Abstract
We have previously reported that short-term (48–72 h) exposure to the GABA-modulatory steroid 3α-OH-5α-pregnan-20-one (3α,5α-THP) increases expression of the α4 subunit of the GABAA receptor (GABAR) in the hippocampus of adult rats. This change in subunit composition was accompanied by altered pharmacology and an increase in general excitability associated with acceleration of the decay time constant (τ) for GABA-gated current of pyramidal cells acutely isolated from CA1 hippocampus similar to what we have reported following withdrawal from the steroid after chronic long-term administration. Because GABAR can be localized to either synaptic or extrasynaptic sites, we tested the hypothesis that this change in receptor kinetics is mediated by synaptic GABAR. To this end, we evaluated the decay kinetics of TTX-resistant miniature inhibitory postsynaptic currents (mIPSCs) recorded from CA1 pyramidal cells in hippocampal slices following 48-h treatment with 3α,5α/β-THP (10 mg/kg, ip). Hormone treatment produced a marked acceleration in the fast decay time constant (τfast) of GABAergic mIPSCs. This effect was prevented by suppression of α4-subunit expression with antisense (AS) oligonucleotide, suggesting that hormone treatment increases α4-containing GABAR subsynaptically. This conclusion was further supported by pharmacological data from 3α,5β-THP-treated animals, demonstrating a bimodal distribution of τs for individual mIPSCs following bath application of the α4-selective benzodiazepine RO15–4513, with a shift to slower values. Because 40–50% of the individual τs were also shifted to slower values following bath application of the non–α4-selective benzodiazepine agonist lorazepam (LZM), we suggest that the number of GABAR synapses containing α4 subunits is equivalent to those that do not following 48-h administration of 3α,5β-THP. The decrease in GABAR-mediated charge transfer resulting from accelerated current decay may then result in increased excitability of the hippocampal circuitry, an effect consistent with the increased behavioral excitability we have previously demonstrated.
INTRODUCTION
In the hippocampus, input from a diverse array of GABAergic interneurons produces inhibitory synaptic drive onto pyramidal cells in the CA1 region (Hajos and Mody 1997). The postsynaptic GABAA receptors (GABARs) on these cells are composed of a heterogeneous population of GABAR subunit isoforms, with α1 and α2-containing receptors predominating (Wisden et al. 1992), each localized to specific subsynaptic sites (Nusser et al. 1996). Many positive modulators of the GABAR exist, including the GABA-modulatory metabolite of progesterone, 3α-OH-5α-pregnan-20-one (3α,5α-THP), which acts in a barbiturate-like fashion to enhance GABA-gated currents of hippocampal neurons (Majewska et al. 1986) by increasing the duration of single channel openings and burst frequency of GABAR (Twyman and Macdonald 1992) without changing channel conductance.
It is well known that acute application of positive modulators of GABARs, such as benzodiazepines (BDZs) (Bai et al. 2001; Poisbeau et al. 1997; Zeng and Tietz 1999), anesthetics (Bai et al. 2001; Banks and Pearce 1999), and neuroactive steroids (Brussaard et al. 1997; Cooper et al. 1999; Haage and Johansson 1999; Harrison et al. 1987; Jorge-Rivera et al. 2000) prolong the decay time of miniature inhibitory synaptic currents. This resultant increase in inhibitory current is thought to underlie the sedative effect of these compounds (Bitran et al. 1991; File 1988). Although most studies have focused on acute effects of this steroid, our recent investigations (Gulinello et al. 2001) have demonstrated that alterations in both GABAR subunit expression and anxiety behavior reflect a complex temporal pattern following sustained exposure to 3α,5α-THP: initially, an increase in hippocampal expression of the α4 subunit is seen in correlation with increased anxiety after 48-h exposure to this steroid (Gulinello et al. 2001). These parameters recover to control levels by 5–7 days of continued steroid exposure and remain unaltered until withdrawal from the steroid after 21 days of steroid exposure (Gulinello et al. 2001), when increases in α4 levels and anxiety are again observed (Smith et al. 1998a,b).
In both cases, increased expression of α4-containing GABAR was associated with GABAergic current exhibiting fast decay kinetics (Gulinello et al. 2001; Smith et al. 1998a,b). However, this finding was observed in acutely isolated neurons in response to externally applied GABA and therefore must necessarily reflect contributions from both synaptic and extra-synaptic GABAR populations (Banks and Pearce 2000).
To determine whether hormone-induced upregulation of the α4 subunit results in a change in the composition and function of GABARs localized subsynaptically, analysis of unitary synaptic events is required. Under conditions where action potentials are suppressed with TTX, the recorded miniature inhibitory postsynaptic currents (mIPSCs) are believed to reflect the postsynaptic quantal response from a single vesicle at one synapse. The decay time constant of these unitary events thus reflects the kinetics of postsynaptic GABAR clusters, whereas compound events occur in response to asynchronous release of transmitter at multiple synapses, and are not useful for estimates of postsynaptic GABAR kinetic properties.
Here, we test the hypothesis that the changes in kinetics of GABAergic currents occur at synaptic sites, For this purpose, the amplitude and decay times of mIPSCs were recorded from adult CA1 pyramidal cells in the hippocampal slice after 48-h in vivo exposure of female rats to 3α,5α/β-THP. To test our hypothesis, α4 expression was suppressed by administering antisense oligonucleotide intraventricularly, thereby allowing us to determine whether increases in α4-containing GABAR contribute to observed changes in mIPSC decay. In addition, both α4-selective and non–α4-selective compounds were tested for their ability to modulate the decay and amplitude of recorded mIPSCs.
METHODS
Experimental animals
Adult female Long-Evans rats (Charles River, 140–200 g) were used for all protocols. Animals were housed in groups of three in a University-operated and AALAC-approved animal facility where the light:dark cycle (14:10 h) and room temperature (21°C) were maintained at constant levels. Food and water were available for ad libitum consumption. Animals were killed during the light phase of the cycle (~4–5 h after lights on). Control rats were tested only on the day of diestrus, a low hormone stage, verified by microscopic evaluation of the vaginal lavage, as previously described (Smith and Chapin 1996). All protocols were conducted following guidelines provided by the Institutional Animal Care and Use Committee.
Hormone administration paradigm
Animals were injected intraperitoneally with neurosteroid (10 mg/kg 3α,5α(β)-THP, 3 injections over 48 h) on a daily basis and killed 1–2 h following the last injection. Because both isomers result in similar increases in hippocampal α4 levels (data not shown), most studies were conducted using 3α,5β-THP. This hormone administration protocol has been shown to result in physiological levels of 3α,5α(β)-THP in the hippocampus (Smith et al. 1998b).
Antisense administration
As described previously (Smith et al. 1998a), 18 base pair antisense oligonucleotides were constructed +5 to the codon initiating translation for the α4 GABAR subunit (Genosys/Sigma), phosphorothioated at all positions, and purified with high-pressure liquid chromatography. Missense control oligonucleotides were identical to antisense oligonucleotides, except that every fourth base was scrambled, yielding an identical G:C content. Compounds were administered in the lateral ventricle (−0.8 mm A-P; 1.5 LAT; 3.2 DOWN; Paxinos and Watson 1982) for 72 h, beginning 1 day prior to and terminating at the conclusion of the hormone administration paradigm. The cannula guide had been previously implanted using stereotaxic surgery 1 wk prior to the onset of the experiment. Oligonucleotides were delivered via a subcutaneously implanted osmotic minipump (2001, Alza) at a concentration of 2 µg/d (vehicle, 0.35% bovine serum albumin/0.15 M saline) at a rate of 1 µl/h through 29-gauge tubing attached to the cannula. Successful downregulation of the α4 subunit was determined in 8 of 10 rats tested using Western blot procedures (see Western blot procedures). In all cases, however, successful delivery of oligonucleotides was verified by histological examination of cannula position and an empty minipump chamber. The two cases when downregulation did not occur thus served as sham controls (antisense failure).
Western blot procedures
Successful downregulation of α4 levels in hippocampus was determined with standard Western blot procedures, as described previously (Smith et al. 1998b). To this end, crude hippocampal membranes were first normalized according to protein content and then probed with an antibody developed against a peptide of the rat α4-subunit (amino acids 517–523, with an N-terminal cysteine), from a protocol originally described by Kern and Sieghart (1994). The α4 band (67 kDa) was detected with enhanced chemiluminescence visualization and quantified using a Umax scanner and One-Dscan software. The results were standardized to a glyceraldehyde 3-phosphate dehydrogenase (GAPDH, 36 kDa) control protein.
In vitro slice preparation
Animals were rapidly decapitated, and the brains were removed and cooled using an ice cold solution of artificial cerebrospinal fluid (ACSF) containing (in mM) 124 NaCl, 5 KCl,2 CaCl2,1.25 KH2PO4, 2 MgSO4, 26 NaHCO3, and 10 glucose, saturated with 95% O2-5% CO2 and buffered to a pH of 7.4. The hippocampi were then rapidly removed and cut into 400-µm coronal slices with a McIlwain-type Tissue Chopper. Hippocampal slices were held between two nylon nets in a tissue chamber on the stage of the microscope and perfused with ACSF (2 ml/min) at near-physiological temperature (35°C), with the exception of pharmacological tests, which were performed at a lower temperature (27°C) to increase sensitivity of the analysis (see Electrophysiological recording and analysis). The slices were allowed to incubate in an oxygenated chamber for at least 1 h prior to electrophysiological recording.
