Abstract
OBJECTIVE
Few prospective data exist on the risk of diabetes in individuals serving in the U.S. military. The objectives of this study were to determine whether military deployment, combat exposures, and mental health conditions were related to the risk of newly reported diabetes over 3 years.
RESEARCH DESIGN AND METHODS
Data were from Millennium Cohort Study participants who completed baseline (July 2001–June 2003) and follow-up (June 2004–February 2006) questionnaires (follow-up response rate = 71.4%). After exclusion criteria were applied, adjusted analyses included 44,754 participants (median age 36 years, range 18–68 years). Survey instruments collected demographics, height, weight, lifestyle, military service, clinician-diagnosed diabetes, and other physical and mental health conditions. Deployment was defined by U.S. Department of Defense databases, and combat exposure was assessed by self-report at follow-up. Odds of newly reported diabetes were estimated using logistic regression analysis.
RESULTS
Occurrence of diabetes during follow-up was 3 per 1,000 person-years. Individuals reporting diabetes at follow-up were significantly older, had greater baseline BMI, and were less likely to be Caucasian. After adjustment for age, sex, BMI, education, race/ethnicity, military service characteristics, and mental health conditions, only baseline posttraumatic stress disorder (PTSD) was significantly associated with risk of diabetes (odds ratio 2.07 [95% CI 1.31–3.29]). Deployments since September 2001 were not significantly related to higher diabetes risk, with or without combat exposure.
CONCLUSIONS
In this military cohort, PTSD symptoms at baseline but not other mental health symptoms or military deployment experience were significantly associated with future risk of self-reported diabetes.
The risk of type 2 diabetes continues to increase worldwide, and multiple risk factors for this condition have been identified. Few studies, though, have assessed risk in a younger working population or focused on U.S. military service members, a relatively healthy population that experiences unique occupational exposures, including military deployments. Furthermore, this population is at higher risk for psychiatric disorders such as depression and posttraumatic stress disorder (PTSD) (1,2) that may also be related to higher diabetes risk. Studies have reported an increase in risk of diabetes associated with depression (2,3), and cross-sectional studies have reported an association between PTSD and diabetes (4), although it is not possible without a prospective design to establish whether PTSD preceded or followed its development. Potential mechanisms linking depression and PTSD to diabetes might involve the stress response associated with these conditions contributing to inflammation and insulin resistance (5).
We examined the incidence of and risk factors for diabetes among U.S. service members participating in the Millennium Cohort Study, an ongoing prospective investigation of health outcomes associated with military service. We focused on unique exposures in this population related to deployment and the presence of mental health conditions as potential predictors for the development of new-onset diabetes in this cohort.
RESEARCH DESIGN AND METHODS
The Millennium Cohort Study enrolled its first panel of participants between July 2001 and June 2003. Those initially invited comprised an 11.3% stratified random sample of the 2.2 million men and women in service as of 1 October 2000. A total of 77,047 individuals completed the baseline survey, representing 36% of invited personnel. During the first follow-up survey between June 2004 and February 2006, 55,021 (71.4%) individuals completed the follow-up survey questionnaire.
The primary data for these analyses derive from self-administered surveys and linked Department of Defense (DoD) databases. The Naval Health Research Center conducted the survey independent of the service member's local command by contacting the study participant directly through postal mail or e-mail and not sharing results with the participant's local command. Information collected in the surveys included occurrence of diabetes, cigarette smoking, alcohol consumption, symptoms of depression, panic, and anxiety (via the Patient Health Questionnaire [PHQ]) (6), weight, height, and PTSD symptoms (via the PTSD Checklist-Civilian Version [PCL-C]) (7). The PCL-C is a 17-item self-report rating of PTSD symptoms during the past 30 days on a 5-point Likert scale, ranging from 1 (not at all) to 5 (extremely). PTSD symptoms at baseline were defined as a report of a moderate or higher level of at least one intrusion symptom, three avoidance symptoms, and two hyperarousal symptoms (criteria established by DSM-IV) (8). Other anxiety (6 items) and panic (15 items) symptoms were assessed with the PHQ instrument. Self-reported depression symptoms (9 items) were assessed using the PHQ (sensitivity = 0.93; specificity = 0.89) and correspond to the depression diagnosis based on the DSM-IV (9). Symptoms of depression at baseline were defined as 1) endorsement of depressed mood or anhedonia and 2) response of “more than half the days” or “nearly every day” to at least 5 of the 9 items. Problem drinking was defined as a positive response on ≥1 of 5 PHQ items pertaining to functional impairment due to alcohol or having received medical advice to cut down on drinking. Binge drinking was defined as consuming ≥5 drinks for men or ≥4 for women on at least 1 day in the past year. Nonsmokers were defined as not having smoked ≥100 cigarettes in their lifetime, past smokers as having smoked ≥100 cigarettes but not at baseline, and current smokers had smoked ≥100 cigarettes and were actively smoking at baseline. BMI was calculated from self-reported data as weight in kilograms divided by the square of height in meters (2).
