Abstract
Contaminated heparin was linked to at least 149 deaths and hundreds of adverse reactions. Published report indicates that heparin contaminants were a natural impurity, dermatan sulfate, and a contaminant, oversulfated chondroitin sulfate (OSCS). OSCS was assumed to derive from animal cartilage. By analyzing 26 contaminated heparin lots from different sources, our data indicate that the heparin contaminants were chemically sulfated or chemically sulfated/desulfated glycosaminoglycans (GAGs) consisting of heparan sulfate, chondroitin sulfate, and dermatan sulfate based on monosaccharide quantification, CE, heparin lyase digestion, and liquid chromatography/mass spectrometry analysis. Since currently recommended heparin quality control assays had failed to detect certain heparin contaminants, a simple method that detects most contaminants in heparin was developed. This assay detects specific heparin structures that most contaminants cannot mimic and can be performed in any laboratory equipped with an UV spectrometer.
Keywords: heparin, contaminants, oversulfated chondroitin sulfate, glycosaminoglycan
Introduction
Glycosaminoglycans (GAGs) are linear polysaccharides comprised of repeating hexosamine-containing disaccharides made by all animal cells. Heparin is a GAG made mainly by mast cells. Heparin is widely used as an anticoagulant for treatment and prevention of thromboembolic disorders.1
Contaminated heparin was linked to at least 149 deaths2 and hundreds of adverse reactions in the US and Germany in 2007 and 2008.3 The contaminants in heparin were reported as an impurity, specifically dermatan sulfate, and a contaminant, chemically oversulfated chondroitin sulfate (OSCS), presumed to derive from animal cartilage chondroitin sulfate based on NMR analysis.4
Heparin is prepared from crude heparin that is usually isolated from porcine intestine or bovine lung entrails. Crude heparin consists of ~50% heparin and ~50% less sulfated GAGs, including heparan sulfate, dermatan sulfate, and chondroitin sulfate.5 The less sulfated GAGs are removed from crude heparin during pharmaceutical grade heparin production into two heparin byproducts named GAG waste and tank bottom. 6 GAG waste has a NMR profile highly resembles heparin6 but it consists of less sulfated heparin/heparan sulfate and dermatan sulfate. The tank bottom consists of even less sulfated heparan sulfate, chondroitin sulfate, and dermatan sulfate.
The less sulfated GAG waste and tank bottom have low anticoagulation activities compared to the highly sulfated heparin. However, enriching 3-O-sulfated heparin/heparan sulfate sequences by chemical sulfation/desulfation7 can greatly enhance the anticoagulation activities of the GAG waste enriched in heparin/heparan sulfate. In contrast, only fully chemical sulfation can turn each component of tank bottom, i.e. chondroitin sulfate,8 dermatan sulfate, and heparan sulfate, into better anticoagulants.9 Furthermore, mixing heparin with OSCS could further enhance the anticoagulation activities of the mixture at therapeutic concentrations by an unknown molecular mechanism.10
Since the tank bottom can be a pure chondroitin sulfate if the crude heparin is isolated from bovine lung,11 the possibility exists that the OSCS observed in contaminated heparin might be derived from the tank bottom heparin byproduct rather than from animal cartilage chondroitin sulfate. Similarly, chemically sulfated/desulfated GAG wastes might be present in contaminated heparin if GAG waste was also used for adulteration.
To test the possibilities, we analyzed 26 heparin lots by monosaccharide quantification, CE, heparin lyase digestion, and liquid chromatography/mass spectrometry analysis. The heparin contaminants had faster, slower, or the same migration rate as heparin based on CE analysis. The heparin contaminants that had slowest migration rate indicated by CE analysis was identified as chemically sulfated/desulfated heparin/heparan sulfate and chemically sulfated/desulfated chondroitin/dermatan sulfate by liquid chromatography/mass spectrometry analysis, which suggests that chemically sulfated/desulfated GAG wastes are part of heparin contaminants.
Since currently recommended NMR, CE, and anion exchange HPLC-based heparin quality control assays are not able to identify all heparin contaminants in contaminated heparins, we developed a simple method that quantifies specific heparin structures, and that can detect most heparin contaminants. We recommend that this assay be used for routine heparin quality control purposes.
Materials and Methods
Materials
Contaminated heparins used in this study were supplied by the US FDA. The authentic heparin sample used in this study was purchased from Sigma. The 96 well UV transparent plates used for heparin lyase I digestion were purchased from BD Bioscience. All heparin lyases were either purchased from Sigma or expressed in E. Coli and purified in our laboratory according to the published method.
Uronic acid analysis
Uronic acids were measured using the simplified carbazole assay.12 In brief, a PCR instrument was set at 100 °C for the hydrolysis and carbazole reactions. The reagents used in the carbazole assay were 0.025M sodium tetraborate·10 H2O in sulfuric acid (H2SO4+Borex) and 0.125% carbazole in absolute ethanol (W/V). Two hundred μl of the H2SO4+Borex solution to 40 μl of GAG solution or water (blank control). The tubes were capped and vortexed briefly. The tubes were then put into a PCR instrument with the lid securely locked. The PCR instrument was programmed to run for 15 min at 100 °C and then cool down to 4 °C. To each tube, 8 μl of carbazole solution was added followed by a brief vortex. The tubes were returned to the PCR instrument for the carbazole reaction, which was run at 15 min at 100 °C and then cooled to 4 °C. The samples were vortexed and 200 μl of each sample was transferred to a 96 well plate. The absorbance at 530 nm was measured by a Spectra MAX M2 plate-reading spectrophotometer (Molecular Devices, Sunnyvale, CA). GlcA was used as an external standard, and the standard curve was derived by plotting absorbance against the concentration of GlcA or standard GAG.
