Abstract
One promising option for transdermal delivery of protein- and nucleic acid-based pharmacologic agents involves the use of microneedles. However, microneedle-generated pores may allow microorganisms to penetrate the stratum corneum layer of the epidermis and cause local or systemic infection. In this study, microneedles with antimicrobial functionality were fabricated using two photon polymerization-micromolding and pulsed laser deposition. The antibacterial activity of the silver-coated organically modified ceramic (Ormocer®) microneedles was demonstrated using an agar diffusion assay. Human epidermal keratinocyte (HEK) viability on the Ormocer® surfaces coated with silver was similar to that on uncoated Ormocer® surfaces. This study indicates that coating microneedles with silver thin films using pulsed laser deposition is a useful and novel approach for creating microneedles with antimicrobial functionality.
Keywords: silver, antimicrobial, microneedle, transdermal drug delivery
Microneedles are small-scale medical devices (<1 mm in length) used for delivery of protein-based pharmacologic agents, nucleic acid-based pharmacologic agents, and other pharmacologic agents that are unable to be administered in oral form [1]. These devices create small pores in the stratum corneum layer of the epidermis of the skin that enable the delivery of pharmacologic agents into deeper layers of the skin and into the bloodstream [2]. Unlike conventional hypodermic needles, microneedle administration of a pharmacologic agent does not require medical training. Due to their smaller dimensions, microneedles are associated with less injection site damage and less patient pain than conventional hypodermic needles [1].
One concern that limits microneedle usage is the risk of infection associated with a compromised stratum corneum [3–5]. The stratum corneum layer serves as the outermost limiting barrier that prevents foreign objects, including microorganisms, from entering the body. Pathogenic microorganisms such as Staphylococcus aureus may be transferred from the skin surface by microneedles. One approach to reduce the risk of infection associated with microneedles is to fabricate microneedles that have antimicrobial properties. For example, antimicrobial microneedles may be fabricated by coating microneedles with thin films of antimicrobial materials such as silver. Silver exhibits broad-spectrum antimicrobial properties against viruses, fungi, and bacteria, including Staphylococcus aureus. These properties have been attributed to interruption of DNA replication and disruption of electron transport [3–10]. Silver also serves to reduce local matrix metalloproteinase levels, reduce inflammatory activity in wounds, and facilitate healing in wounds. Recent work by Demling et al. suggests that silver acts to increase re-epithelialization compared to a conventional antimicrobial solution [11–13].
Antimicrobial coatings may be applied to microneedle surfaces using a physical vapor deposition technique known as pulsed laser deposition [14]. Pulsed laser deposition involves vaporization of a solid target using a high energy laser; the average kinetic energy of atomic and molecular species generated by excimer laser ablation is between 100 kT and 1000 kT. In comparison, the equilibrium energy of species generated in thermal deposition techniques is on the order of kT [15, 16]. The high energy of laser-ablated species enhances film adhesion and promotes chemical reactions between the substrate and the film [17]. Pulsed laser deposition is ideally suited for deposition on biomedical polymeric substrates since deposition of many coating materials can take place at room temperature; other methods may involve deposition temperatures close to or above the glass transition temperatures of many biomedical polymers. Thin films prepared using pulsed laser deposition exhibit useful properties for many biomedical applications due to the high adhesion strength and the high density/low porosity nature of deposited films. In this study, microneedle arrays were created from organically-modified ceramic (Ormocer®) materials using a two photon polymerization-micromolding technique. Ormocer® materials are organic-inorganic hybrid materials originally developed by Fraunhofer Institut für Silicatforschung, which containing organically modified silicon alkoxides and organic monomers [1]. Interactions between organic methacrylate groups and inorganic silicon-oxygen-silicon networks create a three-dimensional network. These interactions between the ceramic and polymer components provide Ormocer® materials with chemical and thermal stability as well as prevent separation of Ormocer® material into separate phases. In previous work, Ormocer® surfaces fabricated using two photon polymerization exhibited acceptable cell viability and cell growth profiles against neuroblast-like cells and epithelial cells [18]. In this study, pulsed laser deposition was used to deposit silver thin films on Ormocer® microneedle arrays. An agar diffusion assay was used to determine the antimicrobial activity of silver-coated Ormocer® microneedles.
