Abstract
Excess type-I interferons (IFN-I) have been linked to the pathogenesis of systemic lupus erythematosus (SLE). Therapeutic use of IFN-I can trigger the onset of SLE and most lupus patients display upregulation of a group of interferon stimulated genes (ISGs). While this “interferon signature” has been linked with disease activity, kidney involvement, and autoantibody production, the source of IFN-I production in SLE remains unclear. Tetramethylpentadecane (TMPD)-induced lupus is at present the only model of SLE associated with excess IFN-I production and ISG expression. Here we demonstrate that TMPD treatment induces an accumulation of immature Ly6Chi monocytes, which are a major source of IFN-I in this lupus model. Importantly, they were distinct from interferon-producing dendritic cells. The expression of IFN-I and ISGs was rapidly abolished by monocyte depletion whereas systemic ablation of dendritic cells (DCs) had little effect. In addition, there was a striking correlation between the numbers of Ly6Chi monocytes and the production of lupus autoantibodies. Therefore, immature monocytes rather than DCs appear to be the primary source of IFN-I in this model of IFN-I dependent lupus.
Keywords: autoimmunity, systemic lupus erythematosus, monocytes
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disorder affecting multiple organs including the skin, joints, kidneys, lungs, heart and the nervous system (1). Antinuclear antibodies against small ribonucleoproteins (snRNPs) and double-stranded (ds) DNA are pathognomonic of the disease (1). Recent evidence suggests that type-I interferons (IFN-I), a family of anti-viral cytokines, are integral to the pathogenesis of SLE. More than two-thirds of SLE patients display upregulation of a group of IFN-I-stimulated genes (ISGs) (2–4). This “interferon signature” is clinically relevant as it correlates with active disease, presence of certain autoantibodies, and an increased incidence of renal involvement (4, 5). Supporting a causal role of IFN-I, therapeutic use of recombinant IFN-α is linked to a wide array of autoimmune manifestations and there are reports of SLE following treatment (6). Although an association between elevated IFN-I levels and SLE is well established, its origin is unclear.
Experimental lupus induced by 2, 6, 10, 14-tetramethylpentadecane (TMPD) displays key immunological and clinical features of human SLE, including the production of autoantibodies against dsDNA and snRNPs, and the development of immune complex-mediated glomerulonephritis and arthritis (7). We recently reported that TMPD-induced lupus is associated with excess IFN-I production and upregulation of ISGs (8). This is at present the only murine model reported to have the “interferon signature”. IFN-I signaling is critically required in this model as shown by the absence of lupus autoantibodies and kidney disease in IFN-I receptor deficient mice (9). The source of the excess IFN-I, however, has not been examined. In this study, we aimed to identify and characterize the cell population(s) responsible for increased IFN-I production in TMPD-induced lupus.
Materials and Methods
Mice
Wild-type BALB/cJ, and B6.FVB-Tg.Itgax-DTR/EGFP.57Lan/J backcrossed to a BALB/c background, referred to as CD11c-DTR (diphtheria toxin receptor) mice (10, 11), were purchased from Jackson Laboratories (Bar Harbor, ME) and housed in a conventional facility. CD11c-DTR mice were maintained as heterozygote crosses and littermates not expressing the transgene were used as controls. 129Sv/Ev Type I interferon receptor α-chain deficient (IFNAR −/−) mice and wild type controls (129Sv/Ev) were purchased from B&K Universal Limited (Grimston, Aldbrough, England). Eight-week-old mice received a single intraperitoneal (i.p.) injection of 0.5 ml TMPD (Sigma-Aldrich, St. Louis, MO), mineral oil (Harris Teeter, Mathews, NC), 4% thioglycollate (BD Bioscience, San Jose, CA), squalene (Sigma), n-hexadecane (Sigma), or PBS. Peritoneal cell isolation, and cecal ligation and puncture were performed as described (8, 11). These studies were approved by the Institutional Animal Care and Use Committee.
PCR
Real-time PCR (RT-PCR) and conventional PCR were performed as described (8). Briefly, total RNA was extracted from 106 peritoneal cells using Trizol reagent (Invitrogen, Carlsbad, CA) and cDNA was synthesized using Superscript II First-Strand Synthesis Kit (Invitrogen). RT-PCR was performed using the SYBR Green Core Reagent Kit (Applied Biosystems, Foster City, CA) with an Opticon II thermocycler (MJ Research, Waltham, MA). Amplification conditions were: 95°C for 10 min, followed by 45 cycles of 94°C for 15 s, 60°C for 25 s, and 72°C for 25 s. After the final extension (72°C for 10 min), a melting-curve analysis was performed to ensure specificity of the products. IFN-I genes were amplified by conventional PCR using a PTC-100 programmable thermal controller (MJ Research). Amplification conditions were: 95°C for 5 min, followed by 40 cycles of 94°C for 30 s, 60°C for 1 min, and 72°C for 1 min. After a final extension (72°C for 10 min), PCR products were analyzed by agarose gel electrophoresis. Primers used in this study include Mx1 (forward: GATCCGACTTC ACTTCCAGATGG; reverse: CATCTCAGTGGTAGTCAACCC), MCP-1 (Forward: AGGTCCCTGTCATGCTTCTG; reverse: GGATCATCTTGCTGGTGAAT), IP-10 (forward: CCTGCAGGATGATGGTCAAG,; reverse: GAATTCTTGCTTCGGCAGTT), consensus IFN-α (forward: ATGGCTAGRCTCTGTGCTTTCCT; reverse: AGGGCTC TCCAGAYTTCTGCTCTG), IFN-α5 (forward: TGTGACCTTCTTCAGACTC; reverse: CTCCTCCTTGCTCAATC), IFN-β (forward: AGCTCCAAGAAAGGACGAACAT; reverse: ATTCTTGCTTCGGCAGTTAC), TNF-α (forward: GGCAGGTCTACTTTG GAGTCATTGC; reverse: ACATTCGAGGCTGCTCCAGTGAATTCGG), iNOS (forward: ATCGACCCGTCCACAGTATG; reverse: GATGGACCCCAAGCAAGACT), IL-12p40 (forward: GAGTGGACTGGACTCCCGA, reverse: CAAGTTCTTGGGCGG GTCTG), β-actin (forward: CCCACACTGTGCCCATCTAC; reverse: CGCTCGGT CAGGATCTTCAT), and 18S RNA (forward: CGGCTACCACATCCAAGGAA; reverse: GCTGGAATTACCGCGGCT).