Glucosamine- and galactosamine-quantification of heparin samples
Glucosamine- and galactosamine-containing GAGs in heparin was analyzed by reversed-phase HPLC separation using the fluorescent isoindole derivatization method.13 Two × 1 μl of 2 mg/ml stock solutions of heparin samples were subjected to acid hydrolysis, sodium borohydride reduction, precolumn derivatization with o-phthaldialdehyde (OPA) and 3-mercaptopropionic acid (3MPA), and reversed phase HPLC separation with fluorescence detection of the isoindole derivatives. GAG aliquots containing 360 pmol of norleucine as an internal standard were dried in pyrolized glass vials (Agilent, Palo Alto, CA, part 5181–8872) before hydrolysis with HCl vapor in N2 gas at 100 °C for 3 hours. The samples were rehydrated in 45 μl of 0.56% NaBH4 to reduce the glucosamine and galactosamine liberated by acid hydrolysis into glucosaminitol and galactosaminitol, respectively. After an overnight incubation at room temperature, the reaction was terminated by adding 5 μl of 2N acetic acid to each vial. The sample was dried by centrifugation under vacuum and dissolved in 5 μl water for precolumn derivatization with 35 μl of 7.5 mM OPA, 375 mM 3MPA, in 0.4N borate adjusted to pH 9.3 with NaOH. Half of this reaction mixture was injected onto a 4.6 × 250 mm C-12 column, a Synergi 4 μMAX-RP 80 Å (Phenomenex, Torrance, CA, part 00G-4337-E0), and heated to 35 °C. The column was equilibrated with Buffer A, consisting of 0.05 M (monobasic and dibasic) sodium phosphate, pH 7.2 in 25% methanol, at a flow rate of 0.8 ml/min. Buffer B consisted of methanol, water, and tetrahydrofuran at 70:30:3 volume ratios. After injection, Buffer B was increased from 0 to 8% by a linear gradient between 0 and 3 min, was maintained at 8% between 3 and 18 min, at 55% between 18 and 30.5 min, at 100% between 30.5 and 32.5 min, and at 0% between 32.5 and 35 min. A 5 min post-run interval at 0% B preceded the initiation of the next precolumn derivatization injection sequence. The fluorescent derivatives of glucosaminitol, galactosaminitol, and the amino acids contained in the GAG lots were excited at 337 nm and detected at 454 nm. All analyses were repeated twice. A more detailed description including statistical analysis of the assay can be found in the original publication.13
Low pH nitrous acid treatment
An established low pH nitrous treatment procedure was used to degrade heparin and contaminated heparin samples.14 In brief, samples were treated with HNO2 at pH 1.5 for 10 minutes on ice, followed by either NaBH4 or NaBD4 reduction. The residual NaBH4 and NaBD4 were destroyed by acetic acid. The samples were lyophilized and dried for liquid chromatography/mass spectrometry.
Enzymatic digestion of heparin/heparan sulfate and chondroitin sulfate/dermatan sulfate
One hundred μg of heparin or contaminated heparin (2 mg/ml in water) were digested with ~1 mU heparin lyases I, II, and III, 1 mU heparin lyase I, or 1 mU heparin lyase I in 200 μl of buffer containing 50 mM ammonium acetate and 0.125 mM calcium acetate pH 7. Chondroitin/dermatan sulfate was digested by chondroitinase ABC (Seikagaku, Tokyo) in 200 μl of buffer containing 50 mM Tris and 50 mM sodium acetate, pH 8.0. The extent of digestion was monitored every 2 min for 120 min at 37 °C by reading absorbance at 232 nm in a 96 well plate reader (Molecular dynamics).
Aniline tagging
Ten μl aniline or D5-aniline was added to heparin lyase digested contaminated heparin samples followed by addition 7 μl 1 M NaBH4 in DMSO and 3 μl glacial acetic acid. The labeling reaction was carried out at 65 °C for 2 h. The samples were dried by lyophilization and dissolved in 10 μl of water.
Liquid chromatography/mass spectrometry
The methods used in the studies were published elsewhere.15–17 Heparin lyase digested samples were labeled with H-aniline or D5-aniline by reductive amination. In contrast, low pH nitrous acid degraded samples were labeled with NaBH4 or NaBD4. Tagging samples with different stable isotopes allow simultaneous comparison of two samples, which eliminates variation during and between analyses associated with liquid chromatography/mass spectrometry methodology. Separations were performed on a 0.3 × 250 mm C18 column (Zorbax 300SB, 5 μm, Agilent) using an Agilent 1100 series capillary HPLC workstation (Agilent, Palo Alto, CA) with Chemstation software for data acquisition, analysis, and management. The capillary HPLC was directly coupled to the mass spectrometer. Mass spectra were acquired on a Mariner BioSpectrometry Workstation ESI time-of-flight mass spectrometer (PerSeptive Biosystems; Framingham, MA) in the negative-ion mode. Total ion current chromatograms and mass spectra were processed with Data Explorer software version 3.0.
CE
CE was conducted on a Hewlett Packard 3D-CE instrument equipped with a diode array detector set at a wavelength of 200 nM (band width 10 nM) as described previously.18 Separations were performed in a bare fused silica capillary, internal diameter 50 mM, 64.5 cm-total length, 56 cm-effective length with a column temperature of 25 °C. The polarity was negative with a voltage of 30 kV. Samples were dissolved in Milli-Q water at a concentration of approximately 10 mg/ml and filtered through 0.2 mM cellulose acetate membrane filters (Micro-Spin filter tubes, Alltech Associates, Deer-field, IL, USA). The sample solutions were injected using hydrodynamic pressure at 50 mbar for 10 s. The electrolyte solution was 36 mM phosphate buffer (pH 3.5) filtered with a 0.2 mM cellulose acetate syringe filter (Grace, Deer- field, IL, USA). The capillary column was preconditioned at the beginning of each day by flushing with 1 M NaOH, 0.1 M NaOH, and water, each for 2 min, and prior to running each sample by flushing with water for 2 min and electrolyte solution for 2 min.
Results
Detect heparin contaminants by monosaccharide analysis
A simple way to distinguish GAG contaminants from other possible contaminants is to determine the amounts of glucosamine/galactosamine relative to uronic acids. Heparin/heparan sulfate consists of repeating glucosamine- and uronic acid-containing disaccharides whereas chondroitin/dermatan sulfate consists of repeating galactosamine- and uronic acid-containing disaccharides. Analysis of 26 lots of contaminated heparin from different sources showed similar amounts of uronic acid per gram dry weight compared to authentic heparin by a carbazole assay,12 indicating that heparin was most likely contaminated with GAGs. By using a glucosamine and galactosamine quantification assay,12,13 we found variable amounts of galactosamine-containing contaminants in the 26 heparin lots (from 2–37%, Table 1) compared to 0.23% in the 26 authentic heparin lots. Although the glucosamine/galactosamine quantification assay quantifies the level of chondroitin/dermatan sulfate contamination, the assay could not exclude the possibility that heparin was also contaminated with heparan sulfate and/or oversulfated heparan sulfate. This assay also could not distinguish low-sulfated from over-sulfated chondroitin/dermatan sulfate.