Electrophysiological recording and analysis
Spontaneous mIPSCs were recorded blind from the pyramidal cell layer of the CA1 hippocampus in the presence of 0.5–1 µM tetrodo-toxin (TTX) using whole cell patch-clamp procedures and low-pass filtering (2-kHz 4-pole Bessel filter) at a holding potential of −60 mV with an Axopatch 1D amplifier (Axon Instruments). Patch pipettes were fabricated from borosilicate glass using a Flaming-Brown puller, and the tips were fire-polished to yield open tip resistances of 2–4 MΩ. [Internal solution (in mM): 130 CsCl, 2 MgCl2, 10 HEPES, 0.2 BAPTA, 5 QX-314, and 2 Mg-ATP, pH 7.2, 290 mOsm]. The bath solution contained ACSF with 2 mM kynurenic acid added to block currents gated by excitatory amino acid transmitters. The GABAergic nature of the recorded currents was verified by blockade with bicu-culline methiodide (20 µM, data not shown) and reversal at ECl−. Only data collected under conditions with pipette access resistance <15 MΩ and 80% series resistance compensation were included in the analysis.
Data were recorded at a 44-kHz sampling frequency on a Vetter VCR and digitized at a 25-kHz sampling frequency using Trace Analyzer (M. Volaski, Albert Einstein College of Medicine, Bronx, NY). Data were filtered digitally at 1–2 kHz with a 4-pole Bessel filter (−3 dB), and events were detected with an automated software program (Ankri et al. 1994). Only currents with fast (<1 ms) rise times and stable baselines were analyzed. Preselection of unitary events with rapid (<1 ms) rise times precludes events distorted by dendritic filtering. The range of values for mIPSC amplitude observed here is consistent with what has been reported (Rudick and Woolley 2001) for female rat hippocampus, recorded under similar conditions. However, the recordings were not of high enough resolution to detect single channel openings, as has been reported (Kraszewski and Grantyn 1992).
The kinetics of mIPSCs recorded following hormone exposure were then analyzed with respect to their decay time constant using mono- and biexponential decay functions applied by nonlinear curve fitting routines from Origin software (Microcal). Biexponential decay functions are described by the following equation: I(t) = If exp(−t/τf) + Is exp(−t/τs); (I = amplitude; τ = decay time constant; f = fast s = slow component), fit between 10% and 90% of peak amplitude. Goodness-of-fit was determined with the least-squares method using Levenburg-Marquardt fitting routines or simplex algorithms as determined by the level of background noise. The F test was used to distinguish mono- versus biexponential decay functions; significance was noted when P < 0.05. In some cases, weighted averages (τw) were determined using the following equation: τw = biexponential fraction × [(fraction – fast decay) × (τfast + fraction-slow decay) × τslow] + monoexponential fraction × τmono. Because τmono was not significantly different from τw, τ was calculated as a monoexponential function in the pharmacology studies that sought to compare drug responses between groups.
The decay time constants, amplitude, and integrated current (total charge transfer) were determined for individual mIPSCs recorded from each neuron. Averaged values were calculated for each cell, and these values were averaged across hormone-treatment groups and states of α4 up- or downregulation. Furthermore, to quantify these changes, composite event frequency histograms of these parameters analyzed for individual mIPSCs (1,000–3,000 events) from the entire population of cells were constructed, using 80–100 mIPSCs/cell, to examine the distributions of the values. Decay time distributions are also presented for sample cells (Fig. 1) to illustrate changes in decay time constants with hormone treatment. All histograms were analyzed for Gaussian distribution, with single (Origin Labs) or multiple peaks (Origin or SEMMAC program; Ankri et al. 1994). In the latter case, an analytical algorithm was used that treats composite amplitude distributions as mixtures of Gaussians of unknown separations or variances (Korn et al. 1993). The frequency of events was also calculated for each cell, and values were averaged per group. In some cases, it was not possible to accurately analyze mIPSCs with amplitudes close to or within the background noise; therefore this population may be underrepresented in these distributions. In addition, although selection of rapid rise times is necessary to eliminate the possibility of dendritic filtering, mIPSCs with slower kinetics (Banks et al. 1998) may also be underrepresented in this analysis.
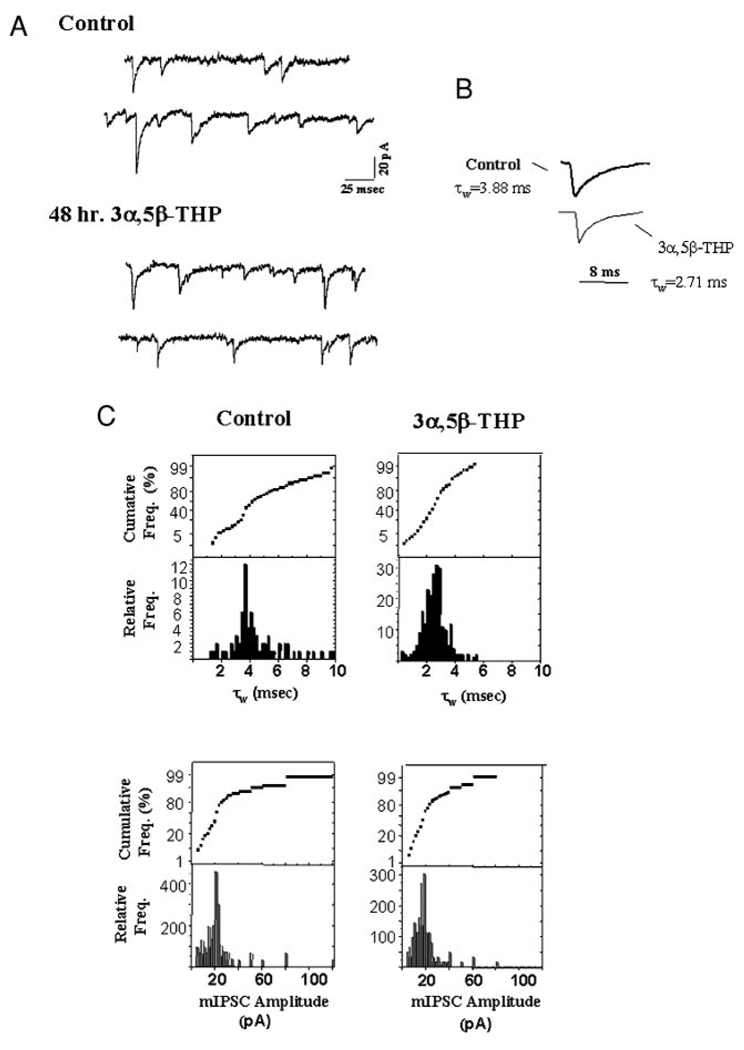
FIG. 1.
Neurosteroid exposure accelerates the decay time constant for GABAergic minature inhibitory postsynaptic currents (mIPSCs). mIPSCs recorded at 35°C following 48 h of 3α,5β-THP exposure are compared with those recorded under control, diestrous conditions. A: individual representative traces of hippocampal recordings from control (top) and 48 h 3α,5β-THP-treated (bottom) animals. B: averaged currents (25–30) from the hormone-treated group (48 h 3α,5β-THP) are presented with averaged control current traces, scaled to peak values. Significant decreases in τw are observed compared with control values (P < 0.05, n = 25, control, 30, 48 h THP). C: histograms and cumulative probability plots of decay time constants (τw, top) and amplitude (bottom) for individual mIPSCs recorded from a single cell/hormone group (n = 700, control; n = 1,200, THP). (These results are representative of those recorded from 80–100 mIPSCs/cell, 20–25 cells/group, 6–10 animals/group.)
Drug application
To distinguish the GABAR subunit composition of recorded mIPSCs, two selective GABAR modulators were tested for their ability to prolong τ of recorded mIPSCs. We chose modulatory drugs that would distinguish between GABAR containing the α4 subunit from those that do not to test the hypothesis that neurosteroid exposure increases the synaptic population of α4βγ2 receptors. Lorazepam (LZM), a BDZ agonist at non–α4-containing synaptic GABARs, is without effect at α4-containing GABARs (Wisden et al. 1991). This class of BDZs routinely increases τ (Poisbeau et al. 1997) of mIPSCs recorded from control hippocampal slices. It was bath applied at a concentration (10 µM) previously shown (Costa et al. 1995; Smith et al. 1998a) to produce robust increases in the amplitude of GABA(EC20)-gated current from acutely dissociated pyramidal cells from female rats recorded at room temperature. In contrast, a BDZ partial inverse agonist, RO15–4513 (10 µM) (Suzdak et al. 1998), acts as a selective BDZ agonist at α4-containing GABARs (Wafford et al. 1996). It was also bath applied for 15–20 min following consistent recording of control predrug responses at room temperature. For these studies, recordings were carried out at room temperature, because recent studies (Perrais and Ropert 1998) suggest that increases in BDZ affinity occur at lower temperatures and magnify changes induced by modulatory states, such as the hormone paradigm employed here. In addition, our previous concentration-response tests comparing drug responses across hormone state have been carried out at room temperature (Gulinello et al. 2001; Smith et al. 1998a,b). In all cases, mIPSCs were analyzed as described above before and during application of these BDZ ligands, and τw was calculated before and after application of these selective GABA-modulatory drugs.
