Abstract
Oral melatonin can improve daytime sleep, but the hormone's short elimination half-life limits its use as a hypnotic in shift workers, jet-lag and other situations. Here we show in healthy subjects that transdermal delivery of melatonin during the daytime can elevate plasma melatonin and reduce waking after sleep onset by promoting sleep in the latter part of an 8-hour sleep opportunity. Thus, transdermal melatonin may have advantages over fast-release oral melatonin in improving sleep maintenance at adverse circadian phases.
Keywords: transdermal melatonin, daytime sleep, sleep maintenance, hypnotic, EEG spectra, circadian wake drive, body temperature, alertness
The modern 24-hour society requires many individuals to seek sleep during the daytime. As evidenced by the prevalence of premature awakenings in night workers, however, maintaining sleep during the daytime is difficult.1-3 This difficulty has a biological basis in an altered relationship between the circadian and homeostatic sleep-regulatory processes: when sleep is attempted during the daytime, the homeostatic sleep pressure that has built up during wakefulness may be high enough to initiate sleep, but its gradual dissipation occurs in the presence of increasing circadian wake drive, which can result in premature awakenings.
Administration of melatonin during the daytime, i.e. outside of the phase of its endogenous secretion, can facilitates sleep.4-10 Fast-release oral melatonin, however, has limitations if the treatment goal is to maintain daytime sleep for ∼8 h: due to the short elimination half-life (∼40 min), melatonin levels are already decreased at a time when the sleep-promoting effect is needed most, i.e. when sleep pressure has decreased and the circadian wake drive is increasing. Therefore, a relatively high oral dose of melatonin has to be administered, which in turn may lead to desensitization of the melatonin receptors11 and potentially contribute to the development of tolerance.8
To overcome the limitations of fast-release oral melatonin, controlled-release, transmucosal and transdermal delivery have been tested.8,9,12-15 Testing of transdermal delivery has not included polysomnography and quantitative electroencephalogram (EEG) analysis and thus its effectiveness to improve sleep maintenance is unknown. The purpose of the present study was to test an experimental skin patch designed to deliver melatonin such that plasma levels steadily increase for 6-8 h, and thereby counteract the increasing circadian wake drive and improve daytime sleep.
Results
Eight healthy subjects received a skin patch (2.1 mg melatonin or placebo, randomized, double-blind, crossover) one hour before an 8-h daytime sleep opportunity (09:00-17:00 h), referred to as melatonin (MEL) or placebo (PL) sleep (see Methods for details).
Plasma melatonin and core body temperature
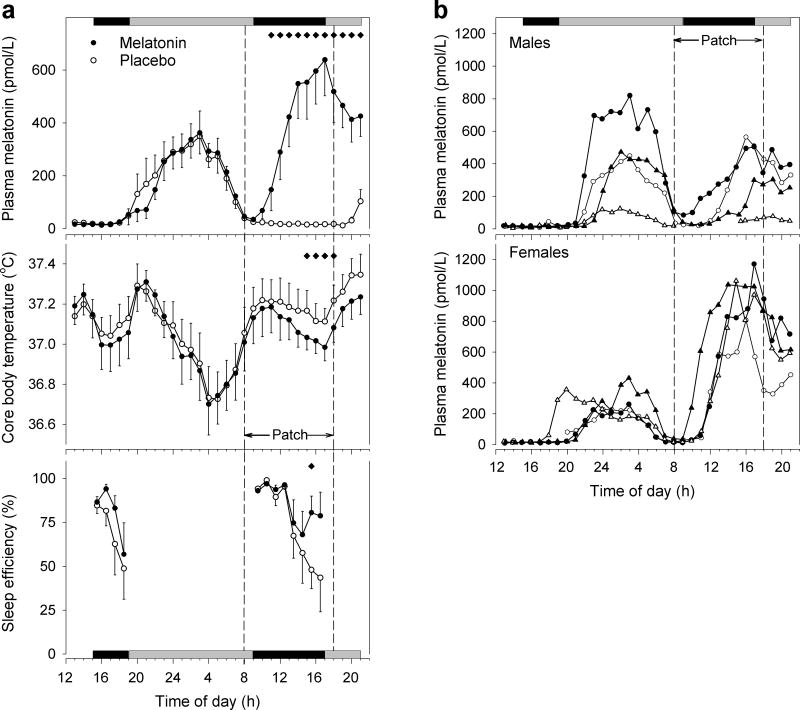
After a lag following application of the active patch, plasma melatonin levels increased steadily, reached statistical significance over placebo after 3 h, and peak concentration (Cmax ± SEM) of 690.4±138.8 pmol/L after (tmax) 8.58±0.63 h (Figure 1a, top). Melatonin reduced body temperature by ∼0.13°C during the last 4 h of administration (Figure 1a, middle).
