Abstract
OBJECTIVE
The accurate quantification of human diabetic neuropathy is important to define at-risk patients, anticipate deterioration, and assess new therapies.
RESEARCH DESIGN AND METHODS
A total of 101 diabetic patients and 17 age-matched control subjects underwent neurological evaluation, neurophysiology tests, quantitative sensory testing, and evaluation of corneal sensation and corneal nerve morphology using corneal confocal microscopy (CCM).
RESULTS
Corneal sensation decreased significantly (P = 0.0001) with increasing neuropathic severity and correlated with the neuropathy disability score (NDS) (r = 0.441, P < 0.0001). Corneal nerve fiber density (NFD) (P < 0.0001), nerve fiber length (NFL), (P < 0.0001), and nerve branch density (NBD) (P < 0.0001) decreased significantly with increasing neuropathic severity and correlated with NDS (NFD r = −0.475, P < 0.0001; NBD r = −0.511, P < 0.0001; and NFL r = −0.581, P < 0.0001). NBD and NFL demonstrated a significant and progressive reduction with worsening heat pain thresholds (P = 0.01). Receiver operating characteristic curve analysis for the diagnosis of neuropathy (NDS >3) defined an NFD of <27.8/mm2 with a sensitivity of 0.82 (95% CI 0.68–0.92) and specificity of 0.52 (0.40–0.64) and for detecting patients at risk of foot ulceration (NDS >6) defined a NFD cutoff of <20.8/mm2 with a sensitivity of 0.71 (0.42–0.92) and specificity of 0.64 (0.54–0.74).
CONCLUSIONS
CCM is a noninvasive clinical technique that may be used to detect early nerve damage and stratify diabetic patients with increasing neuropathic severity.
Established diabetic neuropathy leads to pain and foot ulceration. Detecting neuropathy early may allow intervention with treatments to slow or reverse this condition (1). Recent studies suggested that small unmyelinated C-fibers are damaged early in diabetic neuropathy (2–4) but can only be detected using invasive procedures such as sural nerve biopsy (4,5) or skin-punch biopsy (6–8). Our studies have shown that corneal confocal microscopy (CCM) can identify early small nerve fiber damage and accurately quantify the severity of diabetic neuropathy (9–11). We have also shown that CCM relates to intraepidermal nerve fiber loss (12) and a reduction in corneal sensitivity (13) and detects early nerve fiber regeneration after pancreas transplantation (14). Recently we have also shown that CCM detects nerve fiber damage in patients with Fabry disease (15) and idiopathic small fiber neuropathy (16) when results of electrophysiology tests and quantitative sensory testing (QST) are normal.
In this study we assessed corneal sensitivity and corneal nerve morphology using CCM in diabetic patients stratified for the severity of diabetic neuropathy using neurological evaluation, electrophysiology tests, and QST. This enabled us to compare CCM and corneal esthesiometry with established tests of diabetic neuropathy and define their sensitivity and specificity to detect diabetic patients with early neuropathy and those at risk of foot ulceration.
RESEARCH DESIGN AND METHODS
A total of 101 diabetic patients and 17 nondiabetic healthy volunteers participated in the study. Patients were excluded if they had another cause of neuropathy, had absent pedal pulses, wore contact lenses, or had a history of corneal trauma or surgery. The protocol was approved by the local research ethics committee of the Greater Manchester Health Authority, and all subjects gave written informed consent.
Evaluation of neuropathic severity
The neuropathy deficit score (NDS) was established by neurological examination and the severity of neuropathy was determined: NDS 0–2, no neuropathy; NDS 3–5, mild neuropathy; NDS, 6–8, moderate neuropathy; and NDS, 9–10, severe neuropathy (17,18). QST included assessment of vibration perception threshold (VPT), using a neurothesiometer (Horwell Scientific Laboratory Supplies, Wilford, Nottingham, U.K.); heat as pain thresholds (C-fibers); and cooling detection thresholds (A-δ fibers) using a CASE IV system (WR Medical Electronics, Stillwater, MN) with the thresholds for abnormality set at the 95th percentile. The DANTEC Keypoint electromyography system (software version 1.4) was used to quantify sural sensory and peroneal motor nerve conduction velocity and amplitude in all subjects.
