Abstract
OBJECTIVE
Paternal and maternal type 2 diabetes, exclusive of gestational diabetes, may influence risk factors in the offspring differently (through possible epigenetic effects of parental diabetes) and are difficult to identify without accurate dates of diagnosis. We aimed to examine a metabolic phenotype in three different groups of offspring to see distinct paternal versus maternal effects.
RESEARCH DESIGN AND METHODS
We examined body composition and insulin action (M) in nondiabetic subjects and insulin secretion tested via acute insulin response (AIR) in normal glucose-tolerant full-heritage Pima Indian adults categorized by disparate parental diabetes status: 1) offspring of fathers with early-onset diabetes (age <35 years) and nondiabetic mothers (ODF; n = 10), 2) offspring of mothers with early-onset diabetes (age <35 years), not exposed to diabetes in utero with nondiabetic fathers (OMED; n = 11), and 3) a control group of offspring of parents without diabetes until >50 years of age (CON; n = 15).
RESULTS
ODFs were leaner than CONs and OMEDs (percent of body fat [%BF]: least-squares means adjusted for age and sex [95% CI]: 27.3 [23.3–31.3] in ODFs vs. 35.4 [32.2–38.5] in CONs and 32.4 [28.8–36.1] in OMEDs, P = 0.04). ODFs were more insulin sensitive (had a higher M) than OMEDs or CONs, but not after adjustment for age, sex, and %BF. AIR adjusted for M, age, sex, and %BF was lower in ODFs versus CONs and OMEDs (P < 0.05).
CONCLUSIONS
Adult ODFs were leaner and had lower early insulin secretion, despite being equally insulin sensitive after adjustment for body fat compared to the other groups, indicating a paternal imprinted effect.
Previous studies of heredity established parental history of type 2 diabetes as one of the dominant risk factors for the development of type 2 diabetes (1,2). The offspring phenotype may vary depending on which parent is affected and whether the offspring was exposed to diabetes in utero. Low birth weight (LBW), thought to result from in utero maternal undernutrition, is one such phenotypic feature and has been shown to predict the development of type 2 diabetes (3,4). However, in Pima Indians, LBW predicts diabetes only when paternal (not maternal) diabetes is present (3). In fact, offspring's lower birth weight predicted subsequent development of diabetes in the fathers. These data indicated a possible paternally transmitted imprinted effect. Gautier et al. (5) reported that insulin secretion was positively associated with age of diabetes onset in the mother. However, this was in a mixed cohort in which either or both parents could have had diabetes.
Recent studies in mice demonstrated that although a LBW phenotype, first generated using maternal undernutrition, confers impaired glucose tolerance via both subsequent parental lines, the LBW phenotype is passed on via paternal inheritance only (6). As these mice reach maturity, paternal offspring continue to have lower body weight but similar degrees of impairment in glucose tolerance and impaired insulin secretion.
We hypothesized that offspring of individuals with early-onset paternal diabetes would have a different metabolic phenotype than those with early-onset maternal diabetes (exclusive of exposure to diabetes in utero) and control subjects. To disentangle the separate parental effects in offspring, we carefully selected three groups of individuals: 1) offspring of fathers with early onset of diabetes, 2) offspring of mothers with early onset of diabetes, and 3) offspring in which neither parent developed diabetes. We compared body composition (percentage of body fat [%BF]), insulin action (M), and acute insulin response (AIR) among these groups.
RESEARCH DESIGN AND METHODS
Determination of parental diabetes status
Parental diabetes status was determined from a longitudinal study of health conducted within the Gila River (Pima) Indian Community in which residents ≥5 years old have been invited for research examinations consisting of the measurement of height and weight and a 75-g oral glucose tolerance test (OGTT) approximately every 2 years. Diabetes was diagnosed according to World Health Organization 1999 criteria (7) or by review of the medical record if made in a clinical setting.