Figure 1.
Plasma melatonin, core body temperature, and sleep efficiency before and during transdermal melatonin administration during daytime. Data in (a) represent hourly means ± 1 SEM (N=8 for melatonin and body temperature; N=7 for sleep efficiency). Application and removal of the dermal patch are indicated by dashed vertical lines. Note that elevated melatonin levels prior to patch application are due to endogenous secretion. Horizontal black bars on top and bottom indicate scheduled sleep opportunities. Gray bars indicate scheduled wake episodes in ∼1.8 lux. Subjects maintained a semi-recumbent position (upper part of bed at 45°) for the 6 h before and for the hours after the 8-h daytime sleep opportunity to allow for measurements of body temperature undisturbed by changes in body position. During the sleep episodes subjects were supine. Note that at 20:00 h and 21:00 h in the placebo condition blood samples could only be obtained from 6 subjects. Mixed-model ANOVA on log-transformed hourly melatonin values following patch application was significant for Condition, Time, and Condition × Time (P<0.0001 in all cases). ANOVA on temperature data was significant for Condition and Time (P<0.0001), but not for their interaction (P=0.99). Black diamonds indicate significant differences from placebo [P<0.05, t-tests (melatonin, body temperature) or Wilcoxon signed rank test (sleep efficiency) for planned comparisons]. (b) Individual melatonin profiles in males (top) and females (bottom). Note that one male subject with very low exogenous plasma melatonin levels also had very low endogenous melatonin levels.
Individual plasma profiles revealed marked gender differences in melatonin delivery (Figure 1b). The exogenous maximum in women (1018.7±76.5 pmol/L) was higher (P=0.002, t-test) than in men (362.0±110.2 pmol/L). This difference was unlikely to be caused by the use of oral contraceptives, as the maxima in the two users (1037.0, 806.3 pmol/L) and the two non-users (1170.5, 1061.1 pmol/L) were similar. The rise rate in plasma concentrations as estimated by linear regression of the mean levels in the interval beginning 1 h after patch application and ending with the maximum 8 h later was 129.3±12.0 pmol/L/h (r2=0.94) and 39.6±3.2 pmol/L/h (r2=0.96) for women and men, respectively.
While the maxima of the endogenous melatonin profiles before treatment correlated strongly between the two conditions (r2=0.89, P=0.0004), the maxima of the endogenous and exogenous profiles did not correlate (r2=0.003, P=0.89).
Sleep and EEG spectra
Data from seven subjects were used for sleep and EEG analysis. Transdermal melatonin increased rapid-eye-movement (REM) sleep by 20.7 min (women: 14.5, men: 25.4 min), and decreased wakefulness after sleep onset by 55.6 min (women: 59.3, men: 52.8 min) (Table 1) by promoting sleep in the latter part of the sleep opportunity (Figure 1a, bottom). Whereas no significant difference between conditions was found for any sleep parameter in the first and second one-third of time in bed (TIB), melatonin increased sleep efficiency (MEL: 77.2±7.8 vs. PL: 48.5±10.6 %), total sleep time (123.4±12.5 vs. 77.6±16.9 min), stage 2 (67.8±8.3 vs. 39.9±10.1 min), and REM sleep (30.9±4.4 vs. 22.0±6.1 min) during the last one-third of TIB (P<0.05, Wilcoxon).
