Abstract
OBJECTIVE
Both gestational diabetes mellitus (GDM) and mild glucose intolerance in pregnancy identify women at increased risk of future type 2 diabetes. In this context, we queried whether metabolic changes that occur in the 1st year postpartum vary in relation to gestational glucose tolerance status.
RESEARCH DESIGN AND METHODS
Three-hundred-and-ninety-two women underwent glucose challenge test (GCT) and oral glucose tolerance test (OGTT) in pregnancy followed by repeat OGTT at both 3 months' postpartum and 12 months' postpartum. The antepartum testing defined four gestational glucose tolerance groups: GDM (n = 107); gestational impaired glucose tolerance (GIGT) (n = 75); abnormal GCT with normal glucose tolerance (NGT) on OGTT (abnormal GCT NGT) (n = 137); and normal GCT with NGT on OGTT (normal GCT NGT) (n = 73).
RESULTS
The prevalence of dysglycemia progressively increased across the groups from normal GCT NGT to abnormal GCT NGT to GIGT to GDM at both 3 months' postpartum (2.7% to 10.2% to 18.7% to 34.6%, P < 0.0001) and 12 months' postpartum (2.7% to 11.7% to 17.3% to 32.7%, P < 0.0001). Between 3 and 12 months' postpartum, the groups did not differ with respect to changes in waist circumference, weight, or insulin sensitivity. Importantly, however, they exhibited markedly different changes in β-cell function (Insulin Secretion-Sensitivity Index-2 [ISSI-2]) (P = 0.0036), with ISSI-2 declining in both the GDM and GIGT groups. Furthermore, on multiple linear regression analysis, both GDM (t = −3.06, P = 0.0024) and GIGT (t = −2.18, P = 0.03) emerged as independent negative predictors of the change in ISSI-2 between 3 and 12 months' postpartum.
CONCLUSIONS
Women with GDM and GIGT exhibit declining β-cell function in the 1st year postpartum that likely contributes to their future diabetic risk.
The diagnosis of gestational diabetes mellitus (GDM) identifies a population of young women who are at high risk of developing type 2 diabetes on the order of 20–60% in the first 5 years following an index pregnancy (1–3). A systematic review of studies evaluating the risk of progression to type 2 diabetes following GDM has demonstrated that the cumulative incidence of diabetes increases markedly in the first 5 years' postpartum and appears to plateau after 10 years (3). Thus, events in the early postpartum years are likely to be important in determining diabetic risk in this patient population. At present, however, little is known about the pathophysiologic changes that take place in these early years following a pregnancy complicated by GDM.
A recent series of reports have demonstrated that even women with mild glucose intolerance in pregnancy (i.e., less severe than GDM) have an increased risk of ultimately developing pre-diabetes and diabetes (4–9). The magnitude of this risk is proportional to the degree of gestational dysglycemia, with the highest risk in women with GDM and proportionately lower risk in women with milder abnormalities of gestational glucose tolerance (4). It thus emerges that the spectrum of abnormal glucose homeostasis in pregnancy identifies a continuum of risk for future diabetes and, based on the temporal findings pertaining to GDM, pathophysiologic changes that occur in the early postpartum years may be relevant to the manifestation of this risk potential. Therefore, in the current study, our objective was to perform a longitudinal evaluation of the metabolic changes that take place in the 1st year postpartum in a well-characterized cohort of women representing the full spectrum of glucose tolerance in pregnancy and hence a broad range of future diabetic risk.
RESEARCH DESIGN AND METHODS
This analysis was conducted in the context of an ongoing observational study of early events in the natural history of type 2 diabetes in which a cohort of women recruited at the time of antepartum GDM screening is undergoing longitudinal metabolic characterization in pregnancy and at 3 months' postpartum, 12 months' postpartum, and every 2 years thereafter for 10 years. The study protocol has previously been described in detail (4,5,8,10). Standard obstetrical practice at our institution involves universal screening for GDM in all pregnant women at 24–28 weeks' gestation by a glucose challenge test (GCT), followed by referral for a diagnostic oral glucose tolerance test (OGTT) if the GCT result is abnormal (defined as plasma glucose ≥7.8 mmol/l at 1 h following the ingestion of 50 g of glucose). In this study, regardless of the GCT result, all participants underwent a 3-h 100-g OGTT for determination of glucose tolerance status in pregnancy. Recruitment was performed either before or after the GCT, but prior to the OGTT. It should be noted that the recruitment of women following an abnormal GCT was designed to enrich the study population for women with varying degrees of antepartum glucose intolerance (4,10). At 3 months' postpartum and 1 year postpartum, participants returned for reassessment including evaluation of glucose tolerance by 2-h 75-g OGTT. The study protocol was approved by the Mount Sinai Hospital Research Ethics Board, and all participants provided written informed consent. The study presents data on the first 392 women that have completed their 12-month postpartum visit as of March 2009, as part of this 10-year prospective observational cohort study.
