Abstract
OBJECTIVE
To synthesize the cost-effectiveness (CE) of interventions to prevent and control diabetes, its complications, and comorbidities.
RESEARCH DESIGN AND METHODS
We conducted a systematic review of literature on the CE of diabetes interventions recommended by the American Diabetes Association (ADA) and published between January 1985 and May 2008. We categorized the strength of evidence about the CE of an intervention as strong, supportive, or uncertain. CEs were classified as cost saving (more health benefit at a lower cost), very cost-effective (≤$25,000 per life year gained [LYG] or quality-adjusted life year [QALY]), cost-effective ($25,001 to $50,000 per LYG or QALY), marginally cost-effective ($50,001 to $100,000 per LYG or QALY), or not cost-effective (>$100,000 per LYG or QALY). The CE classification of an intervention was reported separately by country setting (U.S. or other developed countries) if CE varied by where the intervention was implemented. Costs were measured in 2007 U.S. dollars.
RESULTS
Fifty-six studies from 20 countries met the inclusion criteria. A large majority of the ADA recommended interventions are cost-effective. We found strong evidence to classify the following interventions as cost saving or very cost-effective: (I) Cost saving— 1) ACE inhibitor (ACEI) therapy for intensive hypertension control compared with standard hypertension control; 2) ACEI or angiotensin receptor blocker (ARB) therapy to prevent end-stage renal disease (ESRD) compared with no ACEI or ARB treatment; 3) early irbesartan therapy (at the microalbuminuria stage) to prevent ESRD compared with later treatment (at the macroalbuminuria stage); 4) comprehensive foot care to prevent ulcers compared with usual care; 5) multi-component interventions for diabetic risk factor control and early detection of complications compared with conventional insulin therapy for persons with type 1 diabetes; and 6) multi-component interventions for diabetic risk factor control and early detection of complications compared with standard glycemic control for persons with type 2 diabetes. (II) Very cost-effective— 1) intensive lifestyle interventions to prevent type 2 diabetes among persons with impaired glucose tolerance compared with standard lifestyle recommendations; 2) universal opportunistic screening for undiagnosed type 2 diabetes in African Americans between 45 and 54 years old; 3) intensive glycemic control as implemented in the UK Prospective Diabetes Study in persons with newly diagnosed type 2 diabetes compared with conventional glycemic control; 4) statin therapy for secondary prevention of cardiovascular disease compared with no statin therapy; 5) counseling and treatment for smoking cessation compared with no counseling and treatment; 6) annual screening for diabetic retinopathy and ensuing treatment in persons with type 1 diabetes compared with no screening; 7) annual screening for diabetic retinopathy and ensuing treatment in persons with type 2 diabetes compared with no screening; and 8) immediate vitrectomy to treat diabetic retinopathy compared with deferred vitrectomy.
CONCLUSIONS
Many interventions intended to prevent/control diabetes are cost saving or very cost-effective and supported by strong evidence. Policy makers should consider giving these interventions a higher priority.
The cost of diabetes in the U.S. in 2007 was $174 billion (1). Many interventions can reduce the burden of this disease. However, health care resources are limited; thus, interventions for diabetes prevention/control should be prioritized. We wanted to compare the effectiveness and costs of various interventions to find those that were the most effective for the least expense. Cost-effective analysis is a useful tool for this purpose. Such analyses consist of compiling incremental cost-effectiveness ratios (ICERs), which are calculated as a ratio of the difference in costs to the difference in effectiveness between the intervention being evaluated and the comparison intervention.
With the same health outcome indicator, ICERs of interventions are comparable. Therefore, these ICERs can make it easier to decide how to allocate resources. Although many cost-effectiveness (CE) analyses of diabetes interventions have been published, their qualities and conclusions vary. A systematic review, which appraises individual studies and summarizes results, would aid policy makers and clinicians in prioritizing interventions to prevent or treat diabetes and its complications.
Few investigators have conducted systematic reviews of the CE of diabetes interventions (2–5). The systematic review presented here, following the Cochrane Collaboration's protocol (6), includes all English language studies available from 1985 to May 2008. The interventions included only those recommended by the 2008 American Diabetes Association (ADA) Standards of Medical Care in Diabetes (7).
RESEARCH DESIGN AND METHODS
Study selection and protocols for review
We searched the Medical Literature Analysis and Retrieval System Online (MEDLINE), Excerpta Medica (EMBASE), Cumulative Index to Nursing and Allied Health Literature (CINAHL), PsycINFO, Sociological Abstracts (Soc Abs), Web of Science (WOS), and Cochrane databases to identify relevant studies. We created a search strategy involving medical subject headings. The key words—and what each indicated—were:
Indicating diabetes: 26 key words indicating the disease of diabetes, such as “type 1 diabetes,” “type 2 diabetes,” “impaired glucose tolerance,” and “insulin resistance”;
Indicating costs: (“cost or expenditure”) OR (“costs and cost analysis”) OR (“health care costs”) OR (“cost of illness”);
Indicating effectiveness: (“benefit”) OR (“life years”) OR (“quality-adjusted life years”) OR (“disability adjusted life years”);
Indicating CE analysis: [(key words for costs) AND (keywords for effectiveness)] OR (“cost-benefit analysis”) OR (“cost-effectiveness analysis”) OR (“cost-utility analysis”) OR (“economic evaluation”).
Database searches were based on matches in all four keyword categories. Reference lists of all the included articles were screened for additional citations, and Diabetes Care was reviewed manually, issue by issue, as the journal was expected to be highly relevant.
Criteria for inclusion in the review were 1) original CE analysis; 2) intervention directed toward patients with type 1, type 2, or gestational diabetes mellitus (GDM) and recommended in the 2008 ADA standards for medical care (7); 3) outcomes were measured as life years gained (LYGs) or quality-adjusted life years gained (QALYs); and 4) publication in the English language occurred between January 1985 and May 2008 (2). To ensure that only studies with acceptable quality were included, we limited the analysis to studies considered good or excellent according to a 13-item quality-assessment tool based on the British Medical Journal authors' guide for economic studies (8).
To make ICERs comparable across the studies, all costs are expressed as 2007 U.S. dollars with adjustment from other currencies, as needed, using the Federal Reserve Bank's annual foreign exchange rates (9) and from other cost years using the Consumer Price Index (10). If a study did not mention the year used in cost calculations, we assumed cost was as of one year before publication. ICERs were expressed as dollars per QALY or dollars per LYG and were rounded to the nearest hundred dollars per QALY or LYG.
Classification of cost-effectiveness of interventions
Interventions were classified based on the level of CE by convention as described in the literature (2,11,12)—cost saving (an intervention generates a better health outcome and costs less than the comparison intervention) or cost neutral (ICER = 0); very cost-effective (0 < ICER ≤ $25,000 per QALY or LYG); cost-effective ($25,000 < ICER ≤ $50,000 per QALY or LYG); marginally cost-effective ($50,000 < ICER ≤ $100,000 per QALY or LYG); or not cost-effective (>$100,000 per QALY or LYG)—and whether evidence for the intervention's CE was strong, supportive, or uncertain as described below.
There were two grades of evidence included in the “strong” group. Grade 1 was defined as 1) CE of the intervention was evaluated by two or more studies; 2) study quality was rated good or excellent; 3) effectiveness of interventions based on well-conducted, randomized clinical trials with adequate power and generalizable results or meta-analysis or a validated simulation model; 4) effectiveness of interventions rated as level A (clear evidence from well-conducted, generalizable, randomized controlled trials that were adequately powered; compelling nonexperimental evidence, i.e., the all or none rule developed by the Centre for Evidence-Based Medicine at the University of Oxford, U.K.) or level B (supportive evidence from well-conducted cohort studies or supportive evidence from a well-conducted case-control study) according to the 2008 ADA standards of medical care (7); and 5) similar ICERs reported across the studies. Grade 2 was defined as the same as Grade 1 except that the CE was based on only one study and the study was rated as excellent.
We called the level of evidence “supportive” if only one study, rated lower than excellent, evaluated the CE of the intervention or if the effectiveness of the intervention was supported by either level C evidence (supportive evidence from poorly controlled or uncontrolled studies, or conflicting evidence with the weight of evidence supporting the recommendation) or expert consensus (level E) in ADA recommendations (7). The term “uncertain” was used to describe interventions with inconsistent evidence about CE across studies.
Reporting the results of the systematic review
We reported the study results in two ways: 1) summarizing the key features and results for each included study; and 2) synthesizing the CE of the interventions based on the classification criteria described above. For the summary, we grouped interventions based on their intended purposes: a) preventing type 2 diabetes among high-risk persons; b) screening for undiagnosed type 2 diabetes and GDM; c) management of diabetes and risk factors for complications; d) screening for and early treatment of complications; and e) treatment of complications and comorbidities. We considered cases where the same intervention was applied to different populations or was compared with different interventions as different specific interventions and reported the ICERs separately. This was because both incremental costs and effectiveness of an intervention, and thus the ICERs, varied if the population and/or comparison group differed. If the CE of an intervention was evaluated from different study perspectives, we report the ICERs separately. We presented the ICERs in subgroups if their ICERs differed substantially from base-case analysis, and original studies reported the ICERs this way. If the study reported the ICERs only for population subgroups, we provided a range and, when available, trend of the ICERs. Finally, if a study used both LYGs and QALYs as study outcome measures, we reported the ICER in both costs per LYG and QALY.
In reporting the synthesized results, we applied the following rules: 1) We used the median ICER to represent the CE of an intervention if the intervention was evaluated by more than one study. 2) We reported the ICERs from the longer analytical time horizon if the intervention was evaluated from both short- and long-term perspectives. This was appropriate since many of the benefits of most diabetes prevention and control interventions would come from preventing diabetic complications, which occur later in life. 3) We chose the health care system as our primary study perspective for the purpose of cross-study and cross-intervention comparisons. This study perspective included all the medical costs incurred no matter who paid. 4) If the ICERs of an intervention differed substantially between the U.S. and other developed countries (mainly European countries, Australia, and Canada), we reported the summary results separately by labeling the ICER for the U.S. or for the other countries. 5) If the trial on which the CE of an intervention was based was conducted in a mixed population with type 1 or type 2 diabetes, we assumed the CE was the same for both types of diabetes.
RESULTS
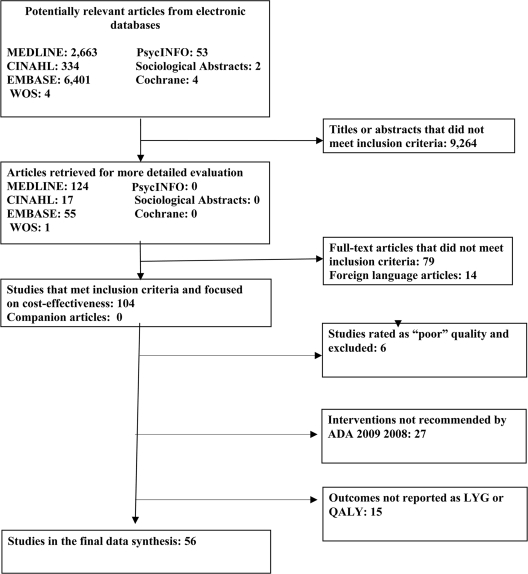
The search yielded 9,461 abstracts. After reviewing the abstracts and subsequent reference tracking, we narrowed the focus to 197 possible original CE studies. Further review of the full text resulted in 56 CE studies that met our inclusion criteria. Figure 1 depicts the data abstraction process.
Figure 1.
Selection of cost-effectiveness studies for systematic review of interventions to prevent and control diabetes.
Table 1 shows the detailed description of the CE studies that we included according to intervention type (13–70). We first grouped similar interventions together, then arranged them chronologically and by the first author's last name. Some studies that evaluated multiple interventions appear in more than one category. The information used to describe each study included the intervention being evaluated; comparison intervention, population, and country setting; data sources for the effectiveness of the intervention; study methods; quality of the study; analytical time horizon; discount rate (a rate that is used to convert future costs and benefits into their present values); and ICER.