TABLE 1.
Sleep parameters during placebo and melatonin treatment
| Placebo | Melatonin | P | |
|---|---|---|---|
| Total sleep time | 356.5 ± 16.6 | 408.9 ± 18.4 | 0.063 |
| Sleep efficiency (%) | 74.3 ± 3.5 | 85.2 ± 3.8 | 0.063 |
| Sleep latency (to stage 2) | 5.8 ± 1.0 | 7.6 ± 1.1 | 0.297 |
| REM latency (from stage 2) | 46.0 ± 8.8 | 53.6 ± 6.3 | 0.938 |
| Waking after sleep onset | 119.6 ± 17.1 | 64.0 ± 18.8 | 0.047 |
| Stage 1 | 31.5 ± 7.0 | 36.9 ± 5.0 | 0.469 |
| Stage 2 | 171.5 ± 13.2 | 200.4 ± 13.8 | 0.219 |
| Slow wave sleep | 75.1 ± 13.4 | 72.6 ± 10.2 | 0.688 |
| REM sleep | 78.4 ± 11.3 | 99.1 ± 10.4 | 0.016 |
Data represent means (in minutes unless indicated otherwise) ± SEM (N=7). Placebo and Melatonin refer to 8-h daytime sleep opportunity (09:00-17:00 h) following application of placebo patch and melatonin patch, respectively. Sleep efficiency corresponds to total sleep time expressed as a percentage of time in bed. P-values are derived from Wilcoxon signed rank tests. Sleep parameters did not differ between conditions in evening nap (15:00-19:00 h) prior to treatment. Sleep data from one female subject were excluded from analysis since this subject exhibited extremely low sleep efficiency (17%; no REM sleep; 1 min of slow wave sleep) during the preceding evening nap, and by far the highest sleep efficiency (97%) during subsequent placebo sleep, indicating that she was sleep deprived. Melatonin data from this subject are represented by open triangles in Figure 1b (bottom).
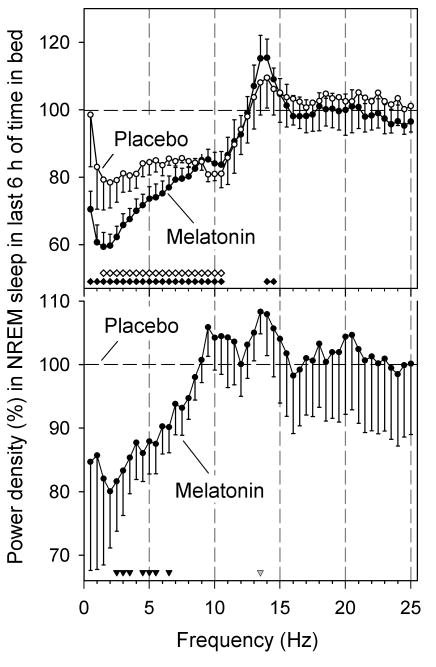
The EEG spectra in non-rapid-eye-movement (NREM) sleep did not differ between conditions during the first two hours of TIB. In both MEL and PL, the EEG underwent the typical changes associated with the dissipation of homeostatic sleep pressure: compared to the first two hours of TIB, power density in the remaining six hours was reduced in the lower frequencies, with the largest reduction being present in the delta (0.75-4.5 Hz) range (Figure 2, top). Compared to PL, melatonin delivery further decreased power density in the delta/theta frequencies (2.5-6.5 Hz) and tended to increase it (P=0.058) within the frequency range of sleep spindles (13.25-13.5 Hz) (Figure 2, bottom). Additional analyses revealed that the EEG changes were not simply a consequence of melatonin's effects on NREM sleep duration and associated dissipation of sleep pressure, and that they were unrelated to the hormone's effect on body temperature (see Supplemental Material online). Melatonin did not affect EEG power spectra during REM sleep (data not shown).
Figure 2.