Flow cytometry
All antibodies were purchased from BD Bioscience with the exceptions of anti-Ly6C-FITC, anti-Ly6C-biotin, avidin-APC (eBioscience, San Diego, CA), anti-F4/80-FITC, anti-Moma2-FITC (Serotec, Raleigh, NC), and anti-CD11b-Pacific blue (Caltag Laboratories, Burlingame, CA). Cell staining was performed as described (8). Propidium iodide (Invitrogen) staining was performed following manufacturer’s protocol. Fifty thousand events per sample were acquired by a CYAN ADP flow cytometer (Dako, Fort Collins, CO) and analyzed with FCS Express 3 (De Novo Software, Ontario, Canada).
Cell sorting
Peritoneal cells from TMPD-treated mice (107) were stained with anti-Ly6G-PE, washed, and incubated with magnetic bead-conjugated anti-PE (Miltenyi Biotec, Auburn, CA). Granulocytes (>99% purity) were positively selected using MS columns while the negative fraction was stained with anti-CD11b-APC, washed, and incubated with magnetic bead-conjugated anti-APC (Miltenyi Biotec). Positive selection using MS columns yielded >85% Ly6Chi monocytes. The negative fraction consisted of lymphocytes and dendritic cells (DCs). Cell sorting using a FACS DIVA flow cytometer (BD Bioscience) yielded similar results with a higher purity of Ly6Chi monocytes (>95%). For morphological analysis, 3 × 104 sorted cells were cytospun onto glass slides and stained using the Hema3 kit (modified Wright stain; Fisher Scientific, Pittsburg, PA).
Monocyte labeling and depletion
Clodronate (a gift from Roche Diagnostics) liposomes (Clo-lip) and 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine perchlorate (DiD)-liposomes were produced as described (12, 13). To label Ly6C− monocytes, 150 μL of DiD-liposomes were injected i.v. into TMPD-treated mice. Clo-lip (200 μl) was delivered i.v. 24 hr prior to the injection of DiD-liposomes for the labeling of Ly6Chi monocytes. Analysis was performed 24 hr after labeling. To deplete peritoneal monocytes, 200 μL of clodronate-containing liposomes were injected i.p. in wild-type BALB/c mice treated with TMPD two weeks earlier.
Dendritic cell depletion
DC ablation was performed in TMPD-treated CD11c-DTR mice by injecting 4 ng/g body weight of diphtheria toxin (DT; Sigma) i.p. Mice were sacrificed for analysis 48 hr after clo-lip or DT administration.
Statistical Analysis
For quantitative variables, diffe rences between groups were analyzed by Student’s unpaired t-test. Bivariate correlations were assessed using Spearman’s correlation coefficient. Data were presented as mean ± s.d. All tests were two-sided and a p-value less than 0.05 was considered statistically significant. Statistical analyses were performed using GraphPad Prism 4.0 (GraphPad Software, San Diego, CA).
Results
The “interferon signature” precedes lupus disease onset
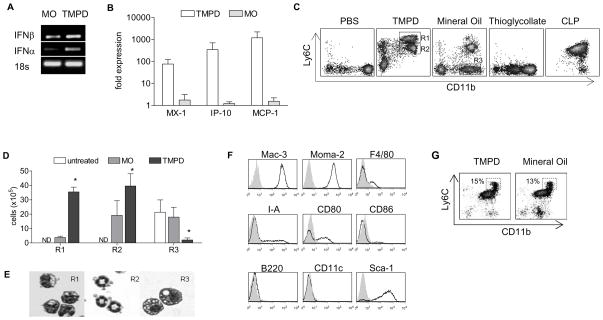
Although the link between SLE and IFN-I is firmly established, it remains unclear whether the “interferon signature” precedes disease manifestations. In the TMPD model of SLE, production of lupus autoantibodies (anti-Sm, -nRNP and -dsDNA) and development of immune-complex-mediated glomerulonephritis occur approximately four to six months after peritoneal exposure to the hydrocarbon oil (7). Upregulation of IFN-α and IFN-β, on the other hand, was observed in peritoneal exudate cells (PECs) within two weeks of TMPD treatment (Fig. 1A). Although consensus PCR primers can amplify most IFN-α subtypes, quantification of ISG expression allows assessment of total IFN-I proteins, since all IFN-I subtypes bind to single receptor complex to activate ISG expression. Accordingly, a panel of ISGs including myxoma resistance protein-1 (Mx-1), macrophage chemoattractant protein-1 (MCP-1/CCL2), and IP-10/CXCL10 were also highly upregulated (Fig. 1B) two weeks after TMPD treatment. In contrast, ISG expression was only modestly increased in mice treated with mineral oil, a control hydrocarbon oil that triggers chronic inflammation without features of lupus. Supporting the gene expression data, elevated levels of IFN-β and MCP-1 protein along with the inflammatory cytokines tumor necrosis factor-α (TNF-α) and interleukin (IL)-12 were demonstrated in peritoneal lavage fluid of TMPD-treated mice (not shown). Thus, the “interferon signature” induced by TMPD was established within 2 weeks of treatment, long before the appearance of lupus autoantibodies and kidney pathology.
Figure 1. TMPD induces elevated expression of IFN-I and accumulation of Ly6Chi monocytes.
