Abstract
The first suggestion that physical exercise results in free radical-mediated damage to tissues appeared in 1978, and the past three decades have resulted in a large growth of knowledge regarding exercise and oxidative stress. Although the sources of oxidant production during exercise continue to be debated, it is now well established that both resting and contracting skeletal muscles produce reactive oxygen species and reactive nitrogen species. Importantly, intense and prolonged exercise can result in oxidative damage to both proteins and lipids in the contracting myocytes. Furthermore, oxidants can modulate a number of cell signaling pathways and regulate the expression of multiple genes in eukaryotic cells. This oxidant-mediated change in gene expression involves changes at transcriptional, mRNA stability, and signal transduction levels. Furthermore, numerous products associated with oxidant-modulated genes have been identified and include antioxidant enzymes, stress proteins, DNA repair proteins, and mitochondrial electron transport proteins. Interestingly, low and physiological levels of reactive oxygen species are required for normal force production in skeletal muscle, but high levels of reactive oxygen species promote contractile dysfunction resulting in muscle weakness and fatigue. Ongoing research continues to probe the mechanisms by which oxidants influence skeletal muscle contractile properties and to explore interventions capable of protecting muscle from oxidant-mediated dysfunction.
I. INTRODUCTION
Regular physical exercise has many health benefits including a lowered threat of all-cause mortality along with a reduced risk of cardiovascular disease, cancer, and diabetes (45, 77, 283). Paradoxically, it is also clear that contracting skeletal muscles generate free radicals and that prolonged and intense exercise can result in oxidative damage to cellular constituents (10, 86, 98, 107, 165, 321, 325). During the past three decades, our knowledge about the biological implications of exercise-induced oxidative stress has expanded rapidly. Indeed, it is now appreciated that while high levels of free radicals can damage cellular components, low-to-moderate levels of oxidants play multiple regulatory roles in cells such as the control of gene expression, regulation of cell signaling pathways, and modulation of skeletal muscle force production (102, 317, 318, 368).
Because of recent advances in the field of exercise and oxidative stress, this is an appropriate time to summarize some of the major principles of exercise-induced oxidative stress and its impact on skeletal muscle function. Our approach will be to provide a critical synopsis of major concepts rather than a detailed analysis of data. We will begin with an overview of oxidant species, antioxidant systems, and the concept of oxidative stress. This will be followed with a historical synopsis of research in the field of exercise-induced oxidative stress and a discussion of cellular sources of oxidants during exercise. We will also discuss the redox modulation of muscle force production/fatigue and address redox-sensitive targets within skeletal muscle. Finally, we will suggest future directions for research in this field. Although this review will focus on a broad range of issues related to exercise-induced oxidative stress, it is impossible for a single report to address all aspects of this expansive field of study. For topics not covered in this report, the interested reader is referred to several reviews on specific aspects of exercise and oxidative stress (8, 27, 75, 90, 92, 106, 112, 164, 168, 179, 181, 216, 252, 302–304, 314, 350, 358, 394).
II. MAJOR REACTIVE SPECIES IN CELLS
Although it is a relatively recent discovery, the occurrence of free radicals in biological processes is now widely accepted (71). The nature and properties of the common free radicals, reactive oxygen species, and reactive nitrogen species found in cells will be discussed in this section of the review.
If an atom/molecule contains one or more unpaired electrons and is capable of independent existence, it is referred to as a “free radical”(136). Atoms possess electrons that are usually associated in pairs. Each pair moves in a defined space around the nucleus referred to as the atomic/molecular orbital. One electron of the pair has spin quantum number +1/2 and the other −1/2. When the electrons are in opposite spins, the electronic state is singlet and referred to as “ground state.” Electrons with the same spin are “triplet state,” but if singlet molecules absorb energy without changing spin, the molecule is in an “excited singlet state” (209). Free radicals can be generated as products of homolytic, heterolytic, or redox reactions, producing either charged or uncharged radical species. Note that reactive oxygen species (ROS) is a general term that refers to not only oxygen-centered radicals but also includes nonradical but reactive derivatives of oxygen (e.g., hydrogen peroxide) (136). Similarly, the term reactive nitrogen species (RNS) refers to both nitrogen radicals along with other reactive molecules where the reactive center is nitrogen. The term reactive oxygen and nitrogen species (RONS) is also used as a collective term for both ROS and RNS and includes both free radical and non-free radical species.
The primary free radicals generated in cells are superoxide (O2•−) and nitric oxide (NO). Superoxide is generated through either incomplete reduction of oxygen in electron transport systems or as a specific product of enzymatic systems, while NO is generated by a series of specific enzymes (the nitric oxide synthases). Both superoxide and NO are reactive and can readily react to form a series of other ROS and RNS. Thus superoxide dismutates rapidly to form hydrogen peroxide and subsequently hydroxyl radicals in the presence of catalytic transition metals (292) while superoxide and NO rapidly react to form peroxynitrite and subsequently other RNS (69, 292). An overview of major and secondary ROS and RNS follows.
A. Superoxide
Superoxide is primarily formed as an intermediate in biochemical reactions (134). This anion is negatively charged and is relatively membrane impermeable. Nonetheless, compared with other free radicals, superoxide has a relatively long half-life that enables diffusion within the cell and, hence, increasing the number of the potential targets. Inflammatory cells produce relatively large amounts of superoxide as part of the process by which they resist invading organisms (116). Although superoxide is generally considered relatively unreactive compared with other radical species, it can react rapidly with some radicals such as NO and with some iron-sulfur clusters in proteins (136). Interest has surrounded the possibility that protonation of superoxide to produce the hydroperoxyl radical (HO2·) may occur to at physiological pH, facilitating the transfer of superoxide across membranes (344). Nonetheless, the probability and circumstances required for the protonation of superoxide in cells remains a topic of debate. As a redox active species, superoxide can reduce some biological materials (e.g., cytochrome c) and oxidize others such as ascorbate. Dismutation of superoxide, both spontaneous and catalyzed by the superoxide dismutases, provides a major source of hydrogen peroxide in cells (reaction 1)
| (reaction1) |
B. Hydrogen Peroxide
Hydrogen peroxide (H2O2) is a reactive compound that can readily generate free radicals such as the hydroxyl radical in specific circumstances. Hydrogen peroxide is stable, permeable to membranes, and has a relatively long half-life within the cell. Hydrogen peroxide is cytotoxic but is considered a relatively weak oxidizing agent. In addition to formation from dismutation of O2•−, a number of enzyme systems also generate H2O2 including urate and amino acid oxidases. Hydrogen peroxide is unable to oxidize DNA or lipids directly but can inactivate some enzymes (136). The cytotoxicity of hydrogen peroxide primarily occurs through its ability to generate hydroxyl radical through metal-catalyzed reactions, such as the Fenton reaction (reaction 2)
| (reaction2) |
In biology it seems likely that this reaction is particularly important as part of the Haber-Weiss reaction, where iron (or copper) is maintained in a reduced form by superoxide and hence capable of catalyzing the formation of the hydroxyl radical from hydrogen peroxide (134). The net reaction is
| (reaction2a) |
In practice the reactions involved are
| (reaction2b) |
| (reaction2) |
or
| (reaction2c) |
| (reaction2d) |
C. Hydroxyl Radicals
Hydroxyl radicals (·OH) are highly reactive with a strong oxidizing potential. Hydroxyl radicals damage molecules close to their site of their generation, and due to their high reactivity, they are not membrane permeable. Hydroxyl radicals appear to combine with cell components at a rate constant of 109–1010 M−1 ·s−1 (134). They are potentially the most damaging ROS present in biological materials, and their reactivity is such that it is virtually impossible to confirm that they exist in living organisms other than through demonstration of the presence of specific products of their reactions.
D. Singlet Oxygen
Another reactive form of oxygen, singlet oxygen, can also be generated in some biological materials. Singlet oxygen has a very short half-life but is capable of diffusion and is permeable to membranes. Singlet oxygen is an electronically excited form of oxygen and is not a radical since no electrons are unpaired. Singlet oxygen exists in one of two states, the first excited state (1ΔgO2) or the more reactive second excited state (2ΣgO2) (134). Singlet oxygen has no spin restriction, and hence, the oxidizing ability is greatly increased (136). Dismutation of the superoxide anion in water can lead to the formation of singlet oxygen in biological systems. At present, it is unclear if singlet oxygen is produced in contracting skeletal muscles during exercise.
E. Nitric Oxide
Nitric oxide (NO·) is synthesized from the amino acid L-arginine by many cell types. Synthesis occurs through nitric oxide synthases (NOS) of three main types: neuronal NOS (NOS1), which was originally found in neural tissue but is also present in most cell types; endothelial NOS (NOS3), originally described in endothelial cells; and inducible NOS (NOS2) that is predominantly found in inflammatory conditions, but now recognized to be more widespread. The nitric oxide synthases convert L-arginine into NO and L-citrulline utilizing NADPH.
NO readily binds to some transition metals, and its major actions in cells relate to its ability to bind to the ferrous ion in guanyl cyclase activating this enzyme and resulting in the formation of cGMP. This binding of NO to iron not only comprises a major mechanism of action, but also appears to play a major role in its inactivation and removal through binding to the iron in hemoglobin. Nitric oxide is a weak reducing agent, reacts with oxygen to form nitric dioxide, and reacts very rapidly with superoxide to produce peroxynitrite (133).
F. Peroxynitrite
The reaction of superoxide with NO to produce peroxynitrite (reaction 3) occurs approximately three times faster than the dismutation of superoxide to produce hydrogen peroxide and even faster than the reaction of NO with heme proteins; hence, this is the primary reaction when both are present
| (reaction3) |
Peroxynitrite (or its protonated form ONOOH) is a strong oxidizing agent and can lead to depletion of thiol groups, damage to DNA, and nitration of proteins. A further effect of the formation of peroxynitrite is a reduced bioavailability of superoxide and NO. Peroxynitrite is classified as RNS that also includes NO and N2O3 and some nitrogencentered radicals, but much less is known about the occurrence and roles of these latter species in biology.
G. Hyperchlorite
Hyperchlorite is formed by the action of myeloperoxidase utilizing hydrogen peroxide (reaction 4). Hyperchlorite is predominantly formed by neutrophils and can damage various biomolecules by oxidizing thiols, lipids, ascorbate, and NADPH with the generation of various secondary products (136). Moreover, in the acid form (i.e., hypochlorous acid), this oxidant can cross cell membranes and can cause fragmentation and aggregation of proteins by multiple reactions (136)
| (reaction4) |
H. Secondary Radical Species
Since free radicals can initiate radical chain reactions in some biological molecules, a number of other free radical species occur in cells as part of these processes. A primary example of these secondary radical species include the intermediary radicals formed during lipid peroxidation (i.e., oxidative degradation of polyunsaturated lipids). The lipid bilayer is the basic structure of all biological membranes and is composed of lipids and proteins. Polyunsaturated fatty acids (PUFAs) are a large constituent of the membrane around cells and their organelles (136). Lipid peroxidation of PUFAs such as arachidonic acid occurs via several stages. The hydroxyl radical typically initiates peroxidation by abstracting a hydrogen atom from a methylene carbon side chain, initially forming a carbon-centered, lipid radical and water (135). For example, arachidonic acid can undergo hydrogen abstraction at carbons 13, 10, and 7 (136). Monoun-saturated and saturated fatty acids have an increased resistance to oxidation compared with PUFAs, since the double bonds in the PUFAs are more susceptible to oxidation (100, 135).
The carbon-centered lipid radical has an unpaired electron, and the molecule stabilizes by molecular rearrangement (393); that is, two lipid radicals can cross-link and form a conjugated diene or usually, the lipid radical donates an electron to oxygen, forming the peroxyl radical (designated LOO·, where L = polyunsaturated fatty acid) and propagating the chain reaction. Generation of a lipid radical is terminated by the formation of a cyclic peroxide or cyclic endoperoxide and other termination products (Fig. 1). Lipid peroxidation can theoretically result in degeneration of membrane structure and loss of membrane protein function (121). Structural derangement of the lipid bilayer alters the fluidity and increases the rigidity of the membrane. This enables proteins within the membrane to be more directly attacked, impairing essential membrane functions such as the activity of intrinsic enzymes and transporters and/or decreasing the rate of carrier coupled flow of ATP and ADP.
FIG. 1.
Arachidonic acid undergoing initiation and propagation stages of lipid peroxidation. MDA, malondialdehyde. [Adapted from Halliwell and Gutteridge (136).]
III. ANTIOXIDANT DEFENSE SYSTEMS: OVERVIEW AND RESPONSE TO CHRONIC EXERCISE
Later sections of this review will discuss the fact that contracting skeletal muscle fibers can produce ROS via a variety of different pathways (86, 165, 321, 325) and outline how ROS are implicated in the etiology of exercise-induced skeletal muscle fatigue (22, 34, 105, 183, 184, 232, 321, 399, 400). Hence, given the importance of maintaining redox homeostasis in muscle fibers, it is not surprising that myocytes contain a network of antioxidant defense mechanisms to reduce the risk of oxidative damage during periods of increased ROS production. The term antioxidant has been defined in many ways, but in the context of our discussion, antioxidants will be broadly defined as any substance that delays or prevents the oxidation of a substrate (i.e., all molecules found in vivo) (136).
To protect against oxidative stress, a well-organized system of antioxidants works in a coordinated fashion to resist redox disturbances in the cell. In this section, we provide an overview of cellular antioxidants and summarize how antioxidants function to protect muscle fibers against oxidative injury. Moreover, we will sum up the literature related to chronic exercise-induced changes in both enzymatic and nonenzymatic antioxidants in skeletal muscle.
A. Cellular Strategies to Regulate Reactive Species
Muscle fibers contain both enzymatic and nonenzymatic antioxidants that work as a complex unit to regulate ROS. Within the fiber, these antioxidants are strategically compartmentalized throughout the cytoplasm and within various organelles (e.g., mitochondria). Moreover, enzymatic and nonenzymatic antioxidants exist in both the extracellular and vascular space. Collectively, these antioxidants protect muscle fibers from oxidative injury during periods of increased oxidant production (e.g., intense or prolonged exercise).
Numerous antioxidant strategies exist and can be used to protect against ROS toxicity. For example, some agents (i.e., catalase) convert ROS into less active molecules and prevent the transformation of these less active species to a more deleterious form. Another antioxidant strategy is to minimize the availability of pro-oxidants such as iron and copper ions via metal binding proteins. Moreover, numerous low-molecular-weight agents are capable of scavenging ROS species. Examples of this antioxidant strategy include endogenously synthesized molecules such as glutathione, uric acid, and bilirubin along with agents found in the diet such as ascorbic acid and vitamin E.