Effect of transdermal melatonin administration on EEG power density during NREM sleep during daytime. Top: for both melatonin and placebo, power densities in the last 6 h of time in bed were expressed as percentage of first 2 h. Data represent means ± 1 SEM (N=7). Filled (melatonin) and open (placebo) diamonds above abscissa indicate frequency bins for which difference from the first 2 h was significant (P<0.05, paired t-tests on log-transformed values). Power density values and significance are plotted at upper limit of 0.5-Hz frequency bins. Two-way mixed-model ANOVA on log-transformed power densities was significant for Condition, and EEG Frequency bin (P<0.0001), but not for their interaction (P=0.23). Bottom: power densities in last 6 h of melatonin condition were expressed relative to corresponding interval of placebo. Filled triangles refer to significant differences from placebo (P<0.05, t-tests), and gray triangle to a statistical tendency (P=0.058).
Alertness and attention after patch removal
While plasma melatonin remained elevated in the MEL condition after daytime sleep, it did not reduce subjective alertness (Karolinska Sleepiness Scale), or visual attention (Psychomotor Vigilance Task) during that time (see Supplemental Material online).
Discussion
Transdermal melatonin delivery was effective in elevating plasma melatonin for an extended duration during the daytime. The plasma profile differed greatly from a typical profile following fast-release oral melatonin administration and resembled the endogenous nocturnal profile more closely.
Consistent with previous reports12,14 we found considerable inter-individual variation in melatonin delivery. The current data suggest gender as a significant source of variation: plasma levels in women were higher than in men. Pharmacokinetic studies16 have indicated that clearance of melatonin does not differ systematically between genders. Moreover, given the absence of a correlation between endogenous and exogenous plasma melatonin levels in the present study, it is more likely that differences in transdermal absorption possibly arising from gender differences in the physiology of the stratum corneum17 rather than disparities in clearance played a role.
Dermal deposition of melatonin is another factor that influences the plasma profile during transdermal delivery. This is evident from a comparison with a study14 that used a higher dose (8 mg) and a larger patch surface (20 cm2). Our data indicate that in order to minimize dermal deposition and approach an endogenous melatonin profile, a lower dose and a smaller patch surface are advantageous. It should be noted however that since our protocol was not long enough and towards the end overlapped with the onset of endogenous melatonin secretion, it was not possible to quantify the kinetics of melatonin's disappearance from the blood.
Transdermal melatonin delivery was effective in improving daytime sleep, inducing changes the spectral composition of the NREM sleep EEG, and lowering core body temperature. This profile of effects is consistent with other melatonin studies.18 Many of these studies – the vast majority of which used oral administration – focused on immediate or short-term action, such as the reduction of sleep latency, and the facilitation of nap sleep. In contrast, the present results highlight melatonin's efficacy to facilitate sleep late within an 8-h daytime sleep opportunity, similar to a sustained-release preparation.8 Transdermal delivery may be of particular use when there is a need for sleep to begin in the morning and last until the afternoon/early evening, as typically desired by night workers and rotating shift workers.19,20 Importantly, the added sleep in the last third of the sleep opportunity consisted of stage 2 and REM sleep, i.e., precisely those sleep stages that are reduced in shift workers.2 Judiciously timed melatonin delivery may also hold promise as a countermeasure against early morning awakenings, a sleep problem associated with old age.21
The present results corroborate the reported small reduction of EEG power density in NREM sleep in the low frequency range and increase within the range of sleep spindles.22 As noted before, these effects are reminiscent of those induced by hypnotics binding to the type-A gamma-aminobutyric acid (GABAA) receptor, in particular benzodiazepines and their analogs.23,24 However, while GABAA receptors may mediate some of melatonin's effects,25 EEG studies with the GABAA-benzodiazepine receptor antagonist flumazenil have suggested the involvement of other receptors.26 The differences in EEG power spectra between MEL and PL are also similar to the differences between nighttime and daytime sleep, indicating that melatonin administration during the day induces sleep which is more similar to nighttime sleep both with respect to its spectral composition as well as its duration.
