Abstract
The initiation of normal embryo development depends on the completion of all events of egg activation. In all species to date, egg activation requires an increase(s) in the intracellular concentration of calcium ([Ca2+]i), which is almost entirely mediated by inositol 1,4,5-trisphosphate receptor 1 (IP3R1). In mammalian eggs, fertilization-induced [Ca2+]i responses exhibit a periodic pattern that are called [Ca2+]i oscillations. These [Ca2+]i oscillations are robust at the beginning of fertilization, which occurs at the second metaphase of meiosis, but wane as zygotes approach the pronuclear stage, time after which in the mouse oscillations cease altogether. Underlying this change in frequency are cellular and biochemical changes associated with egg activation, including degradation of IP3R1, progression through the cell cycle, and reorganization of intracellular organelles. In this study, we investigated the system requirements for IP3R1 degradation and examined the impact of the IP3R1 levels on the pattern of [Ca2+]i oscillations. Using microinjection of IP3 and of its analogs and conditions that prevent the development of [Ca2+]i oscillations, we show that IP3R1 degradation requires uniform and persistently elevated levels of IP3. We also established that progressive degradation of the IP3R1 results in [Ca2+]i oscillations with diminished periodicity while a near complete depletion of IP3R1s precludes the initiation of [Ca2+]i oscillations. These results provide insights into the mechanism involved in the generation of [Ca2+]i oscillations in mouse eggs.
In all species investigated to date, the fertilizing sperm triggers an increase in the egg’s intracellular concentration of free Ca2+ ([Ca2+]i) that provides the cellular cue required to induce egg activation (Miyazaki et al., 1993; Stricker, 1999). The [Ca2+]i signal evokes egg activation by initiating a series of biochemical changes that enable the egg to prevent polyspermy and exit meiosis, and the newly formed zygote to transition through the cell cycle and commence the mitotic cleavages that will unfold the developmental program (Schultz and Kopf, 1995; Ducibella et al., 2002).
Despite its universality, the shape and patterns of [Ca2+]i responses associated with egg activation vary widely among species (Stricker, 1999). For instance, in mammals, which ovulate their eggs arrested at the metaphase stage of the second meiosis (MII), the [Ca2+]i signal consists of brief but periodic increases in [Ca2+]i that are commonly referred to as oscillations (Stricker, 1999). In these species, the cumulative impact of [Ca2+]i oscillations underlies egg activation, although the initiation and completion of individual events of egg activation appear to be established by distinct numbers of [Ca2+]i rises (Ozil and Huneau, 2001; Ducibella et al., 2002). Remarkably, the understanding of the cellular and molecular mechanisms that control the periodicity, and eventual termination of these [Ca2+]i rises remain obscure.
The type 1 IP3R1, or its homolog in lower species, is responsible for the majority of [Ca2+]i increases associated with fertilization (Yoshida et al., 1998; Runft et al., 1999; Iwasaki et al., 2002). Confirmation of the essential role of IP3R1 in fertilization was obtained using function-blocking antibodies, the injection of which precluded the [Ca2+]i responses and egg activation that are normally associated with sperm entry (Miyazaki et al., 1992; Runft et al., 1999). IP3R1s, which are mostly located on the membrane of the endoplasmic reticulum (ER), the main Ca2+ store in somatic cells and eggs (Petersen et al., 2001; Stricker, 2006), are tetrameric Ca2+ channels where each monomer contains six transmembrane regions where the channel pore is located, a large cytosolic N-terminal structure that includes the ligand-binding domain and a small but equally cytosolic C-terminus (Patel et al., 1999; Berridge et al., 2000; Berridge, 2002; Tang et al., 2003; Bosanac et al., 2004). Gating of IP3R1 and Ca2+ release requires binding of IP3 (Mignery and Sudhof, 1990), a product of the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) by a phospholipase C (PLC) (Rebecchi and Pentyala, 2002). In mammals, evidence suggests that the only known sperm-specific PLC, PLCZ1 (henceforth identified with the Greek symbol ζ), is responsible for IP3 production during fertilization (Saunders et al., 2002; Yoon et al., 2008).
Fertilization-associated [Ca2+]i oscillations characteristically occur during M-phase stages of the cell cycle (Stricker, 1999). For example, in mouse eggs [Ca2+]i oscillations persist for 3–4 h but as zygotes progress into interphase, also known as the pronuclear (PN) stage, oscillations become attenuated and cease altogether (Jones et al., 1995a; Day et al., 2000; Marangos et al., 2003). Concurrent with this, PN stage zygotes showed reduced responses to injection of IP3 or other related agonists (Jones and Whittingham, 1996; Parrington et al., 1998; FitzHarris et al., 2003; Jellerette et al., 2004). It is therefore apparent that fertilization-associated [Ca2+]i oscillations undergo precise temporal regulation, although the understanding of the mechanisms that enforce this temporal control remains unclear, at least in part, by the similar inactivation time course that the purportedly involved mechanisms undergo after fertilization (Lee et al., 2006). To wit, fertilization is accompanied by a decline in the activity of the kinases that promote the MII cell-cycle arrest, which are the same kinases involved in ER organization (FitzHarris et al., 2003) as well as in IP3R1 phosphorylation (Jellerette et al., 2004; Lee et al., 2006). Consequently, egg activation results in overt changes in the distribution and phosphorylation status of the IP3R1. Fertilization also typically induces a steady decline in the amount of IP3R1 (Jellerette et al., 2000) and, in mouse zygotes, PLCζ undergoes nuclear translocation following PN formation (Larman et al., 2004).
In this study we focused on the role of the total amount of IP3R1s on [Ca2+]i responses, as the dynamic changes that IP3R1 concentration undergoes during oocyte maturation and following sperm entry suggest an important function in shaping the pattern of [Ca2+]i oscillations. Specifically, IP3R1s steadily increase during maturation and this coincides with maximal [Ca2+]i oscillatory ability at the MII stage (Chiba et al., 1990; Fujiwara et al., 1993; Mehlmann and Kline, 1994). Further, over half of the receptor total mass is lost by the PN stage (Parrington et al., 1998; Brind et al., 2000; Jellerette et al., 2000), which corresponds to the termination at this stage of sperm-initiated oscillations (Day et al., 2000; Jones, 2005). Importantly, the contribution of these changes to the overall pattern of [Ca2+]i oscillations remains to be established. Similarly, we remain unaware of the type of IP3 production that underlies receptor degradation. While it is widely known that IP3R1 degradation requires IP3 binding (Zhu et al., 1999; Zhu and Wojcikiewicz, 2000), whether persistently elevated intracellular concentrations of IP3 ([IP3]i) or transient increases in [IP3]i or both are responsible for signaling receptor degradation during fertilization remains to be thoroughly studied. Progress in this area has been hampered, at least in part, by the difficulty in synchronizing fertilization and [Ca2+]i oscillations in a population of mammalian eggs, and by the limitation of current fluorescent techniques, which are unable to detect the seemingly modest increases in [IP3]i during mouse fertilization (Shirakawa et al., 2006). Thus, to ascertain the pattern of IP3 production that underlies sperm-induced IP3R1 degradation, we increased the egg’s [IP3]i in two distinct patterns and compared the rate of IP3R1 degradation that they promoted. To investigate how IP3R1 degradation impacts [Ca2+]i oscillations, we examined [Ca2+]i oscillations under conditions where only the concentration of IP3R1 was permitted to change. We also generated eggs with different IP3R1 levels and used them to evaluate [Ca2+]i responses induced by strontium chloride (SrCl2). Our results reveal that steady production of IP3 underlies the majority of IP3R1 degradation during mouse fertilization and that this decline in IP3R1 numbers contributes to the decreased periodicity of [Ca2+]i oscillations that precedes their termination at the PN stage.
Materials and Methods
Recovery of oocytes/eggs and culture conditions
CD-1 mice between 6 and 12 weeks of age were used for most experiments except for ICSI studies where B6D2F1 (C57BL/6J × DBA/2J) mice of the same age were used. Germinal vesicle (GV) oocytes and MII eggs were collected from ovaries and oviducts of 6- to 8-week-old female mice, respectively. Females were super stimulated with an injection of 5 IU pregnant mare serum gonadotrophin (PMSG; Sigma, St. Louis, MO; all chemicals from Sigma unless otherwise specified). GV oocytes were recovered 40 h post-PMSG in HEPES-buffered Tyrode’s-lactate solution (TL-HEPES) supplemented with 5% heat-treated fetal calf serum (FCS; Gibco, Grand Island, NY) and 100 μM 3-isobutyl-1-methylxanthine (IBMX). GV oocytes were matured in CZB medium (Chatot et al., 1990) containing 3 mg/ml bovine serum albumin (BSA) under paraffin oil at 36.5°C and 6% CO2 for 14–18 h, the time at which most oocytes had reached the MII stage. In vivo MII eggs were recovered from mice induced to ovulate by an injection of 5 IU human chorionic gonadotropin (hCG) 44–48 h after PMSG stimulation. Cumulus masses were recovered 12–14 h post-hCG and collected in TL-HEPES supplemented with 5% FCS. Cumulus cells were removed by a brief incubation with 0.1% bovine testis hyaluronidase in TL-HEPES. Eggs showing no degeneration and extrusion of the first polar body were transferred into 50 μl drops of potassium simplex optimized medium (KSOM; Specialty Media, Phillipsburg, NJ) under paraffin oil or in four-well plates (Nunc, Roskilde, Denmark). Eggs were incubated at 36.5°C in a humidified atmosphere containing 6% CO2.