Corneal sensitivity
Corneal sensitivity was assessed using a noncontact corneal aesthesiometer (NCCA), which uses a puff of air on the center of the cornea, lasting 0.9 s and exerting a force expressed in millibars (13). The coefficient of variation for NCCA is 5.6%.
CCM
One eye of each subject was selected at random and examined with a Tomey ConfoScan model P4 using previously described methodology (10,12). Three to five high-quality images of the sub-basal nerve plexus from the center of the cornea were assessed from each diabetic patient and control subject in a randomized masked fashion.
Three parameters were quantified (9,10,12): corneal nerve fiber density (NFD), the total number of major nerves per square millimeter; nerve fiber length (NFL), the total length of all nerve fibers and branches (millimeters per square millimeter); and nerve branch density (NBD), the number of branches emanating from major nerve trunks per square millimeter.
Statistical methods
SPSS (version 11.05.0) was used to compute the results. Data are presented as means ± SEM. The data were not normally distributed; hence, ANOVA with Scheffé post hoc tests were used to establish differences among the five (control, none, mild, moderate, and severe diabetic neuropathy) groups. The Pearson coefficient test was used to analyze correlations between variables. Receiver operating characteristic (ROC) curve analysis established the area under the curve (AUC) to determine the optimal threshold, sensitivity, and specificity values to estimate precision for NFD, NBD, NFL, and NCCA in defining the presence of neuropathy (NDS >3) and risk of foot ulceration (NDS >6). The ROC curves were used to compare the four tests and to define the optimum cutoff points, whereby sensitivity and specificity were equally weighted.
RESULTS
A total of 101 diabetic patients aged 58 ± 2.0 years and 17 age-matched (55 ± 4.8 years) control subjects were studied (Table 1). Diabetic patients were stratified according to NDS: none (1.4 ± 0.1, n = 34), mild (3.8 ± 0.1, n = 37), moderate (6.5 ± 0.1, n = 16), and severe (9.7 ± 0.1, n = 14). Age, duration of diabetes, and A1C did not differ among groups. and there was no correlation between NDS and A1C (r = 0.098, P = 0.36).
Table 1.
Clinical demographics, clinical neuropathy evaluation, QST, electrophysiology tests, and corneal sensitivity with NCCA and CCM of the nerve fibers in Bowman layer of the cornea in control subjects and diabetic patients with increasing neuropathic severity
| Parameter | Control | Neuropathy |
|||
|---|---|---|---|---|---|
| No | Mild | Moderate | Severe | ||
| n | 17 | 34 | 37 | 16 | 14 |
| Age (years) | 55 ± 4.8 | 55 ± 1.9 | 58 ± 2.1 | 59 ± 2.5 | 61 ± 2.05 |
| Diabetes duration (years) | 0 | 10.7 ± 1.82 | 15.5 ± 2.08 | 18.6 ± 3.06 | 19.3 ± 2.85 |
| Diabetes type (1/2) | — | 2/32 | 9/28 | 4/12 | 2/12 |