Five-by-five arrays of out-of-plane microneedles were fabricated with Ormocer® materials using a two photon polymerization-micromolding technique. In the first step, two photon polymerization was used to fabricate a master structure of the microneedle array on a silanized glass coverslip (Figure 1). The microneedle array was fabricated using SR 259 polyethylene glycol dimethacrylate (Sartomer, Paris, France) with 2% weight Irgacure® 369 initiator (Ciba Specialty Chemicals, Basel, Switzerland). Femtosecond laser pulses (60 fs, 300 mW, 780 nm) from a titanium: sapphire laser (Kapteyn-Murnane, Boulder, CO) were focused using a 5 × microscope objective. Three C-843 linear translational stages (Physik Instrumente, Karlsruhe, Germany) were used to direct the laser focus position in three dimensions. A HurrySCAN galvo-scanner (Scanlab AG, Puchheim, Germany) was used to control polymerization of the photosensitive resin in the X-Y plane. Control of the laser was determined by an .STL format drawing of the microneedle. The .STL format drawing that was used to fabricate the microneedle arrays contained microneedles with base diameter values of 200 µm and lengths of 500 µm. In addition, the microneedles demonstrated 500 µm microneedle center-to-microneedle center spacing. Arrays of microneedles enable delivery of pharmacologic agents to be provided over a wider area and at higher rates than solitary needles [19]. In the second step, replication of the microneedle array was performed by means of a micromolding process. The master structure was sputter- coated with gold in order to improve separation of the mold from the master structure. Sylgard® 184 silicone elastomer and curing agent (Dow Corning, Midland, MI) were mixed in a 10:1 weight ratio. This silicone elastomer-curing agent mixture was degassed in vacuum and poured into an aluminum seal, which had been placed over the master microneedle array. The unpolymerized mold was placed under vacuum in order to eliminate residual air pockets in the mold. After degassing, the liquid mold was placed on a hotplate in order to initiate polymerization of the silicone elastomer. The hotplate temperature was increased from 25° C to 100° C over thirty minutes. The hotplate temperature was subsequently maintained at 100° C for an additional thirty minutes. The master structure substrate was then clamped to a table. The silicone mold was attached to a vertical actuator. By raising the vertical actuator, the silicone mold was separated from the polyethylene glycol master structure. In the third step, Ormocer® microneedle arrays were photopolymerized within the silicone mold. Ormocore® (Ormocer b59) (Microresist Technology, Berlin, Germany) is a commercially available Ormocer® material with 1.8% Irgacure 369 photoinitiator (Ciba Specialty Chemicals, Basel, Switzerland). 50 µL of liquid Ormocer® material was placed on the silicone mold. The silicone mold containing the liquid Ormocer® was placed under vacuum in order to remove air pockets and completely fill all mold parts with Ormocer® material. The mold with Ormocer® material was subsequently pressed against a glass slide and attached to the vertical actuator. The construct was subsequently exposed for two minutes to a curing lamp that provides visible and ultraviolet light emission (Thorlabs, Newton, NJ) in order to polymerize the Ormocer® material. The silicone mold and the Ormocer® microneedle array were then separated from one another using the vertical actuator. In addition, silicone culture wells were utilized as molds in order to fabricate cylindrical (6 mm diameter × 1 mm thick) Ormocer® wafers for use in the MTT assay. In the fourth step, silver thin films were grown on Ormocer® microneedle arrays and Ormocer® wafers using pulsed laser deposition. A high-purity silver target was obtained from a commercial source (Alfa Aesar, Ward Hill, MA). A krypton fluoride excimer laser (λ = 248 nm, repetition rate = 10 Hz) was used to ablate the high-purity silver target. The thin films were grown under a background pressure of 5 × 10−6 Torr at room temperature for two minutes. Recent work by Warrender et al. indicates that the deposition rate for silver thin films using a KrF laser under similar conditions is ~0.06 nm/s [15].