The DoD Manpower Data Center in Seaside, California, provided information on demographic and occupational information as of 1 October 2000, when the sample was pulled, including sex, birth year, educational level, marital status, pay grade (enlisted or officer), race/ethnicity, service component (active duty or Reserve/Guard), service branch (Army, Air Force, Navy/Coast Guard, or Marine Corps), service occupation, and deployment experience in support of Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) between 2001 and 2004. We further categorized deployment status as deployed with combat exposure if a subject self-reported at least one combat experience in the past 3 years, including witnessing death, trauma, prisoners of war, or refugees.
Outcomes
Occurrence of self-reported diabetes at follow-up was assessed with the following question “In the last 3 years, has your doctor or other health care professional told you that you have any of the following conditions?” [A list of medical conditions followed that included “Diabetes or sugar diabetes (Yes/No).”] The aim of this analysis was to identify risk factors for type 2 diabetes. Therefore, DoD medical record databases were used to identify and exclude individuals who developed type 1 diabetes during follow-up as reflected by the appearance of ICD-CM codes 250.x1 or 250.x3. Survey data rather than medical encounter data were used as our primary sources for capturing new-onset diabetes because members of the Reserves and National Guard do not generally have access to the DoD health care system between periods of activation. Thus, instances of medical care and diagnoses may not be captured by a review of these databases.
Statistical analysis
We compared baseline characteristics and deployment of study subjects by the occurrence of self-reported diabetes at follow-up. Logistic regression analysis to estimate odds ratios (ORs), 95% CIs, and P values was conducted. Characteristics from Table 1 were then separately examined in logistic models that adjusted for known risk factors for diabetes available from the survey or DoD databases (age, sex, ethnicity, educational attainment, and BMI) (Table 2). A final multivariable model was developed using age, sex, ethnicity, educational attainment, and BMI; in addition, backward stepwise regression was used that initially included all variables from the models in Table 2 that were significant at P < 0.10 (Table 3). We assessed the presence of interaction between each psychiatric condition with sex and separation from service (separation assessed at follow-up). Variables were retained in the final model if P < 0.10 before the inclusion of interaction terms. Regression diagnostics included examining covariates for multicollinearity. We conducted all data analyses using SAS software (version 9.1.3; SAS Institute, Cary, NC).
Table 1.
Baseline characteristics among nondiabetic individuals by presence of new-onset diabetes at follow-up
| No | Yes | Unadjusted OR (95% CI)* | P value | |
|---|---|---|---|---|
| n | 44,378 | 376 | ||
| Sex | ||||
| Male | 31,895 (73.6) | 264 (72.3) | 1.00 | 0.7082 |
| Female | 11,450 (26.4) | 101 (27.7) | 1.04 (0.83–1.31) | |
| Race/ethnicity | ||||
| Caucasian | 31,127 (71.8) | 238 (65.2) | 1.00 | 0.0006 |
| African American | 5,042 (11.6) | 69 (18.9) | 1.81 (1.39–2.36) | |
| Hispanic | 2,493 (5.8) | 23 (6.3) | 1.29 (0.85–1.95) | |