Table 1.
Description of the cost-effectiveness studies for diabetes interventions*
| Source/study quality†/country | Study population | Intervention‡ | Comparison | Effectiveness data | Methodology‖/analytical horizon/discount rate | Cost-effectiveness ratios (2007 U.S. $) |
|---|---|---|---|---|---|---|
| Preventing type 2 diabetes among high-risk individuals | ||||||
| Segal et al. 1998 (59)§ Australia | Seriously obese or seriously obese with IGT | Intensive diet and education | Standard care | Literature review | 25 years 5% | Cost saving |
| Overweight or obese IGT or NGT and IGT | Group education in workplace on diet and physical activity for men | Standard care | Cost saving | |||
| High-risk adults IGT or NGT and IGT | General practitioner advice on healthy lifestyle | Standard care | $1,000–$2,500/LYG | |||
| Overweight adults in general population | Community-supported media campaign on obesity/sedentary lifestyle | No campaign | Cost saving | |||
| Women with GDM history + NGT or IGT | Intensive diet and behavioral modification | Standard care | $1,300–$2,500/LYG | |||
| DPP 2003 (66) U.S. | IGT | Intensive lifestyle modification | Standard advice on lifestyle | DPP Multicenter RCT (n = 3,234) | 3 years 0% | $32,900/QALY; if in 10-person group, $11,100/QALY |
| IGT | Metformin | Standard advice on lifestyle | $134,000/QALY; if metformin cost reduced 50%, $76,500/QALY | |||
| IGT | Intensive lifestyle modification‡‡ | Standard advice on lifestyle | $69,400/QALY; if in 10-person group, $36,000/QALY | |||
| IGT | Metformin‡‡ | Standard advice on lifestyle | $133,400/QALY | |||
| Caro et al. 2004 (15) Canada | IGT | Intensive lifestyle modification | No intervention | DPP (n = 3,234), FDPS (n = 52) | 10 years 5% | $700/LYG |
| IGT | Metformin | No intervention | Cost saving in LYG and QALY | |||
| Palmer et al. 2004 (50) Australia, France, Germany, Switzerland, U.K. | IGT | Intensive lifestyle modification | Standard advice on lifestyle | DPP Multicenter RCT (n = 3,234) | Lifetime 5% except U.K.: cost 5%, effectiveness, 1.5% | Cost saving except U.K.; U.K.: $8,300/LYG |
| IGT | Metformin | Standard advice on lifestyle | Cost saving, except UK; UK: $6,500/LYG | |||
| Eddy et al. 2005 (25) U.S. | IGT | Intensive lifestyle modification‡‡ | No intervention | DPP Multicenter RCT (n = 3,234) | 30 years 3% | $84,700/QALY; in 10-person group, $16,000/QALY |
| IGT | Intensive lifestyle modification# | No intervention | $192,600/QALY; in 10-person group, $36,400/QALY | |||
| IGT | Metformin‡‡ | No intervention | $47,900/QALY | |||
| Herman et al. 2005 (34) U.S. | IGT | Intensive lifestyle modification | Standard advice on lifestyle | DPP Multicenter RCT (n = 3,234) | Lifetime 3% | $1,500/QALY; in 10-person group, cost saving |
| Intensive lifestyle modification‡‡ | Standard advice on lifestyle | $11,800/QALY | ||||
| Metformin | Standard advice on lifestyle | $42,000/QALY | ||||
| Generic | Standard advice on lifestyle | $2,400/QALY | ||||
| Metformin‡‡ | $40,200/QALY | |||||
| Lindgren et al. 2007 (41) Sweden | IGT Age 60 years BMI >25 kg/m2, FPG >6.1 mmol/l | Intensive lifestyle intervention (6 years)‡‡ | General lifestyle advice | FDPS (n = 52) | Lifetime 3% | Cost saving not considering cost of extended life; $2,600/QALY including cost of extended life |
| Hoeger et al. 2007 (36) U.S. | U.S. population age 45–74 years, overweight and obese (BMI ≥ 25 kg/m2) Groups | Screening for IGT and IFPG, DPP lifestyle intervention with IGT + IFPG | No screening and no lifestyle intervention | DPP (n = 3,234) | Lifetime 3% | $10,600/QALY; in group settings, cost saving |
| Screening for IGT and IFPG, DPP lifestyle intervention with IFPG or IGT + IFPG | No screening and lifestyle intervention | $12,300/QALY; in group settings, $344/QALY | ||||
| Screening for IGT and IFPG, DPP lifestyle intervention with IGT + IFPG | Screening for IGT and IFPG, following DPP lifestyle intervention with IFPG, IGT, or IFPG + IGT | $13,100/QALY | ||||
| Screening and metformin treatment with IGT + IFPG | No screening and treatment | $26,600/QALY | ||||
| Screening and metformin treatment with IGT, IFPG, or IGT + IFPG | No screening and treatment | $26,000/QALY | ||||
| Screening for undiagnosed type 2 diabetes and gestational diabetes | ||||||
| Centers for Disease Control and Prevention 1998 (16) U.S | U.S. population 25 years and older One-time | Opportunistic screening for undiagnosed type 2 diabetes starting at age 25 years, then treatment (universal screening) | No screening and treatment until clinical diagnosis of type 2 diabetes | Lifetime 3% | $374,900/LYG or $89,800/QALY; increasing with age (age ≥ 25 years) treatment (universal screening) | |
| . | $57,100/LYG or $21,400/QALY (age 25–34 years) | |||||
| $103,200/LYG or $29,700/QALY (age 35–44 years) | ||||||
| 293,900/LYG or $70,100/QALY (age 45–54 years) | ||||||
| $1 million/LYG or $185,000/QALY (age 55–64 years) | ||||||
| $928,000/QALY (age ≥65) | ||||||
| African Americans: | ||||||
| age 25–34 years | $3,500/LYG or $1,300/QALY | |||||
| age 35–44 years | $10,200/LYG or $3,100/QALY | |||||
| age 45–54 years | $95,400/LYG or $19,600/QALY | |||||
| age 55–64 years | $764,100/LYG or $112,600/QALY | |||||
| age ≥65 years | $2 million/LYG or $500,000/QALY | |||||
| Hoerger et al. 2004 (35) U.S. | Persons with hypertension | Targeted screening for undiagnosed diabetes among persons with hypertension | No screening or treatment until clinical diagnosis of type 2 diabetes | Lifetime 3% | $46,800–$130,500/QALY decreasing with age $70,500/QALY for age 45 years | |
| U.S. population | One-time opportunistic screening, then treatment (universal screening) | No screening or treatment until clinical diagnosis of type 2 diabetes | $72,200–$189,100/QALY decreasing with age $183,500/QALY for age 45 years | |||
| U.S. population | One-time opportunistic screening, then treatment (universal screening) | Targeted screening, then treatment | $215,600–$699,800/QALY increasing with age | |||
| Nicolson et al. 2005 (44) U.S. | 30-year-old pregnant women between 24–28 weeks' gestation | Sequential method (50-g GCT + 100-g GTT)‡‡ | No screening 75-g GTT | A few unidentified RCTs | <1 year** 0% | Cost saving |
| 100-g GTT‡‡ | No screening or 75-g GTT method | Cost saving | ||||
| 100-g GTT‡‡ | Sequential method | $35,200/QALY for maternal outcomes, $9,000/QALY for neonatal outcomes | ||||
| Intensive glycemic control | ||||||
| DCCT 1996 (65) U.S. | Type 1 diabetes | Intensive glycemic control through insulin management, self-monitoring, and outpatient visits. The goal was to achieve A1C level as normal as possible (6%) | Conventional therapy (less intensive) | DCCT Multicenter RCT (n = 1,441) | Lifetime 3% | $47,600/life year gained, $50,800/QALY |
| Palmer et al. 2000 (46) Switzerland | Type 1 diabetes | Intensive insulin therapy | Conventional insulin therapy | Literature review | Lifetime 3%, 5%, 6% Reported results at 3% in the table | $46,600/LYG |
| Scuffham et al. 2003 (58) U.K. | Type 1 diabetes | Continous subcutaneous insulin intervention for persons using insulin pump | Multiple daily insulin injections | 1 systematic review 1 meta-analysis | 8 years 6% | $10,200/QALY |
| Roze et al. 2005 (56) U.K. | Type 1diabetes | Continuous subcutaneous insulin infusion | Multiple daily insulin injections | DCCT (n = 1,441) mainly meta-analysis | 60 years 3% | $18,500/QALY |
| Eastman et al. 1997 (24) U.S. | Newly diagnosed type 2 diabetes | Intensive treatment targeting maintenance of A1C level at 7.2% | Standard antidiabetic treatment targeting A1C level at 10% | DCCT (n = 1,441) | Lifetime 3% | $17,400/QALY; sensitive to age at diabetes onset; CER <33,000 for age <50 years; $371,700/QALY for age 70–80 years |
| Gray et al. 2000 (30) U.K. | Type 2 diabetes | Intensive management with insulin or sulfonylurea aiming at FPG <6 mmol/l | Conventional management (mainly through diet) aiming at FPG <15 mmol/l | UKPDS Multicenter RCT (n = 5,120) | 10 years** 6% | Cost saving in trial; $1,100/event-free year gained in clinic setting |
| Wake et al. 2000 (70) Japan | Type 2 diabetes | Intensive insulin therapy through multiple insulin injections A1C <7% | Conventional insulin injection therapy | Kumamoto study RCT (n = 110) | 10 years** 3% | Cost saving |
| Clarke et al. 2001 (18) U.K. | Newly diagnosed type 2 diabetes Overweight | Intensive blood glucose control with metformin aiming at FPG <6 mmol/l | Conventional treatment primarily with diet | UKPDS (n = 5,120) | Median 10.7 years** 6% | Cost saving |
| Centers for Disease Control and Prevention 2002 (17) U.S. | Newly diagnosed type 2 diabetes | Intensive glycemic control with insulin or sulfonylurea aiming at FPG of 6 mmol/l | Conventional glucose control (mainly diet) | UKPDS (n = 5,120) | Lifetime 3% | $62,000/QALY; increasing rapidly with age at diagnosis: $14,400/QALY for age 25–34 years; $27,500–$56,000/QALY for age 35–54 years; > $100,000–$3.1 million for age 55–94 years |
| Cost saving under UKPDS cost scenario (no case management cost, much less self-testing, slightly fewer physician visits) but using U.S. unit cost | ||||||
| Clarke et al. 2005 (19) U.K. | Newly diagnosed type 2 diabetes requiring insulin | Intensive glycemic control with insulin or sulfonylurea at FPG <6 mmol/l | Conventional glucose control therapy (mainly diet) | UKPDS (n = 5,120) | Lifetime 3.5% | $3,400/QALY |
| Newly diagnosed type 2 diabetes Overweight | Intensive glycemic control with metformin | Conventional glucose control therapy (mainly diet) | Cost saving | |||
| Eddy et al. 2005 (25) U.S. | Newly diagnosed type 2 diabetes | Intensive DPP lifestyle with FPG >125 mmol/l Target: A1C level of 7% ‡‡ | Dietary advice | DPP (n = 3,234) | 30 years 3% | $33,100/QALY |
| Almbrand et al. 2000 (13) Sweden | Type 2 diabetes with acute MI | Insulin-glucose infusion for at least 24 h, then subcutaneous multidose insulin for ≥3 months | Standard antidiabetic therapy | DIGAMI study, RCT 1-year intervention, 4-year follow-up (n = 620) | 5 years** 3% | $8,700/LYG, $12,400/QALY |
| Self-monitoring blood glucose | ||||||
| Tunis 2008 (67) U.S. | Type 2 diabetes treated with oral agents in a large HMO | SMBG 1 time/day 40-year horizon public payer | No SMBG | Kaiser Permanente longitudinal study of cohort of “new antidiabetic drug users” | 40 years 3% | $8,200/QALY; 52.6% probability less than $50,000/QALY |
| SMBG 3 times/day 40-year horizon | No SMBG | $6,900/QALY; 60.7% probability less than $50,000/QALY | ||||
| SMBG 1 time/day 5-year horizon 10-year horizon | No SMBG | $24,200/QALY $9,700/QALY |
