Abstract
In the book Epidemiology of Diabetes and Its Vascular Lesions (1978), Kelly West summarized extant knowledge of the distribution and causes of diabetes. The 30 years of epidemiological research that followed have seen remarkable advances in the understanding of obesity as a risk factor for type 2 diabetes, and diabetes and pre-diabetes as risk factors for cardiovascular disease. Increasingly detailed understanding of these relationships has, unfortunately, been accompanied by an alarming increase in the prevalence of obesity, diabetes, and cardiovascular disease. West recognized that pre-diabetes is recognizable as what we now call metabolic syndrome. He predicted that novel insight into diabetes pathogenesis would come from biochemical and genetic epidemiology studies. He predicted that type 2 diabetes could be prevented by healthy lifestyle change. The challenge now is for us to translate these insights into effective strategies for the prevention of the modern epidemic of diabetes and vascular disease.
In his landmark book, Epidemiology of Diabetes and Its Vascular Lesions (1), Kelly West reviewed and summarized the extant scientific knowledge relevant to the distribution and causes of type 1 and type 2 diabetes. Epidemiology of Diabetes was published in 1978. The methodological foundations of modern epidemiology and clinical trials had been laid, and on these foundations West consolidated the major questions that we have sought to answer over subsequent decades. West recognized that obesity, type 2 diabetes, and cardiovascular disease (CVD) are intimately linked. He pointed out that the state of pre-diabetes is readily identifiable, and that type 2 diabetes should be preventable. He hypothesized that new biochemical and genetic markers of diabetes risk would be identified and reveal mechanisms linking obesity, type 2 diabetes, and CVD. Most importantly, he emphasized that the science of clinical epidemiology provides the foundation for the prevention of diabetes and its vascular complications.
The Framingham Heart Study (FHS), a longitudinal, multigenerational cohort study of CVD and its risk factors, has provided a rich resource to address the questions raised by West. Despite the fact that the FHS participants are mostly white and of European ancestry, their health experience reflects the health experience of other populations in the U.S. and other developed countries around the world. The prevalence of obesity (BMI ≥30 kg/m2) in FHS has risen inexorably in both men and women over the past 30 years from just a few percent among men in the 1970s to 25–30% among men in the 1990s (Fig. 1A) (2). A similar trend occurred in women. Over the same time frame, the prevalence of type 2 diabetes in FHS rose steadily (Fig. 1B) (3). Alarmingly, virtually the entire rise in type 2 diabetes has occurred in individuals with obesity, from about 6% in the 1970s to over 12% in the 1990s. This association clearly demonstrates that the widespread rise in obesity is pushing a rising tide of type 2 diabetes. West was among the first to strongly emphasize that CVD is the principal cause of morbidity and mortality in type 2 diabetes. Although the absolute risk of CVD in FHS (defined by fatal and nonfatal myocardial infarction, stroke, and intermittent claudication) has declined between the 1950s and the 1990s by 35% in people without diabetes and by 49% in those with diabetes, the relative risk among those with diabetes to develop CVD has persistently remained about twofold higher relative to those without diabetes (4). The rising prevalence of type 2 diabetes, combined with a constant relative risk for CVD, has translated into a 60% increase in the attributable risk ratio for CVD associated with diabetes, even while the attributable risk for CVD associated with other risk factors like hypertension and smoking has held constant or fallen (Fig. 1C) (5). Again the experience of FHS shows us that the rising tide of obesity is pushing a rising tide of type 2 diabetes, which in turn is pushing a rising tide of CVD and death. West envisioned this, and the challenge for prevention is clear.
Figure 1.
Increasing rates of obesity are associated with increasing rates of diabetes, which in turn are associated with increasing rates of CVD in FHS. A: Shows the dramatically rising 30-year mortality-adjusted cumulative incidence rate of obesity (BMI ≥30 kg/m2) for men in different age-groups; the experience was similar among women (adapted from Vasan et al. [2]). B: Shows that the 8-year incidence rate of diabetes increased primarily in those with obesity (among both men and women) (adapted from Fox et al. [3]). C: Shows a 1.6-fold increase over time in the attributable risk percent for CVD associated with diabetes (arrows); among common CVD risk factors, only diabetes contributed significantly to increased rates of CVD (adapted from Fox et al. [5]). HTN, hypertension.
From a clinical perspective, CVD is often viewed as a consequence of diagnosed type 2 diabetes. However, the experience of FHS and other cohorts has shown that pre-diabetes (defined by blood glucose levels below the diagnostic threshold for diabetes, but nonetheless not normal) is also associated with risk for cardiovascular disease (6). This observation has given rise to the notion that type 2 diabetes and CVD may share a common pathogenesis. Indeed, these diseases share many common risk factors, including obesity (especially central obesity), hyperinsulinemia (reflecting, in part, insulin resistance), hyperglycemia, a dyslipidemia characterized by low levels of HDL cholesterol and elevated levels of triglycerides, and elevated blood pressure. These measurable clinical traits are intercorrelated, co-occur to a far greater degree than would be expected by change alone, and cluster together in an identifiable pattern linked by obesity and insulin resistance (Fig. 2A and B) (7,8). This phenomenon of risk factor clustering is now called, for better or worse, metabolic syndrome (Fig. 2C). There are a variety of enthusiastically contested definitions of metabolic syndrome; in FHS, the definition proposed by the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATPII) is the one most often studied (9).
