Abstract
OBJECTIVE
Plasma protein growth arrest–specific 6 (Gas6) is important to the inflammatory process and is involved in the development of diabetic renal and vascular complications. We set out to determine whether plasma Gas6 levels are associated with altered glucose tolerance, insulin sensitivity, inflammation, and endothelial dysfunction.
RESEARCH DESIGN AND METHODS
A total of 278 adults, including 96 with normal glucose tolerance (NGT), 82 with impaired glucose tolerance (IGT), and 100 with type 2 diabetes were recruited. Plasma Gas6 concentration and biochemical, proinflammatory, and endothelial variables were determined. Insulin sensitivity was examined by homeostasis model assessment.
RESULTS
Plasma Gas6 concentration was significantly lower among patients with type 2 diabetes compared with subjects with NGT (P < 0.001). The plasma Gas6 value was inversely correlated with fasting glucose, tumor necrosis factor (TNF)-α, interleukin (IL)-6, and vascular cell adhesion molecule (VCAM)-1. In multivariate logistic regression analysis, after adjustment for established diabetes risk factors, higher plasma Gas6 concentrations were significantly associated with a decreased risk of type 2 diabetes. Moreover, the association became slightly stronger after further adjustment for TNF-α, IL-6, high-sensitive C-reactive protein, E-selectin, intercellular adhesion molecule-1, and VCAM-1.
CONCLUSIONS
Plasma Gas6 is associated with altered glucose tolerance, inflammation, and endothelial dysfunction. It also may represent a novel independent risk factor of type 2 diabetes and a potential surrogate marker of inflammation and endothelial dysfunction.
The epidemic of type 2 diabetes and impaired glucose tolerance (IGT) is one of the main causes of morbidity and mortality worldwide (1). In both disorders, tissues such as muscle, fat, liver, and endothelial cells become less responsive or, in some cases, resistant to insulin (2). Although it is well established that insulin resistance and impaired insulin secretion are central to the pathogenesis of type 2 diabetes, it has been unclear how these abnormalities arise and how they are related to many different clinical and biochemical features common in type 2 diabetes, including central obesity, hypertension, accelerated atherosclerosis, chronic inflammation, dyslipidemia, and disordered hemostasis.
Protein growth arrest–specific 6 (Gas6) was the last addition to the family of plasma vitamin K–dependent proteins. Gas6 was cloned and characterized in 1993 and found to be similar to plasma anticoagulant protein S (3). Soon after, it was recognized as a growth factor–like molecule, as it interacted with receptor tyrosine kinases of the TAM (Tyro-3, Axl, Mer) family (4). The Gas6/TAM system regulates an intriguing mix of processes, including cell survival and proliferation, cell adhesion and migration, blood clot stabilization, and inflammatory cytokine release (5–8). Therefore, the role of the Gas6/TAM system has been found to be important in inflammation; hemostasis; autoimmune disease; nervous, reproductive, and vascular systems; and cancer (9).
Recently, several reports (10–12) revealed that the Gas6/TAM system was involved in the pathogenesis of diabetic renal and vascular disease. Expression of Gas6/TAM was increased in the glomerulus of diabetic rats, which led to mesangial and glomerular hypertrophy (10). In vascular smooth muscle cells (VSMCs), Gas6/TAM signaling increased cell survival in the presence of low glucose and increased cell migration in the presence of high glucose (11). VSMC migration was increased in patients with diabetes, and diabetes accelerated the accumulation of VSMCs in atherosclerotic lesions (12). These preclinical studies indicate that Gas6/TAM likely represents an important pathogenic mechanism for renal and cardiovascular complications associated with diabetes. However, little is known about the clinical significance of the Gas6/TAM system in patients with diabetes and its association with various biochemical variables that are common in diabetic patients. We have addressed this issue by conducting a cross-sectional study to determine whether plasma Gas6 levels are associated with altered glucose tolerance, insulin sensitivity, inflammatory, and endothelial dysfunction markers in humans.