||||
| SMBG 3 times/day 5-year horizon 10-year horizon | No SMBG | $30,300/QALY $540/QALY |
||||
| Intensive hypertension control | ||||||
| UKPDS 1998 (68) U.K. | Type 2 diabetes Hypertension | Tight control of hypertension, BP <150/<80 mmHg, ACE inhibitor, β-blocker, and other agents | Less tight control of BP (mmHg), Initially <200/105, Later 180/105 | UKPDS (n = 5,120) | Lifetime 6% | Cost saving in trial; $960/year free from occurrence of diabetes endpoint in clinic |
| Elliot et al. 2000 (26) U.S. | Type 2 diabetes Hypertension, initially free of CVD or ESRD | Reduction of BP to 130/85 mmHg Medications not mentioned | Reduction of BP to 140/90 mmHg | Meta-analysis of data from epidemiological studies and clinical trials | Lifetime 3% | |
| Start of treatment | ||||||
| Age 50 years | $1,200/LYG | |||||
| Age 60 years | Cost saving | |||||
| Age 70 years | Cost saving | |||||
| Centers for Disease Control and Prevention 2002 (17) U.S. | Type 2 diabetes Hypertension | Intensified hypertension control ACE inhibitor β-blocker Average BP 144/82 mmHg | Moderate hypertension control, Average BP 154/86 mmHg | UKPDS (n = 5,120) | Lifetime 3% | Cost saving |
| Clarke et al. 2005 (19) U.K. | Type 2 diabetes Hypertension | Tight BP control BP <150/85 mmHg, ACE inhibitor (captopril) or β-blocker (atenolol) | Less tight control of BP (mmHg), Initial <200/105, Later <180/105 | UKPDS (n = 5,120) | Lifetime 3.5% | $200/QALY |
| Cholesterol control | ||||||
| Herman et al. 1999 (33) U.S. | Type 2 diabetes Dyslipidemia, Previous MI or angina | Simvastatin | Placebo | 4S study, Double-blind randomized, placebo-controlled, multicenter, multicountry trial (n = 4,444) | 5 years** 3% for cost, 0% for benefit | Cost saving |
| Jonsson et al. 1999 (39) European countries | Type 2 diabetes Dyslipidemia, Previous MI or angina | Simvastatin | Placebo | 4S study (n = 4,444) | Lifetime 3% | CS-$9,400/LYG in different countries, Median: $2,800/LYG |
| Grover et al. 2000 (31) Canada | Type 2 diabetes Dyslipidemia CVD history, Men and women 60 years old | Simvastatin | Placebo | 4S study (n = 4,444) CARE (n = 4,159) | Lifetime 5% | $6,100–$12,300/LYG Increasing with pretreatment of LDL cholesterol level |
| Type 2 diabetes Dyslipidemia, No CVD history | Simvastatin | Placebo | ||||
| Men Pretreatment LDL cholesterol level: | ||||||
| 5.46 mmol/l (211 mg/dl) | $6,100–$15,000/LYG | |||||
| 3.5 mmol/l (135 mg/dl) | $10,700–$23,000/LYG | |||||
| Women Pretreatment LDL cholesterol level: | ||||||
| 5.46 mmol/l | $15,300–$27,600/LYG | |||||
| 3.5 mmol/l | $36,800–$61,300/LYG | |||||
| Centers for Disease Control and Prevention 2002 (17) U.S. | Type 2 diabetes Dyslipidemia, No CVD history | Pravastatin | Placebo | West Scotland Coronary Prevention Study (n = 6,595 men) | Lifetime 3% | U-shape for age, $77,800/QALY |
| Raikou et al. 2007 (54) U.K. Ireland | Type 2 diabetes, No CVD history, No elevated LDL cholesterol level ≥1 CVD risk factor: retinopathy, microalbuminuria or macroalbuminuria, current smoking, or hypertension | Atorvastatin | Placebo | CARDS, Randomized, controlled, multicenter trial 94% white (n = 2,838) | Lifetime 3.5% | $2,800/LYG, $3,500/QALY Using UKPDS risk engine Low risk: $11,300/QALY; Medium risk: $4,700/QALY; High risk: $2,200/QALY |
| Smoking cessation | ||||||
| Earnshaw et al. 2002 (23) | Newly diagnosed type 2 diabetes | Smoking cessation, Standard antidiabetic care | Standard antidiabetic care | Lifetime 3% | ||
| United States | Smokers | |||||
| Aged 25–84 years | <$25,000/QALY | |||||
| Aged 85–94 years | $89,800/QALY | |||||
| Educational program | ||||||
| Shearer et al. 2004 (61)§ Germany | Type 1 diabetes | Structured treatment and teaching program: educational course of training to self-manage diabetes and enjoy dietary freedom | Usual care (daily insulin injection) | Rosiglitazone trial CODE2 study of prevalence of complications, not an RCT | Lifetime 6% | Cost saving |
| Gozzoli et al. 2001 (29) Switzerland | Type 2 | Standard antidiabetic care plus educational program, Self-monitoring, Recommendations on diet and exercise, Self-management of diabetes and complications, General health education | Standard antidiabetic care | Literature review (quality) | Lifetime 3% | $4,000/LYG |
| Diabetes disease management | ||||||
| Mason et al. 2005 (43) England | Type 2 diabetes Hypertension | Policy to implement clinics led by specialist nurses to treat and control hypertension through consultation, medication review, condition assessment, and lifestyle advice | Usual care | SPLINT RCT (n = 1,407) UKPDS (n = 5,120) | Lifetime 5% | $4,800/QALY |
| Diagnosed diabetes Dyslipidemia | Policy to implement clinics led by specialist nurses to treat and control hyperlipidemia by usual care | Usual care | $23,600/QALY | |||
| Gilmer et al. 2007 (27) San Diego County, California | Diabetes 48% Latinos | Culturally sensitive case management and self-management training program led by bilingual/bicultural medical assistant and registered dietitian stepped-care pharmacologic management of glucose and lipid levels and hypertension‡‡‡ | Standard care | Project Dulce Observational cohort study with controls Average follow-up, 289 days (n = 3,893) | 40 years 3% | $9,400/LYG or $12,000/QALY for uninsured; 100% probability to be less than $50,000 and $100,000/QALY, respectively |
| $22,400/LYG or $29,100/QALY for patients in County Medical Services; | ||||||
| 92% or 98% probability to be cost-effective if willingness to pay was $50,000 or $100,000/QALY, respectively | ||||||
| $42,600/LYG or $53,120/QALY for patients in Medi-Cal; | ||||||
| 57% or 81% probability to be cost-effective if willingness to pay was $50,000 and $100,000/QALY, respectively | ||||||
| $68,400/LYG or $82,300/QALY for patients with commercial insurance; | ||||||
| 31% and 62% probability to be cost-effective if willingness to pay was $50,000 and $100,000/QALY, respectively | ||||||
| Preventing diabetic complications Eye complications | ||||||
| Javitt et al. 1994 (37)§ U.S. | Newly diagnosed type 2 diabetes | 8 strategies for eye screening with dilation: Screening every 1, 2, 3, or 4 years and | No screening | Cross-sectional and longitudinal studies | Lifetime 5% | All 8 strategies were cost saving |
| More frequent follow-up screening for diabetes patients with background retinopathy†† | ||||||
| Javitt et al. 1996 (38) U.S. | Newly diagnosed type 1 and type 2 diabetes | Annual eye screening with dilation for all patients with diabetes but no retinopathy | Eye screening in 60% of diabetes patients | Cross-sectional and longitudinal studies | Lifetime 5% | $3,800/person-year of sight saved, $6,900/QALY |
| Type 1 diabetes | $4,300/QALY | |||||
| Type 2 diabetes | Examination every 6 months for those with retinopathy | $6,900/QALY | ||||
| Palmer et al. 2000 (46) Switzerland | Type 1 diabetes | Annual eye screening and treatment, Conventional insulin therapy | Conventional insulin therapy | Literature review | Lifetime 3% | Cost saving |
| Vijan et al. 2000 (69) U.S. | Type 2 | Eye screening for diabetes patients every 5 years Subsequent annual screening for those with background retinopathy | No screening | Epidemiological studies | Lifetime 3% | $23,500/QALY |
| Eye screening for diabetes patients every 3 years Subsequent annual screening for those with background retinopathy | No screening | $27,000/QALY | ||||
| Eye screening for diabetes patients every 2 years Subsequent annual screening for those with background retinopathy | No screening | $30,700/QALY | ||||
| Eye screening annually for diabetes patients Subsequent annual screening for those with background retinopathy | No screening | $39,500/QALY | ||||
| Eye screening for diabetes patients every 3 years | 5-year intervals | $32,800/QALY | ||||
| Eye screening for diabetes patients every 2 years | 3-year intervals | $54,000/QALY | ||||
| Annual eye screening for diabetes patients | 2-year intervals | $116,800/QALY | ||||
| Maberley et al. 2003 (42) Western James Bay, Victoria, British Columbia, Canada | Type 1 diabetes and Type 2 diabetes | Screening using digital camera Immediate assessment of quality or electronically transferred to a remote reading center | Retina specialists visit Moose Factory every 6 months to examine people with diabetes, and patients in outlying communities are flown to Moose Factory, Canada | 10 years 5% | Cost saving | |
| Foot ulcers | ||||||
| Tennval et al. 2001 (64) Sweden | Type 1 diabetes and Type 2 diabetes | Optimal prevention of foot ulcer including foot inspection, appropriate footwear, treatment, and education | Usual care | Clinical and epidemiological data | 5 years** 0% | |
| High risk: Previous foot ulcer Previous amputation | Cost saving | |||||
| Moderate risk: Neuropathy, PVD, and/or foot deformity | Cost saving | |||||
| Low risk: No specific risk factor | >$100,000/QALY | |||||
| Ortegon et al. 2004§ (45) The Netherlands | Newly diagnosed type 2 diabetes | Intensive glycemic control Optimal foot care | Standard care | UKPDS (n = 5,120) | Lifetime 3% | $44,900/QALY |
| Foot ulcer | Literature review on trials and epidemiological studies | Assuming 10% reduction of foot lesion, $308,300/QALY | ||||
| Assuming 90% reduction of foot lesion, $17,000/QALY | ||||||
| Intensive glycemic control plus optimal foot care | Standard care | Assuming 10% reduction of foot lesion, $34,400/QALY | ||||
| Assuming 90% reduction of foot lesion, $11,010/QALY | ||||||
| End-stage renal disease | ||||||
| Borch-Johnsen et al. 1993 (14)§ Germany | Type 1 diabetes | Annual screening for microalbuminuria at 5 years after diabetes onset, ACEI treatment | Treatment of macroalbuminuria | Danish cohort (n = 2,890) | 30 years 6% | Cost saving |
| Kiberd et al. 1996 (40)§ Canada | Type 1 diabetes | Screening for microalbuminuria ACEI treatment | Treatment of hypertension and/or macroproteinuria | Clinical trial | Lifetime 5% | $58,400/QALY |
| Palmer et al. 2000 (46) Switzerland | Type 1 diabetes High total cholesterol level High systolic BP | Microalbuminuria monitoring, ACE treatment, Conventional insulin therapy | Conventional insulin therapy | Literature review | Lifetime 3% | Cost saving |
| Dong et al. 2004 (22) U.S. | Type 1 diabetes | ACEI treatment starting at 1 year after diagnosis | Annual screening for microalbuminuria ACE treatment | DCCT (n = 1,441) | Lifetime 3% | $38,000/QALY, Increased with lowering A1C level; at A1C level 9%, <25,000/QALY |
| Sakthong et al. 2001 (57)§ Thailand | Type 2 diabetes Microalbuminuria but normal BP | ACE inhibitors | Placebo | 7-year RCT in Israel (n = 94) | 25 years 8% | Cost saving |
