Abstract
An effective oxidative protocol for the liberation of ketones from SAMP hydrazones employing peroxyselenous acid under aqueous buffered conditions (pH 7) has been developed. The procedure proceeds without epimerization of adjacent stereocenters or dehydration, respectively, in representative SAMP alkylation and aldol reaction adducts.
Keywords: SAMP, oxidative cleavage, aldol reactions, ketones, SeO2
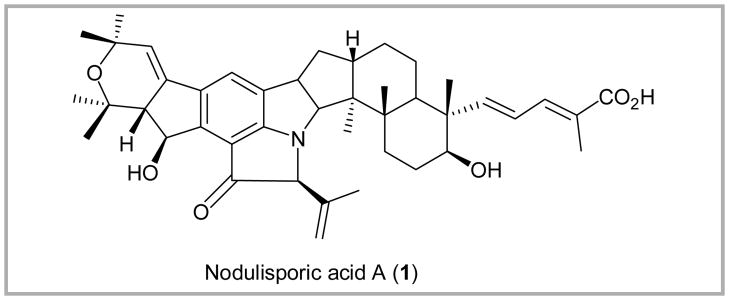
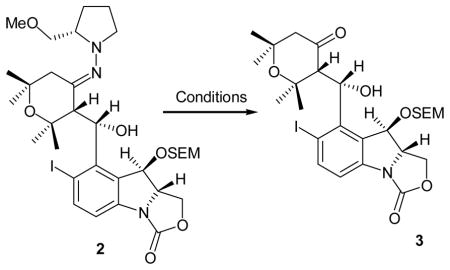
(S)- and (R)- Amino-2-methoxypyrrolidines (SAMP and RAMP), effective chiral auxiliaries introduced by the Enders group,1 have found wide use in asymmetric alkylation and aldol reactions.2 Consequently, a large number of methods have been developed to liberate the resultant aldehydes and ketones from the SAMP/RAMP hydrazone products.3 In conjunction with an ongoing synthetic program directed towards the total synthesis of (+)-nodulisporic acid A (1, Figure 1), a wide variety of known oxidative, hydrolytic, or reductive methods were explored to liberate ketone 3 from advanced SAMP intermediate 2, albeit with limited success (Table 1 entries 1–12). We eventually discovered that peroxyselenous acid, generated in situ from SeO2 and 30% H2O2 (1:4 equiv) was a superior oxidant for the removal of the chiral auxiliary in 2 accompanied by a small amount of epimerization at the α-position of ketone 3 (entry 13).
Figure 1.
Structure of Nodulisporic acid A.
Table 1.
Attempted conditions to cleave SAMP hydrazone 2.
 | ||
|---|---|---|
| Entry | Conditions | Observations |
| 1 | Oxalic acid, Et2O at 23 °C, 15 h | Epimerization |
| 2 | CuCl2, THF/H2O at 23 °C, 15 h | Epimerization |
| 3 | SnCl2, Pd(OAc)2 DMF/H2O at 23 °C, 15 h |
No Reaction |
| 4 | SnCl2, Pd(OAc)2 DMF/H2O at 50 °C, 15 h |
Epimerization |
| 5 | SnCl2, DME/H2O at 23 °C, 15 h | No Reaction |
| 6 | SnCl2, DME/H2O at 50 °C, 15 h | Epimerization |
| 7 | O3, CH2Cl2 at −78 °C, 1 min. | 21% |
| 8 | NaBO3, AcOH, 23 °C, 2 h | Decomposition |
| 9 | NaBO3, pH 7 buffer/t-BuOH at 23 °C 5 h | Decomposition |
| 10 | MMPP, pH 7 buffer/MeOH at 23 °C, 24 h | No Reaction |
| 11 | 30%°C H2O2, pH 7 buffer/MeOH at 23 to 70 °C, 19 h | Decomposition |
| 12 | NaIO4, THF/H2O/pH 7 buffer at 23 °C, 15 h | Decomposition |
| 13 | 30%H2O2/SeO2, MeOH, 48 h at 23 °C | 76% |
| 14 | 30% H2O2/SeO2, MeOH/pH 7 buffer, 24 h at 23 °C | 91% |
Pleasingly, the epimerization problem could be alleviated simply by the introduction of a pH buffer 7 (entry 14). Importantly, no epimerization or retro aldol fragmentation, which was observed with several of the alternative protocols, occurred under these optimized conditions. To the best of our knowledge, this report presents the first examples exploiting SeO2 and H2O2 under buffered conditions for the oxidative deprotection of ketone-derived hydrazones.
