Abstract
OBJECTIVE
Gestational diabetes mellitus (GDM) is associated with high birth weight in the offspring. This may lead to overweight and insulin resistance during childhood. The aim of the study was to assess the impact of GDM on overweight risk and insulin resistance in offspring.
RESEARCH DESIGN AND METHODS
BMI measurements were collected at age 2, 8, and 11 years from 232 offspring of mothers with GDM (OGDM) and compared with those from 757 offspring of mothers with type 1 diabetes (OT1D) and 431 offspring of nondiabetic mothers (ONDM) born between 1989 and 2000. Insulin resistance (homeostasis model assessment of insulin resistance [HOMA-IR]) was determined at age 8 and 11 years in 751 children (74 OGDM). Overweight was defined as BMI percentile ≥90; insulin resistance was defined by HOMA-IR.
RESULTS
Overweight prevalence was increased in OGDM compared with OT1D and to ONDM throughout childhood (age 11 years 31.1, 15.8, and 15.5%; P = 0.005). Maternal obesity was an important predictor of overweight risk in children (age 11 years odds ratio 7.0 [95% CI 1.8–27.7]; P = 0.006); birth size and maternal smoking during pregnancy were inconsistently associated with and treatment of GDM during pregnancy did not affect overweight risk. HOMA-IR was increased in OGDM compared with offspring of ONDM mothers (P = 0.01, adjusted for sex and age) and was associated with the child's BMI (P = 0.004).
CONCLUSIONS
Overweight and insulin resistance in children is increased in OGDM compared with OT1D or ONDM. The finding that overweight risk is associated mainly with maternal obesity suggests that familial predisposition contributes to childhood growth in these offspring.
The increasing prevalence of obesity in children is a major burden not only for affected individuals but also for the health economy. To develop preventive strategies, it is useful to identify subjects at high risk and factors that predict overweight risk. It is widely accepted that gestational and perinatal factors influence weight development in childhood, and several studies indicated that intrauterine exposure to maternal diabetes conveys high risk for obesity and type 2 diabetes in offspring of mothers with diabetes regardless of maternal diabetes type (1–3). Furthermore, an association between increasing hyperglycemia in pregnancy and increasing risk of childhood obesity has been reported (4). The findings have led to the hypothesis that fetal overnutrition leads to increased risk of obesity and insulin resistance later in life (5).
Not all studies, however, show a direct relationship between childhood obesity and diabetes. Our own studies show that maternal type 1 diabetes is unlikely to be a primary association with obesity in offspring, but that factors such as high birth size predispose offspring of mothers with type 1 diabetes (OT1D) to overweight during childhood (6). In addition, others report that maternal pregravid BMI is the strongest predictor of childhood obesity independent of maternal glucose status or birth weight (7). The aim of our study was to determine whether maternal diabetes per se is a risk factor for childhood obesity and insulin resistance by comparing outcome in offspring of mothers with gestational diabetes mellitus (OGDM) and OT1D. Because a previous study reported age-dependent associations of gestational diabetes mellitus (GDM) with higher child weight status (1), a secondary objective was to examine whether associations between offspring weight and peri- or postnatal factors are consistent over time.
RESEARCH DESIGN AND METHODS
Prevalence and risk of overweight were assessed in 232 children who were enrolled in the prospective German GDM offspring study between 1989 and 2000. All children were from singleton births, had a mother with GDM diagnosed according to criteria of the German Diabetes Association using an oral glucose tolerance test (OGTT) with a 75-g glucose load. Women were considered to have GDM if two of three capillary blood glucose values exceeded the following limits: >5 mmol/l (fasting) before an oral glucose tolerance test, >10.0 mmol/l after 60 min, and 8.6 mmol/l after 120 min. Prevalence of overweight was compared with that of a cohort of OT1D (n = 757) and offspring of nondiabetic mothers (ONDM) (n = 431) followed in the BABYDIAB study (6). In both the GDM and BABYDIAB studies, mothers and their offspring were recruited between 1989 and 2000 Germany-wide through the same network of collaborating obstetric departments and pediatricians and entered into the study before the offspring reached the age of 3 months. All children were followed with similar follow-up visits until the age of 14 years. In both studies, >98% of the families were Caucasian. In families with more than one offspring participating in the study, only the oldest one was included in the analyses.