Gestational glucose tolerance status
The GCT and 3-h 100-g OGTT in pregnancy stratified participants into the following four gestational glucose tolerance groups: GDM (defined as ≥2 glucose values above the National Diabetes Data Group [NDDG] diagnostic criteria on the OGTT [11]); gestational impaired glucose tolerance (GIGT) (defined as only 1 glucose value above NDDG thresholds); normal glucose tolerance (NGT) on the antepartum OGTT with an abnormal preceding GCT (abnormal GCT NGT); and normal glucose tolerance on the OGTT with a normal preceding GCT (normal GCT NGT).
Postpartum study visits
Participants returned to the clinical investigation unit for a 2-h 75-g OGTT at both 3 and 12 months' postpartum. At both visits, interviewer-administered questionnaires were completed, and physical examinations were performed. No specific clinical advice was systematically provided in this observational study. The OGTT characterized postpartum glucose tolerance at both visits into one of the following five categories per Canadian Diabetes Association clinical practice guidelines (12): diabetes, impaired glucose tolerance (IGT), impaired fasting glucose (IFG), combined IFG/IGT, and normal glucose tolerance. Pre-diabetes refers to IGT, IFG, and combined IFG/IGT (12).
Laboratory measurements and physiologic indexes
All OGTTs were performed in the morning with venous blood samples drawn for the measurement of glucose and specific insulin at fasting and at 30, 60, and 120 min following the ingestion of the glucose load as previously described (4,5).
Insulin sensitivity was measured using the Matsuda index (ISOGTT), a well-established measure of whole-body insulin sensitivity that has been validated against the euglycemic-hyperinsulinemic clamp (13). ISOGTT is defined as 10,000/√ [(FPG × FPI) × (G × I)], where FPG = fasting plasma glucose, FPI = fasting plasma insulin, G = mean glucose during the OGTT, and I = mean insulin (13). As a secondary measure of insulin sensitivity (largely hepatic), we also calculated the reciprocal of the homeostasis model assessment of insulin resistance (1/HOMA-IR). HOMA-IR (14) was calculated as FPG × FPI/22.5.
The primary measure of β-cell function was the Insulin Secretion-Sensitivity Index-2 (ISSI-2), an OGTT-derived measure that is analogous to the disposition index obtained from the frequently sampled intravenous glucose tolerance test (15,16). ISSI-2 is defined as the product of 1) insulin secretion measured by the ratio of the area under the insulin curve to the area under the glucose curve and 2) insulin sensitivity measured by ISOGTT (15,16). As a secondary measure of β-cell function, we also calculated the insulinogenic index (IGI) divided by HOMA-IR (IGI/HOMA-IR). IGI was calculated as the ratio of the incremental change in insulin during the first 30 min of the OGTT to the incremental change in glucose over the same time period (17). ISSI-2 exhibits stronger correlation with the disposition index than does IGI/HOMA-IR (16) and thus was used as the primary measure of β-cell function in this study.
Statistical analyses
All analyses were conducted using SAS (version 9.1; SAS Institute, Cary, NC). Continuous variables were tested for normality of distribution, and natural log transformations of skewed variables were used, where necessary, in subsequent analyses. Univariate differences across the four gestational glucose tolerance groups were assessed at 3 months' postpartum and at 12 months' postpartum using the Wilcoxon rank sum test for continuous variables and the χ2 test for categorical variables (Table 1). The changes within each study group between 3 and 12 months' postpartum in waist circumference, weight, insulin sensitivity, and β-cell function, respectively, were compared between groups (Fig. 1 and supplemental Fig. 2, available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc10-0315/DC1). Since the magnitude of change of these parameters within each group will be limited by their respective baseline values at 3 months' postpartum (which differed between the groups), these comparisons were adjusted for the baseline values. Multiple linear regression analyses of the change in β-cell function between 3 and 12 months' postpartum (dependent variable) were performed to identify independent predictors of this change with β-cell function measured by either ISSI-2 (Table 2) or IGI/HOMA-IR. Covariates in these models included age, ethnicity, family history of diabetes, breastfeeding, baseline β-cell function at 3 months' postpartum, waist circumference, change in weight between 3 and 12 months' postpartum, and gestational glucose tolerance status.
Table 1.