We defined three groups of offspring according to parental diabetes status: 1) offspring of diabetic fathers (ODF) consisting of subjects whose father developed diabetes at <35 years of age, but whose mother remained nondiabetic until >35 years of age; 2) offspring of mothers with early onset of diabetes (OMED) consisting of subjects whose mother developed diabetes at <35 years of age, but after the date of birth of the subject documented by a normal OGTT following delivery (mean ± SD time between subject's date of birth and mothers date of diagnosis, 8.9 ± 4.2 years), and whose fathers remained nondiabetic until >35 years of age; and 3) a control group (CON) consisting of offspring whose parents were known to be nondiabetic until >50 years of age. The third group can be considered a control group because of previous evidence that offspring of parents who do not develop diabetes until age >50 years have a risk for developing diabetes comparable with that of offspring whose parents never developed diabetes (8).
For additional characterization of the parents, we determined the diabetic cumulative incidence (CI) score of each parent in the sample (8). Briefly, the diabetes score was derived by first calculating the specific cumulative incidence of diabetes as a function of age (CIa) in the Pima population. If diabetes was present, then the score is calculated by 1 − CIa at the age of first diagnosis of diabetes. If diabetes was not present, then score is calculated as 0 − CIa at the time of the last biennial examination. The score thus contains information on whether an individual developed diabetes and the age of onset of diabetes and is positive if diabetes was ever present and greater if diabetes developed at an earlier age. Conversely, a negative score is calculated if the individual was nondiabetic at the last biennial examination and is most negative in those who remain nondiabetic into old age.
Study subjects
Volunteers also participated in a study of metabolic determinants for the development of type 2 diabetes and obesity. At the time of their participation, all subjects were in good health and without evidence of diabetes (by OGTT), as determined by a comprehensive medical evaluation including medical history, physical examination, and routine laboratory testing. Subjects were admitted for 10–15 days to the Clinical Research Unit of the National Institute of Diabetes and Digestive and Kidney Diseases in Phoenix, Arizona, and were provided a standard weight-maintaining energy needs diet containing 50% of calories as carbohydrate, 30% as fat, and 20% as protein for at least 3 days before metabolic testing. After at least 3 days, volunteers underwent a 75-g OGTT after a 12-h overnight fast to exclude diabetes. Volunteers were then assessed for body composition and insulin action in vivo. Only subjects who were full-heritage Pima Indian or related Tohono O'odham were included in the analysis.
Dual-energy X-ray absorptiometry
Body composition was measured by dual-energy X-ray absorptiometry using a total body scanner (DPX-L; Lunar, Madison, WI). Percentage body fat (%BF), fat mass (FM), and fat-free mass (FFM) were calculated as previously described (9).
Measurement of insulin action
Insulin action was assessed at physiological insulin concentrations during the hyperinsulinemic-euglycemic clamp technique (10). Briefly, after an overnight fast, a primed (30 μCi) continuous (0.3 μCi/min) infusion of [3-3H] glucose was started to determine endogenous glucose production (EGP). At least 2 h after starting the isotope infusion, a primed continuous intravenous insulin infusion was administered for 100 min at a constant rate of 40 mU/m2/min. Blood samples for measurement of [3-3H] glucose specific activity were collected at the end of the basal period and every 10 min during the final 40 min of insulin infusion. Under basal (i.e., fasting) conditions, EGP was calculated as the [3-3H] glucose infusion rate divided by the steady-state plasma 3-[3H] glucose specific activity. The rate of total insulin-stimulated glucose disposal was calculated for the last 40 min of the insulin infusion and was corrected for the rate of EGP calculated from the Steele equation (11). Individual variation in plasma glucose and insulin concentrations during the clamp were taken into account in the calculation of insulin action (M-low) (12). All measurements derived from the clamp were normalized to estimated metabolic body size (EMBS), which is directly derived from FFM but takes into account the fact that the intercept of the relationship between FFM and resting metabolic rate is not zero (−17.7 kg in our laboratory [i.e., EMBS = FFM + 17.7 kg]) (12).
Insulin secretion
Insulin secretion was measured as the response to a 25-g intravenous glucose tolerance test (IVGTT). The AIR was calculated as the average increment in plasma insulin concentration above basal in samples obtained 3, 4, and 5 min after the bolus injection of glucose (13).