B. Antioxidant Enzymes
Principal antioxidant enzymes include superoxide dismutase, glutathione peroxidase, and catalase (Fig. 2). Additional antioxidant enzymes such as peroxiredoxin, glutaredoxin, and thioredoxin reductase also contribute to cellular protection against oxidation.
FIG. 2.
Locations of primary cellular enzymatic and nonenzymatic antioxidants. GPX, glutathione peroxidase; SOD1, superoxide dismutase 1; SOD2, superoxide dismutase 2.
1. Superoxide dismutase
Superoxide dismutase (SOD) was discovered in 1969 by McCord and Fridovich (256) and forms the first line of defense against superoxide radicals as SOD dismutates superoxide radicals to form hydrogen peroxide (H2O2) and oxygen (O2). In mammals, three isoforms of SOD (SOD1, SOD2, SOD3) exist, and all require a redox active transition metal in the active site to accomplish the catalytic breakdown of the superoxide anion (82, 385). Two of the SOD isoforms are located within cells, whereas the third SOD isoform is found in the extracellular space (385). SOD1 requires copper-zinc as a cofactor and is primarily located in the cytosol and the mitochondrial intermembrane space. SOD2 uses manganese as a cofactor and is located in the mitochondrial matrix. Finally, SOD3 requires copper-zinc as a cofactor and is located in the extracellular space. A summary of the properties of human SOD isoenzymes is contained in Table 1.
TABLE 1.
Properties of human SOD isoenzymes
| Property | SOD1 | SOD2 | SOD3 |
|---|---|---|---|
| Cellular location | Cytosol and mitochondrial IMS | Mitochondrial matrix | Extracellular |
| Metal/monomer | 1 Cu, 1 Zn | 1 Mn | 1 Cu, 1 Zn |
| Molecular mass, kDa | 32.5 | 24.7 | 30 |
| Subunit | Dimer | Tetramer | Tetramer |
| Inhibition by CN− | Yes | No | Yes |
| Inhibition by H2O2 | Yes | Yes | Yes |
| Rate constant for reaction with O2− | 0.62 × 109 | 1.2 × 109 | 0.72 × 109 |
Although superoxide radicals are not highly toxic, they can extract electrons from biological membranes or other cellular components, resulting in a chain of radical reactions. Superoxide radicals are also toxic through their ability to participate in the formation of hydroxyl radicals (see sect. IIB) and react with NO to form peroxynitrite (see sect. IIF). Therefore, it is essential for cells to keep superoxide radicals in check. Direct evidence to support this notion is illustrated by the fact that the mutagenesis of SOD1 in humans promotes apoptosis of spinal neurons resulting in amyotrophic lateral sclerosis (115).
The relative allocation of the SOD1 and SOD2 isoenzymes varies across tissues. In skeletal muscle, 15–35% of the total SOD activity is in the mitochondria, and the remaining 65–85% is in the cytosol (182, 299). In rat skeletal muscle, SOD activity is highest in oxidative muscles that contain a high percentage of type I and type IIa fibers compared with muscles with low mitochondrial volumes (i.e., type IIx or IIb fibers) (78, 299).
SOD activity in skeletal muscle is not constant and can be modified by activity patterns. Although some studies suggest that chronic (weeks to months) endurance exercise training does not increase SOD activity in muscle (9, 145, 224, 227), most investigators conclude that endurance exercise training promotes 20–112% increases in the activities of both SOD1 and SOD2 in the exercised muscles (78, 155, 229, 234, 235, 284, 299, 300, 311, 399, 400). Analysis of these individual reports does not provide definitive explanation for the variance between studies. Nonetheless, methodological differences in the assay of SOD activity, variations in the exercise training paradigm, and differences in fiber types between the muscles studied could be contributory factors to the differences reported in the literature. In reference to assay techniques, a 10-fold difference exists in the relative sensitivity between common methods used to assay SOD activity (287). Hence, SOD assay techniques with low sensitivity could fail to detect small-to-moderate group differences in SOD activity, and therefore, this shortcoming could explain the failure to observe exercise-induced increases in SOD activity in some reports. Furthermore, the magnitude of exercise-mediated changes in skeletal muscle SOD activity increases as a function of the intensity and duration of exercise, and therefore, studies exercising animals at higher intensities and/or longer daily durations of exercise typically report a larger percent increase in SOD activity (299, 300). Finally, the magnitude of the exercise-induced increase in SOD activity in muscle fibers is greatest in skeletal muscles composed of highly oxidative fibers (e.g., type I and type IIa) (78, 299). The explanation for this blueprint of adaptation could be due to the recruitment patterns of muscle fibers during submaximal endurance exercise whereby highly oxidative fibers are more actively recruited compared with less oxidative fibers (i.e., type IIx and IIB) (342).
2. Glutathione peroxidase
Analysis of the selenoproteome has identified five glutathione peroxidases in mammals (GPX1-GPX5; Table 2) (54, 101). All of these GPX enzymes catalyze the reduction of H2O2 or organic hydroperoxide (ROOH) to water (H2O) and alcohol (ROH), respectively, using reduced glutathione (GSH) or in some cases thioredoxin or glutaredoxin as the electron donor (43, 44, 161). When GSH is the electron donor, it donates a pair of hydrogen ions and GSH is oxidized to glutathione disulfide (GSSG) as follows
TABLE 2.
Physical characteristics and tissue locations of the multiple GPX proteins in humans
| Property | GPX1 | GPX2 | GPX3 | GPX4 | GPX5 |
|---|---|---|---|---|---|
| Cellular location | Cytosol and mitochondria | Cytosol | Extracellular space and cytosol | Membrane-bound nuclei and mitochondria | Extracellular and membrane bound |
| Subunit | Tetrameric | Tetrameric | Tetrameric | Monomeric | Dimeric |
| Molecular mass, kDa | 21 | 22 | 22.5 | 19 | 24 |
| Tissue location | All tissues | Stomach, intestine | All tissues | Testes, spermatozoa, heart, brain | Epididymis, spermatozoa, liver, kidney |
Although the reaction catalyzed by all GPXs appears to be the same, individual GPXs differ in substrate specificity (i.e., varying range of hydroperoxides) and cellular localization (i.e., cytosol vs. mitochondria) (55). The varying substrate specificity and cellular location across GPX isoforms appears to optimize GPX’s function as a cellular antioxidant enzyme. Indeed, the fact that many GPX isoenzymes will reduce a wide range of hydroperoxides ranging from H2O2 to complex organic hydroperoxides makes GPX an important intracellular antioxidant to protect against ROS-mediated damage to membrane lipids, proteins, and nuclei acids (184). Moreover, although all GPX isoenzymes are efficient peroxidases, various GPX isoforms may also exert a variety of specific roles in metabolic regulation such as silencing lipoxygenases (55).
To function, most GPX isoforms require a supply of GSH to provide electrons. Since GSH is oxidized by GPX to form GSSG, cells must possess a pathway capable of regenerating GSH. The reduction of GSSG back to GSH is accomplished by glutathione reductase, a flavin containing enzyme whereby NADPH provides the reducing power (263). Many tissues produce NADPH by glucose-6-phosphate dehydrogenase via the pentose pathway, but skeletal muscles produce NADPH primarily via isocitrate dehydrogenase (228, 230, 262).
Identical to SOD, the relative amount of GPX present in skeletal muscle fibers differs across fiber types; that is, highly oxidative fibers (i.e., type I) contain the highest GPX activity, whereas rodent muscle fibers with low oxidative capacity (i.e., type IIB) possess the lowest levels of GPX (231, 299). Identical to SOD, GPX is inducible in skeletal muscles and GPX is reported to increase in skeletal muscle fibers that are actively recruited during regular exercise. Indeed, numerous studies confirm that endurance exercise training promotes an increase (20–177%) in GPX activity in skeletal muscles (78, 145, 182, 197, 224, 229, 234, 235, 299, 300, 307, 352, 397, 398). Moreover, endurance exercise increases both cytosolic and mitochondrial GPX activity (182). Similar to SOD, the extent of the exercise-induced increase in GPX in skeletal muscle is a function of both the exercise intensity and exercise duration. Specifically, compared with low-intensity exercise, both moderate- and high-intensity exercise produce a larger increase in muscle GPX activity (299). Moreover, long-duration exercise training sessions (e.g., ≥60 min/day) are superior to short-duration (≤30 min/day) exercise bouts in increasing muscle GPX activity (299). Finally, exercise training-induced increases in muscle GPX activity are typically limited to highly oxidative muscles containing primarily type I and IIa fibers (78, 299). Similar to the aforementioned scenario with exercise-induced induction of SOD, the explanation for this adaptive pattern of GPX is likely due to the fact that, compared with fast type IIX or IIb fibers, highly oxidative muscle fibers (i.e., type I) are more actively recruited during submaximal endurance exercise (342).
3. Catalase
Catalase (CAT) serves several biochemical functions, but the principal purpose of CAT is to catalyze the break-down of H2O2 into H2O and O2
Catalase is a homotetramer with a molecular mass of 240 kDa and is widely distributed within the cell (206). Iron is a required cofactor attached to the active site of the enzyme (64, 206, 416). Although CAT and GPX share common substrates, CAT has a much lower affinity for H2O2 at low concentrations compared with GPX (i.e., GPX Km = 1 μM vs. CAT Km = 1 mM) (363).
CAT protein levels are highest in highly oxidative muscle fibers and lowest in fibers with low oxidative capacity (227, 234, 293, 299). Whether or not CAT expression in skeletal muscle increases in response to chronic exercise is controversial with studies reporting an increase (311, 400), decrease (227, 234, 299), or no change (299) in muscle CAT activity following training. The ambiguity of these findings may be due to a variety of factors including issues associated with assaying CAT activity. For example, CAT activity assays typically involve the addition of its substrate (i.e., H2O2), and the degradation of H2O2 is followed spectrophotometrically. However, interestingly, the Vmax of catalase increases as a function of the concentration of H2O2 present in the assay medium (184). Therefore, using this technique, the assessed CAT activity is dependent on not only the amount of active CAT protein in the assay medium, but also the concentration of H2O2 used as substrate (178). Hence, failure to carefully standardize the conditions of the assay may contribute to the wide variation of CAT activities reported within and between studies.
4. Accessory antioxidant enzymes
In addition to the aforementioned primary antioxidant enzymes, cells contain several other enzymes that can contribute directly or indirectly to the maintenance of redox balance. Among these, the thioredoxin, glutaredoxin, and peroxiredoxin systems are important contributors. The thioredoxin antioxidant system is composed of thioredoxin (TRX) and thioredoxin reductase (25, 41, 161, 413). The mammalian TRX is a highly conserved 12-kDa protein and cells contain two TRX systems, the cytosolic (TRX1) and the mitochondrial (TRX2) (41). Functionally, TRX is the major ubiquitous disulfide reductase responsible for maintaining proteins in their reduced state (25). Oxidized TRX is then reduced by electrons from NADPH via thioredoxin reductase (159). Along with the prevention of protein oxidation, numerous other physiological functions of TRX have been described including reduction of transcription factors, protection against oxidative stress, and control of apoptosis (25). Moreover, thioredoxin reductase also contributes as an antioxidant enzyme by reducing hydroperoxides and functioning as a NADPH-dependent dehydroascorbate reductase to recycle vitamin C (25).
Similar to TRX, glutaredoxin (GRX) is a thiodisulfide oxidoreductase that is involved in the protection and repair of protein and non-protein thiols during periods of oxidative stress (41, 158). GRX protects thiols by the transfer of electrons from NADPH to disulfide substrates, and this catalytic cycle is coupled with glutathione and glutathione reductase (41). Human cells contain three different GRXs; GRX1 is located in cytosol, whereas both GRX2 and GRX5 are located in the mitochondria (122, 240, 407).
Although TRX and GRX both control the redox state of thiol groups of cysteinyl side chains, their simultaneous presence in cells suggests different functions for each protein (248). Indeed, while TRX and GRX have some overlapping functions, GRXs are uniquely reactive with glutathione-mixed disulfides (160). Hence, it appears that GRX and TRX function in a cooperative manner to maintain the reduced redox state of both protein and non-protein thiols (374).
Peroxiredoxin (PRX) was discovered in 1988 and is a novel peroxidase capable of reducing both hydroperoxides and peroxynitrate with the use of electrons provided by a physiological thiol like TRX (204, 205, 327). Mammalian cells express six isoforms of PRX (PRX I–VI) that are distributed differentially within the cell: PRX I, II, and VI are found in the cytosol; PRX III is located in the mitochondrion; PRX IV is located in the extracellular space; and PRX V is located in both mitochondria and peroxisomes (327). Unfortunately, a kinetic analysis of PRX reaction rates at physiological concentrations of substrates has not been reported for any of the PRX isoforms. Nonetheless, the molar efficiencies of PRXs are generally smaller than GPX or CAT by several orders of magnitude (113). Therefore, although PRXs may defend against cellular oxidative stress, the importance of their antioxidant role in mammalian cells remains unclear (113). Moreover, growing evidence suggests that in addition to antioxidant properties, these peroxidases may also play a role in the regulation of H2O2 as a second messenger in receptor-mediated signaling (192, 328, 329).
Finally, the effects of regular exercise on the TRX, GRX, and PRX systems in skeletal muscles remain unknown. Nonetheless, it is conceivable that exercise-induced changes in one or all of these antioxidant systems could contribute to the redox adaptation to exercise. This topic remains an interesting area for future work.
C. Nonenzymatic Antioxidants
Numerous nonenzymatic antioxidants exist in cells (e.g., GSH, uric acid, bilirubin, etc.), and a detailed discussion of this topic is beyond the scope of this review. Nonetheless, in this segment, we will provide a brief overview of selected nonenzymatic antioxidants located within skeletal muscle fibers. For a detailed discussion of nonenzymatic antioxidants, the reader is referred to numerous published reviews (97, 123, 177, 178, 184, 298, 303, 408).
Clearly, one of the most important nonenzymatic antioxidants in muscle fibers is GSH. Glutathione is a tripeptide and is the most abundant nonprotein thiol in cells (263). This antioxidant is primarily synthesized in the liver and transported to tissues via the circulation. Glutathione concentration in cells is in the millimolar range for most tissues, and GSH content varies across organs depending on their function (263). For example, tissues with high exposure to oxidants (e.g., lens of the eye and liver) contain high levels of GSH. Similarly, the concentration of GSH found in skeletal muscle fibers varies across fiber types with type I fibers in rats containing 400–600% higher GSH content (i.e., 2–3 mM) compared with type IIb fibers (i.e., ~0.5 mM) (235).