Comparisons of A) IFN-I expression (conventional PCR) and B) ISG expression (RT-PCR) in peritoneal cells after TMPD or MO treatment. C) Flow cytometry of peritoneal cells in wild-type 129Sv mice treated with PBS, TMPD (2 weeks), mineral oil (2 weeks), thioglycollate (3 days), and cecal-puncture ligation (3 days). Boxes indicate Ly6Chi immature monocytes (R1), CD11b+ Ly6Cmid granulocytes (R2), and Ly6C− mature monocytes/macrophages (R3). D) Quantification and E) morphologic analysis of peritoneal cell populations. F) Flow cytometry of surface makers on Ly6Chi immature monocytes. G) Flow cytometry of peritoneal cells from wild-type 129Sv mice treated with TMPD or mineral oil for 1 day. Shaded region (panel F) represents isotype control staining and boxes indicate Ly6Chi immature monocytes. Each bar (panels B and D) represents the mean of 4 animals and error bars indicate standard deviation (s.d.). * p < 0.05 (Student’s t-test).
TMPD induces an accumulation of Ly6Chi monocytes
To identify the source of IFN-I production, we analyzed different cell populations in the peritoneal exudate two weeks following TMPD treatment. More than 80% of the cells in the inflammatory exudate were CD11b+ (not shown). Since CD11b is expressed by monocytes/macrophages, granulocytes and peritoneal B1 cells, we utilized the marker Ly6C to distinguish these populations (14, 15). In untreated or PBS-treated animals, resting peritoneal cells predominantly consisted of macrophages (CD11bhi Ly6C−) and B1 cells (CD11bmid Ly6C−; Fig. 1C). As described previously (14), peritoneal macrophages expressed the F4/80 antigen while B1 cells were positive for the B cell markers B220 and CD5 (not shown). Interestingly, both resting cell types were depleted two weeks following TMPD treatment while two distinct populations emerged: CD11b+ Ly6Chi cells (R1), consistent with the phenotype of immature monocytes (15), and CD11b+ Ly6Cmid cells (R2) characteristic of granulocytes (Fig. 1C, D). The latter (R2) also expressed the neutrophil marker Ly6G (not shown). These populations each represented ~ 30% of cells in the peritoneal inflammatory infiltrate. Morphology of the monocyte and granulocyte populations was consistent with their surface marker profile (Fig. 1E). These findings were consistent in other wild-type strains (C57/BL6 and BALB/c), except that these strains showed a greater influx of granulocytes (not shown).
Treatment with mineral oil, which does not induce the interferon signature, resulted in similar accumulation of granulocytes, but few Ly6Chi monocytes were present (Fig. 1C, D). Instead, mature monocytes resembling resting peritoneal macrophages were found (R3, Fig. 1C, D). Compared to the Ly6Chi monocytes, these cells were larger, more vacuolated, and displayed rounded nuclei, suggestive of a more differentiated phenotype (Fig. 1E). The accumulation of Ly6Chi monocytes was specific to TMPD treatment as this pattern was not seen in sterile peritonitis induced by thioglycollate or septic peritonitis induced by cecal ligation and puncture (CLP; Fig. 1C).
Flow cytometric analysis of Ly6Chi monocytes from TMPD-treated animals demonstrated intense expression of the myeloid markers Mac-3 and Moma-2 (Fig. 1F). Supporting their immature phenotype, only a small fraction of Ly6Chi monocytes expressed the macrophage marker F4/80, major histocompatibility complex (MHC) class II (I-A), and the costimulatory molecules CD80 and CD86 (Fig. 1F). This was in sharp contrast to mature peritoneal macrophages, which expressed these markers at high levels (not shown). Ly6Chi monocytes also lacked markers of dendritic cells (CD11c and CD205), B cells (B220 and CD19), T cells (CD3), granulocytes (Ly6G) or NK cells (Pan-NK; Fig. 1F and not shown). The absence of B220 and CD11c expression distinguishes these cells from the previously described natural interferon-producing cells, which also express Ly6C, albeit at much lower levels compared to Ly6Chi monocytes (16, 17). Curiously, these monocytes exhibited strong expression of Sca-1 (Ly6A/E; Fig. 1F), a marker normally found on hematopoietic stem cells and certain T-cell subsets (18).
While the accumulation of Ly6Chi monocytes was evident after two weeks, these cells appeared in the peritoneal cavity as early as one day after TMPD treatment (Fig. 1G). However, this observation was not limited to TMPD as a similar pattern of acute peritoneal inflammation was elicited by mineral oil (Fig. 1G). But the appearance of Ly6Chi monocytes in response to mineral oil was transient compared to TMPD. Only a small population of Ly6Chi monocytes was found in the peritoneal cavity two weeks after mineral oil treatment whereas the response was maintained for several months with TMPD treatment (Fig. 1C and not shown).
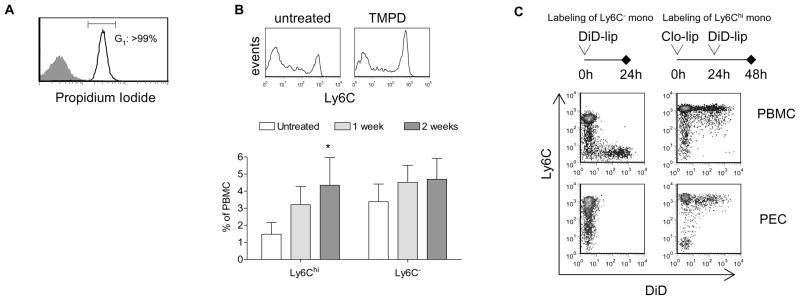
To investigate the source of the accumulating Ly6Chi monocytes, we first performed a cell cycle analysis to determine whether these cells were proliferating in the peritoneal cavity. Propidium iodide staining revealed that virtually all Ly6Chi monocytes in the peritoneal exudate elicited by TMPD were in G1 phase (Fig. 2A), indicating that extramedullary myelopoiesis at the site of inflammation is not a likely explanation for the monocyte accumulation.
Figure 2. TMPD induces the direct recruitment of circulating Ly6Chi monocytes.
A) Propidium idodide cell cycle analysis of peritoneal cells two weeks after TMPD treatment. B) Flow cytometry of Ly6C expression on peripheral blood monocytes (gated on CD11b+Ly6G− cells) and quantification of peripheral blood Ly6Chi and Ly6C− monocytes following TMPD treatment (n = 4 per group). C) Flow cytometry of peripheral blood and peritoneal monocytes (gated on CD11b+Ly6G− cells) in TMPD-treated mice 24 hr after administration of DID-liposomes. Data are representative of 3 independent experiments.