In vitro fertilization (IVF)
In vitro fertilization was performed using human tubal fluid (HTF) medium (Quinn et al., 1985) supplemented with 3 mg/ml BSA. Egg–cumulus complexes were recovered as indicated above and incubated in HTF at 36.5°C and 6% CO2. Cauda epididymides sperm were collected into pre-equilibrated HTF medium and incubated for 1–2 h to attain capacitation. Capacitated sperm were added to HTF medium containing egg–cumulus complexes to final concentrations of 1–3 × 105 sperm/ml. Sperm and eggs were co-cultured for 30 min to 2 h, according to experimental design, after which eggs were washed and either placed in KSOM medium and cultured to observe activation events or monitored for [Ca2+]i oscillations. Rates of second polar body (2PB) extrusion and PN formation were used to assess egg activation, which occur approximately 2 and 4 h post-sperm entry, respectively.
Intracytoplasmic sperm injection (ICSI)
ICSI was performed using Narishige manipulators (Medical System Corp., Great Neck, NY) mounted on a Nikon Diaphot microscope (Nikon, Inc., Garden City, NY), as previously described (Kimura and Yanagimachi, 1995; Kurokawa and Fissore, 2003). ICSI was carried out in HEPES-buffered CZB (HEPES-CZB) medium at room temperature (RT). To separate heads from tails, sperm were lightly sonicated for 10 sec at 4°C (XL2020; Heat Systems, Inc., Farmingdale, NY) after which sperm were washed in microinjection buffer (MIB; 75 mM KCl and 20 mM HEPES, pH 7.0) and mixed with MIB containing 12% polyvinylpyrrolidone (PVP; MW 360 kDa; Sigma) at a 1:1 ratio. Sperm heads were injected into the cytoplasm of eggs using a piezo micropipette-driving unit (Piezodrill; Burleigh Instruments, Inc., Rochester, NY). Piezo pulses of different strengths were applied to first penetrate the zona pellucida and then to pierce the plasma membrane. Following ICSI, eggs were cultured in KSOM or monitored for [Ca2+]i responses; egg activation was assessed as described above.
Microinjection procedures
Eggs were injected as previously described (Wu et al., 1997; Kurokawa et al., 2004) in 50 μl drops of TL-HEPES supplemented with 20% FCS under paraffin oil. For MII eggs, TL-HEPES injection drops were supplemented with 2.5% sucrose (w/v). Glass micropipettes were filled by suction from a microdrop containing the reagents, which were injected into the ooplasm by pneumatic pressure (PLI-100, picoinjector, Harvard Apparatus, Cambridge, MA). All reagents were diluted in MIB. Injection volumes were approximately 5–10 pl, which resulted in final intracellular concentrations of approximately 1.5–3% of the concentrations in the injection pipette.
Fluorescence recordings, [Ca2+]i determinations, and caged IP3 experiments
[Ca2+]i measurements were performed using the Ca2+-sensitive indicator dye, Fura-2, as previously reported by our laboratory (Kurokawa et al., 2005). Eggs were loaded with 1 μM Fura-2/acetoxymethyl ester (Molecular Probes, Eugene, OR) supplemented with 0.02% Pluronic F-127 (Molecular Probes) in TL-HEPES for 20 min at RT. Multiple eggs (up to 10) were simultaneously monitored using a 20× objective on an inverted microscope (Nikon Corp., Tokyo, Japan) fitted for fluorescence measurements and with a temperature-controlled stage (20/20 Technology, Inc., Wilmington, NC). A high-speed filter wheel (Ludl Electronic Products, Hawthorne, NY) controlled by Simple PCI software (C-Imaging System, Cranberry Township, PA) made possible the rotation between 340 and 380 nm excitation wavelengths. Illumination was provided by a 75 W Xenon arc lamp and emitted light above 510 nm was collected by a cooled Photometrics SenSys CCD camera (Roper Scientific, Tucson, AZ). Fluorescence ratios of 340/380 nm were obtained every 20–30 sec. Caged IP3 (1 mM) was injected into eggs previously loaded with Fluo 4 (1 μM Fluo 4-AM or Fluo 4-dextran, MW 10,000; Molecular Probes). Fluo 4 was excited by exposure to 488 nm wavelength, and release of IP3 controlled by pulses of UV light of 360 nm wavelength and 0.005 sec duration.
Generation of IP3R1 knockdown (KD) eggs
GV oocytes recovered in TL-HEPES supplemented with 5% FCS and 100 μM IBMX 40 h post-PMSG were then cultured in CZB medium containing 3 mg/ml BSA without IBMX, which allowed resumption of meiosis. Within 3 h, interval that allowed most oocytes to undergo GV breakdown (GVBD), oocytes were injected with 1, 5, and 10 μM adenophostin A (AdA); AdA-injected oocytes were returned to the incubator and cultured for 12 h, at which time most oocytes had reached the MII stage. The amount of IP3R1s was verified by Western blotting.
Parthenogenetic egg activation and preparation of agonists and inhibitors
Release of MII arrest and generation of interphase zygotes was accomplished by exposing MII eggs to SrCl2 as previously described by our laboratory (Jellerette et al., 2000). MII eggs were incubated for 2 h in Ca2+-free CZB medium supplemented with 10 mM SrCl2 followed by washes in TL-HEPES and subsequently transferred to KSOM medium microdrops; eggs were monitored for signs of activation, such as extrusion of 2PB (2 h) and PN formation (6 h).
AdA, porcine sperm factor (pSF; prepared as in Kurokawa et al., 2005), IP3 (Calbiochem, San Diego, CA), and inositol 2,4,5-trisphosphate (2-IP3; Si-Chem, Bremen, Germany), a non-hydrolysable analog of IP3, were all injected into MII eggs. Thimerosal was prepared on the day of experiment and added to KSOM. Stock solution of 1,2-bis(o-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid/acetoxymethyl ester (BAPTA-AM; Calbiochem) was prepared in DMSO and diluted to working solutions in KSOM. Colcemid was prepared in water and also added to KSOM. Latrunculin B (LatB; Calbiochem), was dissolved in DMSO and final dilutions were attained in TL-HEPES or in KSOM. It is worth noting that for experiments where different concentrations of IP3 or 2-IP3 were injected to monitor their effects on IP3R1 degradation, we did not closely examine egg activation events after the injection. However, we observed that single or double injections of 500 μM IP3 and single injections of 2-IP3 caused 2PB extrusion in the majority of treated eggs.
In vitro transcription of PLCζ
The pBluescript II SK(+) plasmid containing the full-length coding sequence of mouse PLCζ (a gift from Dr. Kiyoko Fukami, Tokyo University of Pharmacy and Life Science, Tokyo, Japan) downstream of the T7 promoter was linearized at a site beyond the 3′ end of the coding sequence. mRNA was transcribed in vitro using the T7 mMessage mMachine kit (Ambion, Austin, TX) and a poly(A) tail was added to the 3′ end of the coding sequence (Ambion). mRNA was purified according to manufacturer’s instructions and used at a concentration of 1 μg/μl.
Western blotting
Cell lysates from 15 to 35 eggs were mixed with 15 μl of 2× Laemmli sample buffer (Laemmli, 1970) and stored at −80°C until the time of Western blotting. Samples were boiled for 3 min and loaded onto NuPAGE Novex 3–8% Tris–acetate gels (Invitrogen, Carlsbad, CA). Proteins were separated by electrophoresis and transferred onto nitrocellulose membranes (Micron Separations, Westboro, MA). Membranes were then blocked in phosphate-buffered saline (PBS) containing 0.1% Tween-20 (PBST) supplemented with 6% non-fat dry milk for 1.5 h and incubated overnight with Rbt03 at 1:1,000 dilution. Following washes in PBST, membranes were incubated for 1.5 h with a goat anti-rabbit secondary antibody coupled to horseradish peroxidase (Bio-Rad Laboratories, Hercules, CA). Membranes were washed and incubated for 1 min in chemiluminescence reagent (NEN Life Science Products, Boston, MA) and developed according to manufacturer’s instructions. The intensities of immunoreactive bands were assessed using a Kodak 440 Image Station (Rochester, NY) and plotted using Sigma Plot (Jandel Scientific Software, San Rafael, CA). The intensity of the immunoreactive bands from MII eggs was arbitrarily given the value of 1 and values in other lanes were expressed relative to this band. IP3R1 was immunodetected using the Rbt03 polyclonal antibody, which was raised against C-terminal amino acids 2735–2749 of mouse IP3R1 (Parys et al., 1995). Figures were prepared from the TIFF files using Image J software (Rasband, W.S., ImageJ, NIH; http://rsb.info.nih.gov/ij/) and Microsoft Powerpoint.
Statistical analysis
Values from two or more experiments performed on different batches of eggs or zygotes were used for evaluation of statistical significance. Statistical comparisons of IP3R1 band intensity and of [Ca2+]i parameters were performed using Student’s t-test or one-way ANOVA followed by the Tukey–Kramer test if differences between groups were found (JMP-IN, SAS Institute, Cary, NC). Significance was set at P <0.05 and is denoted in bar graphs by different superscripts.