As an antioxidant, GSH serves multiple roles in cells. First, GSH can directly react with a variety of radicals by donating a hydrogen atom (414). Second, as mentioned earlier, one of the key antioxidant actions of GSH is to serve as a substrate for GPX to eliminate H2O2 and organic hydroperoxides (263). Furthermore, GSH is also involved in reducing other antioxidants in the cell including vitamins E and C. In this regard, GSH has been shown to reduce vitamin E radicals that are formed in chain-breaking reactions with alkoxyl or lipid peroxyl radicals. Similarly, GSH can reduce the semidehydroascorbate radical (vitamin C radical) derived from the recycling of vitamin E. Collectively, these GSH-mediated reactions assist in maintaining the limited resources of vitamin E and C in the reduced state at the expense of GSH (184).
Studies indicate that skeletal muscle fibers adapt to regular bouts of high-intensity endurance exercise by increasing the levels of GSH (234, 235, 246, 285, 352). It has been suggested that the exercise-induced increase in GSH within muscle fibers is due to increased activity of a key enzyme involved in GSH synthesis (178). Specifically, the activity of the rate-limiting enzyme for GSH biosynthesis (i.e., γ-glutamylcysteine synthase) is increased in exercise-trained muscles and probably plays an important role in exercise-induced increases in GSH synthesis in skeletal muscle (221, 246, 352).
During the past 15 years, α-lipoic acid has received much attention as a cellular antioxidant. α-Lipoic acid is a naturally occurring compound and can be obtained in the diet from a variety of foods (189, 289, 291, 335). Functionally, α-lipoic acid serves as a cofactor for α-dehydrogenase complexes and participates in S-O transfer reactions (189, 289). Normally, α-lipoic acid is present in very small quantities in animal tissues and is generally bound to an enzyme complex which limits its function as an antioxidant (291). However, unbound and reduced α-lipoic acid (dihydrolipoic acid) and several of its metabolites are effective antioxidants (189, 291). Furthermore, numerous studies support the notion that α-lipoic acid can provide beneficial antioxidant effects in a physiological context and can play a role in recycling vitamin C (24, 46, 62, 72, 117, 189, 291, 412, 423). Although an acute bout of exercise appears to increase α-lipoic acid levels in skeletal muscle, chronic exercise training does not alter muscle levels of α-lipoic acid (200).
Uric acid is a by-product of purine metabolism in humans and other primates and is potentially an important low-molecular-mass antioxidant in human biological fluids (14, 148). At physiological pH, almost all uric acid is converted to urate (365). The antioxidant role of uric acid/urate was first reported in 1960, and additional evidence for its antioxidant properties grew in the 1980s by experiments demonstrating that uric acid was a powerful scavenger of peroxyl radicals, hydroxyl radicals, and singlet oxygen (14, 87, 162, 356). As an antioxidant, urate is able to protect against oxidative damage by acting as an electron donor (136). Urate is also able to chelate metal ions such as iron and copper and prevent them from catalyzing hydroxyl radicals via the Fenton reaction (136). The impact of chronic exercise on muscle urate levels remains unknown, but it appears that urate could be an antioxidant scavenger in skeletal muscle during exercise (147, 148).
Bilirubin is the final product of hemoprotein catabolism as heme oxygenase cleaves the heme ring to form biliverdin; biliverdin is then reduced by biliverdin reductase to form bilirubin (376). Although both biliverdin and bilirubin are reducing species, bilirubin is considered to be the best physiological antioxidant (33). Indeed, bilirubin possesses strong antioxidant potential against peroxyl radicals and has been shown to protect cells from toxic levels of hydrogen peroxide (33, 377, 378). It has been suggested that the powerful physiological antioxidant actions of bilirubin are a result of an amplification cycle whereby bilirubin acting as an antioxidant, is itself oxidized back to biliverdin and then recycled back to bilirubin via biliverdin reductase (33). Although intense exercise increases blood levels of bilirubin (111, 258), the impact of chronic exercise on bilirubin levels in skeletal muscle remains unknown.
Another nonenzymatic antioxidant worthy of discussion is coenzyme Q10. Conenzyme Q10 (ubiquinone) is synthesized in cells and is essential in mitochondrial electron transport and is also located in cell membranes (136). In vitro, coenzyme Q10 can function as a nonenzymatic antioxidant by scavenging radicals and inhibiting lipid peroxidation. Nonetheless, the contribution of coenzyme Q10 to antioxidant defense in vivo remains uncertain. Although several studies have investigated the effects of coenzyme Q10 supplementation on skeletal muscle function during exercise (146, 190, 264, 334, 359, 386), the impact of endurance exercise training on coenzyme Q10 levels in muscle remains relatively unknown (84).
D. Dietary Antioxidants
Numerous dietary antioxidants may also contribute to cellular protection against radicals and other ROS. Important dietary antioxidants include vitamin E, vitamin C, and carotenoids. Vitamin E is one of the most widely distributed antioxidants in nature, and it is the primary chain-breaking antioxidant in cell membranes (171, 290). The generic term vitamin E refers to at least eight structural isomers of tocopherols or tocotrienols (171, 347). Among these, α-tocopherol is the best known and possesses the most antioxidant activity (171). In addition to its direct antioxidant properties, growing evidence suggests that some of the beneficial effects of vitamin E in cells resides in its ability to regulate gene expression of proteins (30, 31, 139, 349).
Several studies have investigated the effects of acute and chronic exercise on vitamin E levels in skeletal muscles of rodents. Unfortunately, the results are not consistent with some studies reporting an exercise-induced decrease in muscle vitamin E concentration (49, 125), whereas others conclude that both acute and chronic muscular activity does not alter muscle vitamin E levels (74, 343, 375). Finally, studies investigating the impact of chronic exercise on vitamin E levels in human skeletal muscle suggest that exercise does not alter vitamin E levels (389, 390).
Similar to vitamin E, carotenoids (e.g., β-carotene) are lipid-soluble antioxidants located primarily in the membranes of tissues. The antioxidant properties of carotenoids come from their structural arrangement consisting of long chains of conjugated double bonds; this arrangement permits the scavenging of several different ROS species including superoxide and peroxyl radicals (108, 222, 372). Because of their cellular location and their radical scavenging capacity, carotenoids are efficient biological antioxidants against lipid peroxidation (222). To date, the effects of chronic exercise on muscle levels of carotenoids have not been investigated.
In contrast to both vitamin E and the carotenoids, vitamin C (ascorbic acid) is hydrophilic and functions better in an aqueous environment. Because the pKa of ascorbic acid is 4.25, the ascorbate anion is the predominant form existing at physiological pH (414). Ascorbate is widely distributed in mammalian tissues, and its role as an antioxidant is twofold. First, vitamin C can directly scavenge superoxide, hydroxyl, and lipid hydroperoxide radicals (63). Second, vitamin C plays an important role in the recycling of vitamin E, a process that results in the formation of a vitamin C (semiascorbyl) radical (288). Nonetheless, this semiascorbyl radical can be reduced back to vitamin C by NADH semiascorbyl reductase, or cellular thiols such as glutathione or dihydrolipoic acid (290).
IV. OXIDATIVE STRESS
The term oxidative stress was first defined in 1985 as “a disturbance in the pro-oxidant-antioxidant balance in favor of the former” (363, 364). Although this definition has been widely used for over two decades, the definition of oxidative stress will likely evolve and undergo modification in the future. Indeed, because of the complexity associated with the assessment of cellular redox balance, it has been argued that the term oxidative stress defies a simple pro-oxidant versus antioxidant definition and that the description of an “oxidant stress” is only useful if the molecular details of the imbalance are known (29, 186). In an effort to refine the meaning of oxidative stress, Dean Jones has proposed that this term should be redefined as “a disruption of redox signaling and control” (186). Regardless of whether this new definition gains widespread acceptance, it can be anticipated that the description of oxidative stress will undergo future modifications as the field of redox biology advances.
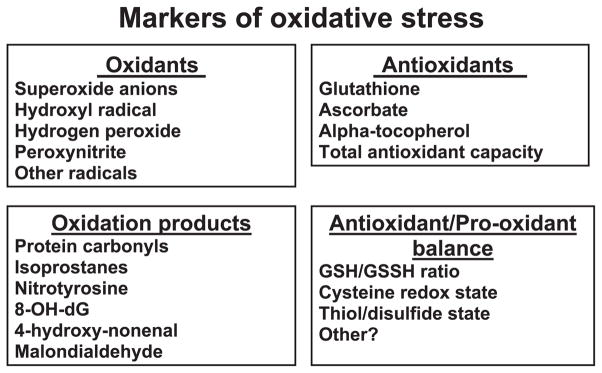
Regardless of how oxidative stress is defined, a persistent pro-oxidant environment in cells can modify redox-sensitive molecules. A common approach to assess oxidative stress in biological systems involves the measurement of the increase or decrease in a redox-sensitive molecule that responds to oxidative stress. In general, reliable markers of oxidative stress possess the following qualities: 1) chemically unique and detectable, 2) increased or decreased during periods of oxidative stress, 3) possess relatively long half-lives, and 4) not impacted by other cellular processes (e.g., cell cycle, energy metabolism, etc.) (136).
Many molecules that fit one or more of these criteria have been identified, and techniques to measure these biomarkers have been reported (88, 99, 138, 163, 186, 233, 331, 338). During periods of oxidative stress, pro-oxidants overwhelm the antioxidant defenses in cells and damage cellular constituents. Thus oxidative stress in biological systems is often characterized by the following parameters: 1) increase in the formation of radicals and other oxidants, 2) decrease in small-molecular-weight and/or lipid-soluble antioxidants, 3) disturbance in cellular redox balance, and 4) oxidative damage to cellular components (i.e., lipids, proteins, and/or DNA). Hence, biomarkers of oxidative stress typically fall into one of four categories (Fig. 3). The first category of biomarkers involves the detection of oxidants. Unfortunately, direct measurement of radical production in living cells is difficult because radicals are highly reactive and have a short half-life. Therefore, exogenous molecules such as fluorescent probes or spin traps are commonly used to measure oxidant production in cells. When added to a biological system, the probe or spin trap is converted into a unique modified radical product with a relatively long half-life that can be quantified as a measurement of oxidant production (138, 163). Nonetheless, because an increase in oxidant production does not necessarily define a pro-oxidant condition, measures of increased oxidant production alone are not definitive markers of oxidative stress. Moreover, a potential disadvantage of using probes or spin traps is that these molecules may perturb the biological system being investigated (136). Another drawback with this approach is that many spin traps and fluorescent probes are toxic to cells (136).
FIG. 3.
The four broad classes of biomarkers used to assess cellular oxidative stress in tissues. These categories include the measurement of oxidant production, cellular levels of antioxidants, oxidation products, and the antioxidant/pro-oxidant balance. 8-OH-dG, 8-hydroxydeoxyguanosine; GSH/GSSG, ratio of reduced glutathione to oxidized glutathione.
A second group of oxidative stress biomarkers incorporates the measurement of antioxidants in tissues. In theory, decreases in antioxidants (e.g., glutathione, ascorbate, α-tocopherol, etc.) are common occurrences during oxidative insults, and therefore, measurement of a decline in tissue antioxidant levels has been used as a biomarker of oxidative stress. Although assessment of tissue antioxidant levels has merit as a biomarker of oxidative stress, this approach is not without weaknesses. For example, other factors such as changes in cellular metabolism and diet can influence antioxidant levels in cells. Another concern associated with the measurement of tissue antioxidants is the potential for auto-oxidation during sample handling resulting in antioxidant depletion in the tissue (136).
A third class of biomarkers of oxidative stress involves the evaluation of oxidatively modified molecules. Indeed, ROS attack of lipids, protein, or DNA generates uniquely oxidized biomolecules that can be used as “fingerprints” to detect oxidative stress in cells. Common measures of bio-oxidation include the measurement of protein carbonyls as an indicator of protein oxidation; assessment of isoprostanes, malondialdehyde, and 4-hydroxyl-2-nonenol as signs of lipid peroxidation; and evaluation of DNA oxidation by assaying the levels of the oxidized base, 8-hydroxy-2′-deoxyguanosine (8-OH-dG) (88, 163, 198, 233). Although it can be argued that oxidized molecules are the most important biomarker of oxidative stress, their measurement in biological systems is often difficult because oxidized molecules exist in limited amounts in cells even during periods of oxidative stress. Moreover, identical to other biomarker methodologies, measurement of oxidation products is subject to assay artifacts if tissue samples are handled improperly.
The fourth and final category of oxidative stress biomarkers involves the measurement of cellular redox balance. One of the most commonly measured markers of cellular redox balance is the ratio of GSH to GSSH. This assay is useful because increased oxidant production results in a decrease in the GSH/GSSH ratio, indicating lower levels of reduced GSH in favor of increased oxidized GSH (i.e., GSSG). Nonetheless, although this assay is conceptually simple, experimental artifacts are common and can occur during tissue removal and sample processing due to improper tissue handling permitting auto-oxidation (136).
In summary, numerous approaches to assess oxidative stress in biological systems have been reported in the literature. Unfortunately, each category of oxidative stress biomarkers has limitations. Therefore, although there are many parameters to quantify oxidative stress, the development of a single and ideal biomarker has proven to be a difficult task. Hence, it appears that no one biomarker best assesses oxidative stress and that in most cases, the measurement of multiple biomarkers is required to confirm the presence of oxidative stress in tissues (136).
V. HISTORICAL OVERVIEW OF EXERCISE-INDUCED OXIDATIVE STRESS
Although Commoner et al. (71) recognized that cells contained free radical intermediates in the 1950s, the first suggestion that exercise was associated with an increase in the formation of lipid peroxidation by-products did not appear until the late 1970s. This section will briefly describe the directions that the field took over the ensuing 30 years and identify several of the key scientists involved. Inevitably this historical account is written from our personal points of view, and hence, we apologize to colleagues who completed studies that contributed to this field but have been inadvertently omitted.
Using the techniques available in the 1970s to analyze lipid peroxidation, Brady et al. (51) and Dillard et al. (98) reported increased lipid peroxidation during exercise in both rats and humans. These data were subsequently confirmed and expanded by Kelvin Davies and colleagues working in Lester Packer’s laboratory at the University of California-Berkeley (86). This 1982 paper is commonly cited as the first evidence that contracting skeletal muscles produce free radicals and that ROS production during exercise is potentially damaging to tissues. The role of mitochondria in generation of superoxide and hydrogen peroxide had been originally reported by researchers in Britton Chance’s laboratory in the 1970s (48) and appeared to provide a ready explanation for the source of radical production in skeletal muscle reported by Davies et al. (86).