| Asian | 3,748 (8.6) | 27 (7.4) | 1.03 (0.70–1.50) | |
| Other | 935 (2.2) | 8 (2.2) | 1.10 (0.54–2.23) | |
| Education | ||||
| No high school diploma | 2,236 (5.2) | 19 (5.2) | 1.00 | 0.0400 |
| High school diploma/equivalent | 16,292 (37.6) | 141 (38.6) | 1.04 (0.65–1.69) | |
| Some college | 11,497 (26.5) | 118 (32.3) | 1.26 (0.77–2.04) | |
| Bachelor's degree | 8,343 (19.2) | 56 (15.3) | 0.80 (0.47–1.35) | |
| Master's/postdoctoral degree | 4,977 (11.5) | 31 (8.5) | 0.83 (0.47–1.46) | |
| Continuous demographic variables | ||||
| Age (years) at baseline submission | 36.3 ± 8.9 | 41.7 ± 10.3 | 1.07 (1.05–1.08) | <.0001 |
| BMI | 26.0 ± 3.3 | 28.8 ± 3.9 | 1.22 (1.19–1.25) | <.0001 |
| Deployment before 2001 | ||||
| No previous deployments | 26,896 (62.1) | 239 (65.5) | 1.00 | 0.6983 |
| 1991 Gulf War only | 3,428 (7.9) | 29 (7.9) | 0.97 (0.66–1.42) | |
| Bosnia/Kosovo/Southwest Asia only | 10,797 (24.9) | 78 (21.4) | 0.87 (0.68–1.11) | |
| Both contingencies | 2,224 (5.1) | 19 (5.2) | 1.06 (0.67–1.65) | |
| Deployment to OEF/OIF before baseline | ||||
| No | 41,716 (96.2) | 356 (97.5) | 1.00 | 0.3902 |
| Yes | 1,629 (3.8) | 9 (2.5) | 0.77 (0.42–1.40) | |
| Deployed to OEF/OIF between baseline and follow-up | ||||
| Nondeployed | 33,624 (77.6) | 310 (84.9) | 1.00 | 0.0038 |
| Deployed without combat exposures | 4,908 (11.3) | 24 (6.6) | 0.54 (0.36–0.81) | |
| Deployed with combat exposures | 4,813 (11.1) | 31 (8.5) | 0.72 (0.50–1.03) | |
| Number of deployments between baseline and follow-up | ||||
| 0 | 33,624 (77.6) | 310 (84.9) | 1.00 | 0.0055 |
| 1 | 4,639 (10.7) | 31 (8.5) | 0.77 (0.54–1.10) | |
| 2 | 2,965 (6.8) | 11 (3.0) | 0.39 (0.21–0.72) | |
| 3 | 2,117 (4.9) | 13 (3.6) | 0.65 (0.37–1.14) | |
| Separated from military as of follow-up | ||||
| No | 37,504 (86.5) | 273 (74.8) | 1.00 | <.0001 |
| Yes | 5,841 (13.5) | 92 (25.2) | 2.20 (1.74–2.78) | |
| Branch of service | ||||
| Army | 19,980 (46.1) | 183 (50.1) | 1.00 | 0.0685 |
| Navy/Coast Guard | 8,177 (18.9) | 79 (21.6) | 1.05 (0.81–1.36) | |
| Marines | 1,843 (4.3) | 8 (2.2) | 0.52 (0.27–1.03) | |
| Air Force | 13,345 (30.8) | 95 (26.0) | 0.80 (0.63–1.03) | |
| Component | ||||
| Reserve/Guard | 19,214 (44.3) | 202 (55.3) | 1.00 | <.0001 |
| Active duty | 24,131 (55.7) | 163 (44.7) | 0.65 (0.53–0.79) | |
| Alcohol consumption | ||||
| None | 23,129 (53.4) | 235 (64.4) | 1.00 | 0.0007 |
| Problem drinking only | 297 (0.7) | 1 (0.3) | 0.64 (0.16–2.60) | |
| Binge drinking only | 15,505 (35.8) | 99 (27.1) | 0.63 (0.50–0.80) | |
| Both of the above | 4,414 (10.2) | 30 (8.2) | 0.68 (0.47–0.99) | |
| Smoking status | ||||
| Nonsmoker | 25,862 (59.7) | 208 (57.0) | 1.00 | 0.6771 |
| Past smoker | 10,898 (25.1) | 102 (27.9) | 1.11 (0.88–1.40) | |
| Current smoker | 6,585 (15.2) | 55 (15.1) | 1.00 (0.75–1.35) | |
| Major depressive disorder | ||||
| No | 42,209 (97.4) | 347 (95.1) | 1.00 | 0.0047 |
| Yes | 1,136 (2.6) | 18 (4.9) | 1.95 (1.23–3.11) | |
| Panic disorder | ||||
| No | 42,898 (99.0) | 353 (96.7) | 1.00 | <.0001 |
| Yes | 447 (1.0) | 12 (3.3) | 3.19 (1.78–5.71) | |
| Anxiety disorder | ||||
| No | 42,594 (98.3) | 353 (96.7) | 1.00 | 0.0130 |
| Yes | 751 (1.7) | 12 (3.3) | 2.03 (1.16–3.54) | |
| PTSD | ||||
| No | 41,781 (96.4) | 334 (91.5) | 1.00 | <.0001 |
| Yes | 1,564 (3.6) | 31 (8.5) | 2.56 (1.78–3.67) |
Data are n (%) or mean ± SD. N = 44,754.
*Bold values indicate statistical significance at P < 0.05.
Table 2.
