Abstract
Objective
Systemic lupus erythematosus (SLE) is diagnosed by a spectrum of clinical manifestations and autoantibodies associated with abnormal expression of Type I interferon (IFN-I) stimulated genes (ISGs). The role of IFN-I in the pathogenesis of SLE remains uncertain, partly due to the lack of suitable animal models. The objective of this study was to examine the role of IFN-I signaling in the pathogenesis of murine lupus induced by 2, 6, 10, 14 tetramethylpentadecane (TMPD).
Methods
129Sv IFN-I receptor deficient (IFNAR−/−) and control 129Sv mice were treated i.p. with TMPD. The expression of ISGs was measured by real-time PCR. Autoantibody production was evaluated by immunofluorescence and ELISA. Proteinuria and renal glomerular cellularity were measured and renal immune complexes were examined by immunofluorescence.
Results
Increased ISG expression was seen in peripheral blood of TMPD-treated wild type but not IFNAR−/− mice. TMPD did not induce lupus-specific autoantibodies (anti-nRNP/Sm, -dsDNA) in IFNAR−/− mice, whereas 129Sv controls developed these specificities. Although glomerular immune complexes were present in IFNAR−/− mice, proteinuria and glomerular hypercellularity did not develop, unlike TMPD-treated controls. Thus, consistent with the association of increased ISG expression with lupus-specific autoantibodies, and nephritis in humans, these clinical and serological manifestations were strongly dependent on IFNAR signaling in TMPD-treated mice.
Conclusion
Signaling via the IFNAR is central to the pathogenesis of autoantibodies and glomerulonephritis in TMPD-lupus, consistent with a similar role in human SLE. TMPD-lupus is the first animal model shown to recapitulate the interferon signature in peripheral blood.
Systemic lupus erythematosus (SLE) is a multi-organ autoimmune disease with protean clinical manifestations accompanied by a highly characteristic subset of antinuclear autoantibodies reactive with small ribonucleoproteins such as the U1 small nuclear ribonucleoprotein (snRNP) and double-stranded (ds) DNA (1). Accumulating evidence suggests that dysregulated production of Type I interferons (IFN-I) reflected in the over-expression of IFN-I stimulated genes (ISGs) in peripheral blood mononuclear cells (PBMCs) is associated with SLE (2;3). This “interferon signature” is clinically important, correlating with disease activity, renal involvement, and the presence of autoantibodies against dsDNA and the Sm/RNP antigens, components of the U1 snRNP (4;5).
Initially recognized for their role in antiviral responses, IFN-I modulate immunity and autoimmunity by promoting dendritic cell (DC) maturation, T cell survival, and antibody production (6–9). The onset of SLE and other autoimmune disorders has been reported in patients undergoing treatment with recombinant IFNα for hepatitis C infection or neoplastic diseases (10–12), suggesting that IFN-I may have a causal role in the pathogenesis of autoimmune disease. In addition, patients with a chromosomal translocation resulting in trisomy of the short arm of chromosome 9 (which bears the IFN-I gene cluster) have high levels of IFN-I production and develop lupus-like disease (13). However, there is at present no direct proof that dysregulation of IFN-I has a causal role in SLE.
Murine lupus induced by 2, 6, 10, 14 tetramethylpentadecane (TMPD) is associated with high levels of IFN-I production (14) as well as key immunological and clinical features of SLE, such as the production of anti-dsDNA and anti-Sm autoantibodies and the development of glomerulonephritis, arthritis, and pulmonary vasculitis (15–18). In this study, we investigated whether IFN-I signaling plays a causal role in the pathogenesis of lupus-like disease in this model.
MATERIALS AND METHODS
Mice
Four-week-old 129Sv/Ev Type I interferon receptor α-chain deficient (IFNAR−/−) mice (19) and wild type (WT) control (129Sv/Ev) breeding pairs (B&K Universal Limited (Grimston, Aldbrough, England) were housed in an SPF facility in barrier cages. Eight-week-old mice received 0.5 ml i.p. of TMPD (Sigma-Aldrich, St. Louis, MO). Peritoneal cells, granulomas, spleen, kidneys, and blood were harvested 6–8 months later. These studies were approved by the Institutional Animal Care and Use Committee.
PCR
Total RNA from murine peripheral blood was isolated using the PAXgene RNA kit (Qiagen, Valencia, CA). RNA (1 µg) was treated with DNase I (Invitrogen Life Technologies, Carlsbad, California) to remove genomic DNA and reverse transcribed to cDNA using the Superscript First-Strand Synthesis System (Invitrogen) for RT-PCR. Amplification was carried out in a PTC-100 Programmable Thermal Controller (MJ Research, Inc., Waltham, MA) as described (14). IFNα consensus and IFNβ primers were as described previously (20).
Real-time PCR was performed using SYBR Green core reagents (Applied Biosystems, Foster City, CA) and specific primer pairs as described (14). MCP-1 primers were AGGTCCCTGTCATGCTTCTG (forward) and GGATCATCTTGCTGGTGAAT (reverse). Transcripts were quantified using the comparative (2 −ΔΔCt) method.
ELISA
Anti-chromatin antibodies were detected by ELISA using chicken erythrocyte chromatin as described previously (21) using serum samples diluted 1: 500. Anti-nRNP/Sm and -Su antigen-capture ELISAs were performed as described using serum diluted 1:500 (21). Levels of anti-dsDNA and -ssDNA antibodies in sera were tested at a 1:250 dilution using S1 nuclease treated calf thymus DNA as antigen (heat denatured for ssDNA) (21). Total immunoglobulin levels were measured by ELISA as described previously (22).
Cytokine ELISA
Levels of IFNβ, IL-6, TNFα and IL-12 in peritoneal lavage fluid were measured using hamster monoclonal and rabbit polyclonal antibodies (TNFα) or rat monoclonal antibody pairs (IL-12) from BD Biosciences (San Jose, CA). After incubation with biotinylated cytokine-specific antibodies, streptavidin-alkaline phosphatase (1:1000 dilution, Southern Biotechnology) was added for 30 min at 22°C, and the reaction was developed with p-nitrophenyl phosphate substrate in diethanolamine buffer and read in a VERSAmax microtiter plate reader (Molecular Devices Corporation, Sunnyvale, CA).
Antinuclear antibodies (ANA)
Levels of serum ANA were determined 6 months after TMPD treatment by indirect immunofluorescence using HEp-2 cells (Immunoconcepts, Sacramento, CA). Sera were screened at a 1:40 dilution and the titers were determined using an Image Titer titration emulation system (Rhigene, Inc., Des Plaines, IL).
Immunoprecipitation and western blot analysis of autoantibodies
Autoantibodies to cellular proteins in sera (5 µl per sample) were analyzed by immunoprecipitation of [35S] labeled proteins from K562 cell extract and analyzed by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and autoradiography as described (23). Autoantibody specificities also were analyzed for reactivity with K562 cell extract by western blotting (23) using a serum dilution of 1:1000 followed by 1:1000 alkaline phosphatase-labeled goat anti-mouse IgG (Southern Biotechnology) for 1 h. The reaction was developed using Immun-Star AP substrate (Bio-Rad Laboratories, Hercules, CA).
Flow cytometry
Lipogranulomas were dissociated with collagenase and a single-cell suspension was stained with anti-B220 and anti-CD4 antibodies (BD Biosciences) and analyzed by flow cytometry as described (14).
Immunohistochemistry
Lipogranulomas were excised from the peritoneal wall, fixed in 4% paraformaldehyde, and embedded in paraffin. Immunohistochemistry was carried out by the Molecular Pathology and Immunology Core at University of Florida using the DAKO Autostainer protocol. Briefly, 4 µm serial sections were deparaffinized and then blocked with Sniper (Biocare Medical, Walnut Creek, CA). Sections were incubated with rat anti-mouse CD45R (B220, BD Biosciences) or anti-CD3 (Serotec, Raleigh, NC) for 1 h followed by incubation with non-biotinylated rabbit anti-rat immunoglobulin antibodies (Vector, Burlingame, CA) for 30 min. Staining was visualized using Mach Gt × Rb HRP polymer (Biocare Medical, Walnut Creek, CA), the chromagen Cardassian DAB (Biocare Medical) and Mayer’s hematoxylin counterstain.
Assessment of glomerulonephritis
Proteinuria was measured at the time of euthanasia using Albustix (Miles Laboratories, Elkhart, IN). Glomerular cellularity was evaluated by counting the number of nuclei per glomerular cross-section (20–30 glomerular cross-sections per mouse) after hematoxylin & eosin staining (24). For assessment of renal immune complex deposition, 4 µm frozen sections were stained with FITC-conjugated goat anti-mouse IgG antibodies or rabbit anti-mouse C3 antiserum, and examined by fluorescence microscopy (16). Glomerular staining intensity was quantified using the Image Titer titration emulation system (Rhigene), which approximates intensity by acquiring series of images taken at different exposure times. These exposures create an image titer equivalent to serial dilutions from which an endpoint is selected using a standard curve. Arbitrary units are designated according to staining intensity based on a standard curve. Renal tissue from three WT and three IFNAR−/− mice treated with TMPD was stained as above. Glomerular fluorescence intensity was determined in three kidney sections per mouse.