Recent studies have demonstrated that Ly6Chi monocytes egress from the bone marrow and that expression of Ly6C diminishes as they mature in the circulation (12). We therefore examined whether TMPD treatment alters the maturation profile of peripheral blood monocytes. In untreated animals, mature circulating monocytes (Ly6C−) outnumbered their immature Ly6Chi counterparts by about 2:1 (Fig. 2B). Two weeks after TMPD treatment, the frequency of Ly6Chi monocytes in the peripheral blood doubled while the Ly6C− subset remained constant (Fig. 2B).
To demonstrate the migration of Ly6Chi monocytes into the peritoneal cavity following TMPD injection, we selectively labeled monocyte subsets in vivo. As described by previously (12), intravenous injection of liposomes containing the fluorescent dye DiD labeled strictly Ly6C− monocytes in the circulation (Figure 2C left). Consistent with the paucity of Ly6C− monocytes in the response to TMPD, DiD+ cells were not recovered from the peritoneal cavity. Using clodronate-containing liposomes first to deplete mature monocytes (12), circulating Ly6Chi monocytes were specifically labeled by the subsequent administration of DiD-liposomes (Figure 2C right). After 24 hours, more than one-third of Ly6Chi monocytes in the peritoneal cavity were DiD+, indicating that TMPD treatment induces rapid and specific recruitment of this monocyte subset. Taken together, the data suggest that TMPD treatment results in the export of Ly6Chi monocytes from the bone marrow into the circulation and followed by their specific recruitment and accumulation in the peritoneal cavity.
Ly6Chi monocytes are a major source of IFN-I production
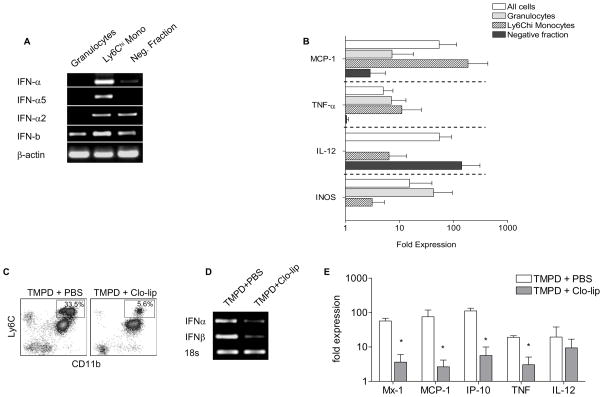
Using magnetic bead sorting, we separated PECs from TMPD-treated mice into Ly6Chi monocytes, Ly6G+ granulocytes, and a negative fraction consisting of lymphocytes and dendritic cells. Although the Ly6Chi monocyte fraction contained a small percentage of contaminating Ly6G+ granulocytes, DCs were found only in the negative fraction (not shown). PCR analysis revealed that Ly6Chi monocytes were the predominant source of IFN-α and IFN-β expression (Fig. 3A). IFN-I transcripts also were detected in other populations, albeit at significantly lower levels (Fig. 3A). Ly6Chi monocytes expressed high levels of the interferon-stimulated chemokine MCP-1 and moderate levels of TNF-α and IL-12 (Fig. 3B) as well. Granulocytes accounted for the remaining TNF-α transcripts and the majority of inducible nitric oxide synthase (iNOS) expression. Not surprisingly, IL-12 transcripts were found predominantly in cells of the negative fraction as this cytokine was most likely derived from DCs (Fig. 3B). Similar results were obtained when this experiment was repeated using flow cytometric cell sorting to increase the purity of sorted populations (not shown).
Figure 3. Ly6Chi monocytes are major producers of IFN-I.
Analysis of A) IFN-I expression (conventional PCR) and B) ISG expression (RT-PCR) in magnetic bead-sorted peritoneal Ly6Chi monocytes, granulocytes and the negative fraction (lymphocytes and DCs) from wild-type 129sv mice treated with TMPD (2 weeks). C) Flow cytometry of TMPD-elicited peritoneal cells 2 days following treatment with clodronate-containing liposomes (clo-lip). Box indicates Ly6Chi monocytes. D) IFN-I expression (conventional PCR) and E) ISG expression (RT-PCR) in peritoneal cells after clodronate-liposome or PBS treatment. Each bar represents the mean of 5 animals and error bars indicate s.d. * p < 0.05 (Student’s t-test).
To confirm that Ly6Chi monocytes were major producers of IFN-I, we asked whether their depletion abolishes the interferon signature. Treatment with clodronate-containing liposomes (clo-lip) effectively eliminates monocytes in vivo (13). Indeed, a single dose of clo-lip i.p. was sufficient to eliminate about 80% of peritoneal Ly6Chi monocytes in mice pretreated with TMPD (Fig. 3C). There were also slight reductions in the number of DCs and lymphocytes (not shown) whereas the number of CD11b+ Ly6Cmid Ly6G+ granulocytes was unaffected by clo-lip treatment.
Concomitant with the depletion of Ly6Chi monocytes, the expression of IFN-α, IFN-β, and ISGs was drastically reduced (Fig. 3D, E). Similarly, the expression of TNF-α, which was highly expressed by Ly6Chi monocytes, also diminished upon their depletion. The expression of IL-12, which was expressed mainly by the negative cell fraction comprised of lymphocytes and DCs, did not change significantly after clo-lip treatment (Fig. 3E). The effect of clo-lip was transient as the number of Ly6Chi monocytes and the expression of ISGs returned to pre-treatment levels after four days (not shown).