Figure 2.
Type 2 diabetes and CVD risk factor clustering is called metabolic syndrome. A: Shows that the observed co-occurrence of two or more of elevated fasting glucose (FG), fasting insulin (FI), triglycerides (TG), blood pressure (BP), BMI, or low HDL cholesterol (all defined in A as the extreme 20th percentile, light gray bars for women and dark gray bars for men) co-occur to a far greater degree than would be expected by change alone (compared with a binomial distribution, dashed line, P < 0.0001). B: Shows that the pattern of risk factor clustering for these risk factors (including, as well, glucose levels 2 h after an oral glucose tolerance test [2-h G] and waist-to-hip ratio [WHR]) represent three clinically identifiable phenotypes: impaired glucose tolerance (IGT), hypertension (HTN), and central obesity-dyslipidemia, linked together by obesity (in this figure, BMI) and fasting hyperinsulinemia (reflecting, in part, insulin resistance). C: Illustrates that obesity and insulin resistance constitute the common physiological antecedents leading to increased risk for the development of both type 2 diabetes (T2D) and CVD; the name currently applied to this phenomenon is metabolic syndrome. A and B are adapted from Wilson et al. (7) and Meigs et al. (8). DBP, diastolic blood pressure; SBP, systolic blood pressure.
A fundamental hypothesis raised by the concept of metabolic syndrome is that its presence increases future risk for both type 2 diabetes and CVD, even after accounting for other disease-specific risk factors. In FHS, metabolic syndrome increases the 7- to 11-year risk for CVD in men by about threefold relative to those without metabolic syndrome, and for type 2 diabetes, the increase is about sevenfold (Fig. 3A) (10). Associations among women are similar. Risk rises steadily in a dose-response relationship as the number of component traits increases. Further, risk is increased regardless of which of the various heterogeneous combinations of specific traits are present and even in the absence of impaired glycemia. For instance, in individuals with the combination of low HDL cholesterol and elevated triglycerides and blood pressure but without impaired glycemia, the relative risk for CVD is about twofold increased relative to those without this trait combination, and for type 2 diabetes, the relative risk is 3.5-fold increased (Fig. 3B). The data suggest that beyond the specific risk factors that may be present, it is something about the phenomenon of risk factor clustering itself that appears to account, at least in part, for subsequent disease risk. Metabolic syndrome also accounts for some of the heterogeneity for future disease risk observed in individuals with obesity. Among FHS individuals with BMI <25 kg/m2 who meet the criteria for metabolic syndrome, the 7-year cumulative incidence of type 2 diabetes was about 7%, while among those with BMI ≥30 kg/m2 but without metabolic syndrome, the rate was only about 3% (11). These individuals can be considered to represent “metabolically obese, normal weight” and “metabolically healthy obese” subphenotypes, respectively. Similar albeit less dramatic patterns were seen for risk of CVD. If there is one obvious lesson that can be drawn from the studies of metabolic syndrome, it is that that metabolic syndrome is a far more powerful risk factor for type 2 diabetes than for CVD.
Figure 3.
Metabolic syndrome (MetS) is a risk factor for both type 2 diabetes (T2D) and CVD. A: Shows that among men in FHS, the 7- to 11-year risk for CVD increases from 1.5 for those with one or two MetS risk factors (RFs) to 4.0 for those with three or more (that is, with MetS) relative to those with no MetS risk factors, even after accounting for other CVD–specific risk factors. The bars in the figure represent the odds ratio and its 95% confidence bounds. The relative risk for CVD is 2.9 comparing MetS vs. no MetS. Risk for type 2 diabetes increases from 4.2 for men with one or two MetS risk factors to 24 for those with MetS relative to those with no MetS risk factors, even after accounting for other type 2 diabetes–specific risk factors. The relative risk for type 2 diabetes is 6.9 comparing MetS vs. no MetS. Patterns are similar for FHS women. Risk rises steadily in a dose-response relationship as the number of component traits increases and is increased regardless of which of the various heterogeneous combinations of specific traits are present, and even in the absence of impaired glycemia (B) (adapted from Wilson et al. [10]). BP, blood pressure; FG, fasting glucose; TG, triglycerides.