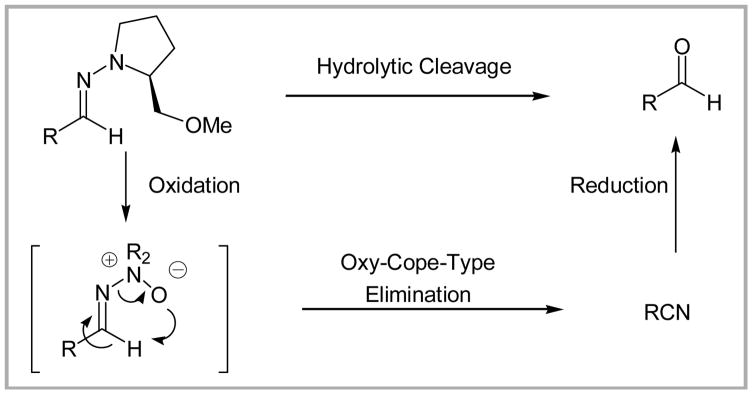
Previous reports have, however, recorded the oxidative cleavage of aldehyde SAMP hydrazones to furnish the corresponding nitriles, via an oxy-Cope-like elimination (Scheme 1),4 with oxidants such as m-CPBA,5 MMPP (magnesium monoperoxyphthalate), SeO2, or 2-nitrobezeneselenic acid with H2O2,6 and H2O2.7 A similar oxidative fragmentation is of course not an option with SAMP ketone hydrazones.
Scheme 1.
Nitrile formation from aldehyde-derived hydrazones.
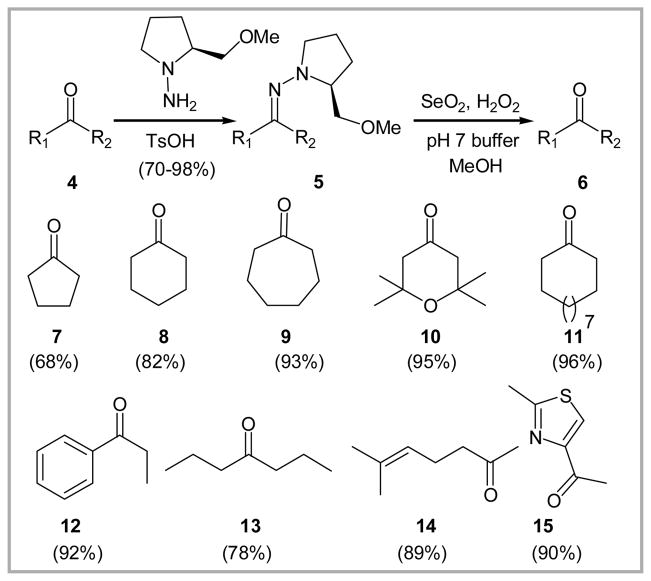
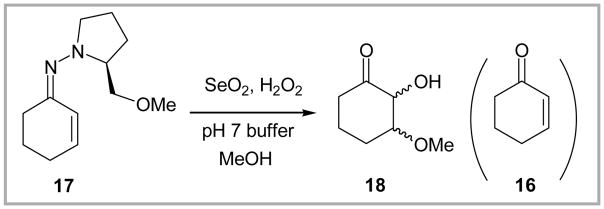
To explore the scope and viability of the pH 7 buffered peroxyselenous acid conditions, a series of ketone SAMP hydrazones were readily prepared from simple ketones 7–16 (Figure 2)8 using SAMP hydrazine and a catalytic amount of TsOH in cyclohexane at reflux; yields ranged from 70–98%. Application of the pH 7 buffered SeO2/H2O2 protocol, optimized during the (+)-nodulisporic acid A synthetic program, regenerated the corresponding ketones 7–15 in 68–96% yields.9 Liberation of cyclohexenone 16 from SAMP hydrazone 17 however was not successful. Instead, an epimeric mixture of 2-hydroxy-3-methoxyclohexanone 18 was isolated in 85% yield (Scheme 2).