Clinical, demographic, and metabolic characteristics of study population (n = 392) at 3 months' and 12 months' postpartum, stratified by glucose tolerance status in pregnancy
| Normal GCT | Abnormal GCT | ||||
|---|---|---|---|---|---|
| NGT | NGT | GIGT | GDM | P | |
| n | 73 | 137 | 75 | 107 | |
| At 3 months' postpartum | |||||
| Age (years) | 35.6 [32.7–39.1] | 34.8 [32.2–37.5] | 35.4 [33.3–38.5] | 35.2 [32.3–38.2] | 0.5725 |
| Ethnicity | 0.2091 | ||||
| White (%) | 82.2 | 81.0 | 74.7 | 74.8 | |
| Asian (%) | 5.5 | 9.5 | 16.0 | 8.4 | |
| Other (%) | 12.3 | 9.5 | 9.3 | 16.8 | |
| Family history of diabetes (%) | 39.7 | 48.5 | 49.3 | 61.7 | 0.0294 |
| Current smoking (%) | 5.5 | 3.7 | 8.0 | 3.7 | 0.5005 |
| Current breastfeeding (%) | 94.5 | 92.0 | 88.0 | 97.2 | 0.0961 |
| BMI (kg/m2) | 25.1 [22.6–28.5] | 25.0 [23.1–28.9] | 26.1 [23.3–30.1] | 26.7 [23.5–30.7] | 0.2400 |
| Waist circumference (cm) | 88.0 [80.0–95.0] | 85.4 [80.0–92.0] | 88.0 [83.0–97.0] | 89.4 [81.0–99.0] | 0.0337 |
| Systolic BP (mmHg) | 108 [101–113] | 108 [101–114] | 108 [103–115] | 111 [105–118] | 0.0539 |
| Diastolic BP (mmHg) | 66 [60–70] | 64 [59–70] | 63 [60–70] | 66 [60–72] | 0.3293 |
| Insulin sensitivity | |||||
| ISOGTT | 13.4 [9.2–17.4] | 12.2 [8.4–16.9] | 8.6 [5.9–12.9] | 9.0 [5.7–13.1] | <0.0001 |
| 1/HOMA-IR | 1.4 [1.0–1.9] | 1.3 [0.8–1.8] | 1.1 [0.6–1.6] | 1.0 [0.6–1.6] | 0.0005 |
| β-Cell function | |||||
| ISSI-2 | 1,123 [850–1,371] | 1,003 [815–1,212] | 868 [733–1,148] | 834 [628–1,062] | <0.0001 |
| IGI/HOMA-IR | 13.8 [7.9–20.0] | 10.1 [7.0–16.2] | 8.4 [5.2–12.8] | 8.0 [4.4–13.2] | <0.0001 |
| OGTT | |||||
| Fasting glucose (mmol/l) | 4.4 [4.1–4.6] | 4.4 [4.2–4.7] | 4.7 [4.3–5.0] | 4.7 [4.3–5.0] | <0.0001 |
| 2-h glucose (mmol/l) | 5.7 [4.8–6.1] | 5.9 [5.0–6.9] | 6.3 [5.2–7.3] | 6.7 [5.5–8.5] | <0.0001 |
| Glucose tolerance status | |||||
| NGT (%) | 97.3 | 89.8 | 81.3 | 65.4 | <0.0001 |
| Isolated IFG (%) | 0 | 0 | 1.3 | 0.9 | |
| Isolated IGT (%) | 1.4 | 10.2 | 10.7 | 28.0 | |
| Combined IFG and IGT (%) | 1.4 | 0 | 1.3 | 0 | |
| Diabetes (%) | 0 | 0 | 5.3 | 5.6 | |
| At 12 months' postpartum | |||||
| Current smoking (%) | 5.5 | 6.7 | 9.5 | 3.7 | 0.4586 |
| Current breastfeeding (%) | 95.9 | 89.8 | 88.0 | 88.8 | 0.3364 |
| BMI (kg/m2) | 24.2 [21.5–27.8] | 24.5 [21.9–27.9] | 25.0 [22.5–28.8] | 26.4 [22.5–30.5] | 0.1478 |
| Waist circumference (cm) | 82.6 [76.5–90.0] | 84.0 [77.2–91.0] | 85.5 [80.0–95.0] | 87.8 [80.0–96.0] | 0.0162 |
| Systolic BP (mmHg) | 109 [100–115] | 109 [102–117] | 110 [101–118] | 110 [103–119] | 0.4896 |
| Diastolic BP (mmHg) | 64 [59–70] | 65 [59–70] | 64 [60–70] | 66 [60–71] | 0.2260 |
| Insulin sensitivity | |||||