During the early 1980s, Lester Packer’s laboratory investigated the role of antioxidant nutrients in the protection of cells and organelles from radical-mediated oxidative damage (85, 313). A summary of these ground-breaking studies from the Packer group was reported at a Ciba Foundation Symposium in London in 1983 (312). At this same meeting, Jackson and colleagues from University College (London) also presented their contemporary studies examining the role of free radicals as damaging agents to muscle and the possible beneficial effects of vitamin E in reducing exercise-induced damage (167). Other work by Jackson and colleagues focused on the role of calcium influx in degradative processes in muscle (166, 185). This research was complimented by studies indicating that depletion of the antioxidant vitamin E from rats increased the risk of contraction-induced membrane damage in skeletal muscles (165, 167). These results complemented earlier reports describing muscle degeneration in vitamin E-deficient animals (86). Collectively, these studies stimulated interest in many laboratories to investigate whether vitamin E (and subsequently other antioxidant nutrients) could retard both tissue damage and muscle contractile dysfunction that occurred during some forms of muscular exercise. These studies have continued to the present day. Nonetheless, although some reports have concluded that vitamin E supplementation decreases the markers of oxidation in tissues (e.g., Refs. 126, 195, 223), positive effects of dietary antioxidants against contraction-induced muscle damage and muscular fatigue are not commonly observed. Moreover, the enthusiasm for further research in this area has also undoubtedly been influenced by the disappointing results of large-scale intervention studies using high doses of antioxidants in many chronic disorders (6, 315).
The techniques used to examine free radical production in skeletal muscles in the early studies utilized the approaches available at that time, mainly measurements of the products of free radical reaction with lipids (51, 98). However, Davies et al. (86) and Jackson et al. (165) also utilized electron-spin resonance spectroscopy (ESR) to examine the relatively stable species that is observed by direct ESR analysis of tissues. Subsequent studies examined other measures of ROS activity, but the elucidation of which free radical species are generated by contracting skeletal muscle occurred only after a further ~10 yr of research. Specifically, ensuing work revealed superoxide release from the contracting diaphragm (321, 322, 325), the demonstration of NO generation by skeletal muscle (32, 207), and the detection of hydroxyl radical formation by contracting muscle tissue (94, 280).
As mentioned previously, the key antioxidant enzyme SOD was characterized in 1969 by Joe McCord and Irwin Fridovitch. The role of SOD and other antioxidant enzymes in regulation of free radical activity during exercise and the variation in tissues during and following exercise were investigated by numerous investigators in the 1980s (10, 155, 173, 194). The dramatic ability of the body to increase antioxidant capacity with acute and chronic exercise was subsequently described in detail in heart, respiratory, and locomotor skeletal muscles. Key contributors to this work were Li Li Ji and colleagues at the University of Wisconsin (175, 180, 182) and Scott Powers and colleagues at the University of Florida (78, 137, 232, 299–301).
Subsequent studies identified the key role that glutathione plays in regulating free radical activity in exercising subjects. A key contributor to this work was Chandan Sen and colleagues who described the effects of varying levels of glutathione on exercise responses and the effects of exercise on glutathione content and redox status (351, 352). José Viña and colleagues from the University of Valencia (Spain) also contributed significantly to this area (345) and built on the expertise developed by José Viña during fundamental work on glutathione metabolism undertaken with Sir Hans Krebs in Oxford, UK.
The recognition that contraction-induced free radicals can influence muscle function and fatigue can be attributed to several investigators including Gerald Supinski, Michael Reid, Jack Barclay, and their colleagues as much of this work was published in the early 1990s (34, 321, 322, 325, 361). This initial work set the stage for subsequent studies to explore redox-sensitive targets in skeletal muscle. Specific details regarding the free radical influence on skeletal muscle function will be addressed in detail in a subsequent segment of this review.
Chandan Sen and Lester Packer also played a key role in highlighting the potential job that free radicals play in modulating cell signaling processes (353) and helped initiate the interest in redox-signaling that continues to the current day. This work paved the way to the increased realization that free radicals play a crucial role in activating degradatory pathways leading to loss of muscle mass that has been developed in studies of diaphragm muscle fatigue and degeneration by Gerald Supinski (360, 361, 382), Thomas Clanton (94, 96), and others (211–215, 255, 357).
Thus, during a period of <30 yr, the field of “exercise” redox biology moved from relatively simple studies that examined changes in markers of “oxidative damage” and their prevention to the current status of a growing understanding of the “redox biology” of skeletal muscle and exercise. Most importantly, instead of being considered a peripheral area of exercise physiology, redox signaling in contracting skeletal muscle is now viewed as a basic element in exercise biology. Inevitably this brief historical review has excluded many individual papers since the field has expanded dramatically within the last 30 yr. A brief examination of the number of papers published in the area of free radicals and exercise indicates that in 1982–1983, 10 papers were published, compared with almost 1,000 reports in 2006, and this growth of the literature shows no sign of decline.
VI. SOURCES OF RADICALS DURING EXERCISE
There are many potential tissue sources from which ROS and RNS may be produced during exercise, but surprisingly few studies have investigated the predominant tissues responsible for this production. This is likely due to both the restricted access to most tissues in humans and the complex nature of exercise that involves many organ systems that are linked through the increased metabolic requirement of skeletal muscles. Hence, although many studies have examined whole body indicators of free radical activity [such as the release of expired pentane originally reported by Dillard et al. (98)], the majority of these reports have assumed that skeletal muscle provides the major source of free radical and ROS generation during exercise. It is however entirely feasible that in some situations other tissues such the heart, lungs, or white blood cells may contribute significantly to the total body generation of ROS.
Because of the invasive nature of obtaining tissue samples from exercising humans, many studies have examined whole body indicators of oxidation. Despite the many variations in the exercise model examined, many studies have confirmed that increased lipid oxidation (e.g., Ref. 26), DNA oxidation (e.g., Ref. 405), and oxidation of other components (e.g., Ref. 355) can be observed in blood, although inevitably this has not been observed by all authors (e.g.. see Ref. 339). Some authors have suggested that common metabolic changes that occur during most exercise protocols such as the increased release of catecholamines may play a role in the increased ROS generation (75), but the general consensus has been that ROS generation occurs predominantly by contracting skeletal and heart muscle. An exception to this rule is an experiment whereby muscle damage occurs, and in this situation, inflammatory processes may play an important role in radical production. In the following three segments, we will discuss several potential production sites of superoxide radicals and NO along with sources of ROS production in muscle following damage.
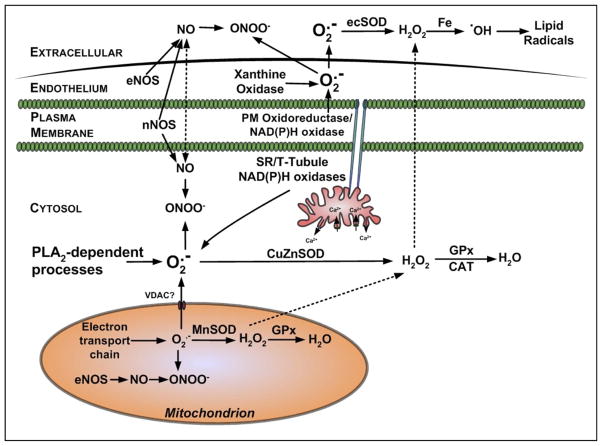
A. Endogenous Sites for Superoxide Generation in Skeletal Muscle
Skeletal muscle generates superoxide at multiple subcellular sites, several of which increase in activity during muscle contractions. Numerous potential sites for superoxide production exist in skeletal muscle and are summarized in the following segments (Fig. 4).
FIG. 4.
Potential sites for the production of superoxide and nitric oxide in skeletal muscle.
1. Mitochondria
Mitochondria have generally been cited as the predominant source of ROS in muscle cells (e.g., Refs. 85, 217), and many authors have reiterated early reports that 2–5% of the total oxygen consumed by mitochondria may undergo one electron reduction with the generation of superoxide (48, 239). More recent research has identified the major site(s) of superoxide generation within mitochondria, and most data now indicate that complexes I and III of the electron transport chain are the main sites of mitochondrial superoxide production (36, 272). In complex I, the main site of electron leakage to oxygen appear to be the iron-sulfur clusters, and in complex III, it appears to be the Q10 semiquinone (272). Furthermore, complex III releases superoxide from both sides of the inner mitochondrial membrane (272). It is unclear if this superoxide crosses the outer mitochondrial membrane or is dismutated by Cu/Zn-SOD located in the mitochondrial intermembrane space where it may minimize this possibility. During exercise, a number of researchers have assumed that the increased ROS generation that occurs during contractile activity is directly related to the elevated oxygen consumption that occurs with increased mitochondrial activity, implying potentially a 50- or 100-fold increase in superoxide generation by skeletal muscle during aerobic contractions (e.g., see Refs. 193, 392). However, a recent finding suggests that mitochondria may not be the dominant source of ROS during exercise (170), and future studies will be required to fully elucidate the role that mitochondria play in contraction-induced production of ROS in skeletal muscle.
Brand and colleagues (371) have recently reassessed the rate of production of ROS by mitochondria and have concluded that the upper estimate of the total fraction of oxygen utilized that forms superoxide was ~0.15%; this value is several orders of magnitude lower than the original estimate of 2–5% (371). This low rate of superoxide production may include a role for uncoupling proteins (specifically UCP3 in skeletal muscle) as regulators of mitochondrial production of ROS (52, 53) acting to protect mitochondria against oxidative damage. In addition, there has been considerable debate about the effect of changes in the respiratory state on ROS generation by mitochondria, and growing evidence reveals that mitochondria produce more ROS during state 4 (basal) respiration compared with state 3 (maximal ADP-stimulated respiration) (4, 93, 149, 219). This is significant because during aerobic contractile activity, skeletal muscle mitochondria are predominantly in state 3, and this limits their generation of ROS during contractions (93, 149, 219). Collectively, these findings suggest that mitochondria are not the primary source of ROS production in skeletal muscle during exercise.
Finally, recent evidence suggests that compared with type I fibers, type II skeletal muscle fibers possess unique properties that promote mitochondrial ROS production. Specifically, using an in situ approach to measure H2O2 release from mitochondria in permeabilized rat muscle fiber bundles, a recent study reported that mitochondrial ROS leak (i.e., H2O2 release/O2 consumed) was two- to threefold greater in type II fibers compared with type I (15). The mechanism responsible for this observation remains unclear but does not appear to be due to differences in mitochondrial GPX activities between type I and II fibers.
2. Sarcoplasmic reticulum
Studies have identified NAD(P)H oxidase enzymes associated with the sarcoplasmic reticulum (SR) of both cardiac (66) and skeletal muscle (409). The superoxide generated by these enzymes appears to influence calcium release by the SR through oxidation of the ryanodyne receptor (66). The skeletal muscle enzyme described appears to preferentially use NADH as substrate (409). Some inhibitor studies have indicated that extracellular superoxide release from stimulated myotubes was reduced by treatment with diphenyleneiodonium (DPI), a nonspecific inhibitor of NAD(P)H oxidases (292), although the NADH oxidase described by Xia et al. (409) is localized to the SR and hence seems unlikely to contribute to the extracellular release.
3. Transverse tubules
Recent data indicate that the transverse tubules of skeletal muscle contain a NADP(H) oxidase whose activity is increased by depolarization (109, 154). This enzyme contains some of the classical subunits found in the NADP(H) oxidase of phagocytic cells and appears to release superoxide to the cytosol of skeletal muscle cells. Finally, this skeletal muscle specific NADP(H) oxidase is inhibited by nonspecific inhibitors of this class of enzyme and by prevention of membrane depolarization.
4. Plasma membrane
Numerous studies reveal that skeletal muscle cells release superoxide into the extracellular space (e.g., see Refs. 253, 292, 321, 325, 427). All cells contain plasma membrane redox systems capable of undertaking electron transfer across the plasma membrane. A NAD(P)H oxidase complex has been reported to be constitutively expressed in diaphragm and limb muscles of the rat and localized to the region of the plasma membrane (172). The enzyme contains four of the subunits that are found in the enzyme in phagocytic cells (gp91phox, p22phox, p47phox, and p67phox), all of which were associated with the cell membranes (236). Whether this complex predominantly releases superoxide to the inside or the outside of the plasma membrane cannot be ascertained from the experiments reported (172), and any potential overlap with the recent discovery of the NAD(P)H oxidase located in the transverse tubules (109, 154) has not been investigated.
There are other plasma membrane redox systems that are capable of transferring electrons from intracellular reductants to appropriate extracellular electron acceptors, although no such system has been described in skeletal muscle (346). Morré (269) has described external NADH oxidase (ECTO-NOX) proteins that exhibit a hydroquinone (NADH) oxidase activity and a protein disulfidethiol exchange activity. The current understanding of these systems is that they accept electrons from the hydroquinones of the plasma membrane and can reduce a number of nonphysiological (e.g., ferricyanide and WST-1) and physiological (e.g., protein thiols or oxygen) electron acceptors outside the cell, although oxygen is likely to be a major acceptor in vivo (89). Transfer of electrons from cytosolic NAD(P)H to the plasma membranes has been proposed to occur through either NADH-cytochrome b5 oxidoreductase or NAD(P)H quinone oxidoreductase (NQO1) (89). Thus, through a series of linked steps, intracellular NAD(P)H can act as substrate for superoxide generation on the cell surface. The relevance of these processes to skeletal muscle contractions has not been established, but it is feasible that these systems are activated during contractile activity or that the substrate level rises to increase electron transfer across the membrane through these systems. The characteristics of the release of superoxide from skeletal muscle are compatible with the involvement of such a system.
5. Phospholipase A2-dependent processes
Phospholipase A2 (PLA2) is an enzyme that cleaves membrane phospholipids to release arachidonic acid, which is a substrate for ROS-generating enzyme systems such as the lipoxygenases (426). Also, activation of PLA2 can stimulate NAD(P)H oxidases (421), and increased PLA2 activity has been reported to stimulate ROS generation in muscle mitochondria (276) and cytosol (130) and release ROS into the extracellular space (426). Both calcium-dependent and independent forms of PLA2 are reported to play a role in muscle ROS generation. The calcium-independent enzymes (iPLA2) have been claimed to modulate cytosolic oxidant activity in skeletal muscle cells (130), while a 14-kDa calcium-dependent isoform (sPLA2) located within mitochondria has been reported to stimulate intracellular ROS generation during contractile activity (277). In nonmuscle cells, activity of the third major type of PLA2, cytosolic (cPLA2) that is activated by micromolar concentrations of calcium, has been linked to ROS generation (273). Reid and colleagues (130) hypothesized that the calcium-independent PLA2 was a major determinant of ROS activity under resting conditions, whereas during contractions, heat stress, or other processes elevating intracellular calcium, the calcium-dependent PLA2 was activated and stimulates ROS production at supranormal rates.