A significant shift in the distribution of values for τ calculated for individual mIPSCs following drug application gives an indication of the percentage of currents (i.e., synapses) that respond to the drug. The percentage of currents that are shifted to slower values of τ following exposure to RO15–4513 versus LZM thus indicates the ratio of α4 and non–α4-containing GABARs within the recorded synaptic population for hormone-treated and control groups. (All chemicals were obtained from Sigma/RBI or Calbiochem.)
Statistical analysis
For all parameters, averaged values and SE were calculated and are presented in the results (mean ± SE). The unpaired Student’s t-test was implemented to determine statistical significance (P < 0.05) between two groups. For drug administration studies, differences between predrug and postdrug values were analyzed using the paired t-test. Differences between more than two groups were determined using one-way ANOVA followed by the Student-Newman-Keuls posthoc analysis, when the data followed a normal distribution. In cases where the data did not follow a normal distribution, the non-parametric Kolmogorov-Smirnov procedure was implemented to determine the degree of significance. In all cases, significance was determined when P < 0.05. The statistical significance of peak values identified by Gaussian analysis was determined using the Maximum Likelihood Estimate and the Wilke’s test (Korn et al. 1993).
RESULTS
Alterations in mIPSC characteristics following 48 h of 3α,5β-THP treatment
To determine if 48-h neurosteroid administration alters mIPSC characteristics, TTX-resistant synaptic currents were recorded from CA1 hippocampal pyramidal cells at near physiological temperature (35°C) using the slice preparation, and the results from steroid-treated and control animals were compared. In vivo exposure to the neurosteroid 3α,5β-THP for 48 h resulted in a significantly 30% faster (P <0.05) mIPSC decay time constant (τw), weighted for the relative contribution of fast and slow components and averaged from the mean values for each of the 25 cells recorded (Table 1) compared with control. Representative traces from single cells are shown in Fig. 1, where similar decreases in τw are noted for individual and averaged traces (Fig. 1, A and B, respectively).
TABLE 1.
Effects of 48 h in vivo 3α,5β-THP administration on mIPSC characteristics of pyramidal cells in CA1 hippocampus
| Control | 48 h of 3α,5β-THP | |
|---|---|---|
| 10–90% Rise time (ms) | 0.85 ± 0.04 | 0.78 ± 0.12 |
| τw (35°) | 3.42 ± 0.45 | 2.66 ± 0.32* |
| τw (27°) | 11.6 ± 2.1 | 8.23 ± 1.65* |
| Monoexponential τ (ms) | 3.3 ± 0.63 | 2.84 ± 0.46 |
| Biexponential τfast (ms) | 0.9 ± 0.16 | 0.5 ± 0.12* |
| (%) | (38 ± 6.7) | (58 ± 11.1) |
| τslow (ms) | 5.2 ± 0.5 | 5.0 ± 1.2 |
| Percent of mIPSCs with a biexponential decay | 10 | 38* |
| mIPSC frequency (Hz) | 12.5 ± 4.5 | 11.2 ± 3.1 |
Unless otherwise indicated, recordings were carried out at 35°C (n = 1,800/group, 35°C; n = 2,000/group, 27°C; representative of 80–100 mIPSCs/ cell, 20–25 cells/group, 6–10 animals/group.)
P < 0.05 vs. control values.
The distribution of values of τw recorded from a single cell after short-term hormone treatment (Fig. 1C) reflects a single mode, with a peak value (2.55 ± 0.04 ms) significantly (P < 0.05) less than that of the control distribution, which in this case was either bimodal (peaks at 3.8 ms, wt. 0.42; 5.0 ms, weight 0.58) or somewhat skewed to the right. Overall, when current deactivation rates were analyzed with respect to the number of exponentials, a greater fraction of mIPSCs recorded from hormone-treated animals were found to decay biexponentially (Table 1), with a markedly accelerated fast component of decay (τfast < 1.0 ms) compared with control values, but no significant difference in the slow component of decay.
Consistent with the observed decreases in τw, the total charge transfer was also significantly (P < 0.01) decreased following 48-h 3α,5β-THP treatment compared with control (Figs. 4B and 5B). However, the range of values for mIPSC amplitude was not significantly altered, although there was a slight shift to lower values after 3α,5β-THP treatment versus control (Fig. 1C). The frequency of mIPSC occurrence did not vary across hormone state (48-h 3α,5β-THP exposure, 11.2 ± 3.1 Hz, n = 1,200 vs. control, 12.5 ± 4.5 Hz, n = 700).
FIG. 4.
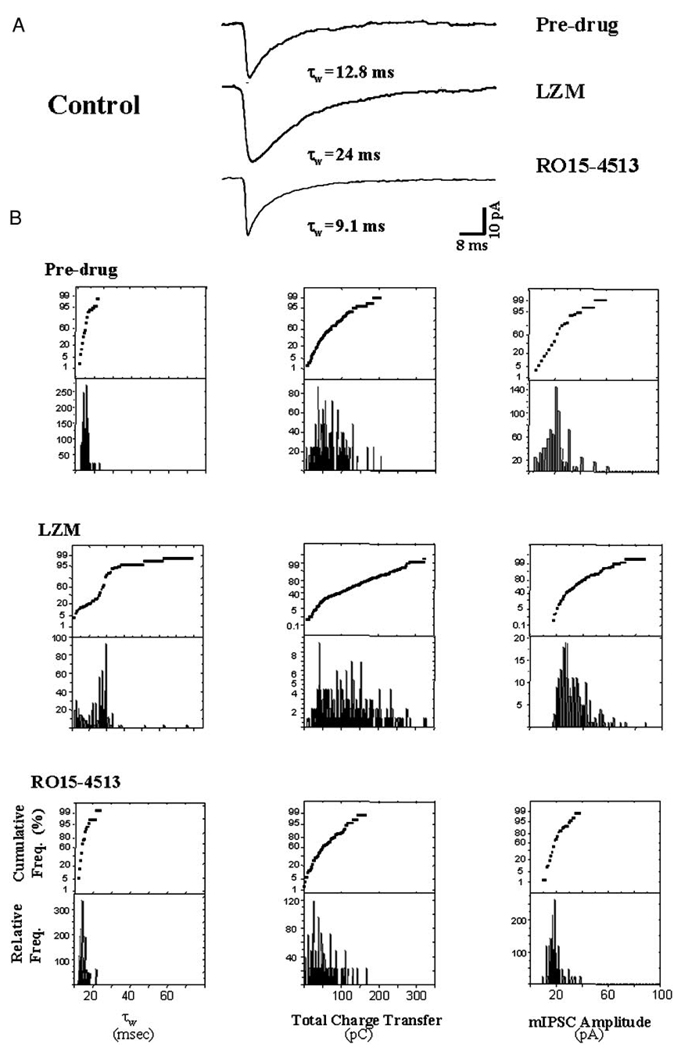
Pharmacological evaluation: control data. In this figure, benzodiazepine modulation of synaptic current recorded from hippocampal slices of control, untreated animals is presented, while in the following figure similar pharmacological tests are carried out in slices from hormone-treated animals. For this and the following figure, 2 differentially selective BDZs, LZM, a non-α4 BDZ agonist, and RO15–4513, which responds as a BDZ agonist only at α4-containing GABAR, were used to test the presence of α4-containing GABAR at synaptic sites. To this end, either LZM (10 µM) or RO15–4513 (10 µM) was bath applied for 20 min following 15–20 min of predrug recording, and possible changes in τw, total charge transfer and amplitude of individual mIPSCs recorded at room temperature (27°C) across the entire population of cells were assessed. Under control conditions, mIPSCs responded robustly to bath application of LZM. Analysis of the averaged current revealed a 2-fold increase in τw, total charge transfer (integrated total current), and current amplitude following LZM administration. In contrast, application of the BDZ partial inverse agonist RO15–4513 under control conditions resulted in minimal decreases in the mIPSC τ, but no significant change in the total charge transfer or mIPSC amplitude. A: superimposed averaged (20–30) current traces. B: histograms and cumulative probability plots of the distribution of the values for τw (left), total charge transfer (middle), and amplitude (right) of individual mIPSCs recorded under the indicated conditions (n = 2,000 events/group; 80–100 mIPSCs/cell, 2–5 cells/animal, 5–6 animals/ group).