Statistical analysis
All statistical analyses employed the two-tailed Mann-Whitney test.
RESULTS
Interferon signature in peripheral blood cells from TMPD-treated mice
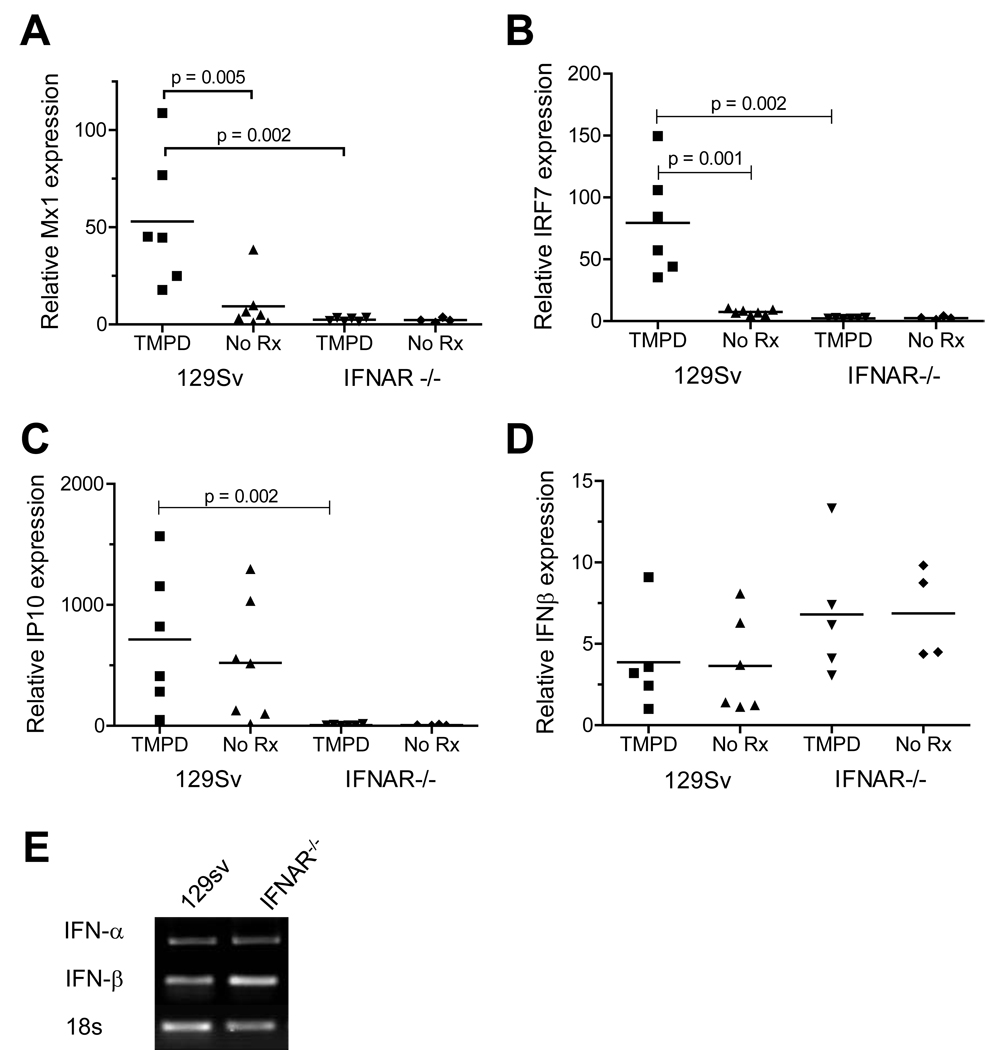
PBMCs from most SLE patients show increased expression of a group of ISGs (2;3). This “interferon signature” also is seen in the peritoneal cells and ectopic lymphoid tissue of mice treated with TMPD, which express significantly higher levels of Mx1, oligoadenylate synthetase (OAS), IP-10, IRF-7, and other ISGs than peritoneal cells from mice treated with a mineral oil that does not induce lupus (14). As shown in Figure 1, PBMCs from 129Sv mice treated with TMPD exhibited increased expression of the ISGs Mx1 (Fig. 1A) and IRF-7 (Fig. 1B) compared with untreated controls. Expression of these genes in IFNAR−/− mice was substantially lower. In contrast, expression of IP-10 (CXCL10) was comparable in both TMPD-treated and untreated 129Sv mice (Fig. 1C). There was little difference between IFNAR−/− and 129Sv mice in the expression of IFNβ, either with or without TMPD treatment (Fig. 1D). IFNα also was expressed comparably in PBMCs from IFNAR−/− mice and 129Sv controls (Fig. 1E).
Figure 1. Interferon signature in PBMCs of TMPD-treated mice.
WT (129Sv) and 129Sv IFNAR−/− mice were treated with either TMPD (0.5 ml i.p.) or left untreated. Peripheral blood was collected in PAXgene tubes 6–8 months later for RNA isolation. Expression of the IFN-I inducible genes Mx1 (A), IRF-7 (B), and IP-10 (C) and of IFNβ (D) was measured by real-time PCR, normalized to β-actin. Gene expression was compared by the Mann-Whitney test. E, RT-PCR analysis of IFNα and IFNβ gene expression by peripheral blood cells from TMPD-treated WT and IFNAR−/− mice (18S RNA expression is shown as a control).
Expression of IFN-regulated genes in other locations
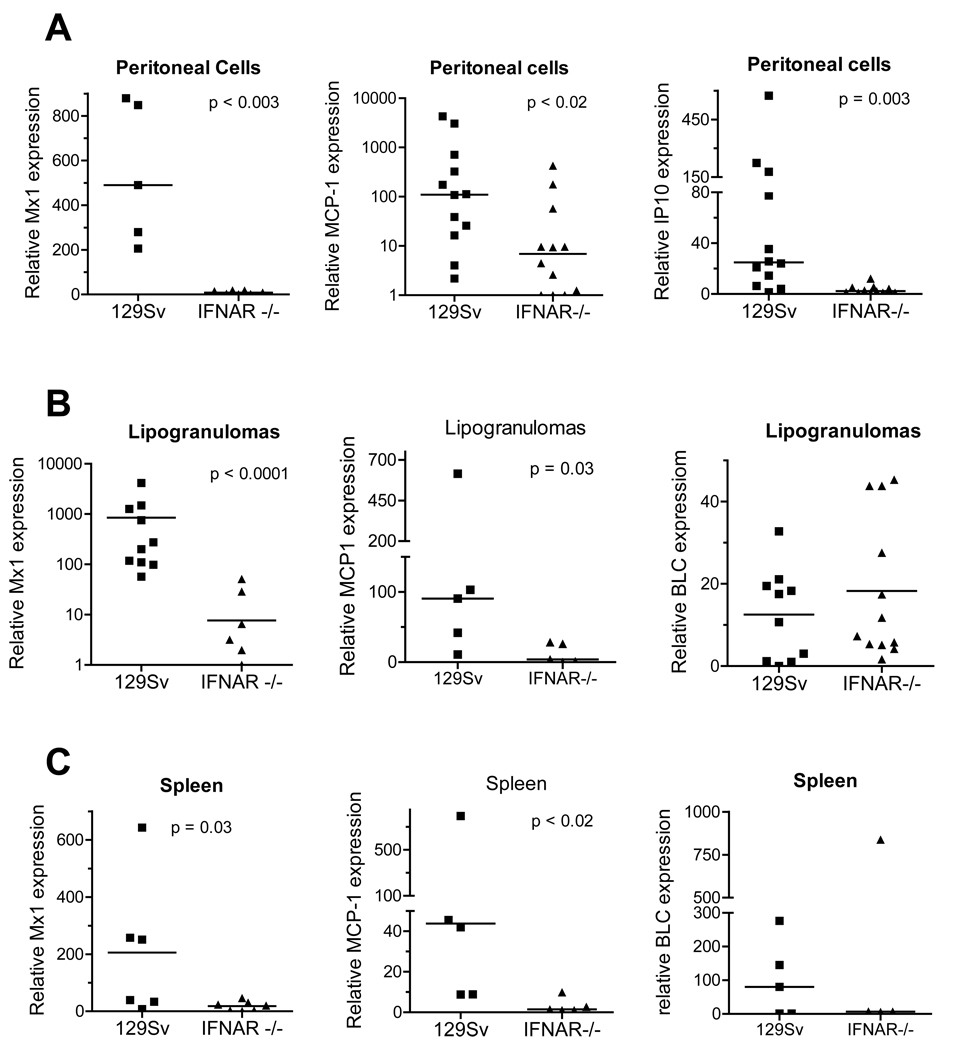
Expression of Mx1 and the IFN-I regulated chemokines IP-10 (CXCL10, which is also IFNγ-regulated) and MCP-1 (CCL2) (25) by peritoneal exudate cells of IFNAR−/− mice treated i.p. with TMPD was ~100-fold than controls (Fig. 2A) as was IRF-7 expression (not shown). A similar picture was seen in the ectopic lymphoid tissue (“lipogranulomas”) induced by TMPD treatment (Fig. 2B). Expression of ISGs Mx1 and MCP-1 was substantially higher in WT mice than in IFNAR−/− mice, whereas expression of BLC (CXCL13), a chemokine that is not IFN-I regulated, was comparable in the ectopic lymphoid tissue from WT and IFNAR−/− mice (Fig. 2B). Thus, the high expression of ISGs seen in peripheral blood (Fig. 1) was also apparent at the site of the TMPD-induced inflammatory response in WT, but not IFNAR−/−, mice. In addition, the expression of Mx1 and MCP-1, but not BLC, was decreased ~100-fold in the spleen of IFNAR−/− mice vs. controls (Fig. 2C). Together with evidence that IFNα/β production by peritoneal and lipogranuloma cells is greatly increased by TMPD treatment (14), these data suggest that the over-expression of a variety of ISGs, including chemokines implicated in the recruitment of inflammatory cells in TMPD-treated mice, requires signaling via the IFNAR. Thus TMPD-lupus recapitulates the interferon signature seen in human SLE.