6. Xanthine oxidase
There has been considerable speculation concerning a role for xanthine oxidase in superoxide generation by skeletal muscle. At present, this speculation is primarily based on the effects of the xanthine oxidase inhibitors allopurinol or oxypurinol (e.g., see Refs. 129, 151). Although rat skeletal muscles contain significant levels of xanthine oxidase (188), human skeletal muscle cells per se appear to possess low amounts of xanthine dehydrogenase or oxidase (145), although these enzymes will inevitably be present in associated endothelial cells. Clearly, additional research is required to determine the role that xanthine oxidase plays in exercise-induced ROS production.
Thus there is clear evidence that superoxide and hydrogen peroxide are generated in muscle cells during contractions, and more limited data indicate that hydroxyl radicals may be generated under more specific circumstances. Despite the initial indications that mitochondria are the predominant site for ROS generation during activity, a number of alternative potential sites have been identified. It is still unclear whether all of these multiple sites contribute to the increased ROS activities that are observed during contractions or whether one site predominates. It is entirely feasible that the multiple sites of generation are active in differing situations and that the effects of the ROS generated are relatively localized and important for disparate functions. For example, localized ROS generation by the sarcoplasmic reticulum or t-tubule systems may be much more important in regulation of sarcoplasmic reticulum calcium handling (see sect. VIIIA1) than ROS generated by mitochondria or extracellular oxidases.
B. Endogenous Sites for NO Production
NO is generated continuously by skeletal muscle, a production that is increased by contractions (32, 207). Skeletal muscle normally expresses the neuronal (type I or nNOS) and the endothelial (type III or eNOS) isoforms of NOS. nNOS is strongly expressed in fast-twitch muscle fibers and localized to the muscle sarco-lemma where it is associated with the dystrophin-glycoprotein complex (DGC). eNOS is localized to the muscle mitochondria (208). iNOS (type II) is also expressed in skeletal muscle in some inflammatory conditions, but it does not play a significant role in normal muscle (373). Analysis of myotubes in culture has confirmed that skeletal muscle cells per se release increased amounts of NO during contractile activity (292), a release that was greatly reduced by the NOS inhibitor L-NAME. nNOS appears to be the prime source of the NO released from skeletal muscle (156). Passive stretching of muscle has also been shown to increase NO release from rat skeletal muscle in vitro (388) and to increase nNOS expression.
C. Generation of ROS in Muscle Following Damage
Nonmuscle sources may play a major role in modifying muscle redox state where tissue damage has occurred, notably through the role played by phagocytic white cells. Substantial injury to muscle fibers is accompanied by invasion of the area with macrophages and other phagocytic cells (e.g., see Ref. 254), and although this process appears to be essential for preparation of the tissue to allow effective regeneration to occur, it also involves the release of substantial amounts of ROS from the phagocytic cells (242). The magnitude of this release can be such that damage to previously undamaged muscle cells may result (417).
VII. RADICALS AND MUSCULAR FATIGUE
In the preceding section we discussed potential sources of ROS and RNS in skeletal muscle and highlighted the fact that skeletal muscles continuously produce ROS and RNS. This segment will address the influence of both RNS and ROS on skeletal muscle force production. Because of the voluminous literature on this topic, it is necessary to condense the material presented in this section. Readers seeking more details should consult the following comprehensive reviews (11, 67, 245, 316–318, 320, 368, 373, 381, 383).
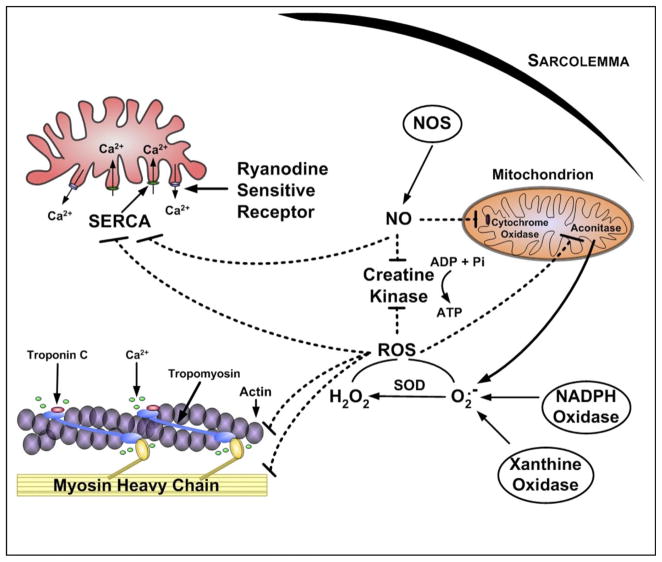
A. Influence of NO on Force Production in Unfatigued Muscle
As discussed previously, NO is produced in mammalian cells via the enzymatic action of a family of NO synthases that are differentially expressed across cell types (207, 208, 336). Furthermore, it is well established that isolated skeletal muscle fibers produce low levels of NO during resting conditions but NO production increases during periods of contractions (207, 310, 319) and that endogenous production of NO can modulate skeletal muscle force production. Indeed, studies using excised bundles of muscle fibers reveal that force production during submaximal tetanic contractions is depressed by NO donors (7, 18, 208, 294, 330) and increased by NOS inhibitors and NO scavengers (18, 207). Interestingly, NO production does not influence maximal tetanic force production. Hence, endogenous NO production shifts the force-frequency curve to the right without decreasing maximal tetanic force production. Furthermore, because NOS3 deficiency does not alter muscle function, it appears that the impact of NO on skeletal muscle contractile properties is likely mediated by NOS1 (156).
B. ROS Modulation of Force Production in Unfatigued Muscle
Similar to NO and muscle force production, it is also established that ROS have an important influence on force production in unfatigued skeletal muscle. For example, the low levels of ROS present in skeletal muscle during basal conditions are a requirement for normal force production (317, 318, 383). Indeed, antioxidant-mediated depletion of ROS from unfatigued skeletal muscle results in a depression of muscle force production (73, 322–324). In contrast, a modest increase in ROS in skeletal muscle fibers results in an increase in force production (322). The positive impact of ROS on muscle force production is reversed at higher ROS concentrations as force production decreases in both a time- and dose-dependent manner (322).
Although numerous investigators have contributed to our understanding of the influence of ROS on muscle force production, Reid et al. (322) were the first to develop a theoretical model to describe the relationship between muscle redox balance and isometric force production. This model is illustrated in Figure 5. Their model assumes that the muscle redox state is a physiologically regulated variable that is balanced by matching the rates of ROS production with cellular antioxidant buffering capacity. The paradigm of Reid et al. (317, 322) predicts that an optimal cellular redox state exists whereby conditions are ideal for muscle force production. It follows that a deviation from the optimal redox balance leads to a loss of force production.
FIG. 5.

A theoretical model proposed by Reid et al. (322) that describes the biphasic effect of ROS on skeletal muscle force production. Point 1 represents the force production by unfatigued muscle exposed to antioxidants or a reducing agent. Point 2 illustrates the force generated by muscle in its basal state (i.e., no antioxidants or oxidants added). Point 3 illustrates the force produced by unfatigued skeletal muscle exposed to low levels of oxidants; this represents the optimal redox state for force production. Point 4 illustrates the deleterious effects of excessive ROS on skeletal muscle force. [Redrawn from Reid (317).]
C. Radicals Contribute to Exercise-Induced Muscular Fatigue
As outlined in previous sections, redox disturbances in skeletal muscle can significantly reduce force production. This observation has stimulated much interest in the possibility that free radicals contribute to muscular fatigue during prolonged exercise. Indeed, the role of oxidants in muscular fatigue has been investigated using a variety of animal models via in vitro and in situ muscle preparations as well as during whole body exercise. Moreover, studies have explored the role of antioxidant supplementation in preventing muscular fatigue during prolonged exercise in humans. In this segment, we summarize the evidence indicating that free radicals contribute to muscular fatigue. For the purpose of this discussion, we will define muscular fatigue as “an exercise-induced reduction in muscle force generation” (120, 404).
1. Role of redox disturbances in muscle fatigue: in vitro and in situ animal studies
As discussed throughout in this review, there is abundant evidence that production of ROS increases in contracting skeletal muscle. Moreover, animal studies provide convincing evidence that ROS contribute to muscle fatigue induced by prolonged muscular contractions. The earliest report indicating that ROS contribute to muscle fatigue appeared in 1990. This early study demonstrated that the ROS scavenger N-acetylcysteine delayed muscle fatigue in an in situ diaphragm muscle preparation (361). Since this initial report, numerous studies using both in vitro and in situ preparations have investigated the role that redox disturbances play in the development of muscular fatigue. Although one study using dietary antioxidants to scavenge ROS failed to demonstrate a role for ROS in the development of muscle fatigue (73), the vast majority of studies have reported that scavenging ROS via enzymatic and nonenzymatic antioxidants delays muscle fatigue during submaximal contractions (22, 34, 203, 267, 321, 322, 384). In contrast, it appears that antioxidant scavengers do not appear to be effective in delaying fatigue when muscle contractions are near maximum (250, 321). Finally, studies examining the effects of antioxidants on muscle performance during recovery from fatiguing exercise are inconsistent with one report indicating a faster recovery of force production (95), whereas others fail to observe a faster recovery time (203, 321, 325).
In contrast to the literature regarding the impact of ROS on muscle fatigue, evidence that NO production directly contributes to the development of muscle fatigue is limited. In theory, because of the effects of NO on muscle proteins involved in force production (i.e., contractile and calcium handling proteins), it could be predicted that NO should contribute to muscle fatigue (368). Nonetheless, a consensus of literature does not exist to support the notion that NO production promotes muscular fatigue. For example, although one report suggests that NOS inhibition does delay fatigue in electrically stimulated diaphragm muscle (118), other studies conclude that NO does not directly influence skeletal muscle fatigue resulting from prolonged submaximal contractions (65, 207). Furthermore, in perfused in situ muscle preparations, NOS blockade appears to accelerate muscle fatigue due to a dysregulation of blood flow (7, 13). Collectively, these data do not support the concept that muscle-derived NO promotes skeletal muscle fatigue.
2. Radicals and muscle fatigue: in vivo studies
Numerous reports document the failure of supplementation with antioxidant vitamins to improve endurance exercise performance in humans (28, 59, 119, 302, 333, 370). Collectively, these results lead to the conclusion that common dietary antioxidants such as vitamin E and C do not improve human exercise performance (68, 196, 302). Moreover, some authors have interpreted these experiments as evidence that ROS do not contribute to muscular fatigue during submaximal exercise in humans. This is not the case as numerous mechanistic studies using a variety of pharmacological antioxidants have clearly demonstrated that exercise-induced production of ROS in skeletal muscle contributes to muscle fatigue. For example, an early animal study using both spin traps and vitamin E demonstrated that scavenging ROS in muscle during exercise delays the onset of muscular fatigue (279). Moreover, a growing number of reports indicate that administration of the antioxidant N-acetylcysteine (NAC) delays muscle fatigue during submaximal exercise in humans (250, 257, 260, 261, 326, 391). As an antioxidant, NAC acts as a reduced thiol donor that supports glutathione resynthesis and may exhibit other antioxidant properties (91, 199). Table 3 illustrates some of the published reports indicating that NAC administration delays muscular fatigue during a variety of submaximal exercise tasks including electrically stimulated fatigue of human limb muscle (326), breathing against an inspiratory load (391), cycling exercise (257, 260, 261), and repetitive handgrip exercise (250). The improvements in exercise performance in these experiments ranged from 15 to 50% (Table 3). Indistinguishable from these positive findings in humans, NAC administration has also been shown to delay fatigue in animal experiments using both in vitro and in situ muscle preparations (94, 203, 361, 384). Nonetheless, NAC does not appear to retard muscle fatigue during exercise at near-maximal intensities (94, 250, 259).
TABLE 3.
A list of selected human studies indicating that N-acetylcysteine delays muscular fatigue during prolonged submaximal exercise
| Mode of Exercise | Subject Pool | NAC Treatment | Exercise-Dependent Measure | Performance Improvement | Reference Nos. |
|---|---|---|---|---|---|
| Cycling to fatigue | Adult male endurance athletes | Multiple iv doses before and during exercise | Time to fatigue | +24% | McKenna et al. (257) |
| Breathing against inspiratory load | Adult men | Single iv dose preexercise | Time to task failure | +50% | Travaline et al. (391) |
| Repetitive handgrip exercise | Adult men and women | Single iv dose preexercise | Time to task failure | +15% | Matuszczak et al. (250) |
| Repeated electrical stimulation of limb muscle | Adult men | Single iv dose preexercise | Force decline during 30 min of contractions | +15% | Reid et al. (326) |
iv, Intravenous.
VIII. REDOX-SENSITIVE SITES IN SKELETAL MUSCLE
The previous section highlighted the effects of ROS and NO on muscle force production and fatigue. Although it is widely stated that free radicals can damage lipids, proteins, and DNA, overt oxidant-induced damage to skeletal muscle caused by ROS or RNS appears to be relatively rare, but the functions and actions of free radicals, ROS, and RNS are more usually associated with oxidation of critical, redox-sensitive sites within skeletal muscle. This section will describe these sites commencing with those that appear to be important in the actions of ROS and NO in modifying force generation in skeletal muscle. This will be followed by an outline of the manner in which ROS and NO modify cellular signaling pathways and activate proteolytic systems that affect muscle maintenance of muscle mass.
A. Redox-Sensitive Targets in Skeletal Muscle That Impact Force Production
There are various potential mechanisms by which NO and ROS might influence skeletal muscle force production. Figure 6 illustrates putative molecular targets and processes involved in skeletal muscle contraction that are influenced by NO and/or ROS. In addition to affecting calcium regulation, and/or myofilament function, NO might theoretically impact on force production through lowering ATP production. The NOS3 isoform is closely associated with skeletal muscle mitochondria, and NO production from this site could impair muscle force production by limiting mitochondrial ATP production via inhibition of cytochrome oxidase (56, 58) or other mitochondrial enzymes (47). NO derivatives also inhibit the activity of both glyceraldehyde-3-phosphate dehydrogenase (265, 266) and creatine kinase (131) that could limit ATP production. Nonetheless, there is currently no evidence that NO limits ATP production in muscle during repetitive contractions.
FIG. 6.