RESEARCH DESIGN AND METHODS
A total of 278 adults were recruited from the outpatient clinics of Tri-Service General Hospital, Taipei, Taiwan. Criteria for inclusion into this study were as follows: 20–75 years of age; BMI <35 kg/m2; absence of infection within the previous weeks; absence of taking oral anticoagulants and antidiabetes therapy, including oral hypoglycemic agents, insulin, and glucagon-like peptide 1; and absence of malignant tumor history. Exclusion criteria included women who were pregnant or breast feeding; patients with impaired renal function (serum creatinine ≥132.6 μmol/l); patients with abnormal serum aspartate aminotransferase or alanine aminotransferase (2.5 times above the upper normal ranges); patients with acute or chronic pancreatitis; patients with a history of cerebrovascular accident, myocardial infarction, or heart failure; patients with autoimmune disorders or psychiatric diseases, including mood disorders and alcoholism; and patients taking concomitant drugs such as β-blockers, diuretics, cholestyramine, or systemic steroids. A 75-g oral glucose tolerance test (OGTT) was performed in all subjects after they had fasted for at least 10 h. According to the American Diabetes Association criteria, participants were divided into normal glucose tolerance (NGT; n = 96, fasting glucose <5.6 mmol/l with a 2-h postload plasma glucose of <7.8 mmol/l), IGT (n = 82, fasting glucose <7.0 mmol/l and 2-h postload glucose between 7.8 and 11.1 mmol/l), and previously unknown type 2 diabetes (n = 100, fasting glucose ≥7.0 mmol/l or 2-h postload glucose >11.1 mmol/l). The institutional review board of the Tri-Service General Hospital approved the protocol, and all subjects gave written informed consent.
Analytic methods
After 10 h of fasting, blood samples were obtained to determine plasma glucose, insulin, creatinine, and lipid profiles. Plasma circulating high-sensitive C-reactive protein (hsCRP), tumor necrosis factor (TNF)-α, and interleukin (IL)-6 levels; E-selectin; intercellular adhesion molecule (ICAM)-1; and vascular cell adhesion molecule (VCAM)-1 were subsequently measured. Serum total cholesterol, triglycerides, and LDL cholesterol were measured using the dry, multilayer analytical slide method in the Fuji Dri-Chem 3000 analyzer (Fuji Photo Film, Tokyo, Japan). The intra-assay and interassay coefficients of variation (CVs) for LDC cholesterol were 0.8 and 2.5%, respectively. Serum levels of HDL cholesterol were determined by an enzymatic cholesterol assay method after dextran sulfate precipitation. The intra-assay and interassay CVs for HDL cholesterol were 1.1 and 1.7%, respectively. The levels of A1C were evaluated by the ion-exchange high-pressure liquid chromatography method (Variant II; Bio-Rad, Los Angeles, CA). The intra-assay and interassay CVs for A1C were 1.3 and 2.2%, respectively. Plasma glucose concentrations were determined by the glucose oxidase method on a Beckman Glucose Analyzer II (Beckman Instruments, Fullerton, CA). The intra-assay and interassay CVs for glucose were 0.6 and 1.5%, respectively. Plasma insulin was measured with a commercial immunoradiometric kit (BioSource Europe, Nivelles, Belgium). The intra-assay and interassay CVs for insulin were 2.2 and 6.5%, respectively. Plasma hsCRP levels were measured using the Tina-quant (Latex) high-sensitivity assay (Roche, Mannheim, Germany). The intra-assay and interassay CVs for hsCRP were 3.7 and 4.9%, respectively. Serum IL-6 concentrations were determined by a human high-sensitivity enzyme-linked immunosorbent assay (ELISA) (Besancon, France). The intra-assay and interassay CVs for IL-6 were 1.5 and 5.3%, respectively. Serum TNF-α was measured with the Biotrak high-sensitivity human ELISA kit from Amersham Biosciences (Buckinghamshire, U.K.). The intra-assay and interassay CVs for TNF-α were 3.5 and 5.3%, respectively. Levels of E-selectin, ICAM-1, and VCAM-1 were measured by commercial ELISA (R&D Systems, Minneapolis, MN). The intra-assay and interassay CVs for E-selectin were 4.5 and 6.2%, respectively; for ICAM-1 were 3.5 and 7.1%, respectively; and for VCAM-1 were 5.0 and 8.7%, respectively. All concentrations of the above biochemical variables were determined in duplicate, and the values of the two samples were averaged. Insulin sensitivity was assessed using the homeostasis model assessment (HOMA), in which the HOMA of insulin resistance (HOMA-IR) = ([fasting insulin {μU/ml}] × [fasting glucose {mmol/l}])/22.5 (13).