Meal test
At ∼0730 h, subjects were fed a mixed-meal breakfast (consisting of a bacon-and-egg sandwich on toast accompanied by orange juice) containing 10% of calories from protein, 45% from fat, and 45% from carbohydrates and providing ∼20% of daily energy requirements for each subject. The meal was consumed within 15 min, and subjects rested quietly in bed throughout the study. Blood samples for insulin and glucose were drawn before and 30, 60, 90, 120, 150, and 180 min after initiation of the meal test.
Analytic measurements
Plasma glucose concentrations were measured by the glucose oxidize method (Beckman Instruments, Fullerton, CA). Plasma insulin concentrations were measured by the Herbert modification of the method of Yalow and Berson (14), by an automated auto-analyzer (ICN Radiochemicals, Costa Mesa, CA), or by an automated immunoassay (Access, Beckman Instruments). Values from the final two assays were regressed to the original assay.
All studies were approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Disease. All volunteers gave written, informed consent.
Statistical analysis
Statistical analyses were performed using the procedures of the SAS statistical package (version 8.2; SAS Institute, Cary, NC). AIR and M-low were log10 transformed to normalize their distributions before parametric analyses. Unless otherwise specified, all data are expressed as means ± SD. General, anthropometric, and metabolic characteristics in Table 1 were evaluated using Student's t test, ANOVA, or χ2 analyses for continuous and categorical variables, respectively. Since even mild degrees of glucose intolerance can be associated with impairments in insulin secretion (15) only subjects with normal glucose tolerance were included for further analyses of AIR. The glucose and insulin responses during the OGTT, IVGTT, and meal test by group were compared by ANCOVA using the MIXED procedure in SAS. The general linear models were used to adjust %BF for age and sex; M-low (mg · kg−1 · estimated metabolic body size (EMBS) · min−1) for age, sex, and %BF, and AIR for age, sex, %BF, and M-low (10). Adjusted least-squares means (lsmeans) were used to compare groups by parental diabetes status using post hoc t test, with Tukey's honestly significant difference adjustment for multiple comparisons. For all groups, further analyses were conducted and adjusted for the same covariates using general estimating equations to account for family membership.
Table 1.
General, anthropometric, and body composition parameters of subjects according to the age of diabetes onset in the parents
| CON | ODF | OMED | |
|---|---|---|---|
| n | 15 | 10 | 11 |
| Female/male (n) | 6/9 | 2/8 | 6/5 |
| Age (years) | 28.4 ± 8.2 | 22.9 ± 6.1 | 26.4 ± 6.9 |
| Mother's CI | −0.440 ± 0.495 | −0.577 ± 0.347 | 0.851 ± 0.047 |
| Father's CI | −0.637 ± 0.373 | 0.901 ± 0.059 | −0.438 ± 0.185 |
| Birth weight (g)* | 3,367 ± 359 | 3,412 ± 528 | 3,413 ± 325 |
| Body weight (kg) | 104.5 ± 30 | 79.6 ± 19.6§ | 98.4 ± 12.2 |
| Height (cm) | 169 ± 7 | 172 ± 6 | 167 ± 8 |
| Body fat (%) | 35.5 ± 8.2 | 25.1 ± 9.1§ | 34.4 ± 7.8 |
| BMI (kg/m2) | 36.4 ± 9.1 | 27.6 ± 6.9§ | 35.9 ± 5.2 |
| Fasting plasma glucose (mmol/l)† | 5.19 ± 0.40 | 4.55 ± 0.55§ | 5.22 ± 0.43 |
| 2-h plasma glucose (mmol/l)† | 6.58 ± 1.28 | 5.88 ± 1.33 | 7.33 ± 1.55 |
| NGT/IGT (n) | 13/2 | 9/1 | 6/5 |
| Log10M-low (mg · kg−1EMBS · min−1) | 0.27 ± 0.04 | 0.52 ± 0.15§ | 0.34 ± 0.06 |
| BGO (mg · kgEMBS · min−1) | 2.33 ± 0.33 | 2.62 ± 0.09 | 2.43 ± 0.48 |
| Log10AIR (pmol/l)‡ | 2.39 ± 0.30 | 2.09 ± 0.28§ | 2.41 ± 0.15 |
Data are means ± SD.
