Abstract
To date, 37 genes have been identified for nonsyndromic hearing impairment (NSHI). Identifying the functional sequence variants within these genes and knowing their population-specific frequencies is of public health value, in particular for genetic screening for NSHI. To determine putatively functional sequence variants in the transmembrane inner ear (TMIE) gene in Pakistani and Jordanian families with autosomal recessive (AR) NSHI, four Jordanian and 168 Pakistani families with ARNSHI that is not due to GJB2 (CX26) were submitted to a genome scan. Two-point and multipoint parametric linkage analyses were performed, and families with logarithmic odds (LOD) scores of 1.0 or greater within the TMIE region underwent further DNA sequencing. The evolutionary conservation and location in predicted protein domains of amino acid residues where sequence variants occurred were studied to elucidate the possible effects of these sequence variants on function. Of seven families that were screened for TMIE, putatively functional sequence variants were found to segregate with hearing impairment in four families but were not seen in not less than 110 ethnically matched control chromosomes. The previously reported c.241C>T (p.R81C) variant was observed in two Pakistani families. Two novel variants, c.92A>G (p.E31G) and the splice site mutation c.212–2A>C, were identified in one Pakistani and one Jordanian family, respectively. The c.92A>G (p.E31G) variant occurred at a residue that is conserved in the mouse and is predicted to be extracellular. Conservation and potential functionality of previously published mutations were also examined. The prevalence of functional TMIE variants in Pakistani families is 1.7% [95% confidence interval (CI) 0.3–4.8]. Further studies on the spectrum, prevalence rates, and functional effect of sequence variants in the TMIE gene in other populations should demonstrate the true importance of this gene as a cause of hearing impairment.
Keywords: Autosomal recessive nonsyndromic hearing impairment, Jordan, Pakistan, Prevalence, TMIE
Introduction
In the past decade, there has been great progress in the mapping and identification of hearing impairment (HI) genes in different populations worldwide. To date, more than 100 HI loci have been mapped and 37 HI genes have been identified [1]. It is predicted that there may be as many as 300 genes involved in the HI phenotype [2]. The large-scale effort to identify HI genes was partially driven by the hope that genetic screening will contribute greatly to earlier diagnosis of genetic HI and allow for early therapeutic and rehabilitative management. However, the clinical usefulness of knowing hundreds of HI genes will remain limited until individual genes are studied for their spectrum of sequence variants, prevalence rates of mutations in various populations, and functional significance in the auditory system.
The transmembrane inner ear (TMIE; MIM no. 607237) gene is one of the more recently identified HI genes [3], the function of which is yet unknown. In this study, two novel sequence variants of the TMIE gene were found in Pakistani and Jordanian families with autosomal recessive nonsyndromic (ARNS) HI. Additionally the prevalence of putatively functional TMIE variants was estimated in Pakistani families with ARNSHI. The occurrence of sequence variants in predicted protein domains and at evolutionarily conserved residues was studied for both previously published and novel TMIE variants to determine whether these variants are involved in the etiology of HI.
Materials and methods
Ascertainment of study subjects
The study was approved by the Institutional Review Boards of Quaid-I-Azam University, Jordan University of Science and Technology, and Baylor College of Medicine and Affiliated Hospitals. Informed consent was obtained from all family members who participated in the study.
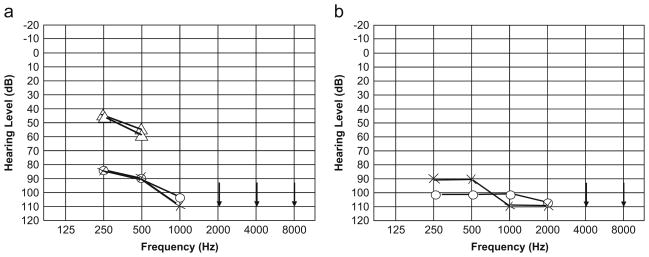
For this study, 192 unrelated Pakistani families with at least two ARNSHI individuals were ascertained from various regions of Pakistan. In addition, five families from Jordan were recruited for ARNSHI research. Medical and family history and information on pedigree structure was obtained from multiple family members. Pure tone audiometry at 250–8,000 Hz was performed for selected subjects. All hearing-impaired family members underwent physical examination. The clinical history indicates that the hearing impairment in all four families is prelingual and probably congenital. There is no evidence that HI is due to inflammatory middle ear disease or specific environmental factors. Within these families, no clinical features that would indicate that the HI is part of a syndrome, including mental retardation, were observed. The audiograms clearly demonstrate that the hearing impairment due to TMIE is bilateral, profound, and affects all frequencies (Fig. 1). No additional diagnostic procedures for vestibular and temporal bone abnormalities were performed, although from the clinical history and physical examination, there were no reports of delay in ambulation during development or of positive findings during vestibular testing and otoscopy.