Measurement of Gas6
The Gas6 protein was measured with a sandwich ELISA. The method has been validated according to the Food and Drug Administration guidelines in a previous study (intra-assay and interassay CVs were 6.5 and 8.5%, respectively, mean recovery on 10 patients of 97%, lower limit of quantification 0.26 ng/ml) (14). Briefly, a 96-well microtitre plate was coated overnight at room temperature with 4 μg/ml of polyclonal mouse anti-human Gas6 antibody (R&D Systems, Lille, France). After three washes with 0.05% Tween 20 in PBS, wells were blocked with 1% BSA in PBS for 1 h at room temperature. Three additional washes were then performed and 100 μl plasma or standards (recombinant human Gas6; R&D Systems) were added for 2 h at room temperature. Washes were repeated and 100 ng/ml biotinylated monoclonal goat anti-human Gas6 antibody (R&D Systems) was added for 2 h at room temperature. Detection was performed with peroxydase-conjugated streptavidin. Measurements were repeated three times.
Statistical methods
Descriptive results of continuous variables were expressed as means ± SE. Before statistical analysis, normal distribution and homogeneity of the variables were evaluated using Levene test for quality of variance, and variables were then given a base logarithmic transformation if necessary. The parameters HOMA-IR, fasting insulin, triglycerides, TNF-α, IL-6, hsCRP, and Gas6 were analyzed and tested for significance on a log scale. We used unpaired t test and ANOVA test (with post hoc least significant difference test) for comparisons of quantitative variables. Bonferroni adjustment for multiple comparisons among subgroups was analyzed, and the corrected P value of 0.0025 was considered as statistical significance. Relationships between variables were tested using Spearman rank-order correlations and partial correlation analysis after adjusting for age. Multivariate logistic regression analysis, with type 2 diabetes as dependent variable, was used to study the independent determinants of plasma Gas6 and other covariates. The statistical analyses were performed using SPSS (version 13.0; SPSS, Chicago, IL).
RESULTS
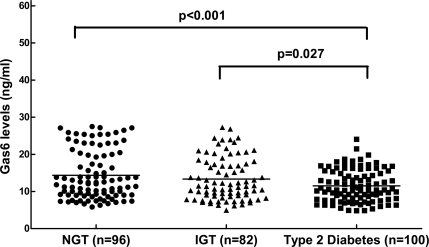
Characteristics of the subjects according to glucose tolerance status are shown in Table 1. Patients with type 2 diabetes had higher BMI, waist-to-hip ratio, blood pressure, HOMA-IR, triglycerides, hsCRP, E-selectin, and ICAM-1 and lower HDL cholesterol than subjects with NGT. Plasma Gas6 concentrations were significantly lower among patients with type 2 diabetes (11.5 ± 0.42 ng/ml) compared with subjects with NGT (14.3 ± 0.66 ng/ml) (P < 0.001), as illustrated in Fig. 1. In all subjects as a whole, the plasma Gas6 value was significantly inversely correlated with fasting, TNF-α, IL-6, and VCAM-1 after adjustment for age (Table 2).
Table 1.