Figure 2.
Oxidative cleavage of SAMP hydrazones to simple ketones.
Scheme 2.
Oxidative hydrolysis of the SAMP hydrazone generated from cyclohexenone.
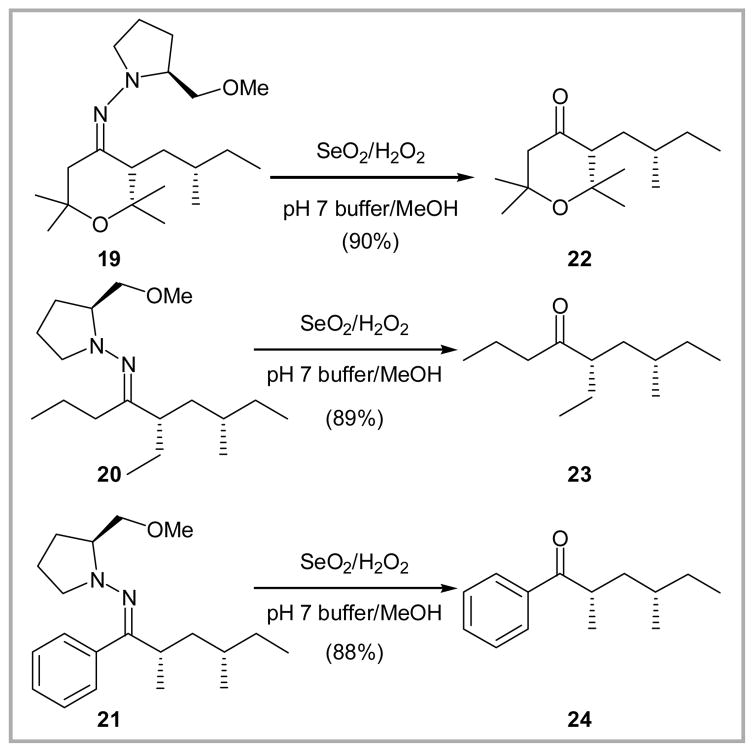
We next turned our attention toward to SAMP hydrazones possessing an α-stereogenic center. The requisite substrates were readily prepared by alkylation of a series of ketone SAMP hydrazones with (S)-(+)-1-iodo-2-methylbutane (Scheme 3);10 yields for the alkylated-SAMP hydrazones (19–21) were again excellent (90–96%). Stereochemical assignments at the α-center were based on the Enders precedent.11 Treatment of the hydrazones with SeO2 and H2O2, again employing an aqueous buffer (pH 7), led to clean removal of the SAMP moiety to furnish ketones (22–24) in 88–90% yield. Importantly, no epimerization (>20:1, 500 MHz NMR) at the α-center was observed.
Scheme 3.
Cleavage of SAMP hydrazones to regenerate chiral ketones.
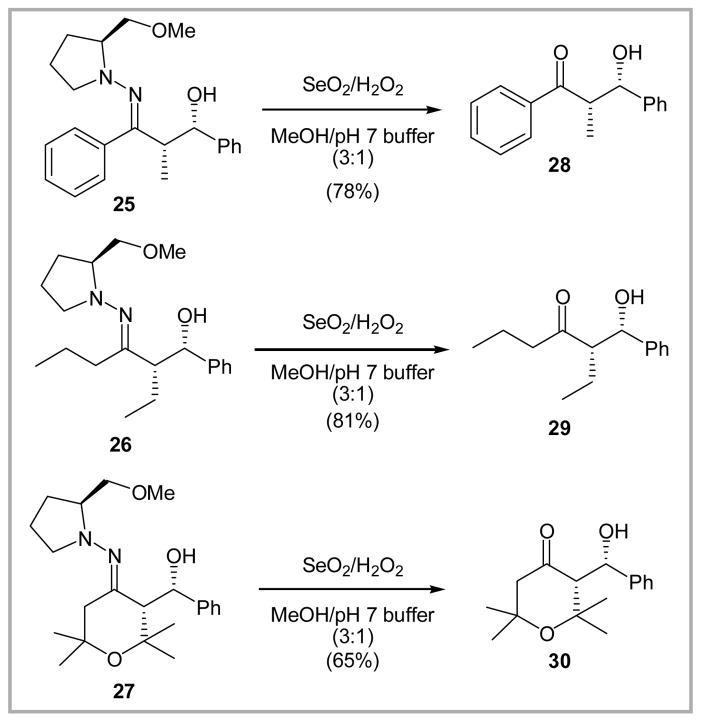
In addition to the SAMP alkylation products, a third series of hydrazones was examined involving the products derived from an aldol reaction with benzaldehyde (cf. 25–27).12 As these aldol products are generally sensitive to acid, we were not surprised initially to observe significant elimination (i.e., dehydration) and/or retro-aldol fragmentation. Pleasingly, such side reactions could be suppressed by increasing the amount of pH 7 buffer (i.e., from 1:18 to 1:3 v/v; buffer: methanol) to afford β-hydroxy ketones 28–30 in 65–81% yield (Scheme 4).13
Scheme 4.