Fig. 1.
Audiograms of hearing-impaired individuals from families 4043 (a) and 4139 (b). Circles and crosses represent air conduction for right and left ears, respectively. Triangles represent masked air conduction thresholds. Both audiograms exhibit bilateral, profound hearing impairment that affects all frequencies. Family 4043 has the p.E31G variant, while family 4139 carries the p.R81C substitution
Genome scan
DNA was isolated from venous blood samples following a standard protocol [4], quantified by spectrophotometry at optical density 260, and stored at −20°C. Of the 197 families, one Jordanian and 12 Pakistani families were positive for GJB2 (MIM no. 121011; GenBank accession no. NM_004004.3) mutations. Twelve other Pakistani families were not included for genome scan due to an insufficient number of DNA samples to carry out a linkage analysis. DNA samples from 128 families were diluted to 40 ng/μl and sent to the Center for Inherited Disease Research (CIDR) for genome scan, while diluted DNA samples from 44 families were sent to the National Heart, Lung and Blood Institute (NHLBI) Mammalian Genotyping Service (Center for Medical Genetics, Marshfield, WI, USA) for genotyping. From 2000 to 2004, samples were sent in six batches, with an average of 395 short tandem repeat (STR) markers spaced at ~10 cM apart for each genome scan that was done on all 22 autosomes and the X and Y chromosomes.
Linkage analysis
Linkage analyses were performed under a fully penetrant AR model with a disease allele frequency of 0.001. The MLINK program of the FASTLINK computer package was utilized for two-point linkage analysis [5], while multipoint analysis was performed using ALLEGRO [6] with map distances from the Marshfield genetic map [7], DeCode genetic map [8], or estimated using Map-O-Mat [9]. Some of the families were too large to analyze in their entirety using ALLEGRO and were therefore broken into two or more branches for the analysis, then the logarithmic odds (LOD) scores from each branch of the family were summed. Marker allele frequencies were estimated from the marker data by means of both observed and reconstructed genotypes of founders from each pedigree and other pedigrees from the same population that were genotyped in the same genome scan.
Sequencing TMIE
The TMIE (GenBank accession no. NM_147196.1) gene was sequenced in one hearing and two hearing-impaired members of families showing linkage to the 3p21.31 region after two-point and multipoint linkage analyses. Primer3 software [10] was used to design primers for the four exons and 1,000 bp of promoter region of the TMIE gene. After DNA amplification and purification with ExoSAP-IT (USB Corp., Cleveland, OH, USA), sequencing was performed with the BigDye Terminator v3.1 Cycle Sequencing Kit together with an Applied Biosystems 3700 DNA Analyzer (Applera Corp., Foster City, CA, USA). Sequence variants were identified via Sequencher Version 4.1.4 software (Gene Codes Corp., Ann Arbor, MI, USA). When a potentially functional sequence variant was found, the exon in which the variant was found was sequenced in all other family members for whom DNA was available. When the identified sequence variant was shown to segregate with HI status within a family, a minimum of 55 unrelated ethnically matched control individuals were also screened for the same exon.
Protein sequence analysis
For the protein analyses, transmembrane domains were predicted with TMHMM v.2.0 [11]. Similarity search was performed through the ExPASy/UniProt database and the NCBI BLASTP 1.5.4-Paracel program [12] using default settings, then the identified eukaryotic proteins were aligned with CLUSTAL W [13]. The PROSITE database [14] was scanned for known patterns and motifs.