Table 1. Monosaccharide composition of contaminated heparin lots.
Twenty-six suspect heparin samples were acid hydrolyzed and analyzed chemically for galactosamine, glucosamine, and uronic acid content. The recovery of galactosamine and glucosamine relative to uronic acid was used to determine the relative amount of chondroitin/dermatan sulfate and heparin/heparan sulfate, respectively.
| Contaminated heparin | Galactosamine Content (%) | Uronic acid (% of heparin) |
|---|---|---|
| LSCH1 | 2 ± 1.4 | 114 ± 6% |
| LSCH2 | 8.0 ± 1.0 | 114 ± 7% |
| LSCH3 | 9.6 ± 0.6 | 108 ± 7% |
| LSCH4 | 10.5 ± 2.1 | 124 ± 3% |
| LSCH5 | 13.8 ± 4.1 | 115 ± 3% |
| LSCH6 | 15.7 ± 0.6 | 122 ± 3% |
| OSCH1 | 10.5 ± 1.5 | 100 ± 20% |
| OSCH2 | 13.0 ± 1.0 | 109 ± 1% |
| OSCH3 | 13.9 ± 0.6 | 110 ± 10% |
| OSCH4 | 23.6 ± 1.5 | 108 ± 0.7% |
| OSLSCH1 | 9.6 ± 0.1 | 110 ± 13% |
| OSLSCH2 | 11.0 ± 0.4 | 100 ± 16% |
| OSLSCH3 | 18.9 ± 0.8 | 110 ± 23% |
| OSLSCH4 | 21.0 ± 1.2 | 110 ± 16% |
| OSLSCH5 | 22.8 ± 1.4 | 120 ± 13% |
| OSLSCH6 | 23.3 ± 0.6 | 107 ± 3% |
| OSLSCH7 | 23.7 ± 0.2 | 90 ± 10% |
| OSLSCH8 | 29.1 ± 0.8 | 101 ± 3% |
| OSLSCH9 | 29.3 ± 3.5 | 108 ± 0.7% |
| OSLSCH10 | 37.1 ± 0.14 | 90 ± 10% |
| S1 | 2.5 ± 0.6 | 92 ± 5% |
| S2 | 3.5 ± 0.5 | 95 ± 3% |
| S3 | 3.8 ± 1.6 | 102 ± 3% |
| G1 | 8.7 ± 2.1 | 100 ± 2% |
| G2 | 7.3 | 110 ± 2% |
| G3 | 6.5 ± 0.6 | 107 ± 8% |
The individual lots were analyzed for uronic acid, galactosamine and glucosamine after acid hydrolysis. The recovery of each hexosamine was used to determine the relative amount of chondroitin/dermatan sulfate and heparin/heparan sulfate, respectively.
Abbreviations: LSCH, low-sulfated contaminant containing heparin; OSCH, oversulfated contaminant containing heparin; OSLSCH, both oversulfated and low-sulfated contaminant containing heparin; S (suspected) lots passed both CE and NMR screen; G (Germany) lots passed screening by NMR but not by CE.
Detect heparin contaminants by CE analysis
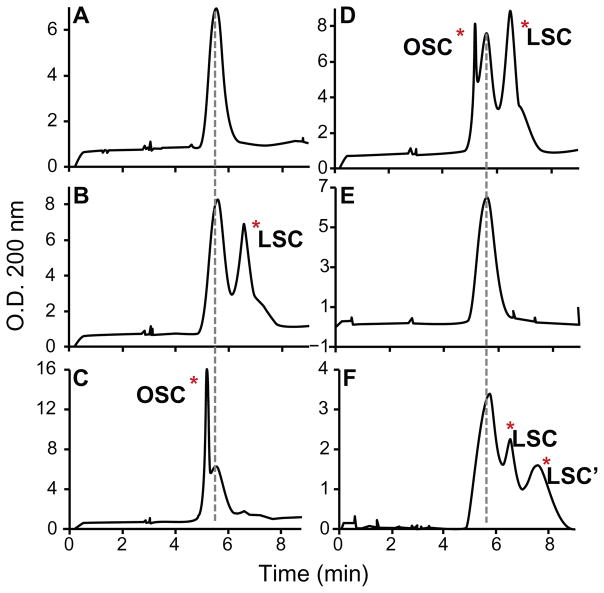
CE separates GAGs mainly by negative charge contributed by the uronic acids and sulfate groups. Thus, less sulfated GAGs migrate slower than heparin, whereas oversulfated GAGs migrate faster than heparin. Since heparin is the most sulfated naturally occurring GAG, any contaminant that migrates faster than or co-migrates with heparin is not natural, but rather represents one or more chemically sulfated contaminant(s). As shown in Figure 1A, authentic heparin eluted at 5.6 minutes. Six of the contaminated lots (lots LSCH1-6) contained a contaminant that eluted later at 6.5 minutes (Fig. 1B), which indicates that the contaminant was less sulfated than heparin. Four samples (OSCH1-4) had a contaminant that eluted earlier than heparin at 5.2 minutes, indicating it was more sulfated than heparin (Fig. 1C). Ten contaminated lots (OSLSCH1-10) had both more sulfated and less sulfated contaminants (OSC and LSC, respectively; Fig. 1D). Three lots (S1-3) containing 3 to 5% galactosamine-containing GAGs had no contaminant detectable by CE (Fig. 1E). Another three lots from Germany (G1-3) had an additional low-sulfated contaminant peak eluting at 7.2 minutes (Fig. 1F). Thus, these heparin lots contained various mixtures of low-sulfated and oversulfated GAG contaminants.
Figure 1. CE profiles of normal and contaminated heparin.
CE was performed using the FDA recommended protocol (See material and methods). A, authentic heparin; B, heparin lot LSCH6 containing lowsulfated contaminant (LSC); C, heparin lot OSCH4 containing an oversulfated contaminant (OSC); D, heparin lot OSLSCH5 containing both oversulfated and low-sulfated contaminants; E, heparin lot S1, which we show contains oversulfated heparan sulfate; and F, heparin lot G1 containing two low-sulfated contaminants. More detailed information about these samples is shown in Table 1 and in the text. CE reliably resolved abnormal peaks in most heparin lots (B-D, and F) but did not resolve contaminants in some (E).