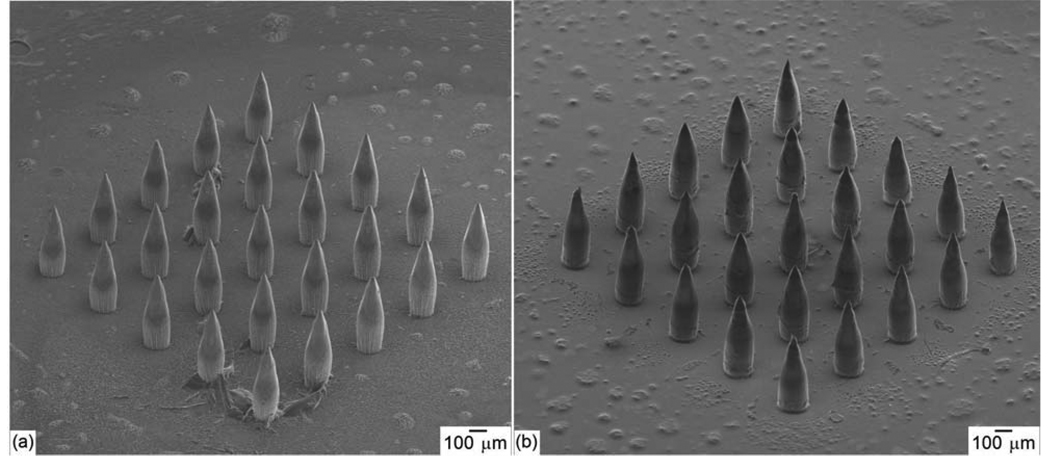
Figure 1.
(a) Scanning electron micrograph of a SR 259 polyethylene glycol dimethacrylate microneedle array, which served as a master structure in the two photon polymerization-micromolding process. (b) Scanning electron micrograph of a silver-coated Ormocer® microneedle array fabricated using two photon polymerization-micromolding and pulsed laser deposition.
Cell proliferation on silver-coated and uncoated Ormocer® materials was examined using neonatal human epidermal keratinocytes (HEK). The MTT assay (3-(4,5-dimethylthiazol-2-yl)2,5-diphenyl tetrazolium bromide) test was used to assess cell viability [20]. In the MTT assay, reduction of a yellow tetrazolium salt (MTT) to a purple formazan dye by mitochondrial succinnic dehydrogenase is observed. Four silver-coated Ormocer® wafers and four uncoated Ormocer® wafers were placed in a 96-well plate and exposed to ultraviolet-B light for three hours in order to sterilize the surfaces prior to seeding with human epidermal keratinocytes. The wafers were rinsed with 200 µl of keratinocyte growth media (KGM-2). The human epidermal keratinocytes (Lonza, Walkersville, MD) were seeded onto the wafers and grown to 80% confluency in a humidified environment containing 5% CO2 at 37° C. Twenty-four hours later, the human epidermal keratinocytes were incubated in the MTT medium (0.5mg/ml KGM-2) for three hours, the formazan dye was extracted with isopropyl alcohol, and the extracted solution in each well was transferred to a new plate in order to prevent the physical presence of the wafers from influencing the data. The absorbance of the solutions was quantified on a Multiskan RC ELISA plate reader (Labsystems, Helsinki, Finland) at λ=550 nm. The silver-coated Ormocer® data was normalized to the uncoated Ormocer® (control) data. Mean values for percent viability were calculated and significant differences (p<0.05) were determined using the PROC GLM Procedure (SAS 9.1 for Windows) (SAS Institute, Cary, NC). When significant differences were found, multiple comparisons were performed using Tukey’s Studentized Range HSD test at p<0.05 level of significance.
An agar plating method was used to assess microbial growth on the silver-coated microneedle array as well as the uncoated microneedle array. Staphylococcus aureus ATCC 25923 (American Type Culture Collection, Manassas, VA) was cultured overnight in tryptic soy broth (VWR International, West Chester, PA). The cell suspensions were then centrifuged at 4500 rpm for ten minutes. The cell pellet was then resuspended in 1X phosphate-buffered saline (VWR International, West Chester, PA) in order to obtain a cell density of ~108 CFU/ml. Tryptic soy agar plates (VWR International, West Chester, PA) were inoculated with a lawn of S. aureus suspension using a sterile swab. Glass cover slips with uncoated and silver-coated microneedle arrays were placed on the inoculated plates with the needles projecting into the agar. The plates were then incubated at 37°C for twenty-four hours. The microneedle arrays were subsequently removed. Optical microscopy was performed in order to determine the extent of microbial growth.