Relative odds of incident self-reported diabetes by each baseline characteristic of interest adjusted for age, sex, race/ethnicity, education, and BMI
| Baseline characteristic | OR (95% CI)* | P value |
|---|---|---|
| Deployment before 2001 | 0.5839 | |
| No previous deployments | 1.00 | |
| 1991 Gulf War only | 0.78 (0.53–1.16) | |
| Bosnia/Kosovo/Southwest Asia only | 1.06 (0.82–1.38) | |
| Both contingencies | 0.98 (0.62–1.55) | |
| Deployment to OEF/OIF before baseline | 0.8744 | |
| No | 1.00 | |
| Yes | 0.95 (0.52–1.75) | |
| Deployed to OEF/OIF between baseline and follow-up | 0.1444 | |
| Nondeployed | 1.00 | |
| Deployed without combat exposures | 0.66 (0.44–1.00) | |
| Deployed with combat exposures | 0.99 (0.68–1.42) | |
| Number of deployments between baseline and follow-up | 0.2272 | |
| 0 | 1.00 | |
| 1 | 0.94 (0.66–1.34) | |
| 2 | 0.53 (0.29–0.97) | |
| 3 | 0.90 (0.51–1.59) | |
| Separated from military as of follow-up | <0.0001 | |
| No | 1.00 | |
| Yes | 1.86 (1.45–2.39) | |
| Branch of service | 0.4113 | |
| Army | 1.00 | |
| Navy/Coast Guard | 1.16 (0.88–1.52) | |
| Marines | 0.97 (0.49–1.93) | |
| Air Force | 0.87 (0.66–1.15) | |
| Component | 0.9435 | |
| Reserve/Guard | 1.00 | |
| Active duty | 0.99 (0.78–1.26) | |
| Alcohol consumption | 0.5004 | |
| None | 1.00 | |
| Problem drinking only | 0.72 (0.18–2.93) | |
| Binge drinking only | 0.83 (0.65–1.06) | |
| Both of the above | 0.92 (0.63–1.35) | |
| Smoking status | 0.3185 | |
| Nonsmoker | 1.00 | |
| Past smoker | 0.87 (0.68–1.11) | |
| Current smoker | 1.12 (0.82–1.51) | |
| Depressive disorder | 0.0838 | |
| No | 1.00 | |
| Yes | 1.54 (0.94–2.50) | |
| Panic disorder | 0.0005 | |
| No | 1.00 | |
| Yes | 2.88 (1.58–5.24) | |
| Other anxiety disorder | 0.0488 | |
| No | 1.00 | |
| Yes | 1.77 (1.00–3.13) | |
| PTSD | <0.0001 | |
| No | 1.00 | |
| Yes | 2.24 (1.54–3.26) |
N = 44,754.
*Bold values indicate statistical significance at P < 0.05.
Table 3.
Adjusted relative odds of incident self-reported diabetes
| Baseline characteristic* | OR (95% CI)† | P value |
|---|---|---|
| Age in years at baseline submission | 1.06 (1.05–1.08) | <0.001 |
| BMI | 1.20 (1.17–1.23) | <0.001 |
| Race/ethnicity | 0.016 | |
| Caucasian | 1.00 | |
| African American | 1.46 (1.10–1.94) | |
| Hispanic | 1.35 (0.89–2.05) | |
| Asian | 1.67 (1.11–2.52) | |
| Other | 1.07 (0.52–2.19) | |
| Education | 0.005 | |
| No high school diploma | 1.00 | |
| High school diploma or equivalent | 1.26 (0.77–2.06) | |
| Some college | 1.28 (0.78–2.11) | |
| Bachelor's degree | 0.84 (0.49–1.43) | |
| Master's/postdoctoral degree | 0.65 (0.36–1.19) | |
| Separated from military as of follow-up | <0.001 | |
| No | 1.00 | |
| Yes | 2.18 (1.61–2.93) | |
| Component | 0.034 | |
| Reserve/Guard | 1.00 | |
| Active duty | 0.74 (0.56–0.98) | |
| Panic disorder | 0.067 | |
| No | 1.00 | |
| Yes | 1.86 (0.96–3.60) | |
| PTSD | 0.002 | |
| No | 1.00 | |
| Yes | 2.07 (1.31–3.29) | |
| Depression and sex interaction | ||
| Nondepressed men | 1.00 | |
| Depressed men | 1.14 (0.60–2.15) | 0.695 |
| Nondepressed women | 1.63 (1.26–2.13) | <0.001 |
| Depressed women | 0.51 (0.15–1.70) | 0.273 |
N = 44,754.