| ISOGTT | 12.3 [8.5–16.8] | 10.4 [7.1–14.9] | 7.7 [4.9–13.6] | 7.6 [4.8–11.1] | <0.0001 |
| 1/HOMA-IR | 1.1 [0.6–1.7] | 1.0 [0.6–1.6] | 0.9 [0.5–1.5] | 0.8 [0.5–1.4] | 0.0134 |
| β-Cell function | |||||
| ISSI-2 | 1,055 [866–1,433] | 977 [763–1,238] | 842 [706–1,053] | 787 [599–1,047] | <0.0001 |
| IGI/HOMA-IR | 14.1 [9.4–22.8] | 10.6 [6.3–16.1] | 7.2 [4.3–11.8] | 7.2 [4.5–11.3] | <0.0001 |
| OGTT | |||||
| Fasting glucose (mmol/l) | 4.5 [4.4–4.7] | 4.6 [4.4–5.0] | 4.8 [4.5–5.1] | 4.8 [4.5–5.2] | <0.0001 |
| 2-h glucose (mmol/l) | 5.4 [4.7–6.0] | 5.8 [5.0–7.0] | 6.0 [5.1–7.1] | 6.9 [5.6–8.3] | <0.0001 |
| Glucose tolerance status | |||||
| NGT (%) | 97.3 | 88.3 | 82.7 | 67.3 | 0.0002 |
| Isolated IFG (%) | 0 | 0.7 | 1.3 | 0 | |
| Isolated IGT (%) | 2.8 | 10.2 | 13.3 | 29.0 | |
| Combined IFG and IGT (%) | 0 | 0 | 0 | 0.9 | |
| Diabetes (%) | 0 | 0.7 | 2.7 | 2.8 |
Continuous variables are presented as median followed by interquartile range in parentheses and categorical variables are presented as percentages. P values refer to overall differences across groups as determined by Wilcoxon rank sum test for continuous variables or χ2 test for categorical variables. BP, blood pressure.
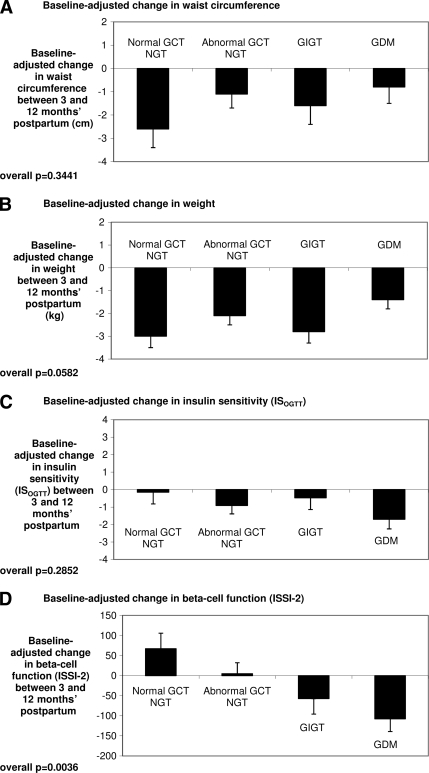
Figure 1.
Baseline-adjusted changes in waist circumference (A), weight (B), insulin sensitivity (C), and β-cell function (D) between 3 months' and 12 months' postpartum by gestational glucose tolerance group. To account for the fact that the magnitude of change of these parameters within each group will be limited by their respective baseline values at 3 months' postpartum (which differed between the groups), these comparisons are adjusted for the baseline values.
Table 2.
Multiple linear regression analyses of the relationships between gestational glucose tolerance status and (dependent variable) the change in β-cell function (ISSI-2) between 3 and 12 months' postpartum
| β | SEM | t | P | Model r2 | |
|---|---|---|---|---|---|
| Model I* | 32.3% | ||||
| Abnormal GCT NGT | −61.71 | 47.12 | −1.31 | 0.191 | |
| GIGT | −118.83 | 55.00 | −2.16 | 0.031 | |
| GDM | −176.99 | 50.94 | −3.47 | 0.0006 | |
| Model II** | 32.7% | ||||
| Abnormal GCT NGT | −63.58 | 47.14 | −1.35 | 0.178 | |
| GIGT | −117.54 | 55.03 | −2.14 | 0.033 | |
| GDM | −173.07 | 50.99 | −3.39 | 0.0008 | |
| Model III*** | 34.1% | ||||
| Abnormal GCT NGT | −54.52 | 46.80 | −1.16 | 0.245 | |
| GIGT | −118.79 | 54.51 | −2.18 | 0.030 | |
| GDM | −155.67 | 50.87 | −3.06 | 0.0024 |
*Covariates in Model I: age, ethnicity, family history of diabetes, breastfeeding status, β-cell function at 3 months' postpartum, and gestational glucose tolerance group.