Illustration of putative redox-sensitive targets in skeletal muscle that can influence force production. SOD, superoxide dismutase; NOS, nitric oxide synthase; NO, nitric oxide; SERCA, sarcoplasmic reticulum calcium ATPase. [Redrawn from Smith and Reid (368).]
A considerable amount of work has examined whether NO acts to influence force production through its usual second messenger, cGMP, or through non-cGMP-dependent processes. Both muscle contractions and NO donors increase cGMP concentration in skeletal muscle, and inhibition of NOS depresses cGMP levels (226). Immunohistochemical studies reveal that cGMP content is not fiber type specific and that cGMP is located near the subsarcolemma and in close proximity to NOS1 (286). Although it is not universally accepted (35, 50), several authors argue that interventions that increase cGMP signaling (e.g., NO donors, cGMP analogs, etc.) reduce skeletal muscle force production (1, 38, 207, 244, 245, 319). In contrast, contractile force is increased by treatments that decrease cGMP levels (e.g., NOS inhibitors and guanylate cyclase inhibitors) (207). Nonetheless, the magnitude of the cGMP-dependent changes in muscle contraction is consistently smaller than the aggregate effect of NO on muscle force production (318). Specifically, it appears that ~50% of the NO-induced depression in muscle force production is mediated via cGMP action (317). Hence, NO-induced depression of muscle force production occurs via both a cGMP-dependent and cGMP-independent mechanism (319).
Collectively, the current data suggest that the predominant effects of ROS and NO on muscle force production are due to changes in calcium handling by muscle cells or due to a change in the sensitivity of myofilaments to calcium. A discussion of the redox influence on SR calcium handling and redox modifications to myofibrillar proteins follows.
1. Redox influence on SR calcium regulation
The redox influence on SR function has been studied extensively in both cardiac and skeletal muscle (2, 3, 21, 247, 340, 409, 415, 424). The ryanodine receptor (RyR) calcium release channel in the SR is a large homotetramer composed of 565-kDa subunits; each subunit contains sulfhydryl groups that are sensitive to redox modulation (281, 380, 418, 425) and is activated by both exposure to ROS and by changes in the environmental redox status of key cellular thiols such as glutathione (424). In situ, the RyR appears to be in close association with the NADP(H) oxidase(s) found in the SR, and locally generated superoxide appears to be the major ROS capable of influencing this channel (409).
Exposure of skeletal muscle fibers and isolated SR proteins to exogenous ROS reveals that regulatory proteins of the SR calcium release channels are oxidized (2, 21, 295). In isolated systems, high levels of ROS increase the probability of opening the ryanodine-sensitive calcium release channel, resulting in increased calcium release from the SR (21, 110). In contrast, reducing agents and antioxidants have the opposite effect and retard calcium release from the SR (322). Although ROS exposure has been shown to alter the release of calcium from the SR in isolated systems, research using mechanically skinned muscle fibers suggests that the redox potential across the SR does not influence excitation-contraction coupling in rat muscle (297). Moreover, oxidizing the calcium release channels in the SR does not alter the amount of calcium released during twitch contractions (297). Andrade and colleagues (16, 19) have also investigated this issue using intact single muscle fibers. Their work suggests that exposure of fibers to ROS results in a significant increase in myoplasmic calcium levels in resting (i.e., noncontracting) muscle (16, 19). This increase in resting calcium levels in fibers appeared to reflect a slowing of SR calcium uptake rather than an increase in calcium release from the SR. Similar to the work of Posterino et al., Andrade and co-workers (16, 19) report that exposure of muscle fibers to ROS has a limited impact on SR calcium release during submaximal tetanic contractions. Therefore, based on these collective experiments, it appears that exposure of muscle fibers to ROS does not have a significant impact on myoplasmic calcium transients during both twitch and tetanic muscular contractions.
High levels of ROS also inhibit SR calcium ATPase (SERCA) activity (132, 410), but paradoxically, reductive stress inhibits SERCA function by reducing sulfhydryl groups on SERCA that are required for ATP hydrolysis (83). SERCA1 and SERCA2 proteins contain critical thiol residues and are influenced by both cellular redox status and ROS exposure. ROS inhibit SERCA function by both interfering with the ATP binding site and by uncoupling calcium uptake from ATP hydrolysis (348, 410). There is some variability in the responses of different SERCA isoforms to inhibition by ROS (37).
NO also has significant effects on SR calcium handling, although the specific target of NO/cGMP signaling remains unclear. Inhibition of soluble cGMP in isolated single muscle fibers results in increased cytosolic calcium transients during a tetanic contraction (18). A potential mechanism for the influence of NO on both skeletal and cardiac muscle is through the inhibition of phospholamban, a protein that retards SERCA activity (337, 419, 420). However, phospholamban is expressed only in cardiac and type I skeletal muscle fibers, and therefore, this mechanism cannot explain the NO/cGMP inhibition of muscle force production in fast type II fibers (275). As previously indicated, SERCA contain a small number of sulfhydryl groups that regulate SERCA activity (401, 402), and exposure to high levels of NO inhibits SERCA activity via thiol oxidation (402) and nitration of tyrosine residues (401). Overall, NO-mediated changes in SERCA sulfhydryl groups result in reduced calcium uptake into the SR and increases in cytosolic calcium levels.
NO donors have complex effects of the RyR (153), which shows a biphasic response to NO (341, 373). At low levels, NO prevents oxidative activation of the RyR by acting as an antioxidant (i.e., scavenging superoxide) without directing modifying channel function (142), but in contrast, prolonged exposure to high levels of NO inhibits ryanodine activity and retards calcium release (142, 152).
2. ROS and NO impact on myofibrillar proteins
Although it is clear that ROS and NO can modify SR calcium handling, growing evidence also indicates that ROS can also influence myofilaments structure and function. In this regard, Michael Reid and colleagues have meticulously investigated potential pathways that might be involved in modification of skeletal muscle force generation and have concluded that the most likely explanation lies in ROS influencing the calcium sensitivity of myofilaments (for a review, see Ref. 368). They based this conclusion on observations indicating that 1) high levels of ROS exposure can alter myofilament structure (60, 61, 79, 143), 2) exogenous ROS diminish calcium sensitivity of myofilaments (17, 19), 3) ROS exposure alters cross-bridge kinetics (19), and 4) muscle-derived ROS have been shown to potentiate the increasing calcium insensitivity of fatiguing muscle (267). Several myofilament proteins are oxidized during exposure to ROS, and myofilament function is impaired by prolonged exposure to high levels of ROS (70, 411). For example, myosin heavy chain molecules are targets of ROS, and oxidation of myosin heavy chain proteins promotes an impairment in myosin function (70, 132, 411). Troponin C is also sensitive to ROS-mediated oxidation, and oxidation of troponin C may alter the function of this protein (191, 296).
Studies also indicate that exposure of isolated muscle fibers to NO donors results in impaired myofilament function (150, 294). Several myofilament proteins are candidates to explain this response. Myosin heavy chains contain several sulfhydryl residues that are redox sensitive; however, modification of these thiol groups may not impair function greatly (79). In contrast, actin and tropomyosin do not appear to be redox sensitive (237, 406), and limited information exists regarding the effects of NO on other skeletal muscle contractile proteins.
3. Levels of ROS required to activate redox-sensitive targets
Note that the above discussion extensively uses terms such as “low” or “high” level of ROS, and this reflects the difficulty in designing and evaluating studies in this area. Currently, we lack reliable data to quantify ROS within cells, even where these substances are relatively stable such as in the case of hydrogen peroxide. Few studies of the hydrogen peroxide content of body fluids have been undertaken, but microdialysis studies indicate that the skeletal muscle interstitial hydrogen peroxide concentration is on the order of 10–15 μM (395). Nonmuscle cells have been shown to respond to concentrations of hydrogen peroxide of this order (15 μM), with adaptive changes in stress gene expression (202). Muscle cells have been reported to activate adaptive changes in gene expression when exposed to 100 μM extracellular hydrogen peroxide. These authors also reported that muscle cells lose viability when exposed to >1 mM hydrogen peroxide (251, 254). These data contrast with experiments performed with levels of exogenous oxidants that appear excessively high. In this regard, concentrations of hydrogen peroxide of up to 50 mM have been used to mimic the effects of the rise in ROS activities seen following contractions on components of skeletal muscle (308). Antunes and Cadenas (20) have examined the concentration difference across membranes when hydrogen peroxide is used in such situations and calculated that an approximately sevenfold drop occurs across biological membranes, implying that an intracellular concentration of 2–15 μM hydrogen peroxide may be sufficient to induce adaptive changes in gene expression dependent on the cell type under study.
Some studies with these more physiological levels of oxidant have been undertaken with, for instance, Reid and colleagues confirming effects on the calcium sensitivity of myofibrils (17, 19) and some recent data supporting the hypothesis that more physiological levels of hydrogen peroxide (10 μM) can activate the RyR in situ (282).
B. Cellular Signaling Pathways
ROS can act through several different pathways of signaling transduction including calcium-dependent pathways, protein tyrosine kinases and protein tyrosine phosphatases, serine threonine kinases, and phospholipases (169). According to Allen and Tresini (12), almost half of the ROS effects reported involve members of the mitogen-activated protein kinase (MAP kinase) and nuclear factor κ-B (NFκB) pathways, but effects are not limited to these processes. While it is also generally assumed that these effects are modulated by ROS-induced oxidation, antioxidants can also stimulate some specific gene expression pathways (12). Lander (225) proposed that the cellular responses stimulated by ROS could be classified into the following catergories: modulation of cytokine, growth factor, hormone release and action, ion transport, transcription, neuromodulation, and apoptosis. In contrast, Jackson et al. (169) concluded that the predominant effects of ROS on cells related to increasing or decreasing cell proliferation, changing immune function, and/or induction of apoptotic cell death. Unfortunately, it is currently unknown if ROS influence all of these processes in skeletal muscle as the effects of ROS on contractile properties have been the predominant area of study.
Ji (176) has extensively studied the role of ROS activation of NFκB and MAP kinases in the adaptations of muscle cells to oxidative stress and concluded that these pathways are critical cellular responses to maintain muscle homeostasis through upregulation of the expression of antioxidant enzymes and other cytoprotective proteins. The initial adaptive responses that occur in skeletal muscle following an increase in ROS activity induced by contractions appear to be in the antioxidant defense enzymes and the stress or heat shock proteins (HSPs), and increased muscle content of several of these proteins has been associated with significant protection against subsequent cellular damage (157, 251, 254). A single isometric contraction protocol in mouse muscle, which had been previously shown to increase muscle ROS generation, also increases the activity of muscle antioxidant defense enzymes such as SOD and catalase together with HSP60 and HSP70 content (253), changes which were replicated in studies of human muscle (201). Presupplementation with vitamin C (202) or other antioxidants (168) reduced these adaptive responses, supporting the possibility that these adaptations were regulated by ROS. Complimentary data from Gomez et al. (128) indicate that treatment of rats with antioxidants prevented several exercise-induced changes in skeletal muscle gene expression.
NFκB and activator protein-1 (AP-1) appear to be key transcription factors in the upregulation of antioxidant enzymes such as SOD and catalase in response to oxidative stress (169, 176, 422). HSP expression in response to acute stress in eukaryotic cells is primarily regulated by the transcription factor heat shock factor 1 (HSF1; Ref. 76). Studies have shown that the activation of this latter transcription factor is dependent on the increased production of ROS and/or the presence of oxidized or unfolded cellular proteins (114). Furthermore, evidence exists that the increased muscle production of ROS during a period of contractions results in the transient oxidation of protein sulfhydryl groups, and this oxidation is reversible within 60 min following the contraction protocol (253). Activation of NFκB, AP-1, and HSF transcription factors in skeletal muscle has been demonstrated following an isometric contraction protocol that also caused increased ROS generation and resulted in increased content of HSPs, SOD, and catalase (396).
The processes by which ROS regulate these pathways continue to be investigated, but current data suggest that site-specific oxidation of thiol groups play an important role. Cellular thiols are readily oxidized by ROS and, although they have been generally thought to play a role in protection of cells against oxidative damage, this oxidation leads to a change in the thiol-to-disulfide ratios. Site-specific changes in these ratios (also described as the local redox potential) are important in regulation of the activities of some signaling molecules and transcription factors within the cell. Particularly important in this respect is the redox status of GSH and thioredoxin (both TRx1 and TRx2) in different parts of the cell. There is evidence that thiols in different subcellular locations have differential susceptibilities to oxidation and are not in equilibrium. Thus the redox potential differs between subcellular compartments. The GSH and TRx content of mitochondria and nuclei appear to be regulated independently of cytosolic contents with, for instance, a small pool of GSH within nuclei that does not exchange freely with cytosolic GSH (39, 174, 366). Assessment of the redox potential of GSH/GSSG within organelles is complex, but a modification of western blotting allows quantification of reduced and oxidized TRx in subcellular fractions (124). TRx1 is found within the cytoplasm and nuclei of cells and TRx2 within mitochondria (124). The redox potential of the different pools of thiols have been calculated for nonmuscle cells and show that mitochondrial TRx2 is the most reduced (approximate value −340 mV), nuclear TRx1 is intermediate (approximately −300 mV), and cytosolic TRx1 is most oxidized (approximately −280 mV) (403). For GSH/GSSG, the mitochondrial redox potential has been calculated to be approximately −280 mV and the cytosolic potential to be −260 to −200 mV (141).
A detailed discussion of redox control of gene expression is beyond the scope of this review, and therefore, only a brief summary will be outlined here. For detailed reviews of this topic, the reader is referred to recent reviews focused on this topic (103, 176, 220, 238, 243, 278). Many transcription factors are redox sensitive, but NFκB has been cited as a typical redox-regulated factor and ROS have been claimed to be principal regulators of NFκB activation in many situations (268). NFκB exists in the cytoplasm as an inactive heterodimer when bound to an inhibitory subunit IκB. When activated by dissociation of IκB, NFκB migrates to the nucleus and binds to NFκB binding sites in the promoter sequences of a variety of genes. Activation by ROS appears to involve oxidation of key cysteine residues in the upstream activators of NFκB and in many situations is prevented by antioxidants or reducing agents (141). A further level of redox control is also evident within the nucleus where binding of the NFκB to DNA requires a reducing environment (187). Analogous redox control occurs for AP-1 and other transcription factors where ROS-induced oxidation stimulates cytosolic activation, but a reducing environment is required for nuclear DNA binding and activation of transcription (169).