*The multivariable model has been adjusted for each baseline characteristic listed in the table and also military occupation as shown in the supplementary Tables 1–3, available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc10-0296/DC1.
†Bold values indicate statistical significance at P < 0.05.
RESULTS
Among the 55,021 subjects who completed the 3-year follow-up survey, exclusions for this analysis were made for the following reasons: 1 person withdrew permission to use his or her data, 454 had missing responses to the diabetes question at baseline, 722 reported diabetes at baseline, 29 had type 1 diabetes during follow-up, 3,538 had missing data on any covariate, 3,284 deployed before the baseline survey, and 2,239 had missing diabetes response at follow-up. Among the 44,754 subjects available for this analysis, 376 reported developing diabetes within the 3-year follow-up period. A univariate comparison of baseline characteristics by diabetes status at follow-up revealed that individuals who developed diabetes were significantly older, had a higher BMI, and were more likely to be African American (Table 1). Certain military service characteristics differed by whether diabetes developed, including separation from military service since baseline, deployment without combat exposure, and active duty as opposed to Reserve/National Guard status (Table 1). Binge and/or problem drinking was less frequent at baseline among individuals who developed diabetes, whereas symptoms of all four psychiatric conditions were more common (Table 1).
Comparison of baseline characteristics by diabetes occurrence at follow-up adjusted for age, sex, ethnicity, education, and BMI reduced the magnitude of the associations between most exposures and odds of diabetes (Table 2). Statistically significant associations remained only with the following exposures: two deployments since baseline, separation from service, panic disorder, other anxiety disorder, and PTSD at baseline.
A multivariable model to predict newly reported diabetes was constructed, and assessment of interaction was conducted as described previously (Table 3). Only the interaction between sex and depression was significant (P = 0.048). Results for the association between odds of diabetes and depression are shown stratified by sex (Table 3). Significant interactions were not found between having separated from service and symptoms of psychiatric conditions.
The final multivariable model included the variables shown in Table 3. Older age or larger BMI, non-Caucasian ethnicity, having separated from the military, and PTSD were significantly associated with an increased odds of incident diabetes. The OR for panic disorder remained elevated but was no longer statistically significant. Higher educational level and being on active duty were associated with a lower odds of diabetes. The interaction between sex and depression revealed that depressed men and women did not have a significantly higher odds of diabetes compared with nondepressed men (Table 3). Nondepressed women, though, had higher odds of diabetes than nondepressed men.
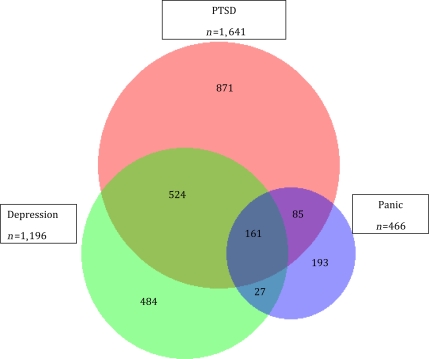
The Venn diagram displays the co-occurrence of symptoms of PTSD, depression, and panic in this population (Fig. 1). There is moderate overlap of these conditions. The majority of individuals who met the criteria for symptoms of depression also were found to have PTSD symptoms (57%). A smaller proportion of individuals with PTSD symptoms had coexisting symptoms of depression (42%).
Figure 1.
Co-occurrence of PTSD, panic, and depression among subjects at baseline.
CONCLUSIONS
PTSD symptoms were independently related to the occurrence of self-reported diabetes in this population. All psychiatric conditions considered were associated with a higher risk in unadjusted analyses (Table 1). The association with depression was diminished in magnitude and no longer statistically significant after adjustment for baseline factors commonly associated with type 2 diabetes (Table 2). Only PTSD symptoms remained strongly and significantly related to diabetes occurrence in a multivariable model that considered all psychiatric conditions and characteristics of military service (Table 3).
Several features of military service were also associated with diabetes risk, including Reserve status and having separated from the military between baseline and follow-up. Active-duty service may be associated with higher energy expenditure that would be expected to lower diabetes risk. We could not assess this factor directly because we did not measure physical activity at the baseline survey. The reasons for the association between separation from military service and higher diabetes risk are also unknown but might plausibly reflect medical discharge due to uncontrolled diabetes, failure to reenlist due to declining health, or higher diagnostic intensity associated with the process of being discharged from military service.