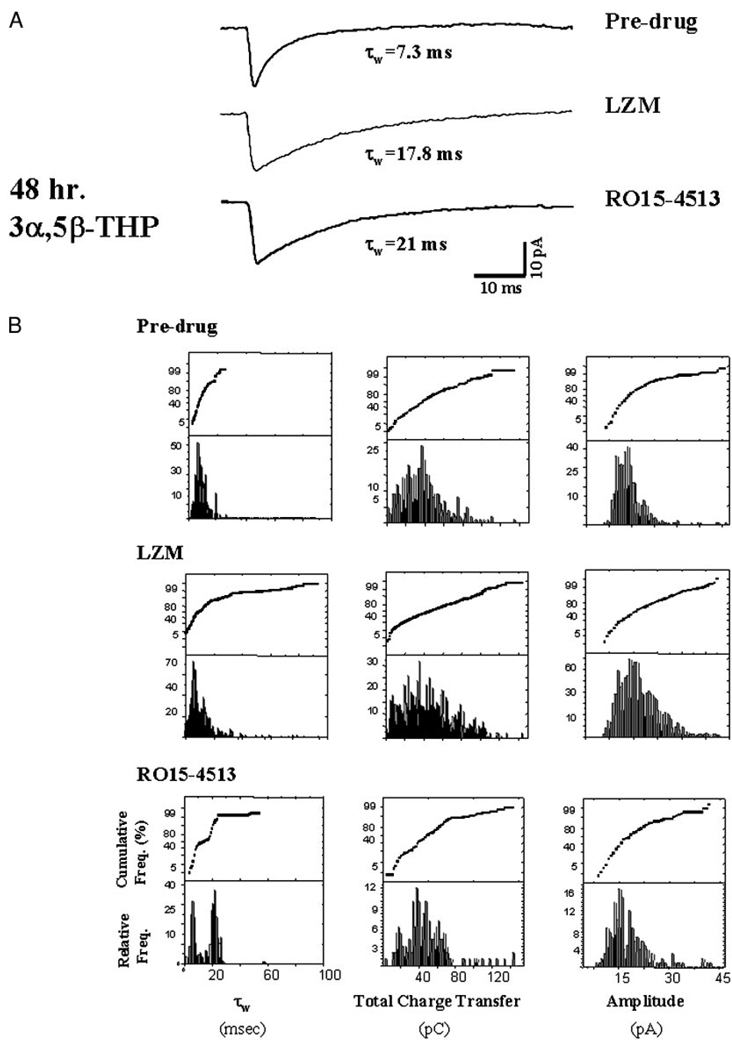
FIG. 5.
Pharmacological evaluation: hormone treatment. Forty-eight hour neurosteroid treatment alters subunit-selective pharmacological responses of mIPSCs. Superimposed averaged (20–30) current traces from A and histograms/probability plots of individual mIPSC characteristics (B) reveal that both the non-α4 selective LZM (10 µM) and the α4-selective RO15–4513 (10 µM) significantly (P < 0.05) prolong τw compared with predrug values of pyramidal cells recorded from hormone-treated rats. B: postdrug (RO15–4513) values of τw reveal a bimodal distribution (left), with 1 peak significantly greater than the predrug value. This pattern suggests an equivalent distribution between populations of cells responsive to and unresponsive to modulation by this compound. Similarly, the distribution of values for τw following bath application of LZM reveals bimodal peaks, suggesting heterogeneity of synaptic responses to this compound. The values for total charge transfer reflect a similar distribution (middle). However, in contrast to the control results (see Fig. 4), postdrug amplitude distributions (right) were not significantly different from predrug values for either drug tested (n = 2,000 events/group; 80–100 mIPSCs/cell, 2–5 cells/animal, 20–25 animals/group).
α4 GABAR subunit antisense administration
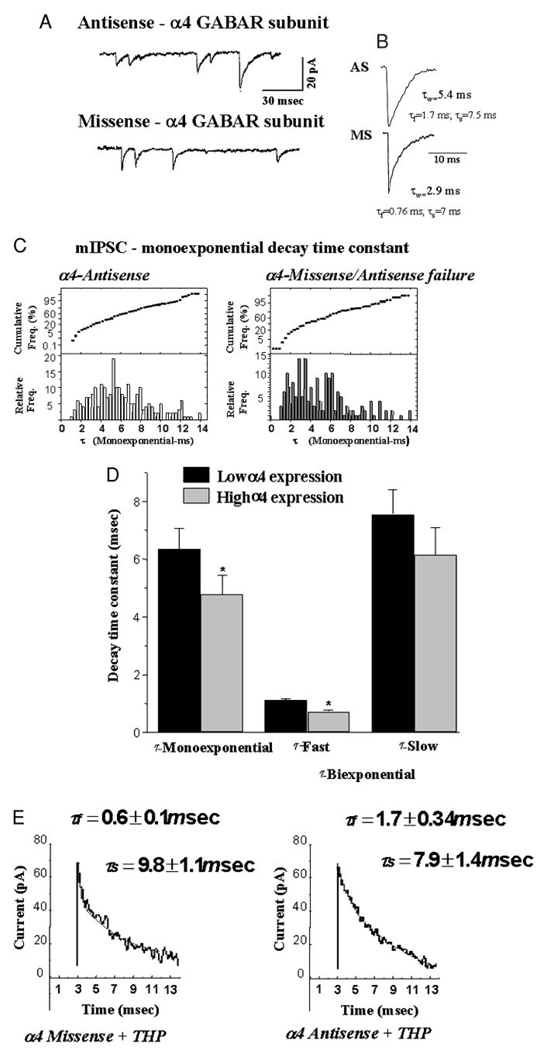
Our previous work established that 48-h exposure to 3α,5α-THP (Gulinello et al. 2001) increases hippocampal levels of the α4 GABAR subunit by two- to threefold. To test the possibility that α4-containing GABAR at the synapse contribute to the acceleration in mIPSC τ observed following hormone exposure, hormone-treated animals were continuously administered α4 antisense or missense oligonucleotide intraventricularly across the final 72-h period of the respective hormone paradigm. Administered in this way, α4 antisense oligonucleotide significantly (P < 0.001) reduced hippocampal levels of the GABAR α4 subunit from a 180% increase to almost undetectable levels (92 ± 5% reduction) in 8 of 10 animals following 48-h neurosteroid exposure (Fig. 2). Using this approach, a significantly (P < 0.05) slower τw was observed under conditions of low α4 expression (5.52 ± 0.45 ms) than was seen with high α4 expression (2.87 ± 0.32 ms).
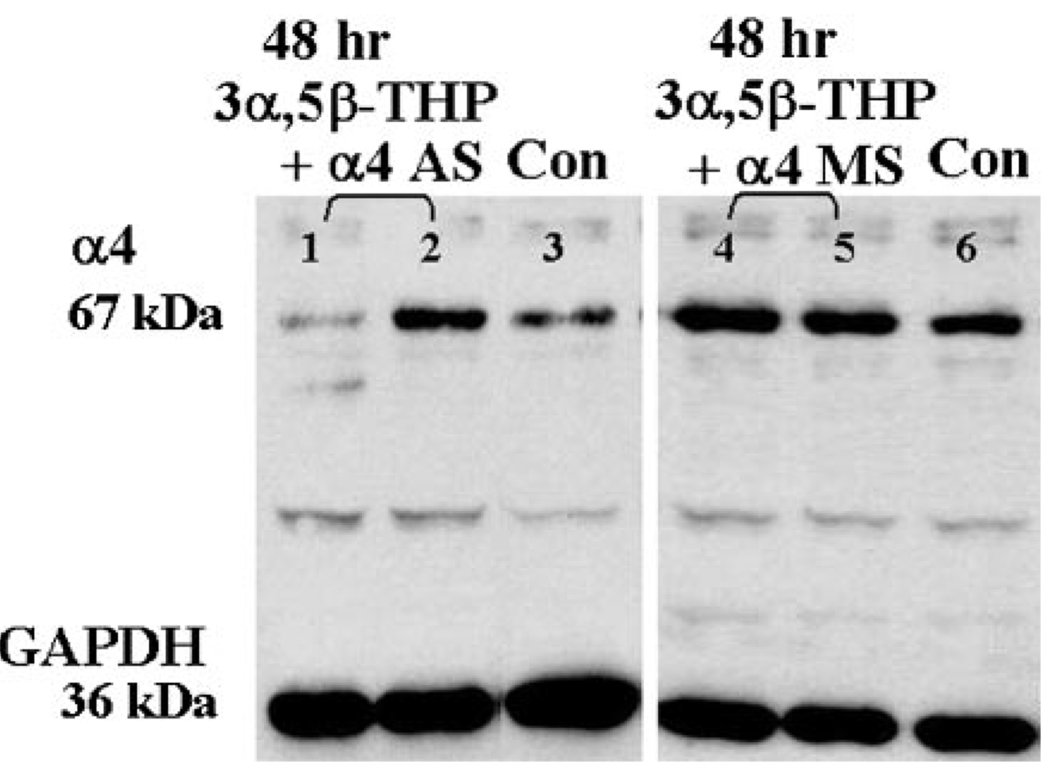
FIG. 2.
Antisense treatment prevents α4 subunit upregulation by chronic neurosteroid exposure. A representative Western blot demonstrates both successful (8/10, 1) and unsuccessful (2/10, 2) suppression of α4 subunit expression by intraventricular administration of α4 antisense oligonucleotide compared with untreated control (3). In contrast, missense treatment (10/10) did not prevent robust increases in α4 expression (4, 5) compared with control (6) (performed in triplicate at 2 different protein concentrations).