Figure 2. Expression of IFN-I regulated genes in TMPD-treated mice.
Gene expression by cells from different peripheral sites in WT and IFNAR−/− mice was measured by real-time PCR, normalized to β-actin expression. Gene expression levels were compared using the Mann-Whitney test. A. Peritoneal exudate cells. Expression of Mx1, MCP-1, and IP-10 by peritoneal cells from WT and IFNAR−/− mice was measured. B. Ectopic lymphoid tissue (lipogranulomas). Expression of the IFN-I inducible genes Mx1 and MCP-1 and the non-IFN-regulated gene BLC in ectopic lymphoid tissue was determined. C. Spleen. Expression of Mx1, MCP-1, and BLC were measured.
Ectopic lymphoid tissue in TMPD-treated IFNAR −/− mice
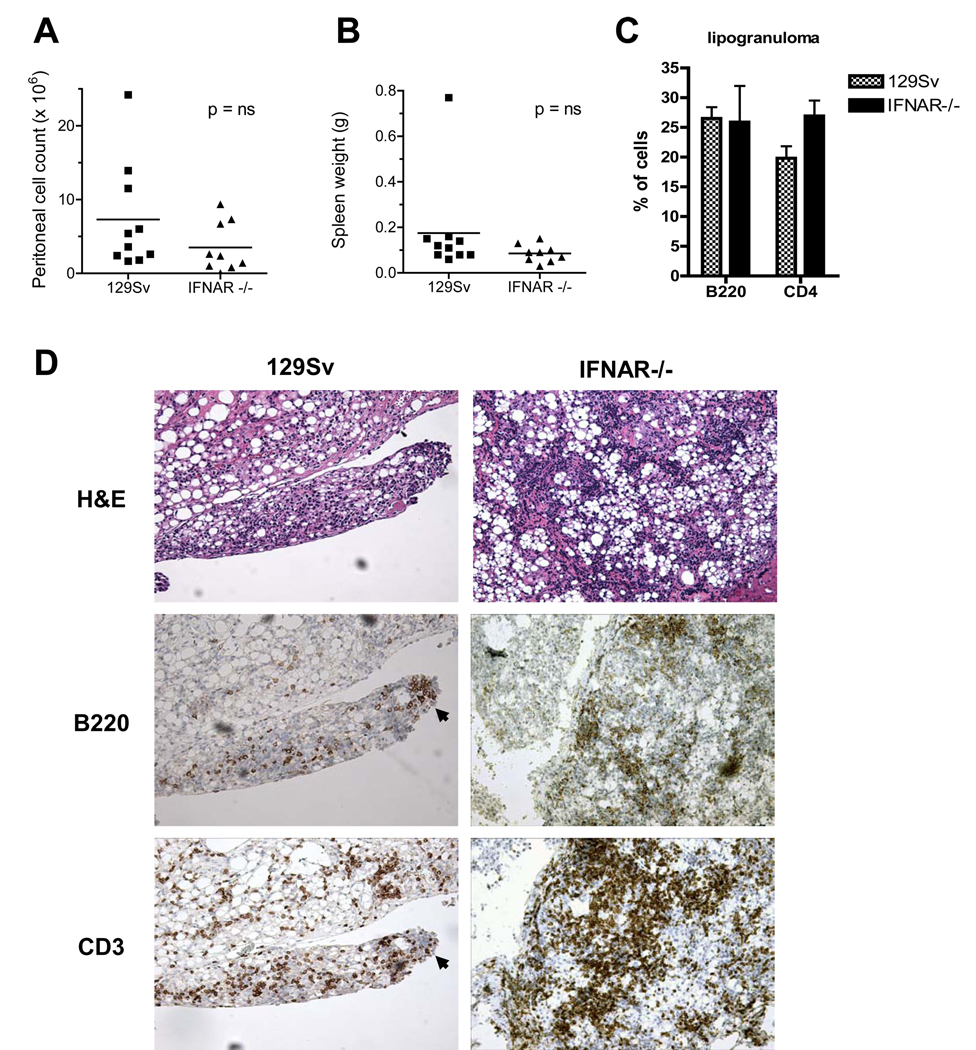
In WT mice, TMPD causes chronic peritoneal inflammation culminating in the development of ectopic lymphoid tissue, the site of a germinal center-like reaction giving rise to autoantibody-secreting cells (D Nacionales, et al. Submitted). Therefore, it was of interest to examine whether peritoneal inflammation and ectopic lymphoid tissue develop in IFNAR−/− mice. Absence of the IFNAR did not diminish the peritoneal inflammatory response substantially at 6 months, since the total number of peritoneal inflammatory cells in TMPD-treated WT and IFNAR−/− mice were comparable (Fig. 3A). Similarly, spleen weight was not significantly different in WT vs. IFNAR−/− mice (Fig. 3B).
Figure 3. Inflammatory response to TMPD in WT vs. IFNAR −/− mice.
A, Peritoneal cell counts Peritoneal lavage was performed at 6–8 months in TMPD-treated WT or IFNAR−/− mice. Total cells were counted using a hemocytometer. B, Spleen weight. Spleens of WT or IFNAR−/− mice were weighed at 6–8 months after TMPD treatment. C, Flow cytometry of lipogranuloma cells. T and B cells were stained using anti-CD4 and anti-B220, respectively. Staining is represented as a percentage of total isolated lipogranuloma cells. D. H&E staining and immunohistochemistry. Top, Hematoxylin and eosin staining of ectopic lymphoid tissue from WT and IFNAR−/− mice treated 6–8 months earlier with TMPD. Bottom, Immunoperoxidase staining of ectopic lymphoid tissue from WT and IFNAR−/− mice treated 6–8 months earlier with TMPD. Anti-B220 and anti-CD3 are shown.
The peritoneal cavities of WT and IFNAR−/− mice both contained “lipogranulomas” [ectopic lymphoid tissue, (14)]. Flow cytometry of isolated lipogranuloma cells confirmed the presence of a similar percentage of B (B220+) and T (CD4+) lymphocytes in the ectopic lymphoid tissue from TMPD-treated WT vs. IFNAR−/− mice (Fig. 3C). H&E staining of paraffin sections revealed that the lipogranulomas from IFNAR−/− and WT mice had a similar histological appearance, with numerous oil droplets surrounded by mononuclear cell infiltrates (Fig. 3D). Consistent with the findings in BALB/c mice (14), ectopic lymphoid tissue in TMPD-treated 129Sv mice contained B220+ B-cells and CD3+ T cells (Fig. 3D, left). Serial sections revealed groups of B cells within more diffuse T cell infiltrates (arrows, B220 and CD3). Ectopic lymphoid tissue from TMPD-treated IFNAR−/− mice had a similar appearance (Fig. 3D, right).