*Birth weight in ODF group available only in seven subjects.
†In ODF group, data available only in eight subjects.
‡NGT subjects only.
§P < 0.05 ODF vs. CON (ANOVA; sex differences χ2 analysis). BGO, basal glucose output; IGT, impaired glucose tolerance; NGT, normal glucose tolerance.
RESULTS
General, anthropometric, and metabolic characteristics of the study population are shown in Table 1. We found 10 ODF; 8 of 10 fathers from the ODF group had diabetes preconception, and 2 fathers had diagnoses of diabetes 3 and 4 years after the offspring birth. Onset age of diabetes for fathers was lower in the ODF group than for mothers in the OMED group (26.6 ± 4.2 vs. 30.4 ± 3.0 years, P = 0.02). This slight difference in age was due to exclusion of mothers in the OMED group who did not have a nondiabetic OGTT following childbirth. Consistent with this age difference, fathers' CI score in the ODF group was higher than mothers' CI score in the OMED group (P = 0.04, Table 1). There was no significant difference between mothers CI score in the ODF group and fathers CI score in the OMED group, indicating no difference in relative age of onset of diabetes in the opposite parent. The CI score was comparable between mothers and fathers in the CON group (Table 1). The birth weight and height was not different among the three groups (Table 1).
ODFs had lower mean %BF than CONs or OMEDs (Table 1). After adjustment for age and sex, %BF remained lower in the ODF group than in the CON group (lsmeans [95% CI]: 27.3% [23.3–31.3] in ODF vs. 35.4% [32.2–38.5] in CON and 32.4% [28.8–36.1] in OMED, P = 0.04). The FFM, adjusted for age and sex, tended to be lower in ODF in comparison to CON and OMED, but did not reach statistical significance (57.9kg [50.4–65.3] in ODF vs. 65.8 kg [60.0–71.7] in CON, and 67.2 kg [60.4–74.1] in OMED, P = 0.1).
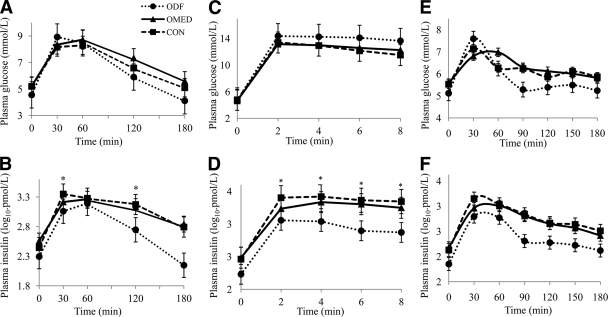
ODFs had lower fasting glucose, but 2-h plasma glucose concentrations were comparable with those of CONs or OMEDs (P = 0.01 and P = 0.1, respectively, ANOVA; Table 1). Despite similar fasting plasma insulin concentrations and glucose responses during the OGTT, plasma insulin concentrations at 30 and 120 min during OGTT (INS30, INS120) were lower in the ODF group than in the CON and OMED groups (F = 4.38, P = 0.02; F = 3.81, P = 0.04, respectively; Fig. 1). After adjustment for age, sex, 30 min glucose concentration, and M-low, only INS30 remained lower in the ODF group than in both the CON and OMED groups (lsmeans [95% CI]: 722 pmol/l [132–1,582] in ODF vs. 2,699 pmol/l [2,047–3,352] in CON and 1,790 pmol/l [1,367–2,206] in OMED, P = 0.01).
Figure 1.