| Souchet et al. 2003 (62) France | Type 2 diabetes Nephropathy | Losartan | Placebo | RENAAL study Multicenter international trial (n = 1,513) | 4 years** Cost discounted at 8% Benefits not discounted | Cost saving |
| Szucs et al. 2004 (63)§ Switzerland | Type 2 diabetes Nephropathy | Losartan | Placebo | RENAAL study Multicenter international trial (n = 1,513) | 3.5 years** 0% | Cost saving |
| Palmer et al. 2003 (47) Belgium, France | Type 2 diabetes Macroalbuminuria Hypertension | Irbesartan | Standard therapy for hypertension | IDNT study Multicenter, double-blind placebo controlled trial (n = 1,715) | Lifetime 3% | Cost saving |
| Palmer et al. 2004 (49) U.K. | Type 2 diabetes Hypertension Nephropathy | Irbesartan | Standard therapy for hypertension | IDNT study (n = 1,715) | 10 years 6% for costs 1.5% for benefits | Cost saving |
| Palmer et al. 2005 (51) Spain | Type 2 diabetes Microalbuminuria Hypertension | Irbesartan | Standard therapy for hypertension, No ACEI, AIIRA, or β-blockers | IDNT study (n = 1,715) IRMA-2 trial Randomized controlled study (n = 582) | 25 years 3% | Cost saving |
| Palmer et al. 2007 (52) Hungary | Type 2 diabetes Microalbuminuria | Adding irbesartan | Placebo + standard therapy for hypertension | IDNT study (n = 1,715) IRMA-2 trial (n = 582) | 25 years 5% | Cost saving |
| Palmer et al. 2004 (48) U.S. | Type 2 diabetes Hypertension | Irbesartan at stage of microalbuminuria | Standard therapy for hypertension | IDNT study (n = 1,715) | 25 years 3% | Cost saving |
| Microalbuminuria | Irbesartan at stage of macroalbuminuria | Standard therapy for hypertension | Cost saving | |||
| Irbesartan at stage of microalbuminuria | Irbesartan at stage of macroalbuminuria | Cost saving | ||||
| Palmer et al. 2007 (53) U.K. | Type 2 diabetes Hypertension | Irbesartan at stage of microalbuminuria | Standard therapy for hypertension | IDNT study (n = 1,715) IRMA-2 trial (n = 582) | 25 years 3.5% | Cost saving |
| Microalbuminuria | Irbesartan at stage of macroalbuminuria | Standard therapy for hypertension | Cost saving | |||
| Irbesartan at stage of microalbuminuria | Irbesartan at stage of macroalbuminuria | Cost saving | ||||
| Coyle et al. 2007 (21) Canada | Type 2 diabetes Hypertension Macronephropathy or Micronephropathy | Irbesartan added at stage of microalbuminuria | Conventional treatment for diabetes and hypertension, No ACEI or AIIRAs | IDNT study (n = 1.715) IRMA-2 trial (n = 582) | Lifetime 5% | Cost saving |
| Irbesartan added at stage of overt nephropathy | Conventional treatment for diabetes and hypertension | Cost saving | ||||
| Irbesartan added at stage of microalbuminuria | Irbesartan added at stage of overt nephropathy | Cost saving | ||||
| Golan et al. 1999 (28) U.S. | Newly diagnosed type 2 diabetes | Treat patients with new diagnosis with ACEI | Screening for macroalbuminuria and treatment with ACEI | U.S.-Canada Collaborative study for type 1 diabetes, RCT (n = 207) 2 RCT for type 2 diabetes in Israel (n = 94 and 156, respectively) | Lifetime 3% | Cost saving |
| Screening for microalbuminuria and treatment with ACEI | Screening for macroalbuminuria and treatment with ACEI | Cost saving | ||||
| Treat patients with new diagnosis with ACEI | Screening for microalbuminuria and treatment with ACEI | $10,900/QALY | ||||
| Clarke et al. 2000 (20) Canada | Type 1 diabetes | Province or territory paying for ACEI | Pay from out-of-pocket | Collaborative observational study using administrative data base (N=8.4 million) | 21 years 5% | Cost saving Compliance rate and cost of ACEI affected ICER greatly |
| Rosen et al. 2005 (55) US | Medicare population Type 1 and type 2 diabetes | Medicare full-payment for ACEI | Pay from out-of-pocket | HOPE Trial | Lifetime 3% | Cost saving if ACEI use increased by at least 7.2% |
| Medicare paying for ACEI | Current Medicare Modernization Act | Multinational RCT | If use increased by 2.9%, <$20,000/QALY | |||
| Cost saving if ACEI use increased by at least 6.2% | ||||||
| If use increased by 2.2%, <$20,000/QALY | ||||||
| Comprehensive interventions | ||||||
| Palmer et al. 2000 (46) Switzerland | Type 1 diabetes | C + ACEI therapy + eye screening and treatment (EYE) | Conventional glycemic control (C) | Literature review | Lifetime 3% | Cost saving |
| Intensive insulin therapy (I) + ACEI therapy | I | $46,500/LYG | ||||
| I + EYE | I | $50,600/LYG | ||||
| I + ACEI therapy + EYE | I | $49,800/LYG | ||||
| Gozzoli et al. 2001 (29) Switzerland | Type 2 diabetes | Added education program, nephropathy screening, and ACEI therapy to standard antidiabetic care | Standard antidiabetic care | Literature review | Lifetime 0%, 3% | Cost saving |
| Added education program, nephropathy screening, ACEI therapy, and retinopathy screening and laser therapy to standard antidiabetic care | Standard antidiabetic care | Cost saving | ||||
| Multifactorial intervention included educational program, screening for nephropathy and retinopathy, control of CVD risk factors, early diagnosis and treatment of complications, and health education | Standard antidiabetic care | Cost saving | ||||
| Treatment of diabetes-related complications Retinopathy | ||||||
| Sharma et al. 2001 (60) U.S. | Diabetic retinopathy Health maintenance organization | Immediate vitrectomy for management of vitreous hemorrhage secondary to diabetic retinopathy | Deferral of vitrectomy | DRVS | Lifetime 6% | $2,900/QALY |
| Foot ulcer | ||||||
| Habacher et al. 2007 (32) Austria | Newly diagnosed diabetic foot ulcer | Intensified treatment by international consensus on diabetic foot care | Standard treatment | Retrospective study of patient records on 119 consecutive ulcerations in 86 patients at tertiary outpatient clinic specializing in treatment of diabetic foot ulcers | 15 years 0–8% | Cost saving |
4S, Scandinavian Simvastatin Survival Study; ACEI, angiotensin converting enzyme inhibitors; AHT, arterial hypertension; AIIRA, angiotensin II receptor antagonists; BP, blood pressure; C, conventional glycemic control; CAD, coronary artery disease; CARDS, Collaborative Atorvastatin Diabetes Study; CARE, Cholesterol and Recurrent Events; CDC, Centers for Disease Control and Prevention; CODE2 = the cost of diabetes type 2 in Europe; CORE, Center for Outcomes Research; CVD, cardiovascular disease; DAIS, Diabetes Atherosclerosis Intervention Study; DCCT, Diabetes Control and Complications Trial; DIGAMI, Diabetes Mellitus Insulin Glucose Infusion in Acute Myocardial Infarction; DiGEM, diabetes glycemic education and monitoring; DPN, diabetic peripheral neuropathy; DPP, diabetes prevention program; DRVS, Diabetic Retinopathy Vitrectomy Study; DTTP, diabetes treatment and teaching program; EYE, screening for retinopathy and ensuing treatment; FDPS, Finish Diabetes Prevention Study; FPG, fasting plasma glucose; HMO, Health Maintenance Organization; HOPE, Heart Outcome Prevention Evaluation; I, intensive glycemic control; ICER, incremental cost effectiveness ratio; IDNT, Irbesartan Type II Diabetic Nephropathy Trial; IFPG, impaired fasting plasma glucose; IGT, impaired glucose tolerance; IMPACT, Improving Mood-Promoting Access to Collaborative Treatment; KORA, Cooperative Research in the Region of Augsburg; MI, myocardial infarction; NGT, normal glucose tolerance; NIDDM, Non-Insulin Dependent Diabetes Mellitus; OGTT, oral glucose tolerance test; PHN, postherpetic neuralgia; PROactive, PROspective pioglitAzone Clinical Trial in macroVascular Events; PROPHET, Prospective Population Health Event Tabulation; PVD, peripheral vascular disease; RCT, randomized clinical trial; RENAAL, Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan; ROSSO, RetrOlective Study Self-Monitoring of Blood Glucose and Outcome; RPG, random plasma glucose; SMBG, self-monitoring blood glucose; SPECT, single proton emission computed tomography; SPLINT, specialist nurse-led intervention to treat and control hypertension and hyperlipidemia in diabetes; QALY, quality adjusted life year; VA-HIT, VA-HDL Intervention Trial.
*The studies were ordered by grouping similar interventions together, then follow the year and alphabetical order of the first author's last name; the numbers in the parenthesis are the reference number.
†The study was rated as “excellent” quality unless otherwise indicated.
§The study was rated as “good” quality.
‖The study is based on simulation modeling unless otherwise indicated.
**Within trial or within epidemiological study.
‡The study was done from the perspective of the health system unless otherwise indicated.
‡‡The study was done from the societal perspective.
#The study done from the perspective of the health plan.
††The study was done from the federal budget perspective.
†††Third party payer perspective.
Thirty-nine of the 56 studies took a long-term analytical time horizon, such as 20–30 years or lifetime. Nearly all of the studies with the long-term horizon used simulation modeling. Only one study was conducted in a developing country (Thailand) (57). There were 48 excellent studies and 8 good studies. Only three studies took perspectives other than the health care system.
The interventions evaluated in these CE studies covered a wide range: lifestyle and medication therapy to prevent type 2 diabetes among high-risk individuals (eight studies); screening for undiagnosed type 2 diabetes or GDM (three studies); intensive glycemic control (12 studies); self-monitoring of blood glucose (one study); intensive hypertension control (four studies); statin therapy for cholesterol control (five studies); smoking cessation (one study); diabetic health education program (two studies); diabetes disease management program (two studies); screening to prevent diabetic retinopathy (five studies); optimal foot care to prevent foot ulcer and amputation (two studies); ACE inhibitor (ACEI) or angiotensin receptor blocker (ARB) therapy to prevent diabetic end-stage renal diseases (ESRD) (15 studies); comprehensive interventions using a combination of several of the above secondary prevention interventions (two studies); and interventions treating diabetic retinopathy and foot ulcers (two studies).
The classification of the interventions based on their level of CE and strength of evidence is presented in Table 2. For each intervention, we also described the number of studies that evaluated the CE of this intervention, its comparison intervention, and the study population in which the intervention was implemented. We reported the median and range of the ICERs across the studies.
Table 2.