NMR had failed to detect G1, G2, G3, S1, S2, and S3 as contaminated heparin lots whereas currently recommended NMR, CE, and anion exchange HPLC analyses (data not shown) had failed to detect S1, S2, and S3 as contaminated heparin lots.
Identify heparin contaminants in G1, G2, and G3 by liquid chromatography/mass spectrometry analysis
The three contaminated heparin lots (G1, G2, and G3) were retrieved from Germany. These heparin lots had low amounts of galactosamine-containing GAGs by monosaccharide analysis (6%–8%, Table 1). The galactosamine-containing GAGs were susceptible to chondroitinase ABC digestion, but had an unusual composition compared to cartilage chondroitin sulfate and porcine mucosal dermatan sulfate (Table 2). In these experiments, the products derived from chondroitinase digestion, consisting mostly of disaccharides, were analyzed by NaBH4 reductive amination with isotope-tagged aniline and liquid chromatography/mass spectrometry.15 This technique allows determination of the number of sulfate groups (and their position) on each disaccharide. As shown in Table 2, the chondroitin-like material in G1, G2, and G3 consisted of non-sulfated disaccharides (55%–57% of the total), monosulfated disaccharides (18%–20%) and disulfated disaccharides (24%–27%). This pattern differed significantly from shark cartilage chondroitin sulfate (97% monosulfated disaccharides) and porcine mucosal dermatan sulfate (18% non-sulfated disaccharides, 78% monosulfated disaccharides, and only 4% disulfated disaccharides).
Table 2. Sulfated di- and tetra-saccharides of three contaminated heparin lots from Germany.
Samples were digested with chondroitinase ABC or a mixture heparin lyases I, II and III and the products were derivatized by reductive amination with aniline.15 The tagged products were mixed with differentially isotope-tagged aniline derivatized standards and the mixture was analyzed by liquid chromatography/mass spectrometry.16 The recovery of the standards was used to determine the recovery of each disaccharide and tetrasaccharide from the sample.
| Lyase digestion products | Samples (Galactosamine %) |
Standards |
||||
|---|---|---|---|---|---|---|
| G1 (8.7%) | G2 (7.3%) | G3 (6.5%) | Dermatan Sulfate | Chondroitin sulfate-A | Heparin | |
| Chondroitin/Dermatan sulfate | ||||||
| D-a | 57% | 56% | 55% | 18% | 2% | |
| D-a + 1S | 19% | 20% | 18% | 78% | 97% | |
| D-a + 2S | 25% | 24% | 27% | 4% | 1% | |
| Heparin/Heparan sulfate | ||||||
| D-A | 23% | 18% | 11% | 5% | ||
| D-H + 3S | 73% | 61% | 70% | 95% | ||
| D-H-U-H + 6S | 4% | 21% | 19% | 1% | ||
The modified version of the disaccharide structure code was used to designate the core structure of the individual disaccharides and tetrasaccharides.21 Although the position of the sulfate groups was determined by co-elution of standards, the isobaric species containing the indicated number of sulfate groups were summed together to simplify the presentation of the data. D, Δ4,5-unsaturated uronic acid; a, N-acetylgalactosamine; A, N-acetylglucosamine; H, glucosamine; U, GlcA or IdoA; S, sulfate.
As shown in Figure 1F, the heparin lots from Germany had substantial amounts of two low-sulfated contaminants that resolved from heparin by CE. The relatively low amounts of galactosamine-containing GAG (Table 1) suggested that these peaks contained mainly glucosamine-containing GAG. Liquid chromatography/mass spectrometry of products generated by combined heparin lyase I, II, and III digestion showed non-sulfated disaccharides (11%–23%) and trisulfated disaccharides (61%–73%) typical of heparin, and unusually large amounts of lyase-resistant hexasulfated tetrasaccharides (4%–21%) compared to heparin (Table 2) CE analysis (Fig. 1) suggests that the contaminants In G1, G2, and G3 are naturally occurring heparin by product. However, liquid chromatography/mass spectrometry analysis (Table 2) indicates that the heparin byproduct might have been chemically oversulfated then desulfated to enhance its anticoagulant activities.7 Indeed, G1, G2, and G3 had better anticoagulation activities compared to un-adulterated heparin based on 4 independent anticoagulation assays (unpublished results). Therefore, chemical oversulfation/desulfation might be the scheme employed to turn the GAG waste heparin byproduct into a better anticoagulant and then added to heparin to create the contaminated heparin preparations.
Identify chemically modified heparin/heparan sulfate structures in contaminated heparins by liquid chromatography/mass spectrometry analysis
Since chemical sulfation/desulfation reactions are accompanied by GAG structure modifications through different side reactions,7 identifying structures that are not present in native heparan sulfate/heparin but present in chemically modified heparin byproducts would provide direct evidence that heparin byproducts were modified to mimic heparin. Indeed, one abnormal acetylated heparin/heparan sulfate structure that might be generated through an established N-O intramolecular acetyl migration reaction19 was reported.20
We decided to test if other chemically modified heparan sulfate/heparin structures are also present in contaminated heparin preparations. To this end, we systematically compared several heparin lots containing low sulfated contaminants (LSCH2 and LSCH4), oversulfated heparin contaminants (OSCH2), both low and oversulfated contaminants (OSLSCH4), or heparin-like contaminants that have passed both CE and NMR-based heparin quality control assays (S1, S2, and S3) by liquid chromatography/mass spectrometry analysis. Representative data are shown in Figure 2.
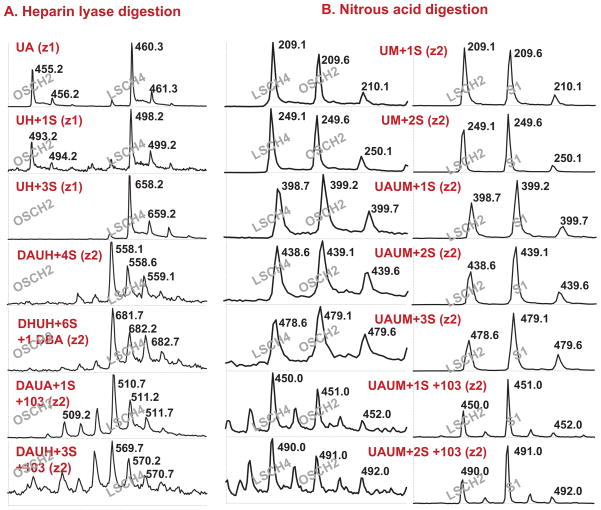
Figure 2. Liquid chromatography/mass spectrometry analysis of reduced di- and tetrasaccharides.