Anthropometric and biochemical variables among different glucose tolerance subjects
| NGT | IGT | Type 2 diabetes | P value* | |
|---|---|---|---|---|
| n | 96 | 82 | 100 | |
| Age (years) | 50.2 ± 1.54 | 56.2 ± 1.45 | 52.4 ± 1.53 | 0.026 |
| Sex (male/female) | 43/53 | 26/56 | 57/43 | 0.003 |
| BMI (kg/m2) | 23.9 ± 0.37 | 25.4 ± 0.50 | 26.0 ± 0.39 | 0.002 † |
| Waist-to-hip ratio | 0.85 ± 0.01 | 0.86 ± 0.01 | 0.90 ± 0.01 | <0.001 † |
| Blood pressure (mmHg) | ||||
| Systolic | 118.8 ± 1.67 | 125.0 ± 1.77 | 126.2 ± 1.80 | 0.006† |
| Diastolic | 76.9 ± 0.91 | 78.1 ± 1.16 | 81.1 ± 0.91 | 0.008† |
| OGTT glucose (mmol/l) | ||||
| Fasting glucose | 5.05 ± 0.06 | 5.42 ± 0.07 | 8.30 ± 0.44 | <0.001 † |
| 2-h glucose | 6.32 ± 0.09 | 9.29 ± 0.10 | 16.9 ± 0.54 | <0.001 † |
| OGTT insulin (pmol/l) | ||||
| Fasting insulin‡ | 54.0 ± 3.85 | 75.5 ± 11.82 | 84.1 ± 6.19 | 0.013† |
| 2-h insulin‡ | 388.2 ± 32.5 | 659.1 ± 61.49 | 528.2 ± 52.05 | 0.001 |
| A1C (%) | 5.6 ± 0.03 | 6.0 ± 0.05 | 8.2 ± 0.33 | <0.001 † |
| HOMA-IR‡ | 2.04 ± 0.15 | 3.19 ± 0.61 | 4.85 ± 0.38 | <0.001 † |
| Triglycerides (mmol/l)‡ | 2.94 ± 0.17 | 3.64 ± 0.27 | 4.97 ± 0.30 | <0.001 † |
| HDL cholesterol (mmol/l) | 1.49 ± 0.05 | 1.45 ± 0.06 | 1.18 ± 0.03 | <0.001 † |
| Inflammatory markers | ||||
| TNF-α (ng/ml)‡ | 3.19 ± 0.20 | 3.20 ± 0.21 | 3.31 ± 0.23 | 0.914 |
| IL-6 (pg/ml)‡ | 2.6 ± 0.45 | 4.5 ± 1.31 | 5.9 ± 1.44 | 0.113 |
| hsCRP (mg/l)‡ | 0.70 ± 0.08 | 0.92 ± 0.09 | 1.15 ± 0.10 | 0.002 † |
| Endothelial dysfunction markers | ||||
| E-selectin (ng/ml) | 45.1 ± 1.92 | 47.4 ± 2.54 | 60.9 ± 3.00 | <0.001 † |
| VCAM-1 (ng/ml) | 527.6 ± 31.08 | 506.7 ± 38.72 | 660.2 ± 40.38 | 0.006† |
| ICAM-1 (ng/ml) | 248.8 ± 9.08 | 244.9 ± 8.36 | 293.6 ± 10.39 | <0.001 † |
| Gas6 (ng/ml)‡ | 14.3 ± 0.66 | 13.3 ± 0.63 | 11.5 ± 0.42 | 0.002 † |
Data are means ± SE.
*Assessed by one-way ANOVA, data shown as mean ± standard error mean.
†P < 0.0025, NGT vs. type 2 diabetes. All assessed by post hoc least significant difference test.
‡The logarithms of these variables were used for the analysis. Boldface indicates statistical significance.
Figure 1.
Plasma Gas6 concentrations in subjects with NGT, subjects with IGT, and patients with type 2 diabetes. The lines represent the median values in each group. The type 2 diabetic group had significantly lower plasma Gas6 levels than those with NGT (P < 0.001).
Table 2.
Age-adjusted Spearman partial correlation coefficients between plasma Gas6 concentration and biochemical variables
| Spearman partial correlation coefficient (n = 278)* |
||
|---|---|---|
| r | P | |
| BMI | −0.074 | 0.222 |
| Waist-to-hip ratio | −0.131 | 0.031 |
| OGTT glucose (mmol/l) | ||
| Fasting glucose | −0.195 | 0.001 |
| 2-h glucose | −0.157 | 0.009 |
| OGTT insulin (pmol/l) | ||
| Fasting insulin† | −0.031 | 0.612 |
| 2-h insulin† | 0.002 | 0.975 |
| HOMA-IR† | −0.107 | 0.076 |
| A1C (%) | −0.152 | 0.027 |
| TNF-α (ng/ml)† | −0.221 | <0.001 |
| IL-6 (pg/ml)† | −0.230 | <0.001 |
| hsCRP (mg/l)† | −0.071 | 0.242 |
| E-selectin (ng/ml) | −0.157 | 0.009 |
| VCAM-1 (ng/ml) | −0.269 | <0.001 |
| ICAM-1 (ng/ml) | −0.026 | 0.675 |
*Corrected for age.
†The logarithms of these variables were used for the analysis. Boldface indicates statistical significance.
