treatment intensity within the 5-year period.
Our models were developed by first including age and sex and then testing the effect of additional covariates. All variables potentially related to GHb were then included to establish their association. Nonsignificant variables were removed one at a time (in decreasing P value order) until only significant variables remained. BMI and the exposure variables were included. Violations of the regression assumptions were checked in the final model using a residual-versus-fitted plot. Variance inflation factors were assessed to check for the presence of multicollinearity.
In exploratory multivariate analysis, we tested for interactions separately between periodontal care and each of the following variables: diabetes complication severity, age (40–49 or 50–70 years), sex, any insulin use (2002–2006), BMI, and smoking status (2002–2006). We tested for effect modification by BMI and smoking based on the hypothesis that inflammation associated with obesity or smoking may obscure the association between periodontitis and glycemic control. We hypothesized that the association between periodontitis and glycemic control may differ by diabetes severity (treatment, complication) because glycemic control in more advanced disease may resist influence. We stratified by age based on findings from a previous study (13).
RESULTS
During the study period (2002–2006), the mean GHb for our dual-insured population with diabetes (n = 5,103) was 7.66%, the average age was 55 years, and 38% received periodontal care (Table 1). Compared with participants without periodontal care, those with periodontal care were younger, used ambulatory services less, and were more likely to smoke, have lower BMI, and be men or be on government-based insurance.
Table 1.
Select characteristics by periodontitis status of our analysis sample (n = 5,103) of a continuously enrolled diabetic population, age 40–70 years, with medical and dental insurance and at least one dental and one medical visit during the study years 2002–2006
| Total | No periodontal care | Periodontal care | P * | |
|---|---|---|---|---|
| n | 5,103 | 3,153 | 1,950 | — |
| Average 5-year GHb % | 7.66 ± 1.3 | 7.62 ± 1.3 | 7.72 ± 1.4 | 0.01 |
| Average number of GHb tests/5 years | 7.56 ± 4.4 | 7.62 ± 4.4 | 7.46 ± 4.3 | 0.19 |
| Age (years) | ||||
| 40–49 (n = 1,308) | 26 | 25 | 26 | 0.01 |
| 50–59 (n = 2,466) | 48 | 47 | 50 | |
| 60–70 (n = 1,329) | 26 | 28 | 24 | |
| Sex (female) | 45 | 48 | 40 | <0.001 |
| Smoking status during 5-year study period†‡ | ||||
| Nonsmoker | 86 | 87 | 83 | <0.001 |
| Intermittent smoker | 9 | 8 | 10 | |
| Continuous smoker | 5 | 5 | 6 | |
| Preventive service use | ||||
| Average number well-care visits/5 years | 1.10 ± 1.1 | 1.10 ± 1.1 | 1.09 ± 1.1 | 0.65 |
| Average number retinal eye exams/5 years | 4.04 ± 4.2 | 4.13 ± 4.2 | 3.91 ± 4.2 | 0.08 |
| Ambulatory service use | ||||
| Average primary care visits/5 years | 21.90 ± 16.6 | 22.65 ± 17.4 | 20.70 ± 15.2 | <0.001 |
| Average specialty visits/5 years | 11.99 ± 11.8 | 12.47 ± 12.2 | 11.21 ± 11.2 | <0.001 |
| Insurance type 2002‡ | <0.001 | |||
| Medicare | 12 | 14 | 10 | |
| Individual | 2 | 2 | 2 | |
| Commercial | 24 | 24 | 26 | |
| Government | 61 | 60 | 63 | |
| Other | 0 | 0 | 0 | |
| Diabetes Complication Severity Index 2002–2003 | ||||
| 0:0 | 49 | 49 | 49 | 0.1 |
| 1:1 | 23 | 23 | 25 | |
| 2:>1 | 28 | 28 | 26 | |
| Diabetes treatment intensity 2002–2006 | 0.93 | |||
| 0:No hypoglycemic medication | 23 | 22 | 23 | |
| 1:Oral hypoglycemics only | 46 | 46 | 46 | |
| 2:Any insulin | 31 | 32 | 31 | |
| RxRisk (comorbidity score) 2002† | ||||
| 201–1,440 | 25 | 24 | 27 | 0.004 |
| 1,441–2,750 | 25 | 25 | 25 | |
| 2,751–4,560 | 25 | 25 | 25 | |
| ≥4,561 | 25 | 26 | 23 | |
| Average median BMI (first three measurements in 2002–2006)† | 33.72 ± 7.3 | 33.95 ± 7.4 | 33.36 ± 7.0 | 0.006 |
Data are means ± SD or percent unless otherwise indicated.