Results
Bolus injection of IP3 initiates [Ca2+]i oscillations without IP3R1 degradation
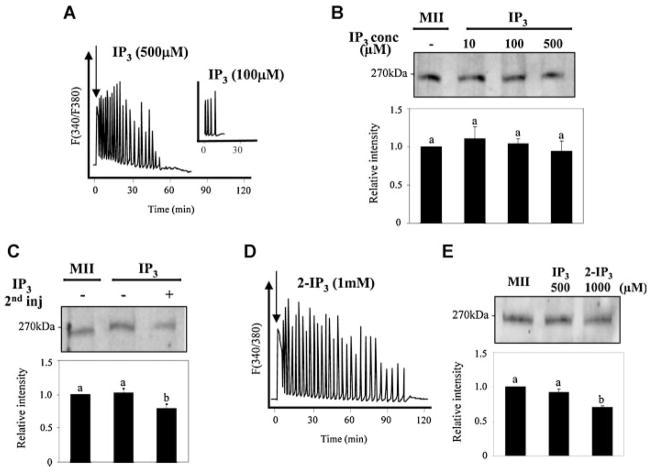
The type of IP3 production that underlies IP3R1 degradation, and possibly [Ca2+]i oscillations, during mammalian fertilization is presently unknown due to a combination of factors, which include the asynchrony of mammalian fertilization, the purported modest increases in [IP3]i induced by fertilization in these species and the detection limit of the fluorescent probes currently available to measure [IP3]i. Consequently, to gain insight into the prevailing [IP3]i during fertilization, we made use of the finding that IP3R1 degradation depends on IP3 binding and that ~50% of IP3R1 mass is lost by 4 h post-fertilization. We therefore generated widely dissimilar patterns of [IP3]i and examined how closely they resembled sperm-induced IP3R1 degradation. Hence, we first induced transient increases in [IP3]i by bolus injections of different concentrations of IP3 and 4 h later collected the eggs to monitor the IP3R1 degradation generated by those IP3 injections. As reported by other groups (Miyazaki, 1988), injection of IP3 into mouse eggs initiated [Ca2+]i responses and, expectedly, higher concentrations, such as 500 μM, induced longer lasting [Ca2+]i oscillations (Fig. 1A). Remarkably, despite prolonged [Ca2+]i responses, these IP3 injections failed to induce noticeable levels of IP3R1 degradation (Fig. 1B).
Fig. 1.
Bolus injection of IP3 that generates transient increases in [IP3]i induces [Ca2+]i oscillations that fail to promote substantive IP3R1 degradation. Injection of IP3 initiated dose-dependent [Ca2+]i responses in mouse eggs (A; 500 μM IP3; inset, 100 μM IP3). These IP3 concentrations (10, 100, and 500 μM; lanes 2, 3, and 4, respectively) did not induce noticeable receptor degradation in eggs collected 4 h post-injection (B). A double injection of 500 μM IP3, the second injection of which was delivered 2 h after the first injection, resulted in an ~20% loss of IP3R1 mass (C). Injection of a metabolically stable form of IP3, 2-IP3, at 1 mM initiated long-term oscillations (D) without inducing significant receptor degradation (E). The intensity of the immunoreactive bands in all parts in this figure (B, C, E; bar graphs) and elsewhere in the article was normalized to that of uninjected MII eggs, which was given the value of 1; bars with different superscripts represent values with significant differences (P < 0.05). Arrows in(A) and(D) denote time of injection. In(C), the minus sign under the IP3 treatment indicates single injection of IP3.
The modest rates of IP3R1 degradation observed after bolus injections of IP3 could be due to the transient nature of [IP3]i increases accomplished by this method. We therefore sought to extend the time during which [IP3]i levels were elevated. To accomplish this, we first resorted to a second injection of 500 μM IP3 ~2 h after the first injection. Our results reveal that this approach only induced an ~15–20% decrease in IP3R1 concentrations (Fig. 1C), which is markedly less than the rates of IP3R1 degradation induced by fertilization (Brind et al., 2000; Jellerette et al., 2000). We also used a metabolically stable form of IP3, 2-IP3, which shows longer half-life than IP3, although it has less affinity for the receptor than IP3 and serves only as a partial agonist. While injection of 2-IP3 (1 mM) induced [Ca2+]i oscillations that lasted for over 90 min (Fig. 1D), it had only a marginal effect on the amount of IP3R1 (Fig. 1E). Taken together, these results indicate that transient, albeit high, increases in [IP3]i do not lead to an IP3R1 degradation comparable to that observed during fertilization.
Periodic increases in [IP3]i fail to induce IP3R1 degradation
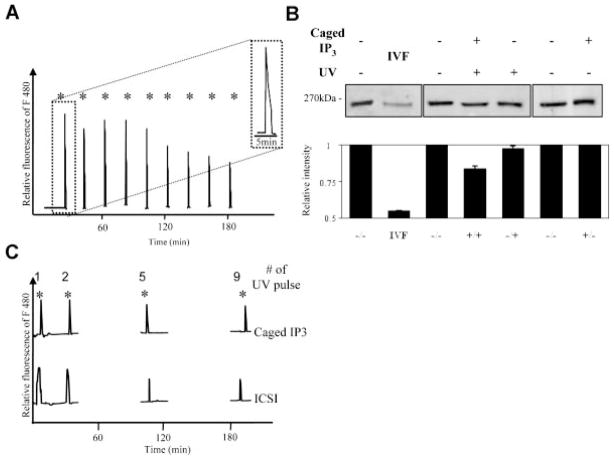
The described transient increases in [IP3]i did not take into account the periodicity at which [IP3]i may oscillate during fertilization, expected to be every 20 min if it occurs in synchrony with [Ca2+]i oscillations, nor the total duration of these [IP3]i oscillations, expected to last for ~3 h if it spans the duration of sperm-induced [Ca2+]i oscillations (Deguchi et al., 2000; Ito et al., 2008a). While oscillations in [IP3]i during fertilization have not yet been reported, they probably occur given that the sperm-specific PLCζ is highly sensitive to Ca2+, reaching high levels of activity even at ~100 nM [Ca2+]i, which is the baseline value in eggs (Kouchi et al., 2004; Nomikos et al., 2005). Accordingly, to mimic the presumptive pulsatile pattern of [IP3]i, we injected eggs with caged IP3 and proceeded to release IP3 by brief UV light pulses every 20 min for 3 h (Deguchi et al., 2000; Kurokawa and Fissore, 2003). As expected, each pulse of IP3 induced a single and sharp increase in [Ca2+]i, although the amplitude of the rises showed a slight decrease after the fifth pulse (Fig. 2A). It is worth noting that the amplitude and duration of the [Ca2+]i rises induced by caged IP3 was very similar to those induced by the sperm (Fig. 2C). Importantly, this periodical release of IP3 resulted in less than 20% decrease in IP3R1 concentration, which was clearly lower than the loss in receptor concentration observed in IVF control eggs (Fig. 2B, lanes 4 and 2, respectively). Together, these results support the concept that transient increases of [IP3]i do not constitute the main, and probably are not the only, stimulus for IP3R1 degradation during fertilization.
Fig. 2.
Discrete IP3 pulses from caged IP3 cause single [Ca2+]i rises and minor IP3R1 degradation. MII mouse eggs injected with caged IP3 were exposed to UV light for 0.005 sec at intervals of 20 min to cause pulsatile release of IP3. Each UV flash induced a [Ca2+]i rise that was detected in a [Ca2+]i monitoring system; 9 pulses were delivered per egg (A). The inset in (A) shows an enlarged version of the first rise so that the greater duration of the Ca2+ spike can be appreciated. The IP3R1 degradation induced by periodic release of IP3 was compared to that induced by IVF (B). Eggs were collected 4 h after the beginning of the uncaging protocol or 5 h after insemination. The intensity of IP3R1 degradation was quantified as described above. Simultaneous Ca2+ monitoring of [Ca2+]i rises #1, 5, and 9 induced by caged IP3 and by ICSI reveals that both procedures triggered[Ca2+]i rises of similar amplitude and duration (C). IP3R1-immunoreactive bands within each box (B) denote samples that were collected and processed for Western blotting at the same time. Given the number of samples needed for each experiment and the number eggs needed to perform Western blots, it was technically not possible to perform all sample collections in the same experiment.