Lack of lupus-associated autoantibodies in IFNAR −/− mice
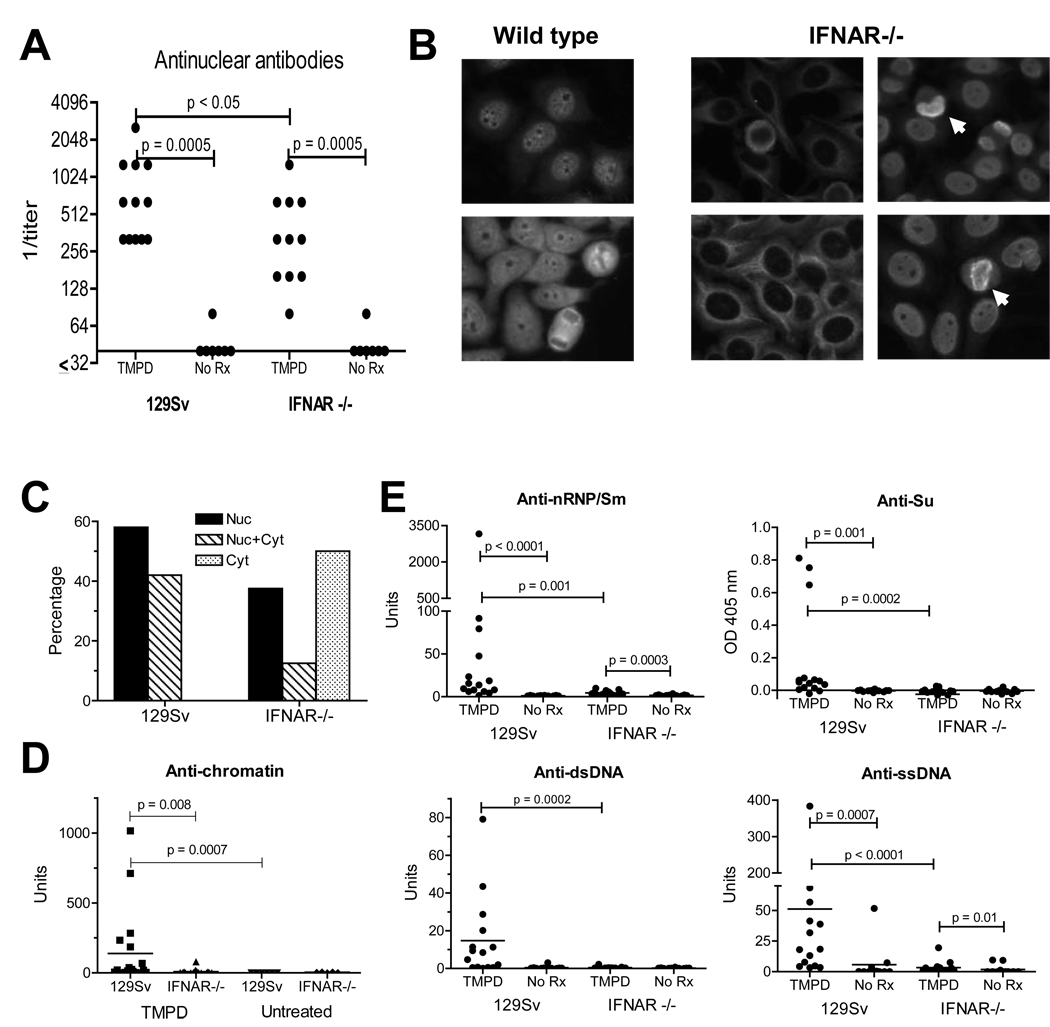
ANA, one of the hallmarks of SLE, are produced at high levels by TMPD-treated BALB/c mice. Not surprisingly, sera from TMPD-treated 129Sv mice also were strongly ANA positive (Fig. 4A). ANA were readily detectable in sera from TMPD-treated IFNAR−/− mice as well, but the mean titer was lower than in 129Sv controls (Fig. 4A). Untreated mice were negative or had only low titers. All sera from TMPD-treated WT mice gave a nuclear staining pattern, sometimes accompanied by cytoplasmic staining (Fig. 4B–C). In contrast, half of the TMPD-treated IFNAR−/− mice sera had cytoplasmic staining and the rest displayed nuclear or nuclear + cytoplasmic staining (Fig. 4B–C). Although sera from both WT and knockout mice sometimes stained mitotic figures, consistent with the presence of anti-chromatin antibodies (Fig. 4B, arrows), only the sera from TMPD-treated WT mice were positive for anti-chromatin antibodies by ELISA (Fig. 4D).
Figure 4. Autoantibody production.
A, ANA in TMPD treated mice Sera from WT or IFNAR−/− mice treated 6–8 months earlier with TMPD or untreated mice were tested for ANA by immunofluorescence (1:40 dilution). ANA levels were compared using the Mann-Whitney test. B, Immunofluorescence pattern. HEp-2 cells were incubated with sera (1:40 dilution) from representative TMPD-treated WT or IFNAR−/− mice (left and right panels, respectively). Arrows indicate staining of mitotic chromosomes by sera from IFNAR−/− mice. C, frequencies of immunofluorescence staining. Shown is the % of WT and IFNAR−/− sera with nuclear (Nuc), cytoplasmic (Cyt) or both (Nuc + Cyt) staining patterns by indirect immunofluorescence. D, Anti-chromatin ELISA. Sera from TMPD-treated or untreated IFNAR−/− mice or 129Sv controls were tested by ELISA for reactivity with chicken erythrocyte chromatin. D, Decreased lupus autoantibodies in IFNAR −/− mice. Serum levels of IgG anti-nRNP/Sm, anti-Su, anti-dsDNA, and anti-ssDNA autoantibodies were measured by ELISA 6–8 months after treatment with TMPD (15 per group) or no treatment (12 per group). Autoantibody levels in WT and IFNAR −/− mice were compared using the Mann-Whitney test.
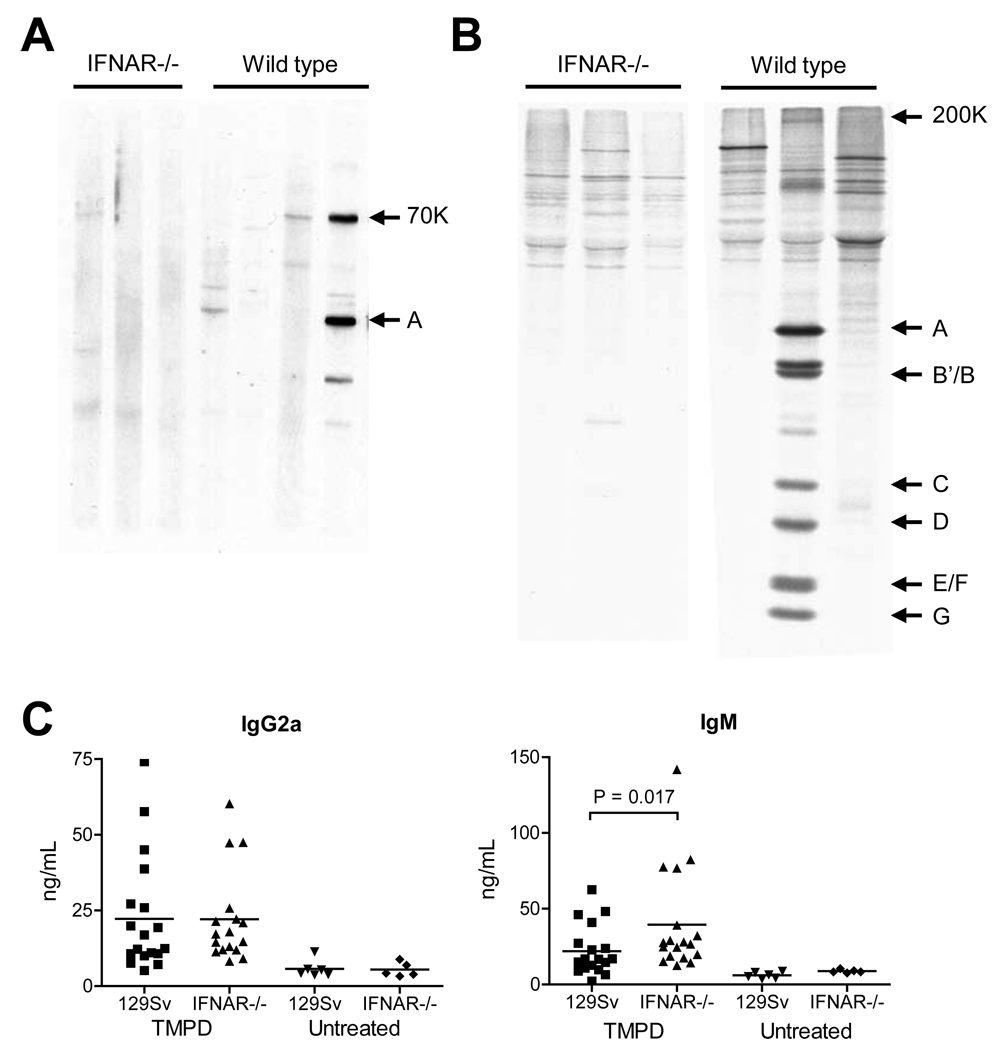
In striking contrast to the immunofluorescence results, lupus-associated autoantibodies (IgG anti-dsDNA, anti-ssDNA, anti-Sm/RNP, and anti-Su) were nearly undetectable in the sera of TMPD-treated IFNAR−/− mice by ELISA but were present at high concentrations in sera from 129Sv controls (Fig. 4E). Longitudinal studies up to 8 months indicated that the absence of this subset of autoantibodies in IFNAR−/− mice was not explained by delayed onset of autoantibody production (not shown). Moreover, these autoantibodies were absent in IFNAR−/− mice with high titer ANA (Fig. 4E), suggesting that the absence of interferon signaling affected the production of only a subset of ANA while having little effect on another subset. The specificities of the ANA in IFNAR−/− mice could not be determined by western blotting or immunoprecipitation, whereas anti-RNP/Sm autoantibodies were readily detectable in sera from WT mice by both techniques (Fig. 5A–B). Interestingly, total serum IgG2a (the primary isotype of the anti-nRNP/Sm, dsDNA, and other autoantibodies induced by TMPD) was comparable in TMPD-treated IFNAR−/− and WT mice, whereas total IgM was higher in IFNAR−/− mice (Fig. 5C), indicating that the effect of IFNAR deficiency on autoantibody production was not mediated at the level of IgG2a production or isotype switching.