| Sex (male/female) | 8/9 | 19/15 | 32/5 | 12/4 | 10/4 |
| A1C (%) | <6.5 | 8.1 ± 0.27 | 7.9 ± 0.23 | 8.4 ± 0.37 | 8.3 ± 0.38 |
| NDS* | 0 | 1.4 ± 0.15¶ | 3.8 ± 0.11¶ | 6.5 ± 0.18¶ | 9.7 ± 0.11¶ |
| SNCV (≥40) (m/s)* | 47.85 ± 2.62 | 42.88 ± 0.92 | 41.10 ± 0.83† | 36.78 ± 1.88‡ | 37.26 ± 2.60† |
| Sural amplitude (μA) (>5)* | 18.62 ± 2.55 | 13.74 ± 1.46 | 6.13 ± 0.73¶ | 4.05 ± 0.67¶ | 6.16 ± 3.25† |
| PMNCV (≥40) (m/s)* | 49.26 ± 1.63 | 44.60 ± 0.65 | 41.21 ± 0.72¶ | 35.77 ± 1.68¶ | 33.82 ± 2.60¶ |
| Peroneal amplitude (>2)* | 5.58 ± 1.02 | 3.58 ± 0.28† | 2.10 ± 0.25¶ | 1.62 ± 0.28¶ | 1.16 ± 0.50¶ |
| VPT (V)* | 9.58 ± 0.93 | 9.56 ± 0.84 | 18.18 ± 1.96† | 25.35 ± 2.85¶ | 42.29 ± 3.83¶ |
| CDT (percentile) | — | 54.64 ± 3.89 | 76.08 ± 4.1‡ | 89.60 ± 4.9¶ | 98.40 ± 0.89¶ |
| NCCA (mbar)* | 0.72 ± 0.36 | 1.16 ± 0.07 | 1.34 ± 0.10 | 1.49 ± 0.20 | 2.23 ± 0.51¶ |
| NFD (no./mm2)* | 45.60 ± 4.47 | 31.63 ± 2.33† | 28.36 ± 1.95¶ | 18.57 ± 3.63¶ | 17.84 ± 2.49¶ |
| NBD (no./mm2)* | 25.38 ± 2.99 | 17.42 ± 2.02† | 13.28 ± 1.79¶ | 5.63 ± 1.33¶ | 4.95 ± 1.79¶ |
| NFL (mm/mm2)* | 11.21 ± 0.88 | 8.05 ± 0.71 | 5.48 ± 0.45¶ | 3.01 ± 0.39¶ | 2.99 ± 0.34¶ |
Data are means ± SEM for diabetic patients and control subjects. CDT, cold detection threshold; PMNCV, peroneal motor nerve conduction velocity; SNCV, sural nerve conduction velocity.
*Statistically significant difference between diabetic patients and controls using ANOVA: P < 0.001. Post hoc results with significant difference between control subjects and diabetic patients with differing severity of neuropathy:
†P < 0.05;
‡P < 0.01,
¶P < 0.001.
Peroneal and sural nerve conduction velocities did not differ in patients without neuropathy but were significantly reduced in those with mild (P < 0.001 and P < 0.05), moderate (P < 0.001 and P < 0.01), and severe (P < 0.001 and P < 0.001) neuropathy. Likewise, sural nerve amplitude did not differ in diabetic patients without neuropathy but was significantly reduced in those with mild, moderate, and severe (P < 0.001) neuropathy. Peroneal nerve amplitude was significantly reduced in diabetic patients without (P < 0.05) and with mild, moderate, and severe (P < 0.001) neuropathy.
VPT increased significantly with severity of neuropathy (P < 0.0001) but did not differ in patients without neuropathy (P = 1.0) and was only significant in those with mild (P = 0.01), moderate (P < 0.0001), and severe (P < 0.0001) neuropathy. Cold detection threshold was significantly increased in those with mild (P = 0.01), moderate (P < 0.0001), and severe (P < 0.0001) neuropathy. Heat pain perception thresholds increased significantly with increasing neuropathic severity (P < 0.05).
Corneal sensitivity decreased in diabetic patients compared with that in control subjects (P < 0.0001). However, this was significant only in patients with severe neuropathy (P < 0.001) (Table 1).