Summary of the cost-effectiveness studies by intervention*
| Intervention | Comparison | Intervention population | Number of studies | Level of recommendation by ADA | Median of the cost-effectiveness ratios | Range of the cost-effectiveness ratios |
|---|---|---|---|---|---|---|
| Strong evidence | ||||||
| Cost saving | ||||||
| ACEI therapy for intensive hypertension control | Standard hypertension control | Type 2 | 4 | B | Cost saving | Cost saving-$1,200/LYG $230/QALY |
| Addition of ACEI or ARB therapy to prevent ESRD | No ACEI or ARB therapy | Type 2 | 7 | A | Cost saving | Cost saving |
| Irbesartan therapy at the stage of microalbuminuria | Irbestartan therapy at the stage of macroalbuminuria | Type 2 | 3 | A | Cost saving | Cost saving |
| Comprehensive foot care to prevent ulcer† | Usual care | Mixed population of type 1 and type 2 | 1 | B | Cost saving | Cost saving |
| Multi-component interventions (conventional insulin control, ACEI treatment, eye screening, and treatment) | Conventional insulin control | Type 1 | 1 | A: ACEI treatment B: eye screening and ensuing treatment |
Cost saving | Cost saving |
| Multi-component interventions (standard antidiabetic care plus education, nephropathy screening, ACEI treatment, retinopathy screening) | Standard antidiabetic care | Type 2 | 1 | B: education E: nephropathy screening B: ACEI therapy B: retinopathy screening |
Cost saving | Cost saving |
| Very cost-effective | ||||||
| Intensive lifestyle modification | Standard lifestyle recommendation or no intervention | IGT | 8 | B: medical nutritional therapy | $1,500/QALY | Cost saving-$84,700/QALY‡ |
| A: physical activity | ||||||
| Universal opportunistic screening for undiagnosed type 2 diabetes in African Americans between 45 and 54 years old | No screening | African Americans aged 45–54 years | 1 | B | $19,600/QALY | $19,600/QALY |
| Intensive glycemic control as in UKPDS setting | Conventional glycemic control | Type 2 newly diagnosed | 6 | A, B | ||
| $3,400/QALY | Cost saving-$12,400/QALY | |||||
| Statin therapy | No statin therapy | Type 2, with hyperlipidemia, with CVD history | 3 | A | $2,800/LYG | Cost saving-$12,300/LYG |
| Smoking cessation | No smoking cessation | Type 2 | 1 | A, B | <$25,000/QALY | <$25,000/QALY-$89,800/QALY (aged 85–94 years) |
| Annual screening for diabetic retinopathy | No screening | Type 1 | 2 | B | $2,150/QALY | Cost saving-$4,300/QALY |
| Annual screening for diabetic retinopathy | No screening | Type 2 | 3 | B | $6,900/QALY | Cost saving-$39,500/QALY |
| Immediate vitrectomy to treat diabetic retinopathy | Deferral of vitrectomy | Mixed population of type 1 and type 2 | 1 | Mentioned but not explicitly provided level, supported by trials | $2,900/QALY | $2,900/QALY |
| Cost-effective | ||||||
| Targeted screening for undiagnosed type 2 diabetes | No screening | U.S. population with hypertension 45 years and older | 1 | B: in adults of any age who are overweight or obese and who have one or more additional risk factors for diabetes. In those without these risk factors, testing should begin at age 45 | $49,200/QALY | $46,800–$70,500/QALY starting at different age |
| Intensive insulin treatment | Conventional glycemic control | Type 1 | 4 | A, B | $28,900/QALY | $10,200–$50,800/QALY |
| Intensive glycemic control as in the U.S. setting | Conventional glycemic control | Type 2 newly diagnosed at 25–54 years old | 1 | A, B | $27,500/QALY | $14,400-$56,000/QALY |
| Intensive glycemic control through lifestyle modification | Conventional glycemic control | Type 2 newly diagnosed | 1 | A, B | $33,100/QALY | $33,100/QALY |
| Statin therapy | No statin therapy | Type 2, with hyperlipidemia, without CVD history | 3 | A: statin therapy for diabetic patients without CVD who are older than 40 years and have one or more other CVD risk factors | $38,200/LYG§ | $6,100/LYG–$61,300/LYG $77,800/QALY |
| Multi-component interventions (intensive insulin control, ACEI treatment, eye screening and ensuing treatment) | Intensive insulin control | Type 1 | 1 | A, B: intensive insulin control A: ACEI therapy B: eye screening |
$49,800/LYG (non U.S.) | $46,500-$50,600/LYG |
| Marginally cost-effective | ||||||
| Intensive glycemic control as in the U.S. setting | Conventional glycemic control | Type 2 newly diagnosed All age group diagnosed of diabetes at 25 years and older | 1 | A, B | $62,000/QALY | $14,400–$3 million/QALY |
| Eye screening every 2 years | Eye screening every 3 years | Type 2 | 1 | B: annual eye screening recommended, less frequent exams (every 2–3 years) may be considered following one or more normal eye exams | $54,000/QALY | $54,000/QALY |
| Not cost-effective | ||||||
| Universal opportunistic screening for undiagnosed type 2 diabetes | Targeted screening in persons with hypertension | U.S. population 45 years and older | 1 | B | >$100,000/QALY | $70,100-$928,000/QALY |
| Universal opportunistic screening for undiagnosed type 2 diabetes and ensuing treatment | No screening | U.S. population 45 years and older | 2 | B | >$100,000/QALY | $70,100-$1 million |
| Intensive glycemic control as in the U.S. setting | Conventional glycemic control | Type 2 Newly diagnosed at 55–94 years | 1 | A, B | >$100,000/QALY | >$100,000–$3 million/QALY |
| Eye screening every year | Eye screening every 2 years | Type 2 | 1 | B | $116,800/QALY | $116,800/QALY |
| Supportive evidence | ||||||
| Cost saving | ||||||
| Screening for GDM with sequential method‖ | No screening | 30-year-old pregnant women between 24–28 weeks' gestation | 1 | C | Cost saving | Cost saving |
| Screening for GDM with 100-g GTT | No screening | 30-year-old pregnant women between 24–28 weeks' gestation | 1 | C | Cost saving | Cost saving |
| Screening for GDM with sequential method | 75-g GTT | 30-year-old pregnant women between 24–28 weeks' gestation | 1 | C | Cost saving | Cost saving |
| Screening for GDM with 100-g GTT | 75-g GTT | 30-year-old pregnant women between 24–28 weeks' gestation | 1 | C | Cost saving | Cost saving |
| Diabetes self-management education | No education | Type 1 | 1 | B | Cost saving | Cost saving |
| Reimbursement for ACEI by public insurance | Paying out-of-pocket | Type 1 | 1 | E | Cost saving | Cost saving |
| Reimbursement for ACEI by public insurance | Paying out-of-pocket | Type 2 | 1 | E | Cost saving | Cost saving |
| Screening using mobile camera and electronically transmitted to a data reading center and read by trained personnel | Retina-specialists visit | Mixed population of type 1 and type 2 at a remote area | 1 | Recommended but not leveled, assume level E | Cost saving | Cost saving |
| Screening for diabetic nephropathy and ensuing ACEI or ARB therapy | Treat until macroalbuminuria | Type 1 | 3 | E: screening A: ACEI treatment | Cost saving | Cost saving-$58,400/QALY |
| Intensified foot ulcer treatment | Standard treatment | A mixed population of type 1 and type 2 | 1 | B | Cost saving | Cost saving |
| Very cost-effective | ||||||
| Intensive diet and education | Standard antidiabetic care | Women with GDM history, currently IGT | 1 | A, B | $2,500/LYG | $2,500/LYG |
| Universal opportunistic screening for type 2 diabetes in younger and certain ethnic groups | No screening | African Americans, aged 25–44 years | 1 | B: if overweight or obese | $3,100/QALY | $1,300–$19,600/QALY |
| Screening for GDM 100-g GTT | Sequential method | 30-year-old pregnant women between 24–28 weeks' gestation | 1 | E | $35,200/QALY for maternal outcomes, $9,000/QALY for neonatal outcomes | $9,000–$35,200/QALY |
| Diabetes self-management education | No education | Type 2 | 1 | B | $4,000/LYG | $4,000/LYG |
| Disease management | No disease management program | Type 2 or mixed types | 2 | Mentioned but not provided level, assume level E | $23,350/QALY | $4,800–$68,400/QALY for groups with different insurance |
| SMBG 3 times/day¶ | No SMBG | Type 2 treated with oral agents in a large HMO | 1 | E | $6,900/QALY | $540–$30,300/QALY for different time horizon |
| SMBG 1 time/day¶ | No SMBG | 1 | E | $9,700/QALY | $8,200–$24,200/QALY for different time horizon | |
| Cost-effective | ||||||
| Metformin | Placebo | IGT | 6 | E | $26,600/QALY | Cost saving-$47,900/QALY‡ |
| Marginally cost-effective | ||||||
| NA | ||||||
| Not cost-effective | ||||||
| NA | ||||||
| Uncertain | ||||||
| Optimal screening for type 2 diabetes starting age | U.S. population 45 years and older | 2 | B: recommend starting screening for type 2 diabetes at age 45 years if no other risk factors | |||
ACEI, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blocker; CVD, cardiovascular disease; ESRD, end stage renal disease; GDM, gestational diabetes; GTT, glucose tolerance test; IGT, impaired glucose tolerance; LYG, life year gained; NA, not available; QALY, quality adjusted life years; SMBG, self-monitoring blood glucose. A, as defined in Standards of Medical Care in Diabetes—2008: clear evidence from well-conducted, generalizable, randomized controlled trials that are adequately powered; compelling non-experimental evidence, i.e., “all or none” rule developed by the Centre for Evidence-Based Medicine at Oxford; supportive evidence from well-conducted randomized controlled trials that are adequately powered. B, as defined in Standards of Medical Care in Diabetes–2008: supportive evidence from well-conducted cohort studies; supportive evidence from a well-conducted case-control study. C, as defined in Standards of Medical Care in Diabetes–2008: supportive evidence from poorly controlled or uncontrolled studies; conflicting evidence with the weight of evidence supporting the recommendation. E, as defined in Standards of Medical Care in Diabetes–2008: expert consensus or clinical experience.
*, the same interventions applied to different populations or compared with different comparison interventions were treated as different specific interventions.
†, including foot exams, appropriate footwear, treatment, and education.
‡, the study for within trial and the results from health plan perspective are not used for determining the cost-effectiveness of the intervention.
§, get this number by taking the median for women in one study (conservative, women > men) as the results for that study, then take the median of the three study.
‖, 50-g GTT + 100-g GTT.
¶, the evidence was very weak: there was an over 40% probability that the intervention would cost more than $50,000/QALY in a long-term.
Twenty-six interventions were classified as supported by strong evidence concerning their CE (Table 2). Among these, six interventions were cost saving, eight were very cost-effective, six were cost-effective, two were marginally cost-effective, and four were not cost-effective. These interventions consisted of primary prevention, screening for undiagnosed type 2 diabetes, diabetic risk factor control, early prevention of diabetes complications, and treatment of diabetes complications.
The six cost-saving interventions with strong evidence were 1) ACEI therapy for intensive hypertension control, as in the UK Prospective Diabetes Study (UKPDS), in persons with type 2 diabetes compared with standard hypertension control; 2) ACEI or ARB therapy to prevent ESRD for type 2 diabetes compared with no ACEI or ARB therapy; 3) early irbesartan therapy at the stage of microalbuminuria to prevent ESRD in people with type 2 diabetes compared with treatment at the stage of macroalbuminuria; 4) comprehensive foot care to prevent ulcers in mixed population with either type 1 or type 2 diabetes compared with usual care; 5) multi-component interventions for diabetic risk factor control and early detection of complications compared with conventional insulin therapy for persons with type 1 diabetes; and 6) multi-component interventions for diabetic risk factor control and early detection of complications compared with standard glycemic control for persons with type 2 diabetes.
Of the eight very cost-effective interventions with strong evidence, six were for persons with type 2 diabetes, one for persons with type 1 diabetes, and one for a mixed population with type 1 or type 2 diabetes. Interventions for type 2 diabetes included: 1) primary prevention through intensive lifestyle modification; 2) universal opportunistic screening for undiagnosed type 2 diabetes in African Americans between 45 and 54 years old; 3) intensive glycemic control as implemented in UKPDS; 4) statin therapy for secondary prevention of cardiovascular disease; 5) smoking cessation; and 6) annual screening for diabetic retinopathy and early treatment of it. The intervention for type 1 diabetes was annual screening for diabetic retinopathy and treating the positive cases. The intervention for mixed population of type 1 and type 2 diabetes was immediate vitrectomy to treat diabetic retinopathy compared with deferral of vitrectomy.