Data collection
Data on weight and height were collected at birth and at age 2, 8, and 11 years by physicians. At birth, length was measured on a measuring board and weight on a calibrated scale by health care professionals. Weight and height were obtained for 215 children at age 2 years, 89 children at age 8 years, and 74 children at age 11 years. Birth weight was adjusted for sex and gestational age and is expressed as a percentile of the German reference population (8). Offspring less than the 10th percentile were defined as small for gestational age, those ≥90th percentile as large for gestational age and those between >10th and <90th percentile as appropriate for gestational age. From age 2 years, height and weight were measured by physicians at regular clinical visits using a standard protocol. Weight was measured on a calibrated scale with the subject wearing light clothing, and height was measured with a fixed tape standing barefoot. Height, weight, and BMI from age 2 years were expressed as percentiles adjusted for age and sex according to German reference data (9). Overweight was defined as BMI percentile ≥90.
Maternal height and weight in early pregnancy were collected by physicians at the first pregnancy visit, BMI was calculated and categorized as obese (≥30 kg/m2), overweight (25.0–29.9 kg/m2), or normal weight (<25 kg/m2). Data on diabetes treatment (categorized as treated by insulin injections or diet alone) and maternal smoking behavior during pregnancy (defined as < or ≥1 cigarette/day) were obtained from a questionnaire at birth.
Written informed consent was obtained from the parents. The study was approved by the Ethics Committee of Bavaria (Bayerische Landesärztekammer no. 95357).
Insulin resistance measurements substudy
Since 2003, fasting blood samples were collected for the determination of insulin resistance in OGDM as well as in offspring from the BABYDIAB study. Insulin resistance was measured by homeostasis model assessment of insulin resistance (HOMA-IR). A total of 751 children (including 74 OGDM and 425 OT1D) with data on HOMA-IR either at age 8 or 11 years were included in this substudy. Fasting insulin was determined centrally using an automated immunoassay analyzer (AIA 360; Tosoh, San Francisco, CA). The interassay coefficient of variation of the insulin assay is 8.4% at a concentration of 22.6 μU/ml and the lower limit of detection is 0.5 μU/ml.
Statistical analysis
Overweight prevalence in offspring at age 2, 8, and 11 years was compared between groups using the χ2 test. Logistic regression analyses were performed to test the association of maternal BMI at early pregnancy, birth size, maternal smoking during pregnancy, treatment modality during pregnancy, and breast-feeding duration as univariate covariates with overweight risk in OGDM, giving the odds ratio (OR) and 95% CI for overweight for each of the covariates. Factors that were significantly associated with overweight risk in the univariate analysis were included as covariates in the multivariate logistic regression analysis. Missing data were not considered in the univariate analyses but were included as a separate categorical value for each of the variables included in the multivariate analysis. Linear regression was used to compare HOMA-IR in OGDM with those in OT1D and in ONDM adjusted for age and sex and to analyze factors associated with HOMA-IR in OGDM. For all analyses, two-tailed P < 0.05 was considered significant. All statistical analyses were performed using SPSS 17.0 (SPSS, Chicago, IL).
RESULTS
Prevalence and predictors of overweight in OGDM
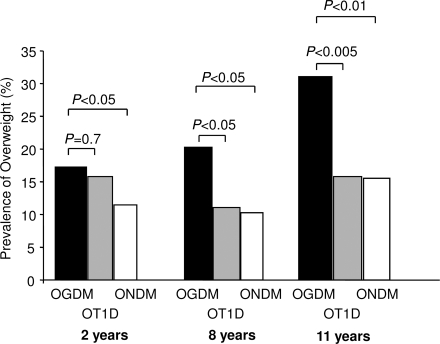
Prevalence of overweight in OGDM was 17.2% at age 2 years, 20.2% at age 8 years, and 31.1% at age 11 years. Prevalence of overweight was increased in OGDM compared with OT1D (15.8, 11.0, and 15.8% at age 2, 8, and 11 years, respectively; P < 0.05, P = 0.03, and P < 0.01, respectively) and with ONDM (11.4, 10.3, and 15.5%; P = 0.7, P = 0.02, and P = 0.005, respectively (Fig. 1).
Figure 1.