In many ways metabolic syndrome is synonymous with pre-diabetes. This fact has been exploited to develop highly reliable clinical prediction rules for type 2 diabetes risk prediction. In FHS and other studies, the combination of age, sex, family history of diabetes, and metabolic syndrome traits can correctly discriminate an individual at risk to develop type 2 diabetes in the next 7 years in about 85% of cases (12). The majority of research on outcomes associated with metabolic syndrome has been conducted in rigorously phenotyped, formal epidemiological studies like FHS. Nonetheless, risk factor clustering defined with imprecise phenotyping, such as is available in usual care clinical data, effectively predicts adverse outcomes. If medical records of patients without clinical diabetes or CVD contain any evidence of obesity or elevated blood pressure, causal glucose, triglycerides, or total cholesterol, those with none, one to two, or three or more of these traits have a 3-year incidence of type 2 diabetes of 1.4, 4, and 11%, respectively (13). Incidence rates of CVD are 3.1, 5.3, and 6.4%, annual total medical costs are about $3,000, $4,000, and $5,000, and inpatient length-of-stay are 4, 5, and 6 days, respectively. Despite increased risk for adverse outcomes associated with risk factor clustering, few patients have ever heard of metabolic syndrome, those with risk factor clustering do not perceive themselves to be at increased risk of type 2 diabetes, and even those that do perceive themselves to be at risk of diabetes are not more likely than those who do to have intentions to adopt a more healthy lifestyle in the coming year (14). Again, the challenge for prevention is clear. The concept of risk factor clustering as embodied in metabolic syndrome appears to afford a readily available clinical approach to identify individuals at elevated risk for type 2 diabetes, CVD, and increased health care costs and utilization. The overall utility of metabolic syndrome has been strongly contested, due, appropriately, to the lack of direct evidence that it arises from a unifying pathophysiology or that its diagnosis alters clinical outcomes. It does seem to have obvious value for risk identification and prevention of type 2 diabetes, but this remains to be tested formally in a clinical trial.
The concept of metabolic syndrome has lead to a vigorous search for molecular and physiological mechanisms to explain the phenomenon of risk factor clustering. Abnormal adipocyte signaling, impaired endothelial function, and systemic subclinical inflammation have been hypothesized to play a role that unifies excess adiposity with insulin resistance, impaired β-cell function, and small and large vessel arterial disease (15). The adipocytokines adiponectin, resistin, and tumor necrosis factor-α (TNF-α) are all associated with both obesity and insulin resistance in free-living humans (16). However, beyond these robust cross-sectional associations, the inter-relationships of adipokine biomarkers with incident diabetes and CVD have been difficult to untangle. For instance, low levels of adiponectin (but not resistin or TNF-α) are associated with the increased risk for type 2 diabetes after accounting for confounding risk factors including obesity (17), whereas resistin but not adiponectin are associated with the risk for heart failure, and neither are associated with the risk for coronary heart disease (18). Insulin resistance, on the other hand, is associated in FHS and many other studies with both type 2 diabetes and CVD, even after accounting for confounding risk factors (19). Plasma biomarkers of endothelial dysfunction including levels of E-selectin, plasminogen activator inhibitor-1 (PAI-1), and von Willebrand factor are associated with the risk for new cases of type 2 diabetes even after considering concurrent obesity, impaired glycemia, family history of type 2 diabetes, and other common risk factors (Fig. 4A) (20,21). Plasma levels of von Willebrand factor antigen also predict incident CVD, especially in individuals with type 2 diabetes, pointing to endothelial dysfunction as a risk factor common to both type 2 diabetes and CVD and perhaps a unifying factor underlying risk factor clustering (Fig. 4B) (22).
Figure 4.
Plasma biomarkers of endothelial dysfunction predict both type 2 diabetes (T2D) and CVD. A: Shows the 7-year cumulative incidence of type 2 diabetes by quartile (Q1-Q4) of plasminogen activator inhibitor-1 (PAI-1) or von Willebrand factor (vWF). The type 2 diabetes–risk factor adjusted relative risk (RR) for type 2 diabetes per interquartile (IQR) increase in vWF was 1.4 (95% CI 1.1–1.8, P = 0.009) (adapted from Meigs et al. [21]). B: Shows survival free of CVD events in FHS as a function of the presence or absence of type 2 diabetes and by low (Q1-Q3) or high (Q4) levels of vWF. Risk of CVD is increased in individuals with elevated levels of vWF, especially in individuals with type 2 diabetes and elevated vWF (P < 0.0001) (adapted from Frankel et al. [22]). DM, diabetes mellitus.
For the most part, data to support abnormal adipokine signaling, endothelial dysfunction, or inflammation as central to risk factor clustering come from observational studies, where bias or residual confounding could account for the associations observed. Mendelian randomization is a methodological approach that has been proposed to address this problem, where genetic variants associated with intermediate traits (for instance, adiponectin) can be used as unconfounded markers of risk for disease end points (like type 2 diabetes) associated with the intermediate trait (23). The demonstration that common genetic variants associated with variation in LDL cholesterol levels also increase the risk of coronary heart disease provide proof-of-principal for the approach (24). In the case of adiponectin, however, variants in regulatory regions of ADIPOQ, the gene-encoding adiponectin, are associated with variation adiponectin levels, while a different coding variant in ADIPOQ appears to be associated with type 2 diabetes risk (25). It is not entirely clear that in every instance Mendelian randomization will allow observational data to reveal causality. Indeed, West's vision that biochemical and genetic markers of diabetes risk will reveal mechanisms linking obesity, type 2 diabetes, and CVD has proved to be true. However, defining novel methods to define causal links among the many factors that have been discovered remains a puzzle yet to be solved.