Because the primary change in mIPSC characteristics observed as a consequence of neurosteroid exposure was acceleration of the fast component of decay, individual mIPSCs recorded from both treatment groups were evaluated for mono-and biexponential fit. To this end, the coefficient of determination (r2) and the F test were used to distinguish between fits. Using this approach, conditions of high α4 expression (missense/antisense failure + THP) were associated with a greater fraction of mIPSCs best fit with a biexponential decay compared with low (antisense + THP) α4 expression (42.3% vs. 16.3%, respectively; Fig. 3; Table 2). The distribution of values for the fast component of τ (τfast) exhibited two peaks with values of 0.54 ± 0.007 ms (75%) and 1.08 ± 0.04 ms (25%, mean = 0.67 ± 0.03 ms, P < 0.05) under conditions of high α4 expression, with the fast component accounting for 62% of the total current. In contrast, τfast was significantly slower (1.14 ± 0.06 ms), and it accounted for a smaller fraction of the total current (47.0%) under conditions of low α4 expression (P < 0.05). Values for τslow were not significantly different between high and low α4 expression groups (Table 2).
FIG. 3.
Suppression of α4 GABAA receptor (GABAR) subunit expression alters the kinetics of GABA-gated current following neurosteroid exposure. For this study, animals were administered 3α,5β-THP over a 48-h period (10 mg/kg, ip), in conjunction with either α4 antisense or missense oligonucleotide administered intraventricularly to manipulate α4 subunit expression (see Fig. 2). Representative traces (A) and averaged, scaled currents (B) from both α4 antisense (top) and α4 missense-treated (bottom) animals during the 48-h 3α,5α-THP administration paradigm. (n = 30, AS; n = 34, MS). C: histograms and probability plots for mIPSCs best fit with a monoexponential τ recorded under conditions favoring high α4 expression (α4-Missense/Antisense failure,right, n = 1,731) vs. low α4 expression (α4-Antisense, left, n = 2,511). D: summary diagram: conditions of high α4 expression resulted in an accelerated τfast compared with values recorded under conditions of low α4 expression, but no significant change in τslow (n = 489, AS; n = 1,269, MS). Significant 30% decreases in τmono are also observed under conditions of high α4 expression. (n = 80–100 mIPSCs/cell, 4–8 cells/animal, 8–12 rats/group). E: representative current trace from α4 missense (left) and α4 antisense (right) groups demonstrate a faster τfast under conditions of high α4 expression (α4 Missense + THP). (These results are representative of those recorded from 4–8 cells/animal, 8–12 animals/group.)
TABLE 2.
mIPSC characteristics of pyramidal cells in CA1 hippocampus under conditions of high and low α4 expression
| LOW α4 | HIGH α4 | |
|---|---|---|
| 10–90% Rise time (ms) | 0.88 ± 0.07 | 0.82 ± 0.08 |
| τw | 5.52 ± 0.45 | 2.87 ± 0.32* (MS) |
| 2.56 ± 0.24* (AS failure) | ||
| Monoexponential τ | 6.02 ± 0.05 | 3.40 ± 0.37 |
| (ms) | (n = 2,511) | (n = 1,731) |
| Distribution of τmono | 2.73 ± 0.07 (80%) | |
| (%) | 5.96 ± 0.17 (20%) | |
| Biexponential τfast | 1.14 ± 0.06 | 0.67 ± 0.03* |
| (ms) | (n = 489) | (n = 1,269) |
| (%) | (47 ± 2.6) | (61.6 ± 1.7) |
| Distribution of τfast | 0.54 ± 0.007 (75%) | |
| (%) | 1.08 ± 0.04 (25%) | |
| τslow (ms) | 5.2 ± 0.5 | 5.0 ± 1.2 |
| Percent of current with a biexponential decay | 16.3 | 42.3* |
| Frequency (Hz) | 10.1 ± 2 | 11.6 ± 3 |
Either antisense (LOW α4) or α4 missense (HIGH α4) oligonucleotide was administered intraventricular for 72 h concomitant with 48-h 3α,5β-THP administration (ip) to female rats. In two cases, antisense administration failed to suppress α4 expression (AS failure), and these values are included in the HIGH α4 group, because the findings were statistically similar to the missense data. In all cases, α4 levels in the hippocampus were verified by Western blot. All recordings were carried out at 35°C. (n = 3,000 total mIPSCs/group).
P < 0.05 vs. control values.
Conditions of low α4 expression resulted in a majority of mIPSCs best fit as monoexponential decay functions (Fig. 3; Table 2). The distribution of values for τmono revealed a single peak around 5 ms, with an average τmono = 6.02 ± 0.05 ms. In contrast, high α4 conditions (missense/antisense failure) produced a bimodal distribution of τmono, with peaks at 2.73 ± 0.07 and 5.96 ± 0.17 ms (P < 0.05), yielding an average τmono = 3.40 ± 0.37 ms. From the total population of mIPSCs sampled following 48-h 3α,5β-THP treatment (both mono-and biexponential decays), the total percentage of current decaying with a faster rate than control currents under conditions of α4 upregulation was 47%, thus suggesting that approximately one-half of the synaptic GABAR clusters exhibit faster rates of deactivation following short-term neurosteroid exposure.
Benzodiazepine modulation of synaptic current after 48-h 3α,5β-THP treatment: LZM
To pharmacologically and quantitatively distinguish between α4-containing and non–α4-containing subsynaptic GABARs following hormone treatment, synaptic responses were recorded after application of the BDZ ligands LZM or RO15–4513, which elicit different responses at α4βγ2 versus non-α4βγ2 GABARs (Wafford et al. 1996; Wisden et al. 1994). LZM is a selective BDZ agonist at GABAR isoforms that lack the α4 or α6 type subunit (Wafford et al. 1996; Wisden et al. 1994). That is, α4-containing GABARs are insensitive to modulation by this compound. Therefore distributions of τw (weighted decay time constant) for individual mIPSCs were analyzed before and after bath application of 10 µM LZM to compare responses of slices from hormone-treated versus control animals and to estimate the percentage of non–α4-containing GABARs subsynaptically. This compound yielded robust two- to threefold increases in τw of individual mIPSCs recorded at 27°C in slices from control animals compared with predrug responses (Fig. 4, A and B; Table 3). In contrast, synaptic currents recorded following 48-h 3α,5β-THP treatment (Fig. 5; Table 3) responded to LZM with at most a 70–100% increase in τw. While the frequency distributions of τw suggest that 90% of control values were shifted to slower values after exposure to LZM, only 30–40% of individual mIPSC τws from hormone-treated slices were shifted to slower values following LZM application.
TABLE 3.
Weighted decay τw of mIPSCs analyzed before and after bath application of the non-α4 selective BDZ agonist LZM (10 µM) or the α4 selective BDZ agonist RO15-4513 (10 µM) to hippocampal slices from control or 48 h 3α,5β-THP-treated female rats
| Pre-drug τw, ms | Post-drug τw, ms | |
|---|---|---|
| Control | ||
| LZM | 11.2 ± 3.0 | 22.4 ± 1.30* |
| RO 15-4513 | 11.2 ± 3.0 | 8.7 ± 0.6* |
| 48 h | ||
| 3α,5β-THP | ||
| LZM | 8.79 ± 1.2 | 13.1 ± 0.9* |
| RO 15-4513 | 8.79 ± 1.2 | 20.3 ± 3.3* |
All recordings were carried out at 27°C. Values were averaged from individual cell means for each condition (n = 2,000 mIPSCs, 15–25 cells/group, 6–15 rats/group). τw, time constants; LZM, lorazepam.
P < 0.05 vs. control values.
Under control conditions, bath application of LZM also increased the mIPSC amplitude twofold under control conditions (Fig. 4A), an effect observed in more than 60% of the recorded cells. In contrast, mIPSC amplitude was increased by 50% in only 10–15% of the recorded mIPSCs following 48-h neurosteroid exposure (Fig. 5A). In both cases, total charge transfer was increased by LZM administration (Figs. 4 and 5), but this effect was significant (P < 0.05) only for mIPSCs recorded from control slices.
RO15– 4513
The second compound used, RO15–4513, is a BDZ partial inverse agonist at GABARs lacking the α4/6 subunit (Suzdak et al. 1988) and a full positive agonist at receptors containing the α4 subunit (Wafford et al. 1996). Thus estimating the percentage of synaptic currents responsive to this compound should give an indication of the prevalence of α4-containing GABAR located sub-synaptically. mIPSCs recorded following 48-h 3α,5β-THP treatment exhibited a significant (P < 0.05) prolongation of decay time (τw) following bath application of 10 µM RO15–4513 compared with decay of currents recorded prior to drug application (τw = 20.3 ± 3.3 vs. 8.79 ± 1.2 ms, predrug, Fig. 5, Table 3). In contrast, the decay of mIPSCs recorded in slices from untreated rats was significantly accelerated (Fig. 4; Table 3) after exposure to this drug, an effect consistent with its properties as a BDZ partial inverse agonist at non–α4-containing GABARs. Analysis of the individual currents across the entire population of cells from hormone-treated animals sampled before and during application of RO15–4513 revealed a bimodal distribution. The primary peak, which accounted for approximately 60% of the recorded current, represented values of τw around 20 ms, a value significantly greater than the 8.45 ms average calculated for predrug values. In contrast, the secondary peak around 5 ms represented a slightly decreased range of values for τw compared with predrug conditions. Individual values calculated for total charge transfer also exhibited a bimodal distribution, with peaks similar to predrug values as well as higher (56 pC) than the range of predrug values for this parameter. In contrast, mIPSC amplitude was unaffected by bath application of RO15–4513. These results suggest that approximately 50% of the GABAergic currents recorded respond to α4-selective compounds following neurosteroid exposure.