Plasma glucose and insulin concentrations during OGTT (A and B), during IVGTT (C and D), and during meal test (E and F) in ODF ●, CON ■, and OMED ▴. Statistical significance as revealed by two-way ANOVA for the time and group factors and their interactions (time × group). OGTT: Post hoc tests for significant differences in time versus group interactions (effect of time [P < 0.0001 for all models]); effect of group (glucose P = 0.02, insulin P = 0.001); effect of time × groups interaction (glucose P = 0.3, insulin P = 0.1). IVGTT: Post hoc tests for significant differences in time versus group interactions (effect of time [P < 0.0001 for all models]); effect of group (glucose P = 0.02, insulin P < 0.0001); effect of time × groups interaction, (glucose P = 0.3, insulin P < 0.0001). IVGTT data available only in six subjects in the ODF, six in the CON, and seven in the OMED group. Meal test: Post hoc tests for significant differences in time versus group interactions (effect of time [P < 0.0001 for all models]); effect of group (glucose P = 0.2, insulin P < 0.0001); effect of time × groups interaction (glucose P = 0.3, insulin P = 0.002). Meal test data available only in six subjects in the ODF, eight in the CON, and eleven in the OMED group. Data are expressed as means ± SE. *P < 0.05 for specific time intervals.
Intravenous glucose administration resulted in a comparable increase in plasma glucose levels in all three groups. The insulin responses were significantly lower in ODF than in CON subjects (effect of diagnosis × time P < 0.0001; ANOVA; Fig. 1). In addition, log-transformed AIR was lower in the ODF group than the CON group (Table 1), and it remained significant after adjustment for age, sex, %BF, and M-low (lsmeans [95% CI]: 2.08 pmol/l (1.96–2.20) in ODF vs. 2.55 pmol [2.24–2.75] in CON and 2.40 pmol/l [2.13–2.55] in OMED, P < 0.05).
During the meal test, there was no difference in glucose responses between groups. The insulin responses during the meal test were significantly lower in ODF than in CON subjects (effect of diagnosis × time × sex P = 0.02; Fig. 1). Plasma insulin concentrations at 30 min of the meal test were slightly lower in the ODF group compared to both the CON and OMED groups, but it did not reach statistical significance prior to adjustment (1,084 ± 462 pmol/l in ODF vs. 2090 ± 1,081 pmol/l in CON and 1,472 ± 673 pmol/l in OMED, P = 0.07; Fig. 1), or following adjustment for age, sex, 30-min plasma glucose concentration, and M-low (lsmeans [95% CI]: 1,422 pmol/l [489–2,355] in ODF vs. 2,087 pmol/l (1,372–2,803) in CON and 1,492 pmol/l [1,059–1924] in OMED, P = 0.1).
ODFs were on average leaner (having a lower %BF); therefore the mean log transformed M-lows were higher in the ODF group than in the CON and OMED groups (Table 1), but after adjustment for age, sex, and %BF, M-lows were comparable in all three groups (lsmeans [95% CI]: 2.68 [1.80–3.55] mg · kg−1 · EMBS · min−1 in ODF vs. 2.34 [1.73–2.96] mg · kg−1 · EMBS · min−1 in CON, 2.28 [1.87–2.68] mg · kg−1 · EMBS · min−1 in OMED, P = 0.4). For all analyses, results did not differ after accounting for family membership.
CONCLUSIONS
Our analyses indicate that adult nondiabetic offspring of fathers with early onset of diabetes are leaner than offspring of either mothers with early onset of type 2 diabetes or control subjects (neither parent developed type 2 diabetes by age 50 years). ODFs had lower insulin secretion, demonstrated by multiple tests (IVGTT, OGTT, and meal test), but after adjusting for body size, ODFs showed comparable insulin action in vivo. Previous studies have shown that the offspring of individuals with early-onset type 2 diabetes are at increased risk for developing diabetes (5,8). Some studies report that the risk associated with maternal and paternal early onset of diabetes is approximately additive (16), while others do not (17). The present analysis was undertaken to clarify the separate effects of parental diabetes on the offspring's phenotype. To our knowledge, this is the first study to show a paternal influence on adult body composition. While previous studies have shown that offspring of diabetic fathers have lower birth weights (3,18), these studies did not explore parameters for body composition at later ages.