A) Heparin lyase digestion and liquid chromatography/mass spectrometry analysis. LSCH4 and OSCH2 were digested by a mixture of heparin lyase I, II, and III. OSCH2 was tagged with H-aniline and LSCH4 was tagged with D-aniline. Both tagged samples were reduced with NaBH4. An equal amount of samples was mixed and analyzed simultaneously by liquid chromatography/mass spectrometry. DA refers to a disaccharide containing an Δ4,5-unsaturated uronic acid (U) linked to N-acetylglucosamine (A). UAUH refers to a tetrasaccharide composed of uronic acid (U, GLCA or IDOA)-N-acetylglucosamine (A)-uronic acid (U)-glucosamine (H). S refers to sulfate. B) Low pH nitrous acid degradation and liquid chromatography/mass spectrometry analysis. Samples LSCH2, LSCH4, OSCH2, and S1 were degraded with nitrous acid at pH 1.5 and the resulting saccharides of LSCH2 and LSCH4 were reduced with NaBH4. OSCH2 and S1 were treated similarly and reduced with NaBD4. LSCH4 was mixed with OSCH2 whereas LSCH2 was mixed with S1. The mixtures were analyzed by liquid chromatography/mass spectrometry. UM refers to a disaccharide containing an unspecified uronic acid (U) linked to anhydromannitol (M). UAUM refers to a tetrasaccharide composed of uronic acid (U)-N-acetylglucosamine (A)-uronic acid (U)-anhydromannitol (M). S refers to sulfate.
In Figure 2A, LSCH4 and OSCH2 were digested with a combination of heparin lyase I, II, and III. The digested LSCH4 was tagged with D-aniline and the digested OSCH2 with H-aniline followed by NaBH4 reduction. Then an equal amount of the D-aniline tagged LSCH4 and H-aniline tagged OSCH2 were mixed and analyzed by liquid chromatography/mass spectrometry simultaneously. Seven di- and tetra-saccharides (designated DA, DH+1S, DH+2S, DH+3S, DAUH+3S, DAUH+4S, DHUH+6S) along with two novel heparin/heparan sulfate structures (designated DAUA+1S+103 and DAUH+3S+103) in the LSCH4 are shown in Figure 2A. (D stands for unsaturated uronic acid21 (ΔUA), U stands for either glucuronic or iduronic acid, A stands for N-acetylglucosamine, H stands for glucosamine, S stands for sulfate, and 103 stands for an unknown structure with a mass of 103 Daltons). In contrast, only DA and DH+1S were observed in the OSCH2 (Fig. 2A), which indicates that oversulfated heparin byproducts inhibited heparin lyase I but not heparin lyase III digestion since a comparable amount of non-sulfated and N-sulfated disaccharides was produced in the OSCH2.
To determine if the novel DAUA+1S+103 and DAUH+3S+103 structures were artifacts associated with heparin lyase digestion/aniline labeling/NaBH4 reduction of LSCH4, contaminated heparin samples LSCH2, LSCH4, OSCH2, LSOSCH4, S1, S2, and S3 were treated with nitrous acid at pH 1.5. Cleavage in this way removes N-sulfate groups and generates 2,5-anhydromannose at the reducing end.22 The identity of the nitrous acid generated di-, tetra-, and other saccharides was assessed by reducing the anhydromannose residues to 2,5-anhydromannitol with sodium borohydride (NaBH4) or sodium borodeuteride (NaBD4), respectively. Then an equal amount of the H-NaBH4 tagged LSCH2 or LSCH4 and D-NaBD4 tagged OSCH2 or S1 were mixed and analyzed by liquid chromatography/mass spectrometry simultaneously (Fig. 2B). Both OSCH2 and S1 showed enrichment in multiply sulfated disaccharides (UM+2S) and tetrasaccharides (UAUM+1 to 3 S) compared to LSCH2 and LSCH4, suggesting that heparin/heparan sulfate in OSLSCH4 and S1 are more sulfated than that in LSCH2 and LSCH4, which was consistent with the CE analysis result. Most importantly, UAUM+1S+103 and UAUM+2S+103 for H-NaBH4 tagged LSCH2 and LSCH4 and UAUM+1S+104 and UAUM+2S+104 for D-NaBD4 tagged OSLSCH4 and S1 were observed (Fig. 2B). We found that the unknown structures generated by H-NaBH4 tagging (103) or D-NaBD4 tagging (104) after low pH nitrous acid digestion was a common feature of all the contaminated heparins tested (LSCH2, OSCH4, LSOSCH4, S1, S2, and S3). Therefore, independent heparin lyase and nitrous acid treatments followed by NaBH4 or NaBD4 reduction generated the same unknown heparin/heparan sulfate structures. These results indicate that the DAUA+1S+103 and DAUH+3S+103 structures (ion current intensities are shown in Table 3) were not artifacts of the assay systems but represented chemically modified heparin/heparan sulfate because such structures were not detected in un-adulterated heparin or in other naturally occurring heparan sulfate by using the same liquid chromatography/mass spectrometry analysis approach.15
Table 3. Detection of oversulfated heparan sulfate in contaminated heparin by liquid chromatography/mass spectrometry.
A. Heparin lyase digestion and liquid chromatography/mass spectrometry analysis. LSCH4 and OSCH2 were digested by a mixture of heparin lyase I, II, and III. OSCH2 was tagged with H-aniline and LSCH4 was tagged with D-aniline. Both tagged samples were reduced with NaBH4. An equal amount of samples was mixed and analyzed simultaneously by liquid chromatography/mass spectrometry. The elution time and total ion current for the disaccharides, tetrasaccharides, and novel tetrasaccharides corresponding to the proposed structures were listed. The ratio of each structure in the two differentially tagged samples was determined.
| Proposed structures | A. Total Ion Current |
|||
|---|---|---|---|---|
| Elution time (min) | OSCH2 | LSCH4 | Ratio | |
| DA+0S | 15.6 | 7559 | 13000 | 0.58 |
| DH+1S | 17.0 | 453 | 846 | 0.54 |
| DH+3S | 20.1 | 0 | 37000 | |
| DAUH+4S | 23.3 | 0 | 1092 | |
| DHUH+6S | 24.7 | 0 | 1675 | |
| DAUA+1S+103 | 25.8 | 0 | 4885 | |
| DAUH+3S+103 | 28.0 | 0 | 1003 | |
DA refers to a disaccharide containing an Δ4,5-unsaturated uronic acid (U) linked to N-acetylglucosamine (A). UAUH refers to a tetrasaccharide composed of uronic acid (U, GlcA or IdoA)-N-acetylglucosamine (A)-uronic acid (U)-glucosamine (H). S refers to sulfate.