DISCUSSION
The results from this study suggest that increased expression of α4βγ2 GABARs at CA1 pyramidal cell synapses produced by a 48-h neurosteroid exposure results in current with an accelerated decay time constant. This change was accompanied by significant decreases in total charge transfer, an effect that would decrease inhibition following hormone treatment. In contrast, there were insignificant decreases in mIPSC amplitude and no change in event frequency, suggesting a specific action on the postsynaptic component of GABAergic synapses. The resulting decrease in inhibitory synaptic input to CA1 hippocampal neurons as a consequence of hormone exposure is consistent with the increased behavioral excitability we observe at this time (Gulinello et al. 2001).
α4 Subunit upregulation
The results from the present study suggest that (α4-containing GABARs localized to synaptic sites are responsible, at least in part, for the observed decrease in decay time for GABAergic mIPSCs following short-term neurosteroid exposure. The most compelling evidence for this conclusion is that suppression of expression of α4 subunit levels prevented the decrease in mIPSC τ following hormone exposure. In contrast, marked decreases in τ were observed under conditions where α4 upregulation was not suppressed following hormone exposure.
In addition, the pharmacological specificity of mIPSC response observed after 48-h neurosteroid treatment also suggests a sub-synaptic localization of α4-containing GABARs: the distribution of decay time constants recorded at this time shifted to slower values after bath application of the α4-selective BDZ agonist RO15–4513 (Wafford et al. 1996). This shift in τ distribution is consistent with a prolongation in τ for about 50% of the responses. In contrast, this compound produced slight decreases in τ for mIPSCs recorded under control conditions, an effect consistent with its role as a BDZ partial inverse agonist at non-α4/α6 GABAR (Suzdak et al. 1988). mIPSC τ distributions calculated after 48-h 3α,5β-THP treatment also revealed a population of slower τs for 50% of the recorded population in response to LZM application, reflective of non–α4-containing GABARs, because the α4 subunit is BDZ-insensitive (Wafford et al. 1996; Wisden et al. 1991). It is noteworthy that both the pharmacology and antisense protocols revealed changes in approximately one-half of the synaptic events recorded from cells following hormone treatment. This suggests a 1:1 expression of α4- and non–α4-containing GABARs subsynaptically following 48-h neurosteroid exposure.
Although α4-containing GABARs have not been localized to the synapse heretofore, due to problems with antibody affinity, the α4 subunit has been shown to coexpress with the γ2 subunit in CA1 hippocampus (Sur et al. 1999), which is required for synaptic localization (Essrich et al. 1998). In addition, however, 3α,5β-THP withdrawal following chronic administration of its parent compound progesterone increases expression of α4βδ GABAR (Sundstrom-Poromaa et al. 2002), a receptor isoform that is believed to be extrasynaptic (Nusser et al. 1998). These results suggest the possibility that α4-containing receptors may be differentially distributed between synaptic versus extrasynaptic GABAR populations, depending on the hormone exposure paradigm.
Current deactivation
The mIPSC decay time constant is an approximate measure of the deactivation rate of synaptically localized GABARs, given that the GABA released at synapses on the pyramidal cell soma, the locus of most inhibitory activity (Soltesz et al. 1995), is quickly (<1 ms) removed from the synaptic cleft (Maconochie et al. 1994; Williams et al. 1998). The most striking effect of neurosteroid exposure was to accelerate τfast, an effect blocked by prior administration of antisense oligonucleotide to prevent α4 subunit upregulation, while τslow was either unchanged, or in some cases, prolonged in comparison to control values. The fast component of decay is thought to represent the initial closing of channels within a burst (Jones and Westbrook 1996; McClellan and Twyman 1999) while τslow is more likely to represent final channel closing and unbinding of ligand (Jones and Westbrook 1996). In the present study, currents were preselected for rapid rise times (<1 ms); thus analyzed currents would be less likely to be contaminated with the effects of dendritic filtering, which would have produced a more heterogeneous range of values for τfast (Edwards et al. 1990). The acceleration in τfast following 48-h 3α,5β-THP exposure is consistent with recent findings demonstrating a shorter mean open time for α4-containing GABAR, assessed using fluctuation analysis (Maric et al. 1999). In fact, rates of GABAR deactivation are known to be influenced by the expression of particular GABAR α subunits; α1-containing GABARs deactivate with a time constant sixfold faster than α2-containing GABARs, an outcome demonstrated both using excised neuronal membrane patches and synaptic current recording (Lavoie et al. 1997; Vicini et al. 2001).
In addition to producing decreases in τ, 48-h exposure to 3α,5β-THP also increased the percentage of currents decaying biexponentially from 16% (Poisbeau et al. 1999) to 42%. Interestingly, in addition to reflecting a change in GABAR subunit composition, this phenomenon may be a result of post-translational modification such as receptor phosphorylation. In fact, increases in phosphorylation (Poisbeau et al. 1999) produce a number of changes that are strikingly similar to those we observe following short-term hormone treatment, including 1) an increase in currents with a biexponential decay, 2) an acceleration in τ (Jones and Westbrook 1997), and 3) a decrease in mIPSC amplitude. These intriguing similarities suggest that alterations in phosphorylation state may also play a role in mediating the changes in synaptic current we observe following neurosteroid exposure.
Transmitter saturation
Our data suggest that mIPSC amplitude is increased by bath application of the BDZ agonist LZM in slices from control animals, an effect that was markedly attenuated following 48-h exposure to neurosteroid. This finding is most likely to represent a difference in postsynaptic receptor saturation between the two experimental conditions. In acutely isolated CA1 hippocampal neurons from control female rats, 10 µM LZM prolongs the decay but fails to increase the amplitude of currents gated by saturating concentrations of GABA at room temperature (unpublished data), consistent with an effect of this drug on increasing the frequency of single channel bursts as previously reported (Twyman et al. 1989). Therefore an increase in mIPSC amplitude produced by LZM may reflect a lack of receptor saturation at these synapses under control conditions. Recent studies have demonstrated that mIPSCs recorded at room temperature from CA1 hippocampus in young male rodents are increased in amplitude following application of BDZ type I agonists such as zolpidem (Cohen et al. 2000; Hajos et al. 2000; Perrais and Ropert 1999), suggesting that these synapses do not receive saturating concentrations of agonist during quantal release. Although synapses in adult, male rat CA1 hippocampus have been shown to receive saturating concentrations of transmitter (Cohen et al. 2000), the present study is the first to evaluate these synapses in the female and to suggest a gender-specific effect. However, after short-term neurosteroid exposure, BDZ agonists produced only minor increases in the amplitude of mIPSCs, suggesting that one consequence of short-term neurosteroid administration is saturation of synaptic GABARs. This effect is most likely due to a decrease in GABAR density subsynaptically rather than to an increase in released GABA, because mIPSC amplitude was slightly decreased under predrug conditions compared with mIPSC amplitude recorded from the diestrous control animals. However, presynaptic mechanisms (Frerking et al. 1995) cannot be completely ruled out.
Functional consequences of kinetic changes
Chronic exposure to and withdrawal from other GABA modulatory compounds such as the benzodiazepines also produces changes in hippocampal synaptic current by decreasing mIPSC amplitude (Poisbeau et al. 1997; Zeng and Tietz 1999), and in some cases, this results in “silent” synapses in hippocampal neurons (Poisbeau et al. 1997). In all cases, these changes would be expected to decrease inhibitory tone in this region, an effect that would be expected to produce hyperexcitability of the circuit. In the present study, decreases in total charge transfer resulting from the faster τ produced by neuro-steroid administration would also lead to hyperexcitability of the hippocampal circuitry.
This decrease in inhibition may underlie the increases in anxiety observed after 48-h exposure to neurosteroid when BDZ-resistant increases in anxiety are observed (Gulinello et al. 2001). In addition, the present results may be comparable to chronic treatment or withdrawal from other GABA-modulatory drugs (Devaud et al. 1997; Holt et al. 1996; Mahmoudi et al. 1997) and kindling models of epilepsy, when circuit hyperexcitability and α4 subunit upregulation (Brooks-Kayal et al. 1998; Holt et al. 1996; Mahmoudi et al. 1997) occur in conjunction with BDZ insensitivity (Kapur 2000; Mtchedlishvili et al. 2001).