Deployment without combat exposure was associated with a lower risk of diabetes in unadjusted analyses compared with nondeployment, but this association was only borderline significant after adjustment for known diabetes risk factors (Table 2). This lower risk is probably due to the screening process used to select deployers, an aim of which is to exclude individuals who do not meet medical and/or fitness criteria for this assignment (10). A 14-year follow-up survey of 1991 Gulf War era veterans did not find a statistically significant difference in prevalence of diabetes associated with deployment in this conflict (11).
Research on the biological correlates of PTSD and diabetes revealed some intriguing similarities and differences. Common findings include higher levels of inflammatory markers and endothelial dysfunction (12,13). Neuroanatomical similarities have been reported, with lower hippocampal volumes demonstrated in relation to PTSD and diabetes (14,15). The hippocampus plays an important role in learning and memory and may also regulate food intake (16). Whether these brain-imaging findings precede or follow the development of diabetes and PTSD is not known. Abnormalities of the hypothalamic-pituitary-adrenal axis have been reported in both pre-diabetes and diabetes, as demonstrated by excess cortisol production (17). Björntorp et al. (17) proposed that stressful experiences activate the hypothalamic-pituitary axis, leading to excessive cortisol production, central body fat distribution, insulin resistance, and associated metabolic abnormalities. Some but not all investigations of cortisol production in individuals with PTSD, though, have reported paradoxically low cortisol levels compared with those in control subjects (18). Individuals with PTSD frequently have sleep disorders and shorter sleep duration, both of which have been found to be risk factors for type 2 diabetes (19,20). We could not address this issue directly because the survey did not include questions specifically designed to address sleep quality or duration.
In contrast to our findings, a meta-analysis of multiple observational studies has reported a higher risk of diabetes associated with depression (2). In common with PTSD, depression is associated with hippocampal atrophy and sleep disorders but differs in that higher cortisol production is often observed (21,22). No significant association between depression and the new onset of diabetes was seen after adjustment for covariates, suggesting that the depression-diabetes association in this cohort can be explained by other known risk factors. A likely explanatory variable is being overweight, a major diabetes risk factor and more common in those with depression. In addition, the high co-occurrence of PTSD and depression in this population may indicate that in other studies not directly measuring both of these conditions, depression may serve as a surrogate marker for PTSD and possibly not be otherwise independently associated with diabetes. To our knowledge, no prior study of depression and diabetes has adjusted for the presence of PTSD. Furthermore, although our subjects were younger than those included in several previous studies of depression and diabetes, it does not seem that this age difference can explain the absence of an association, because studies have reported that the risk of diabetes associated with depression was higher in individuals younger than age 50 years (23).
A significant interaction between sex and depression was observed: nondepressed women were at significantly higher risk of diabetes than nondepressed men, whereas the risk in depressed women was not increased. We are not aware of a plausible explanation for this finding, and further research will be needed to determine whether this is a chance association.
There are several limitations to this study. Self-reported diabetes status was used without direct testing or medical record confirmation. A review of the validity of self-reported diabetes based on survey data compared with clinical assessment or review of medical encounter data demonstrated sensitivities ranging from 70 to 99% (median 81%) and specificities from 92 to 99% in 11 studies (24). Therefore, it is likely that we captured considerably more than half of the cases of diabetes at both baseline and follow-up; there is a low probability of falsely classified cases given the high specificity of self-report. The 36% participation rate at baseline could potentially have led to bias only if nonparticipation was linked to both mental health conditions and higher (or lower) future diabetes risk. Loss to follow-up (28.6%) is a potential source of bias if an association existed between the likelihood of being lost to follow-up and both the outcome and exposure. The filter used to determine deployment eligibility resulted in apparently healthier individuals receiving this assignment, as reflected by the lower risk of diabetes in this group in unadjusted analyses. Whether our adjusted models adequately accounted for this confounding is uncertain. Mental health conditions were assessed using self-reported survey data that presumably represent clinician diagnosis but were not validated here. The definitions of mental health conditions were based on established survey methods that have high levels of sensitivity and specificity. The PCL-C has been shown to have high discrimination with an area under the receiver operating characteristic curve of 0.88 compared with the gold standard Clinician-Administered PTSD Scale (25). We cannot eliminate the possibility that subjects underreported mental health symptoms because of concern of adverse consequences regarding their service record. To ease this concern, we did not involve local command in the distribution or processing of surveys and provided assurances of confidentiality in the consent form that the information provided would be used for research purposes only. We did not account for use of psychoactive medications in this cohort, several of which have been reported to be associated with a higher risk of diabetes (26), and we cannot exclude the possibility that medications prescribed for treatment of PTSD symptoms led to diabetes. However, we did ask the following general question at baseline about the use of these medications: “Are you currently taking any medication for anxiety, depression, or stress?” Of individuals meeting criteria for PTSD, 21.4% responded affirmatively to this question. This low exposure to psychoactive pharmaceuticals argues against use of these medications potentially explaining the observed association between PTSD and diabetes risk. The potential exists for diabetes detection bias in individuals under medical treatment for PTSD. Because we did not identify PTSD through medical encounters the likelihood of this bias should not be high. It is not clear whether PTSD symptoms reported at baseline were the result of experiences that occurred as a result of military service or during civilian life. Because development of type 1 diabetes results in medical discharge from military service, we believe that we were able to identify all such individuals and exclude them from this analysis.