The general effect of family history on offspring phenotype has been extensively examined, but attempts to separate different parental effects have been more limited. Lindsay et al. (3) found that paternal history of diabetes was associated with lower birth weight. In fact, only those with LBW and paternal history of diabetes had increased diabetes risk, and LBW predicted diabetes risk in the father. Our results are in agreement with a previous study also in Pima Indians (5) in which lower AIR was associated with earlier onset of diabetes in either parent. In that study, early onset was defined as age of onset below the median age for the cohort (age <41 years for mothers and <46 years for fathers). In those whose mothers developed diabetes at <35 years of age (exclusive of exposure to diabetes in pregnancy), insulin secretion rate was also lower than in those whose parents developed type 2 diabetes at a later age. However, it is not clear to what extent differences in glucose tolerance (i.e., greater impaired glucose tolerance in group of offspring of the mothers with early onset of type 2 diabetes) may have affected these results. Our results extend and add to these findings as we are able to demonstrate in different tests (IVGTT, OGTT, and meal tests) done on different days a consistently lower AIR. During the OGTT, the 30-min insulin remained lower in ODF compared to both OMED and CON, even after adjustment for age, sex, 30-min glucose, and M-low. In contrast, the 120-min insulin was no longer significantly lower after the same adjustments. These results indicate that the primary effect of early paternal diabetes is on early insulin secretion, rather than insulin action. This is supported by the relatively lower fasting plasma glucose in ODFs and the lack of a difference in M-low after adjustment for body size.
Our results imply an imprinted effect identified by early-onset paternal diabetes. Imprinting is the expression of only a single copy of a gene depending on parent-of-origin and is commonly found in genes that affect fetal growth. There are at least two distinct mechanisms through which epigenetic information can be inherited: DNA methylation (commonly associated with gene silencing and a contributor to X chromosomal inactivation) and histone modification (19). Numerous genes have been found to be imprinted, and some of them are also involved in longitudinal and skeletal growth and may also be involved in organ growth (20). Notable among them are genes such as IGF-2, a growth hormone signaling gene (21). In the human liver, the major production source of circulating IGF-2, the IGF-2 gene is maternally imprinted during fetal life, whereas postnatally IGF-2 is primarily transcribed from the P1 promoter, which is biallelically active in the liver. In other adult tissue (including the pancreas), IGF-2 is mainly transcribed from the paternally active promoters (22). Animal studies have demonstrated that genes involved directly in pancreatic β-cell development might be imprinted, e.g., the gene for insulin receptor subunit Sur1 (6,21). Another study demonstrates that genes essential to pancreatic development, such as pancreatic homeobox transcription factor-1 (Pdx-1) are susceptible to epigenetic modifications (23). In humans, an independent association between paternal insulin resistance and cord insulin concentrations (24) also indicates indirect evidence of imprinting.
Genes related to adipocyte development, specifically preadipocyte factor 1 (Pref1), Necdin, and paternally expressed gene 1 (Peg1) can be imprinted (6). Pref1 (an inhibitor of adipogenesis) is expressed from the paternally inherited chromosome, and imprinting of Pref1 is under complex control by both maternal and paternal alleles (6). Although a study in mice overexpressing Pref1 showed reduced fat content (25), additional studies are required to elucidate the potential role of epigenetic effects in adipose tissue development in humans.
Because of our stringent group criteria, our sample size is small. However, our findings of a leaner phenotype in the ODF group are robust and consistent with the previous findings of LBW in this group. Our results could have been affected by the fact that these individuals were studied in adulthood, and so any individuals who developed type 2 diabetes prior to age 18 years were excluded. However, this would have, if anything, reduced our power to see group differences, particularly in an important diabetes risk factor such as AIR. Although the age of onset of diabetes in the parents was quite young, there was a slight (3.8 ± 4.0 years) but statistically significant difference in the age of onset of diabetes in parents of the ODF versus OMED group. However, the AIR in ODF was markedly lower across different tests on different days, implying that the paternal effect was more important than this relatively small difference in age of diagnosis.
In conclusion, the results of the present analyses indicate that offspring of fathers with early-onset diabetes are leaner and have lower early insulin secretion compared to individuals in whom both parents remained without diabetes up to 50 years of age. Insulin action, after adjustment for body size, was comparable in all three groups of offspring. These findings indicate an important role of paternal heritability in body composition and β-cell dysfunction. Paternal transmission patterns for susceptibility to diabetes indicate that epigenetic mechanisms are involved in the predisposition to diabetes. Whether epigenetic markers are associated with parent-of-origin effects needs further investigation.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health (NIH), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
No potential conflicts of interest relevant to this article were reported.