TMPD-induced IFN-I production is not dependent on DCs
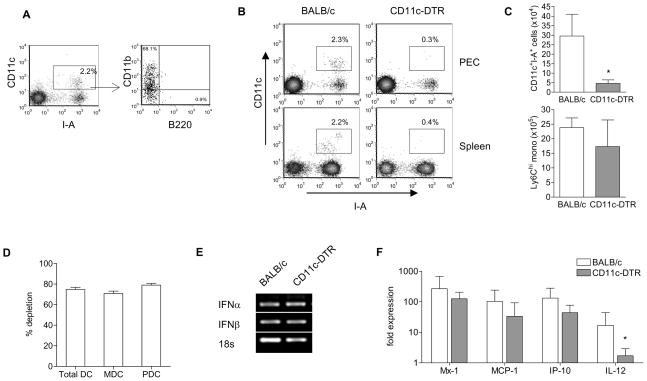
Plasmacytoid DCs (PDCs) are capable of secreting large amounts of IFN-I during viral infection and are thought to be primary interferon producers in SLE (17, 19). In the peritoneal cavity of TMPD-treated animals, CD11c+ I-A+ DCs comprised of ~ 2% of the infiltrating inflammatory cells. Most peritoneal DCs expressed CD11b but not B220 (Fig. 4A), consistent with the phenotype of myeloid DCs (MDCs). However, PDCs may home to other secondary lymphoid tissue following activation (16, 17). To elucidate the extent to which DCs contribute to IFN-I production in the TMPD model, we utilized transgenic mice carrying a simian diphtheria toxin receptor under the control of the CD11c promoter (CD11c-DTR)(10). Injection of diphtheria toxin (DT) rapidly ablates both PDCs and MDCs systemically in CD11c-DTR mice whereas wild-type mice are unaffected by the toxin (10).
Figure 4. Dendritic cells are not required for IFN-I production induced by TMPD.
A) Flow cytometry of peritoneal DCs after TMPD treatment. Box indicate CD11c+ I-A+ DCs. B) Depletion in CD11c-DTR mice 2 days after diphtheria toxin (DT) injection. Box indicates CD11c+ I-A+ DCs. C) Quantification of peritoneal dendritic cells and Ly6Chi monocytes. D) Quantification of splenic DC depletion. MDCs were defined as CD11chi I-A+ CD11b+ cells and PDCs were defined as CD11c+ B220+ PDCA-1+. E) IFN-I expression (conventional PCR) and F) ISG expression (RT-PCR) in peritoneal exudates cells. Each bar represents the mean of 6 animals and error bars indicate s.d. * p < 0.05 (Student’s t-test).
Two days following DT injection, TMPD-treated CD11c-DTR mice showed >85% depletion of CD11c+ I-A+ DCs in the peritoneal exudate compared to wild-type controls (Fig. 4B,C). In line with previous reports (11, 20), DC depletion was systemic as splenic MDCs and PDCs were also depleted by 70–80% (Figure 4B,C). In contrast, there was no significant difference in the peritoneal accumulation of Ly6Chi monocytes, granulocytes, and lymphocytes (Fig. 4C and not shown). Both CD11chiCD11b+I-A+ MDCs and CD11c+B220+PDCA-1+ PDCs were depleted to a similar degree in the spleen (Figure 4D) and lymph nodes (not shown). Systemic depletion of DCs did not affect TMPD-induced IFN-I production as the expression of IFN-I and ISGs were unaffected in CD11c-DTR animals (Fig. 4E,F). The expression of TNF-α and iNOS was also unchanged (not shown). In contrast, IL-12 expression was drastically reduced in the absence of DCs (Fig. 4F), consistent with the cell sorting experiment (Fig. 3E). Taken together, the data indicate that DCs were the primary source of IL-12 but not IFN-I.
We also tried to deplete PDCs using the recently described PDC-specific antibody 120G8 (21). Treatment with 120G8 i.p. resulted in ~70% depletion of splenic PDCs after 24 hr, comparable to the levels seen in CD11c-DTR mice. However, peritoneal Ly6Chi monocytes and T lymphocytes were also reduced by >50% (not shown). While the antigen bound by 120G8 and PDCA-1 is normally expressed on PDCs, its expression can be induced by IFN-I in other cell types (21, 22). Indeed, elevated IFN-I production in TMPD mice is associated with the expression of this PDC antigen on Ly6Chi monocytes, granulocytes and T lymphocytes (not shown), making selective antibody-mediated depletion of PDCs unfeasible in this model.
Accumulation of Ly6Chi monocytes is associated with autoantibody production
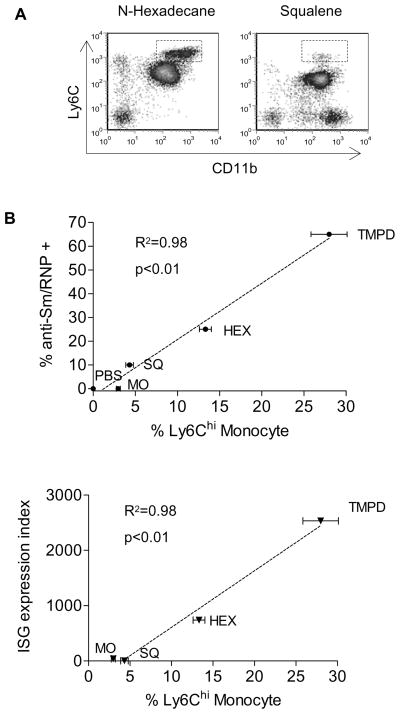
Expression of ISGs is associated with autoantibodies against Sm/RNP in SLE patients (4, 5). In the TMPD model, 60–70% of treated BALB/c mice exhibit anti-Sm/RNP antibodies after 4–6 months vs. 0% of IFN-I receptor deficient mice (9). These autoantibodies either appear less frequently or are completely absent in mice treated with other adjuvant oils such as n-hexadecane, squalene, or mineral oil (23). Interestingly, treatment with n-hexadecane (which induces anti-Sm/RNP in about 25% of treated animals) elicited the accumulation of Ly6Chi monocytes, albeit the response was milder than with TMPD, whereas squalene (which induces a weak anti-Sm/RNP response in <10% of treated animals) recruited mostly mature monocytes/macrophages and few Ly6Chi monocytes, resembling the pattern seen with MO (Fig. 5A). Since Ly6C hi monocytes were the major source of IFN-I in TMPD-treated mice, we examined whether the frequency of anti-Sm/RNP autoantibodies correlates with the number of these cells. Indeed, the adjuvant oils’ ability to elicit anti-Sm/RNP antibodies was highly correlated (r2=0.98) with the accumulation of Ly6Chi monocytes (Fig. 5B). Numbers of Ly6Chi monocytes also correlated with ISG expression (Fig. 5C), supporting our finding that these cells are major IFN-I producers. In contrast, the recruitment of granulocytes was similar among all treatment groups and more DCs were present in the PECs following MO or squalene treatment than TMPD (not shown).