The dazzling pace at which genetic risk factors for type 2 diabetes have been discovered in recent years only adds to the excitement and challenge in the field. Inexpensive, high-density single nucleotide polymorphism (SNP) arrays combined with extensive, careful phenotyping in large numbers of individuals have opened a new era in genetic discovery for common disease. In FHS, millions of SNPs linked to thousands of traits in over 8,000 individuals have been made publicly available (with appropriate permissions) as the Framingham Heart Study SNP Health Association Resource (SHARe) at the National Center for Biotechnology Information Genotypes and Phenotypes (NCBI dbGaP) web site (www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?id=phs000007). At least as exciting has been the collaboration among FHS SHARe and similar studies to form large consortia for genetic discovery. The Meta-Analysis of Glucose and Insulin-related traits Consortium (MAGIC), for instance, has pooled data including over 120,000 individuals of European ancestry without clinical diabetes from over 50 cohort studies for collaborative research involving over 300 investigators. Already, MAGIC has identified 16 common SNPs associated with levels of fasting glucose, fasting insulin, and levels of glucose 2 h after an oral glucose tolerance test (26–28). Eleven of these loci are completely novel and eight are also associated with the risk for type 2 diabetes, suggesting some genetic control over glucose homeostasis that is distinct from genetic factors in type 2 diabetes pathogenesis. Many new loci can be localized to diverse aspects of β-cell mass, life span, and function. Several others have revealed or confirmed novel mechanistic pathways underlying diabetes physiology, including variants that localize in or near genes involved in the Circadian (MTNR1B, CRY2) and alpha-adrenergic systems (ADRA2), fatty acid metabolism (FADS1), and the incretin system (GIPR). West would no doubt have been excited by this demonstration of the awesome power of large-scale epidemiology to reveal novel aspects of type 2 diabetes physiology and pathogenesis.
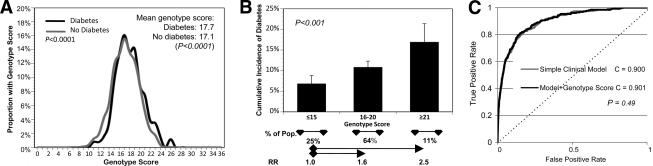
The possibility to use new biochemical and genetic discoveries for type 2 diabetes prediction and prevention would perhaps have most intrigued West. It is now possible for an individual to purchase a diabetes genetic risk profile from several different companies for just a few hundred dollars. However, the ability of these profiles to discriminate future disease risk remains, at present, somewhat uncertain. In FHS, a genetic risk score comprised of 18 confirmed type 2 diabetes risk SNPs was higher among individuals who developed type 2 diabetes over 28 years compared with those who remained free of diabetes, although the absolute magnitude of the difference was minute (about 0.6 risk alleles, although the probability that there was no difference was <0.0001) (Fig. 5A and B) (29).
Figure 5.
A genetic risk score of 18 SNPs predicts type 2 diabetes. A: Shows the distribution of an 18-SNP genetic risk score, where FHS individuals were scored with a 0 if they had no risk alleles at a given SNP, one if they were heterozygous, and two if they were homozygous for the risk allele. Individuals who developed type 2 diabetes over 28 years of follow-up have about 0.6 more risk alleles than those who remain free of diabetes (P < 0.0001). B: Shows that risk for type 2 diabetes increases with increasing genetic risk burden. Those with 16–20 risk alleles have a risk of diabetes of 1.6, and those with ≥21 risk alleles have a risk of 2.5 relative to those with ≤15 risk alleles (P < 0.001). However, the genetic risk score does not discriminate those who will develop type 2 diabetes from those who will not after accounting for common clinical risk factors (age, sex, family history of diabetes, and metabolic syndrome traits). C: Shows that the area under the receiver operating characteristic curve (the C statistic) was 0.900 for a simple clinical model including clinical risk factors (gray line), and 0.901 for a model including clinical risk factors and the genetic risk score (black line) (adapted from Meigs et al. [[29]).