A.P. contributed to the discussion and wrote the manuscript. J.C.B. researched data, contributed to the discussion, and reviewed/edited the manuscript. C.B. researched data, contributed to the discussion, and reviewed/edited the manuscript. J.K. researched data, contributed to the discussion, and reviewed/edited the manuscript.
The authors acknowledge the help of the nursing and dietary staffs of the NIH metabolic unit for the care of the volunteers. The authors are grateful to the members and leaders of the Gila River Indian Community for their continuing cooperation in these studies. The authors thank Dr. William C. Knowler of the Phoenix Epidemiology and Clinical Research Branch, NIDDK, NIH, Phoenix, Arizona, for help with calculation of cumulative incidence of type 2 diabetes (CI).
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Lillioja S, Mott DM, Zawadzki JK, Young AA, Abbott WG, Knowler WC, Bennett PH, Moll P, Bogardus C: In vivo insulin action is familial characteristic in nondiabetic Pima Indians. Diabetes 1987;36:1329–1335 [DOI] [PubMed] [Google Scholar]
- 2. Sakul H, Pratley R, Cardon L, Ravussin E, Mott D, Bogardus C: Familiality of physical and metabolic characteristics that predict the development of non-insulin-dependent diabetes mellitus in Pima Indians. Am J Hum Genet 1997;60:651–656 [PMC free article] [PubMed] [Google Scholar]
- 3. Lindsay RS, Dabelea D, Roumain J, Hanson RL, Bennett PH, Knowler WC: Type 2 diabetes and low birth weight: the role of paternal inheritance in the association of low birth weight and diabetes. Diabetes 2000;49:445–449 [DOI] [PubMed] [Google Scholar]
- 4. McCance DR, Pettitt DJ, Hanson RL, Jacobsson LT, Knowler WC, Bennett PH: Birth weight and non-insulin dependent diabetes: thrifty genotype, thrifty phenotype, or surviving small baby genotype? BMJ 1994;308:942–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gautier JF, Wilson C, Weyer C, Mott D, Knowler WC, Cavaghan M, Polonsky KS, Bogardus C, Pratley RE: Low acute insulin secretory responses in adult offspring of people with early-onset type 2 diabetes. Diabetes 2001;50:1828–1833 [DOI] [PubMed] [Google Scholar]
- 6. Jimenez-Chillaron JC, Isganaitis E, Charalambous M, Gesta S, Pentinat-Pelegrin T, Faucette RR, Otis JP, Chow A, Diaz R, Ferguson-Smith A, Patti ME: Intergenerational transmission of glucose intolerance and obesity by in utero undernutrition in mice. Diabetes 2009;58:460–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization, Department of Noncommunicable Disease Surveillance. Definition, Diagnosis and Classification of Diabetes and its Complications: Report of a WHO Consultation. Part 1: Diagnosis and classification of diabetes. Geneva, Switzerland, World Health Org., 1999, p. 1–59 [Google Scholar]
- 8. Hanson RL, Knowler WC: Analytic strategies to detect linkage to a common disorder with genetically determined age of onset: diabetes mellitus in Pima Indians. Genet Epidemiol 1998;15:299–315 [DOI] [PubMed] [Google Scholar]
- 9. Tataranni PA, Ravussin E: Use of dual-energy X-ray absorptiometry in obese individuals. Am J Clin Nutr 1995;62:730–734 [DOI] [PubMed] [Google Scholar]
- 10. Bogardus C, Lillioja S, Howard BV, Reaven G, Mott D: Relationships between insulin secretion, insulin action, and fasting plasma glucose concentration in nondiabetic and noninsulin-dependent diabetic subjects. J Clin Invest 1984;74:1238–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Steele R: Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci 1959;82:420–430 [DOI] [PubMed] [Google Scholar]