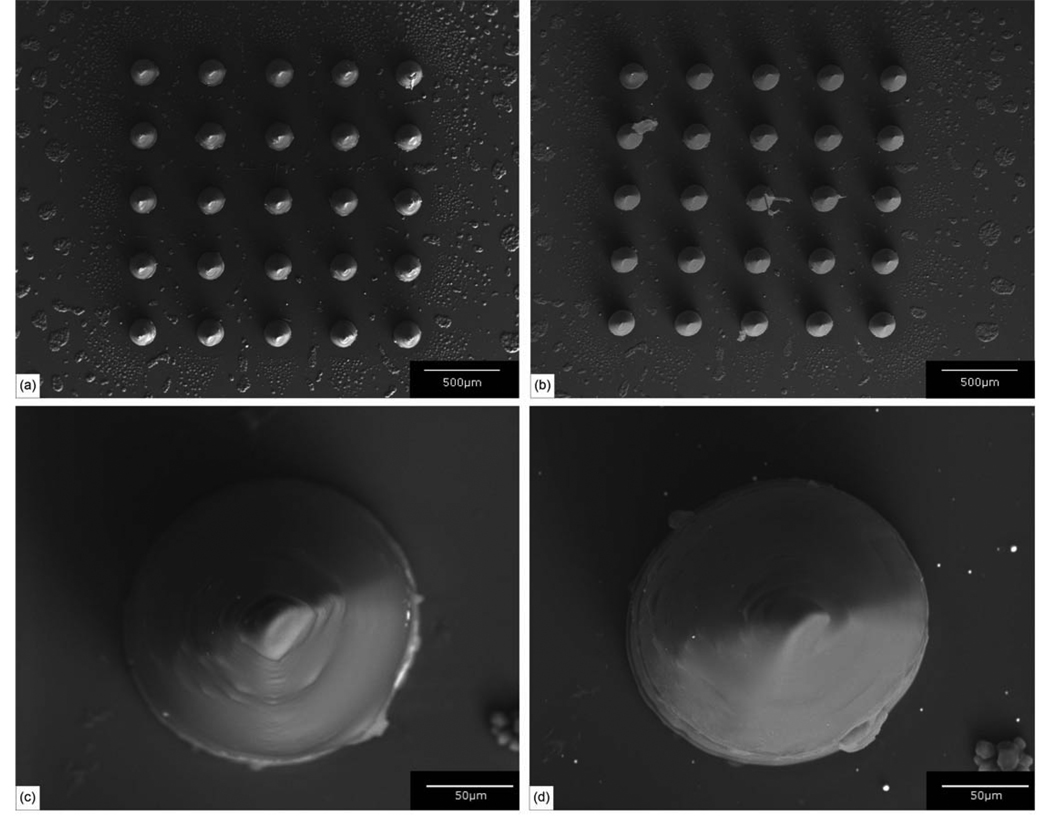
Silver-coated microneedles within the arrays demonstrated sharp (<10 µm diameter) tips, tip angle values of 45°, and good microneedle-to-microneedle uniformity (Figure 1(b)). A few deviations from the dimensions specified in the STL file were observed. For example, the base diameter of the microneedles (181.3 ± 4.7 µm) was slightly smaller than the base diameter that was specified in the .STL format drawing. This decrease in diameter is due to shrinkage of the Ormocer® material during polymerization [21]. Shrinkage of the Ormocer® material is beneficial in the micromolding component of the two photon polymerization-micromolding process since it enhances separation between the mold and the fabricated device. Iteration of microneedle materials and processing parameters may enable the development of microneedle arrays with more uniform features. In general, these images indicate that the features observed in the microneedles were consistent with the dimensions specified in the .STL format drawing. No pinholes, pores or nonuniformities in the silver thin film were noted in the scanning electron micrograph of the individual silver-coated microneedle (Figure 2). Energy-dispersive X-ray spectra indicated that silver was present on the microneedles and the glass substrate. Silicon and carbon were also identified in the spectra; these elements were attributed to the glass substrate and the Ormocer® microneedle. No trace amounts of other elements were observed.