Results
Of 172 Pakistani and Jordanian families that underwent two-point and/or multipoint linkage analyses, seven families had two-point and multipoint LOD scores of 1.0 or greater between markers flanking the TMIE gene. Table 1 shows the LOD scores per family, along with ethnolinguistic information and sequence variants that were found in homozygosity in hearing-impaired individuals but not in unaffected family members.
Table 1.
Families screened for TMIE (GenBank accession no. NM_147196.1) mutations
| Family | Place of origin | Language | Two-point LOD | Multipoint LOD | Sequence variant |
|---|---|---|---|---|---|
| 4009 | DG Khan, Punjab | Sairiki | 1.3; 2.9 at chromosome 7 | 2.2; 6.6 at chromosome 7 | None |
| 4019 | Rawalpindi, Punjab | Punjabi | 3.5 | 5.5 | c.241C>T (p.R81C) |
| 4043 | Rahim Yar Khan, Punjab | Punjabi | 1.5 | 2.8 | c.92A>G (p.E31G) |
| 4051 | Bahwalpur, Punjab | Sairiki | 1.8; 1.0 at chromosome 10 | 1.8; 1.9 at chromosome 10 | none |
| 4139 | Kotli Kshmir, AJK | Kashmiri | 1.9 | 2.0 | c.241C>T (p.R81C) |
| 4197 | Quetta, Baloochistan | Pushto | 1.1; 1.6 at chromosome 4 | 1.8; 1.8 at chromosome 4 | None |
| 3002 | Jordan | Arabic | 4.1 | 6.1 | c.212 –2A>C |
LOD Logarithmic odds, AJK Azad Jammu and Kashmir
Four families were positive for putatively functional sequence variants. Two Pakistani families had a previously published variant, c.241C>T (p.R81C) [3], which occurs at a highly conserved cytoplasmic amino acid residue (Table 2). Two other families possess novel sequence variants. One Pakistani family had an A to G transition at position 92, thus resulting in the replacement of the charged polar amino acid side chain by a hydrogen molecule (p.E31G). The amino acid residue is predicted to occur at the extracellular region and is conserved in the mouse Tmie protein but not in Tmie-like proteins of Drosophila melanogaster and Caenorhabditis elegans. The splice site mutation c.212 –2A>C that was found in a Jordanian family is predicted to cause skipping of the third exon and subsequent removal of part of the second transmembrane segment and half of the long C-terminal tail of the protein. Neither p.R81C nor p.E31G was identified with a known protein sequence motif. All three variants were not observed in at least 110 control chromosomes.
Table 2.
TMIE sequence variants found to segregate with hearing impairment status within families
| Exon | Nucleotide change | Protein change | Domaina | Evolutionary conservationb | Allele frequencies among control chromosomesc |
|---|---|---|---|---|---|
| 1 | c.92A>G | p.E31G | EC | Conserved in mouse only | 0/110 |
| 3 | c.212 –2A>C | Exon skipping predicted | N/A | N/A | 0/176 |
| 3 | c.241C>T | p.R81C | IC | Identical | 0/122 |
IC Intracellular, EC extracellular, N/A not applicable
TMHMM v.2.0 predicted two membrane-spanning domains for the TMIE protein [11]
Human TMIE sequence compared with mouse Tmc1, CG15130-PA protein of Drosophila melanogaster, and hypothetical protein Y39A1C.1 of Caenorhabditis elegans
Control individuals were matched by country of origin. The c.212 –2A>C mutation was also negative in 122 Pakistani control chromosomes, and the c.241C>T (p.R81C) variant was not found in 176 control chromosomes from Jordan
Several polymorphisms were observed in the seven families, but none of these variants segregated with HI status within the families (Table 3). Four TMIE sequence variants that were identified in a previous study [3] were not found in the families that are reported here but were also examined in terms of conservation and occurrence of amino acid residues at functional sites (Table 4). Three missense changes at arginine residues, including the known variant p.R81C, were predicted to occur within the intracellular domain at the C-terminal tail. Two substitutions, p.R81C and p.R84W, are at highly conserved residues, while p.R92W occurs at a putative tyrosine kinase phosphorylation site.