Figure 5. TMPD-induced autoantibodies in IFNAR−/− mice.
A, Western blot Total proteins from K562 cell extract were probed with sera from IFNAR−/− or WT mice 6 months after TMPD treatment. Sera from the IFNAR−/− mice were negative, whereas sera from 2/4 WT mice exhibited reactivity with the U1-70K (anti-RNP) antigen and 1/4 with the U1-A protein, as determined by comparison with human reference sera and mAb 2.73 (anti-U1-70K) (not shown). B, Immunoprecipitation. Sera from TMPD-treated IFNAR−/− or WT mice were tested for reactivity with radiolabeled K562 cell extract. Positions of U snRNP proteins U5-200K (indicative of anti-Sm reactivity), U1-A, B’/B, C, D, E/F, and G are indicated on the left. C, Total IgG2a and IgM levels. Levels of total IgG2a (the predominant isotype of TMPD-induced autoantibodies) and IgM in sera from TMPD-treated or untreated IFNAR−/− mice or WT controls were measured by ELISA. IgM was significantly different between the IFNAR−/− and control groups (P = 0.017, Mann Whitney test).
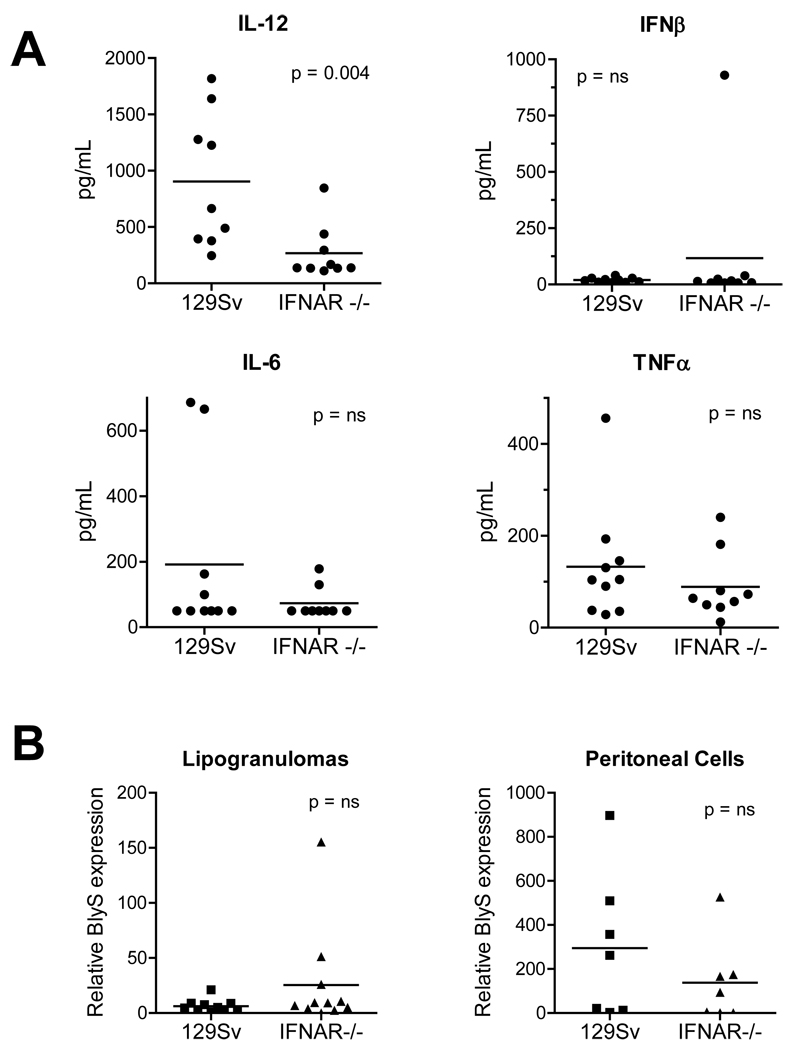
To further explore how autoantibodies in TMPD-treated mice were generated, we measured the expression of several cytokines implicated in germinal center B cell expansion and differentiation, including IL-12 (26), IFNβ, IL-6 (9), TNFα (27), and BlyS/BAFF (28–30). TMPD-induced lupus is milder (decreased autoantibody production and/or less severe nephritis) in IL-6 or IL-12 deficient mice (21;31), whereas the effect of TNFα or BlyS/BAFF deficiency has not been investigated. Consistent with the known effect of IFN-I on maturation of myeloid dendritic cells (major IL-12 producers) IL-12 levels in peritoneal lavage fluid were reduced in TMPD-treated IFNAR−/− mice vs. controls. In contrast, there was no difference in IFNβ, IL-6, or TNFα (Fig. 6A).
Figure 6. Cytokine expression.
A, IL-12, IFNβ, IL-6, and TNFα. Cytokines were measured in peritoneal lavage fluid by ELISA in WT and IFNAR−/− mice. B. BlyS/BAFF. BlyS/BAFF mRNA was quantified (normalized to β-actin) in lipogranulomas and peritoneal cells from WT and IFNAR−/− mice by real-time PCR.
BlyS/BAFF over-expression in mice increases polyclonal IgG, IgA, and IgE, leading to autoantibody production and a lupus-like syndrome (32). Although IFN-I regulates BlyS/BAFF expression (33), its expression by both peritoneal exudate cells and lipogranuloma cells was comparable in IFNAR−/− vs. WT mice (Fig. 6B), suggesting that BlyS/BAFF expression is regulated at least partly through IFN-I independent mechanisms and that its expression is not sufficient for the production of the lupus autoantibodies anti-Sm/RNP, anti-Su, and anti-dsDNA.