Prevalence of overweight at 2, 8, and 11 years of age in OGDM (■), OT1D ( ), and ONDM (□).
), and ONDM (□).
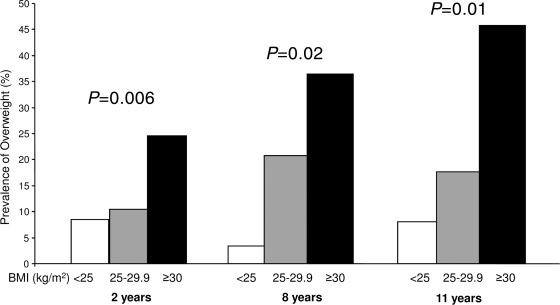
In OGDM, maternal obesity was a strong predictor of overweight in children at age 2, 8, and 11 years in the univariate and multivariate logistic regression model (Tables 1 and 2). The prevalence of overweight in children at 2, 8, and 11 years of age was 24.6, 36.4, and 45.8% in children of obese mothers compared with 9.2, 11.3, and 11.9% of children born to nonobese mothers (P = 0.01, P = 0.02, and P = 0.003, respectively) (Fig. 2). Birth size, therapy of GDM during pregnancy, and maternal smoking during pregnancy showed inconsistent associations with overweight risk in the offspring (Tables 1 and 2).
Table 1.
Associations of maternal BMI in early pregnancy, birth size, therapy modality, and smoking behavior during pregnancy on overweight-risk (BMI ≥ 90th percentile) in OGDM at 2, 8, and 11 years of age: univariate analysis
| 2 years of age |
8 years of age |
11 years of age |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| n | OR (95% CI) | P value | n | OR (95% CI) | P value | n | OR (95% CI) | P value | |
| Maternal BMI in early pregnancy | |||||||||
| Normal weight (BMI <25.0 kg/m2) | 71 | Reference | 29 | Reference | 24 | Reference | |||
| Overweight (BMI 25.0–29.9 kg/m2) | 48 | 1.3 (0.4–4.4) | 0.7 | 24 | 7.4 (0.8–68.2) | 0.08 | 18 | 3.1 (0.5–19.5) | 0.2 |
| Obesity (BMI ≥30 kg/m2) | 57 | 3.5 (1.3–9.9) | 0.02 | 22 | 16.0 (1.8–140.9) | 0.01 | 24 | 9.3 (1.8–48.7) | 0.008 |
| Birth size | |||||||||
| AGA | 115 | Reference | 48 | Reference | 39 | Reference | |||
| SGA | 10 | 1.1 (0.1–9.1) | 1.0 | 2 | — | 1 | — | ||
| LGA | 59 | 5.2 (2.3–11.8) | <0.0001 | 21 | 3.1 (0.96–9.8) | 0.06 | 21 | 2.6 (0.9–8.1) | 0.09 |
| Therapy modality in pregnancy | |||||||||
| Diet | 138 | Reference | 55 | Reference | 43 | Reference | |||
| Insulin | 72 | 2.4 (1.2–4.9) | 0.02 | 31 | 0.6 (0.2–1.9) | 0.4 | 28 | 1.0 (0.4–2.7) | 1.0 |
| Maternal smoking during pregnancy | |||||||||
| No | 111 | Reference | 61 | Reference | 48 | Reference | |||
| ≥1 cigarette/day | 18 | 1.8 (0.5–6.3) | 0.3 | 7 | 0.7 (0.08–6.2) | 0.7 | 5 | 17.3 (1.7–174) | 0.02 |
| Breast-feeding | |||||||||
| Fully breast-fed <3 months | 94 | 1.0 | 43 | 1.0 | 0.7 | 45 | 1.0 | ||
| Fully breast-fed ≥3 months | 51 | 0.7 (0.2–1.9) | 0.5 | 23 | 0.9 (0.2–3.6) | 0.9 | 18 | 0.2 (0.02–1.6) | 0.1 |
AGA, appropriate for gestational age; LGA, large for gestational age; SGA, small for gestational age.
Table 2.