Table 3.
TMIE polymorphisms that do not segregate with hearing impairment status within families
| Exon | Nucleotide change |
|---|---|
| Promoter | g.1 –1017T>C |
| Promoter | g.[1 –804T>C (+) 1 –74A>T] |
| Promoter | g.1 –300C>A |
| 2 | c.94 –46C>A |
| 2 | c.94 –25C>T |
| 3 | c.212 –63G>A3 |
| 3 | c.321G>A (synonymous) |
| 4 | c.388_390dupAAG (p.K9dup) |
| 4 | g.1598G>A (at +975 of the TGA) |
Table 4.
Previously published sequence variants in the TMIE genea
| Exon | Nucleotide change | Protein change | Domain | Evolutionary conservation |
|---|---|---|---|---|
| Intron 1–exon 2 | c.94 –2_98del AGCCCAGinsCb | Exon skipping predicted | N/A | N/A |
| 2 | c.125_126dup CGCCb | Frameshift results in truncation | N/A | N/A |
| 3 | c.250C>T | p.R84W | IC | Identical |
| 3 | c.274C>T | p.R92Wc | IC | Nonconserved |
N/A Not applicable
TMIE sequence variants are from Naz et al. [3]. Membrane-spanning domains, evolutionary conservation, and functional effect were determined as described in Table 2
These sequence variants were originally reported as IVS1-2_98del AGCCCAGinsC and 125-126insCGCC. The nomenclature has been modified in accordance with recommendations from the Human Genome Variation Society (http://www.genomic.unimelb.edu.au/mdi/)
The arginine residue at position 92 belongs to a putative tyrosine kinase phosphorylation site according to the PROSITE database [14]
Discussion
Putatively functional TMIE variants were observed in 3 of the 168 Pakistani families that underwent a genome scan. Twelve additional Pakistani families in the study did not undergo a genome scan but are known to have functional variants in the GJB2 gene [15]. The prevalence of functional TMIE variants in these Pakistani families is therefore 1.7% [95% confidence interval (CI) 0.3–4.8]. This is the first report of the population-specific prevalence of TMIE variants in individuals with ARNSHI. In this population of Pakistani families with ARNSHI, the prevalence of putatively functional variants in the GJB2 gene is 6.1% (95% CI 3.2–10.4) [15], while the prevalence of putatively functional TMC1 (MIM no. 606706) variants is 4.4% (95% CI 1.9–8.6) [16]. The importance of the TMIE gene as a cause of HI cannot be known until other populations are studied. Due to insufficient number of families, it is not possible to accurately estimate the prevalence rate of functional TMIE variants in the Jordanian population.
Among seven families in which the TMIE gene was sequenced, a potentially functional variant was not identified in three families (Table 1). A conservative criterion of a LOD score of 1.0 or greater was used in choosing the families for sequencing. It is therefore not surprising that for families 4009, 4051, and 4197 which had multipoint LOD scores of 6.6, 1.9, and 1.8, respectively, to other chromosomal regions, the TMIE gene could not be implicated as the cause of HI. For family 4009, there is highly significant evidence of linkage to DFNB44 [17]; the gene that causes HI in this family almost certainly lies in this region. It was probably overly conservative to rule out the involvement of TMIE in the HI phenotype in this family by sequencing of the gene. For both families 4051 and 4197, a multipoint LOD score of 1.8 was obtained within the interval of the TMIE gene. However, a multipoint LOD score of 1.9 was achieved on chromosome 10 for family 4051, while a LOD score of 1.8 was attained on chromosome 4 for family 4197. In these two families, it was not possible to a priori rule out TMIE being involved in HI since there is no statistically significant evidence to map the gene segregating in this family to any chromosomal region.