Uniform IP3 production in the absence of [Ca2+]i oscillations induces IP3R1 degradation
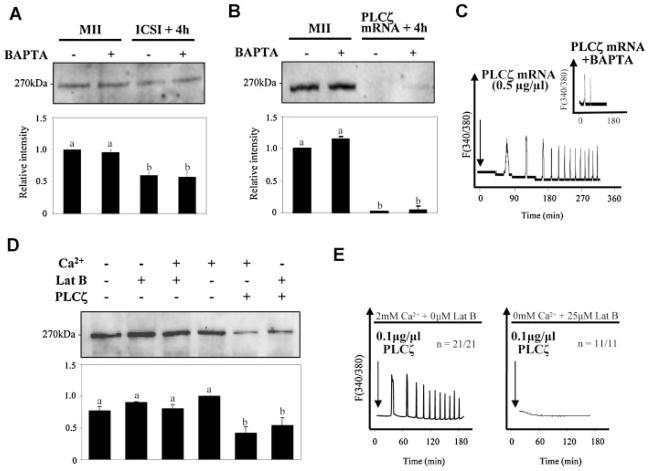
In the absence of, or in conjunction with, transient or pulsatile increases in [IP3]i, the sperm’s PLCζ during fertilization may persistently elevate basal [IP3]i inducing, in turn, a low but continuous rate of IP3R1 degradation. To uniformly elevate [IP3]i it was necessary to stimulate IP3 production while precluding [Ca2+]i rises, which could stimulate PLCζ and trigger pulsatile IP3 production. To induce IP3 production, eggs were injected with either sperm, using the ICSI procedure, or with mPLCζ mRNA. To prevent [Ca2+]i rises, eggs were either cultured in the presence of the Ca2+ chelating agent BAPTA-AM or in nominal Ca2+-free KSOM medium supplemented with LatB, an actin depolymerizing agent that reportedly modifies the organization of the egg cortex (Schatten et al., 1986; McAvey et al., 2002). Using BAPTA-AM, an earlier study had shown that fertilization-induced IP3R1 degradation was not affected by the absence of [Ca2+]i oscillations, although in that study unfertilized eggs cultured in the presence of 10 μM BAPTA-AM showed significant spontaneous rates of IP3R1 degradation (Brind et al., 2000). We therefore optimized BAPTA-AM concentrations and found that 2 μM BAPTA-AM did not spontaneously induce IP3R1 degradation (Fig. 3A) but effectively prevented the initiation of egg activation by the sperm, as judged by the absence of 2PB extrusion (n = 20), and greatly diminished the [Ca2+]i responses induced by injection of 0.5 μg/μl PLCζ mRNA (Fig. 3C). IP3R1 degradation induced by either of these treatments, which ranged from ~40% for ICSI and ~90% for PLCζ mRNA, was seemingly unhindered by the presence of BAPTA-AM (Fig. 3A,B). Similarly, IP3R1 degradation induced by injection of 0.1 μg/μl mPLCζ mRNA was not affected in eggs cultured in nominal Ca2+-free medium plus LatB (Fig. 3D) even though this treatment prevented [Ca2+]i responses (Fig. 3E); lower concentrations of mPLCζ mRNA were used in this study to approximate the rates of IP3R1 degradation induced by fertilization. While we still do not precisely know how the LatB + Ca2+-free media combination prevents the initiation of oscillations, we speculate that by disorganizing the egg cortex, LatB decreases the function of IP3R1s, which are organized in clusters at this location in MII eggs, or affects the location/function of Ca2+ channels involved in influx, and that these changes together prevent the initiation of oscillations. Collectively, these results show that uniform production of [IP3]i, which we assume occurred in our conditions as [Ca2+]i oscillations were suppressed, efficiently supports IP3R1 degradation and suggest that persistently elevated levels of [IP3]i might underlie the majority of IP3R1 degradation during mouse fertilization.
Fig. 3.
IP3R1 degradation occurs in the absence of [Ca2+]i oscillations. MII eggs were incubated in medium containing 2 μ MBAPTA-AM for 30 min prior to ICSI or injection of mPLCζ mRNA. Despite precluding egg activation, BAPTA-AM treatment did not affect IP3R1 degradation by ICSI (A). BAPTA-AM treatment also did not affect IP3R1 degradation induced by injection of PLCζ mRNA (0.5 μg/μl) (B), although it greatly reduced the[Ca2+]i oscillations induced by them RNA (inset) (C). IP3R1 degradation in response to PLCζ mRNA(0.1 μg/μl) injection (D) was not prevented or modified by the absence of [Ca2+]i oscillations, a condition that in this case was accomplished by culturing eggs innominal Ca2+-free medium and LatB (E). Eggs were injected with PLCζ mRNA in normal media, but within 10 min of injection eggs were moved to Ca2+-free media supplemented with LatB. Arrows in (C) and (E) denote time of injection.
IP3R1 numbers influence the pattern of [Ca2+]i oscillations
Fertilization-associated [Ca2+]i oscillations become attenuated as eggs exit meiosis (Jones et al., 1998; Day et al., 2000; Deguchi et al., 2000) and this occurs concomitantly with an ~60% reduction in IP3R1 mass (Parrington et al., 1998; Brind et al., 2000; Jellerette et al., 2000). Nonetheless, whether the protracted loss of IP3R1s independently impacts the pattern of [Ca2+]i oscillations has not been thoroughly investigated. To evaluate this it is necessary to isolate the decrease in receptor mass from the cell-cycle transition and other egg activation events that change with progression of the cell cycle. Consequently, an obvious strategy is to initiate [Ca2+]i oscillations while eggs are arrested at the MII stage. Colcemid, a microtubule polymerization inhibitor, could be used for this purpose, as by disrupting spindle integrity, which prevents cyclin B degradation and inactivation of maturation promoting factor (MPF) (Hashimoto and Kishimoto, 1988; Jones et al., 1995b), it maintains the MII arrest in fertilized eggs while supporting long-lasting [Ca2+]i oscillations (Winston et al., 1995; Jones et al., 1995a). Importantly, it is unknown whether colcemid affects IP3R1 degradation. To first ascertain this, eggs were treated with 100 ng/ml colcemid for 1 h and then subjected to ICSI or injection with 10 μM AdA followed by examination of IP3R1 degradation 4 h post-injection. While colcemid-treated eggs remain arrested in a MII-like stage, as evidenced by the absence of 2PB extrusion and PN formation, IP3R1 degradation occurred unhindered after each treatment (Fig. 4 and data not shown).
Fig. 4.
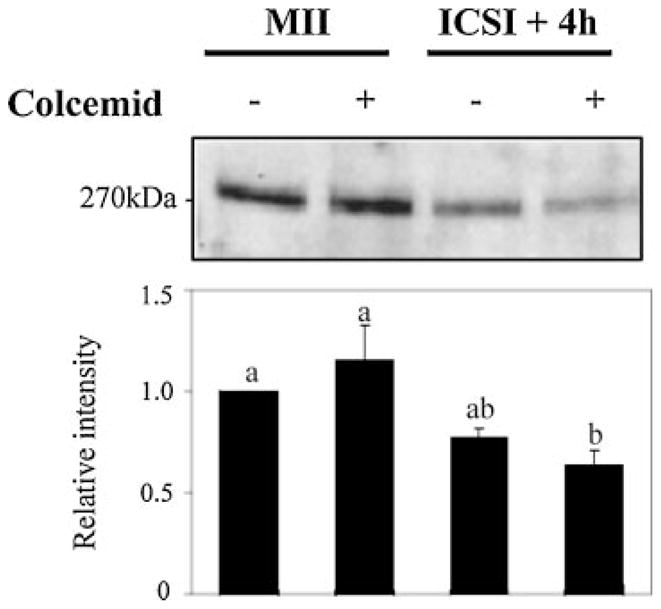
IP3R1 degradation does not require spindle integrity. Mouse eggs were subjected to ICSI and immediately placed in culture medium in the presence or absence of colcemid (100 ng/ml). Colcemid did not alter the rate of IP3R1 degradation despite preventing cell-cycle progression.
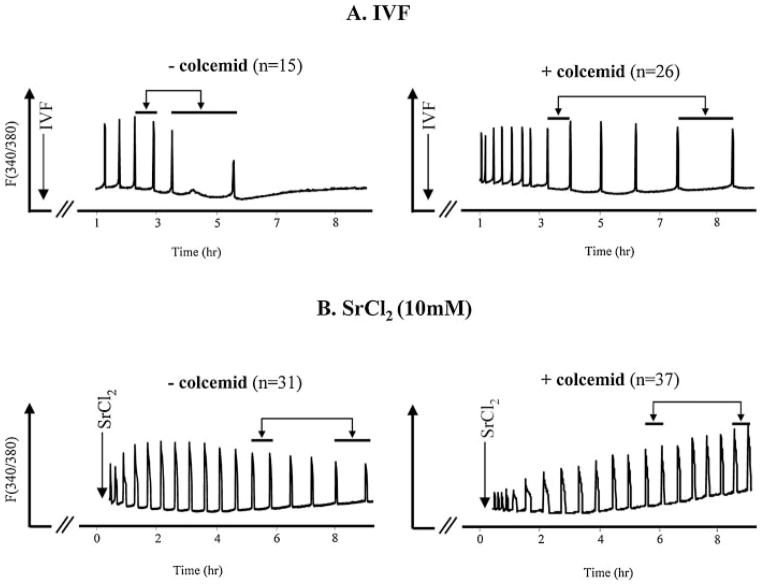
To then address the question of how IP3R1 degradation affects [Ca2+]i oscillations during fertilization, mouse eggs were pretreated with colcemid as before and subjected to IVF. Under this experimental protocol, all egg activation events dependent on cell-cycle progression are precluded while [Ca2+]i oscillations occur unperturbed. In the absence of colcemid, all sperm-initiated [Ca2+]i oscillations stopped by around the time of PN formation, a cessation that in some cases was preceded by a marked lengthening of the last interval (Fig. 5A). Nevertheless, in the presence of colcemid, fertilization-induced oscillations persisted well beyond 4 h, although, after ~3 h, the intervals between [Ca2+]i rises progressively increased (Fig. 5A; right part); we interpret this lengthening of the intervals, which exhibited a mean duration of 12 ± 0.6 (mean ± SEM) min 1–2 h after fertilization versus 31 ± 2.4 min 4–6 h post-fertilization (P <0.05), to be due to IP3R1 degradation. To validate this assumption, we followed the same protocol but instead of IVF, eggs were exposed to SrCl2, which induces oscillations without causing IP3R1 degradation. The assumption here is that in the absence of IP3R1 degradation, oscillations should occur at stable intervals even after prolonged monitoring. As shown in Figure 5B, left part, in the absence of colcemid, SrCl2-initiated oscillations persisted in excess of 6 h, although intervals increased between rises by 6 h, as eggs progressed into interphase. Importantly, SrCl2-triggered oscillations in eggs arrested at MII remained unchanged for the duration of the monitoring period (Fig. 5B, right part; mean duration of intervals of 22 ± 0.8 min 1–2 h after initiation of oscillations vs. 18 ± 1.2 min 4–6 h after initiation of oscillations; P >0.05). These results suggest that IP3R1 degradation influences the parameters of sperm-induced [Ca2+]i oscillations, and that this effect is manifested as an increase in the interval between [Ca2+]i rises.