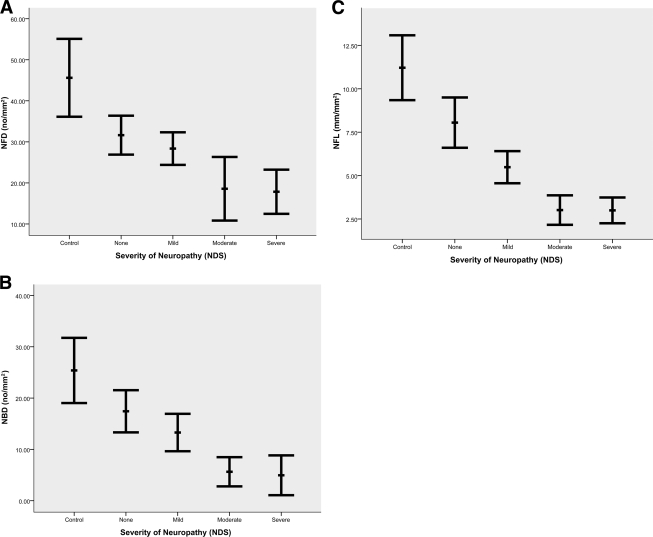
Qualitatively there was a reduction in nerve fiber and branch density in diabetic patients compared with that in control subjects (Fig. 1). Intraindividual variability was established by repeating CCM in 15 subjects on two occasions, and the coefficient of variation was 12% for NFD, 9% for NFL, and 24% for NBD. Corneal nerve fiber density (P < 0.0001), branch density (P < 0.0001). and length (P < 0.0001) were significantly and progressively reduced in diabetic patients (Table 1, Fig. 2). NFD was significantly reduced in diabetic patients with no (P < 0.02), mild (P < 0.001), moderate (P < 0.0001), and severe (P < 0.0001) neuropathy compared with that in control subjects (Fig. 2A). Similarly, NBD was significantly reduced in diabetic patients with no (P < 0.03), mild (P < 0.001), moderate (P < 0.0001), and severe (P < 0.0001) neuropathy compared with that in control subjects (Fig. 2B). NFL was reduced in diabetic patients with no (P = 0.07), mild (P < 0.0001), moderate (P < 0.0001), and severe (P < 0.0001) neuropathy compared with that in control subjects (Fig. 2C). NDS correlated significantly with corneal sensitivity (r = 0.44, P = 0.0001), NFD (r = −0.44, P < 0.0001), NBD (r = −0.44, P < 0.0001), and NFL (r = −0.57, P < 0.0001).
Figure 1.
Images of corneal nerves in Bowman layer, showing abundant nerve fibers and adequate branching in a control subject (A) with a typical image from a diabetic patient with mild (B), moderate (C), and severe (D) neuropathy showing a progressive loss of nerve fibers.
Figure 2.
Corneal nerve morphology in control subjects and diabetic patients with increasing neuropathic severity: A: NFD (P < 0.0001); B: NBD (P < 0.0001); C: NFL (P < 0.0001).
According to the heat pain threshold (HPT), patients were classified into four groups: normal (percentile 0–25); mild (percentile 26–50); moderate (percentile 51–75), and severe (percentile 76–100) neuropathy (Table 2). NCCA increased and NFD decreased with increasing HPTs but were not significant. However, NBD and NFL demonstrated a progressive reduction with worsening HPT (P = 0.01).
Table 2.
Results of corneal nerve parameters in diabetic patients stratified for severity of neuropathy according to the HPT
| Neuropathy |
P value | ||||
|---|---|---|---|---|---|
| No | Mild | Moderate | Severe | ||
| n | 34 | 37 | 16 | 15 | |
| NFD (no./mm2) | 28.63 ± 2.51 | 25.73 ± 2.27 | 22.00 ± 4.99 | 23.26 ± 3.26 | 0.44 |
| NBD (no./mm2) | 15.13 ± 2.09 | 13.79 ± 2.13 | 8.60 ± 2.20 | 5.65 ± 1.37 | 0.01 |
| NFL (mm/mm2) | 6.54 ± 0.65 | 5.75 ± 0.74 | 4.90 ± 1.18 | 3.27 ± 0.24 | 0.01 |
| NCCA (mbar) | 1.19 ± 0.07 | 1.38 ± 0.11 | 1.83 ± 0.43 | 1.75 ± 0.38 | 0.11 |
CCM sensitivity and specificity (Table 3)
Table 3.
Diagnostic efficiency of corneal nerve parameters presented as AUC and P values with CCM and NCCA cutoffs with sensitivity and specificity for diagnosis of patients with neuropathy (NDS >3) and for diagnosis of patients at risk of foot ulceration (NDS >6)
| Variable | NDS >3 |
NDS >6 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AUC | P value | Optimum cutoff | Sensitivity | Specificity | AUC | P value | Optimum cutoff | Sensitivity | Specificity | |
| NFD | 0.764 | <0.0001 | <27.81 | 0.82 (0.68–0.92) | 0.52 (0.40–0.64) | 0.751 | <0.0001 | <20.82 | 0.71 (0.42–0.92) | 0.64 (0.54–0.74) |
| NBD | 0.801 | <0.0001 | <13.89 | 0.91 (0.79–0.98) | 0.45 (0.34–0.57) | 0.783 | <0.0001 | <6.94 | 0.71 (0.42–0.92) | 0.71 (0.61–0.80) |
| NFL | 0.849 | <0.0001 | <3.39 | 0.64 (0.49–0.78) | 0.79 (0.68–0.88) | 0.813 | <0.0001 | <3.29 | 0.64 (0.35–0.87) | 0.71 (0.61–0.80) |
| NCCA | 0.678 | 0.001 | >1.12 | 0.60 (0.44–0.75) | 0.61 (0.48–0.72) | 0.720 | 0.003 | >2.1 | 0.23 (0.05–0.54) | 0.89 (0.80–0.94) |
Data in parentheses are 95% CI.