*We used Pearson χ2 for categorical variables and ANOVA F tests for continuous variables to test differences in percents and means of population characteristics by periodontitis status.
†Counts for missing data: smoking n = 2, RxRisk n = 2, BMI n = 187 (3.6%).
‡Percentages do not add up to 100 because of rounding.
As hypothesized, multivariate analyses linked periodontal care (a proxy for periodontitis) with higher GHb levels. In the unadjusted model, annual mean GHb was 0.11 percentage points higher for people with diabetes who received periodontal care than for those receiving no periodontal care (P = 0.005) (Table 2). The magnitude of the association decreased but remained statistically significant after controlling for age, BMI, and sex (GHb 0.09 percentage points higher; P = 0.02) as well as other variables related to diabetes control, including comorbidity, smoking, medical utilization (primary care visits, specialty care visits, and GHb tests), and number of preventive well-care visits (0.08 percentage points higher; P = 0.04). The association was independent of diabetes severity (0.08 percentage points higher; P = 0.02).
Table 2.
Multivariate linear regression analysis: annual mean GHb modeled as a function of periodontitis status in diabetic patients with medical and dental insurance, age 40–77 years, and at least one medical and dental visit during the 5-year study period
| n | Difference in mean GHb among patients who did and did not receive periodontal care | P | Lower CI | Upper CI | |
|---|---|---|---|---|---|
| Unadjusted | 5,103 | 0.11 | 0.005 | 0.03 | 0.19 |
| Age, BMI, and sex adjusted | 5,102 | 0.09 | 0.02 | 0.01 | 0.17 |
| Multiple adjustment* | 5,099 | 0.08 | 0.04 | 0.005 | 0.16 |
| Multiple adjustment and controlling for severity of diabetes† | 5,099 | 0.08 | 0.02 | 0.01 | 0.15 |
*Covariates included in model: age, BMI, sex, RxRisk in 2002, smoking status, number of primary care visits (square root), number of specialty care visits (square root), number of well visits (square root), and number of GHb tests (square root).
†In addition to covariates mentioned above, this model also included diabetes treatment level (no medication for diabetes, oral glycemic medication only, or any insulin) and Diabetes Complication Severity Index variables.
The periodontitis-GHb association was similar within categories of smoking status, BMI, and insulin use. However, the magnitude of the association appeared greater in women than men (interaction term P = 0.002) (Table 3). There was also suggestion that the magnitude of the association may be greater at younger ages (40–49 years old).
Table 3.