Figure 5. Accumulation of Ly6Chi monocytes is correlated with the frequency of anti-Sm/RNP autoantibodies and upregulation of ISG expression.
A) Flow cytometry of peritoneal cells in wild-type 129Sv mice treated with n-hexadecane (2 weeks) or squalene (2 weeks). Boxes indicate Ly6Chi immature monocytes. Correlation of the levels of Ly6Chi monocyte accumulation with B) the frequency of anti-Sm/RNP autoantibodies and C) ISG expression induced by TMPD, n-hexadecane (HEX), squalene (SQ), and mineral oil (MO) and PBS. Autoantibody frequencies represent the mean of >10 mice per group from previous studies. Percentage of Ly6Chi monocytes and ISG expression index represent the mean of 4 mice. Error bars indicate s.d. ISG expression index was calculated as follows: mean relative expression of (Mx-1 + MCP + IP-10)/3.
Surface expression of Ly6C on monocytes is not IFN-I-dependent
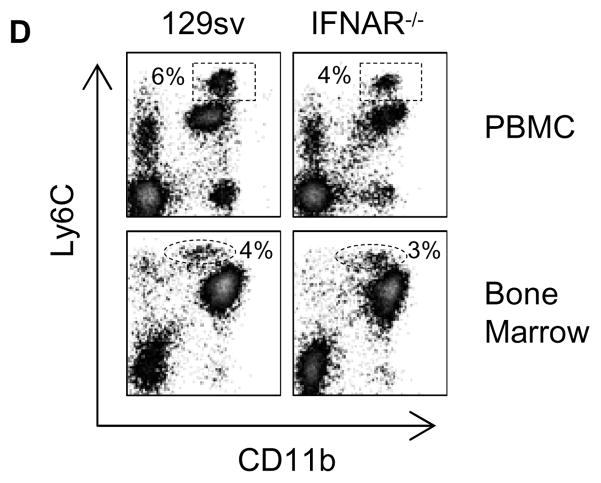
Since Ly6C is an interferon-inducible gene, it is possible that IFN-I production triggered by TMPD treatment contributes to the Ly6Chi monocyte phenotype. To address this issue, we analyzed monocyte subsets in IFN-I receptor-deficient (IFNAR−/−) mice. Compared to wild type controls, untreated IFNAR−/− mice showed similar levels of Ly6Chi and Ly6C− monocytes in the circulation, accounting for approximately 2 and 5% of PBMC, respectively (not shown). Two weeks following TMPD treatment, IFNAR−/− mice also displayed increased numbers of circulating Ly6Chi monocytes, albeit the response was milder than that of wild type controls (Fig. 6). Importantly, Ly6Chi and Ly6C− monocytes, as well as the Ly6Cmid granulocyte populations in the peripheral blood were clearly discernible even in the absence of IFN-I signaling. The number of bone marrow precursors of Ly6Chi monocytes was also comparable between wild-type and IFNAR−/− mice (Fig. 6). These data suggest that although IFN-I have been shown to induce Ly6C expression, the Ly6Chi phenotype of the immature monocyte population in naïve or TMPD-treated mice was not dependent on IFN-I signaling.
Figure 6. Ly6C expression on immature monocytes is not dependent on IFN-I signaling.
Flow cytometry analysis of monocyte subsets in the peripheral blood (upper) and bone marrow (lower) of TMPD-treated wild-type 129sv and IFN-I receptor-deficient mice. Boxes and ovals indicate peripheral blood Ly6Chi monocytes and bone marrow Ly6Chi monocyte precursors, respectively.
Discussion
Elevated serum IFN-I was first associated with SLE over two decades ago (24). Recent studies using microarrays and RT-PCR further defined a panel of ISGs over-expressed in the peripheral blood of SLE patients (2–4). While this interferon signature has been linked to disease activity, kidney involvement, and autoantibody levels, the source of IFN-I responsible for the interferon signature remains speculative. This issue has been difficult to address using animal models as most of them do not exhibit upregulation of IFN-I.
We recently reported that murine lupus induced by TMPD is associated with elevated IFN-I production and ISG expression (8). Disruption of IFN-I signaling completely abrogates the development of kidney disease and the onset of autoantibody production (9). In this study, we show that Ly6Chi monocytes are a major source of IFN-I in the TMPD model of lupus. Upregulation of IFN-I and ISGs occurred long before the clinical manifestations of lupus and coincided with an accumulation of Ly6Chi monocytes. These immature monocytes expressed large amounts of IFN-I and the “interferon signature” was rapidly abolished upon depletion of these cells by clo-lip. Moreover, the abundance of Ly6Chi monocytes was highly associated with the ability of various adjuvant oils to induce anti-Sm/RNP antibodies. In contrast, systemic depletion of DCs did not alter IFN-I production triggered by TMPD.