According to the ROC curves (Fig. 3) for the three CCM parameters for NDS >3, AUC was 0.76 for NFD, 0.79 for NBD, and 0.84 for NFL and for NDS >6 AUC was 0.76 for NFD, 0.79 for NBD, and 0.81 for NFL. Because the ROC curve (Fig. 3A) for NFL was greater than those for the other two variables, it is considered to be the better test for diagnosing diabetic neuropathy, although for high values of specificity NFD and NBD may be considered better tests. For those at risk of foot ulceration, the ROC curves for the three CCM parameters (Fig. 3B) are more comparable, although NFL has a higher specificity than the other tests. For the diagnosis of neuropathy, the sensitivity and specificity were, respectively, 82 and 52% for NFD, 91 and 45% for NBD, 64 and 79% for NFL, and 60 and 61% for NCAA. For detecting those at risk of foot ulceration the sensitivity and specificity were 71 and 64% for NFD, 71 and 71% for NBD, 64 and 71% for NFL, and 23 and 89% for NCCA.
Figure 3.
ROC curves for NFD, NBD, and NFL for (A) NDS >3 and (B) NDS >6. Diagonal segments are produced by ties.
CONCLUSIONS
It is important to detect nerve damage at the earliest stage of diabetic neuropathy as intervention at this stage with improved glycemic control (Diabetes Control and Complications Trial) (19) or improvement of other risk factors (20,21) may prevent nerve degeneration or promote regeneration. Although diabetic patients with established neuropathy have increased vibration and thermal perception and decreased nerve conduction velocity, and early detection of neuropathy is difficult (22). Recent studies show significant intraepidermal nerve fiber (IENF) loss in skin biopsies, despite normal results for electrophysiology tests and QST (2,3), suggesting that IENF assessment may be important in the early diagnosis of neuropathy (23). However, because skin biopsy is an invasive procedure, in this study we assessed the utility of two novel noninvasive measures of neuropathy, namely corneal esthesiometry and corneal confocal microscopy.
An early study of type 1 diabetic patients demonstrated a reduction in corneal sensitivity and the number of corneal nerve fiber bundles, which correlated significantly with the severity of neuropathy (24). We have previously demonstrated a significant reduction in corneal sensitivity using two independent measures and both correlated with neuropathic severity (13). Our studies (9,10) using CCM demonstrated corneal nerve fiber abnormalities, which were related to the severity of somatic neuropathy. More recently, we have shown that CCM reflects IENF loss in skin biopsies from the dorsum of the foot in diabetic patients (12) and may also show nerve repair after pancreas transplantation (14). Furthermore, we have recently demonstrated that CCM detects small fiber damage in patients with Fabry disease (15) and idiopathic small fiber neuropathy (16), indicating that CCM is a direct surrogate of peripheral neuropathy.
We now demonstrate a progressive reduction in corneal sensitivity and increasing corneal nerve degeneration with increasing severity of diabetic neuropathy. Importantly, corneal nerve fiber damage was present in patients deemed to have no evidence of neuropathy based on neurological evaluation, QST, and neurophysiology tests, consistent with the recent studies showing IENF loss in diabetic patients without neuropathy (2,3). We also establish for the first time that corneal esthesiometry and CCM have reasonable sensitivity and specificity to detect diabetic patients with minimal neuropathy and those at risk of foot ulceration. A limitation of this study is that the data are derived from a cross-sectional study. Ideally a longitudinal study would provide more robust data regarding the ability of CCM to identify patients at risk of developing neuropathy.