Stratified analysis: annual mean GHb modeled as a function of periodontitis status in multivariate linear regression models in diabetic patients, age 40–70 years, with medical and dental insurance and at least one medical and dental visit during the 5-year study period
| Stratification variable* | n | Difference in GHb among patients who did and did not receive periodontal care | Lower CI | Upper CI | Interaction P value |
|---|---|---|---|---|---|
| Sex | 0.002 | ||||
| Female | 2,261 | 0.18 | 0.08 | 0.28 | |
| Male | 2,564 | −0.01 | −0.10 | 0.08 | |
| Age-group (years)† | 0.10 | ||||
| 40–49 | 1,223 | 0.20 | 0.04 | 0.36 | |
| 50–70 | 3,602 | 0.04 | −0.03 | 0.11 | |
| Diabetes Complication Severity Index | 0.20 | ||||
| 0 | 2,466 | 0.01 | −0.08 | 0.10 | |
| 1 | 1,208 | 0.16 | 0.03 | 0.29 | |
| ≥2 | 1,425 | 0.09 | −0.04 | 0.23 | |
| BMI (kg/m2) | 0.36 | ||||
| ≤24.9 | 410 | 0.18 | −0.06 | 0.41 | |
| 25–29.9 | 1,180 | 0.09 | −0.04 | 0.21 | |
| 30–39.9 | 2,363 | 0.10 | 0.004 | 0.20 | |
| ≥40 | 872 | −0.06 | −0.24 | 0.12 | |
| Insulin use | 0.47 | ||||
| No insulin use | 3,297 | 0.04 | −0.04 | 0.11 | |
| Insulin use | 1,528 | 0.12 | −0.014 | 0.25 | |
| Smoking | 0.34 | ||||
| Nonsmoker | 4,145 | 0.10 | 0.03 | 0.17 | |
| Intermittent smoker | 434 | −0.06 | −0.28 | 0.17 | |
| Continuous smoker | 246 | −0.01 | −0.37 | 0.36 |
*Covariates included in models: age, BMI, sex, RxRisk in 2002, smoking status, number of primary care visits (square root), number of specialty care visits (square root), number of well visits (square root), number of A1C tests (square root), treatment level (no medication for diabetes, oral glycemic medication only, or any insulin), and Diabetes Complication Severity Index variables.
†Controlled for age within age-group.
Among diabetic patients with periodontal care (n = 1,950), 44% received more than seven nonsurgical periodontal services including periodontal maintenance, local antimicrobials, and other nonsurgical treatments. Surgical care was relatively uncommon; 93% had no periodontal surgeries, 4% had one, and 3% had two or more. As hypothesized, among individuals with periodontal care, those who received greater treatment intensity had lower GHb levels. In the adjusted model, people with more than the median number (7) of nonsurgical visits had GHb 0.13 percentage points lower than individuals with seven or fewer nonsurgical visits (95% CI −0.24 to −0.03; P = 0.01). Compared with people with no periodontal surgeries, those with 1 and 2 or more surgeries had GHb levels that were 0.25 (95% CI −0.49 to −0.01; P = 0.04) and 0.36 (95% CI −0.58 to −0.13; P = 0.002) percentage points lower, respectively. Similar findings were observed in analyses limited to nonsmokers. Among nonsmokers, people with more than the median number (7) of nonsurgical visits had GHb 0.14 percentage points lower than individuals with fewer visits (P = 0.02). Compared with people with no periodontal surgeries, those with one and two or more surgeries had GHb levels that were 0.26 (P = 0.05) and 0.29 (P = 0.006) percentage points lower, respectively.
CONCLUSIONS
In our cross-sectional study of 5,103 patients with diabetes, we observed a small association between higher GHb and receipt of any periodontal care provided by community dentists or periodontists, after accounting for confounders. The association between periodontal care and glycemic control can reflect either periodontitis itself or treatment for the disease. Treatment for periodontitis could worsen certain markers of glycemic control possibly linked to a short-term increase of inflammatory markers (21) or due to the increase in fasting plasma glucose that may happen upon resolution of certain infections. If periodontal care is a marker of periodontitis (which is our primary hypothesis), our results are consistent with a small body of observational evidence linking periodontitis and poor glycemic control in adjusted analyses. A Swedish population-based study of 179 participants with type 2 diabetes reported a 0.6% difference in GHb between patients with and without periodontitis defined by the percentage of teeth with 30% bone loss (9). One multivariate analysis found an association between GHb and probing depth but not attachment loss (11), while another linked GHb to attachment loss but not to probing depth (14). In a cohort study of 88 participants of Pima ancestry with type 2 diabetes, attachment loss was associated with a sixfold increase (95% CI 1.5–25) in the odds of GHb ≥9% after 2 years of follow-up (13).