The EEG changes occurred at melatonin concentrations that are lower than previously reported. Whereas the doses used in other EEG studies produce plasma levels that are at least 5-10 times above the endogenous levels, the concentrations observed here were within or close to the physiological range. These observations have important implications for understanding the influence of endogenous melatonin on the sleep EEG; they provide new evidence that the circadian variation in plasma melatonin concentration may indeed be large enough to contribute to the circadian variation of EEG activity in the low-frequency range,27 and spindle frequency range.27-29
In conclusion, transdermal melatonin delivery may provide important advantages over oral administration as a countermeasure for certain sleep problems: 1) it can elevate plasma melatonin for an extended duration while maximal concentrations remain relatively close to the physiological range; 2) the shape of the induced plasma melatonin profile with its gradual rise and delayed maximum is well suited to counteract the circadian wake drive during the biological day. Thus, transdermal melatonin delivery may represent a more effective way to help shift workers and individuals in jet-lag situations to maintain sleep at adverse circadian phases.
Methods
Subjects
Eight volunteers (mean age ± SD: 27.8±3.6, range: 24-34 years; 4 women) completed the study. The protocol was approved by the Human Research Committee of Partners Healthcare System. Subjects gave written informed consent to all procedures prior to participation. They were in good health as established by medical history, psychological questionnaires, physical exam, electrocardiography, blood and urine tests, and overnight clinical polysomnography. Subjects' body weight (mean ± SD) was 66.9±8.7 kg (women: 60.8±3.9, men: 73.0±8.0 kg).
Protocol
The protocol consisted of two inpatient visits that were 7-16 days apart. For three weeks prior to the first visit and between visits, subjects were instructed to refrain form alcohol, caffeine, nicotine and any drug except oral contraceptives. Compliance was verified at each admission by urine toxicology. Subjects maintained a fixed sleep/rest schedule (23:00-07:00 h) as verified with actigraphy (Actiwatch-L, Minimitter Inc., Bend, OR) during one week prior to the first visit, and between visits. Each visit lasted ∼36 h and included a nap opportunity (15:00-19:00 h) on day 1, and a daytime sleep opportunity (09:00-17:00 h) on day 2. The sleep opportunities were scheduled such in order to approximate a sleep/wake schedule typically recommended to shift workers as they rotate to a night duty.19 Subjects wore a patch (melatonin or placebo) between 08:00 and 18:00 h on day 2. During the scheduled wake episodes, the light intensity was ∼0.0048 W/m2 (∼1.8 lux; horizontal angle). Subjects were accompanied by a staff member between 00:00 and 09:00 h on day 2 to ensure wakefulness. The study was carried out in an environment free of time cues (no windows, clocks, TV, radio, telephone, or internet).
Drug
Patches (Biotek Inc., Wellesley, MA) contained 2.1 mg melatonin or no active drug (placebo) in a solvent formulation of water, ethyl alcohol, propylene glycol, lauric acid, and hydroxypropylcellulose. They had a porous, non-rate-limiting membrane and an active contact area of 1.2 cm2. Extensive ex-vivo experiments with human skin indicated that the chosen dose was best suited to produce the intended plasma profile (see Introduction). Patches were attached medially on the forearm after the site was briefly washed with regular soap and dried with a towel.
Melatonin measurements
Blood samples were obtained through an indwelling intravenous catheter in the forearm. Extended plastic tubing permitted drawings during sleep from an adjacent room without disturbing the subjects. A heparinized solution (5 IU heparin/ml 0.45% NaCl) was infused (40 ml/h) between samples. Plasma was analyzed for melatonin by radioimmunoassay (Pharmasan Inc., Osceola, WI). Intra-assay and inter-assay coefficients of variation were ≤12.1% and ≤13.2%, respectively.
Core body temperature measurements
Temperature recordings were made with an indwelling rectal sensor at 1-min intervals (Yellow Springs Instruments Company, Yellow Springs, OH).