**Covariates in Model II: covariates in Model I + waist circumference at 3 months' postpartum.
***Covariates in Model III: covariates in Model II + change in weight between 3 months' and 12 months' postpartum.
RESULTS
Characteristics of study population at baseline and follow-up
Table 1 shows the characteristics of the study population at baseline (3 months' postpartum) and at follow-up (12 months' postpartum) stratified into the following four groups based on glucose tolerance status in pregnancy: normal GCT NGT (n = 73), abnormal GCT NGT (n = 137), GIGT (n = 75), and GDM (n = 107). At 3 months' postpartum, there were no significant differences between the groups with respect to age, ethnicity, smoking, breastfeeding, and blood pressure. Although BMI did not differ between the groups, waist circumference increased with worsening gestational glucose tolerance (P = 0.0337). As anticipated, both insulin sensitivity (ISOGTT and 1/HOMA-IR) and β-cell function (ISSI-2 and IGI/HOMA-IR) progressively decreased from the normal GCT NGT group to the abnormal GCT NGT group to GIGT to GDM at 3 months' postpartum (all P ≤ 0.0005). Furthermore, these differences translated into a stepwise increase in the prevalence of dysglycemic states across these four groups (P < 0.0001) (Table 1).
These differences between the groups persisted at 12 months' postpartum (Table 1). Specifically, the earlier differences in waist circumference (P = 0.0162), insulin sensitivity (ISOGTT P < 0.0001; 1/HOMA-IR P = 0.0134), and β-cell function (ISSI-2 P < 0.0001; IGI/HOMA-IR P < 0.0001) all remained. Similarly, the earlier stepwise increase in the prevalence of dysglycemic states from the normal GCT NGT group to the abnormal GCT NGT group to GIGT to GDM also persisted at 12 months' postpartum (P = 0.0002).
Metabolic changes between 3 and 12 months postpartum
In light of their differential future risk of diabetes, we next sought to determine whether the gestational glucose tolerance groups differed with respect to metabolic changes that took place between 3 and 12 months' postpartum. In this regard, it should first be noted that, although the prevalence of dysglycemia (pre-diabetes or diabetes) differed significantly between the groups at both 3 months' (P < 0.0001) and 12 months' postpartum (P < 0.0001), the rates within each group were very similar on both occasions (normal GCT NGT 2.7% at 3 months and 2.7% at 12 months; abnormal GCT NGT 10.2 and 11.7; GIGT 18.7 and 17.3; and GDM 34.6 and 32.7) (supplemental Fig. 1). Thus, in the absence of fluctuation in the prevalence of dysglycemia, we queried whether the groups exhibited differential changes over this 9-month period in physiologic factors affecting glucose homeostasis including obesity, insulin sensitivity, and β-cell function. As shown in Fig. 1A, the baseline-adjusted change in waist circumference between 3 and 12 months' postpartum was not significantly different between the four gestational glucose tolerance groups (P = 0.3441). The baseline-adjusted change in weight suggested possibly lesser weight loss in women with GDM compared with their peers, but this comparison across the groups did not reach statistical significance (P = 0.0582) (Fig. 1B). Importantly, the baseline-adjusted change in insulin sensitivity (ISOGTT) also did not differ between the groups (P = 0.2852) (Fig. 1C). This result was unchanged with insulin sensitivity measured by 1/HOMA-IR (P = 0.3928) (supplemental Fig. 2A). In contrast, however, the groups differed markedly with respect to their respective changes in β-cell function between 3 and 12 months' postpartum. Indeed, whereas women with normal GCT NGT showed an increase in baseline-adjusted ISSI-2 and those with abnormal GCT NGT showed little change, both the GIGT and GDM groups showed a decline in baseline-adjusted ISSI-2 between 3 and 12 months' postpartum (P = 0.0036) (Fig. 1D). These latter findings reflected an overall 2.6% decline in ISSI-2 in the GIGT group and a 4.0% decline in ISSI-2 in the GDM group. Furthermore, measurement of β-cell function by IGI/HOMA-IR revealed the same pattern of differential changes between the groups with GIGT and GDM again both showing a fall in this measure between 3 and 12 months' postpartum (P = 0.0036) (supplemental Fig. 2B).
Multiple linear regression analyses
Having shown that the pattern of change in β-cell function varies according to gestational glucose tolerance group, we next performed multiple linear regression analyses to determine if other factors may account for this relationship. Indeed, on this analysis (Table 2, Model I), both GDM (β = −176.99, t = −3.47, P = 0.0006) and GIGT (β = −118.83, t = −2.16, P = 0.031) emerged as negative independent predictors of the (dependent variable) change in ISSI-2 between 3 and 12 months' postpartum after adjustment for age, ethnicity, family history of diabetes, breastfeeding, and baseline ISSI-2, in a model that reconciled 32.3% of the variance in the dependent variable. These independent associations of GDM (β = −173.07, t = −3.39, P = 0.0008) and GIGT (β = −117.54, t = −2.14, P = 0.033) persisted after further adjustment for waist circumference at 3 months' postpartum (Table 2, Model II). Moreover, additional adjustment for weight change between 3 and 12 months' postpartum (which difference nearly achieved statistical significance) also did not attenuate these relationships (GDM [β = −155.67, t = −3.06, P = 0.0024]; GIGT [β = −118.79, t = −2.18, P = 0.030]) (Table 2, Model III). The same results were observed with adjustment for BMI in models II and III rather than waist circumference (data not shown). Inclusion of prepregnancy weight also did not change the findings (data not shown). In addition, repeating all of the models using the change in IGI/HOMA-IR between 3 and 12 months' postpartum as the dependent variable also supported these findings with both GDM and GIGT consistently emerging as independent negative predictors of the change in β-cell function (data not shown).