Oxidative hydrolysis of SAMP aldol products to regenerate β-hydroxy ketones.
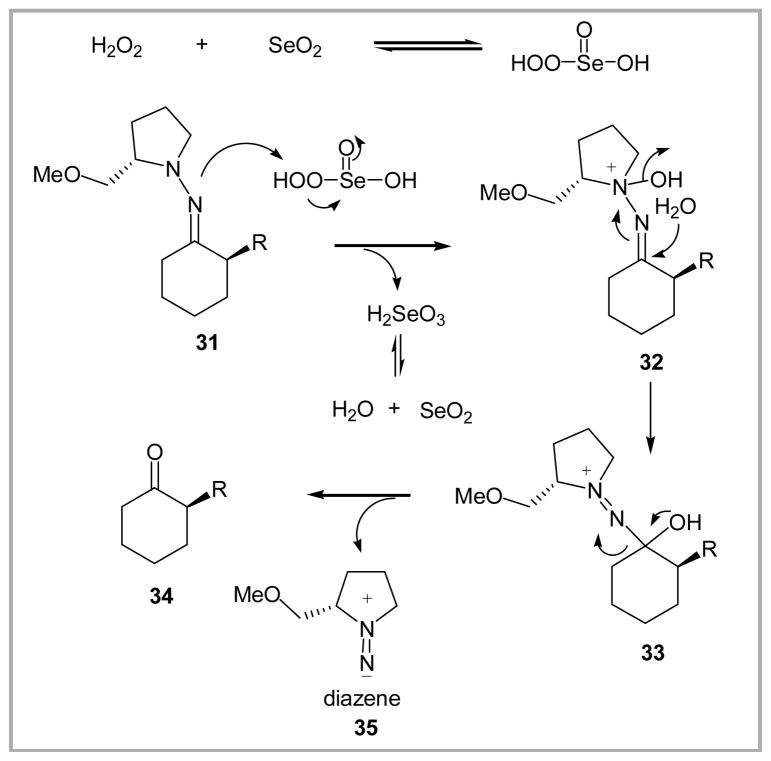
A mechanistic picture of the pH 7 buffered oxidative hydrolysis using SeO2 and H2O2 is proposed in Scheme 5. After initial formation of peroxyselenous acid, oxidation of the pyrrolidine nitrogen in 31 is envisioned to generate intermediate 32, thereby activating the hydrazone toward hydrolysis. Addition of water followed by fragmentation would then deliver the ketone 34 and diazene 35 as a byproduct.
Scheme 5.
Proposed mechanism for the oxidative hydrolysis of SAMP hydrazones using peroxyselenous acid.
In summary, an efficient method to regenerate ketones from SAMP ketone hydrazones employing SeO2 and H2O2 under buffered conditions has been developed. Aldol hydrazone adducts derived from the SAMP hydrazone require additional buffer to suppress side reactions. Given the scope of this protocol, this method holds promise as an effective and mild alternative to the more conventional methods to regenerate ketones from SAMP hydrazones.
Acknowledgments
Support was provided by the National Institutes of Health (Institute of General Medical Sciences) through grant GM-29028 and the University of Pennsylvania. We thank Drs. G. Furst, J. Gu, and R. Kohli (University of Pennsylvania) for assistance in obtaining NMR spectra and high-resolution mass spectra, respectively.