Fig. 5.
IP3R1 down-regulation during fertilization underlies the progressive increase in the interval between [Ca2+]i spikes. In vitro fertilized mouse eggs cultured in the absence (left part) or presence (right part) of colcemid (100 ng/ml) were monitored for [Ca2+]i responses starting ~90 min after insemination to minimize UV light exposure, which delays cell-cycle progression. Colcemid treatment avoided the abrupt termination of oscillations, although it did not prevent the growing gap between [Ca2+]i rises (A). Under the same conditions, SrCl2-induced oscillations exhibited broadening intervals between rises in the absence of colcemid, which was prevented by colcemid (B). The double head arrows in (A) and (B) connect horizontal bars that represent the length of the intervals between [Ca2+]i rises, which changed during the course of oscillations in fertilized eggs treated with colcemid but that remained largely unchanged in SrCl2-treated eggs in the presence of colcemid.
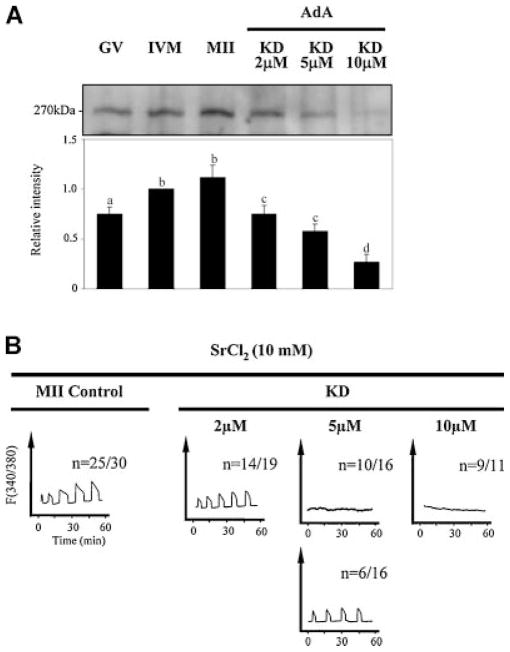
To further investigate the role of IP3R1 concentrations on IP3-induced [Ca2+]i oscillations, we generated eggs with different IP3R1 numbers and evaluated their [Ca2+]i responses after agonist stimulation. To generate IP3R1 KD eggs, GV oocytes were injected with increasing concentrations of AdA (2, 5, or 10 μM) and in vitro matured for 12 h to the MII stage. AdA, a potent non-hydrolyzable agonist of IP3R1 isolated from Penicillium brevicompactum, reportedly has 100-folds greater affinity for IP3R than IP3 and also shows extended binding to the receptor (Takahashi et al., 1994); it might be these properties that make AdA such an efficient inducer of IP3R1 degradation (Brind et al., 2000; Jellerette et al., 2000). In this study, AdA induced dose-dependent IP3R1 degradation without affecting oocyte maturation (Fig. 6A). For instance, injection of 2 μM AdA induced ~25% reduction in receptor mass, whereas 5 μM caused ~40% reduction, and 10 μM induced over 70% reduction of IP3R1 content (lanes 4, 5, and 6, respectively).
Fig. 6.
Down-regulation of IP3R1 mass by injection of adenophostin A (AdA) reduces/eliminates [Ca2+]i responses induced by SrCl2. Injection of AdA induces dose-dependent down-regulation of IP3R1 in mouse oocytes (A). Down-regulation of IP3R1 mass by injection of 2 μM AdA did not affect the number of eggs capable of initiating [Ca2+]i oscillations when exposed to SrCl2 (B, right part, left trace), although injection of 5 μM AdA significantly decreased the number of eggs responding to SrCl2 (right part, center traces), and injection of 10 μM AdA nearly abolished responses in all injected eggs (right part, right trace). A representative [Ca2+]i trace of a control egg is shown in the left part.
To test how different concentrations of IP3R1 affect the parameters of [Ca2+]i oscillations, we exposed IP3R1 KD eggs to 10 mM SrCl2. We selected SrCl2 because it is capable of inducing IP3R1-mediated oscillations without stimulating IP3 production, which otherwise would have caused additional IP3R1 degradation. Our results show that while an ~25% decline in IP3R1 concentration did not affect the number of eggs showing oscillatory responses to SrCl2, an ~40% decline in IP3R1 content greatly reduced the number of eggs capable of oscillating, whereas an ~80% reduction in IP3R1 numbers nearly obliterated the initiation of oscillations in all eggs (Fig. 6B). Altogether, our results show that loss of IP3R1s leads to attenuation or abolition of [Ca2+]i responses and suggest that IP3R1 numbers directly affect [Ca2+]i oscillations parameters during fertilization.
Discussion
The IP3R1 plays an essential role in mammalian fertilization, yet we are unaware of the type of IP3 production that underlies its down-regulation and the impact that its loss has on sperm-initiated [Ca2+]i oscillations. Our results provide insight into these two key points by showing that: (1) IP3R1 degradation requires persistent production and binding of IP3 to the receptor; (2) receptor degradation occurs independently of [Ca2+]i oscillations; (3) IP3R1 degradation does not require an intact spindle; (4) IP3R1 down-regulation contributes to shaping the pattern of sperm-initiated [Ca2+]i oscillations.
It is well established that binding of IP3 to IP3R1 is the main stimulus responsible for receptor degradation. This association was unmistakably established when a form of the receptor with a disabled ligand-binding domain introduced in human neuroblastoma cells failed to undergo degradation following carbachol-induced IP3 production, whereas the wild-type receptor was normally down-regulated (Zhu et al., 1999). IP3 binding stimulates IP3R1 ubiquitination, possibly by exposing a degradation signal for ubiquitination in the receptor, targeting it for degradation by the proteasome (Zhu et al., 1999; Webster et al., 2003; Wojcikiewicz et al., 2003; Wojcikiewicz, 2004). Consistent with these findings, bortezomib, a potent proteasome inhibitor, prevents IP3R1 ubiquitination and degradation (Xu et al., 2004). A similar mechanism seems to operate in mammalian eggs, as following agonist stimulation IP3R1 degradation in eggs was partially blocked by proteasome inhibitors (Brind et al., 2000; Jellerette et al., 2000).
Although in somatic cells it is now possible to assess [IP3]i responses to a variety of agonists in real time, the presumed modest increases in [IP3]i induced by mammalian sperm have thus far eluded detection, thereby delaying our understanding of the pattern of [IP3]i levels that underlie IP3R1 degradation in eggs. For example, using a variety of fluorescence resonance energy transfer (FRET) probes, it has been shown that [IP3]i levels during [Ca2+]i oscillations can either remain steadily elevated or oscillate with each [Ca2+]i rise depending on the somatic cell type and stimuli (Violin et al., 2003). By contrast, one of these probes, fretino-2, failed to detect changes in [IP3]i in mouse eggs despite the presence of sperm-induced [Ca2+]i oscillations (Shirakawa et al., 2006). Nonetheless, this probe protractedly detected pulsatile [IP3]i increases associated with [Ca2+]i rises in eggs injected with mPLCζ mRNA, although, by this time, the increase in basal [IP3]i outpaced the magnitude of the discrete [IP3]i rises (Shirakawa et al., 2006). Thus, results with FRET probes support the view that PLCζ is highly sensitive to Ca2+ and that [Ca2+]i rises may stimulate IP3 production, although to date they do not provide enough evidence to draw conclusions about the prevailing [IP3]i levels during mouse/mammalian fertilization. Therefore, given the reliance of IP3R1 degradation on IP3 production and binding, we examined the loss of endogenous IP3R1 mass induced by two widely dissimilar patterns of [IP3]i and used it to retrospectively estimate the pattern of [IP3]i that regulates IP3R1 degradation during fertilization. Our data show that transient increases in [IP3]i produced by either bolus injections of IP3 or 2-IP3 or by discrete releases of caged IP3 did not approximate the IP3R1 degradation observed after fertilization. Instead, the sperm-induced receptor degradation was closely replicated by increases in [IP3]i induced by ICSI or by injection of mPLCζ mRNA but under conditions that precluded [Ca2+]i oscillations, which we predict resulted in uniform production of the ligand. Hence, our results support the notion that continuous production of IP3 by a constitutively active PLCζ causes increased basal [IP3]i, which in turn sensitizes IP3R1 and leads to its protracted degradation. Our results are in line with data by others who demonstrated that fertilization-like [Ca2+]i oscillations were mimicked most closely by short and repeated uncaging episodes of IP3, which per se do not result in Ca2+ release, than by larger, productive releases of IP3 spaced farther apart (Jones and Nixon, 2000). Our results, however, do not imply that Ca2+-induced pulsatile increases in [IP3]i do not occur during mammalian fertilization, as such increases may be important for wave propagation, signal amplification and may encode spatial information during each [Ca2+]i rise.