Finally, we also repeated the models from Table 2 in only those women with NGT at 3 months' postpartum. This limited dataset (n = 325) consisted of 71 women in the normal GCT NGT group, 123 in the abnormal GCT NGT group, 61 with GIGT, and 70 women with GDM. After adjustment for age, ethnicity, family history of diabetes, breastfeeding, and baseline ISSI-2 (Model I), GDM remained a negative independent predictor of the change in ISSI-2 between 3 and 12 months' postpartum (β = −148.39, t = −2.58, P = 0.0103), while GIGT no longer reached statistical significance (β = −98.93, t = −1.63, P = 0.1048). These results were unchanged after further adjustment 1) for waist circumference (Model II) (GDM [β = −141.99, t = −2.47, P = 0.0142]; GIGT [β = −97.89, t = −1.61, P = 0.1092]) and 2) additionally for weight change between 3 and 12 months' postpartum (Model III) (GDM [β = −124.39, t = −2.15, P = 0.032]; GIGT [β = −96.47, t = −1.59, P = 0.1121]). Thus, even when limited to those women with NGT at 3 months' postpartum, GDM remained an independent predictor of declining β-cell function between 3 and 12 months' postpartum.
CONCLUSIONS
Women with GDM have a chronic defect in β-cell function (18). Although this defect likely antedates the pregnancy (19), it is first detected clinically in the form of insufficient β-cell compensation for the severe acquired insulin resistance of late pregnancy resulting in the gestational hyperglycemia by which GDM is diagnosed. While the gestational hyperglycemia typically resolves following delivery, it is important to recognize that the β-cell defect in women with GDM is still present in the postpartum. Indeed, several studies have confirmed the presence of chronic β-cell dysfunction (and chronic insulin resistance [19]) in this patient population many years after the index pregnancy (4,18–21). Furthermore, it has recently been demonstrated that GIGT and even abnormal GCT NGT are both also characterized by β-cell dysfunction (of proportionately lesser severity than that of GDM), and this dysfunction also persists into the postpartum (4,5,8). Accordingly, the concept has emerged that the spectrum of abnormal glucose homeostasis in pregnancy identifies a gradient of chronic β-cell dysfunction that may antedate the pregnancy and translates into a continuum of future risk for the development of type 2 diabetes, with GDM representing the most extreme element followed, in turn, by GIGT (4). In this context, we sought to determine whether there are metabolic changes in the 1st year postpartum that vary in relation to gestational glucose tolerance status and, hence, may be relevant to this gradient of future diabetic risk.
Four key points arise from the current study. First, the longitudinal design (with two assessments in the 1st year postdelivery) reveals that deterioration of β-cell function is a very early event in women with GDM and GIGT, taking place within the 1st year postpartum. Second, the evaluation of women across the full spectrum of antepartum glucose tolerance shows that the degree of this decline in β-cell function varies in relation to the severity of gestational dysglycemia. Specifically, the decline is greatest in women with GDM followed by those with GIGT, whereas β-cell function does not appear to worsen in women with NGT in pregnancy. Third, this gradient of change in β-cell function in relation to gestational glucose tolerance status occurs in the absence of differential changes between the groups in waist circumference, weight, and insulin sensitivity, respectively (Fig. 1). Last, these data suggest that the deterioration in β-cell function in women with GDM and GIGT appears to precede the worsening of glycemia that must occur for progression to type 2 diabetes. Specifically, β-cell function declined in women with GDM and GIGT despite stable rates of dysglycemia between 3 and 12 months' postpartum (the rates actually decreased slightly in both groups over this period) (supplemental Fig. 1), and GDM remained an independent predictor of declining β-cell function even when the multiple linear regression analyses were limited to only those women with NGT at 3 months' postpartum. Indeed, this observation is consistent with a recent report showing impaired β-cell function at median 7 years' postpartum in 52 women with previous GDM compared with 39 control women with normoglycemia in pregnancy despite similar insulin sensitivity and the maintenance of NGT (22). Taken together with the current findings, these data suggest that chronic progressive β-cell dysfunction is likely the dominant pathophysiologic defect driving the progression to type 2 diabetes in women with a history of GDM. This idea is further supported by evidence linking the early preservation of β-cell function in response to thiazolidinedione therapy with protection from the development of diabetes in women with a history of GDM (23).