Although the function of TMIE in the inner ear remains unknown, there are indications that the protein may have a role in both stereocilia maturation and auditory nerve function. The deaf Tmie-mutant spinner mouse was shown to have progressive hair cell degeneration with irregular apical surfaces of hair cells and extra rows of maturing stereocilia in inner hair cells [18]. Also, the absence of auditory brainstem response in spinner mice correlates with spiral ganglion degeneration [18, 19]. In another mouse model called circling, the mapped locus also includes the Tmie gene [20, 21], and the circling mouse also exhibited hair cell axonal and spiral ganglion degeneration [22]. There is a similar pattern of histologic features in Cdh23+/Pcdh15+ and Ush1C+/+ mice [23, 24]; additionally, the CDH23, PCDH15, and USH1C proteins have been identified as stereocilia bundling proteins. However, it is difficult to surmise whether the hair cell and/or spiral ganglion loss in the Tmie mouse mutants were due to primary stereocilia defects or to secondary responses to another type of inner ear malfunction. The occurrence of putatively functional sequence variants in the extracellular domain and the cytoplasmic carboxyl tail of the TMIE protein may indicate the importance of these segments to cochlear function, but the lack of protein structures that are similar to TMIE inhibits prediction of how these sequence variants may cause hearing impairment. More information on related protein structures should help in building a good model for the pathophysiologic role of TMIE in the inner ear.
Acknowledgments
We are thankful to all family members who participated in this study. This work was made possible through grants from the National Institutes of Health-National Institute of Deafness and other Communication Disorders (NIH-NIDCD; R01-DC03594), the Deanship of Research, Jordan University of Science and Technology, Irbid, Jordan, and Higher Education Commission, Islamabad, Pakistan. Genotyping services were provided by CIDR and the NHLBI Mammalian Genotyping Service. CIDR is fully funded through a federal contract from the National Institutes of Health to The Johns Hopkins University, contract number N01-HG-65403.
Abbreviations
- TMIE
Transmembrane inner ear gene
- HI
Hearing impairment
- NSHI
Nonsyndromic HI
- ARNSHI
Autosomal recessive NSHI
- GJB2
Gap-junction β-2 gene
- TMC1
Transmembrane cochlear 1 gene
- CDH23
Cadherin 23 gene
- PCDH15
Protocadherin 15 gene
- CIDR
Center for Inherited Disease Research
- NHLBI
National Heart, Lung and Blood Institute
Biographies
 Regie Lyn P. Santos is an otorhinolaryngologist from the University of the Philippines Manila. She received her Doctor of Science in Genetic Epidemiology from Erasmus MC Rotterdam, the Netherlands, and is now earning her Ph.D. as a fellow at Dr. Leal’s laboratory in the Department of Molecular and Human Genetics, Baylor College of Medicine. Her main interests are clinical otology and genetic hearing impairment research.
Regie Lyn P. Santos is an otorhinolaryngologist from the University of the Philippines Manila. She received her Doctor of Science in Genetic Epidemiology from Erasmus MC Rotterdam, the Netherlands, and is now earning her Ph.D. as a fellow at Dr. Leal’s laboratory in the Department of Molecular and Human Genetics, Baylor College of Medicine. Her main interests are clinical otology and genetic hearing impairment research.
 Suzanne M. Leal received her M.S. in biostatistics and Ph.D. in epidemiology from Columbia University in New York City, USA. She is currently an Associate Professor in the Department of Molecular and Human Genetics at Baylor College of Medicine. Her research interests include gene mapping of complex and Mendelian traits, the genetics of non-syndromic hearing impairment and the study of methodological problems in statistical genetics.
Suzanne M. Leal received her M.S. in biostatistics and Ph.D. in epidemiology from Columbia University in New York City, USA. She is currently an Associate Professor in the Department of Molecular and Human Genetics at Baylor College of Medicine. Her research interests include gene mapping of complex and Mendelian traits, the genetics of non-syndromic hearing impairment and the study of methodological problems in statistical genetics.
Contributor Information
Regie Lyn P. Santos, Department of Molecular and Human Genetics, Baylor College of Medicine, One Baylor Plaza, Alkek N1619.01, Houston, TX 77030, USA. Genetic Epidemiology Unit, Department of Epidemiology and Biostatistics, Erasmus Medical Centre, Rotterdam, The Netherlands
Hatem El-Shanti, Department of Pediatrics, Division of Medical Genetics, University of Iowa Hospital and Clinics, Iowa City, IA, USA. Department of Applied Biological Sciences, Jordan University of Science and Technology, Irbid, Jordan.