These smaller studies found stronger associations between periodontitis and GHb than we found between periodontal care and GHb. This difference may relate to our use of periodontal care as a marker of periodontitis. However, we believe this assumption is reasonable for several reasons: 1) all participants were examined by a dentist; 2) in our prior validation analysis, the positive predictive value, sensitivity, and specificity were 84, 80, and 44%, respectively, when the periodontal care code set was compared with chart probing depth of ≥5 mm on two or more teeth in a subset with periodontal charts (22); 3) the proportion of patients receiving periodontal care in our study (38%) resembles the Centers for Disease Control and Prevention (CDC) estimate of 30% of people with diabetes having severe periodontal diseases; (23) and 4) when periodontal codes were categorized by intensity and evaluated in patients who received periodontal care, we saw slightly lower GHb among individuals who received higher treatment intensity. However, we could not identify people with periodontitis who did not receive periodontal care. This misclassification would have biased our results toward the null.
The smaller difference in GHb levels between individuals with and without periodontal care in our study may have been due to our ability to adjust more completely for important confounders. Our analyses adjusted for covariates that captured medical care utilization and preventive health behaviors, variables not included in other studies. However, we could not control for some adjusters used in other studies, including antibiotic use, number of teeth, race, socioeconomic status, and use of nonsteroidal anti-inflammatory medications. Although we could not control for these covariates, our analysis may have partially accounted for them because of the following: our cohort was primarily Caucasian (∼75%); most, due to local policy, were using statins, which have anti-inflammatory effects; and the population was dual medical/dental insured, which may reduce differences in socioeconomic status. No study, including our own, has controlled for nutritional status beyond BMI. If poor nutrition is associated with both increased periodontal care (as a marker for periodontitis) and increased GHb, our inability to control for potential confounding by nutritional status could bias our results toward finding an association when none exists.
Our stratified analysis suggested that the magnitude of the association between periodontal care and GHb may be greater in younger diabetic subjects (age 40–49 years); these results must be interpreted cautiously given the 0.10 significance of the interaction term. The study of people of Pima ancestry who had diabetes reported a similar finding. When periodontitis was defined based on bone loss, the association with GHb was observed only in patients age ≤35 years. However, since we could not control for number of teeth, another explanation is that we are not capturing periodontitis as well in older adults, who tend to need less periodontal treatment because they have lost more teeth.
In an exploratory analysis, we observed a higher magnitude of association between periodontal care and GHb level in women than in men. We may have less measurement error in GHb in women because of their higher health care use. Women, compared with men, had slightly higher mean number of GHb tests performed during the 5 years (7.9 vs. 7.5). Men and women did not differ in baseline or 5-year mean GHb levels, diabetes treatment intensity, or diabetes complication severity index. This finding is intriguing because some literature links increases in proinflammatory cytokines with declining estrogen in menopause (24), during which the average age is 51 years, close to our female population's average age of 54 years. Assuming similar measurement error and increased generalized inflammatory burden with each decade of women's age, we might expect to see a lower magnitude of association with increasing age, which our data suggested (age 40–49 years, 0.35 percentage points higher; age 50–59 years, 0.14 higher; age 60–70 years, 0.09 higher); however, the P value for the interaction term was not significant (P = 0.19). Additionally, a recent study, which used survey data and evaluated temporal sequence, reported a stronger association between periodontal disease and incident diabetes in women versus men (25). Our finding needs further corroboration.
In participants with diabetes who received periodontal care, we observed lower GHb with greater intensity of periodontal treatment, but the magnitude of this association was small. These results are consistent with a recent meta-analysis of nine small randomized controlled trials (485 individuals) supporting the idea that periodontal treatment improves glycemic control (15). An earlier meta-analysis of 10 intervention studies reported nonsignificant results (16). The relatively small magnitude of the association in our study may be due to our use of dental claims and the fact that all patients in this group received at least some periodontal care. Our measure of periodontal treatment intensity may have nondifferential measurement error, since it does not precisely measure the scope or type of treatment, which could bias our results. Or, the smaller effect may reflect differences in effectiveness of periodontal treatment delivered in experimental trials compared with periodontal care provided in the community.