A multivariate logistic regression analysis to investigate whether plasma Gas6 values were related to type 2 diabetes independent from other established diabetes risk factors is shown in Table 3. After adjustment for age, sex, BMI, waist-to-hip ratio, blood pressure, smoking, and alcohol consumption, higher plasma Gas6 concentrations were significantly associated with a decreased risk of type 2 diabetes (type 2 diabetes versus NGT, 0.93 [0.87–0.99]; type 2 diabetes versus IGT+NGT, 0.94 [0.89–0.99]). This association remained significant after further adjustment for other covariates (including TNF-α, IL-6, and hsCRP). Moreover, the association became slightly stronger after further adjustment for TNF-α, IL-6, hsCRP, E-selectin, ICAM-1, and VCAM-1 (type 2 diabetes versus NGT, 0.90 [0.83–0.97]; type 2 diabetes versus IGT+NGT, 0.92 [0.86–0.98]).
Table 3.
Multivariate logistic regression analyses of plasma Gas 6 concentration among different glucose tolerance subjects
| Type 2 diabetes vs. NGT | Type 2 diabetes vs. IGT | Type 2 diabetes vs. (IGT+NGT) | |
|---|---|---|---|
| Model 1 | 0.93 (0.87–0.99) | 0.94 (0.88–1.01) | 0.94 (0.89–0.99) |
| Model 2 | 0.92 (0.86–0.99) | 0.93 (0.87–0.99) | 0.94 (0.89–0.99) |
| Model 3 | 0.90 (0.83–0.97) | 0.92 (0.85–0.99) | 0.92 (0.86–0.98) |
Data are odds ratio (95% CI). Model 1: adjusted for age, sex, BMI, waist-to-hip ratio, blood pressure, smoking, and alcohol consumption. Model 2: further adjustment for TNF-α, IL-6, and hsCRP. Model 3: further adjustment for TNF-α, IL-6, hsCRP, E-selectin, ICAM-1, and VCAM-1.
CONCLUSIONS
Numerous studies have shown that the Gas6/TAM system regulates cell survival, proliferation, migration, adhesion, and phagocytosis. Consequently, altered activity/expression of Gas6/TAM components has been detected in a variety of pathologies such as inflammation, coagulopathy, cancer, autoimmune disease, and diabetic vascular and renal diseases (9). However, direct clinical evidence of the Gas6/TAM system is lacking. Our results, described for the first time herein, revealed that plasma Gas6 concentrations were significantly lower among patients with new onset of type 2 diabetes and were associated with glucose levels, inflammation, and endothelial dysfunction markers. These findings demonstrate that Gas6/TAM signaling is associated with type 2 diabetes, inflammation, and endothelial dysfunction, thereby implicating that Gas6/TAM signaling may play a potential role in the pathogenesis of type 2 diabetes, inflammation, and endothelial dysfunction.
Increasing evidence indicates that chronic low-grade inflammation and activation of the innate immune system are closely involved in the pathogenesis of type 2 diabetes (15). Recently, several reports have shown that Gas6/TAM signaling resulted in intrinsic inhibition of the inflammatory response in dendritic cells and macrophages, which indicated a possible role of the Gas6 protein in controlling innate immunity and inflammation processes (16). For example, TAM triple-knockout mice with low Gas6 levels showed hyperactivation of monocytes/macrophages, and their monocytes reacted with an excessive secretion of TNF-α and IL-6 after lipopolysaccharides challenge (17). Our results revealed that plasma Gas6 values were lower in type 2 diabetes and also negatively correlated with inflammation markers including TNF-α and IL-6. Therefore, we hypothesized that inflammatory effects of high glucose may be, at least in part, mediated through low Gas6 levels as well as reduced TAM signaling and, consequently, activated innate immunity.