Shaheen Sikandar, Department of Biological Sciences, Quaid-I-Azam University, Islamabad, Pakistan.
Kwanghyuk Lee, Department of Molecular and Human Genetics, Baylor College of Medicine, One Baylor Plaza, Alkek N1619.01, Houston, TX 77030, USA.
Attya Bhatti, Department of Biological Sciences, Quaid-I-Azam University, Islamabad, Pakistan.
Kai Yan, Department of Molecular and Human Genetics, Baylor College of Medicine, One Baylor Plaza, Alkek N1619.01, Houston, TX 77030, USA.
Maria H. Chahrour, Department of Molecular and Human Genetics, Baylor College of Medicine, One Baylor Plaza, Alkek N1619.01, Houston, TX 77030, USA
Nathan McArthur, Department of Molecular and Human Genetics, Baylor College of Medicine, One Baylor Plaza, Alkek N1619.01, Houston, TX 77030, USA.
Thanh L. Pham, Department of Molecular and Human Genetics, Baylor College of Medicine, One Baylor Plaza, Alkek N1619.01, Houston, TX 77030, USA
Amjad Abdullah Mahasneh, Department of Applied Biological Sciences, Jordan University of Science and Technology, Irbid, Jordan.
Wasim Ahmad, Department of Biological Sciences, Quaid-I-Azam University, Islamabad, Pakistan.
Suzanne M. Leal, Email: sleal@bcm.tmc.edu, Department of Molecular and Human Genetics, Baylor College of Medicine, One Baylor Plaza, Alkek N1619.01, Houston, TX 77030, USA, Tel.: +1-713-7984011, Fax: +1-713-7984373
References
- 1.Van Camp G, Smith RJH. Hereditary Hearing Loss homepage. 2005 http://webhost.ua.ac.be/hhh/
- 2.Nance WE. The genetics of deafness. Ment Retard Dev Disabil Res Rev. 2003;9:109–119. doi: 10.1002/mrdd.10067. [DOI] [PubMed] [Google Scholar]
- 3.Naz S, Giguere CM, Kohrman DC, Mitchem KL, Riazuddin S, Morell RJ, Ramesh A, Srisailpathy S, Deshmukh D, Riazuddin S, Griffith AJ, Friedman TB, Smith RJ, Wilcox ER. Mutations in a novel gene, TMIE, are associated with hearing loss linked to the DFNB6 locus. Am J Hum Genet. 2002;71:632–636. doi: 10.1086/342193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grimberg J, Nawoschik S, Bellusico L, McKee R, Turck A, Eisenberg A. A simple and efficient non-organic procedure for the isolation of genomic DNA from blood. Nucleic Acids Res. 1989;17:83–90. doi: 10.1093/nar/17.20.8390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cottingham RW, Idury RM, Schaffer AA. Faster sequential genetic linkage computations. Am J Hum Genet. 1993;53:252–263. [PMC free article] [PubMed] [Google Scholar]
- 6.Gudbjartsson DF, Jonasson K, Frigge ML, Kong A. Allegro, a new computer program for multipoint linkage analysis. Nat Genet. 2000;25:12–13. doi: 10.1038/75514. [DOI] [PubMed] [Google Scholar]
- 7.Broman KW, Murray JC, Sheffield VC, White RL, Weber JL. Comprehensive human genetic maps individual and sex-specific variation in recombination. Am J Hum Genet. 1998;63:861–869. doi: 10.1086/302011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kong A, Gudbjartsson DF, Sainz J, Jonsdottir GM, Gudjonsson SA, Richardsson B, Sigurdardottir S, Barnard J, Hallbeck B, Masson G, Shlien A, Palsson ST, Frigge ML, Thorgeirsson TE, Gulcher JR, Stefansson K. A high-resolution recombination map of the human genome. Nat Genet. 2002;31:241–247. doi: 10.1038/ng917. [DOI] [PubMed] [Google Scholar]
- 9.Kong X, Matise TC. MAP-O-MAT: Internet-based linkage mapping. Bioinformatics. 2005;21:557–559. doi: 10.1093/bioinformatics/bti024. [DOI] [PubMed] [Google Scholar]