Associations of maternal obesity in early pregnancy, LGA status, and maternal smoking during pregnancy on overweight risk in OGDM at 2, 8, and 11 years of age: multivariate analysis
| 2 years of age |
8 years of age |
11 years of age |
||||
|---|---|---|---|---|---|---|
| Adjusted OR (95% CI) | P value | Adjusted OR (95% CI) | P value | Adjusted OR (95% CI) | P value | |
| n | 215 | 89 | 74 | |||
| Maternal BMI in early pregnancy ≥30 kg/m2 | 2.6 (1.04–6.4) | 0.04 | 5.1 (1.4–18.6) | 0.014 | 7.0 (1.8–27.7) | 0.006 |
| Birth size LGA | 5.4 (2.3–12.4) | 0.0001 | 3.5 (1.0–12.1) | 0.05 | 3.7 (0.9–16.0) | 0.08 |
| Maternal smoking during pregnancy | 2.2 (0.6–8.3) | 0.3 | 0.7 (0.1–6.5) | 0.7 | 22.7 (1.9–278) | 0.015 |
Variables included in the multivariate analysis: maternal BMI before pregnancy, birth size, and maternal smoking during pregnancy. LGA, large for gestational age.
Figure 2.
Prevalence of overweight at 2, 8, and 11 years of age in OGDM in relation to maternal BMI in early pregnancy: BMI <25.0 kg/m2 (□), BMI 25.0–29.9 kg/m2 ( ), and BMI ≥30 kg/m2 (■).
), and BMI ≥30 kg/m2 (■).
Predictors of insulin resistance during childhood (substudy)
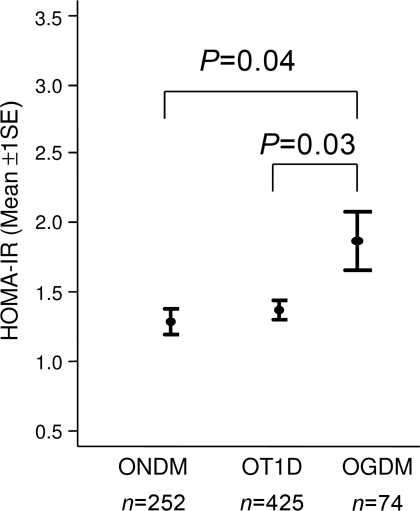
In the substudy on insulin resistance, OGDM had increased HOMA-IR compared with that of children of ONDM and of OT1D (P = 0.04 and P = 0.03, adjusted for age and sex) (Fig. 3). Within the OGDM, HOMA-IR was associated with the child's BMI (P = 0.01, adjusted for age and sex) (supplementary Fig. 1A, available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc10-0139/DC1) but not with maternal obesity in early pregnancy (supplementary Fig. 1B).
Figure 3.
HOMA-IR (mean ± 1 SE) at age 11 years in OGDM compared with OT1D and ONDM.
CONCLUSIONS
This study shows an increased prevalence of overweight at 2, 8, and 11 years of age in OGDM compared with OT1D and ONDM. In OGDM, overweight up to age 11 years was strongly associated with maternal obesity at early pregnancy. Birth size of the child, maternal smoking during pregnancy, or treatment modality of GDM were less consistent predictors of overweight in children of mothers with GDM. OGDM also had higher HOMA-IR than ONDM. Thus, GDM programs a high BMI/insulin resistance phenotype in children.
This GDM offspring study is a prospective study from birth, in which questionnaires addressing in pregnancy-related factors were administered at birth and children's weight and height were measured regularly by a pediatrician according to standard protocols. Recall bias and over- or underestimating of growth that may occur when data are reported by parents are therefore avoided or limited. Our results are based on the definition of overweight as BMI percentile ≥90, which has been proposed by the German association of obesity during childhood and obesity. Findings were consistent when overweight was defined as BMI ≥85 or ≥95 percentile (data not shown). Although not population based, this is the largest prospective study following Caucasian children of mothers with GDM from birth in defined follow-up intervals, enabling a longitudinal analysis of factors affecting overweight risk. Nevertheless, the numbers of children of mothers with GDM remains relatively low at ages 8 and 11 years. As a consequence, CIs for some of the risk estimates are wide, and some associations may change with a larger number of subjects.