Interestingly, both parental history of type 2 diabetes and the genetic risk score were independently associated with type 2 diabetes risk. This suggests both that our genetic understanding of type 2 diabetes remains incomplete and that family history, which is commonly considered to represent inherited genetic risk, likely also represents behaviors and norms that are learned and transmitted in families. However, the genetic risk score did not substantively improve risk discrimination after considering characteristics that are easily and commonly measured in a routine adult check-up, including age, sex, family history of diabetes, and metabolic syndrome traits (Fig. 5C). It appears that, at present, reliance on these standard phenotypic risk factors remains the best way to identify individuals at increased risk for future type 2 diabetes. However, both physicians and patients place high faith in the present and future ability of genetic testing to improve type 2 diabetes prevention and care (30). In a hypothetical scenario, 99% of patients with a genetic test result indicating a high chance of developing diabetes state that they would be more motivated to make recommended lifestyle changes, while 59% of those with a test result indicating low genetic risk would be unmotivated or less motivated to make recommended lifestyle changes. This is highly relevant since healthy lifestyle change appears to reduce the future risk of type 2 diabetes in people at high and low diabetes genetic risk (31), and there is a very clear dose-response relationship with long-term success with healthy lifestyle change and the reduced risk of type 2 diabetes (32). These observations raise the compelling hypothesis that genetic knowledge could be leveraged to improve motivation and adherence to type 2 diabetes prevention interventions.
The 30 years of epidemiology and clinical trials research that have followed the publication of Epidemiology of Diabetes and Its Vascular Lesions have seen remarkable advances in our understanding of the importance of obesity as a risk factor for type 2 diabetes, and of type 2 diabetes and pre-diabetes as risk factors for CVD. Despite increasingly detailed biochemical and genetic understanding of these relationships, the past 30 years have seen an alarming increase in the prevalence of obesity, type 2 diabetes, and CVD. West pointed out that the state of increased risk for diabetes is clearly recognizable—today we call this risk factor clustering metabolic syndrome. He pointed out that type 2 diabetes is readily preventable. His suggestion that the means to this end, healthy lifestyle change through achievement and maintenance of a healthy body weight and regular modest physical activity, has been convincingly proven in many clinical trials. Rigorous epidemiological studies like FHS and many others have provided the foundation for our understanding of the nature and magnitude of the problem at hand. What remains is for us to translate that understanding into clinical practice and public policy that puts that science in the hands of patients and providers to reverse the steadily rising tide of obesity, type 2 diabetes, and CVD.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
References
- 1. West KM: Epidemiology of diabetes and its vascular lesions. New York, Elsevier, 1978. [Google Scholar]
- 2. Vasan RS, Pencina MJ, Cobain M, Freiberg MS, D'Agostino RB: Estimated risks for developing obesity in the Framingham Heart Study. Ann Intern Med 2005;143:473–480 [DOI] [PubMed] [Google Scholar]
- 3. Fox CS, Pencina MJ, Meigs JB, Vasan RS, Levitzky YS, D'Agostino RB, Sr: Trendsin the incidence of type 2 diabetes mellitus from the 1970s to the 1990s: the Framingham Heart Study. Circulation 2006;113:2914–2918 [DOI] [PubMed] [Google Scholar]
- 4. Fox CS, Coady S, Sorlie PD, Levy D, Meigs JB, D'Agostino RB, Sr, Wilson PW, Savage PJ: Trends in cardiovascular complications of diabetes. JAMA 2004;292:2495–2499 [DOI] [PubMed] [Google Scholar]
- 5. Fox CS, Coady S, Sorlie PD, D'Agostino RB, Sr, Pencina MJ, Vasan RS, Meigs JB, Levy D, Savage PJ: Increasing cardiovascular disease burden due to diabetes mellitus: the Framingham Heart Study. Circulation 2007;115:1544–1550 [DOI] [PubMed] [Google Scholar]