References
- 1.Enders D, Eichenauer H. Angew Chem Int Ed. 1976;15:549. [Google Scholar]
- 2.Job A, Janeck CF, Bettray W, Peter R, Enders D. Tetrahedron. 2002;58:2253. and references therein. [Google Scholar]
- 3.Enders D, Wortmann L, Peters R. Acc Chem Res. 2000;33:157. doi: 10.1021/ar990062y. [DOI] [PubMed] [Google Scholar]
- 4.Enders D, Plant A. Synlett. 1994:1054. [Google Scholar]
- 5.Said SB, Skarzewski J, Mlochowski J. Synthesis. 1989:223. [Google Scholar]
- 6.Stupp SI, Son S, Li LS, Lin HC, Keser M. J Am Chem Soc. 1995;117:5212. [Google Scholar]
- 7.Eichenauer H, Friedrich E, Lutz W, Enders D. Angew Chem Int Ed. 1978;17:206. [Google Scholar]
- 8.General procedure for the synthesis of hydrazones: A mixture of SAMP (0.04 mmol), ketone (0.04 mmol) and p-toluenesulfonic acid (0.004 mmol) was heated at reflux in cyclohexane (1 mL) overnight. The mixture was then cooled to room temperature, neutralized with saturated NaHCO3 (3 mL) and the aqueous layer was extracted with ethyl acetate (3 × 5 mL). The combined organic layers were dried over Na2SO4 and concentrated. The residue was purified by flash chromatography to provide the desired hydrazone (70–98%).
- 9.General procedure for synthesis of ketones 7–15 and 22–24: To a room temperature solution of hydrazone (0.18 mmol) and SeO2 (0.14 mmol) in MeOH (2.3 mL) was added pH 7 phosphate buffer (0.066 mL) followed by 30% H2O2 (0.066 mL). After completion of the hydrolysis reaction, saturated NaHCO3 (3 mL) was added and the aqueous layers were extracted with pentane (3 × 3 mL). The combined organic layers were combined, dried over Na2SO4 and concentrated. The residue was purified via flash chromatography to provide the corresponding ketone in 68–96% yield.
- 10.General procedure for the synthesis of alkylated SAMP hydrazones 19–21: To a solution of the corresponding hydrazone (0.26 mmol) in THF (2 mL) at −78 °C was added t-BuLi (1.6 M in pentane, 0.39 mmol). The mixture was kept at this temperature for 2 h before cooling to −100 °C. (S)-(+)-1-Iodo-2-methylbutane (0.52 mmol) was then added via syringe, the solution stirred at −100 °C for 0.5 h, and then at −78 °C for 2 h. The reaction was quenched with saturated NH4Cl (3 mL). The aqueous layers were extracted with ethyl ether (3 × 5 mL) and the combined organic layers were dried over Na2SO4, concentrated and the residue was purified by flash chromatography to furnish the alkylated hydrazone in 90–96% yield.
- 11.Enders D, Bockstiegel B. Synthesis. 1989:493. [Google Scholar]
- 12.General procedure for the synthesis of SAMP aldol products 25–27: To a solution of hydrazone (0.18 mmol) in THF (1.2 mL) at −78 °C was added t-BuLi (1.6 M in pentane, 0.18 mmol). The mixture was maintained at this temperature for 2 h before cooling to −100 °C. Benzaldehyde (0.36 mmol) was then added via syringe, the solution stirred at −100 °C for 0.5 h, and then at −78 °C for 2 h. The reaction was quenched with saturated NH4Cl (3 mL), the aqueous layer extracted with ethyl ether (3 × 5 mL), and the combined organic layers were dried over Na2SO4 and concentrated. The residue was purified by flash chromatography to furnish aldol products in 68–96% yield.
- 13.General procedure for the synthesis of β-hydroxy ketones 28–30: To a room temperature solution of the corresponding hydrazone (0.03 mmol) and SeO2 (0.045 mmol) in MeOH (0.45 mL) was added pH 7 phosphate buffer (0.15 mL) followed by 30% H2O2 (0.015 mL). After completion, saturated NaHCO3 (2 mL) was added to the mixture and the aqueous layers were extracted with ethyl acetate (2 × 3 mL). The combined organic layers were dried over Na2SO4 and concentrated. The residue was purified by flash chromatography (SiO2 deactivated with 18% H2O) to provide β-hydroxy ketones (65–81%).