Polysomnography and EEG analysis
The EEG (C3/A2, C4/A1, O1/A2, O2/A1), electrooculogram (EOG), and submental electromyogram (EMG) were recorded with the Vitaport-3 system (TEMEC Instruments B.V., Kerkrade, The Netherlands). Signals were high-pass filtered [time constants: 0.68 s (EEG, EOG), or 0.015 s (EMG)], low-pass filtered [Bessel, 24 dB/octave; -6 dB at 70.1 Hz (EEG), 34.8 Hz (EOG), or 55.1 Hz (EMG)], digitized (resolution: 12 bit, sampling rate: 256 Hz, storage rate: 128 Hz), and scored on a 30-s basis.30 The EEG (C3/A2) was subjected to an offline fast-Fourier-transform routine (Vitagraph, TEMEC Instruments B.V.) using 4-s epochs (rectangular window). Spectra from up to ten overlapping 4-s epochs were averaged into 30-s epochs after discarding visually identified artifacts.
Supplementary Material
Acknowledgments
We thank Dr. Wei Wang for statistical advice. We would like to express our gratitude to Lisa McCaig for her help with subject recruitment, and the dedicated staff of the General Clinical Research Center and the Division of Sleep Medicine's Chronobiology Core for subject care and carrying out the study protocol, as well as the Sleep & EEG Core for polysomnography support. This work was supported by NIH grants R44-NS43129 (LD Nichols), R01-HL077399 (D Aeschbach), P01-AG009975 (CA Czeisler), and NCRR-GCRC-M01-RR02635 (Brigham and Women's Hospital).
Footnotes
Supplementary Material is linked to the online version of the paper at http://www.nature.com/cpt
Conflict Of Interest Dr. Aeschbach has received research support from Cephalon Inc., and has served as a paid member of the scientific advisory board of Zeo, Inc. Dr. Dijk has received research support from Airforce Office of Scientific Research, Biotechnology and Biological Sciences Research Council UK, GlaxoSmithKline, H Lundbeck A/S, Merck & Co. Inc., Philips Lighting, Organon, Takeda Pharmaceuticals, and Wellcome Trust. Dr. Dijk has served as consultant for Actelion, Cephalon, Inc., GlaxoSmithKline, H Lundbeck A/S, Merck & Co. Inc., Pfizer Inc, Philips Lighting, Sanofi Aventis, Takeda Pharmaceuticals. Dr. Lockley received lecture fees from Takeda Pharmacueticals in 2006. Dr. Nichols was an employee of Biotek Inc. and is currently no longer associated with this company. Dr. Nuwayser is the president and owner of Biotek Inc. Dr. Czeisler has received consulting fees from or served as a paid member of scientific advisory boards for: Actelion, Ltd., Cephalon, Inc., Delta Airlines, Eli Lilly and Co., Garda Síochana Inspectorate, Global Ground Support, Johnson & Johnson, Koninklijke Philips Electronics, N.V., Portland Trail Blazers, Respironics Inc., Sanofi-Aventis Groupe, Sepracor, Inc., Sleep Multimedia Inc., Somnus Therapeutics Inc., Vanda Pharmaceuticals Inc., and Zeo Inc.,. Dr. Czeisler owns an equity interest in Lifetrac Inc., Somnus Therapeutics Inc., Vanda Pharmaceuticals Inc. and Zeo Inc. Dr. Czeisler's research laboratory has received research and/or education funds Cephalon Inc., Koninklijke Philips Electronics, N.V. and ResMed. The Harvard Medical School Division of Sleep Medicine has received Educational Grant funding from Cephalon Inc., Takeda Pharmaceuticals, Sanofi-Aventis Inc. and Sepracor Inc. Dr. Czeisler is the incumbent of an endowed professorship provided to Harvard University by Cephalon, Inc. and holds a number of process patents in the field of sleep/circadian rhythms (e.g., photic resetting of the human circadian pacemaker). Since 1985, Dr. Czeisler has also served as an expert witness on various legal cases related to sleep and/or circadian rhythms. Mr. Lockyer has declared no conflict of interest.