Figure 2.
(a) Scanning electron micrograph of an uncoated Ormocer® microneedle array. (b) Scanning electron micrograph of a silver-coated Ormocer® microneedle array. (c) Scanning electron micrograph of an individual uncoated Ormocer® microneedle. (d) Scanning electron micrograph of an individual silver-coated Ormocer® microneedle.
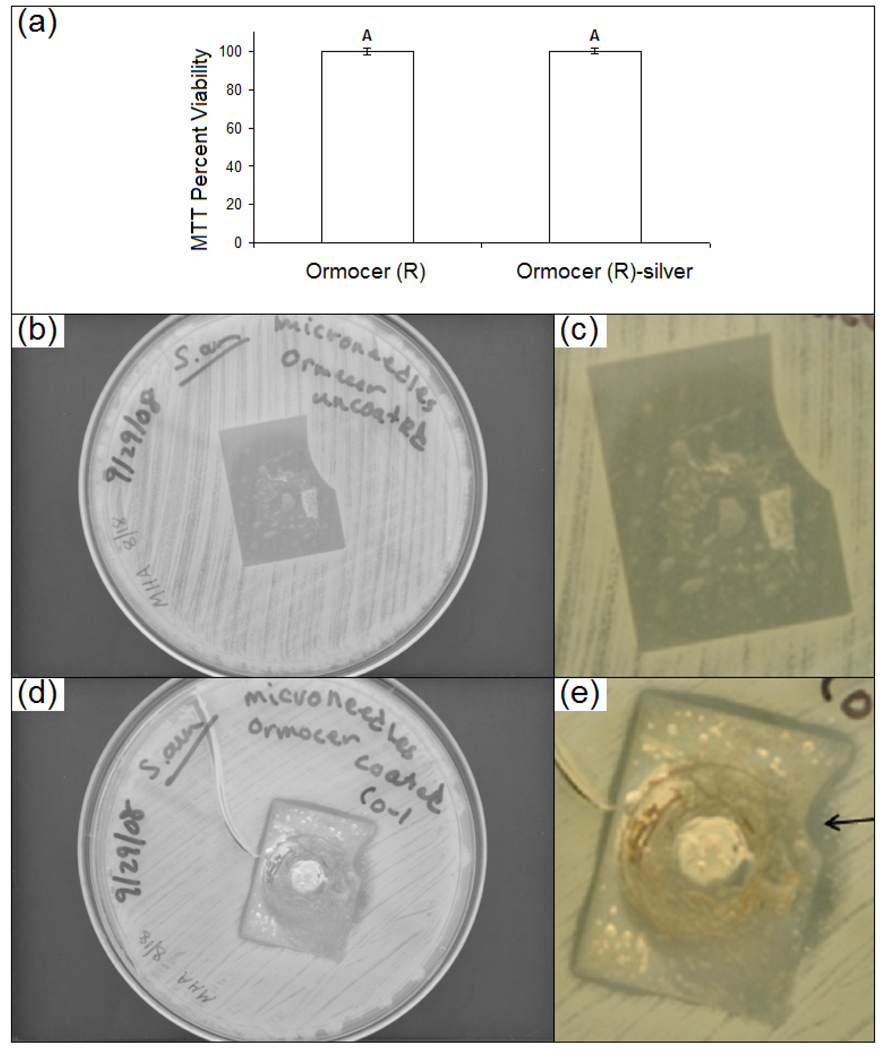
The twenty-four hour MTT assay (Figure 3(a)) indicated that the silver-coated Ormocer® and uncoated Ormocer® wafers supported human epidermal keratinocyte growth. No significant differences were noted between human epidermal keratinocyte growth on the test surfaces. These results suggest that silver-coated Ormocer® processed using two photon polymerization-micromolding and pulsed laser deposition does not impair cell growth or decrease cell viability. Previous studies have indicated that cell growth on Ormocer® surfaces is similar to that on polystyrene control surfaces [22]. The agar plating method provides qualitative data regarding susceptibility of microorganisms to a given antimicrobial agent. This protocol is similar to one that is outlined by the National Committee for Clinical Laboratory Standards [23]. Results of the agar plating study for the uncoated microneedle array and the silver-coated microneedle array are shown in Figure 3(b)–Figure 3(e). On the plate containing the uncoated Ormocer® microneedle array, S. aureus growth was present directly under the microneedle array; no zone of growth inhibition was observed (Figure 3(c)). The plate containing the silver-coated Ormocer® microneedle array exhibited an absence of S. aureus growth under the array. In addition, inhibited growth surrounding the microneedle array was noted (Figure 3(e)). The presence of a zone of inhibition suggests that silver was released into agar adjacent to the microneedle array.