- 6. Levitzky YS, Pencina MJ, D'Agostino RB, Meigs JB, Murabito JM, Vasan RS, Fox CS: Impact of impaired fasting glucose on cardiovascular disease: the Framingham Heart Study. J Am Coll Cardiol 2008;51:264–270 [DOI] [PubMed] [Google Scholar]
- 7. Wilson PW, Kannel WB, Silbershatz H, D'Agostino RB: Clustering of metabolic factors and coronary heart disease. Arch Intern Med 1999;159:1104–1109 [DOI] [PubMed] [Google Scholar]
- 8. Meigs JB, D'Agostino RB, Sr, Wilson PW, Cupples LA, Nathan DM, Singer DE: Risk variable clustering in the insulin resistance syndrome: the Framingham Offspring Study. Diabetes 1997;46:1594–1600 [DOI] [PubMed] [Google Scholar]
- 9. Meigs JB: Metabolic syndrome and risk for type 2 diabetes. Expert Rev Endocrin Metab 2006;1:57–66 [DOI] [PubMed] [Google Scholar]
- 10. Wilson PW, D'Agostino RB, Parise H, Sullivan L, Meigs JB: Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation 2005;112:3066–3072 [DOI] [PubMed] [Google Scholar]
- 11. Meigs JB, Wilson PW, Fox CS, Vasan RS, Nathan DM, Sullivan LM, D'Agostino RB: Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab 2006;91:2906–2912 [DOI] [PubMed] [Google Scholar]
- 12. Wilson PW, Meigs JB, Sullivan L, Fox CS, Nathan DM, D'Agostino RB, Sr: Prediction of incident diabetes mellitus in middle-aged adults: the Framingham Offspring Study. Arch Intern Med 2007;167:1068–1074 [DOI] [PubMed] [Google Scholar]
- 13. Hivert MF, Grant RW, Shrader P, Meigs JB: Identifying primary care patients at risk for future diabetes and cardiovascular disease using electronic health records. BMC Health Serv Res 2009;9:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hivert MF, Warner AS, Shrader P, Grant RW, Meigs JB: Diabetes risk perception and intention to adopt healthy lifestyles among primary care patients. Diabetes Care 2009;32:1820–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yudkin JS, Eringa E, Stehouwer CD: “Vasocrine” signalling from perivascular fat: a mechanism linking insulin resistance to vascular disease. Lancet 2005;365:1817–1820 [DOI] [PubMed] [Google Scholar]
- 16. Hivert MF, Sullivan LM, Fox CS, Nathan DM, D'Agostino RB, Sr, Wilson PW, Meigs JB: Associations of adiponectin, resistin, and tumor necrosis factor-alpha with insulin resistance. J Clin Endocrinol Metab 2008;93:3165–3172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heidemann C, Sun Q, van Dam RM, Meigs JB, Zhang C, Tworoger SS, Mantzoros CS, Hu FB: Total and high-molecular-weight adiponectin and resistin in relation to the risk for type 2 diabetes in women. Ann Intern Med 2008;149:307–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Frankel DS, Vasan RS, D'Agostino RB, Sr, Benjamin EJ, Levy D, Wang TJ, Meigs JB: Resistin, adiponectin, and risk of heart failure: the Framingham offspring study. J Am Coll Cardiol 2009;53:754–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rutter MK, Meigs JB, Sullivan LM, D'Agostino RB, Sr, Wilson PW: Insulin resistance, the metabolic syndrome, and incident cardiovascular events in the Framingham Offspring Study. Diabetes 2005;54:3252–3257 [DOI] [PubMed] [Google Scholar]
- 20. Meigs JB, Hu FB, Rifai N, Manson JE: Biomarkers of endothelial dysfunction and risk of type 2 diabetes mellitus. JAMA 2004;291:1978–1986 [DOI] [PubMed] [Google Scholar]
- 21. Meigs JB, O'Donnell CJ, Tofler GH, Benjamin EJ, Fox CS, Lipinska I, Nathan DM, Sullivan LM, D'Agostino RB, Wilson PW: Hemostatic markers of endothelial dysfunction and risk of incident type 2 diabetes: the Framingham Offspring Study. Diabetes 2006;55:530–537 [DOI] [PubMed] [Google Scholar]
- 22. Frankel DS, Meigs JB, Massaro JM, Wilson PW, O'Donnell CJ, D'Agostino RB, Tofler GH: Von Willebrand factor, type 2 diabetes mellitus, and risk of cardiovascular disease: the Framingham Offspring Study. Circulation 2008;118:2533–2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sandhu MS, Debenham SL, Barroso I, Loos RJ: Mendelian randomisation studies of type 2 diabetes: future prospects. Diabetologia 2008;51:211–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kathiresan S, Melander O, Anevski D, Guiducci C, Burtt NP, Roos C, Hirschhorn JN, Berglund G, Hedblad B, Groop L, Altshuler DM, Newton-Cheh C, Orho-Melander M: Polymorphisms associated with cholesterol and risk of cardiovascular events. N Engl J Med 2008;358:1240–1249 [DOI] [PubMed] [Google Scholar]