In clinical studies, β-cell dysfunction has consistently emerged as an independent predictor of incident type 2 diabetes in several populations (17) including women with previous GDM (24). In this context, the model arising from the current data is one where the previously demonstrated gradient of β-cell dysfunction associated with gestational dysglycemia (4) is mirrored by a gradient of chronic progressive deterioration of β-cell function over time that will ultimately lead to the manifestation of long-term diabetic risk. In other words, as the most extreme element, women with GDM have the most severe β-cell defect at the outset, coupled with the greatest deterioration over the 1st year postpartum, resulting in the highest risk for the development of diabetes. Furthermore, the demonstration of this deterioration within the 1st year postpartum suggests that gestational dysglycemia provides the unique opportunity to identify a patient population in which the early evolution of β-cell dysfunction is unfolding prior to the development of diabetes or even pre-diabetes. It follows from these data that lifestyle modification (previously shown to reduce the risk of type 2 diabetes in women with a remote history of GDM [25]) potentially should be instituted as early as possible in the postpartum to protect β-cell function, although further study is required.
A limitation of this study is the use of surrogate measures of insulin sensitivity and β-cell function. However, clamp studies would be difficult to implement in a study of this size (n = 392) given their cost, invasiveness, and time requirements. These issues may be particularly problematic for performing two measurements in the 1st year postpartum in new mothers, a design feature that was integral to the novel demonstration of early deterioration of β-cell function. Furthermore, we have used two established and validated measures for both insulin sensitivity and β-cell function (13–17) with consistent results observed in each case. A second limitation is that causality cannot be conclusively established in an observational cohort study owing to the possibility of unrecognized confounding (although the biologic plausibility and consistency of the current data are encouraging). There is likely to be phenotypic and genetic variability within the population that will influence the natural history of β-cell function in the postpartum. The third limitation is that the current study does not provide estimates for the overall population prevalence of postpartum pre-diabetes/diabetes due to the nature of the recruitment strategy, which was designed to enrich the study population for varying degrees of antepartum glucose intolerance. The final study limitation is that the current data cannot determine whether similar mechanisms are responsible for chronic β-cell dysfunction and the increased risk of postpartum metabolic syndrome in women with glucose intolerance in pregnancy (10), though it is likely that insulin resistance is relevant to both of these outcomes.
In summary, the pattern of change in β-cell function in the 1st year postpartum varies in relation to glucose tolerance status in pregnancy unlike changes in waist circumference, weight, and insulin sensitivity. Indeed, both GDM and GIGT independently predict declining β-cell function between 3 and 12 months' postpartum. It thus emerges that β-cell dysfunction progresses in the early postpartum in women with a history of gestational dysglycemia and is likely a pathophysiologic factor contributing to the development of type 2 diabetes in this at-risk patient population.
Supplementary Material
Acknowledgments
This study was supported by operating grants MOP-67063 and 84206 from the Canadian Institutes of Health Research (CIHR); OG-3-08-2543-RR from the Canadian Diabetes Association (CDA); and NA6747 from the Heart and Stroke Foundation of Ontario. R.R. holds a CIHR New Investigator Award, CDA Clinician-Scientist incentive funding, and University of Toronto Banting and Best Diabetes Centre New Investigator funding. A.J.G.H. holds a Tier-II Canada Research Chair in Diabetes Epidemiology. B.Z. holds the Sam and Judy Pencer Family Chair in Diabetes Research at Mount Sinai Hospital and the University of Toronto.
No potential conflicts of interest relevant to this article were reported.
R.R. designed the analysis plan, researched the data, and wrote the manuscript. Y.Q. performed the statistical analyses. R.R., M.S., P.W.C., A.J.G.H., and B.Z. were involved in the design/implementation of the overall study. All authors reviewed/edited the manuscript and contributed to the discussion.