Several studies have suggested that the Gas6/TAM system may play a role in vascular diseases, such as atherosclerosis, which are characterized by accumulation of VSMCs. Cavet et al. (11) investigated the effects of varying glucose concentration on Axl signaling in VSMCs and demonstrated a role for glucose in altering Axl signaling through coupling to binding partners. Recently, Jiang et al. (18) demonstrated that the Gas6 plasma concentrations correlated with cardiovascular disease, especially in patients with acute coronary syndrome. In addition, Gas6 c.834 + 7G>A polymorphism was associated with a lower risk for cardiovascular disease. With the exception of VSMCs, prospective evidence linked endothelial dysfunction with atherosclerosis, demonstrating that endothelial dysfunction was the first step in atherosclerosis (19). Endothelial dysfunction contributes to cardiovascular diseases, including hypertension, atherosclerosis, and coronary heart disease, which are also characterized by insulin resistance (20). Two recent studies (21,22) in humans provide evidence that plasma Gas6 originates from endothelial cells and leukocytes. Our results demonstrated that plasma Gas6 values are significantly, but negatively, correlated with the endothelial dysfunction marker VCAM-1. Meanwhile, using in vitro studies (Y.J. Hung, C.H. Lee, Y.S. Shieh, unpublished data), we provided evidence that hyperglycemia can cause endothelial dysfunction with downregulation of Gas6/TAM signaling. Hence, we hypothesize that hyperglycemia will lead to diminished Gas6/TAM receptor signaling, which may result in cross-talk between Gas6/TAM signaling and insulin signaling, thereby inducing an imbalance in the production of nitric oxide and endothelin-1 in endothelial cells.
It can be concluded from this study that plasma Gas6 levels are associated with altered glucose tolerance, inflammation, and endothelial dysfunction. Plasma Gas6 concentration may represent an independent risk factor of type 2 diabetes and a potential surrogate marker of inflammation and endothelial dysfunction. These results support the hypothesis that modulation of Gas6 activity may provide an important point for intervention. Gas6/TAM signaling represents a new class of therapeutic targets. Understanding the nature of the Gas6/TAM interaction would ultimately help in the development of novel small molecules or neutralizing monoclonal antibodies for therapeutic applications for diseases in which the interaction between Gas6 and TAM receptors contributes to their progression or pathology (23).
Acknowledgments
This work was supported by research grants from the National Science Council (NSC 96-2314-B-016-020-MY3, NSC 97-2314-B-016-015), the Office of National Science and Technology Program for Biotechnology and Pharmaceuticals (DOH99-TD-I-111-TM012), and Tri-Service General Hospital (TSGH-C98-23), Taiwan.
No potential conflicts of interest relevant to this article were reported.
We declare that all authors listed have actively participated in the study and met the requirements of the authorship. Y.-J.H. wrote the manuscript and researched data. C.-H.L. researched data and reviewed/edited the manuscript. N.-F.C. contributed to discussion and statistical analyses and reviewed/edited the manuscript. Y.-S.S. supervised the project and reviewed/edited the manuscript. All authors have read and approved the final version of the manuscript.
We are grateful to Dr. Yu-Ching Chou and Dr. Fu-Huang Lin (School of Public Health, National Defense Medical Center, Taipei, Taiwan) for assistance with the statistical analyses.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Zimmet P, Alberti KG, Shaw J: Global and societal implications of the diabetes epidemic. Nature 2001;414:782–787 [DOI] [PubMed] [Google Scholar]
- 2. Stumvoll M, Goldstein BJ, van Haeften TW: Type 2 diabetes: principles of pathogenesis and therapy. Lancet 2005;365:1333–1346 [DOI] [PubMed] [Google Scholar]
- 3. Manfioletti G, Brancolini C, Avanzi G, Schneider C: The protein encoded by a growth arrest-specific gene (gas6) is a new member of the vitamin K-dependent proteins related to protein S, a negative coregulator in the blood coagulation cascade. Mol Cell Biol 1993;13:4976–4985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hafizi S, Dahlback B: Gas6 and protein S: vitamin K-dependent ligands for the Axl receptor tyrosine kinase subfamily FEBS J 2006;273:5231–5244 [DOI] [PubMed] [Google Scholar]