- 25. Hivert MF, Manning AK, McAteer JB, Florez JC, Dupuis J, Fox CS, O'Donnell CJ, Cupples LA, Meigs JB: Common variants in the adiponectin gene (ADIPOQ) associated with plasma adiponectin levels, type 2 diabetes, and diabetes-related quantitative traits: the Framingham Offspring Study. Diabetes 2008;57:3353–3359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Prokopenko I, Langenberg C, Florez JC, Saxena R, Soranzo N, Thorleifsson G, Loos RJ, Manning AK, Jackson AU, Aulchenko Y, Potter SC, Erdos MR, Sanna S, Hottenga JJ, Wheeler E, Kaakinen M, Lyssenko V, Chen WM, Ahmadi K, Beckmann JS, Bergman RN, Bochud M, Bonnycastle LL, Buchanan TA, Cao A, Cervino A, Coin L, Collins FS, Crisponi L, de Geus EJ, Dehghan A, Deloukas P, Doney AS, Elliott P, Freimer N, Gateva V, Herder C, Hofman A, Hughes TE, Hunt S, Illig T, Inouye M, Isomaa B, Johnson T, Kong A, Krestyaninova M, Kuusisto J, Laakso M, Lim N, Lindblad U, Lindgren CM, McCann OT, Mohlke KL, Morris AD, Naitza S, Orrù M, Palmer CN, Pouta A, Randall J, Rathmann W, Saramies J, Scheet P, Scott LJ, Scuteri A, Sharp S, Sijbrands E, Smit JH, Song K, Steinthorsdottir V, Stringham HM, Tuomi T, Tuomilehto J, Uitterlinden AG, Voight BF, Waterworth D, Wichmann HE, Willemsen G, Witteman JC, Yuan X, Zhao JH, Zeggini E, Schlessinger D, Sandhu M, Boomsma DI, Uda M, Spector TD, Penninx BW, Altshuler D, Vollenweider P, Jarvelin MR, Lakatta E, Waeber G, Fox CS, Peltonen L, Groop LC, Mooser V, Cupples LA, Thorsteinsdottir U, Boehnke M, Barroso I, Van Duijn C, Dupuis J, Watanabe RM, Stefansson K, McCarthy MI, Wareham NJ, Meigs JB, Abecasis GR: Variants in MTNR1B influence fasting glucose levels. Nat Genet 2009;41:77–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, Wheeler E, Glazer NL, Bouatia-Naji N, Gloyn AL, Lindgren CM, Mägi R, Morris AP, Randall J, Johnson T, Elliott P, Rybin D, Thorleifsson G, Steinthorsdottir V, Henneman P, Grallert H, Dehghan A, Hottenga JJ, Franklin CS, Navarro P, Song K, Goel A, Perry JR, Egan JM, Lajunen T, Grarup N, Sparsø T, Doney A, Voight BF, Stringham HM, Li M, Kanoni S, Shrader P, Cavalcanti-Proença C, Kumari M, Qi L, Timpson NJ, Gieger C, Zabena C, Rocheleau G, Ingelsson E, An P, O'Connell J, Luan J, Elliott A, McCarroll SA, Payne F, Roccasecca RM, Pattou F, Sethupathy P, Ardlie K, Ariyurek Y, Balkau B, Barter P, Beilby JP, Ben-Shlomo Y, Benediktsson R, Bennett AJ, Bergmann S, Bochud M, Boerwinkle E, Bonnefond A, Bonnycastle LL, Borch-Johnsen K, Böttcher Y, Brunner E, Bumpstead SJ, Charpentier G, Chen YD, Chines P, Clarke R, Coin LJ, Cooper MN, Cornelis M, Crawford G, Crisponi L, Day IN, de Geus EJ, Delplanque J, Dina C, Erdos MR, Fedson AC, Fischer-Rosinsky A, Forouhi NG, Fox CS, Frants R, Franzosi MG, Galan P, Goodarzi MO, Graessler J, Groves CJ, Grundy S, Gwilliam R, Gyllensten U, Hadjadj S, Hallmans G, Hammond N, Han X, Hartikainen AL, Hassanali N, Hayward C, Heath SC, Hercberg S, Herder C, Hicks AA, Hillman DR, Hingorani AD, Hofman A, Hui J, Hung J, Isomaa B, Johnson PR, Jørgensen T, Jula A, Kaakinen M, Kaprio J, Kesaniemi YA, Kivimaki M, Knight B, Koskinen S, Kovacs P, Kyvik KO, Lathrop GM, Lawlor DA, Le Bacquer O, Lecoeur C, Li Y, Lyssenko V, Mahley R, Mangino M, Manning AK, Martínez-Larrad MT, McAteer JB, McCulloch LJ, McPherson R, Meisinger C, Melzer D, Meyre D, Mitchell BD, Morken MA, Mukherjee S, Naitza S, Narisu N, Neville MJ, Oostra BA, Orrù M, Pakyz R, Palmer CN, Paolisso G, Pattaro C, Pearson D, Peden JF, Pedersen NL, Perola M, Pfeiffer AF, Pichler I, Polasek O, Posthuma D, Potter SC, Pouta A, Province MA, Psaty BM, Rathmann W, Rayner NW, Rice K, Ripatti S, Rivadeneira F, Roden M, Rolandsson O, Sandbaek A, Sandhu M, Sanna S, Sayer AA, Scheet P, Scott LJ, Seedorf U, Sharp SJ, Shields B, Sigurethsson G, Sijbrands EJ, Silveira A, Simpson L, Singleton A, Smith NL, Sovio U, Swift A, Syddall H, Syvänen AC, Tanaka T, Thorand B, Tichet J, Tönjes A, Tuomi T, Uitterlinden AG, van Dijk KW, van Hoek M, Varma D, Visvikis-Siest S, Vitart V, Vogelzangs N, Waeber G, Wagner PJ, Walley A, Walters GB, Ward KL, Watkins H, Weedon MN, Wild SH, Willemsen