Glomerulonephritis is abolished in IFNAR−/− mice
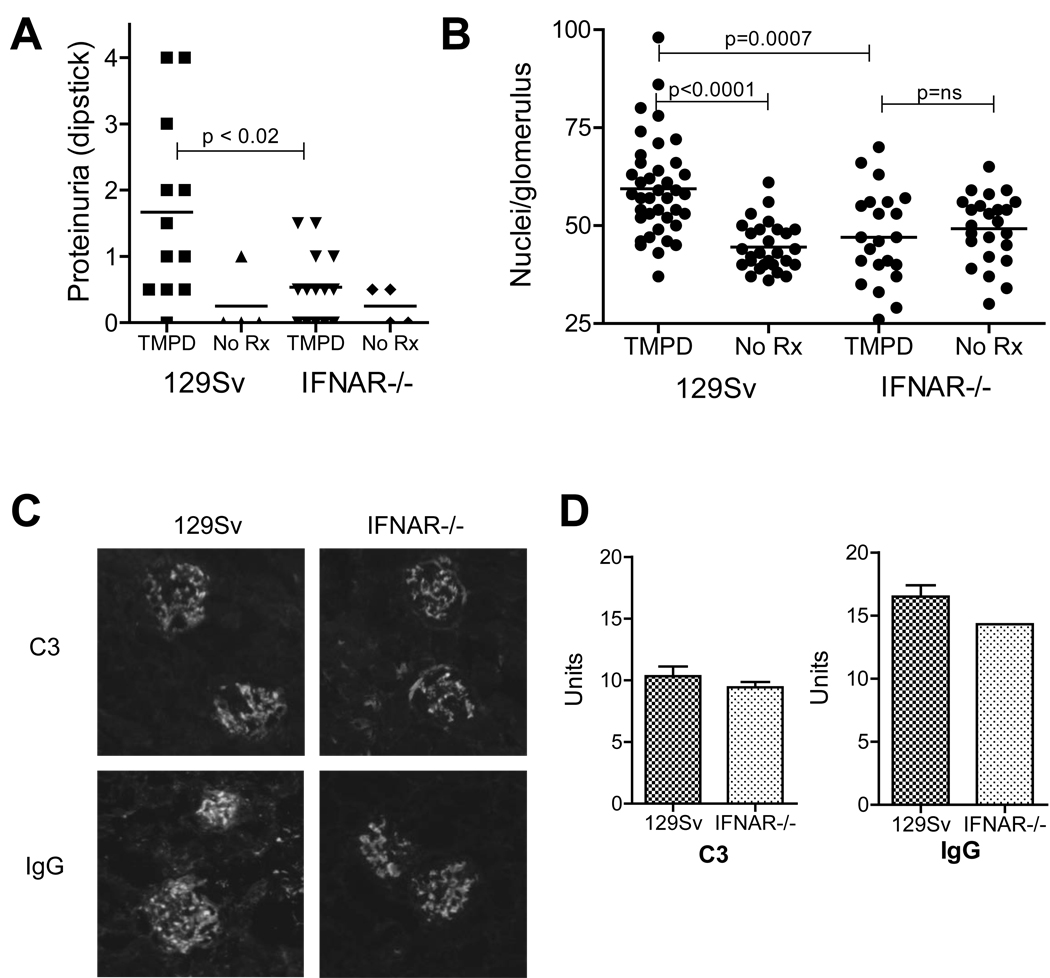
WE next examined the effect of IFNAR deficiency on the induction of lupus nephritis. As shown in Fig. 7A, ≥ 2+ proteinuria was seen 6 months after TMPD treatment in 5/12 female 129Sv controls not in any of the TMPD-treated female IFNAR−/− mice. The number of nuclei per glomerular cross-section, a measure of glomerular cellularity, was increased in TMPD-treated 129Sv mice vs. untreated controls (Fig. 7B). In contrast, there was no difference in glomerular cellularity between TMPD-treated IFNAR−/− mice and untreated controls; moreover, the number of nuclei/glomerulus was comparable to that in untreated WT mice. The number of nuclei/glomerulus was significantly lower in TMPD-treated IFNAR−/− mice than in TMPD-treated 129Sv controls (P = 0.0007, Mann-Whitney). Interestingly, direct immunofluorescence revealed glomerular immune complex deposits consisting of IgG and C3 in TMPD-treated IFNAR−/− as well as 129Sv mice (Fig. 7C). Quantification of the glomerular IgG and C3 staining confirmed that immune complex deposition was not significantly decreased in IFNAR−/− mice vs. controls (Fig. 7D), suggesting that signaling through the IFNAR is not required for immune complex formation and renal deposition. In contrast, IFNAR signaling was necessary for the inflammatory response to renal immune complexes (glomerular hypercellularity and proteinuria).
Figure 7. Absence of renal disease in IFNAR −/− mice.
A, Proteinuria. Absence of proteinuria in TMPD-treated IFNAR−/− mice (p < 0.02 vs. WT (129Sv) controls, Mann-Whitney test). B, Glomerular cellularity. Number of nuclei per glomerular cross-section, WT vs. IFNAR−/− mice (representative of 3 experiments). C, Immune complexes. Direct immunofluorescence of glomeruli for IgG and complement component C3. D, Quantification of IgG and C3 staining. Immunofluorescence staining intensity (IgG and C3) was measured by titration emulation (3 mice/group) in IFNAR−/− and WT mice.
DISCUSSION
Most immunocompetent mouse strains develop ANAs as well as lupus-specific autoantibodies such as anti-Sm, anti-dsDNA, and anti-ribosomal P following intraperitoneal injection of TMPD (15;16;34). Many strains, such as BALB/c and SJL, develop glomerulonephritis and some develop arthritis (e.g. BALB/c) or pulmonary vasculitis (e.g. B6) (17;18). Thus, TMPD induces an autoimmune disease meeting 4 of the ACR criteria for classifying SLE (35). We show here that PBMCs from mice with TMPD-induced lupus over-express ISGs, an abnormality exhibited by most SLE patients (2;3), and that signaling via the IFNAR is central to the pathogenesis of lupus in this model. Strikingly, although IFNAR deficient mice developed ANA, they did not develop lupus-specific autoantibodies such as anti-dsDNA or anti-Sm following TMPD treatment, strongly suggesting that IFN-I dysregulation promotes the production of autoantibodies against a subset of nucleic acid-protein autoantigens. Similarly, although the IFNAR−/− mice did not develop nephritis (defined by proteinuria and/or hematuria), glomerular immune complex deposition was apparent. These studies provide new evidence that IFN-I is involved in the pathogenesis of lupus.
Interferon signature in TMPD-induced lupus
PBMCs from most SLE patients express high levels of multiple ISGs (2;3). However, there is at present no direct proof that abnormal IFN-I production causes human SLE and no animal model that recapitulates the interferon signature. The peritoneal inflammatory response to TMPD is associated with increased ISG expression (14). PBMCs from TMPD-treated mice, like those from lupus patients, also exhibited increased ISG expression (Fig. 1), which was abolished in the absence of IFNAR signaling (Fig. 2). Increased ISG expression has not been reported in other lupus models with the exception of Ifi-202, which is increased in NZB/W mice due to a polymorphism of the promoter region (36). To our knowledge, TMPD-lupus is the first lupus model displaying the IFN-I signature.
There is other evidence implicating IFN-I in the pathogenesis of autoimmune disease. In NZB mice, although IFN-I expression is not elevated spontaneously, autoimmune hemolytic anemia and glomerular immune deposits are ameliorated in IFNAR deficient animals (37). However, functional improvement in renal disease could not be assessed because NZB mice do not generally develop proteinuria or hematuria, nor do they develop arthritis, serositis, skin rashes, or CNS disease (37–39). The frequency of a positive ANA in NZB mice is <10% and they do not generally develop lupus-specific autoantibodies such as anti-Sm and anti-dsDNA (38). In contrast to NZB, which is perhaps best regarded as a model for interferon-mediated autoimmune hemolytic anemia (39), (NZBXNZW)F1 mice develop a florid lupus-like disease with glomerulonephritis, positive ANA, and anti-dsDNA antibodies (39) exacerbated by exogenous IFNα (40). However, it is unclear whether IFNα plays a causal role or merely accelerates pre-existing subclinical inflammatory disease.
In MRL lpr/lpr mice, IFN-I ameliorates lupus (41). Male BXSB mice have a duplication of the TLR7 gene, which enhances responsiveness to RNA ligands capable of stimulating IFN-I production (42), but there is no information on ISG expression in their PBMCs. Thus, at present none of the mouse models of spontaneous lupus have been unequivocally shown to exhibit the interferon signature nor is there direct proof that increased IFN-I production is required for the development of lupus.
IFN-dependent and -independent autoantibodies
SLE patients who produce anti-dsDNA or anti-ribonucleoprotein (Sm, RNP, Ro, La) autoantibodies have higher ISG expression than those who do, suggesting that IFN-I is linked to the pathogenesis of these autoantibodies (5). Although it has been suggested that anti-RNP antibodies in human SLE may drive IFN-I production (43), the induction of anti-dsDNA, -Sm, -RNP, and –chromatin autoantibodies by TMPD was abrogated in IFNAR−/− mice, strongly suggesting that IFN-I signaling drives the production of anti-nucleoprotein autoantibodies (and not vice versa). While the relationship between anti-nucleoprotein antibodies and IFN-I might be intrinsically different in human vs. murine lupus, this is unlikely in view of the production of anti-nucleoprotein autoantibodies by patients with a chromosomal translocation causing over-production of IFN-I (13) and by individuals receiving exogenous IFNα (12).
Interestingly, TMPD-treated IFNAR−/− mice still produced ANA, although at a lower mean titer than WT mice (Fig. 4A). But these were not the typical “lupus” autoantibodies (Figs. 4–5). In many cases, the immunofluorescence pattern displayed by sera from TMPD-treated IFNAR−/− mice suggested reactivity with cytoplasmic filaments (Fig. 4B). In other cases, there was nuclear and chromosomal staining suggestive of anti-chromatin antibodies (Fig. 4B), but reactivity with chromatin, ssDNA, or dsDNA could not be verified (Fig. 4D–E). The autoantibody specificities in IFNAR−/− mice remain to be determined. There may be parallels between the generation of ANA in IFNAR−/− mice and “positive ANAs” in healthy individuals who do not have manifestations of SLE. This subset does not exhibit the interferon signature (5), further suggesting that IFN-I has a unique role in the pathogenesis of lupus-specific autoantibodies in humans as well as mice.