References
- 1.U.S.Congress & Office of Technology Assessment. Biological rhythms: Implications for the worker. U.S. Government Printing Office; Washington, DC: 1991. [Google Scholar]
- 2.Åkerstedt T. Shift work and disturbed sleep/wakefulness. Sleep Med Rev. 1998;2:117–128. doi: 10.1016/s1087-0792(98)90004-1. [DOI] [PubMed] [Google Scholar]
- 3.Rajaratnam SM, Arendt J. Health in a 24-h society. The Lancet. 2001;358:999–1005. doi: 10.1016/S0140-6736(01)06108-6. [DOI] [PubMed] [Google Scholar]
- 4.Tzischinsky O, Lavie P. Melatonin possesses time-dependent hypnotic effects. Sleep. 1994;17:638–645. doi: 10.1093/sleep/17.7.638. [DOI] [PubMed] [Google Scholar]
- 5.Dollins AB, Zhdanova IV, Wurtman RJ, Lynch HJ, Deng MH. Effect of inducing nocturnal serum melatonin concentrations in daytime on sleep, mood, body temperature, and performance. Proc Natl Acad Sci U S A. 1994;91:1824–1828. doi: 10.1073/pnas.91.5.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes RJ, Badia P. Sleep-promoting and hypothermic effects of daytime melatonin administration in humans. Sleep. 1997;20:124–131. [PubMed] [Google Scholar]
- 7.Matsumoto M. The hypnotic effects of melatonin treatment on diurnal sleep in humans. Psychiatry Clin Neurosci. 1999;53:243–245. doi: 10.1046/j.1440-1819.1999.00480.x. [DOI] [PubMed] [Google Scholar]
- 8.Sharkey KM, Fogg LF, Eastman CI. Effects of melatonin administration on daytime sleep after simulated night shift work. J Sleep Res. 2001;10:181–192. doi: 10.1046/j.1365-2869.2001.00256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rajaratnam SM, Middleton B, Stone BM, Arendt J, Dijk DJ. Melatonin advances the circadian timing of EEG sleep and directly facilitates sleep without altering its duration in extended sleep opportunities in humans. J Physiol. 2004;561:339–351. doi: 10.1113/jphysiol.2004.073742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wyatt JK, Dijk DJ, Ritz-De Cecco A, Ronda JM, Czeisler CA. Sleep facilitating effect of exogenous melatonin in healthy young men and women is circadian-phase dependent. Sleep. 2006;29:609–618. doi: 10.1093/sleep/29.5.609. [DOI] [PubMed] [Google Scholar]
- 11.Dubocovich ML. Melatonin receptors: role on sleep and circadian rhythm regulation. Sleep Med. 2007;8 3:34–42. doi: 10.1016/j.sleep.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Lee BJ, Parrott KA, Ayres JW, Sack RL. Preliminary evaluation of transdermal delivery of melatonin in human subjects. Res Commun Mol Pathol Pharmacol. 1994;85:337–346. [PubMed] [Google Scholar]
- 13.Garfinkel D, Laudon M, Nof D, Zisapel N. Improvement of sleep quality in elderly people by controlled-release melatonin. Lancet. 1995;346:541–544. doi: 10.1016/s0140-6736(95)91382-3. [DOI] [PubMed] [Google Scholar]
- 14.Bénès L, et al. Transmucosal, oral controlled-release, and transdermal drug administration in human subjects: A crossover study with melatonin. J Pharm Sci. 1997;86:1115–1119. doi: 10.1021/js970011z. [DOI] [PubMed] [Google Scholar]
- 15.Priano L, et al. Solid lipid nanoparticles incorporating melatonin as new model for sustained oral and transdermal delivery systems. J Nanosci Nanotechnol. 2007;7:3596–3601. doi: 10.1166/jnn.2007.809. [DOI] [PubMed] [Google Scholar]