It is worthwhile noting that in our studies we used two metabolically stable analogs of IP3, 2-IP3, and AdA. Interestingly only AdA induced significant IP3R1 degradation, which is consistent with previous reports by us and others (Brind et al., 2000; Jellerette et al., 2000; Malcuit et al., 2005). The minor IP3R1 degradation induced by 2-IP3 is surprising, given that 2-IP3 injection promoted [Ca2+]i oscillations that lasted in excess of 60 min. Nonetheless, besides highlighting the stability of IP3R1 in eggs, these results support the view that it is not just the binding of IP3 to its receptor that promotes degradation, but also the affinity and duration of the binding (Willars et al., 2001), both of which are greater for AdA than for native IP3 (Adkins et al., 2000; Morris et al., 2002).
Whether the degradation that the egg’s IP3R1 undergoes after fertilization contributes to the well-characterized “slow-down” of the oscillations prior to their cessation at the PN stage, as the interval between [Ca2+]i rises nearly doubles between the initiation and termination of the oscillations in monospermically fertilized mouse eggs, has not been closely examined (Deguchi et al., 2000; Kurokawa and Fissore, 2003). Hence, to elucidate this point, [Ca2+]i oscillations were induced in eggs treated with colcemid, which arrests them at the M-phase stage, thereby preventing the resumption of the cell cycle, the main confounding factor. Under these conditions, fertilization-induced [Ca2+]i oscillations consistently underwent a slow down in frequency, which we attribute to IP3R1 degradation, as colcemid-induced cell-cycle arrest did not affect IP3R1 down-regulation. While it is possible that other changes in the egg’s Ca2+ release mechanisms may also contribute to the deceleration of the oscillations, including inactivation/degradation of PLCζ, it is worth noting that in colcemid-treated eggs fertilization-initiated oscillations can last in excess of 20 h, which makes the latter possibility an unlikely cause of termination of the oscillations (Day et al., 2000). Moreover, under the same conditions, SrCl2-induced oscillations, which occur in the absence of receptor degradation, showed similar persistence albeit without obvious changes in periodicity. Further, our results are in agreement with data showing that the periodicity of sperm-induced oscillations in mouse eggs prevented to exit MII by agents that inhibit microtubule polymerization also resulted in prolongation of the intervals between [Ca2+]i rises, although this change was not noted in those studies (Jones et al., 1995a; Day et al., 2000). Together, these data demonstrate that the decline in IP3R1 numbers is one of the factors that control the periodicity of fertilization-induced [Ca2+]i oscillations. Our results do not imply that IP3R1 degradation is responsible for the termination of oscillations, which occurs in the mouse around PN formation (Jones et al., 1995a). Instead, we propose that the partial desensitization of IP3R1 caused by the reduction of IP3R1 numbers, and possibly by receptor dephosphorylation and re-organization, predisposes early mouse zygotes to premature termination of oscillations, event that in many zygotes is precipitated by decreasing levels of PLCζ. After fertilization, PLCζ levels in the ooplasm are likely to be steadily reduced during PN formation, as it well documented that mouse PLCζ is sequestered into the forming PN (Larman et al., 2004; Yoda et al., 2004). Importantly, termination of the oscillations occurs ahead of complete PN formation, which is consistent with the notion that in IP3R1-desensitized zygotes slight changes in the available levels of PLCζ caused by the early accumulation of the enzyme in the forming PN (Ito et al., 2008b), may be sufficient to signal the termination of oscillations. PLCζ sequestration is unlikely to contribute to the termination of oscillations in rat zygotes, as in this species PLCζ does not undergo translocation to the PN (Ito et al., 2008c).
IP3R1 degradation is also observed in other cell systems as a means to control [Ca2+]i responses and agonist stimulation. A remarkable illustration of this is the rapid and near complete IP3R1 down-regulation observed in gonadotropes stimulated with gonadotropin-releasing hormone, which precludes Ca2+ release and abolishes the subsequent hormone action (Willars et al., 2001). A more controlled reduction of IP3Rs numbers was accomplished in A7r5 cells, which made possible the detection of more subtle effects of IP3R1 numbers on Ca2+ release (Wang et al., 2001). For example, [Ca2+]i responses induced by vasopressin were slightly affected by an ~20% loss in IP3R1 mass, although Ca2+ release was severely reduced when receptors losses amounted to ~40% of total IP3R1 mass, and the [Ca2+]i signal was abolished when losses of IP3R1 reached ~80% (Wang et al., 2001). Notably, using IP3R1 KD eggs, our results show that the initiation of SrCl2-induced [Ca2+]i oscillations was similarly affected by equivalent losses in IP3R1 mass. In agreement with our results, two other studies demonstrated that reduced IP3R1 numbers, accomplished either by RNA interference or by injection of AdA, decreased/eliminated [Ca2+]i responses, although a careful examination of the degree of receptor degradation and impact on oscillations was not performed in those studies (Brind et al., 2000; Xu et al., 2003).
We conclude that continuous production of IP3 during mouse fertilization underlies IP3R1 degradation, which in turn decreases the frequency of [Ca2+]i oscillations. We propose that the persistent production of IP3 induced by the sperm along with the resilience of the eggs’ IP3R1 is required both to overcome the robust MII arrest and to assure the orderly completion of all events of egg activation. Whether the subtle changes in the pattern of [Ca2+]i oscillations caused by receptor degradation, which is possibly just a consequence of the long-term production of IP3, have any practical impact on embryo development remains to be demonstrated.
Acknowledgments
We thank Ms. Changli He for technical assistance. This work was supported in part by a grant HD051872 from the NIH to R.A.F. Work in the laboratory of J.B.P. was supported by Research Program G.0604.07 of the Research Foundation—Flanders (FWO) and by grants G.O.A. 2004/07 and G.O.A. 2009/12 from the Concerted Actions of the K.U. Leuven.
Contract grant sponsor: NIH;
Contract grant number: HD051872.
Contract grant sponsor: Research Foundation—Flanders (FWO);
Contract grant number: G.0604.07.
Contract grant sponsor: Concerted Actions of the K.U. Leuven;
Contract grant numbers: G.O.A. 2004/07, G.O.A. 2009/12.
Literature Cited
- Adkins CE, Wissing F, Potter BV, Taylor CW. Rapid activation and partial inactivation of inositol trisphosphate receptors by adenophostin A. Biochem J. 2000;352:929–933. [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ. The endoplasmic reticulum: A multifunctional signaling organelle. Cell Calcium. 2002;32:235–249. doi: 10.1016/s0143416002001823. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- Bosanac I, Michikawa T, Mikoshiba K, Ikura M. Structural insights into the regulatory mechanism of IP3 receptor. Biochim Biophys Acta. 2004;1742:89–102. doi: 10.1016/j.bbamcr.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Brind S, Swann K, Carroll J. Inositol 1,4,5-trisphosphate receptors are downregulated in mouse oocytes in response to sperm or adenophostin A but not to increases in intracellular Ca(2+) or egg activation. Dev Biol. 2000;223:251–265. doi: 10.1006/dbio.2000.9728. [DOI] [PubMed] [Google Scholar]
- Chatot CL, Lewis JL, Torres I, Ziomek CA. Development of 1-cell embryos from different strains of mice in CZB medium. Biol Reprod. 1990;42:432–440. doi: 10.1095/biolreprod42.3.432. [DOI] [PubMed] [Google Scholar]
- Chiba K, Kado RT, Jaffe LA. Development of calcium release mechanisms during starfish oocyte maturation. Dev Biol. 1990;140:300–306. doi: 10.1016/0012-1606(90)90080-3. [DOI] [PubMed] [Google Scholar]
- Day ML, McGuinness OM, Berridge MJ, Johnson MH. Regulation of fertilization-induced Ca(2+)spiking in the mouse zygote. Cell Calcium. 2000;28:47–54. doi: 10.1054/ceca.2000.0128. [DOI] [PubMed] [Google Scholar]
- Deguchi R, Shirakawa H, Oda S, Mohri T, Miyazaki S. Spatiotemporal analysis of Ca2+ waves in relation to the sperm entry site and animal-vegetal axis during Ca2+ oscillations in fertilized mouse eggs. Dev Biol. 2000;218:299–313. doi: 10.1006/dbio.1999.9573. [DOI] [PubMed] [Google Scholar]