A small number of studies have examined the impact of maternal diabetes on overweight risk in offspring, differentiating between OGDM and offspring of mothers with preexisting type 1 diabetes (10,11). Clausen et al. (11) reported an increased prevalence of overweight in adults who were from GDM pregnancies (40%) or type 1 diabetes pregnancies (41%) compared with control subjects (24%). Lawlor et al. (10) found only a minor increase in the prevalence of overweight in OGDM children (30%) compared with control children (23%) and OT1D (23%). The findings of Lawlor et al. support our previous report that OT1D are not at higher risk for overweight compared with ONDM (6). However, in the current study, we saw a pronounced difference between OGDM and OT1D, whereas differences are not significant in the study of Lawlor et al. To reconcile this discrepancy, it is noted that our study in Germany reported a lower background prevalence of overweight in children compared with the U.S. and that the numbers of OT1D and OGDM are larger in our study. Our findings are unlikely to be biased with respect to the comparisons between OGDM and OT1D children because both cohorts were recruited over the same period and from the same region and ethnic background. They are also supported by expected associations of maternal BMI with overweight risk in the OGDM. However, data for potential confounders such as socioeconomic status, dietary habits during childhood, and physical activity were not collected, and we cannot exclude the possibility that the associations observed could, in some cases, be due to one or more of these confounding variables.
The finding that overweight risk is significantly higher in OGDM compared with OT1D indicates that exposure to hyperglycemia during pregnancy per se can only partly explain the increased prevalence of overweight in these children. Maternal obesity in early pregnancy was the strongest predictor of overweight risk at 2, 8, and 11 years in OGDM. Others have shown that maternal pregravid BMI is the strongest predictor of childhood obesity independent of maternal glucose status or birth weight (7). It has also been shown that childhood BMI correlates more closely with maternal BMI than does paternal BMI, indicating that in addition to genetic influences an obese intrauterine environment per se may contribute to the higher overweight risk in children of obese mothers (12,13). This finding is strengthened by results from animal studies showing that feeding-induced obesity at conception increases the prevalence of obesity in offspring. Programming of obesity in animals was independent of changes in birth weight and was associated with significant changes in metabolic and endocrine parameters and adipose tissue cellularity independent of postnatal caloric intake (13). Finally, the possibility that the influence of maternal obesity on childhood overweight could also be due to similar adverse dietary and physical activity behaviors in obese mothers and their offspring should be considered. Similarly, the observation that maternal smoking during pregnancy is associated with overweight in the child could further indicate an influence via an “unhealthy” postnatal environment. The relationship between childhood obesity and both maternal obesity and smoking during pregnancy became more pronounced with age, further giving support to this hypothesis.
Not unexpectedly given the increased risk for overweight in OGDM, our substudy found higher HOMA-IR in OGDM than in ONDM and also in OT1D. HOMA-IR was associated with the child's BMI in OGDM. This is consistent with the finding of Krishnaveni et al. (14) who also reported a relationship between offspring BMI and insulin resistance in a small group of 23 female OGDM. A recently published study found that in offspring of mothers with pregravid obesity fetal insulin resistance strongly correlated with fetal adiposity (15). We could not find a significant association between insulin resistance in the offspring and maternal obesity in early pregnancy, but numbers were relatively small.
In summary, our results show that prevalence of overweight and, as a consequence, HOMA-IR during childhood is higher in OGDM than in OT1D, indicating that maternal diabetes type affects overweight risk. The finding that overweight risk was associated with maternal obesity and to a lesser extent with birth size suggests that a combination of genetic predisposition, fetal overnutrition and lifestyle factors are likely to contribute to childhood growth in OGDM.
Acknowledgments
This study was supported by the German Federal Ministry for Education and Research (grants BMBF 01KD89030 and 01KD9601), the Juvenile Diabetes Research Foundation (grant 1-2006-665), the German Diabetes Association, the Foundation “Children with Type 1 Diabetes” (Stiftung Das Zuckerkranke Kind), and the German Competence Net for Diabetes (grant 01GI0805).
No potential conflicts of interest relevant to this article were reported.
H.B. researched data, contributed to discussion, and wrote the manuscript. M.P. and L.H. researched data and contributed to discussion. A.-G.Z. and S.H. researched data, contributed to discussion, wrote the manuscript, and reviewed/edited the manuscript.