In summary, we found that symptoms of PTSD but not depression were independently associated with an increased risk of diabetes in military service members. To our knowledge this is the first report of a prospective association between PTSD and diabetes, and confirmation by additional research is needed.
Supplementary Material
Acknowledgments
The Millennium Cohort Study is funded through the Military Operational Medicine Research Program of the U.S. Army Medical Research and Materiel Command, Fort Detrick, Maryland. The Department of Veterans Affairs supported E.J.B.'s involvement with this research.
No potential conflicts of interest relevant to this article were reported.
E.J.B., B.S., M.A.K.R., T.I.H., G.D.G., and T.C.S. developed the study concept and design. E.J.B., I.G.J., B.S., T.I.H., G.D.G., E.B.-C., and T.C.S. analyzed and interpreted data. E.J.B. and I.G.J. performed statistical analysis. M.A.K.R. and T.C.S. supervised the study. E.J.B. and I.G.J. wrote the manuscript. E.J.B., I.G.J., B.S., T.I.H., P.J.A., G.D.G., E.B.-C., and T.C.S. revised/edited the manuscript.
We are indebted to the Millennium Cohort Study participants, without whom these analyses would not be possible. We thank Scott L. Seggerman from the Management Information Division, Defense Manpower Data Center, Seaside, California. In addition, we thank Melissa Bagnell, MPH, Gina Creaven, MBA, James Davies, Lacy Farnell, Nisara Granado, MPH, PhD, Gia Gumbs, MPH, Jaime Horton, Cynthia LeardMann, MPH, Travis Leleu, Jamie McGrew, Amanda Pietrucha, MPH, Teresa Powell, MS, Amber Seelig, MPH, Katherine Snell, Steven Speigle, Kari Welch, MA, Martin White, MPH, James Whitmer, and Charlene Wong, MPH, from the Department of Defense Center for Deployment Health Research, Naval Health Research Center, San Diego, California, and Michelle Stoia, also from the Naval Health Research Center. We also thank the professionals from the U.S. Army Medical Research and Materiel Command, especially those from the Military Operational Medicine Research Program, Fort Detrick, Maryland. We appreciate the support of the Henry M. Jackson Foundation for the Advancement of Military Medicine, Rockville, Maryland. These individuals provided assistance as part of their official duties as employees of the Department of Defense, and none received additional financial compensation.
APPENDIX
In addition to the authors, the Millennium Cohort Study Team includes Gregory C. Gray, MD, MPH, College of Public Health and Health Professions (Gainesville, FL), James R. Riddle, DVM, MPH, and Timothy S. Wells, DVM, PhD.