In conclusion, the results from the present study suggest that short-term in vivo exposure to the GABA-modulatory 3α,5β-THP accelerates the decay time for synaptic current primarily as a result of upregulation of the GABAR α4 subunit. This altered kinetic state would decrease inhibitory synaptic drive to the hippocampal circuitry and may be one mediating factor for the alteration in affective tone observed across naturally occurring fluctuations in endogenous steroids, such as occur during premenstrual syndrome.
Acknowledgments
The authors thank R. S. Markowitz, X. Li, A. Polish, and Y. Ruderman for helpful technical assistance, and A. S. Cohen and J. Celentano for a critical reading of the manuscript.
This work was supported by National Institutes of Health Grants DA-09618 and AA-12958 to S. S. Smith and NS-21848 to D. S. Faber.
REFERENCES
- Ankri N, Legendre P, Faber DS, Korn H. Automatic detection of spontaneous synaptic responses in central neurons. J Neurosci Methods. 1994;52:87–100. doi: 10.1016/0165-0270(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Bai D, Zhu G, Pennefather P, Jackson MF, Macdonald JF, Orser BA. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by GABA-A receptors in hippocampal neurons. Mol Pharmacol. 2001;59:814–824. doi: 10.1124/mol.59.4.814. [DOI] [PubMed] [Google Scholar]
- Banks MI, Li TB, Pearce RA. The synaptic basis of GABA-A, slow. J Neurosci. 1998;18:1305–1317. doi: 10.1523/JNEUROSCI.18-04-01305.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks MI, Pearce RA. Dual actions of volatile anesthetics on GABA(A) IPSCs: dissociation of blocking and prolonging effects. Anesthesiology. 1999;90:120–134. doi: 10.1097/00000542-199901000-00018. [DOI] [PubMed] [Google Scholar]
- Banks MI, Pearce RA. Kinetic differences between synaptic and extra-synaptic GABAA receptors in CA1 pyramidal cells. J Neurosci. 2000;20:937–948. doi: 10.1523/JNEUROSCI.20-03-00937.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitran D, Hilvers RJ, Kellogg CK. Anxiolytic effects of 3α-hydroxy-5α[β]-pregnan-20-one: endogenous metabolites of progesterone that are active at the GABAA receptor. Brain Res. 1991;561:157–161. doi: 10.1016/0006-8993(91)90761-j. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409:88–92. doi: 10.1038/35051086. [DOI] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective changes in single cell GABA(A) receptor subunit expression and function in temporal lobe epilepsy. Nat Med. 1998;4:1166–1172. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- Brussaard AB, Kits KS, Baker RE, Willems WP, Leyting-Vermeulen JW, Voorn P, Smit AB, Bicknell RJ, Herbison AE. Plasticity in fast synaptic inhibition of adult oxytocin neurons caused by switch in GABA(A) receptor subunit expression. Neuron. 1997;19:1103–1114. doi: 10.1016/s0896-6273(00)80401-8. [DOI] [PubMed] [Google Scholar]
- Brussaard AB, Wossink J, Lodder JC, Kits KS. Progesterone-metabolite prevents protein kinase C-dependent modulation of γ -aminobutyric acid type A receptors in oxytocin neurons. Proc Natl Acad Sci USA. 2000;97:3625–3630. doi: 10.1073/pnas.050424697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AS, Lin DD, Coulter DA. Protracted postnatal development of inhibitory synaptic transmission in rat hippocampal area CA1 neurons. J Neurophysiol. 2000;84:2465–2476. doi: 10.1152/jn.2000.84.5.2465. [DOI] [PubMed] [Google Scholar]
- Cooper EJ, Johnston GA, Edwards FA. Effects of a naturally occurring neurosteroid on GABAA IPSCs during development in rat hippocampal or cerebellar slices. J Physiol. 1999;521:437–449. doi: 10.1111/j.1469-7793.1999.00437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa AMN, Spence KT, Smith SS, ffrench-Mullen JMH. Withdrawal from the endogenous steroid progesterone results in GABAA currents insensitive to benzodiazepine modulation in rat CA1 hippocampus. J Neurophysiol. 1995;74:464–469. doi: 10.1152/jn.1995.74.1.464. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Fritschy JM, Sieghart W, Morrow AL. Bidirectional alterations of GABAA receptor subunit peptide levels in rat cortex during chronic ethanol consumption and withdrawal. J Neurochem. 1997;69:126–130. doi: 10.1046/j.1471-4159.1997.69010126.x. [DOI] [PubMed] [Google Scholar]
- Draguhn A, Heinemann U. Different mechanisms regulate IPSC kinetics in early postnatal and juvenile hippocampal granule cells. J Neurophysiol. 1996;76:3983–3993. doi: 10.1152/jn.1996.76.6.3983. [DOI] [PubMed] [Google Scholar]
- Edwards FA, Konnerth A, Sakmann B. Quantal analysis of inhibitory synaptic transmission in the dentate gyrus of rat hippocampal slices: a patch-clamp study. J Physiol. 1990;430:213–249. doi: 10.1113/jphysiol.1990.sp018289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essrich C, Lorez M, Benson JA, Fritschy JM, Luscher B. Postsynaptic clustering of major GABA-A receptor subtypes requires the gamma-2 subunit and gephyrin. Nat Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- Fancsik A, Linn DM, Tasker JG. Neurosteroid modulation of GABA IPSCs is phosphorylation dependent. J Neurosci. 2000;20:3067–3075. doi: 10.1523/JNEUROSCI.20-09-03067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE. The benzodiazepine receptor and its role in anxiety. Br J Psychiatry. 1988;152:599–600. doi: 10.1192/bjp.152.5.599. [DOI] [PubMed] [Google Scholar]
- Follesa P, Serra M, Cagetti E, Pisu MG, Porta S, Floris S, Massa F, Sanna E, Biggio G. Allopregnanolone synthesis in cerebellar granule cells: roles in regulation of GABA(A) receptor expression and function during progesterone treatment and withdrawal. Mol Pharmacol. 2000;57:1262–1270. [PubMed] [Google Scholar]
- Frerking M, Borges S, Wilson M. Variation in GABA mini amplitude is the consequence of variation in transmitter concentration. Neuron. 1995;15:885–895. doi: 10.1016/0896-6273(95)90179-5. [DOI] [PubMed] [Google Scholar]
- Gallo MA, Smith SS. Progesterone withdrawal decreases latency to and increases duration of electrified prod burial: a possible rat model of PMS anxiety. Pharmacol Biochem Behav. 1993;46:897–904. doi: 10.1016/0091-3057(93)90219-j. [DOI] [PubMed] [Google Scholar]
- Greenfield LJ, Jr, Macdonald RL. Whole-cell and single-channel alpha1beta1gamma2S GABA-A receptor currents elicited by a “mul-tipuffer” drug application device. Pfluegers Arch. 1996;432:1080–1090. doi: 10.1007/s004240050238. [DOI] [PubMed] [Google Scholar]
- Gulinello M, Gong QH, Li X, Smith SS. Short-term exposure to a neuroactive steroid increases α4 GABAA receptor subunit levels in association with increased anxiety in the female rat. Brain Res. 2001;910:55–66. doi: 10.1016/s0006-8993(01)02565-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haage D, Johansson S. Neurosteroid modulation of synaptic and GABA-evoked currents in neurons from the rat medial preoptic nucleus. J Neurophysiol. 1999;82:143–151. doi: 10.1152/jn.1999.82.1.143. [DOI] [PubMed] [Google Scholar]
- Hajos N, Mody I. Synaptic communication among hippocampal interneurons: properties of spontaneous IPSCs in morphologically identified cells. J Neurosci. 1997;17:8427–8442. doi: 10.1523/JNEUROSCI.17-21-08427.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajos N, Nusser Z, Rancz EA, Freund TF, Mody I. Cell type- and synapse-specific variability in synaptic GABA-A receptor occupancy. Eur J Neurosci. 2000;12:810–818. doi: 10.1046/j.1460-9568.2000.00964.x. [DOI] [PubMed] [Google Scholar]
- Harrison NL, Vicini S, Barker JL. A steroid anesthetic prolongs inhibitory postsynaptic currents in cultured rat hippocampal neurons. J Neurosci. 1987;7:604–609. doi: 10.1523/JNEUROSCI.07-02-00604.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt RA, Bateson AN, Martin IL. Chronic treatment with diazepam or abecarnil differently affects the expression of GABAA receptor subunit mRNAs in the rat cortex. Neuropharmacology. 1996;35:1457–1463. doi: 10.1016/s0028-3908(96)00064-0. [DOI] [PubMed] [Google Scholar]
- Jones MV, Westbrook GL. The impact of receptor desensitization on fast synaptic transmission. Trends Neurosci. 1996;19:96–101. doi: 10.1016/s0166-2236(96)80037-3. [DOI] [PubMed] [Google Scholar]
- Jones MV, Westbrook GL. Shaping of IPSCs by endogenous calcineurin activity. J Neurosci. 1997;17:7626–7633. doi: 10.1523/JNEUROSCI.17-20-07626.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorge-Rivera JC, McIntyre KL, Henderson LP. Anabolic steroids induce region- and subunit-specific rapid modulation of GABA(A) receptor-mediated currents in the rat forebrain. J Neurophysiol. 2000;83:3299–3309. doi: 10.1152/jn.2000.83.6.3299. [DOI] [PubMed] [Google Scholar]