IFN-I dependence of renal disease
In humans, the interferon signature is associated with lupus nephritis and increased disease severity (2;5). In the TMPD lupus model, nephritis was also strongly dependent on IFN-I (Fig. 7). Interestingly, although there was little difference in immune complex deposition (Fig. 7C–D), IFNAR−/− mice failed to develop proteinuria or glomerular hypercellularity (Fig. 7A–B). The uncoupling of glomerular immune complex deposition from functionally significant nephritis (proteinuria and/or hematuria) is reminiscent of the pattern in NZB/W mice lacking the common γ chain of FcγRI/FcγRIII (44) and in TMPD-treated IL-12 deficient mice, which develop anti-dsDNA antibodies and renal immune complexes, but not nephritis (31). At present the mechanism of protection is not known. Renal expression of the IFN-inducible chemokine MCP-1 (25) was lower in IFNAR−/− mice (data not shown), suggesting that recruitment of CCR2+ inflammatory cells to the glomeruli may be decreased. This would be consistent with the decreased glomerular cellularity seen in the IFNAR−/− mice (Fig. 7), the importance of monocyte/macrophages in the pathogenesis of lupus nephritis (45), and the increased urinary MCP-1 levels in patients with active lupus nephritis (46). Alternatively, since FcγRI is IFNα-inducible (47), there could be a preponderance of renal FcγRIIb (anti-inflammatory) expression over FcγRI (pro-inflammatory) expression in IFNAR−/− mice. Finally, by promoting the generation of autoantibodies against nucleic acid-protein autoantigens, IFNAR signaling might enhance the formation of immunostimulatory immune complexes that trap in the glomeruli causing inflammation (48). Further study will be necessary to distinguish between the possibilities.
Mechanism of IFN-I signaling in lupus
The precise mechanism by which IFN-I promotes autoantibody production remains to be elucidated. Our studies suggest that abnormal IFN-I signaling is an early event in lupus pathogenesis. The interferon signature appeared in PBMCs 2 weeks after TMPD treatment (Fig. 1), long before the onset of autoantibody production (3–4 months) or renal disease (4–6 months) (16). IFN-I could promote autoimmunity through its effects on downstream cytokines such as IL-12 and IFNγ (31). Although IFNγ−/− mice have milder disease, we have not found increased levels of either IFNγ protein or mRNA following TMPD treatment (14). In contrast, IL-12 mRNA and protein levels are elevated. The stimulation of IL-12 production by TMPD was abrogated in IFNAR−/− mice (Fig. 6), suggesting that IL-12 plays a role in SLE downstream of IFNAR signaling. IFN-I acts synergistically with the NFκB pathway and is required for the production of bioactive IL-12p70 following the engagement of Toll-like receptors on myeloid dendritic cells (49). The decreased production of IL-12 in TMPD-treated IFNAR−/− mice may reflect reduced maturation of myeloid DCs, which is promoted by TLR ligation as well as IFN-I (50). This could, in turn, greatly diminish T cell-dependent antibody (autoantibody) responses (7).
IFN-I also could promote the differentiation of autoreactive B cells into plasma cells (9). However, a subset of B cells can differentiate into autoantibody-secreting cells in the absence of IFNAR signaling, since ANA reactive with targets other than the classic lupus autoantigens were produced nearly as efficiently in IFNAR−/− mice as in 129Sv controls (Fig. 4A). Unexpectedly, BlyS/BAFF, an IFN-inducible cytokine implicated in the pathogenesis of autoantibodies and SLE (32;33) as well as plasma cell development (28), was expressed at comparable levels in WT and IFNAR−/− mice (Fig. 6). Although it is unlikely that a lack of BlyS/BAFF expression explains the absence of anti-DNA, RNP, Sm, and Su autoantibodies in the IFNAR−/− mice, this pathway could be involved in the production of ANA in IFNAR−/− mice (Fig. 4A).
In summary, TMPD-lupus is a model of interferon signature-associated SLE. IFN-I signaling is critical for the development of proliferative nephritis and lupus autoantibodies in this model. As the first animal model in which high IFN-I production appears prior to the onset of disease, TMPD-induced lupus will be a useful tool for investigating the both origins of the interferon signature and its relationship to disease pathogenesis.
ACKNOWLEDGEMENT
We thank Mr. Dustin S. Vale-Cruz (Dept. of Anatomy and Cell Biology, University of Florida) for assisting with the fluorescence microscopy.
This research was supported by research grant R01-AR44731 from the US Public Health Service and by generous gifts from Lupus Link, Inc. (Daytona Beach, FL) and Mr. Lewis M. Schott to the UF Center for Autoimmune Disease. Dr. Nacionales is the recipient of an Arthritis Foundation Postdoctoral Fellowship. This work was supported with resources and the use of facilities at the Malcolm Randall VA Medical Center, Gainesville, FL.
REFERENCES
- 1.Reeves WH, Narain S, Satoh M. Autoantibodies in systemic lupus erythematosus. In: Koopman WJ, editor. Arthritis and Allied Conditions, A Textbook of Rheumatology. Philadelphia: Lippincott Williams & Wilkins; 2004. pp. 1497–1521. [Google Scholar]
- 2.Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A. 2003;100(5):2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197(6):711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirou KA, Lee C, George S, Louca K, Papagiannis IG, Peterson MG, et al. Coordinate overexpression of interferon-alpha-induced genes in systemic lupus erythematosus. Arthritis Rheum. 2004;50(12):3958–3967. doi: 10.1002/art.20798. [DOI] [PubMed] [Google Scholar]
- 5.Zhuang H, Narain S, Sobel E, Lee PY, Nacionales DC, Kelly KM, et al. Association of anti-nucleoprotein autoantibodies with upregulation of Type I interferon-inducible gene transcripts and dendritic cell maturation in systemic lupus erythematosus. Clin Immunol. 2005;117(3):238–250. doi: 10.1016/j.clim.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Asselin-Paturel C, Brizard G, Chemin K, Boonstra A, O'Garra A, Vicari A, et al. Type I interferon dependence of plasmacytoid dendritic cell activation and migration. J Exp Med. 2005;201(7):1157–1167. doi: 10.1084/jem.20041930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Bon A, Schiavoni G, D'Agostino G, Gresser I, Belardelli F, Tough DF. Type I interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity. 2001;14(4):461–470. doi: 10.1016/s1074-7613(01)00126-1. [DOI] [PubMed] [Google Scholar]
- 8.Marrack P, Kappler J, Mitchell T. Type I interferons keep activated T cells alive. J Exp Med. 1999;189(3):521–529. doi: 10.1084/jem.189.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19(2):225–234. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 10.Ronnblom LE, Alm GV, Oberg KE. Autoimmunity after alpha-interferon therapy for malignant carcinoid tumors. Ann Intern Med. 1991;115(3):178–183. doi: 10.7326/0003-4819-115-3-178. [DOI] [PubMed] [Google Scholar]
- 11.Dumoulin FL, Leifeld L, Sauerbruch T, Spengler U. Autoimmunity induced by interferon-alpha therapy for chronic viral hepatitis. Biomed Pharmacother. 1999;53(5–6):242–254. doi: 10.1016/S0753-3322(99)80095-X. [DOI] [PubMed] [Google Scholar]
- 12.Ioannou Y, Isenberg DA. Current evidence for the induction of autoimmune rheumatic manifestations by cytokine therapy. Arthritis Rheum. 2000;43:1431–1442. doi: 10.1002/1529-0131(200007)43:7<1431::AID-ANR3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 13.Zhuang H, Kosboth M, Lee P, Rice A, Driscoll DJ, Zori R, et al. Lupus-like disease and high interferon levels corresponding to trisomy of the type I interferon cluster on chromosome 9p. Arthritis Rheum. 2006;54(5):1573–1579. doi: 10.1002/art.21800. [DOI] [PubMed] [Google Scholar]
- 14.Nacionales DC, Kelly KM, Lee PY, Zhuang H, Weinstein JS, Sobel E, et al. Type I interferon production by tertiary lymphoid tissue developing in response to 2, 6, 10, 14 tetramethylpentadecane (pristane) Am J Pathol. 2006;168:1227–1240. doi: 10.2353/ajpath.2006.050125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Satoh M, Reeves WH. Induction of lupus-associated autoantibodies in BALB/c mice by intraperitoneal injection of pristane. J Exp Med. 1994;180:2341–2346. doi: 10.1084/jem.180.6.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Satoh M, Kumar A, Kanwar YS, Reeves WH. Antinuclear antibody production and immune complex glomerulonephritis in BALB/c mice treated with pristane. Proc Natl Acad Sci USA. 1995;92:10934–10938. doi: 10.1073/pnas.92.24.10934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wooley PH, Seibold JR, Whalen JD, Chapdelaine JM. Pristane-induced arthritis. The immunologic and genetic features of an experimental murine model of autoimmune disease. Arthritis Rheum. 1989;32:1022–1030. doi: 10.1002/anr.1780320812. [DOI] [PubMed] [Google Scholar]