G, Witteman JC, Yarnell JW, Zeggini E, Zelenika D, Zethelius B, Zhai G, Zhao JH, Zillikens MC, DIAGRAM Consortium, GIANT Consortium, Global BPgen Consortium. Borecki IB, Loos RJ, Meneton P, Magnusson PK, Nathan DM, Williams GH, Hattersley AT, Silander K, Salomaa V, Smith GD, Bornstein SR, Schwarz P, Spranger J, Karpe F, Shuldiner AR, Cooper C, Dedoussis GV, Serrano-Ríos M, Morris AD, Lind L, Palmer LJ, Hu FB, Franks PW, Ebrahim S, Marmot M, Kao WH, Pankow JS, Sampson MJ, Kuusisto J, Laakso M, Hansen T, Pedersen O, Pramstaller PP, Wichmann HE, Illig T, Rudan I, Wright AF, Stumvoll M, Campbell H, Wilson JF, Anders Hamsten on behalf of Procardis Consortium, MAGIC investigators. Bergman RN, Buchanan TA, Collins FS, Mohlke KL, Tuomilehto J, Valle TT, Altshuler D, Rotter JI, Siscovick DS, Penninx BW, Boomsma DI, Deloukas P, Spector TD, Frayling TM, Ferrucci L, Kong A, Thorsteinsdottir U, Stefansson K, van Duijn CM, Aulchenko YS, Cao A, Scuteri A, Schlessinger D, Uda M, Ruokonen A, Jarvelin MR, Waterworth DM, Vollenweider P, Peltonen L, Mooser V, Abecasis GR, Wareham NJ, Sladek R, Froguel P, Watanabe RM, Meigs JB, Groop L, Boehnke M, McCarthy MI, Florez JC, Barroso I: New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet 2010;42:105–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Saxena R, Hivert MF, Langenberg C, Tanaka T, Pankow JS, Vollenweider P, Lyssenko V, Bouatia-Naji N, Dupuis J, Jackson AU, Kao WH, Li M, Glazer NL, Manning AK, Luan J, Stringham HM, Prokopenko I, Johnson T, Grarup N, Boesgaard TW, Lecoeur C, Shrader P, O'Connell J, Ingelsson E, Couper DJ, Rice K, Song K, Andreasen CH, Dina C, Köttgen A, Le Bacquer O, Pattou F, Taneera J, Steinthorsdottir V, Rybin D, Ardlie K, Sampson M, Qi L, van Hoek M, Weedon MN, Aulchenko YS, Voight BF, Grallert H, Balkau B, Bergman RN, Bielinski SJ, Bonnefond A, Bonnycastle LL, Borch-Johnsen K, Böttcher Y, Brunner E, Buchanan TA, Bumpstead SJ, Cavalcanti-Proença C, Charpentier G, Chen YD, Chines PS, Collins FS, Cornelis M, J Crawford G, Delplanque J, Doney A, Egan JM, Erdos MR, Firmann M, Forouhi NG, Fox CS, Goodarzi MO, Graessler J, Hingorani A, Isomaa B, Jørgensen T, Kivimaki M, Kovacs P, Krohn K, Kumari M, Lauritzen T, Lévy-Marchal C, Mayor V, McAteer JB, Meyre D, Mitchell BD, Mohlke KL, Morken MA, Narisu N, Palmer CN, Pakyz R, Pascoe L, Payne F, Pearson D, Rathmann W, Sandbaek A, Sayer AA, Scott LJ, Sharp SJ, Sijbrands E, Singleton A, Siscovick DS, Smith NL, Sparsø T, Swift AJ, Syddall H, Thorleifsson G, Tönjes A, Tuomi T, Tuomilehto J, Valle TT, Waeber G, Walley A, Waterworth DM, Zeggini E, Zhao JH, GIANT consortium, MAGIC investigators. Illig T, Wichmann HE, Wilson JF, van Duijn C, Hu FB, Morris AD, Frayling TM, Hattersley AT, Thorsteinsdottir U, Stefansson K, Nilsson P, Syvänen AC, Shuldiner AR, Walker M, Bornstein SR, Schwarz P, Williams GH, Nathan DM, Kuusisto J, Laakso M, Cooper C, Marmot M, Ferrucci L, Mooser V, Stumvoll M, Loos RJ, Altshuler D, Psaty BM, Rotter JI, Boerwinkle E, Hansen T, Pedersen O, Florez JC, McCarthy MI, Boehnke M, Barroso I, Sladek R, Froguel P, Meigs JB, Groop L, Wareham NJ, Watanabe RM: Genetic variation in GIPR influences the glucose and insulin responses to an oral glucose challenge. Nat Genet 2010;42:142–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Meigs JB, Shrader P, Sullivan LM, McAteer JB, Fox CS, Dupuis J, Manning AK, Florez JC, Wilson PW, D'Agostino RB, Sr, Cupples LA: Genotype score in addition to common risk factors for prediction of type 2 diabetes. N Engl J Med 2008;359:2208–2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grant RW, Hivert M, Pandiscio JC, Florez JC, Nathan DM, Meigs JB: The clinical application of genetic testing in type 2 diabetes: a patient and physician survey. Diabetologia 2009;52:2299–2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Florez JC, Jablonski KA, Bayley N, Pollin TI, de Bakker PI, Shuldiner AR, Knowler WC, Nathan DM, Altshuler D: Diabetes Prevention Program Research Group. TCF7L2 polymorphisms and progression to diabetes in the Diabetes Prevention Program. N Engl J Med 2006;355:241–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M: Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–1350 [DOI] [PubMed] [Google Scholar]