Establish a simple heparin quality control assay
Since certain contaminants cannot be detected by CE and/or NMR, and liquid chromatography/mass spectrometry analysis is an expensive and time consuming process for routine heparin quality control, we sought a technique to assess the purity of heparin based on its unique chemical structures recognized by heparin lyases that none of the contaminants can mimic. The liquid chromatography/mass spectrometry analysis indicates that oversulfated heparin contaminants specifically inhibited heparin lyase I but not heparin lyase III activities (Fig. 2A). Heparin lyase I specifically digests N-sulfated regions in heparin and heparan sulfate. The N-sulfated regions are more abundant in heparin than in heparan sulfate. Such properties make heparin lyase I digestion more specific for heparin detection compared to the combined heparin lyase I, II, and III digestions that detect both heparin and heparan sulfate equally well.
This assay monitors and quantifies the extent of heparin lyase I digestion by measuring the UV absorption at 232 nm of the unsaturated uronic acids generated during heparin lyase I digestion. The presence of contaminants (indigestible oversulfated GAGs, chondroitin sulfate, dermatan sulfate, non-sulfated regions of heparan sulfate, salts, and other macromolecules) reduces the expected absorbance.
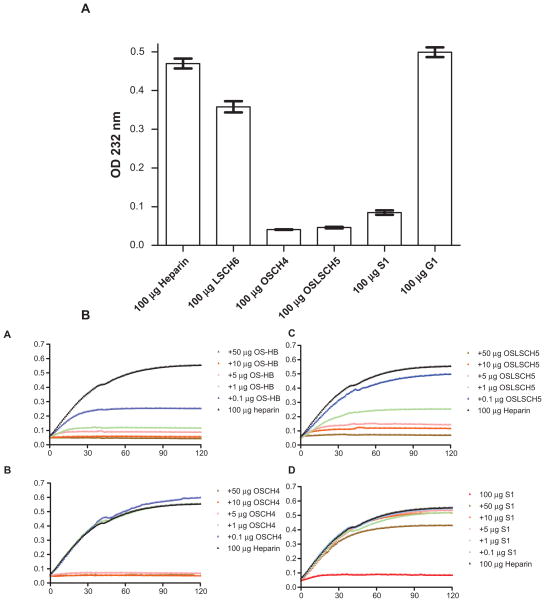
To demonstrate the utility of this approach, the six heparin lots described in Figure 1 were digested by heparin lyase I in triplicate and the extent of digestion was assessed (Fig. 3A). Lower UV absorbance was observed in the heparin lots containing low-sulfated contaminants (LSCH6 ΔOD 0.358 ± 0.025) compared to authentic heparin (ΔOD 0.470 ± 0.022). Heparin lots containing oversulfated contaminants (OSCH4 ΔOD 0.041 ± 0.001) or both oversulfated and low-sulfated contaminants (OSLSCH5 ΔOD 0.049 ± 0.003) were barely digestible. Contaminated heparin lots S1, S2, and S3, which have passed both FDA recommended CE and NMR screening, were only partially digestible by the enzyme (S1 ΔOD 0.085 ± 0.010). Based on this data, we suggest that heparin lots that deviate by 5% from the expected absorbance values of authentic heparin should be tested further for possible contamination.
Figure 3.
A) Detection of contaminated heparin by enzymatic assay. Exactly 100 μg of heparin and the five contaminated heparin lots shown in Figure 1 were digested for 2 hr with 1 mU heparin lyase I in triplicate and the absorbance at 232 nm was recorded for 120 min. The maximum change in absorbance (OD) of 100 μg of digested samples is shown with standard deviation. B) Inhibition heparin digestion by chemically sulfated heparin byproduct and contaminated heparins. Heparin (100 μg) alone or heparin plus chemically oversulfated heparin byproduct (OS-HB) or other contaminated heparins (OSCH4, OSLSCH5, and S1) in duplicates were digested with heparin lyase I and the absorbance at 232 nm was recorded every min for a total 120 min. Each data point of 120 data points shown in each digestion curve was average values of two independent heparin lyase digestion reactions. A) 100 μg heparin plus 0, 0.1, 1, 5, 10, or 50 μg of oversulfated heparin byproduct; B) 100 μg heparin plus 0, 0.1, 1, 5, 10, or 50 μg of contaminated heparin OSCH4; C) 100 μg heparin plus 0, 0.1, 1, 5, 10, or 50 μg of contaminated heparin OSLSCH5; D) 100 μg heparin plus 0, 0.1, 1, 5, 10, or 50 μg of contaminated heparin S1.
We also devised a second method based on the ability of oversulfated GAGs to inhibit heparin lyase I. In this assay, an aliquot of authentic heparin is mixed with different amounts of test sample and the extent of digestion is determined (Fig. 3B). This assay is extremely sensitive in detecting oversulfated contaminants. For example, 0.1% oversulfated heparin byproduct (1000 ppm) inhibited digestion of standard heparin by 50% (Fig. 3B). By using authentic heparin as a control, both of these methods are independent of the specific activities or source of the heparin lyase I. Furthermore, these assays only require access to a UV spectrophotometer, as opposed to expensive NMR and CE equipment.
Discussion
The monosaccharide analysis of 26 contaminated heparin lots indicates that GAGs were the dominant contaminants in heparin (Table 1). However, the heparin contaminants varied from lots to lots in that the contaminants could have faster, slower, or the same migration rate as heparin based on CE analysis (Fig. 1). We provided direct evidence that chemically oversulfated and desulfated GAGs were present in contaminated heparin lots G1, G2, and G3 (Table 2). We further showed the presence of abnormal heparin/heparan sulfate structures in different contaminated heparin lots (LSCH2, LSCH4, OSCH2, OSLSCH4, S1, S2, and S3 (Fig. 2 and Table 3). The abnormal structures, DAUA+1S+103 and DAUH+3S+103, had never been observed in heparin during the past.