- 18.Chowdhary VR, Grande JP, Luthra HS, David CS. Characterization of haemorrhagic pulmonary capillaritis: another manifestation of Pristane-induced lupus. Rheumatology (Oxford) 2007 doi: 10.1093/rheumatology/kem117. In Press. [DOI] [PubMed] [Google Scholar]
- 19.Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, et al. Functional role of type I and type II interferons in antiviral defense. Science (Wash D C ) 1994;264(5167):1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 20.Marie I, Durbin JE, Levy DE. Differential viral induction of distinct interferon-alpha genes by positive feedback through interferon regulatory factor-7. EMBO J. 1998;17(22):6660–6669. doi: 10.1093/emboj/17.22.6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richards HB, Satoh M, Shaw M, Libert C, Poli V, Reeves WH. IL-6 dependence of anti-DNA antibody production: evidence for two pathways of autoantibody formation in pristane-induced lupus. J Exp Med. 1998;188:985–990. doi: 10.1084/jem.188.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshida H, Satoh M, Behney KM, Lee CG, Richards HB, Shaheen VM, et al. Effect of an exogenous trigger on the pathogenesis of lupus in NZB X NZW (F1) mice. Arthritis Rheum. 2002;46:2235–2244. doi: 10.1002/art.10441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reeves WH, Satoh M, Lyons R, Nichols C, Narain S. Detection of autoantibodies against proteins and ribonucleoproteins by double immunodiffusion and immunoprecipitation. In: Detrick B, Hamilton RG, Folds JD, editors. Manual of Molecular and Clinical Laboratory Immunology. Washington, DC: ASM Press; 2006. pp. 1007–1018. [Google Scholar]
- 24.Johnson RJ, Iida H, Alpers CE, Majesky MW, Schwartz SM, Pritzl P, et al. Expression of smooth muscle cell phenotype by rat mesangial cells in immune complex nephritis: α-smooth muscle actin is a marker of mesangial cell proliferation. J Clin Invest. 1991;87:847–858. doi: 10.1172/JCI115089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bauer JW, Baechler EC, Petri M, Batliwalla FM, Crawford D, Ortmann WA, et al. Elevated Serum Levels of Interferon-Regulated Chemokines Are Biomarkers for Active Human Systemic Lupus Erythematosus. PLoS Med. 2006;3(12):e491. doi: 10.1371/journal.pmed.0030491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dubois B, Barthelemy C, Durand I, Liu YJ, Caux C, Briere F. Toward a role of dendritic cells in the germinal center reaction: triggering of B cell proliferation and isotype switching. J Immunol. 1999;162(6):3428–3436. [PubMed] [Google Scholar]
- 27.Jourdan M, Tarte K, Legouffe E, Brochier J, Rossi JF, Klein B. Tumor necrosis factor is a survival and proliferation factor for human myeloma cells. Eur Cytokine Netw. 1999;10(1):65–70. [PMC free article] [PubMed] [Google Scholar]
- 28.Schneider P. The role of APRIL and BAFF in lymphocyte activation. Curr Opin Immunol. 2005;17(3):282–289. doi: 10.1016/j.coi.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Ramanujam M, Wang X, Huang W, Schiffer L, Grimaldi C, Akkerman A, et al. Mechanism of action of transmembrane activator and calcium modulator ligand interactor-Ig in murine systemic lupus erythematosus. J Immunol. 2004;173(5):3524–3534. doi: 10.4049/jimmunol.173.5.3524. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, Park CS, Yoon SO, Li L, Hsu YM, Ambrose C, et al. BAFF supports human B cell differentiation in the lymphoid follicles through distinct receptors. Int Immunol. 2005;17(6):779–788. doi: 10.1093/intimm/dxh259. [DOI] [PubMed] [Google Scholar]
- 31.Calvani N, Satoh M, Croker BP, Reeves WH, Richards HB. Nephritogenic autoantibodies but absence of nephritis in IL-12p35-deficient mice with pristane-induced lupus. Kidney Int. 2003;64:897–905. doi: 10.1046/j.1523-1755.2003.00178.x. [DOI] [PubMed] [Google Scholar]
- 32.Gross JA, Johnston J, Mudri S, Enselman R, Dillon SR, Madden K, et al. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature (Lond ) 2000;404(6781):995–999. doi: 10.1038/35010115. [DOI] [PubMed] [Google Scholar]
- 33.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3(9):822–829. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Satoh M, Richards HB, Shaheen VM, Yoshida H, Shaw M, Naim JO, et al. Widespread susceptibility among inbred mouse strains to the induction of lupus autoantibodies by pristane. Clin Exp Immunol. 2000;121:399–405. doi: 10.1046/j.1365-2249.2000.01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 36.Rozzo SJ, Allard JD, Choubey D, Vyse TJ, Izui S, Peltz G, et al. Evidence for an interferon-inducible gene, Ifi202, in the susceptibility to systemic lupus. Immunity. 2001;15(3):435–443. doi: 10.1016/s1074-7613(01)00196-0. [DOI] [PubMed] [Google Scholar]
- 37.Santiago-Raber ML, Baccala R, Haraldsson KM, Choubey D, Stewart TA, Kono DH, et al. Type-I interferon receptor deficiency reduces lupus-like disease in NZB mice. J Exp Med. 2003;197(6):777–788. doi: 10.1084/jem.20021996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mellors RC. Autoimmune disease in NZB/BL mice. I. Pathology and pathogenesis of a model system of spontaneous glomerulonephritis. J Exp Med. 1965;122:25–40. doi: 10.1084/jem.122.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hahn BH. Animal models of systemic lupus erythematosus. In: Wallace DJ, Hahn BH, Quismorio FP, Klinenberg JR, editors. Dubois' Lupus Erythematosus. Baltimore: Williams & Wilkins; 1996. pp. 339–380. [Google Scholar]
- 40.Mathian A, Weinberg A, Gallegos M, Banchereau J, Koutouzov S. IFN-alpha induces early lethal lupus in preautoimmune (New Zealand Black × New Zealand White) F1 but not in BALB/c mice. J Immunol. 2005;174(5):2499–2506. doi: 10.4049/jimmunol.174.5.2499. [DOI] [PubMed] [Google Scholar]
- 41.Hron JD, Peng SL. Type I IFN protects against murine lupus. J Immunol. 2004;173(3):2134–2142. doi: 10.4049/jimmunol.173.3.2134. [DOI] [PubMed] [Google Scholar]
- 42.Pisitkun P, Deane JA, Difilippantonio MJ, Tarasenko T, Satterthwaite AB, Bolland S. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science (Wash D C ) 2006;312(5780):1669–1672. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- 43.Ronnblom L, Alm GV. An etiopathogenic role for the type I IFN system in SLE. Trends Immunol. 2001;22(8):427–431. doi: 10.1016/s1471-4906(01)01955-x. [DOI] [PubMed] [Google Scholar]
- 44.Clynes R, Dumitru C, Ravetch JV. Uncoupling of immune complex formation and kidney damage in autoimmune glomerulonephritis. Science (Wash D C ) 1998;279(5353):1052–1054. doi: 10.1126/science.279.5353.1052. [DOI] [PubMed] [Google Scholar]
- 45.Bergtold A, Gavhane A, D'Agati V, Madaio M, Clynes R. FcR-Bearing Myeloid Cells Are Responsible for Triggering Murine Lupus Nephritis. J Immunol. 2006;177(10):7287–7295. doi: 10.4049/jimmunol.177.10.7287. [DOI] [PubMed] [Google Scholar]
- 46.Tucci M, Barnes EV, Sobel ES, Croker BP, Segal MS, Reeves WH, et al. Strong association of a functional polymorphism in the monocyte chemoattractant protein 1 promoter gene with lupus nephritis. Arthritis Rheum. 2004;50(6):1842–1849. doi: 10.1002/art.20266. [DOI] [PubMed] [Google Scholar]
- 47.Fultz MJ, Vogel SN. Interferon alpha-induced changes in Fc gamma R-specific mRNA expression and isotype-specific, Fc gamma R-mediated phagocytosis in C3H/OuJ (Lpsn) and C3H/HeJ (Lpsd) macrophages. J Leukoc Biol. 1992;51(3):300–304. doi: 10.1002/jlb.51.3.300. [DOI] [PubMed] [Google Scholar]
- 48.Vollmer J, Tluk S, Schmitz C, Hamm S, Jurk M, Forsbach A, et al. Immune stimulation mediated by autoantigen binding sites within small nuclear RNAs involves Toll-like receptors 7 and 8. J Exp Med. 2005;202(11):1575–1585. doi: 10.1084/jem.20051696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gautier G, Humbert M, Deauvieau F, Scuiller M, Hiscott J, Bates EE, et al. A type I interferon autocrine-paracrine loop is involved in Toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. J Exp Med. 2005;201(9):1435–1446. doi: 10.1084/jem.20041964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tough DF. Type I interferon as a link between innate and adaptive immunity through dendritic cell stimulation. Leuk Lymphoma. 2004;45(2):257–264. doi: 10.1080/1042819031000149368. [DOI] [PubMed] [Google Scholar]