- Ducibella T, Huneau D, Angelichio E, Xu Z, Schultz RM, Kopf GS, Fissore R, Madoux S, Ozil JP. Egg-to-embryo transition is driven by differential responses to Ca(2+) oscillation number. Dev Biol. 2002;250:280–291. [PubMed] [Google Scholar]
- FitzHarris G, Marangos P, Carroll J. Cell cycle-dependent regulation of structure of endoplasmic reticulum and inositol 1,4,5-trisphosphate-induced Ca(2+) release in mouse oocytes and embryos. Mol Biol Cell. 2003;14:288–301. doi: 10.1091/mbc.E02-07-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T, Nakada K, Shirakawa H, Miyazaki S. Development of inositol trisphosphate-induced calcium release mechanism during maturation of hamster oocytes. Dev Biol. 1993;156:69–79. doi: 10.1006/dbio.1993.1059. [DOI] [PubMed] [Google Scholar]
- Hashimoto N, Kishimoto T. Regulation of meiotic metaphase by a cytoplasmic maturation-promoting factor during mouse oocyte maturation. Dev Biol. 1988;126:242–252. doi: 10.1016/0012-1606(88)90135-2. [DOI] [PubMed] [Google Scholar]
- Ito J, Yoon SY, Lee B, Vanderheyden V, Vermassen E, Wojcikiewicz RJ, Alfandari D, De Smedt H, Parys JB, Fissore RA. Inositol 1,4,5-trisphosphate receptor 1, a widespread Ca2+ channel, is a novel substrate of polo-like kinase 1 in eggs. Dev Biol. 2008a;320:402–413. doi: 10.1016/j.ydbio.2008.05.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Shikano T, Kuroda K, Miyazaki S. Relationship between nuclear sequestration of PLCzeta and termination of PLCzeta-induced Ca2+ oscillations in mouse eggs. Cell Calcium. 2008b;44:400–410. doi: 10.1016/j.ceca.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Ito M, Shikano T, Oda S, Horiguchi T, Tanimoto S, Awaji T, Mitani H, Miyazaki S. Difference in Ca2+ oscillation-inducing activity and nuclear translocation ability of PLCZ1, an egg-activating sperm factor candidate between mouse, rat, human, and medaka fish. Biol Reprod. 2008c;78:1081–1090. doi: 10.1095/biolreprod.108.067801. [DOI] [PubMed] [Google Scholar]
- Iwasaki H, Chiba K, Uchiyama T, Yoshikawa F, Suzuki F, Ikeda M, Furuichi T, Mikoshiba K. Molecular characterization of the starfish inositol 1,4,5-trisphosphate receptor and its role during oocyte maturation and fertilization. J Biol Chem. 2002;277:2763–2772. doi: 10.1074/jbc.M108839200. [DOI] [PubMed] [Google Scholar]
- Jellerette T, He CL, Wu H, Parys JB, Fissore RA. Down-regulation of the inositol 1,4,5-trisphosphate receptor in mouse eggs following fertilization or parthenogenetic activation. Dev Biol. 2000;223:238–250. doi: 10.1006/dbio.2000.9675. [DOI] [PubMed] [Google Scholar]
- Jellerette T, Kurokawa M, Lee B, Malcuit C, Yoon SY, Smyth J, Vermassen E, De Smedt H, Parys JB, Fissore RA. Cell cycle-coupled [Ca2+]i oscillations in mouse zygotes and function of the inositol 1,4,5-trisphosphate receptor-1. Dev Biol. 2004;274:94–109. doi: 10.1016/j.ydbio.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Jones KT. Mammalian egg activation: From Ca2+ spiking to cell cycle progression. Reproduction. 2005;130:813–823. doi: 10.1530/rep.1.00710. [DOI] [PubMed] [Google Scholar]
- Jones KT, Nixon VL. Sperm-induced Ca(2+) oscillations in mouse oocytes and eggs can be mimicked by photolysis of caged inositol 1,4,5-trisphosphate: Evidence to support a continuous low level production of inositol 1,4 5-trisphosphate during mammalian fertilization. Dev Biol. 2000;225:1–12. doi: 10.1006/dbio.2000.9826. [DOI] [PubMed] [Google Scholar]
- Jones KT, Whittingham DG. A comparison of sperm- and IP3-induced Ca2+ release in activated and aging mouse oocytes. Dev Biol. 1996;178:229–237. doi: 10.1006/dbio.1996.0214. [DOI] [PubMed] [Google Scholar]
- Jones KT, Carroll J, Merriman JA, Whittingham DG, Kono T. Repetitive sperm-induced Ca2+ transients in mouse oocytes are cell cycle dependent. Development. 1995a;121:3259–3266. doi: 10.1242/dev.121.10.3259. [DOI] [PubMed] [Google Scholar]
- Jones KT, Carroll J, Whittingham DG. Ionomycin, thapsigargin, ryanodine, and sperm induced Ca2+ release increase during meiotic maturation of mouse oocytes. J Biol Chem. 1995b;270:6671–6677. doi: 10.1074/jbc.270.12.6671. [DOI] [PubMed] [Google Scholar]
- Jones K, Soeller C, Cannell M. The passage of Ca2+ and fluorescent markers between the sperm and egg after fusion in the mouse. Development. 1998;125:4627–4635. doi: 10.1242/dev.125.23.4627. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Yanagimachi R. Intracytoplasmic sperm injection in the mouse. Biol Reprod. 1995;52:709–720. doi: 10.1095/biolreprod52.4.709. [DOI] [PubMed] [Google Scholar]
- Kouchi Z, Fukami K, Shikano T, Oda S, Nakamura Y, Takenawa T, Miyazaki S. Recombinant phospholipase Czeta has high Ca2+ sensitivity and induces Ca2+ oscillations in mouse eggs. J Biol Chem. 2004;279:10408–10412. doi: 10.1074/jbc.M313801200. [DOI] [PubMed] [Google Scholar]
- Kurokawa M, Fissore RA. ICSI-generated mouse zygotes exhibit altered calcium oscillations, inositol 1,4,5-trisphosphate receptor-1 down-regulation, and embryo development. Mol Hum Reprod. 2003;9:523–533. doi: 10.1093/molehr/gag072. [DOI] [PubMed] [Google Scholar]
- Kurokawa M, Sato K, Smyth J, Wu H, Fukami K, Takenawa T, Fissore RA. Evidence that activation of Src family kinase is not required for fertilization-associated [Ca2+]i oscillations in mouse eggs. 2004;127:441–454. doi: 10.1530/rep.1.00128. [DOI] [PubMed] [Google Scholar]
- Kurokawa M, Sato K, Wu H, He C, Malcuit C, Black SJ, Fukami K, Fissore RA. Functional, biochemical, and chromatographic characterization of the complete [Ca(2+)](i) oscillation-inducing activity of porcine sperm. Dev Biol. 2005;285:376–392. doi: 10.1016/j.ydbio.2005.06.029. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larman MG, Saunders CM, Carroll J, Lai FA, Swann K. Cell cycle-dependent Ca2+ oscillations in mouse embryos are regulated by nuclear targeting of PLCzeta. J Cell Sci. 2004;117:2513–2525. doi: 10.1242/jcs.01109. [DOI] [PubMed] [Google Scholar]
- Lee B, Yoon SY, Fissore RA. Regulation of fertilization-initiated [Ca2+]i oscillations in mammalian eggs: A multi-pronged approach. Semin Cell Dev Biol. 2006;17:274–284. doi: 10.1016/j.semcdb.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Malcuit C, Knott JG, He C, Wainwright T, Parys JB, Robl JM, Fissore RA. Fertilization and inositol 1,4,5-trisphosphate (IP3)-induced calcium release in type-1 inositol 1,4,5-trisphosphate receptor down-regulated bovine eggs. Biol Reprod. 2005;73:2–13. doi: 10.1095/biolreprod.104.037333. [DOI] [PubMed] [Google Scholar]
- Marangos P, FitzHarris G, Carroll J. Ca2+ oscillations at fertilization in mammals are regulated by the formation of pronuclei. Development. 2003;130:1461–1472. doi: 10.1242/dev.00340. [DOI] [PubMed] [Google Scholar]
- McAvey BA, Wortzman GB, Williams CJ, Evans JP. Involvement of calcium signaling and the actin cytoskeleton in the membrane block to polyspermy in mouse eggs. Biol Reprod. 2002;67:1342–1352. doi: 10.1095/biolreprod67.4.1342. [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Kline D. Regulation of intracellular calcium in the mouse egg: Calcium release in response to sperm or inositol trisphosphate is enhanced after meiotic maturation. Biol Reprod. 1994;51:1088–1098. doi: 10.1095/biolreprod51.6.1088. [DOI] [PubMed] [Google Scholar]
- Mignery GA, Sudhof TC. The ligand binding site and transduction mechanism in the inositol-1,4,5-triphosphate receptor. EMBO J. 1990;9:3893–3898. doi: 10.1002/j.1460-2075.1990.tb07609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S. Inositol 1,4,5-trisphosphate-induced calcium release and guanine nucleotide-binding protein-mediated periodic calcium rises in golden hamster eggs. J Cell Biol. 1988;106:345–353. doi: 10.1083/jcb.106.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S, Yuzaki M, Nakada K, Shirakawa H, Nakanishi S, Nakade S, Mikoshiba K. Block of Ca2+ wave and Ca2+ oscillation by antibody to the inositol 1,4,5-trisphosphate receptor in fertilized hamster eggs. Science. 1992;257:251–255. doi: 10.1126/science.1321497. [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Shirakawa H, Nakada K, Honda Y. Essential role of the inositol 1,4,5-trisphosphate receptor/Ca2+ release channel in Ca2+ waves and Ca2+ oscillations at fertilization of mammalian eggs. Dev Biol. 1993;158:62–78. doi: 10.1006/dbio.1993.1168. [DOI] [PubMed] [Google Scholar]