Limitations are inherent to our study's design. As a cross-sectional study, it cannot establish causality or temporality. In addition, since this study is observational, residual confounding may explain the minimal difference in GHb between people with diabetes who did and did not receive periodontal care. However, this study does support using linked populations from medical and dental providers and the associated automated data to expand research on the association between oral health and other medical conditions.
In this study, based on medical care and dental claims data, we found a small positive association between periodontal care (a marker of periodontitis) and higher GHb. In sub-analyses, these findings were stronger among women than men, and we found that among those who were treated for periodontitis, more intense treatment was associated with lower GHb.
Acknowledgments
Washington Dental Service and Group Health Cooperative, employers of five of our coauthors, funded this study.
No other potential conflicts of interest relevant to this article were reported.
L.S., R.J.R., R.I., and K.M.N. researched the data; L.S., R.J.R., R.I., K.M.N., P.H., M.C., R.J.G., and W.E.B. contributed to discussion; L.S. wrote manuscript; and L.S., R.J.R., R.I., K.M.N., P.H., M.C., R.J.G., and W.E.B. reviewed/edited the manuscript.
Preliminary findings of this study were presented at the 2009 American Diabetes Association 69th Scientific Sessions.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Loos BG. Systemic markers of inflammation in periodontitis. J Periodontol 2005;76:2106–2115 [DOI] [PubMed] [Google Scholar]
- 2. Bretz WA, Weyant RJ, Corby PM, Ren D, Weissfeld L, Kritchevsky SB, Harris T, Kurella M, Satterfield S, Visser M, Newman AB. Systemic inflammatory markers, periodontal diseases, and periodontal infections in an elderly population. J Am Geriatr Soc 2005;53:1532–1527 [DOI] [PubMed] [Google Scholar]
- 3. Festa A, D'Agostino R, Jr, Howard G, Mykkänen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS). Circulation 2000;102:42–47 [DOI] [PubMed] [Google Scholar]
- 4. Donahue RP, Wu T. Insulin resistance and periodontal disease: an epidemiologic overview of research needs and future directions. Ann Periodontol 2001;6:119–124 [DOI] [PubMed] [Google Scholar]
- 5. Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 2007;298:2654–2664 [DOI] [PubMed] [Google Scholar]
- 6. Hujoel P. Dietary carbohydrates and dental-systemic diseases. J Dent Res 2009;88:490–502 [DOI] [PubMed] [Google Scholar]
- 7. Novaes AB, Jr, Gutierrez FG, Novaes AB. Periodontal disease progression in type II non-insulin-dependent diabetes mellitus patients (NIDDM). Part I–Probing pocket depth and clinical attachment. Braz Dent J 1996;7:65–73 [PubMed] [Google Scholar]
- 8. Collin HL, Uusitupa M, Niskanen L, Kontturi-Närhi V, Markkanen H, Koivisto AM, Meurman JH. Periodontal findings in elderly patients with non-insulin dependent diabetes mellitus. J Periodontol 1998;69:962–966 [DOI] [PubMed] [Google Scholar]
- 9. Jansson H, Lindholm E, Lindh C, Groop L, Bratthall G. Type 2 diabetes and risk for periodontal disease: a role for dental health awareness. J Clin Periodontol 2006;33:408–414 [DOI] [PubMed] [Google Scholar]
- 10. Guzman S, Karima M, Wang HY, Van Dyke TE. Association between interleukin-1 genotype and periodontal disease in a diabetic population. J Periodontol 2003;74:1183–1190 [DOI] [PubMed] [Google Scholar]