Somehow, chemical desulfation reserved certain disulfated disaccharides in the modified chondroitin/dermatan sulfate and the hexasulfated tetrasaccharides in the modified heparin/heparin sulfate in G1, G2, and G3 samples (Table 2) by an unknown molecular mechanism. Overall, the non-sulfated disaccharides (Table 2) in the chemically modified chondroitin/dermatan sulfate and heparin/heparin sulfate dominated the overall charge density and gave the contaminants an under-sulfated phenotype based on the CE analysis (Fig. 1).
NMR analysis alone has failed to detect heparin contamination in six contaminated heparin lots (G1, G2, G3, S1, S2, and S3), whereas combined CE and NMR analysis have failed in 3 (S1, S2, and S3) out of 26 contaminated heparin lots in our current studies based on monosaccharide (Fig. 1 and Table 1), liquid chromatography/mass spectrometry (Fig. 2 and Tables 2 and 3), and heparin lyase digestion studies (Figs. 3). Therefore, a reliable assay is needed for future heparin quality control.
Pair wise comparison of test heparin with unadulterated heparin by liquid chromatography/mass spectrometry after enzymatic or chemical degradation (Fig. 2 and Tables 2 and 3) represents a reliable assay for future heparin quality control. However, the sensitivity and simplicity of assaying heparin samples by heparin lyase I digestion represent a practical assay for determining the presence of contaminants in heparin. Moreover, it detects not only oversulfated GAGs but also other possible contaminants in heparin. 23 Some contaminated heparin lots (e.g. G1, G2, and G3) produced higher UV absorbance compared to authentic heparin (Fig. 3). The presence of contaminants in these samples can be confirmed by CE (Fig. 1E) or anion exchange HPLC analysis. Thus, the enzymatic assays can be used independently or in conjunction with CE or DEAE-HPLC method to ensure that heparin is free of contaminants.
Heparin is one of the most widely used medications on a unit basis. Twelve million patients receive heparin treatment annually in the US alone.24 Heparin interacts with 22% of plasma proteins25 and regulates/balances many human physiological processes beyond its anticoagulation capability,26 which makes it an essential drug that people cannot do without. However, in a patient-specific manner, heparin can induce many contradicted side effects that have never been observed with any other drugs in modern medicine.
Despite heparin associated side effects, heparin remains a necessary life saving drug in modern medicine.1 Accumulated knowledge during the past 70 years enables doctors to handle most of heparin-associated side effects. Heparin should be kept clean for this reason alone.
Contaminated heparin has made the rare anaphylactoid reaction into an adverse event.3 Moreover, contaminated heparin is also associated with thrombocytopenia as long term side effects27,28 where affected patients could lose arms, legs, toes, or lives due to thrombosis. Therefore, all forms of contaminated heparin should be detected and eliminated from use. Keeping heparin clean will minimize heparin-associated public health risks considering hundreds of millions patients use heparin annually in the world.
Table 3B. Low pH nitrous acid degradation and liquid chromatography/mass spectrometry analysis.
Samples LSCH2, LSCH4, OSCH2, and S1 were degraded with nitrous acid at pH 1.5 and the resulting saccharides of LSCH2 and LSCH4 were reduced with NaBH4. OSCH2 and S1 were treated similarly and reduced with NaBD4. LSCH4 was mixed with OSCH2 and LSCH2 was mixed with S1. The mixtures were analyzed by liquid chromatography/mass spectrometry. The elution time and total ion current for the disaccharides, tetrasaccharides, and novel tetrasaccharides corresponding to the proposed structures were listed. The ratio of each structure in the two differentially tagged samples was determined.
| Proposed structures | B. Total Ion Current |
||||||
|---|---|---|---|---|---|---|---|
| Elution time (min) | OSCH2 | LSCH4 | Ratio | S1 | LSCH2 | Ratio | |
| UM+1S | 15.0 | 3000 | 3800 | 0.80 | 1180 | 1317 | 0.90 |
| UM+2S | 24.5 | 24000 | 27000 | 0.89 | 41000 | 30000 | 1.37 |
| UAUM+1S | 23.9 | 1440 | 1112 | 1.29 | 702 | 431 | 1.63 |
| UAUM+2S | 30.1 | 2669 | 2368 | 1.13 | 2286 | 1386 | 1.75 |
| UAUM+3S | 31.6 | 1339 | 1059 | 1.26 | 1712 | 914 | 1.87 |
| UAUM+1S+103 | 31.5 | 1743 | 2255 | 0.77 | 1600 | 746 | 2.14 |
| UAUM+2S+103 | 32.5 | 1021 | 1398 | 0.73 | 1794 | 763 | 2.35 |
UM refers to a disaccharide containing an unspecified uronic acid (U) linked to anhydromannitol (M). UAUM refers to a tetrasaccharide composed of uronic acid (U)-N-acetylglucosamine (A)-uronic acid (U)-anhydromannitol (M). S refers to sulfate.
Acknowledgments
The authors thank Dr. Jeffrey D. Esko for his suggestions, comments, and critiques of the manuscript.
Funding
This work is supported in part by National Institutes of Health Grants R01GM069968 to LZ.
Abbreviations
- GAG
glycosaminoglycan
- NMR
nuclear magnetic resonance
- CE
capillary electrophoresis
- OSCS
over-sulfated chondroitin sulfate
Footnotes
Authors’ Claim
The views presented in this article do not represent those of the Food and Drug Administration nor of Libertas Academica.
Competing Interests
The authors have declared that no competing interests exist.
This is an open access article. Unrestricted non-commercial use is permitted provided the original work is properly cited.
References
- 1.Fareed J, Hoppensteadt DA, Fareed D, et al. Survival of heparins, oral anticoagulants, and aspirin after the year 2010. Semin Thromb Hemost. 2008;34:411. doi: 10.1055/s-2008-1066025. [DOI] [PubMed] [Google Scholar]
- 2.FDA. Information on Adverse Event Reports and Heparin. 2009 http://wwwfdagov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatient-sandProviders/ucm112669htm.