The six cost-effective interventions with strong evidence were 1) one-time opportunistic targeted screening for undiagnosed type 2 diabetes in hypertensive persons aged 45 years and older compared with no screening; 2) intensive insulin treatment for persons with type 1 diabetes compared with conventional glycemic control; 3) UKPDS-like intensive glycemic control applied to the U.S. health care system among adults younger than age 54 years with type 2 diabetes compared with conventional glycemic control; 4) intensive glycemic control by a Diabetes Prevention Program (DPP) type of intensive lifestyle intervention in persons with newly diagnosed type 2 diabetes compared with conventional glycemic control; 5) statin therapy for primary prevention of cardiovascular disease in persons with type 2 diabetes compared with no statin therapy; 6) multi-component interventions including insulin therapy, ACEI therapy, and screening for retinopathy in persons with type 1 diabetes compared with intensive insulin therapy.
The two marginally cost-effective interventions with strong evidence were 1) intensive glycemic control for all U.S. residents with type 2 diabetes diagnosed at age 25 years and older compared with usual care; and 2) screening for diabetic retinopathy every two years compared with screening every three years in persons with type 2 diabetes.
The four interventions with strong evidence of not being cost-effective were 1) one-time universal opportunistic screening for undiagnosed type 2 diabetes among those aged 45 years and older compared with no screening; 2) universal screening for type 2 diabetes compared with targeted screening; 3) intensive glycemic control in the U.S. setting for patients diagnosed with diabetes at older ages (55–94 years of age) compared with usual care; and 4) annual screening for retinopathy compared with screening every two years. All these studies were for type 2 diabetes.
There were 18 specific interventions for which their CEs were based only on “supportive” evidence. Among them, 15 were each supported by one CE study, 13 were supported by level C or level E evidence, and five were supported by level A or B evidence as defined in the 2008 ADA standards of medical care in diabetes (7). For those interventions with level A or B evidence, the CE of each intervention was evaluated by one study with a quality of being “good.”
In terms of the level of the CE, 10 of the 18 specific interventions based on “supportive” evidence were cost-saving, including 1) screening using the sequential method (50-g glucose challenge test followed by 100-g glucose tolerance test [GTT]) for GDM in 30-year-old pregnant women between 24–28 weeks' gestation compared with no screening; 2) screening for GDM using the 100-g GTT method compared with no screening; 3) the sequential method compared with 75-g GTT screening for GDM; 4) 100-g GTT compared with 75-g GTT screening for GDM; 5) diabetes self-management education for persons with type 1 diabetes compared with no education; 6) full-reimbursement policy for ACEI for patients with type 1 diabetes compared with patients paying out-of-pocket; 7) full-reimbursement policy for ACEI for patients with type 2 diabetes compared with patients paying out-of-pocket; 8) screening using a mobile camera at a remote area and processing data in a reading center compared with a retina specialist's visit in a mixed population of type 1 and type 2 diabetes; 9) screening for diabetic nephropathy and ensuing ACEI or ARB therapy in persons with type 1 diabetes compared with no screening; and 10) intensified foot ulcer treatment in a mixed population with type 1 or type 2 diabetes compared with standard treatment.
Seven of the 18 specific interventions were very cost-effective: 1) primary prevention of type 2 diabetes in women with GDM history through intensive lifestyle intervention compared with usual care; 2) universal opportunistic screening for type 2 diabetes in African Americans aged 25–44 years compared with no screening; 3) 100-g GTT compared with the sequential screening method for detecting GDM in 30-year-old pregnant women between 24–28 weeks' gestation; 4) diabetes self-management education for persons with type 2 diabetes compared with no education; 5) disease management programs using specialist nurse–led clinics to treat and control hypertension or hyperlipidemia in patients with type 2 diabetes in a city in England or a culturally sensitive case–management training program to control diabetes and its risk factors in a Latino population with both type 1 and type 2 diabetes in a U.S. county compared with usual care only; 6) self-monitoring of blood glucose (SMBG) three times per day compared with no SMBG in type 2 noninsulin users; and 7) SMBG once per day compared with no SMBG in type 2 noninsulin users. One of the 18 specific interventions was cost-effective, i.e., the use of metformin to prevent type 2 diabetes in obese persons with impaired glucose tolerance compared with standard lifestyle intervention. No interventions in the “supportive” evidence category were “marginally cost-effective” or “not cost-effective.”
Current evidence is uncertain on how the CE of screening for undiagnosed type 2 diabetes would change with the age of those screened. Two studies evaluated the CE of screening for undiagnosed type 2 diabetes; one study reported that cost-effectiveness ratios (CERs) increased with initial screening age (16) while the other reported that they decreased with screening age (35).
CONCLUSIONS
Our systematic review showed that, with few exceptions, ADA-recommended interventions for preventing or treating diabetes and its complications were cost saving, very cost-effective, or cost-effective (i.e., with an ICER of less than $50,000 per QALY or LYG), although the strength of evidence varied. Generally, interventions that cost less than $50,000 per QALY are considered an efficient use of resources and worth recommending (11). Interventions with strong evidence for being cost saving, very cost-effective, or cost-effective should be considered for implementation. Interventions with supportive evidence for being cost saving, very cost-effective, or cost-effective should be adopted if extra resources are available or if similar interventions with strong evidence are unavailable or infeasible in a specific setting.
The one intervention recommended by the ADA that was shown as not CE was screening for type 2 diabetes of all U.S. residents aged 45 years and older. When considering allocating resources efficiently, universal screening for undiagnosed diabetes should be undertaken with great caution. The high CE ratio for universal screening for undiagnosed type 2 diabetes was primarily attributable to the small gain in health benefit. For example, screening everyone aged 45 years and older gained only 0.003 QALY per eligible person compared with no screening. However the additional costs associated with screening and early treatments were relatively large ($564 per person). Although detecting and treating diabetes earlier can prevent future diabetes-related complications and their associated medical costs, such savings are relatively small ($57 per person). Combining the health benefit and costs would yield an ICER of more than $1 million per QALY (35). An alternative to broad screening is to focus on screening persons with additional risk factors, such as hypertension. Such targeted screening is shown to be cost-effective when compared with no screening or universal screening.
Intensive glycemic control for all U.S. residents with type 2 diabetes diagnosed at age 25 years and older is marginally CE. However the cost-effectiveness of this intervention varies by age at the time of the diabetes diagnosis. The intervention is cost-effective in persons diagnosed at 25–54 years of age. However, intensive glycemic control for those diagnosed with diabetes at 55 years of age and older is not cost-effective. In fact, this result is consistent with the ADA's recommendation of less stringent A1C goals for patients with limited life expectancies.
The ADA recommended annual eye screening for diabetic retinopathy. This recommended intervention is very cost-effective compared with no screening in persons with type 2 diabetes. If considering the efficient allocation of resources, however, screening every other year might be a better alternative. Screening annually leads to a small health benefit but results in a moderate additional cost. For example, Vijan et al. (69) showed that, compared with a 2-year screening, annual screening among persons at moderate risk (65 years old with A1C level 9%) resulted in an increase of 2–3 days of sight at a cost of $540–690 per person. However the ADA also stated in its recommendation that “less frequent exams (every 2–3 years) may be considered following one or more normal eye exams.”
For the interventions with uncertain CE (including optimal age of starting screening for type 2 diabetes), following the current treatment guidelines may be the best option until more evidence on their CE is available.
The CEs of 43 ADA-recommended interventions were evaluated. Of these, 25 were in the “strong” evidence category. This number would probably have been larger if we had used less stringent criteria to define evidence as being strong. For example, evidence on the CE of using metformin to prevent type 2 diabetes among high-risk individuals was considered “supportive” in our current classification even though the efficacy of the intervention was shown by well-conducted multi-center large clinical trials in different country settings (71,72), and its CE was evaluated by “excellent” CE studies (25,34). This intervention was considered to have supportive evidence because it ranked lower in the ADA recommendations (7).
Among all the interventions considered, evidence for the CE of primary prevention through intensive lifestyle modification was the strongest regarding the quantity and quality of the CE studies and efficacy data. Several well-conducted clinical trials have shown the efficacy of intensive lifestyle modification in preventing diabetes in different country settings, such as the U.S. DPP (71), Finnish Diabetes Prevention Study (73), China Da Qing Diabetes Prevention Study (74), and Indian DPP (72). Eight cost-effectiveness studies (seven of them rated as excellent quality) have been conducted by different groups in different countries based on data from these well-conducted clinical trials (15,25,34,36,41,50,59,66). The results from these studies consistently showed that intensive lifestyle modification in persons with impaired glucose tolerance was cost saving or very cost-effective in the long run (15,25,34,36,41,50,59). Even in a short-term and one-on-one consulting setting, the intervention remained cost-effective (66). The intervention would be more cost-effective than existing studies show if the cost of the lifestyle intervention could be reduced. This might be achieved by changing the setting in which the intervention is provided. Only one study found a DPP-like intervention to be marginally cost-effective (25). Even in this study, however, the intervention would have been very cost-effective (23) if done in the type of group environment that is most likely in a real-world setting. A group-based, DPP-style lifestyle intervention partnership with the YMCA costs $275 to $325 per participant in the first year compared with $1,400 in the one-on-one setting of the DPP trial (75). Preventing diabetes, in particular by lifestyle modification, is not only effective but also a very efficient use of health care resources.
The CE of an intervention can vary by country setting. For example, intensive glycemic control (with a goal A1C level of 7%) in type 2 diabetic patients diagnosed at 25 years of age and older was marginally cost-effective in the U.S. but very cost-effective in other developed countries. Although the efficacy data of all studies of intensive glycemic control in type 2 diabetic patients were based on the same UKPDS data, the cost data were based on how residents of the different countries used health services and the cost of those services. The incremental cost of intensive glycemic control was much higher in the U.S. than in the U.K. because of different practice patterns. Patients outside the U.S. did not receive diabetes disease management services and had less frequent self-testing and physician office visits than their U.S. counterparts at the time these studies were conducted. If using the health services as described in the UKPDS setting but with the U.S. cost of these services, the CE of the intensive glycemic control in the U.S. would resemble that of other developed countries.
Future economic evaluation of diabetes interventions should consider the following. First, more studies are needed to evaluate the CE of interventions that fell in the “supportive” evidence category. For studies with weaker efficacy data, further efficacy studies are needed. Second, there are also 38 interventions recommended by the ADA but they have not been evaluated for their CE or the studies did not meet the inclusion criteria for our review (list is available upon request from the authors). The CE of these interventions should be assessed. Third, more CE studies are needed that address interventions in real-world settings. For example, few studies considered attrition rate, noncompliance, and dropout rates in evaluating CE. Fourth, more studies are needed to evaluate the CE of public policy changes. Only two studies evaluated public insurance reimbursement of ACEI therapy and both found this intervention to be cost saving. Finally, the CE of multiple interventions needs to be evaluated. In most real-world settings, patients receive multiple interventions simultaneously. Nearly all previous studies only evaluated the CE of a single intervention.