- 16.Fourtillan JB, et al. Melatonin secretion occurs at a constant rate in both young and older men and women. Am J Physiol Endocrinol Metab. 2001;280:E11–E22. doi: 10.1152/ajpendo.2001.280.1.E11. [DOI] [PubMed] [Google Scholar]
- 17.Jacobi U, Gautier J, Sterry W, Lademann J. Gender-related differences in the physiology of the stratum corneum. Dermatology. 2005;211:312–317. doi: 10.1159/000088499. [DOI] [PubMed] [Google Scholar]
- 18.Cajochen C, Kräuchi K, Wirz-Justice A. Role of melatonin in the regulation of human circadian rhythms and sleep. J Neuroendocrinol. 2003;15:432–437. doi: 10.1046/j.1365-2826.2003.00989.x. [DOI] [PubMed] [Google Scholar]
- 19.Monk TH. Mini Review: What can the chronobiologist do to help the shift worker? J Biol Rhythms. 2000;15:86–94. doi: 10.1177/074873040001500202. [DOI] [PubMed] [Google Scholar]
- 20.Santhi N, Aeschbach D, Horowitz TS, Czeisler CA. The impact of sleep timing and bright light exposure on attentional impairment during night work. J Biol Rhythms. 2008;23:341–352. doi: 10.1177/0748730408319863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duffy JF, Dijk DJ, Klerman EB, Czeisler CA. Later endogenous circadian temperature nadir relative to an earlier wake time in older people. Am J Physiol. 1998;275:R1478–R1487. doi: 10.1152/ajpregu.1998.275.5.r1478. [DOI] [PubMed] [Google Scholar]
- 22.Dijk DJ, et al. Melatonin effect on daytime sleep in men: suppression of EEG low frequency activity and enhancement of spindle frequency activity. Neurosci Lett. 1995;201:13–16. doi: 10.1016/0304-3940(95)12118-n. [DOI] [PubMed] [Google Scholar]
- 23.Borbély AA, Mattmann P, Loepfe M, Strauch I, Lehmann D. Effect of benzodiazepine hypnotics on all-night sleep EEG spectra. Hum Neurobiol. 1985;4:189–194. [PubMed] [Google Scholar]
- 24.Landolt HP, Gillin JC. GABAA1a receptors: involvement in sleep regulation and potential of selective agonists in the treatment of insomnia. CNS Drugs. 2000;13:185–199. [Google Scholar]
- 25.Wan Q, et al. Differential modulation of GABAA receptor function by Mel1a and Mel1b receptors. Nat Neurosci. 1999;2:401–403. doi: 10.1038/8062. [DOI] [PubMed] [Google Scholar]
- 26.Nave R, Herer P, Haimov I, Shlitner A, Lavie P. Hypnotic and hypothermic effects of melatonin on daytime sleep in humans: lack of antagonism by flumazenil. Neurosci Lett. 1996;214:123–126. doi: 10.1016/0304-3940(96)12899-8. [DOI] [PubMed] [Google Scholar]
- 27.Dijk DJ, Shanahan TL, Duffy JF, Ronda JM, Czeisler CA. Variation of electroencephalographic activity during non-rapid eye movement and rapid eye movement sleep with phase of circadian melatonin rhythm in humans. J Physiol (Lond) 1997;505.3:851–858. doi: 10.1111/j.1469-7793.1997.851ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aeschbach D, Dijk DJ, Borbély AA. Dynamics of EEG spindle frequency activity during extended sleep in humans: Relationship to slow-wave activity and time of day. Brain Res. 1997;748:131–136. doi: 10.1016/s0006-8993(96)01275-9. [DOI] [PubMed] [Google Scholar]
- 29.Knoblauch V, Martens W, Wirz-Justice A, Krauchi K, Cajochen C. Regional differences in the circadian modulation of human sleep spindle characteristics. Eur J Neurosci. 2003;18:155–163. doi: 10.1046/j.1460-9568.2003.02729.x. [DOI] [PubMed] [Google Scholar]
- 30.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. U.S. Government Printing Office; Washington, D.C.: 1968. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.