- 10.Rozen S, Skaletsky HJ. Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S, editors. Bioinformatics methods and protocols: methods in molecular biology. Humana Press; NJ: 2000. pp. 365–386. [DOI] [PubMed] [Google Scholar]
- 11.Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. 2001;305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 12.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sigrist CJ, Cerutti L, Hulo N, Gattiker A, Falquet L, Pagni M, Bairoch A, Bucher P. PROSITE: a documented database using patterns and profiles as motif descriptors. Brief Bioinform. 2002;3:265–274. doi: 10.1093/bib/3.3.265. [DOI] [PubMed] [Google Scholar]
- 15.Santos RLP, Wajid M, Pham TL, Hussan J, Ali G, Ahmad W, Leal SM. Low prevalence of Connexin 26 (GJB2) variants in Pakistani families with autosomal recessive non-syndromic hearing impairment. Clin Genet. 2005;67:61–68. doi: 10.1111/j.1399-0004.2005.00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santos RLP, Wajid M, Khan MN, McArthur N, Pham TL, Bhatti A, Lee K, Irshad S, Mir A, Yan K, Chahrour MH, Ansar M, Ahmad W, Leal SM. Novel sequence variants in the TMC1 gene in Pakistani families with autosomal recessive hearing impairment. Hum Mutat. 2005;26:396. doi: 10.1002/humu.9374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ansar M, Chahrour MH, Amin Ud Din M, Arshad M, Haque S, Pham TL, Yan K, Ahmad W, Leal SM. DFNB44, a novel autosomal recessive non-syndromic hearing impairment locus, maps to chromosome 7p14.1–q11.22. Hum Hered. 2004;57:195–199. doi: 10.1159/000081446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchem KL, Hibbard E, Beyer LA, Bosom K, Dootz GA, Dolan DF, Johnson KR, Raphael Y, Kohrman DC. Mutation of the novel gene Tmie results in sensory cell defects in the inner ear of spinner, a mouse model of human hearing loss DFNB6. Hum Mol Genet. 2002;11:1887–1898. doi: 10.1093/hmg/11.16.1887. [DOI] [PubMed] [Google Scholar]
- 19.Deol MS, Robins MW. The spinner mouse. J Hered. 1962;53:133–136. doi: 10.1093/oxfordjournals.jhered.a107147. [DOI] [PubMed] [Google Scholar]
- 20.Cho KI, Lee JW, Kim KS, Lee EJ, Suh JG, Lee HJ, Kim HT, Hong SH, Chung WH, Chang KT, Hyun BH, Oh YS, Ryoo ZY. Fine mapping of the circling (cir) gene on the distal portion of mouse chromosome 9. Comp Med. 2003;53:642–648. [PubMed] [Google Scholar]
- 21.Mouse Genome Sequencing Consortium. Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;20:520–562. doi: 10.1038/nature01262. May 2004 reference sequence as viewed in: Genome Bioinformatics Group of UC Santa Cruz. UCSC Genome Browser. http://genome.ucsc.edu/cgi-bin/hgGateway. [DOI] [PubMed]
- 22.Lee JW, Lee EJ, Hong SH, Chung WH, Lee HT, Lee TW, Lee JR, Kim HT, Suh JG, Kim TY, Ryoo ZY. Circling mouse: possible animal model for deafness. Comp Med. 2001;51:550–554. [PubMed] [Google Scholar]
- 23.Zheng QY, Yan D, Ouyang XM, Du LL, Yu H, Chang B, Johnson KR, Liu XZ. Digenic inheritance of deafness caused by mutations in genes encoding cadherin 23 and protocadherin 15 in mice and humans. Hum Mol Genet. 2005;14:103–111. doi: 10.1093/hmg/ddi010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson KR, Gagnon LH, Webb LS, Peters LL, Hawes NL, Chang B, Zheng QY. Mouse models of USH1C and DFNB18: phenotypic and molecular analyses of two new spontaneous mutations of the Ush1c gene. Hum Mol Genet. 2003;12:3075–3086. doi: 10.1093/hmg/ddg332. [DOI] [PMC free article] [PubMed] [Google Scholar]