Ideally, a test for detecting neuropathy in the early stages should be noninvasive and quantitative and detect changes with time or in response to therapeutic interventions (2). CCM seems to fulfill these attributes, especially because it is noninvasive, directly quantifies small fiber pathology, and stratifies neuropathic severity. CCM may therefore be an ideal surrogate marker for early diagnosis, stratification of severity, and assessment of therapeutic efficacy of new treatments in human diabetic neuropathy.
Acknowledgments
This work was supported by the Juvenile Diabetes Research Foundation International (grant 5-2002-185) and the National Eye Institute (grant 1-R01-NS46259-01).
No potential conflicts of interest relevant to this article were reported.
M.T. researched data, contributed to discussion, wrote the manuscript, and reviewed/edited manuscript. C.Q., C.A., P.K., A.M., J.F., and P.M. researched data. N.E. contributed to discussion. A.J.M.B. reviewed/edited manuscript. R.A.M. contributed to discussion, wrote the manuscript, and reviewed/edited manuscript.
We thank Annie Herbert from Pennine Acute Hospitals NHS Trust for statistical advice.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Dyck PJ, Norell JE, Tritschler H, Schuette K, Samigullin R, Ziegler D, Bastyr EJ, 3rd, Litchy WJ, O'Brien PC. Challenges in design of multicenter trials: end points assessed longitudinally for change and monotonicity. Diabetes Care 2007;30:2619–2625 [DOI] [PubMed] [Google Scholar]
- 2. Umapathi T, Tan WL, Loke SC, Soon PC, Tavintharan S, Chan YH. Intraepidermal nerve fiber density as a marker of early diabetic neuropathy. Muscle Nerve 2007;35:591–598 [DOI] [PubMed] [Google Scholar]
- 3. Løseth S, Stålberg E, Jorde R, Mellgren SI. Early diabetic neuropathy: thermal thresholds and intraepidermal nerve fibre density in patients with normal nerve conduction studies. J Neurol 2008;255:1197–1202 [DOI] [PubMed] [Google Scholar]
- 4. Malik RA, Tesfaye S, Newrick PG, Walker D, Rajbhandari SM, Siddique I, Sharma AK, Boulton AJ, King RH, Thomas PK, Ward JD. Sural nerve pathology in diabetic patients with minimal but progressive neuropathy. Diabetologia 2005;48:578–585 [DOI] [PubMed] [Google Scholar]
- 5. Malik RA, Veves A, Walker D, Siddique I, Lye RH, Schady W, Boulton AJ. Sural nerve fibre pathology in diabetic patients with mild neuropathy: relationship to pain, quantitative sensory testing and peripheral nerve electrophysiology. Acta Neuropathol 2001;101:367–374 [DOI] [PubMed] [Google Scholar]
- 6. Novella SP, Inzucchi SE, Goldstein JM. The frequency of undiagnosed diabetes and impaired glucose tolerance in patients with idiopathic sensory neuropathy. Muscle Nerve 2001;24:1229–1231 [DOI] [PubMed] [Google Scholar]
- 7. Singleton JR, Smith AG, Bromberg MB. Increased prevalence of impaired glucose tolerance in patients with painful sensory neuropathy. Diabetes Care 2001;24:1448–1453 [DOI] [PubMed] [Google Scholar]
- 8. Sumner CJ, Sheth S, Griffin JW, Cornblath DR, Polydefkis M. The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology 2003;60:108–111 [DOI] [PubMed] [Google Scholar]
- 9. Kallinikos P, Berhanu M, O'Donnell C, Boulton AJ, Efron N, Malik RA. Corneal nerve tortuosity in diabetic patients with neuropathy. Invest Ophthalmol Vis Sci 2004;45:418–422 [DOI] [PubMed] [Google Scholar]
- 10. Malik RA, Kallinikos P, Abbott CA, van Schie CH, Morgan P, Efron N, Boulton AJ. Corneal confocal microscopy: a non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia 2003;46:683–688 [DOI] [PubMed] [Google Scholar]