This review's conclusions should be used with caution. First, our conclusions are based on available information up to May 2008. More studies have been published since then. In addition, data on both the effectiveness and cost of an intervention could have changed since the time the original study was conducted. Using the newly available data could change our current conclusion. For example, in our review, we concluded that the CE of optimal age to start screening for type 2 diabetes was uncertain. A recently published CE study on age at initiation of screening for type 2 diabetes, released after our analysis was complete, might change that conclusion (76). Another example is the large decrease in costs for metformin, statins, and ACEIs. Studies that evaluate CE using current costs might look more favorably on interventions that include statins and ACEIs than those reported here. Reevaluating the costs and benefits of these interventions, using current-day costs, is beyond the scope of this study. Second, when using the results and conclusions of our review, readers need to be certain that terms are understood correctly. For example, “intensive insulin treatment” in our review meant “multiple insulin injection” or “insulin infusion.” Developments in medical technology might make continuous glucose monitoring systems, which record blood glucose levels throughout the day and night, more common. Drugs such as TZD Byetta and Gliptin, not available at the time covered by this review, are increasingly used to achieve intensive glycemic control. The CE of treatment with these and other new devices and drugs are unknown. New CE analyses are needed for these new interventions. Third, not everyone will necessarily agree with our classification criteria. Different classification criteria might have changed some conclusions. Fourth, most of the CE studies are based on simulation modeling. Although good-quality simulation modeling can provide information at a much lower cost than clinical trials, models are based on assumptions and represent a simplification of—and therefore might depart from—reality. Fifth, these CE studies use different methods, which could account for some differences in CERs. If the results from different models were consistent, we would have more confidence in the conclusion on the CE of the intervention. Sixth, we used the same threshold for the classification of the CE of interventions regardless of whether the ICERs were expressed as dollars per LYG or dollars per QALY, although they are different measures. The studies that reported costs per LYG did not incorporate the impact of the intervention on quality of life into the analysis. If they did, the cost per QALY could be higher, lower, or the same depending on the relative magnitude of the health benefit of the intervention on quality of life. Seventh, the interpretation of the CE of an intervention must include consideration of variables such as study population, comparison interventions, and country setting. Lastly, our recommendations are based on the CE of the interventions and not their efficacy; therefore, these recommendations are not necessarily the same as the ADA recommendations.
The importance of CE in decision making should not be overstated. CE is only one aspect to consider. CE analysis does not address the distribution of costs and the benefits of an intervention, societal or personal willingness to pay, social and legal aspects, or ethical issues associated with each intervention. All these aspects are important in formulating public policy. The good news is that our study shows that a majority of the recommended diabetes interventions provide both health benefits and good use of health care resources.
Acknowledgments
The authors conducted this project as part of their jobs as employees of the Centers for Disease Control and Prevention (CDC). The CDC is a federal agency in the U.S. government. The authors have no financial interest in this project.
Parts of this study were presented at the 69th Scientific Sessions of the American Diabetes Association, New Orleans, Louisiana, 5–9 June 2009, and at the Division of Diabetes Translation 2007 Annual Conference, Atlanta, Georgia, April 30–May 3, 2007.
We thank Drs. Sue Kirkman, Richard Khan, William H. Herman, John Anderson, Susan Braithwaite, Dan Lorber, and Vivian Fonseca for reviewing the earlier version of this manuscript and providing valuable comments. We also thank Elizabeth Lee Greene for her invaluable editorial assistance.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily reflect the official positions of the Centers for Disease Control and Prevention.
References
- 1. American Diabetes Association. Economic costs of diabetes in the U.S. in 2007. Diabetes Care 2008;31:1–20 [DOI] [PubMed] [Google Scholar]
- 2. Klonoff DC, Schwartz DM: An economic analysis of interventions for diabetes. Diabetes Care 2000;23:390–404 [DOI] [PubMed] [Google Scholar]
- 3. Raikou M, McGuire A: The economics of screening and treatment in type 2 diabetes mellitus. Pharmacoeconomics 2003;21:543–564 [DOI] [PubMed] [Google Scholar]
- 4. Zhang P, Engelgau MM, Norris SL, Gregg EW, Narayan KM: Application of economic analysis to Diabetes and Diabetes Care. Ann Intern Med 2004;140:972–977 [DOI] [PubMed] [Google Scholar]
- 5. Vijgen SM, Hoogendoorn M, Baan CA, de Wit GA, Limburg W, Feenstra TL: Cost effectiveness of preventive interventions in type 2 diabetes mellitus: a systematic literature review. Pharmacoeconomics 2006;24:425–441 [DOI] [PubMed] [Google Scholar]
- 6. Clarke M, Oxman AD: Cochrane Reviewers' Handbook. Oxford: updated software (October 2001), Chichester, U.K., John Wiley & Sons, Ltd; [Google Scholar]
- 7. American Diabetes Association. Standards of medical care in diabetes–2009. Diabetes Care 2008;31(Suppl. 1):S12–S54 [DOI] [PubMed] [Google Scholar]
- 8. Drummond MF, Jefferson TO: Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ 1996;313:275–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Federal Reserve Bank. Foreign Exchange Rates (annual) [Internet], 2008. Available at http://www.federalreserve.gov/releases/g5a/. Accessed 30 September 2008
- 10. U.S. Department of Labor. Bureau of Labor Statistics: Consumer Price Index: achieved Consumer Price Index detailed report information: U.S. city average, by medical care [Internet], 2008. Available at http://www.bls.gov/cpi/cpi_dr.htm#2007. Accessed 30 September 2008
- 11. Laupacis A, Deeny D, Detsky AS, Tugwell PX: How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. Can Med Assoc J 1992;146:473–481 [PMC free article] [PubMed] [Google Scholar]
- 12. Grosse SD: Assessing cost-effectiveness in health care: history of the $50,000 per QALY threshold. Value Health 2008;8:165–178 [DOI] [PubMed] [Google Scholar]
- 13. Almbrand B, Johannesson M, Sjostrand B, Malmberg K, Ryden L: Cost-effectiveness of intense insulin treatment after acute myocardial infarction in patients with diabetes mellitus; results from the DIGAMI study. Eur Heart J 2000;217:33–39 [DOI] [PubMed] [Google Scholar]
- 14. Borch-Johnsen K, Wenzel H, Viberti GC, Mogensen CE: Is screening and intervention for microalbuminuria worthwhile in patients with insulin dependent diabetes? BMJ 1993;306:1722–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Caro JJ, Getsios D, Caro I, Klittich WS, O'Brien JA: Economic evaluation of therapeutic interventions to prevent type 2 diabetes in Canada. Diabet Med 2004;21:1229–1236 [DOI] [PubMed] [Google Scholar]
- 16. CDC Diabetes Cost-Effectiveness Study Group. The cost-effectiveness of screening for type 2 diabetes. JAMA 1998;280:1757–1763 [PubMed] [Google Scholar]
- 17. CDC Diabetes Cost-Effectiveness Group. Cost-effectiveness of intensive glycemic control, intensified hypertension control, and serum cholesterol level reduction for type 2 diabetes. JAMA 2002;287:2542–2551 [DOI] [PubMed] [Google Scholar]
- 18. Clarke P, Gray A, Adler A, Stevens R, Raikou M, Cull C, Stratton I, Holman R: UKPDS Group. Cost-effectiveness analysis of intensive blood-glucose control with metformin in overweight patients with type II diabetes (UKPDS No. 51). Diabetologia 2001;44:298–304 [DOI] [PubMed] [Google Scholar]
- 19. Clarke PM, Gray AM, Briggs A, Stevens RJ, Matthews DR, Holman RR; United Kingdom Prospective Diabetes Study. Cost-utility analyses of intensive blood glucose and tight blood pressure control in type 2 diabetes (UKPDS 72). Diabetologia 2005;48:868–877 [DOI] [PubMed] [Google Scholar]
- 20. Clarke WF, Churchill DN, Forwell L, Macdonald G, Foster S: To pay or not to pay? A decision and cost-utility analysis of angiolensin-converting-enzyme inhibitor therapy for diabetic nephropathy. CMAJ Canadian Medical Association Journal 2000;162:195–198 [PMC free article] [PubMed] [Google Scholar]
- 21. Coyle D, Rodby R, Soroka S, Levin A, Muirhead N, de Cotret PR, Chen R, Palmer A: Cost-effectiveness of irbesartan 300 mg given early versus late in patients with hypertension and a history of type 2 diabetes and renal disease: a Canadian perspective. Clinical Therapeutics 2007;29:1508–1523 [DOI] [PubMed] [Google Scholar]
- 22. Dong FB, Sorensen SW, Manninen DL, Thompson TJ, Narayan V, Orians CE, Gregg EW, Eastman RC, Dasbach EJ, Herman WH, Newman JM, Narva AS, Ballard DJ, Engelgau MM: Cost effectiveness of ACE inhibitor treatment for patients with type 1 diabetes mellitus. Pharmacoeconomics 2004;22:1015–1027 [DOI] [PubMed] [Google Scholar]
- 23. Earnshaw SR, Richter A, Sorensen SW, Hoerger TJ, Hicks KA, Engelgau M, Thompson T, Narayan KM, Williamson DF, Gregg E, Zhang P: Optimal allocation of resources across four interventions for type 2 diabetes. Med Decis Making 2002;22(Suppl. 5):S80–S91 [DOI] [PubMed] [Google Scholar]
- 24. Eastman RC, Javitt JC, Herman WH, Dasbach EJ, Copley-Merriman C, Maier W, Dong F, Manninen D, Zbrozek AS, Kotsanos J, Garfield SA, Harris M: Model of complications of NIDDM: II. Analysis of the health benefits and cost-effectiveness of treating NIDDM with the goal of normoglycemia. Diabetes Care 1997;20:735–744 [DOI] [PubMed] [Google Scholar]
- 25. Eddy DM, Schlessinger L, Kahn R: Clinical outcomes and cost-effectiveness of strategies for managing people at high risk for diabetes. Ann Intern Med 2005;143:251–264 [DOI] [PubMed] [Google Scholar]
- 26. Elliott WJ, Weir DR, Black HR: Cost-effectiveness of the lower treatment goal (of JNC VI) for diabetic hypertensive patients. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Arch Intern Med 2000;160:1277–1283 [DOI] [PubMed] [Google Scholar]
- 27. Gilmer TP, Roze S, Valentine WJ, Emy-Albrecht K, Ray JA, Cobden D, Nicklasson L, Philis-Tsimikas A, Palmer AJ: Cost-effectiveness of diabetes case management for low-income populations. Health Services Research 2007;42:1943–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Golan L, Birkmeyer JD, Welch HG: The cost-effectiveness of treating all patients with type 2 diabetes with angiotensin-converting enzyme inhibitors. Ann Intern Med 1999;131:660–667 [DOI] [PubMed] [Google Scholar]
- 29. Gozzoli V, Palmer AJ, Brandt A, Spinas GA: Economic and clinical impact of alternative disease management strategies for secondary prevention in type 2 diabetes in the Swiss setting. Swiss Med Wkly 2001;131:303–310 [DOI] [PubMed] [Google Scholar]
- 30. Gray A, Raikou M, McGuire A, Fenn P, Stevens R, Cull C, Stratton I, Adler A, Holman R, Turner R: Cost effectiveness of an intensive blood glucose control policy in patients with type 2 diabetes: economic analysis alongside randomised controlled trial (UKPDS 41). United Kingdom Prospective Diabetes Study Group. BMJ 2000;320:1373–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grover SA, Coupal L, Zowall H, Dorais M: Cost-effectiveness of treating hyperlipidemia in the presence of diabetes: who should be treated? Circulation 2000;102:722–727 [DOI] [PubMed] [Google Scholar]