C. Oxidants and Nonspecific Oxidative Damage to Lipids, Proteins, and DNA
Recent developments have questioned the validity of the original concept of oxidative stress, particularly due to the realization that individual signaling and control events occur through discrete redox pathways rather than through mechanisms that are directly responsive to a change in the global thiol/disulfide balance (186). Hence, measurements of individual ROS and subcellular oxidation targets may be more important than global markers of oxidative damage, but nevertheless there are numerous reports that increased ROS activity during exercise leads to increases in oxidation of markers of lipid peroxidation, protein oxidation, and DNA oxidation (see Ref. 354 for comprehensive reviews). The relationship of increases in these markers of oxidative damage to redox-modulated physiological responses of muscle to exercise is unclear as is the desirability of attempting to suppress these changes through the use of antioxidants. A schematic diagram illustrating the conceptual relationship between markers of oxidation and physiological responses is shown in Figure 7.
FIG. 7.
Hypothetical model depicting muscle responses to increasing oxidation of redox-sensitive components due to increased ROS exposure. It is envisaged that a low-level increase in ROS exposure changes the cellular redox state to initially affect redox-sensitive signaling pathways and if sufficiently large or sustained may lead to cellular damage (middle panel). It seems likely that it will not be possible to clearly demarcate the amounts of oxidation required to invoke differing responses, and hence, the boundaries between these actions are not shown as solid lines. Where in this spectrum of events traditional marker of ROS activity, such as measures of lipid, DNA, or protein oxidation, become abnormal is currently unknown (and hence these are also demarcated by dotted lines in left panel). Contractile activity is known to lead to an increase in ROS generation in muscle, but the factors that govern when this is sufficient to change the redox status and activate redox-sensitive signaling event or lead to damage are not understood (right panel). Possible factors influencing the magnitude of this response include the nature and duration of the contractile activity, the antioxidant status of the muscle, and the basal redox status prior to exercise.
D. ROS-Mediated Activation of Calpains and Caspases
Growing evidence suggests that ROS species can serve as second messengers in cellular signal transduction pathways that promote proteolysis in skeletal muscle during periods of inactivity. The first evidence that oxidants played a signaling role in the regulation of disuse muscle atrophy was provided by Kondo et al. (212). The pioneering work of Kondo and colleagues (211–215) revealed that immobilization of skeletal muscles is associated with increased ROS production resulting in oxidative injury in inactive muscle fibers and that disuse muscle atrophy could be retarded via antioxidants. These early observations have subsequently been confirmed by others(23, 42, 249), and it is now clear that oxidative stress can contribute to the rate of disuse muscle atrophy by promoting the activation of calpain and/or caspase-3 (reviewed in Refs. 305, 306). A brief summary of the redox regulation of each of these proteases follows.
1. ROS and calpain activation
Calpains are Ca2+-dependent cysteine proteases found in all mammalian cells (127). Numerous members of the calpain family exist, but the two best characterized calpains are labeled as μ-calpain and m-calpain; these terms refer to the micromolar and millimolar amounts of calcium required to activate each respective calpain isoform (127). Activation of calpain results in a release of sarcomeric proteins via the cleavage of cytoskeletal proteins (e.g., titin, nebulin) that anchor contractile elements (210, 309).
In vivo calpain activity is regulated by multiple factors including cytosolic free calcium levels and the concentration of the endogenous calpain inhibitor calpastatin (127). In this regard, calpain activity can be elevated by a sustained increase in free calcium in the cytosol and/or a decrease in calpastatin levels (127). In reference to calpain during disuse muscle atrophy, it is clear that skeletal muscle inactivity promotes an increase in both cytosolic calcium levels and calpain activation (214, 241, 357, 379, 387). Although the mechanism responsible for this inactivity-mediated calcium overload is unknown, it is feasible that intracellular production of ROS plays a key role in calcium dyshomeostasis. A potential mechanism to link oxidative stress with calcium overload is that ROS-induced formation of reactive aldehydes (i.e., 4-hydroxy-2,3-trans-nonenal) have been reported to inhibit plasma membrane Ca2+-ATPase activity (362). Hence, an oxidative stress-induced decrease in Ca2+-ATPase activity would hinder Ca2+removal from the cell and encourage intracellular Ca2+accumulation. However, it is currently unknown as to whether this mechanism is the solitary explanation for inactivity-mediated calcium overload in muscle as numerous other possibilities exist (218).
2. ROS regulation of caspase-3 activity
Accumulating evidence indicates that caspase-3 contributes to protein degradation and muscle atrophy (104, 255). More specifically, caspase-3 activation promotes degradation of actomyosin complexes, and inhibition of caspase-3 activity suppresses the overall rate of skeletal muscle proteolysis in diabetes-mediated cachexia and diaphragmatic atrophy following prolonged periods of mechanical ventilation (104, 255).
Control of caspase-3 activity in the cell is complex and involves numerous interconnected signaling pathways. In the case of diabetes-induced muscle atrophy, it seems possible that caspase-3 is activated by caspase-12 (via a calcium release pathway) and/or activation of caspase-9 (via a mitochondrial pathway) (104). A potential interaction between these caspase-3 activation pathways is that both of these can be activated by ROS (306).
IX. SUMMARY AND FUTURE DIRECTIONS
The first evidence that muscular exercise promotes oxidative damage in tissues appeared in 1978. Since this first report, the field of redox biology has evolved significantly, and our understanding of the sources and consequences of exercise-induced free radical production has advanced markedly. Current evidence suggests that contracting muscles produce oxidants from a variety of cellular locations. Furthermore, although mitochondria are a potential source of ROS in cells, growing evidence suggests that these organelles may play a less prominent role in oxidant production in contracting skeletal muscles than was previously thought.
Many early studies investigating exercise and free radical production focused on the damaging effects of oxidants in muscle (e.g., lipid peroxidation). However, a new era in redox biology exists today with an ever-growing number of reports detailing the advantageous biological effects of free radicals. Indeed, it is now clear that ROS and RNS are involved in modulation of cell signaling pathways and the control of numerous redox-sensitive transcription factors. Furthermore, physiological levels of ROS are essential for optimal force production in skeletal muscle. Nonetheless, high levels of ROS promote skeletal muscle contractile dysfunction resulting in muscle fatigue.
While the field of redox biology has advanced rapidly during the past two decades, many questions remain unanswered. Therefore, in an effort to simulate additional research in this field, the remainder of this section will focus on the identification of important redox topics that warrant additional work.
First, we believe that the lack of any true quantitative measures to assess the redox status of muscle cells and organelles is a considerable hindrance to investigators and is an important area where developments are required. Moreover, the development of sensitive and reliable techniques to assess the redox status of individual proteins will be required for scientists to identify specific factors responsible for redox-regulating processes in the cell.
Another technical limitation in the field of redox biology is the lack of tools available to accurately measure (and hence compare) the production of specific radicals in living cells or organelles in vivo. That is, at present, no techniques exist to quantify the specific rate of radical production in cell compartments. While it has been possible for the field to develop thus far using indirect measures of ROS activity, a full evaluation of the generation of specific ROS and redox-regulated responses will only be achieved with the development of new analytical approaches. Current approaches limit research progress in many areas of redox biology including the study of radical production in contracting skeletal muscle fibers. Some new developments in this area offer promise including novel probes for specific ROS, such as HyPer, a genetically encoded probe that can be transfected into mammalian cells and in which yellow fluorescent protein is inserted into the regulatory domain of the prokaryotic hydrogen peroxide sensing protein OxyR (40). This probe may be specifically targeted to organelles, such as mitochondria. A complementary approach in which a common ROS probe, dihydroethidium, has been linked through a hexyl carbon chain to a triphenylphosphonium group to produce a compound (known as MitoSOX or Mito-HE) that accumulates in mitochondria also holds considerable promise (332). New methods are evolving to measure the oxidation of specific thiols in various cellular compartments. With the use of these tools, emerging evidence suggests that changes in redox status within cells may be compartmentalized and that this may serve as a mechanism for regulating specific redox signaling pathways within cells. For example, it is feasible that both mitochondrial and nuclear compartmentalization of redox signaling exists, and this could be important in exercise-induced changes in redox signaling in muscle fibers (140). This is a critical topic for future work because improving our understanding of redox compartmentalization in cells may provide key insight into redox signaling in muscle fibers. In a related area, improving our understanding of the nature of redox-sensitive targets in skeletal muscle is important. It seems highly unlikely that the various potential targets in cells show an equivalent sensitivity to specific ROS and thus there is likely to be a hierarchy for oxidation and functional change that is dependent on factors such as their location compared with the site of generation of the radical species, the nature of the oxidizing species, the local concentration of antioxidants, etc. Knowledge of these processes would undoubtedly aid future growth in this field.
Another important area for further work, which arises due to the increasing use of cell culture and isolated tissue models over the last 25 years, is the effect of the in vitro environment on ROS generation and responses in cells and tissues. This has not been widely studied, but cell culture models are generally undertaken at ~20% environmental oxygen, and isolated muscle tissue models use protocols that involve predominantly bubbling of bathing solutions with 95% oxygen-5% carbon dioxide, although the oxygen environment of muscle cells at rest in vivo is much less oxygen rich and appears to be between 2 and 10% (81, 367). Although limited studies exist, Csete and colleagues (80, 81, 270) propose that high oxygen tensions modify muscle cell fate and phenotype through processes that involve increased ROS generation in the oxygen-rich environments. Clearly, this is an important issue that warrants additional research to determine if muscle cell lines that are maintained at high oxygen tensions develop phenotypes that influence experimental outcomes.
The development of targeted antioxidants remains an important area of research in redox biology. Indeed, the development of a highly effective and targeted antioxidant could provide researchers with a sensitive antioxidant probe to explore mechanistic questions in this field. For example, using a mitochondrial targeted antioxidant, researchers could study specific mitochondrial ROS production and its consequences using organelle specific antioxidants (5, 57, 274, 369).
Increased use of genetically modified experimental animals (e.g., conditional and tissue specific knockout and overexpressers) could prove to be a valuable tool to scientists working on redox-related problems. Also, the use of gene silencing tools (e.g., siRNA) in studies of redox biology provides a unique opportunity for investigators to study the impact of a specific gene product in cells. Allied to this area is the use of experimental animals or cells that are genetically modified to provide a technique to monitor the activity of a specific molecular process in vivo or in vitro. The best known of these are the NFκB reporter mice that are modified to express luciferase when NFκB is activated, such as occurs following ROS exposure. Models such as this provide a new opportunity to study key signaling processes in vivo. This approach has already been utilized to examine the effect of antioxidants on NFκB activation in vivo (271), but the relevance to exercise does not appear to have been examined. There is clearly much more to be learned about this exciting field.