- 12. Lillioja S, Mott DM, Howard BV, Bennett PH, Yki-Järvinen H, Freymond D, Nyomba BL, Zurlo F, Swinburn B, Bogardus C: Impaired glucose tolerance as a disorder of insulin action. Longitudinal and cross-sectional studies in Pima Indians. N Engl J Med 1988;318:1217–1225 [DOI] [PubMed] [Google Scholar]
- 13. Lillioja S, Nyomba BL, Saad MF, Ferraro R, Castillo C, Bennett PH, Bogardus C: Exaggerated early insulin release and insulin resistance in a diabetes-prone population: a metabolic comparison of Pima Indians and Caucasians. J Clin Endocrinol Metab 1991;73:866–876 [DOI] [PubMed] [Google Scholar]
- 14. Yalow RS, Berson SA: Plasma insulin concentrations in nondiabetic and early diabetic subjects. Determinations by a new sensitive immuno-assay technic. Diabetes 1960;9:254–260 [DOI] [PubMed] [Google Scholar]
- 15. Weyer C, Bogardus C, Pratley RE: Metabolic characteristics of individuals with impaired fasting glucose and/or impaired glucose tolerance. Diabetes 1999;48:2197–2203 [DOI] [PubMed] [Google Scholar]
- 16. Meigs JB, Cupples LA, Wilson PW: Parental transmission of type 2 diabetes: the Framingham Offspring Study. Diabetes 2000;49:2201–2207 [DOI] [PubMed] [Google Scholar]
- 17. Mitchell BD, Valdez R, Hazuda HP, Haffner SM, Monterrosa A, Stern MP:Differences in the prevalence of diabetes and impaired glucose tolerance according to maternal or paternal history of diabetes. Diabetes Care 1993;16:1262–1267 [DOI] [PubMed] [Google Scholar]
- 18. Alvarsson M, Efendic S, Grill VE: Insulin responses to glucose in healthy males are associated with adult height but not with birth weight. J Intern Med 1994;236:275–279 [DOI] [PubMed] [Google Scholar]
- 19. Pinney SE, Simmons RA: Epigenetic mechanisms in the development of type 2 diabetes.Trends Endocrinol Metab 2010;21:223–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Knight B, Shields BM, Turner M, Powell RJ, Yajnik CS, Hattersley AT: Evidence of genetic regulation of fetal longitudinal growth. Early Hum Dev 2005;81:823–831 [DOI] [PubMed] [Google Scholar]
- 21. Jimenez-Chillaron JC, Hernandez-Valencia M, Reamer C, Fisher S, Joszi A, Hirshman M, Oge A, Walrond S, Przybyla R, Boozer C, Goodyear LJ, Patti ME: Beta-cell secretory dysfunction in the pathogenesis of low birth weight-associated diabetes: a murine model. Diabetes 2005;54:702–711 [DOI] [PubMed] [Google Scholar]
- 22. Souren NY, Paulussen AD, Steyls A, Loos RJ, Brandao RD, Gielen M, Smeets HJ, Beunen G, Fagard R, Derom C, Vlietinck R, Geraedts JP, Zeegers MP: Parent-of-origin specific linkage and association of the IGF2 gene region with birth weight and adult metabolic risk factors. Int J Obes (Lond) 2009;33:962–970 [DOI] [PubMed] [Google Scholar]
- 23. Bernardo AS, Hay CW, Docherty K: Pancreatic transcription factors and their role in the birth, life and survival of the pancreatic beta cell. Mol Cell Endocrinol 2008;294:1–9 [DOI] [PubMed] [Google Scholar]
- 24. Shields BM, Knight B, Turner M, Wilkins-Wall B, Shakespeare L, Powell RJ, Hannemann M, Clark PM, Yajnik CS, Hattersley AT: Paternal insulin resistance and its association with umbilical cord insulin concentrations. Diabetologia 2006;49:2668–2674 [DOI] [PubMed] [Google Scholar]
- 25. Lee K, Villena JA, Moon YS, Kim KH, Lee S, Kang C, Sul HS: Inhibition of adipogenesis and development of glucose intolerance by soluble preadipocyte factor-1 (Pref-1). J Clin Invest 2003;111:453–461 [DOI] [PMC free article] [PubMed] [Google Scholar]