We thank Mount Sinai Hospital Department of Pathology and Laboratory Medicine and Patient Care Services.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet 2009;373:1773–1779 [DOI] [PubMed] [Google Scholar]
- 2. Feig DS, Zinman B, Wang X, Hux JE. Risk of development of diabetes mellitus after diagnosis of gestational diabetes. CMAJ 2008;179:229–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care 2002;25:1862–1868 [DOI] [PubMed] [Google Scholar]
- 4. Retnakaran R, Qi Y, Sermer M, Connelly PW, Hanley AJ, Zinman B. Glucose intolerance in pregnancy and future risk of pre-diabetes or diabetes. Diabetes Care 2008;31:2026–2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Retnakaran R, Qi Y, Sermer M, Connelly PW, Zinman B, Hanley AJ. Isolated hyperglycemia at 1 hour on oral glucose tolerance test in pregnancy resembles gestational diabetes mellitus in predicting postpartum metabolic dysfunction. Diabetes Care 2008;31:1275–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carr DB, Newton KM, Utzschneider KM, Tong J, Gerchman F, Kahn SE, Heckbert SR. Modestly elevated glucose levels during pregnancy are associated with a higher risk of future diabetes among women without gestational diabetes mellitus. Diabetes Care 2008;31:1037–1039 [DOI] [PubMed] [Google Scholar]
- 7. Vambergue A, Dognin C, Boulogne A, Réjou MC, Biausque S, Fontaine P. Increasing incidence of abnormal glucose tolerance in women with prior abnormal glucose tolerance during pregnancy: DIAGEST 2 study. Diabet Med 2008;25:58–64 [DOI] [PubMed] [Google Scholar]
- 8. Retnakaran R, Qi Y, Sermer M, Connelly PW, Zinman B, Hanley AJ. An abnormal screening glucose challenge test in pregnancy predicts postpartum metabolic dysfunction, even when the antepartum oral glucose tolerance test is normal. Clin Endocrinol 2009;71:208–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Retnakaran R, Shah BR. Abnormal screening glucose challenge test in pregnancy and future risk of diabetes in young women. Diabet Med 2009;26:474–477 [DOI] [PubMed] [Google Scholar]
- 10. Retnakaran R, Qi Y, Connelly PW, Sermer M, Zinman B, Hanley AJ. Glucose intolerance in pregnancy and postpartum risk of metabolic syndrome in young women. J Clin Endocrinol Metab 2010;95:670–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039–1057 [DOI] [PubMed] [Google Scholar]
- 12. Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. 2008 Canadian Diabetes Association Clinical Practice Guidelines. Canadian Journal of Diabetes 2008; 32(Suppl. 1):S10–S13 [DOI] [PubMed] [Google Scholar]
- 13. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 14. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 15. Retnakaran R, Shen S, Hanley AJ, Vuksan V, Hamilton JK, Zinman B. Hyperbolic relationship between insulin secretion and sensitivity on oral glucose tolerance test. Obesity 2008;16:1901–1907 [DOI] [PubMed] [Google Scholar]
- 16. Retnakaran R, Qi Y, Goran MI, Hamilton JK. Evaluation of proposed oral disposition index measures in relation to the actual disposition index. Diabet Med 2009;26:1198–1203 [DOI] [PubMed] [Google Scholar]
- 17. Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia 2003;46:3–19 [DOI] [PubMed] [Google Scholar]
- 18. Buchanan TA, Xiang AH. Gestational diabetes mellitus. J Clin Invest 2005;115:485–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Catalano PM, Huston L, Amini SB, Kalhan SC. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am J Obstet Gynecol 1999;180:903–916 [DOI] [PubMed] [Google Scholar]
- 20. Ryan EA, Imes S, Liu D, McManus R, Finegood DT, Polonsky KS, Sturis J. Defects in insulin secretion and action in women with a history of gestational diabetes. Diabetes 1995;44:506–512 [DOI] [PubMed] [Google Scholar]
- 21. Xiang AH, Kawakubo M, Trigo E, Kjos SL, Buchanan TA. Declining beta-cell compensation for insulin resistance in Hispanic women with recent gestational diabetes mellitus: association with changes in weight, adiponectin, and C-reactive protein. Diabetes Care 2010;33:396–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Seghieri G, Tesi F, Anichini R, De Bellis A, Barsotti E, Mari A, Ferrannini E. Influence of gestational diabetes on the long-term control of glucose tolerance. Diabetologia 2007;50:2234–2238 [DOI] [PubMed] [Google Scholar]
- 23. Buchanan TA, Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, Ochoa C, Tan S, Berkowitz K, Hodis HN, Azen SP. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk Hispanic women. Diabetes 2002;51:2796–2803 [DOI] [PubMed] [Google Scholar]
- 24. Xiang AH, Wang C, Peters RK, Trigo E, Kjos SL, Buchanan TA. Coordinate changes in plasma glucose and pancreatic beta-cell function in Latino women at high risk for type 2 diabetes. Diabetes 2006;55:1074–1079 [DOI] [PubMed] [Google Scholar]
- 25. Ratner RE, Christophi CA, Metzger BE, Dabelea D, Bennett PH, Pi-Sunyer X, Fowler S, Kahn SE: Diabetes Prevention Program Research Group. Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J Clin Endocrinol Metab 2008;93:4774–4779 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.