- Morris SA, Nerou EP, Riley AM, Potter BV, Taylor CW. Determinants of adenophostin A binding to inositol trisphosphate receptors. Biochem J. 2002;367:113–120. doi: 10.1042/BJ20020675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomikos M, Blayney LM, Larman MG, Campbell K, Rossbach A, Saunders CM, Swann K, Lai FA. Role of phospholipase C-zeta domains in Ca2+-dependent phosphatidylinositol 4,5-bisphosphate hydrolysis and cytoplasmic Ca2+ oscillations. J Biol Chem. 2005;280:31011–31018. doi: 10.1074/jbc.M500629200. [DOI] [PubMed] [Google Scholar]
- Ozil JP, Huneau D. Activation of rabbit oocytes: The impact of the Ca2+ signal regime on development. Development. 2001;128:917–928. doi: 10.1242/dev.128.6.917. [DOI] [PubMed] [Google Scholar]
- Parrington J, Brind S, De Smedt H, Gangeswaran R, Lai FA, Wojcikiewicz R, Carroll J. Expression of inositol 1,4,5-trisphosphate receptors in mouse oocytes and early embryos: The type I isoform is upregulated in oocytes and downregulated after fertilization. Dev Biol. 1998;203:451–461. doi: 10.1006/dbio.1998.9071. [DOI] [PubMed] [Google Scholar]
- Parys JB, de Smedt H, Missiaen L, Bootman MD, Sienaert I, Casteels R. Rat basophilic leukemia cells as model system for inositol 1,4,5-trisphosphate receptor IV, a receptor of the type II family: Functional comparison and immunological detection. Cell Calcium. 1995;17:239–249. doi: 10.1016/0143-4160(95)90070-5. [DOI] [PubMed] [Google Scholar]
- Patel S, Joseph SK, Thomas AP. Molecular properties of inositol 1,4,5-trisphosphate receptors. Cell Calcium. 1999;25:247–264. doi: 10.1054/ceca.1999.0021. [DOI] [PubMed] [Google Scholar]
- Petersen OH, Tepikin A, Park MK. The endoplasmic reticulum: One continuous or several separate Ca(2+) stores? Trends Neurosci. 2001;24:271–276. doi: 10.1016/s0166-2236(00)01787-2. [DOI] [PubMed] [Google Scholar]
- Quinn P, Warnes GM, Kerin JF, Kirby C. Culture factors affecting the success rate of in vitro fertilization and embryo transfer. Ann N Y Acad Sci. 1985;442:195–204. doi: 10.1111/j.1749-6632.1985.tb37520.x. [DOI] [PubMed] [Google Scholar]
- Rebecchi MJ, Pentyala SN. Anaesthetic actions on other targets: Protein kinase C and guanine nucleotide-binding proteins. Br J Anaesth. 2002;89:62–78. doi: 10.1093/bja/aef160. [DOI] [PubMed] [Google Scholar]
- Runft LL, Watras J, Jaffe LA. Calcium release at fertilization of Xenopus eggs requires type I IP(3) receptors, but not SH2 domain-mediated activation of PLCgamma or G(q)-mediated activation of PLCbeta. Dev Biol. 1999;214:399–411. doi: 10.1006/dbio.1999.9415. [DOI] [PubMed] [Google Scholar]
- Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, Blayney LM, Swann K, Lai FA. PLC zeta: A sperm-specific trigger of Ca(2+) oscillations in eggs and embryo development. Development. 2002;129:3533–3544. doi: 10.1242/dev.129.15.3533. [DOI] [PubMed] [Google Scholar]
- Schatten G, Schatten H, Spector I, Cline C, Paweletz N, Simerly C, Petzelt C. Latrunculin inhibits the microfilament-mediated processes during fertilization, cleavage and early development in sea urchins and mice. Exp Cell Res. 1986;166:191–208. doi: 10.1016/0014-4827(86)90519-7. [DOI] [PubMed] [Google Scholar]
- Schultz RM, Kopf GS. Molecular basis of mammalian egg activation. Curr Topics Dev Biol. 1995;30:21–62. doi: 10.1016/s0070-2153(08)60563-3. [DOI] [PubMed] [Google Scholar]
- Shirakawa H, Ito M, Sato M, Umezawa Y, Miyazaki S. Measurement of intracellular IP3 during Ca2+ oscillations in mouse eggs with GFP-based FRET probe. Biochem Biophys Res Commun. 2006;345:781–788. doi: 10.1016/j.bbrc.2006.04.133. [DOI] [PubMed] [Google Scholar]
- Stricker SA. Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev Biol. 1999;211:157–176. doi: 10.1006/dbio.1999.9340. [DOI] [PubMed] [Google Scholar]
- Stricker SA. Structural reorganizations of the endoplasmic reticulum during egg maturation and fertilization. Semin Cell Biol. 2006;17:303–313. doi: 10.1016/j.semcdb.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Tanzawa K, Takahashi S. Adenophostins, newly discovered metabolites of Penicillium brevicompactum, act as potent agonists of the inositol 1,4,5-trisphosphate receptor. J Biol Chem. 1994;269:369–372. [PubMed] [Google Scholar]
- Tang TS, Tu H, Wang Z, Bezprozvanny I. Modulation of type 1 inositol (1,4,5)-trisphosphate receptor function by protein kinase a and protein phosphatase 1alpha. J Neurosci. 2003;23:403–415. doi: 10.1523/JNEUROSCI.23-02-00403.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Violin JD, Zhang J, Tsien RY, Newton AC. A genetically encoded fluorescent reporter reveals oscillatory phosphorylation by protein kinase C. J Cell Biol. 2003;161:899–909. doi: 10.1083/jcb.200302125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Chen J, Wang Y, Taylor CW, Hirata Y, Hagiwara H. Crucial role of type 1, but not type 3, inositol 1,4,5-trisphosphate (IP3) receptors in IP3-induced Ca2+ release, capacitative Ca2+ entry, and proliferation of A7r5 vascular smooth muscle cells. Circ Res. 2001;88:202–209. doi: 10.1161/01.res.88.2.202. [DOI] [PubMed] [Google Scholar]
- Webster JM, Tiwari S, Weissman AM, Wojcikiewicz RJ. Inositol 1,4,5-trisphosphate receptor ubiquitination is mediated by mammalian Ubc7, a component of the endoplasmic reticulum-associated degradation pathway, and is inhibited by chelation of intracellular Zn2+ J Biol Chem. 2003;278:38238–38246. doi: 10.1074/jbc.M305600200. [DOI] [PubMed] [Google Scholar]
- Willars GB, Royall JE, Nahorskim SR, El-Gehani F, Everest H, McArdle CA. Rapid down-regulation of the type I inositol 1,4,5-trisphosphate receptor and desensitization of gonadotropin-releasing hormone-mediated Ca2+ responses in alpha T 3-1 gonadotropes. J Biol Chem. 2001;276:3123–3129. doi: 10.1074/jbc.M008916200. [DOI] [PubMed] [Google Scholar]
- Winston NJ, McGuinness O, Johnson MH, Maro B. The exit of mouse oocytes from meiotic M-phase requires an intact spindle during intracellular calcium release. J Cell Sci. 1995;108:143–151. doi: 10.1242/jcs.108.1.143. [DOI] [PubMed] [Google Scholar]
- Wojcikiewicz RJ. Regulated ubiquitination of proteins in GPCR-initiated signaling pathways. Trends Pharmacol Sci. 2004;25:35–41. doi: 10.1016/j.tips.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Wojcikiewicz RJ, Xu Q, Webster JM, Alzayady K, Gao C. Ubiquitination and proteasomal degradation of endogenous and exogenous inositol 1,4,5-trisphosphate receptors in alpha T 3-1 anterior pituitary cells. J Biol Chem. 2003;278:940–947. doi: 10.1074/jbc.M206607200. [DOI] [PubMed] [Google Scholar]
- Wu H, He CL, Fissore RA. Injection of a porcine sperm factor triggers calcium oscillations in mouse oocytes and bovine eggs. Mol Reprod Dev. 1997;46:176–189. doi: 10.1002/(SICI)1098-2795(199702)46:2<176::AID-MRD8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Xu Z, Williams CJ, Kopf GS, Schultz RM. Maturation-associated increase in IP3 receptor type 1: Role in conferring increased IP3 sensitivity and Ca2+ oscillatory behavior in mouse eggs. Dev Biol. 2003;254:163–171. doi: 10.1016/s0012-1606(02)00049-0. [DOI] [PubMed] [Google Scholar]
- Xu Q, Farah M, Webster JM, Wojcikiewicz RJ. Bortezomib rapidly suppresses ubiquitin thiolesterification to ubiquitin-conjugating enzymes and inhibits ubiquitination of histones and type I inositol 1,4,5-trisphosphate receptor. Mol Cancer Ther. 2004;3:1263–1269. [PubMed] [Google Scholar]
- Yoon SY, Jellerette T, Salicioni AM, Lee HC, Yoo MS, Coward K, Parrington J, Grow D, Cibelli JB, Visconti PE, Mager J, Fissore RA. Human sperm devoid of PLC, zeta 1 fail to induce Ca(2+) release and are unable to initiate the first step of embryo development. J Clin Invest. 2008;118:3671–3681. doi: 10.1172/JCI36942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoda A, Oda S, Shikano T, Kouchi Z, Awaji T, Shirakawa H, Kinoshita K, Miyazaki S. Ca2+ oscillation-inducing phospholipase C zeta expressed in mouse eggs is accumulated to the pronucleus during egg activation. Dev Biol. 2004;268:245–257. doi: 10.1016/j.ydbio.2003.12.028. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Sensui N, Inoue T, Morisawa M, Mikoshiba K. Role of two series of Ca2+ oscillations in activation of ascidian eggs. Dev Biol. 1998;203:122–133. doi: 10.1006/dbio.1998.9037. [DOI] [PubMed] [Google Scholar]
- Zhu CC, Wojcikiewicz RJ. Ligand binding directly stimulates ubiquitination of the inositol 1,4 5-trisphosphate receptor. Biochem J. 2000;348:551–556. [PMC free article] [PubMed] [Google Scholar]
- Zhu CC, Furuichi T, Mikoshiba K, Wojcikiewicz RJ. Inositol 1,4,5-trisphosphate receptor down-regulation is activated directly by inositol 1,4,5-trisphosphate binding. Studies with binding-defective mutant receptors. J Biol Chem. 1999;274:3476–3484. doi: 10.1074/jbc.274.6.3476. [DOI] [PubMed] [Google Scholar]