- 11. Tervonen T, Oliver RC. Long-term control of diabetes mellitus and periodontitis. J Clin Periodontol 1993;20:431–435 [DOI] [PubMed] [Google Scholar]
- 12. Cutler CW, Machen RL, Jotwani R, Iacopino AM. Heightened gingival inflammation and attachment loss in type 2 diabetics with hyperlipidemia. J Periodontol 1999;70:1313–1321 [DOI] [PubMed] [Google Scholar]
- 13. Taylor GW, Burt BA, Becker MP, Genco RJ, Shlossman M, Knowler WC, Pettitt DJ. Severe periodontitis and risk for poor glycemic control in patients with non- insulin-dependent diabetes mellitus. J Periodontol 1996;67:1085–1093 [DOI] [PubMed] [Google Scholar]
- 14. Bridges RB, Anderson JW, Saxe SR, Gregory K, Bridges SR. Periodontal status of diabetic and non-diabetic men: effects of smoking, glycemic control, and socioeconomic factors. J Periodontol 1996;67:1185–1192 [DOI] [PubMed] [Google Scholar]
- 15. Darré L, Vergnes JN, Gourdy P, Sixou M. Efficacy of periodontal treatment on glycaemic control in diabetic patients: a meta-analysis of interventional studies. Diabete Metab 2008;34:497–506 [DOI] [PubMed] [Google Scholar]
- 16. Janket SJ, Wightman A, Baird AE, Van Dyke TE, Jones JA. Does periodontal treatment improve glycemic control in diabetic patients? A meta-analysis of intervention studies. J Dent Res 2005;84:1154–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Theis MK, Reid RJ, Chaudhari M, Newton KM, Spangler L, Grossman DC, Inge RE. Case study of linking dental and medical healthcare records. Am J Manag Care 2010;16:e51–e56 [PubMed] [Google Scholar]
- 18. Newton KM, LaCroix AZ, Heckbert SR, Abraham L, McCulloch D, Barlow W. Estrogen therapy and risk of cardiovascular events among women with type 2 diabetes. Diabetes Care 2003;26:2810–2816 [DOI] [PubMed] [Google Scholar]
- 19. Fishman PA, Goodman MJ, Hornbrook MC, Meenan RT, Bachman DJ, O'Keeffe Rosetti MC. Risk adjustment using automated ambulatory pharmacy data: the RxRisk model. Med Care 2003;41:84–99 [DOI] [PubMed] [Google Scholar]
- 20. Young BA, Lin E, Von Korff M, Simon G, Ciechanowski P, Ludman EJ, Everson-Stewart S, Kinder L, Oliver M, Boyko EJ, Katon WJ. Diabetes complications severity index and risk of mortality, hospitalization, and healthcare utilization. Am J Manag Care 2008;14:15–23 [PMC free article] [PubMed] [Google Scholar]
- 21. Ide M, Jagdev D, Coward PY, Crook M, Barclay GR, Wilson RF. The short-term effects of treatment of chronic periodontitis on circulating levels of endotoxin, C-reactive protein, tumor necrosis factor-alpha, and interleukin-6. J Periodontol 2004;75:420–428 [DOI] [PubMed] [Google Scholar]
- 22. Spangler L, Chaudhari M, Barlow WE, Newton KM, Inge R, Hujoel P, Genco R, Reid RJ. Using administrative data for epidemiologic research: case study to identify persons with periodontitis. Periodontology (In press) [DOI] [PubMed] [Google Scholar]
- 23. National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2007. Available from http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2007.pdf. Accessed 18 July 2008
- 24. Pfeilschifter J, Köditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocr Rev 2002;23:90–119 [DOI] [PubMed] [Google Scholar]
- 25. Demmer RT, Jacobs DR, Jr, Desvarieux M. Periodontal disease and incident type 2 diabetes mellitus: results from the First National Health and Nutrition Examination Survey and its epidemiologic follow-up study. Diabetes Care 2008;1373–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]