- 32. Habacher W, Rakovac I, Görzer E, Haas W, Gfrerer RJ, Wach P, Pieber TR: A model to analyse costs and benefit of intensified diabetic foot care in Austria. J Eval Clin Pract 2007;13:906–912 [DOI] [PubMed] [Google Scholar]
- 33. Herman WH, Alexander CM, Cook JR, Boccuzzi SJ, Musliner TA, Pedersen TR, Kjekshus J, Pyörälä K: Effect of simvastatin treatment on cardiovascular resource utilization in impaired fasting glucose and diabetes: findings from the Scandinavian Simvastatin Survival Study. Diabetes Care 1999;22:1771–1778 [DOI] [PubMed] [Google Scholar]
- 34. Herman WH, Hoerger TJ, Brandle M, Hicks K, Sorensen S, Zhang P, Hamman RF, Ackermann RT, Engelgau MM, Ratner RE: Diabetes Prevention Program Research Group. The cost-effectiveness of lifestyle modification or metformin in preventing type 2 diabetes in adults with impaired glucose tolerance. Ann Intern Med 2005;142:323–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hoerger TJ, Harris R, Hicks KA, Donahue K, Sorensen S, Engelgau M: Screening for type 2 diabetes mellitus: a cost-effectiveness analysis. Ann Intern Med 2004;140:689–699 [DOI] [PubMed] [Google Scholar]
- 36. Hoerger TJ, Hicks KA, Sorensen SW, Herman WH, Ratner RE, Ackermann RT, Zhang P, Engelgau MM: Cost-effectiveness of screening for pre-diabetes among overweight and obese U.S. adults. Diabetes Care 2007;30:2874–2879 [DOI] [PubMed] [Google Scholar]
- 37. Javitt JC, Aiello LP, Chiang Y, Ferris FL, 3rd, Canner JK, Greenfield S: Preventive eye care in people with diabetes is cost-saving to the federal government: implications for health-care reform. Diabetes Care 1994;17:909–917 [DOI] [PubMed] [Google Scholar]
- 38. Javitt JC, Aiello LP: Cost-effectiveness of detecting and treating diabetic retinopathy. Ann Intern Med 1996;124:164–169 [DOI] [PubMed] [Google Scholar]
- 39. Jönsson B, Cook JR, Pedersen TR: The cost-effectiveness of lipid lowering in patients with diabetes: results from the 4S trial. Diabetologia 1999;42:1293–1301 [DOI] [PubMed] [Google Scholar]
- 40. Kiberd BA, Jindal KK: Screening to prevent renal failure in insulin dependent diabetic patients: an economic evaluation. BMJ 1995;311:1595–1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lindgren P, Lindström J, Tuomilehto J, Uusitupa M, Peltonen M, Jönsson B, de Faire U, Hellénius ML: DPS Study Group. Lifestyle intervention to prevent diabetes in men and women with impaired glucose tolerance is cost-effective. Int J Technol Assess Health Care 2007;23:177–183 [DOI] [PubMed] [Google Scholar]
- 42. Maberley D, Walker H, Koushik A, Cruess A: Screening for diabetic retinopathy in James Bay, Ontario: a cost-effectiveness analysis. CMAJ 2003;168:160–164 [PMC free article] [PubMed] [Google Scholar]
- 43. Mason JM, Freemantle N, Gibson JM, New JP: SPLINT trial. Specialist nurse-led clinics to improve control of hypertension and hyperlipidemia in diabetes: economic analysis of the SPLINT trial. Diabetes Care 2005;28:40–46 [DOI] [PubMed] [Google Scholar]
- 44. Nicholson WK, Fleisher LA, Fox HE, Powe NR: Screening for gestational diabetes mellitus: a decision and cost-effectiveness analysis of four screening strategies. Diabetes Care 2005;28:1482–1484 [DOI] [PubMed] [Google Scholar]
- 45. Ortegon MM, Redekop WK, Niessen LW: Cost-effectiveness of prevention and treatment of the diabetic foot: a Markov analysis. Diabetes Care 2004;27:901–907 [DOI] [PubMed] [Google Scholar]
- 46. Palmer AJ, Weiss C, Sendi PP, Neeser K, Brandt A, Singh G, Wenzel H, Spinas GA: The cost-effectiveness of different management strategies for type I diabetes: a Swiss perspective. Diabetologia 2000;43:13–26 [DOI] [PubMed] [Google Scholar]
- 47. Palmer AJ, Annemans L, Roze S, Lamotte M, Rodby RA, Cordonnier DJ: An economic evaluation of irbesartan in the treatment of patients with type 2 diabetes, hypertension and nephropathy: cost-effectiveness of Irbesartan in Diabetic Nephropathy Trial (IDNT) in the Belgian and French settings. Nephrol Dial Transplant 2003;18:2059–2066 [DOI] [PubMed] [Google Scholar]
- 48. Palmer AJ, Annemans L, Roze S, Lamotte M, Lapuerta P, Chen R, Gabriel S, Carita P, Rodby RA, de Zeeuw D, Parving HH: Cost-effectiveness of early irbesartan treatment versus control (standard antihypertensive medications excluding ACE inhibitors, other angiotensin-2 receptor antagonists, and dihydropyridine calcium channel blockers) or late irbesartan treatment in patients with type 2 diabetes, hypertension, and renal disease. Diabetes Care 2004;27:1897–1903 [DOI] [PubMed] [Google Scholar]
- 49. Palmer AJ, Annemans L, Roze S, Lamotte M, Rodby RA, Bilous RW: An economic evaluation of the Irbesartan in Diabetic Nephropathy Trial (IDNT) in a UK setting. J Hum Hypertens 2004;18:733–738 [DOI] [PubMed] [Google Scholar]
- 50. Palmer AJ, Roze S, Valentine WJ, Spinas GA, Shaw JE, Zimmet PZ: Intensive lifestyle changes or metformin in patients with impaired glucose tolerance: modeling the long-term health economic implications of the diabetes prevention program in Australia, France, Germany, Switzerland, and the United Kingdom. Clin Ther 2004;26:304–321 [DOI] [PubMed] [Google Scholar]
- 51. Palmer AJ, Annemans L, Roze S, Lapuerta P, Chen R, Gabriel S, Carita P, Rodby RA, de Zeeuw D, Parving HH, De Alvaro F: Irbesartan is projected to be cost and life saving in a Spanish setting for treatment of patients with type 2 diabetes, hypertension, and microalbuminuria. Kidney Int Suppl 2005;93:S52–S54 [DOI] [PubMed] [Google Scholar]
- 52. Palmer AJ, Valentine WJ, Ray JA, Roze S, Muszbek N: Health economic implications of irbesartan treatment versus standard blood pressure control in patients with type 2 diabetes, hypertension and renal disease: a Hungarian analysis. Eur J Health Econ 2007;8:161–168 [DOI] [PubMed] [Google Scholar]
- 53. Palmer AJ, Valentine WJ, Ray JA: Irbesartan treatment of patients with type 2 diabetes, hypertension and renal disease: a UK health economics analysis. Int J Clin Pract 2007;61:1626–1633 [DOI] [PubMed] [Google Scholar]
- 54. Raikou M, McGuire A, Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Charlton-Menys V, Fuller JH: CARDS Investigators. Cost-effectiveness of primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes: results from the Collaborative Atorvastatin Diabetes Study (CARDS). Diabetologia 2007;50:733–740 [DOI] [PubMed] [Google Scholar]
- 55. Rosen AB, Hamel MB, Weinstein MC, Cutler DM, Fendrick AM, Vijan S: Cost-effectiveness of full medicare coverage of angiotensin-converting enzyme inhibitors for beneficiaries with diabetes. Annals of Internal Medicine 2005;143:89–99 [DOI] [PubMed] [Google Scholar]
- 56. Roze S, Valentine WJ, Zakrzewska KE, Palmer AJ: Health-economic comparison of continuous subcutaneous insulin infusion with multiple daily injection for the treatment of type 1 diabetes in the UK. Diabet Med 2005;22:1239–1245 [DOI] [PubMed] [Google Scholar]
- 57. Sakthong P, Tangphao O, Eiam-Ong S, Kamolratanakul P, Supakankunti S, Himathongkam T, Yathavong K: Cost-effectiveness of using angiotensin-converting enzyme inhibitors to slow nephropathy in normotensive patients with diabetes type II and microalbuminuria. Nephrology 2001;6:71–77 [Google Scholar]
- 58. Scuffham P, Carr L: The cost-effectiveness of continuous subcutaneous insulin infusion compared with multiple daily injections for the management of diabetes. Diabet Med 2003;20:586–593 [DOI] [PubMed] [Google Scholar]
- 59. Segal L, Dalton AC, Richardson J: Cost-effectiveness of the primary prevention of non-insulin dependent diabetes mellitus. Health Promot Int 1998;13:197–209 [Google Scholar]
- 60. Sharma S, Hollands H, Brown GC, Brown MM, Shah GK, Sharma SM: The cost-effectiveness of early vitrectomy for the treatment of vitreous hemorrhage in diabetic retinopathy. Curr Opin Ophthalmol 2001;12:230–234 [DOI] [PubMed] [Google Scholar]
- 61. Shearer A, Bagust A, Sanderson D, Heller S, Roberts S: Cost-effectiveness of flexible intensive insulin management to enable dietary freedom in people with type 1 diabetes in the UK. Diabet Med 2004;21:460–467 [DOI] [PubMed] [Google Scholar]
- 62. Souchet T, Durand Zaleski I, Hannedouche T, Rodier M, Gaugris S, Passa P: RENAAL study. An economic evaluation of Losartan therapy in type 2 diabetic patients with nephropathy: an analysis of the RENAAL study adapted to France. Diabete Metab 2003;29:29–35 [DOI] [PubMed] [Google Scholar]
- 63. Szucs TD, Sandoz MS, Keusch GW: The cost-effectiveness of losartan in type 2 diabetics with nephropathy in Switzerland: an analysis of the RENAAL study. Swiss Med Wkly 2004;134:440–447 [DOI] [PubMed] [Google Scholar]
- 64. Tennvall GR, Apelqvist J: Prevention of diabetes-related foot ulcers and amputations: a cost-utility analysis based on Markov model simulations. Diabetologia 2001;44:2077–2087 [DOI] [PubMed] [Google Scholar]
- 65. The DCCT Research Group. Lifetime benefits and costs of intensive therapy as practiced in the Diabetes Control and Complications Trial. JAMA 1996;276:1409–1415 [PubMed] [Google Scholar]
- 66. Diabetes Prevention Program Research Group. Within-trial cost-effectiveness of lifestyle intervention or metformin for the primary prevention of type 2 diabetes. Diabetes Care 2003;26:2518–2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tunis SL, Minshall ME: Self-monitoring of blood glucose in type 2 diabetes: cost-effectiveness in the United States. Am J Manag Care 2008;14:131–140 [PubMed] [Google Scholar]
- 68. UK Prospective Diabetes Study Group. Cost effectiveness analysis of improved blood pressure control in hypertensive patients with type 2 diabetes: UKPDS 40. BMJ 1998;317:720–726 [PMC free article] [PubMed] [Google Scholar]
- 69. Vijan S, Hofer TP, Hayward RA: Cost-utility analysis of screening intervals for diabetic retinopathy in patients with type 2 diabetes mellitus. JAMA 2000;283:889–896 [DOI] [PubMed] [Google Scholar]
- 70. Wake N, Hisashige A, Katayama T, Kishikawa H, Ohkubo Y, Sakai M, Araki E, Shichiri M: Cost-effectiveness of intensive insulin therapy for type 2 diabetes: a 10-year follow-up of the Kumamoto study. Diabetes Res Clin Pract 2000;48:201–210 [DOI] [PubMed] [Google Scholar]
- 71. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM: Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V: Indian Diabetes Prevention Programme (IDPP). The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006;49:289–297 [DOI] [PubMed] [Google Scholar]
- 73. Lindström J, Louheranta A, Mannelin M, Rastas M, Salminen V, Eriksson J, Uusitupa M, Tuomilehto J: Finnish Diabetes Prevention Study Group. The Finnish Diabetes Prevention Study (DPS): lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care 2003;26:3230–3236 [DOI] [PubMed] [Google Scholar]
- 74. Li G, Zhang P, Wang J, Gregg EW, Yang W, Gong Q, Li H, Li H, Jiang Y, An Y, Shuai Y, Zhang B, Zhang J, Thompson TJ, Gerzoff RB, Roglic G, Hu Y, Bennett PH: The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet 2008;371:1783–1789 [DOI] [PubMed] [Google Scholar]
- 75. Ackermann RT, Marrero DG: Adapting diabetes prevention program lifestyle intervention for delivery in the community. Diabetes Educ 2007;33:69. [DOI] [PubMed] [Google Scholar]
- 76. Kahn R, Alperin P, Eddy D, Borch-Johnsen K, Buse J, Feigelman J, Gregg E, Holman RR, Kirkman MS, Stern M, Tuomilehto J, Wareham NJ: Age at initiation and frequency of screening to detect type 2 diabetes: a cost-effectiveness analysis. Lancet 2010;375:1324–1326 [DOI] [PubMed] [Google Scholar]