While Ly6Chi monocytes were found in the inflammatory infiltrate one day after thioglycollate (12, 13) or mineral oil administration, the response was transient as the number of these cells was drastically reduced after 72 hours. The recruitment and accumulation of Ly6Chi monocytes seen in TMPD-treated animals, on the other hand, persisted for as long as 4 months after treatment. A recent study shows that the egression of Ly6Chi monocytes from the bone marrow in response to Listeria monocytogenes infection is dependent on the interaction between the monocyte attractant CCL2/MCP-1 and its receptor CCR2 (25). Interestingly, as confirmed here, MCP-1 is an ISG that is induced by TMPD treatment, raising the possibility that the production of interferon-inducible chemokines may drive the recruitment of Ly6Chi monocytes. This is relevant to human SLE as elevated serum levels of MCP-1 have been associated with the interferon signature (3). How TMPD-treatment maintains the infiltrating monocytes in an immature state is unknown, but a defect intrinsic to the cells is unlikely as Ly6Chi monocytes spontaneously acquired a mature macrophage-like phenotype (Ly6C− F4/80+ I-A+) in vitro (not shown). The immaturity of Ly6Chi monocytes may be important to their ability to produce IFN-I as their mature counterparts elicited by mineral oil displayed significantly lower levels. Analogously, PDCs also are better equipped to secrete large amounts of IFN-I when immature (26).
Prolonged elevation of IFN-I may promote DC maturation (27), T cell survival (28), isotype class-switching (29), and B cell maturation into plasma cells (30), culminating in the loss of tolerance and autoantibody production. It is noteworthy that while IFN-I is essential to TMPD-induced lupus (9), other factors such as IL-12 and IFN-γ play important roles since autoantibody production and kidney disease development are reduced in deficient mice (31, 32).
Our findings challenge the conventional view that PDCs are solely responsible for the elevated IFN-I in SLE. In TMPD-treated mice, the vast majority of the increased IFN-I production is derived from the 20–30% of peritoneal cells that are Ly6Chi CD11b+ B220− CD11c− monocytes. Although, PDCs can synthesize up to 1000-fold more IFN-α than most other cell types (33), the number of circulating PDCs is drastically reduced in SLE patients (4, 34). While it is plausible that PDCs home to tissues following activation, a view supported by the presence of these cells in lupus skin lesions (35), there is little direct evidence connecting tissue PDCs to the excess serum IFN-I seen in SLE patients. Indeed, as tissue (e.g. spleen) PDCs were greatly reduced by DT treatment of CD11c-DTR mice, despite little effect on ISG expression, it is unlikely that PDCs were the main source of IFN-I in TMPD-induced lupus. Also, the elevation of IFN-I in this model occurs within two weeks of treatment, long before the appearance of autoantibodies against dsDNA and snRNP and formation of immune complexes. The proposed mechanism of IFN-I induction in PDCs by endogenous nucleic acids present in immune complexes, therefore, is not a likely explanation of our findings (19). Nevertheless, PDCs may function to amplify IFN-I production once autoantibodies and immune complexes develop. MDCs also play a role in TMPD-induced lupus as they are the major source of IL-12, a cytokine critical for the development of kidney disease in this model (31). The role of DCs in human SLE is more difficult to assess as targeted DC therapy is not yet available.
It is noteworthy that Ly6Chi monocytes also have been recently reported to play a role in atherosclerosis (36). Therefore, these monocyte-like interferon producing cells could play a role in the premature atherosclerosis seen in SLE patients. A CD14hiCD16− monocyte subset (also called “classical monocytes”) is the equivalent of murine Ly6Chi monocytes in terms of migratory properties (15). Whether this or other monocyte subsets produce IFN-I in human SLE warrants detailed investigation.
Acknowledgments
This work was supported by research grants R01-AR44731 from the US Public Health Service and by generous gifts from Lupus Link, Inc. (Daytona Beach, FL) and Mr. Lewis M. Schott to the UF Center for Autoimmune Disease. P.Y.L and J.S.W. are NIH T32 trainees (DK07518 and AR007603).
The work was supported with resources and the use of facilities at the Malcolm Randall VA Medical Center, Gainesville, FL. We thank Neal Benson and the UF Flow Cytometry Core for assistance with cell sorting.
References
- 1.Reeves WH, Narain S, Satoh M. Autoantibodies in systemic lupus erythematosus. In: Koopman LWWJ, Moreland, editors. Arthritis and Allied Conditions. Lippincott Williams & Wilkins; Philadelphia, PA: 2004. pp. 1497–1521. [Google Scholar]
- 2.Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, Shark KB, Grande WJ, Hughes KM, Kapur V, Gregersen PK, Behrens TW. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A. 2003;100:2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, Pascual V. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197:711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhuang H, Narain S, Sobel E, Lee PY, Nacionales DC, Kelly KM, Richards HB, Segal M, Stewart C, Satoh M, Reeves WH. Association of anti-nucleoprotein autoantibodies with upregulation of Type I interferon-inducible gene transcripts and dendritic cell maturation in systemic lupus erythematosus. Clin Immunol. 2005 doi: 10.1016/j.clim.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Kirou KA, Lee C, George S, Louca K, Peterson MG, Crow MK. Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 2005;52:1491–1503. doi: 10.1002/art.21031. [DOI] [PubMed] [Google Scholar]
- 6.Ronnblom LE, Alm GV, Oberg KE. Autoimmunity after alpha-interferon therapy for malignant carcinoid tumors. Ann Intern Med. 1991;115:178–183. doi: 10.7326/0003-4819-115-3-178. [DOI] [PubMed] [Google Scholar]