We thank Marina Zwilling for expert technical assistance and Ezio Bonifacio for critical reading of the manuscript. This study forms part of the dissertation of Lydia Henneberger.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Silverman BL, Rizzo T, Green OH, Cho NH, Winter RJ, Ogata ES, Richards GE, Metzger BE: Long-term prospective evaluation of offspring of diabetic mothers. Diabetes 1991;40(Suppl. 2):121–125 [DOI] [PubMed] [Google Scholar]
- 2. Pettitt DJ, Nelson RG, Saad MF, Bennett PH, Knowler WC: Diabetes and obesity in the offspring of Pima Indian women with diabetes during pregnancy. Diabetes Care 1993;16:310–314 [DOI] [PubMed] [Google Scholar]
- 3. Clausen TD, Mathiesen ER, Hansen T, Pedersen O, Jensen DM, Lauenborg J, Damm P: High Prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: the role of intrauterine hyperglycemia. Diabetes Care 2008;31:340–346 [DOI] [PubMed] [Google Scholar]
- 4. Hillier TA, Pedula KL, Schmidt MM, Mullen JA, Charles MA, Pettitt DJ: Childhood obesity and metabolic imprinting. Diabetes Care 2007;30:2287–2292 [DOI] [PubMed] [Google Scholar]
- 5. Dabelea D: The predisposition to obesity and diabetes in offspring of diabetic mothers. Diabetes Care 2007;30(Suppl. 2):S169–S174 [DOI] [PubMed] [Google Scholar]
- 6. Hummel S, Pflüger M, Kreichauf S, Hummel M, Ziegler AG: Predictors of overweight during childhood in offspring of parents with type 1 diabetes. Diabetes Care 2009;32:921–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Catalano PM, Farrell K, Thomas A, Huston-Presley L, Mencin P, de Mouzon SH, Amini SB: Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr 2009;90:1303–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Voigt M, Schneider KTM, Stillger R, Pildner von Steinburg S, Fusch C, Hesse V: Analyse des Neugeborenenkollektivs der Jahre 1995–1997 der Bundesrepublik Deutschland. Geburtshilfe Frauenheilkd 2005;65:474–481 [Google Scholar]
- 9. Kromeyer-Hauschild K, Wabitsch M, Kunze D, Geller F, Geiß HC, Hesse V, von Hippel A, Jaeger U, Johnsen D, Korte W, Menner K, Müller G, Müller JM, Müller M, Niemann-Pilatus A, Remer T, Schaefer F, Wittchen HU, Zabransky S, Zellner K, Ziegler A, Hebebra J: Perzentile für den Body-mass-Index für das Kinder- und Jugendalter unter Heranziehung verschiedener deutscher Stichproben. Monatsschr Kinderheilkd 2001;8:807–818 [Google Scholar]
- 10. Lawlor DA, Fraser A, Lindsay RS, Ness A, Dabelea D, Catalano P, Davey Smith G, Sattar N, Nelson SM: Association of existing diabetes, gestational diabetes and glycosuria in pregnancy with macrosomia and offspring body mass index, waist and fat mass in later childhood: findings from a prospective pregnancy cohort. Diabetologia 2010;53:89–97 [DOI] [PubMed] [Google Scholar]
- 11. Clausen TD, Mathiesen ER, Hansen T, Pedersen O, Jensen DM, Lauenborg J, Schmidt L, Damm P: Overweight and the metabolic syndrome in adult offspring of women with diet-treated gestational diabetes mellitus or type 1 diabetes. J Clin Endocrinol Metab 2009;94:2464–2470 [DOI] [PubMed] [Google Scholar]
- 12. Danielzik S, Langnäse K, Mast M, Spethmann C, Müller MJ: Impact of parental BMI on the manifestation of overweight 5–7 year old children. Eur J Nutr 2002;41:132–138 [DOI] [PubMed] [Google Scholar]
- 13. Shankar K, Harrell A, Liu X, Gilchrist JM, Ronis MJ, Badger TM: Maternal obesity at conception programs obesity in the offspring. Am J Physiol Regul Integr Comp Physiol 2008;294:R528–R538 [DOI] [PubMed] [Google Scholar]
- 14. Krishnaveni GV, Veena SR, Hill JC, Kehoe S, Karat SC, Fall CH: Intra-uterine exposure to maternal diabetes is associated with higher adiposity and insulin resistance and clustering of cardiovascular risk markers in Indian children. Diabetes Care 2010;33:402–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S: Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care 2009;32:1076–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]