Figure 3.
(a) MTT viability of human epithelial keratinocytes on uncoated Ormocer and silver-coated Ormocer wafers. (b) Low magnification and (c) high magnification agar diffusion assay result for uncoated Ormocer® microneedle array. (d) Low magnification and (e) high magnification agar diffusion assay result for silver-coated Ormocer® microneedle array. The arrow indicates an absence of growth under the microneedle array as well as inhibited growth surrounding the microneedle array.
The results of this study indicate that coating microneedles with silver thin films using pulsed laser deposition is a useful approach for creating microneedles with antimicrobial functionality. Two photon polymerization-micromolding is an indirect rapid prototyping technique that is compatible with high throughput production of microscale medical devices. Pulsed laser deposition produced a pinhole-free, pore-free silver film on an Ormocer® microneedle array. Microneedles fabricated using two photon polymerization-micromolding and pulsed laser deposition were shown to inhibit growth of S. aureus. It is anticipated that microneedles with antimicrobial functionality may find wider uses than conventional microneedles for transdermal delivery of protein- and nucleic-acid based pharmacologic agents.
Acknowledgements
We thank Mr. Al Inman at the Center of Chemical Toxicology Research and Pharmacokinetics at North Carolina State University for technical assistance.
References
- 1.McAllister DV, Wang PM, Davis SP, Park JH, Canatella PJ, Allen MG, Prausnitz MR. Microfabricated Needles for Transdermal Delivery of Macromolecules and Nanoparticles: Fabrication Methods and Transport Studies. Proc. Natl. Acad. Sci. USA. 2003;100:13755–13760. doi: 10.1073/pnas.2331316100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gittard SD, Ovsianikov A, Monteiro-Riviere NA, Lusk J, Morel P, Minghetti P, Lenardi C, Chichkov BN, Narayan RJ. Fabrication of Polymer Microneedles Using a Two-Photon Polymerization and Micromolding Process. J. Diabetes Sci. Technol. 2009;3:304–311. doi: 10.1177/193229680900300211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee SH, Jeong SK, Ahn SK. An Update of the Defensive Barrier Function of Skin. Yonsei Med. J. 2006;47:293–306. doi: 10.3349/ymj.2006.47.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banga AK. Theme Section: Transdermal Delivery of Proteins. Pharm Res. 2007;24:1357–1359. doi: 10.1007/s11095-007-9323-3. [DOI] [PubMed] [Google Scholar]
- 5.Birchall JC. Microneedle Array Technology: The Time is Right But is the Science Ready? Exp. Rev. Med. Dev. 2006;3:1–4. doi: 10.1586/17434440.3.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Bragg PD, Rainnie DJ. The effect of silver ions on the respiratory chain of Escherichia coli. Can. J. Microbiol. 1974;20:883–889. doi: 10.1139/m74-135. [DOI] [PubMed] [Google Scholar]
- 7.Rosenkranz HS, Rosenkranz S. Silver Sulfadiazine: Interaction with Isolated Deoxyribonucleic Acid. Antimicrob. Agents Chemother. 1972;2:373–383. doi: 10.1128/aac.2.5.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stoimenov PK, Klinger RL, Marchin GL, Klabunde KJ. Metal Oxide Nanoparticles as Bactericidal Agents. Langmuir. 2002;18:6679–6686. [Google Scholar]