- 3.Blossom DB, Kallen AJ, Patel PR, et al. Outbreak of Adverse Reactions Associated with Contaminated Heparin. N Engl J Med. 2008;359:2674–84. doi: 10.1056/NEJMoa0806450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guerrini M, Beccati D, Shriver Z, et al. Oversulfated chondroitin sulfate is a contaminant in heparin associated with adverse clinical events. Nat Biotech. 2008;26:669–75. doi: 10.1038/nbt1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keire DA, Trehy ML, Reepmeyer JC, et al. Analysis of crude heparin by (1)H NMR, capillary electrophoresis, and strong-anion-exchange-HPLC for contamination by over sulfated chondroitin sulfate. J Pharm Biomed Anal. 2009;51:921–6. doi: 10.1016/j.jpba.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 6.Guerrini M, Zhang Z, Shriver Z, et al. Orthogonal analytical approaches to detect potential contaminants in heparin. Proc Natl Acad Sci U S A. 2009;106:16956–61. doi: 10.1073/pnas.0906861106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casu B, Naggi A, Torri G. Chemical derivatization as a strategy to study structure-activity relationships of glycosaminoglycans. Semin Thromb Hemost. 2002;28:335–42. doi: 10.1055/s-2002-34302. [DOI] [PubMed] [Google Scholar]
- 8.Maruyama T, Toida T, Imanari T, Yu G, Linhardt RJ. Conformational changes and anticoagulant activity of chondroitin sulfate following its O-sulfonation. Carbohydr Res. 1998;306:35–43. doi: 10.1016/s0008-6215(97)10060-x. [DOI] [PubMed] [Google Scholar]
- 9.Toida T, Maruyama T, Ogita Y, et al. Preparation and anticoagulant activity of fully O-sulphonated glycosaminoglycans. Int J Biol Macromol. 1999;26:233–41. doi: 10.1016/s0141-8130(99)00088-4. [DOI] [PubMed] [Google Scholar]
- 10.Li B, Suwan J, Martin JG, et al. Oversulfated chondroitin sulfate interaction with heparin-binding proteins: new insights into adverse reactions from contaminated heparins. Biochem Pharmacol. 2009;78:292–300. doi: 10.1016/j.bcp.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volpi N. Purification of heparin, dermatan sulfate and chondroitin sulfate from mixtures by sequential precipitation with various organic solvents. J Chromatogr B Biomed Appl. 1996;685:27–34. doi: 10.1016/0378-4347(96)00154-5. [DOI] [PubMed] [Google Scholar]
- 12.Frazier SB, Roodhouse K, Hourcade DE, Zhang L. Glycosaminoglycan Quantification by HPLC, Carbazole, and Alcian Blue Methods. Open Glycoscience. 2008;1:31–9. doi: 10.2174/1875398100801010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Studelska DR, Giljum K, McDowell LM, Zhang L. Quantification of glycosaminoglycans by reversed-phase HPLC separation of fluorescent isoin-dole derivatives. Glycobiology. 2006;16:65–72. doi: 10.1093/glycob/cwj037. [DOI] [PubMed] [Google Scholar]
- 14.Guo YC, Conrad HE. Analysis of oligosaccharides from heparin by reversed-phase ion-pairing high-performance liquid chromatography. Anal Biochem. 1988;168:54–62. doi: 10.1016/0003-2697(88)90009-7. [DOI] [PubMed] [Google Scholar]
- 15.Lawrence R, Olson SK, Steele RE, et al. Evolutionary differences in glycosaminoglycan fine structure detected by quantitative glycan reductive isotope labeling. J Biol Chem. 2008;283:33674–84. doi: 10.1074/jbc.M804288200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Studelska DR, Mandik-Nayak L, Zhou X, et al. High affinity glycosaminoglycan and autoantigen interaction explains joint specificity in a mouse model of rheumatoid arthritis. J Biol Chem. 2009;284:2354–62. doi: 10.1074/jbc.M806458200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dündar M, Müller T, Zhang Q, et al. Loss of dermatan-4-sulfotransferase-1 function results in ‘adducted thumb-clubfoot syndrome’ (ATCS) The American Journal of Human Genetics. 2009;85:873–82. doi: 10.1016/j.ajhg.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trehy ML, Reepmeyer JC, Kolinski RE, Westenberger BJ, Buhse LF. Analysis of heparin sodium by SAX/HPLC for contaminants and impurities. J Pharm Biomed Anal. 2009;49:670–3. doi: 10.1016/j.jpba.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 19.Sohma Y, Hayashi Y, Skwarczynski M, et al. O-N intramolecular acyl migration reaction in the development of prodrugs and the synthesis of difficult sequence-containing bioactive peptides. Biopolymers. 2004;76:344–56. doi: 10.1002/bip.20136. [DOI] [PubMed] [Google Scholar]
- 20.Viskov C. Consequences of OSCS crisis on heparin compliance to USP proposed monograph. 2009 http://wwwusporg/pdf/EN/meetings/workshops/heparin2009Viskov2pdf.
- 21.Lawrence R, Lu H, Rosenberg RD, Esko JD, Zhang L. Disaccharide structure code for the easy representation of constituent oligosaccharides from glycosaminoglycans. Nat Meth. 2008;5:291–2. doi: 10.1038/nmeth0408-291. [DOI] [PubMed] [Google Scholar]
- 22.Shively JE, Conrad HE. Formation of anhydrosugars in the chemical depolymerization of heparin. Biochemistry. 1976;15:3932–42. doi: 10.1021/bi00663a005. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z, Li B, Suwan J, et al. Analysis of pharmaceutical heparins and potential contaminants using (1)H-NMR and PAGE. J Pharm Sci. 2009;98:4017–26. doi: 10.1002/jps.21729. [DOI] [PubMed] [Google Scholar]
- 24.Ahmad S. Heparin-induced thrombocytopenia: impact of bovine versus porcine heparin in HIT pathogenesis. Front Biosci. 2007;12:3312–20. doi: 10.2741/2314. [DOI] [PubMed] [Google Scholar]
- 25.Saito A, Munakata H. Analysis of plasma proteins that bind to glycosaminoglycans. Biochim Biophys Acta. 2007;1770:241–6. doi: 10.1016/j.bbagen.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 26.Lindahl U. What else can ‘Heparin’ do? Haemostasis. 1999;29(Suppl S1):38–47. doi: 10.1159/000054111. [DOI] [PubMed] [Google Scholar]
- 27.Adiguzel C, Bansal V, Litinas E, et al. Increased prevalence of antiheparin platelet factor 4 antibodies in patients may be due to contaminated heparin. Clin Appl Thromb Hemost. 2009;15:145–51. doi: 10.1177/1076029609332802. [DOI] [PubMed] [Google Scholar]
- 28.Greinacher A, Warkentin TE. Contaminated heparin. N Engl J Med. 2008;359:1291–2. doi: 10.1056/NEJMc081387. author reply 1293. [DOI] [PubMed] [Google Scholar]