- Kapur J. Hippocampal neurons express GABA-A receptor insensitive to diazepam in hyperexcitable conditions. Epilepsia. 2000;41:S86–S89. doi: 10.1111/j.1528-1157.2000.tb01563.x. [DOI] [PubMed] [Google Scholar]
- Kern W, Sieghart W. Polyclonal antibodies directed against an epitope specific for the alpha 4-subunit of GABA-A receptors identify a 67-kDa protein in rat brain membranes. J Neurochem. 1994;62:764–769. doi: 10.1046/j.1471-4159.1994.62020764.x. [DOI] [PubMed] [Google Scholar]
- Korn H, Bausela F, Charpier S, Faber DS. Synaptic noise and multi-quantal release at dendritic synapses. J Neurophysiol. 1993;70:1249–1254. doi: 10.1152/jn.1993.70.3.1249. [DOI] [PubMed] [Google Scholar]
- Kraszewski K, Grantyn R. Unitary, quantal and miniature GABA-activated synaptic chloride currents in cultured neurons from the rat superior colliculus. Neuroscience. 1992;47:555–570. doi: 10.1016/0306-4522(92)90165-x. [DOI] [PubMed] [Google Scholar]
- Lavoie AM, Tingey JJ, Harrison NL, Pritchett DB, Twyman RE. Activation and deactivation rates of recombinant GABAA receptor channels are dependent on α-subunit isoform. Biophys J. 1997;73:2518–2526. doi: 10.1016/S0006-3495(97)78280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maconochie DJ, Zempel JM, Steinbach JH. How quickly can GABAA receptors open? Neuron. 1994;12:61–71. doi: 10.1016/0896-6273(94)90152-x. [DOI] [PubMed] [Google Scholar]
- Mahmoudi M, Kang MH, Tillakaratne N, Tobin AJ, Olsen RW. Chronic intermittent ethanol treatment in rats increases GABAA receptor α4 subunit expression: possible relevance to alcohol dependence. J Neurochem. 1997;68:2485–2492. doi: 10.1046/j.1471-4159.1997.68062485.x. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Maric D, Maric I, Wen X, Fritschy JM, Sieghart W, Barker JL, Serafini R. GABA-A receptor subunit composition and functional properties of Cl- channels with differential sensitivity to zolpidem in embryonic rat hippocampal cells. J Neurosci. 1999;19:4921–4937. doi: 10.1523/JNEUROSCI.19-12-04921.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan AM, Twyman RE. Receptor system response kinetics reveal functional subtypes of native murine and recombinant human GABA(A) receptors. J Physiol. 1999;515:711–727. doi: 10.1111/j.1469-7793.1999.711ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody I. Distinguishing between GABA(A) receptors responsible for tonic and phasic conductances. Neurochem Res. 2001;26:907–913. doi: 10.1023/a:1012376215967. [DOI] [PubMed] [Google Scholar]
- Mody I, De Koninck Y, Otis TS, Soltesz I. Bridging the cleft at GABA synapses in the brain. Trends Neurosci. 1994;17:517–525. doi: 10.1016/0166-2236(94)90155-4. [DOI] [PubMed] [Google Scholar]
- Mtchedlishvili Z, Bertram EH, Kapur J. Diminished allopregnanolone enhancement of GABA(A) receptor currents in a rat model of chronic temporal lobe epilepsy. J Physiol. 2001;537:453–465. doi: 10.1111/j.1469-7793.2001.00453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Benke D, Fritschy JM, Somogyi P. Differential synaptic localization of two major γ-aminobutyric acid type A receptor α subunits on hippocampal pyramidal cells. Proc Natl Acad Sci USA. 1996;93:11939–11944. doi: 10.1073/pnas.93.21.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic; 1982. [DOI] [PubMed] [Google Scholar]
- Perrais D, Ropert N. Effect of zolpidem on miniature IPSCs and occupancy of postsynaptic GABA(A) receptors in central synapses. J Neurosci. 1999;19:578–588. doi: 10.1523/JNEUROSCI.19-02-00578.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poisbeau P, Cheney MC, Browning MD, Mody I. Modulation of synaptic GABAA receptor function by PKA and PKC in adult hippocampal neurons. J Neurosci. 1999;19:674–683. doi: 10.1523/JNEUROSCI.19-02-00674.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poisbeau P, Williams SR, Mody I. Silent GABAA synapses during flurazepam withdrawal are region-specific in the hippocampal formation. J Neurosci. 1997;17:3467–3475. doi: 10.1523/JNEUROSCI.17-10-03467.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers CJ, Twyman RE, Macdonald RL. Benzodiazepine and beta-carboline regulation of single GABA-A receptor channels of mouse spinal neurons in culture. J Physiol. 1994;475:69–82. doi: 10.1113/jphysiol.1994.sp020050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudick CN, Woolley CS. Estrogen regulates functional inhibition of hippocampal CA1 pyramidal cells in the adult female rat. J Neurosci. 2001;21:6532–6543. doi: 10.1523/JNEUROSCI.21-17-06532.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Chapin JK. The estrous cycle and the olivo-cerebellar circuit II: Enhanced selective sensory gating of responses from the rostral dorsal accessory olive. Exp Brain Res. 1996;111:385–392. doi: 10.1007/BF00228727. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Hsu FC, Markowitz RS, ffrench-Mullen JMH, Li X. GABAA receptor α4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998a;392:926–930. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Li X, Moran MH, Bitran D, Frye CA, Hsu FC. Withdrawal from 3α-OH-5α-pregnan-20-one using a pseudopregnancy model alters the kinetics of hippocampal GABAA-gated current and increases the GABAA receptor α4 subunit in association with increased anxiety. J Neurosci. 1998b;18:5275–5284. doi: 10.1523/JNEUROSCI.18-14-05275.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltesz I, Smetters DK, Mody I. Tonic inhibition originates from synapses close to the soma. Neuron. 1995;14:1273–1283. doi: 10.1016/0896-6273(95)90274-0. [DOI] [PubMed] [Google Scholar]
- Sundstrom-Poromaa I, Smith DH, Gong QH, Sabado TN, Li X, Light A, Wiedmann M, Williams K, Smith SS. Hormonally-regulated α4β2δ GABAA receptors are a target for alcohol. Nat Neurosci. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur C, Farrar SJ, Kerby J, Whiting PJ, Atack JR, McKernan RM. Preferential coassembly of alpha-4 and delta subunits of the GABA-A receptor in rat thalamus. Mol Pharmacol. 1999;56:110–115. doi: 10.1124/mol.56.1.110. [DOI] [PubMed] [Google Scholar]
- Suzdak PD, Paul SM, Crawley JN. Effects of Ro15–4513 and other benzodiazepine receptor inverse agonists on alcohol-induced intoxication in the rat. J Pharmacol Exp Ther. 1988;245:880–886. [PubMed] [Google Scholar]
- Twyman RE, Macdonald RL. Neurosteroid regulation of GABAA receptor single-channel kinetic properties of mouse spinal cord neurons in culture. J Physiol. 1992;456:215–245. doi: 10.1113/jphysiol.1992.sp019334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twyman RE, Rogers CJ, Macdonald RL. Differential regulation of gamma-aminobutyric acid receptor channels by diazepam and phenobarbital. Ann Neurol. 1989;25:213–220. doi: 10.1002/ana.410250302. [DOI] [PubMed] [Google Scholar]
- Vicini S, Ferguson C, Prybylowski K, Kralic J, Morrow AL, Homanics GE. GABA-A receptor alpha-1 subunit deletion prevents developmental changes of inhibitory synaptic currents in cerebellar neurons. J Neurosci. 2001;21:3009–3016. doi: 10.1523/JNEUROSCI.21-09-03009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wafford KA, Thompson SA, Thomas D, Sikela J, Wilcox AS, Whiting PJ. Functional characterization of human GABA-A receptors containing the α4 subunit. Mol Pharmacol. 1996;50:670–678. [PubMed] [Google Scholar]
- Williams SR, Buhl EH, Mody I. The dynamics of synchronized neurotransmitter release determined from compound spontaneous IPSCs in rat dentate granule neurones in vitro. J Physiol. 1998;510:477–497. doi: 10.1111/j.1469-7793.1998.477bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisden W, Herb A, Wieland H, Keinanen K, Luddens H, Seeburg PH. Cloning, pharmacological characteristics and expression pattern of the rat GABAA receptor α4 subunit. FEBS Lett. 1991;289:227–230. doi: 10.1016/0014-5793(91)81076-k. [DOI] [PubMed] [Google Scholar]
- Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABA-A receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng XJ, Tietz EI. Benzodiazepine tolerance at GABAergic synapses on hippocampal CA1 pyramidal cells. Synapse. 1999;31:263–277. doi: 10.1002/(SICI)1098-2396(19990315)31:4<263::AID-SYN4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]