- 5. Godowski PJ, Mark MR, Chen J, Sadick MD, Raab H, Hammonds RG: Reevaluation of the roles of protein S and Gas6 as ligands for the receptor tyrosine kinase Rse/Tyro 3. Cell 1995;82:355–358 [DOI] [PubMed] [Google Scholar]
- 6. Nagata K, Ohashi K, Nakano T, Arita H, Zong C, Hanafusa H, Mizuno K: Identification of the product of growth arrest-specific gene 6 as a common ligand for Axl, Sky, and Mer receptor tyrosine kinases J Biol Chem 1996;271:30022–30027 [DOI] [PubMed] [Google Scholar]
- 7. Bellosta P, Zhang Q, Goff SP, Basilico C: Signaling through the ARK tyrosine kinase receptor protects from apoptosis in the absence of growth stimulation. Oncogene 1997;15:2387–2397 [DOI] [PubMed] [Google Scholar]
- 8. Collett G, Wood A, Alexander MY, Varnum BC, Boot-Handford RP, Ohanian V, Ohanian J, Fridell YW, Canfield AE: Receptor tyrosine kinase Axl modulates the osteogenic differentiation of pericytes. Circ Res 2003;92:1123–1129 [DOI] [PubMed] [Google Scholar]
- 9. Linger RM, Keating AK, Earp HS, Graham DK: TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv Cancer Res 2008;100:35–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nagai K, Arai H, Yanagita M, Matsubara T, Kanamori H, Nakano T, Iehara N, Fukatsu A, Kita T, Doi T: Growth arrest-specific gene 6 is involved in glomerular hypertrophy in the early stage of diabetic nephropathy. J Biol Chem 2003;278:18229–18234 [DOI] [PubMed] [Google Scholar]
- 11. Cavet ME, Smolock EM, Ozturk OH, World C, Pang J, Konishi A, Berk BC: Gas6-axl receptor signaling is regulated by glucose in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 2008;28:886–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Suzuki LA, Poot M, Gerrity RG, Bornfeldt KE: Diabetes accelerates smooth muscle accumulation in lesions of atherosclerosis: lack of direct growth-promoting effects of high glucose levels. Diabetes 2001;50:851–860 [DOI] [PubMed] [Google Scholar]
- 13. Muniyappa R, Lee S, Chen H, Quon MJ: Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab 2008;294:E15–E26 [DOI] [PubMed] [Google Scholar]
- 14. Alciato F, Sainaghi PP, Castello L, Bergamasco L, Carnieletto S, Avanzi GC: Development and validation of an ELISA method for detection of growth arrest specific 6 (GAS6) protein in human plasma. J Immunoassay Immunochem 2008;29:167–180 [DOI] [PubMed] [Google Scholar]
- 15. Pickup JC: Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care 2004;27:813–823 [DOI] [PubMed] [Google Scholar]
- 16. Lu Q, Lemke G: Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science 2001;293:306–311 [DOI] [PubMed] [Google Scholar]
- 17. Lemke G, Lu Q: Macrophage regulation by Tyro 3 family receptors. Curr Opin Immunol 2003;15:31–36 [DOI] [PubMed] [Google Scholar]
- 18. Jiang L, Liu CY, Yang QF, Wang P, Zhang W: Plasma level of growth arrest-specific 6 (GAS6) protein and genetic variations in the GAS6 gene in patients with acute coronary syndrome. Am J Clin Pathol 2009;131:738–743 [DOI] [PubMed] [Google Scholar]
- 19. Sheetz MJ, King GL: Molecular understanding of hyperglycemia's adverse effects for diabetic complications. JAMA 2002;288:2579–2588 [DOI] [PubMed] [Google Scholar]
- 20. Nigro J, Osman N, Dart AM, Little PJ: Insulin resistance and atherosclerosis. Endocr Rev 2006;27:242–259 [DOI] [PubMed] [Google Scholar]
- 21. Borgel D, Clauser S, Bornstain C, Bieche I, Bissery A, Remones V, Fagon JY, Aiach M, Diehl JL: Elevated growth-arrest-specific protein 6 plasma levels in patients with severe sepsis. Crit Care Med 2006;34:219–222 [DOI] [PubMed] [Google Scholar]
- 22. Gibot S, Massin F, Cravoisy A, Dupays R, Barraud D, Nace L, Bollaert PE: Growth arrest-specific protein 6 plasma concentrations during septic shock. Crit Care 2007;11:R8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fisher PW, Brigham-Burke M, Wu SJ, Luo J, Carton J, Staquet K, Gao W, Jackson S, Bethea D, Chen C, Hu B, Giles-Komar J, Yang J: A novel site contributing to growth-arrest-specific gene 6 binding to its receptors as revealed by a human monoclonal antibody. Biochem J 2005;387:727–735 [DOI] [PMC free article] [PubMed] [Google Scholar]