- 7.Satoh M, Reeves WH. Induction of lupus-associated autoantibodies in BALB/c mice by intraperitoneal injection of pristane. J Exp Med. 1994;180:2341–2346. doi: 10.1084/jem.180.6.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nacionales DC, Kelly KM, Lee PY, Zhuang H, Li Y, Weinstein JS, Sobel E, Kuroda Y, Akaogi J, Satoh M, Reeves WH. Type I interferon production by tertiary lymphoid tissue developing in response to 2,6,10,14-tetramethyl-pentadecane (pristane) Am J Pathol. 2006;168:1227–1240. doi: 10.2353/ajpath.2006.050125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nacionales DC, Kelly-Scumpia KM, Lee PY, Weinstein JS, Lyons R, Sobel E, Satoh M, Reeves WH. Deficiency of the type I interferon receptor protects mice from experimental lupus. Arthritis Rheum. 2007;56:3770–3783. doi: 10.1002/art.23023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scumpia PO, McAuliffe PF, O’Malley KA, Ungaro R, Uchida T, Matsumoto T, Remick DG, Clare-Salzler MJ, Moldawer LL, Efron PA. CD11c+ dendritic cells are required for survival in murine polymicrobial sepsis. J Immunol. 2005;175:3282–3286. doi: 10.4049/jimmunol.175.5.3282. [DOI] [PubMed] [Google Scholar]
- 12.Sunderkotter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, Leenen PJ. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 13.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 14.Cook AD, Braine EL, Hamilton JA. The phenotype of inflammatory macrophages is stimulus dependent: implications for the nature of the inflammatory response. J Immunol. 2003;171:4816–4823. doi: 10.4049/jimmunol.171.9.4816. [DOI] [PubMed] [Google Scholar]
- 15.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 16.Asselin-Paturel C, Boonstra A, Dalod M, Durand I, Yessaad N, Dezutter-Dambuyant C, Vicari A, O’Garra A, Biron C, Briere F, Trinchieri G. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat Immunol. 2001;2:1144–1150. doi: 10.1038/ni736. [DOI] [PubMed] [Google Scholar]
- 17.Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, Colonna M. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 18.Spangrude GJ, Aihara Y, Weissman IL, Klein J. The stem cell antigens Sca-1 and Sca-2 subdivide thymic and peripheral T lymphocytes into unique subsets. J Immunol. 1988;141:3697–3707. [PubMed] [Google Scholar]
- 19.Ronnblom L, Eloranta ML, Alm GV. The type I interferon system in systemic lupus erythematosus. Arthritis Rheum. 2006;54:408–420. doi: 10.1002/art.21571. [DOI] [PubMed] [Google Scholar]
- 20.Kassim SH, Rajasagi NK, Zhao X, Chervenak R, Jennings SR. In vivo ablation of CD11c-positive dendritic cells increases susceptibility to herpes simplex virus type 1 infection and diminishes NK and T-cell responses. J Virol. 2006;80:3985–3993. doi: 10.1128/JVI.80.8.3985-3993.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asselin-Paturel C, Brizard G, Pin JJ, Briere F, Trinchieri G. Mouse strain differences in plasmacytoid dendritic cell frequency and function revealed by a novel monoclonal antibody. J Immunol. 2003;171:6466–6477. doi: 10.4049/jimmunol.171.12.6466. [DOI] [PubMed] [Google Scholar]
- 22.Blasius AL, Giurisato E, Cella M, Schreiber RD, Shaw AS, Colonna M. Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation. J Immunol. 2006;177:3260–3265. doi: 10.4049/jimmunol.177.5.3260. [DOI] [PubMed] [Google Scholar]
- 23.Kuroda Y, Akaogi J, Nacionales DC, Wasdo SC, Szabo NJ, Reeves WH, Satoh M. Distinctive patterns of autoimmune response induced by different types of mineral oil. Toxicol Sci. 2004;78:222–228. doi: 10.1093/toxsci/kfh063. [DOI] [PubMed] [Google Scholar]
- 24.Hooks JJ, Moutsopoulos HM, Geis SA, Stahl NI, Decker JL, Notkins AL. Immune interferon in the circulation of patients with autoimmune disease. N Engl J Med. 1979;301:5–8. doi: 10.1056/NEJM197907053010102. [DOI] [PubMed] [Google Scholar]
- 25.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 26.Kadowaki N, Antonenko S, Lau JY, Liu YJ. Natural interferon alpha/beta-producing cells link innate and adaptive immunity. J Exp Med. 2000;192:219–226. doi: 10.1084/jem.192.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;294:1540–1543. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 28.Marrack P, Kappler J, Mitchell T. Type I interferons keep activated T cells alive. J Exp Med. 1999;189:521–530. doi: 10.1084/jem.189.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Bon A, Schiavoni G, D’Agostino G, Gresser I, Belardelli F, Tough DF. Type i interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity. 2001;14:461–470. doi: 10.1016/s1074-7613(01)00126-1. [DOI] [PubMed] [Google Scholar]
- 30.Finkelman FD, Svetic A, Gresser I, Snapper C, Holmes J, Trotta PP, Katona IM, Gause WC. Regulation by interferon alpha of immunoglobulin isotype selection and lymphokine production in mice. J Exp Med. 1991;174:1179–1188. doi: 10.1084/jem.174.5.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calvani N, Satoh M, Croker BP, Reeves WH, Richards HB. Nephritogenic autoantibodies but absence of nephritis in Il-12p35-deficient mice with pristane-induced lupus. Kidney Int. 2003;64:897–905. doi: 10.1046/j.1523-1755.2003.00178.x. [DOI] [PubMed] [Google Scholar]
- 32.Richards HB, Satoh M, Jennette JC, Croker BP, Yoshida H, Reeves WH. Interferon-gamma is required for lupus nephritis in mice treated with the hydrocarbon oil pristane. Kidney Int. 2001;60:2173–2180. doi: 10.1046/j.1523-1755.2001.00045.x. [DOI] [PubMed] [Google Scholar]
- 33.Coccia EM, Severa M, Giacomini E, Monneron D, Remoli ME, Julkunen I, Cella M, Lande R, Uze G. Viral infection and Toll-like receptor agonists induce a differential expression of type I and lambda interferons in human plasmacytoid and monocyte-derived dendritic cells. Eur J Immunol. 2004;34:796–805. doi: 10.1002/eji.200324610. [DOI] [PubMed] [Google Scholar]
- 34.Scheinecker C, Zwolfer B, Koller M, Manner G, Smolen JS. Alterations of dendritic cells in systemic lupus erythematosus: phenotypic and functional deficiencies. Arthritis Rheum. 2001;44:856–865. doi: 10.1002/1529-0131(200104)44:4<856::AID-ANR142>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 35.Farkas L, Beiske K, Lund-Johansen F, Brandtzaeg P, Jahnsen FL. Plasmacytoid dendritic cells (natural interferon- alpha/beta-producing cells) accumulate in cutaneous lupus erythematosus lesions. Am J Pathol. 2001;159:237–243. doi: 10.1016/s0002-9440(10)61689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]