- 11. Hossain P, Sachdev A, Malik RA. Early detection of diabetic peripheral neuropathy with corneal confocal microscopy. Lancet 2005;366:1340–1343 [DOI] [PubMed] [Google Scholar]
- 12. Quattrini C, Tavakoli M, Jeziorska M, Kallinikos P, Tesfaye S, Finnigan J, Marshall A, Boulton AJ, Efron N, Malik RA. Surrogate markers of small fiber damage in human diabetic neuropathy. Diabetes 2007;56:2148–2154 [DOI] [PubMed] [Google Scholar]
- 13. Tavakoli M, Kallinikos PA, Efron N, Boulton AJ, Malik RA. Corneal sensitivity is reduced and relates to the severity of neuropathy in patients with diabetes. Diabetes Care 2007;30:1895–1897 [DOI] [PubMed] [Google Scholar]
- 14. Mehra S, Tavakoli M, Kallinikos PA, Efron N, Boulton AJ, Augustine T, Malik RA. Corneal confocal microscopy detects early nerve regeneration after pancreas transplantation in patients with type 1 diabetes. Diabetes Care 2007;30:2608–2612 [DOI] [PubMed] [Google Scholar]
- 15. Tavakoli M, Marshall A, Thompson L, Kenny M, Waldek S, Efron N, Malik RA: Corneal confocal microscopy: a novel noninvasive means to diagnose neuropathy in patients with Fabry disease. Muscle Nerve 2009;40:976–984 [DOI] [PubMed] [Google Scholar]
- 16. Tavakoli M, Marshall A, Pitceathly R, Fadavi H, Gow D, Roberts ME, Efron N, Boulton AJ, Malik RA: Corneal confocal microscopy: a novel means to detect nerve fibre damage in idiopathic small fibre neuropathy. Exp Neurol 2010;223:245–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abbott CA, Carrington AL, Ashe H, Bath S, Every LC, Griffiths J, Hann AW, Hussein A, Jackson N, Johnson KE, Ryder CH, Torkington R, Van Ross ER, Whalley AM, Widdows P, Williamson S, Boulton AJ. The North-West Diabetes Foot Care Study: incidence of, and risk factors for, new diabetic foot ulceration in a community-based patient cohort. Diabet Med 2002;19:377–384 [DOI] [PubMed] [Google Scholar]
- 18. Young MJ, Boulton AJ, MacLeod AF, Williams DR, Sonksen PH. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia 1993;36:150–154 [DOI] [PubMed] [Google Scholar]
- 19. Bretzel RG. Intensive insulin regimens: evidence for benefit. Int J Obes Relat Metab Disord 2004;28(Suppl. 2):S8–S13 [DOI] [PubMed] [Google Scholar]
- 20. Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008;358:580–591 [DOI] [PubMed] [Google Scholar]
- 21. Tesfaye S, Chaturvedi N, Eaton SE, Ward JD, Manes C, Ionescu-Tirgoviste C, Witte DR, Fuller JH: EURODIAB Prospective Complications Study Group. Vascular risk factors and diabetic neuropathy. N Engl J Med 2005;352:341–350 [DOI] [PubMed] [Google Scholar]
- 22. Dyck PJ, Karnes JL, O'Brien PC, Litchy WJ, Low PA, Melton LJ, 3rd: The Rochester Diabetic Neuropathy Study: reassessment of tests and criteria for diagnosis and staged severity. Neurology 1992;42:1164–1170 [DOI] [PubMed] [Google Scholar]
- 23. Lauria G, Lombardi R, Camozzi F, Devigili G. Skin biopsy for the diagnosis of peripheral neuropathy. Histopathology 2009;54:273–285 [DOI] [PubMed] [Google Scholar]
- 24. Rosenberg ME, Tervo TM, Immonen IJ, Müller LJ, Grönhagen-Riska C, Vesaluoma MH. Corneal structure and sensitivity in type 1 diabetes mellitus. Invest Ophthalmol Vis Sci 2000;41:2915–2921 [PubMed] [Google Scholar]
- 25. Tavakoli M, Marshall A, Thompson L, Kenny M, Waldek S, Efron N, Malik RA: Corneal confocal microscopy: a novel noninvasive means to diagnose neuropathy in patients with Fabry disease. Muscle Nerve 2009;40:976–984 [DOI] [PubMed] [Google Scholar]