Footnotes
This represents report 09-35, supported by the U.S. Department of Defense, under work unit no. 60002. The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of Veterans Affairs, Department of the Navy, Department of the Army, Department of the Air Force, Department of Defense, or the U.S. Government. This research has been conducted in compliance with all applicable federal regulations governing the protection of human subjects in research (protocol NHRC.2000.0007). The funding organization had no role in the design and conduct of the study; collection, analysis, or preparation of data; or preparation, review, or approval of the manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Hoge CW, Castro CA, Messer SC, McGurk D, Cotting DI, Koffman RL. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med 2004;351:13–22 [DOI] [PubMed] [Google Scholar]
- 2.Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care 2008;31:2383–2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cosgrove MP, Sargeant LA, Griffin SJ. Does depression increase the risk of developing type 2 diabetes? Occup Med (Lond) 2008;58:7–14 [DOI] [PubMed] [Google Scholar]
- 4.Goodwin RD, Davidson JR. Self-reported diabetes and posttraumatic stress disorder among adults in the community. Prev Med 2005;40:570–574 [DOI] [PubMed] [Google Scholar]
- 5.Black PH. The inflammatory consequences of psychologic stress: relationship to insulin resistance, obesity, atherosclerosis and diabetes mellitus, type II. Med Hypotheses 2006;67:879–91 [DOI] [PubMed] [Google Scholar]
- 6.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ Primary Care Study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA 1999;282:1737–1744 [DOI] [PubMed] [Google Scholar]
- 7.Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD Checklist (PCL). Behav Res Ther 1996;34:669–673 [DOI] [PubMed] [Google Scholar]
- 8.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders 4th ed Washington, DC, American Psychiatric Association, 1994 [Google Scholar]
- 9.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Individual protection and individual unit deployment policy, Oct 2001 (with updates through Jan 2009) [policy letter]. MacDill Air Force Base, FL, U.S. Department of Defense, Commander, Central Command, 2009 [Google Scholar]
- 11.Kang HK, Li B, Mahan CM, Eisen SA, Engel CC. Health of US veterans of 1991 Gulf War: a follow-up survey in 10 years. J Occup Environ Med 2009;51:401–410 [DOI] [PubMed] [Google Scholar]
- 12.Goldberg RB. Cytokine and cytokine-like inflammation markers, endothelial dysfunction, and imbalanced coagulation in development of diabetes and its complications. J Clin Endocrinol Metab 2009;94:3171–3182 [DOI] [PubMed] [Google Scholar]
- 13.von Känel R, Hepp U, Kraemer B, Traber R, Keel M, Mica L, Schnyder U. Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. J Psychiatr Res 2007;41:744–752 [DOI] [PubMed] [Google Scholar]
- 14.den Heijer T, Vermeer SE, van Dijk EJ, Prins ND, Koudstaal PJ, Hofman A, Breteler MM. Type 2 diabetes and atrophy of medial temporal lobe structures on brain MRI. Diabetologia 2003;46:1604–1610 [DOI] [PubMed] [Google Scholar]
- 15.Kasai K, Yamasue H, Gilbertson MW, Shenton ME, Rauch SL, Pitman RK. Evidence for acquired pregenual anterior cingulate gray matter loss from a twin study of combat-related posttraumatic stress disorder. Biol Psychiatry 2008;63:550–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davidson TL, Kanoski SE, Schier LA, Clegg DJ, Benoit SC. A potential role for the hippocampus in energy intake and body weight regulation. Curr Opin Pharmacol 2007;7:613–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Björntorp P, Holm G, Rosmond R. Hypothalamic arousal, insulin resistance and type 2 diabetes mellitus. Diabet Med 1999;16:373–83 [DOI] [PubMed] [Google Scholar]
- 18.Yehuda R. Current status of cortisol findings in post-traumatic stress disorder. Psychiatr Clin North Am 2002;25:341–368, vii [DOI] [PubMed] [Google Scholar]
- 19.Mallon L, Broman JE, Hetta J. High incidence of diabetes in men with sleep complaints or short sleep duration: a 12-year follow-up study of a middle-aged population. Diabetes Care 2005;28:2762–2767 [DOI] [PubMed] [Google Scholar]
- 20.Singareddy RK, Balon R. Sleep in posttraumatic stress disorder. Ann Clin Psychiatry 2002;14:183–190 [DOI] [PubMed] [Google Scholar]
- 21.Brown ES, Varghese FP, McEwen BS. Association of depression with medical illness: does cortisol play a role? Biol Psychiatry 2004;55:1–9 [DOI] [PubMed] [Google Scholar]
- 22.Nutt D, Wilson S, Paterson L. Sleep disorders as core symptoms of depression. Dialogues Clin Neurosci 2008;10:329–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown LC, Majumdar SR, Newman SC, Johnson JA. History of depression increases risk of type 2 diabetes in younger adults. Diabetes Care 2005;28:1063–1067 [DOI] [PubMed] [Google Scholar]
- 24.Saydah SH, Geiss LS, Tierney E, Benjamin SM, Engelgau M, Brancati F. Review of the performance of methods to identify diabetes cases among vital statistics, administrative, and survey data. Ann Epidemiol 2004;14:507–516 [DOI] [PubMed] [Google Scholar]
- 25.Yeager DE, Magruder KM, Knapp RG, Nicholas JS, Frueh BC. Performance characteristics of the posttraumatic stress disorder checklist and SPAN in Veterans Affairs primary care settings. Gen Hosp Psychiatry 2007;29:294–301 [DOI] [PubMed] [Google Scholar]
- 26.Rubin RR, Ma Y, Marrero DG, Peyrot M, Barrett-Connor EL, Kahn SE, Haffner SM, Price DW, Knowler WC: Diabetes Prevention Program Research Group Elevated depression symptoms, antidepressant medicine use, and risk of developing diabetes during the diabetes prevention program. Diabetes Care 2008;31:420–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.