- 9.Slawson RM, Van Dyke MI, Lee H, Trevors JT. Germanium and Silver Resistance, Accumulation, and Toxicity in Microorganisms. Plasmid. 1992;27:72–79. doi: 10.1016/0147-619x(92)90008-x. [DOI] [PubMed] [Google Scholar]
- 10.Zhao G, Stevens SE., Jr Multiple Parameters for the Comprehensive Evaluation of the Susceptibility of Escherichia Coli to the Silver Ion. BioMetals. 1998;11:27–32. doi: 10.1023/a:1009253223055. [DOI] [PubMed] [Google Scholar]
- 11.Trengove NJ, Stacey MC, Macauley S, Bennett N, Gibson J, Burslem F, Murphy G, Schultz G. Analysis of the Acute and Chronic Wound Environments: The Role of Proteases and Their Inhibitors. Wound Repair Regen. 1999;7:442–452. doi: 10.1046/j.1524-475x.1999.00442.x. [DOI] [PubMed] [Google Scholar]
- 12.Wright JB, Lam K, Buret AG, Olson ME, Burrell RE. Burrell, R.E., Early Healing Events in a Porcine Model of Contaminated Wounds: Effects of Nanocrystalline Silver on Matrix Metalloproteinases, Cell Apoptosis, and Healing. Wound Repair Regen. 2002;10:141–151. doi: 10.1046/j.1524-475x.2002.10308.x. [DOI] [PubMed] [Google Scholar]
- 13.Demling RH, DeSanti L. The Rate of Re-Epithelialization Across Meshed Skin Grafts is Increased With Exposure to Silver. Burns. 2002;28:264–266. doi: 10.1016/s0305-4179(01)00119-x. [DOI] [PubMed] [Google Scholar]
- 14.Morrison ML, Buchanan RA, Liaw PK, Berry CJ, Brigmon R, Riester L, Jin C, Narayan RJ. Electrochemical and Antimicrobial Properties of Diamondlike Carbon-Metal Composite Films. Diamond Related Mater. 2006;15:138–146. [Google Scholar]
- 15.Warrender JM, Aziz MJ. Kinetic Energy Effects on Morphology Evolution During Pulsed Laser Deposition of Metal-On-Insulator Films. Phys. Rev. B. 2007;75:085433. [Google Scholar]
- 16.Willmott PR. Deposition of Complex Multielemental Thin Films. Prog. Surf. Sci. 2004;76:163–217. [Google Scholar]
- 17.Lackner JM. Industrially-Scaled Large-Area and High-Rate Tribological Coating By Pulsed Laser Deposition. Surf. Coat. Technol. 2005;200:1439–1444. [Google Scholar]
- 18.Doraiswamy A, Jin C, Narayan RJ, Mageswaran P, Mente P, Modi R, Auyeung R, Chrisey DB, Ovsianikov A, Chichkov B. Two Photon Polymerization of Microneedles for Drug Delivery. Acta Biomater. 2006;2:267–275. doi: 10.1016/j.actbio.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Davis SP, Landis BJ, Adams ZH, Allen MG, Prausnitz MR. Insertion of Microneedles Into Skin: Measurement and Prediction of Insertion Force and Needle Fracture Force. J. Biomech. 2004;37:1155–1163. doi: 10.1016/j.jbiomech.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Monteiro-Riviere NA, Inman AO, Zhang LW. Limitations and Relative Utility of Screening Assays to Assess Engineered Nanoparticle Toxicity in a Human Cell Line. Toxicol. Appl. Pharmacol. 2009;234:222–235. doi: 10.1016/j.taap.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 21.Yap AUJ, Soh MS. Post-Gel Polymerization Contraction of “Low Shrinkage” Composite Restoratives. Oper. Dent. 2004;29:317–324. [PubMed] [Google Scholar]
- 22.Ovsianikov A, Chichkov BN, Mente P, Monteiro-Riviere NA, Doraiswamy A, Narayan RJ. Two Photon Polymerization of Polymer-Ceramic Hybrid Materials for Transdermal Drug Delivery. Int. J. Appl. Ceramic Technol. 2007;4:22–29. [Google Scholar]
- 23.Performance standards for antimicrobial disk susceptibility tests (M2-A6) Wayne, PA: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]