References
- 1.Abraham RZ, Kobzik L, Moody MR, Reid MB, Stamler JS. Cyclic GMP is a second messenger by which nitric oxide inhibits diaphragm contraction. Comp Biochem Physiol A Mol Integr Physiol. 1998;119:177–183. doi: 10.1016/s1095-6433(97)00421-2. [DOI] [PubMed] [Google Scholar]
- 2.Abramson JJ, Salama G. Critical sulfhydryls regulate calcium release from sarcoplasmic reticulum. J Bioenerg Biomembr. 1989;21:283–294. doi: 10.1007/BF00812073. [DOI] [PubMed] [Google Scholar]
- 3.Abramson JJ, Zable AC, Favero TG, Salama G. Thimerosal interacts with the Ca2+ release channel ryanodine receptor from skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1995;270:29644–29647. doi: 10.1074/jbc.270.50.29644. [DOI] [PubMed] [Google Scholar]
- 4.Adhihetty PJ, Ljubicic V, Menzies KJ, Hood DA. Differential susceptibility of subsarcolemmal and intermyofibrillar mitochondria to apoptotic stimuli. Am J Physiol Cell Physiol. 2005;289:C994–C1001. doi: 10.1152/ajpcell.00031.2005. [DOI] [PubMed] [Google Scholar]
- 5.Adlam VJ, Harrison JC, Porteous CM, James AM, Smith RA, Murphy MP, Sammut IA. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB J. 2005;19:1088–1095. doi: 10.1096/fj.05-3718com. [DOI] [PubMed] [Google Scholar]
- 6.Albanes D, Heinonen OP, Taylor PR, Virtamo J, Edwards BK, Rautalahti M, Hartman AM, Palmgren J, Freedman LS, Haapakoski J, Barrett MJ, Pietinen P, Malila N, Tala E, Liippo K, Salomaa ER, Tangrea JA, Teppo L, Askin FB, Taskinen E, Erozan Y, Greenwald P, Huttunen JK. α-Tocopherol and beta-carotene supplements and lung cancer incidence in the alpha-tocopherol, beta-carotene cancer prevention study: effects of baseline characteristics and study compliance. J Natl Cancer Inst. 1996;88:1560–1570. doi: 10.1093/jnci/88.21.1560. [DOI] [PubMed] [Google Scholar]
- 7.Albertini M, Lafortuna C, Aguggini G. Effects of nitric oxide on diaphragmatic muscle endurance and strength in pigs. Exp Physiol. 1997;82:99–106. doi: 10.1113/expphysiol.1997.sp004018. [DOI] [PubMed] [Google Scholar]
- 8.Aldred S. Oxidative and nitrative changes seen in lipoproteins following exercise. Atherosclerosis. 2007;192:1–8. doi: 10.1016/j.atherosclerosis.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Alessio HM, Goldfarb AH. Lipid peroxidation and scavenger enzymes during exercise: adaptive response to training. J Appl Physiol. 1988;64:1333–1336. doi: 10.1152/jappl.1988.64.4.1333. [DOI] [PubMed] [Google Scholar]
- 10.Alessio HM, Goldfarb AH, Cutler RG. MDA content increases in fast- and slow-twitch skeletal muscle with intensity of exercise in a rat. Am J Physiol Cell Physiol. 1988;255:C874–C877. doi: 10.1152/ajpcell.1988.255.6.C874. [DOI] [PubMed] [Google Scholar]
- 11.Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008;88:287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- 12.Allen RG, Tresini M. Oxidative stress and gene regulation. Free Radic Biol Med. 2000;28:463–499. doi: 10.1016/s0891-5849(99)00242-7. [DOI] [PubMed] [Google Scholar]
- 13.Ameredes BT, Provenzano MA. Influence of nitric oxide on vascular resistance and muscle mechanics during tetanic contractions in situ. J Appl Physiol. 1999;87:142–151. doi: 10.1152/jappl.1999.87.1.142. [DOI] [PubMed] [Google Scholar]
- 14.Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci USA. 1981;78:6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson EJ, Neufer PD. Type II skeletal myofibers possess unique properties that potentiate mitochondrial H2O2 generation. Am J Physiol Cell Physiol. 2006;290:C844–C851. doi: 10.1152/ajpcell.00402.2005. [DOI] [PubMed] [Google Scholar]
- 16.Andrade FH, Reid MB, Allen DG, Westerblad H. Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J Physiol. 1998;509:565–575. doi: 10.1111/j.1469-7793.1998.565bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrade FH, Reid MB, Allen DG, Westerblad H. Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J Physiol. 1998;509:565–575. doi: 10.1111/j.1469-7793.1998.565bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrade FH, Reid MB, Allen DG, Westerblad H. Effect of nitric oxide on single skeletal muscle fibres from the mouse. J Physiol. 1998;509:577–586. doi: 10.1111/j.1469-7793.1998.577bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrade FH, Reid MB, Westerblad H. Contractile response of skeletal muscle to low peroxide concentrations: myofibrillar calcium sensitivity as a likely target for redox-modulation. FASEB J. 2001;15:309–311. doi: 10.1096/fj.00-0507fje. [DOI] [PubMed] [Google Scholar]
- 20.Antunes F, Cadenas E. Estimation of H2O2 gradients across biomembranes. FEBS Lett. 2000;475:121–126. doi: 10.1016/s0014-5793(00)01638-0. [DOI] [PubMed] [Google Scholar]
- 21.Anzai K, Ogawa K, Ozawa T, Yamamoto H. Oxidative modification of ion channel activity of ryanodine receptor. Antioxid Redox Signal. 2000;2:35–40. doi: 10.1089/ars.2000.2.1-35. [DOI] [PubMed] [Google Scholar]
- 22.Anzueto A, Andrade FH, Maxwell LC, Levine SM, Lawrence RA, Gibbons WJ, Jenkinson SG. Resistive breathing activates the glutathione redox cycle and impairs performance of rat diaphragm. J Appl Physiol. 1992;72:529–534. doi: 10.1152/jappl.1992.72.2.529. [DOI] [PubMed] [Google Scholar]
- 23.Appell HJ, Duarte JA, Soares JM. Supplementation of vitamin E may attenuate skeletal muscle immobilization atrophy. Int J Sports Med. 1997;18:157–160. doi: 10.1055/s-2007-972612. [DOI] [PubMed] [Google Scholar]
- 24.Arend A, Zilmer M, Vihalemm T, Selstam G, Sepp E. Lipoic acid prevents suppression of connective tissue proliferation in the rat liver induced by n-3 PUFAs. A pilot study. Ann Nutr Metab. 2000;44:217–222. doi: 10.1159/000046687. [DOI] [PubMed] [Google Scholar]
- 25.Arner ES, Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem. 2000;267:6102–6109. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- 26.Ashton T, Rowlands CC, Jones E, Young IS, Jackson SK, Davies B, Peters JR. Electron spin resonance spectroscopic detection of oxygen-centred radicals in human serum following exhaustive exercise. Eur J Appl Physiol Occup Physiol. 1998;77:498–502. doi: 10.1007/s004210050366. [DOI] [PubMed] [Google Scholar]
- 27.Atalay M, Lappalainen J, Sen CK. Dietary antioxidants for the athlete. Curr Sports Med Rep. 2006;5:182–186. doi: 10.1097/01.csmr.0000306504.71105.6e. [DOI] [PubMed] [Google Scholar]
- 28.Avery NG, Kaiser JL, Sharman MJ, Scheett TP, Barnes DM, Gomez AL, Kraemer WJ, Volek JS. Effects of vitamin E supplementation on recovery from repeated bouts of resistance exercise. J Strength Cond Res. 2003;17:801–809. doi: 10.1519/1533-4287(2003)017<0801:eoveso>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 29.Azzi A, Davies KJA, Kelly F. Free radical biology: terminology and critical thinking. FEBS Lett. 2004;558:3–6. doi: 10.1016/s0014-5793(03)01526-6. [DOI] [PubMed] [Google Scholar]
- 30.Azzi A, Gysin R, Kempna P, Munteanu A, Villacorta L, Visarius T, Zingg JM. Regulation of gene expression by alpha-tocopherol. Biol Chem. 2004;385:585–591. doi: 10.1515/BC.2004.072. [DOI] [PubMed] [Google Scholar]
- 31.Azzi A, Gysin R, Kempna P, Ricciarelli R, Villacorta L, Visarius T, Zingg JM. The role of alpha-tocopherol in preventing disease: from epidemiology to molecular events. Mol Aspects Med. 2003;24:325–336. doi: 10.1016/s0098-2997(03)00028-1. [DOI] [PubMed] [Google Scholar]
- 32.Balon TW, Nadler JL. Nitric oxide release is present from incubated skeletal muscle preparations. J Appl Physiol. 1994;77:2519–2521. doi: 10.1152/jappl.1994.77.6.2519. [DOI] [PubMed] [Google Scholar]
- 33.Baranano DE, Rao M, Ferris CD, Snyder SH. Biliverdin reductase: a major physiologic cytoprotectant. Proc Natl Acad Sci USA. 2002;99:16093–16098. doi: 10.1073/pnas.252626999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barclay JK, Hansel M. Free radicals may contribute to oxidative skeletal muscle fatigue. Can J Physiol Pharmacol. 1991;69:279–284. doi: 10.1139/y91-043. [DOI] [PubMed] [Google Scholar]
- 35.Barclay JK, Reading SA, Murrant CL, Woodley NE. Inotropic effects on mammalian skeletal muscle change with contraction frequency. Can J Physiol Pharmacol. 2003;81:753–758. doi: 10.1139/y03-031. [DOI] [PubMed] [Google Scholar]
- 36.Barja G. Mitochondrial oxygen radical generation and leak: sites of production in states 4 and 3, organ specificity, relation to aging and longevity. J Bioenerg Biomembr. 1999;31:347–366. doi: 10.1023/a:1005427919188. [DOI] [PubMed] [Google Scholar]
- 37.Barnes KA, Samson SE, Grover AK. Sarco/endoplasmic reticulum Ca2+-pump isoform SERCA3a is more resistant to superoxide damage than SERCA2b. Mol Cell Biochem. 2000;203:17–21. doi: 10.1023/a:1007053802481. [DOI] [PubMed] [Google Scholar]
- 38.Belia S, Pietrangelo T, Fulle S, Menchetti G, Cecchini E, Felaco M, Vecchiet J, Fano G. Sodium nitroprusside, a NO donor, modifies Ca2+ transport and mechanical properties in frog skeletal muscle. J Muscle Res Cell Motil. 1998;19:865–876. doi: 10.1023/a:1005499606155. [DOI] [PubMed] [Google Scholar]
- 39.Bellomo G, Vairetti M, Stivala L, Mirabelli F, Richelmi P, Orrenius S. Demonstration of nuclear compartmentalization of glutathione in hepatocytes. Proc Natl Acad Sci USA. 1992;89:4412–4416. doi: 10.1073/pnas.89.10.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belousov VV, Fradkov AF, Lukyanov KA, Staroverov DB, Shakhbazov KS, Terskikh AV, Lukyanov S. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat Methods. 2006;3:281–286. doi: 10.1038/nmeth866. [DOI] [PubMed] [Google Scholar]
- 41.Berndt C, Lillig CH, Holmgren A. Thiol-based mechanisms of the thioredoxin and glutaredoxin systems: implications for diseases in the cardiovascular system. Am J Physiol Heart Circ Physiol. 2007;292:H1227–H1236. doi: 10.1152/ajpheart.01162.2006. [DOI] [PubMed] [Google Scholar]
- 42.Betters JL, Criswell DS, Shanely RA, Van Gammeren D, Falk D, Deruisseau KC, Deering M, Yimlamai T, Powers SK. Trolox attenuates mechanical ventilation-induced diaphragmatic dysfunction and proteolysis. Am J Respir Crit Care Med. 2004;170:1179–1184. doi: 10.1164/rccm.200407-939OC. [DOI] [PubMed] [Google Scholar]
- 43.Bjornstedt M, Kumar S, Bjorkhem L, Spyrou G, Holmgren A. Selenium and the thioredoxin and glutaredoxin systems. Biomed Environ Sci. 1997;10:271–279. [PubMed] [Google Scholar]
- 44.Bjornstedt M, Xue J, Huang W, Akesson B, Holmgren A. The thioredoxin and glutaredoxin systems are efficient electron donors to human plasma glutathione peroxidase. J Biol Chem. 1994;269:29382–29384. [PubMed] [Google Scholar]
- 45.Blair SN, Cheng Y, Holder JS. Is physical activity or physical fitness more important in defining health benefits? Med Sci Sports Exerc. 2001;33:S379–399. doi: 10.1097/00005768-200106001-00007. [DOI] [PubMed] [Google Scholar]
- 46.Block F, Schwarz M. Effects of antioxidants on ischemic retinal dysfunction. Exp Eye Res. 1997;64:559–564. doi: 10.1006/exer.1996.0244. [DOI] [PubMed] [Google Scholar]
- 47.Bolanos JP, Peuchen S, Heales SJ, Land JM, Clark JB. Nitric oxide-mediated inhibition of the mitochondrial respiratory chain in cultured astrocytes. J Neurochem. 1994;63:910–916. doi: 10.1046/j.1471-4159.1994.63030910.x. [DOI] [PubMed] [Google Scholar]
- 48.Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bowles DK, Torgan CE, Ebner S, Kehrer JP, Ivy JL, Starnes JW. Effects of acute, submaximal exercise on skeletal muscle vitamin E. Free Radic Res Commun. 1991;14:139–143. doi: 10.3109/10715769109094126. [DOI] [PubMed] [Google Scholar]
- 50.Bowman WC, Lam FY, Rodger IW, Shahid M. Cyclic nucleotides and contractility of isolated soleus muscle. Br J Pharmacol. 1985;84:259–264. [PMC free article] [PubMed] [Google Scholar]
- 51.Brady PS, Brady LJ, Ullrey DE. Selenium, vitamin E and the response to swimming stress in the rat. J Nutr. 1979;109:1103–1109. doi: 10.1093/jn/109.6.1103. [DOI] [PubMed] [Google Scholar]
- 52.Brand MD, Affourtit C, Esteves TC, Green K, Lambert AJ, Miwa S, Pakay JL, Parker N. Mitochondrial superoxide: production, biological effects, activation of uncoupling proteins. Free Radic Biol Med. 2004;37:755–767. doi: 10.1016/j.freeradbiomed.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 53.Brand MD, Esteves TC. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2005;2:85–93. doi: 10.1016/j.cmet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 54.Brigelius-Flohe R. Glutathione peroxidases and redox-regulated transcription factors. Biol Chem. 2006;387:1329–1335. doi: 10.1515/BC.2006.166. [DOI] [PubMed] [Google Scholar]
- 55.Brigelius-Flohe R. Tissue-specific functions of individual glutathione peroxidases. Free Radic Biol Med. 1999;27:951–965. doi: 10.1016/s0891-5849(99)00173-2. [DOI] [PubMed] [Google Scholar]
- 56.Brown GC. Nitric oxide regulates mitochondrial respiration and cell functions by inhibiting cytochrome oxidase. FEBS Lett. 1995;369:136–139. doi: 10.1016/0014-5793(95)00763-y. [DOI] [PubMed] [Google Scholar]
- 57.Brown SE, Ross MF, Sanjuan-Pla A, Manas AR, Smith RA, Murphy MP. Targeting lipoic acid to mitochondria: synthesis and characterization of a triphenylphosphonium-conjugated alpha-lipoyl derivative. Free Radic Biol Med. 2007;42:1766–1780. doi: 10.1016/j.freeradbiomed.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 58.Brunori M, Giuffre A, Forte E, Mastronicola D, Barone MC, Sarti P. Control of cytochrome c oxidase activity by nitric oxide. Biochim Biophys Acta. 2004;1655:365–371. doi: 10.1016/j.bbabio.2003.06.008. [DOI] [PubMed] [Google Scholar]
- 59.Bryant RJ, Ryder J, Martino P, Kim J, Craig BW. Effects of vitamin E and C supplementation either alone or in combination on exercise-induced lipid peroxidation in trained cyclists. J Strength Cond Res. 2003;17:792–800. doi: 10.1519/1533-4287(2003)017<0792:eoveac>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 60.Burke M, Reisler F, Harrington WF. Effect of bridging the two essential thiols of myosin on its spectral and actin-binding properties. Biochemistry. 1976;15:1923–1927. doi: 10.1021/bi00654a020. [DOI] [PubMed] [Google Scholar]
- 61.Callahan LA, Nethery D, Stofan D, DiMarco A, Supinski G. Free radical-induced contractile protein dysfunction in endotoxin-induced sepsis. Am J Respir Cell Mol Biol. 2001;24:210–217. doi: 10.1165/ajrcmb.24.2.4075. [DOI] [PubMed] [Google Scholar]
- 62.Cao X, Phillis JW. The free radical scavenger, alpha-lipoic acid, protects against cerebral ischemia-reperfusion injury in gerbils. Free Radic Res. 1995;23:365–370. doi: 10.3109/10715769509065257. [DOI] [PubMed] [Google Scholar]
- 63.Carr A, Frei B. Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J. 1999;13:1007–1024. doi: 10.1096/fasebj.13.9.1007. [DOI] [PubMed] [Google Scholar]
- 64.Chelikani P, Fita I, Loewen PC. Diversity of structures and properties among catalases. Cell Mol Life Sci. 2004;61:192–208. doi: 10.1007/s00018-003-3206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen LE, Seaber AV, Nasser RM, Stamler JS, Urbaniak JR. Effects of S-nitroso-N-acetylcysteine on contractile function of reperfused skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 1998;274:R822–R829. doi: 10.1152/ajpregu.1998.274.3.R822. [DOI] [PubMed] [Google Scholar]
- 66.Cherednichenko G, Zima AV, Feng W, Schaefer S, Blatter LA, Pessah IN. NADH oxidase activity of rat cardiac sarcoplasmic reticulum regulates calcium-induced calcium release. Circ Res. 2004;94:478–486. doi: 10.1161/01.RES.0000115554.65513.7C. [DOI] [PubMed] [Google Scholar]
- 67.Clanton TL, Zuo L, Klawitter P. Oxidants and skeletal muscle function: physiologic and pathophysiologic implications. Proc Soc Exp Biol Med. 1999;222:253–262. doi: 10.1046/j.1525-1373.1999.d01-142.x. [DOI] [PubMed] [Google Scholar]
- 68.Clarkson PM. Antioxidants and physical performance. Crit Rev Food Sci Nutr. 1995;35:131–141. doi: 10.1080/10408399509527692. [DOI] [PubMed] [Google Scholar]
- 69.Close GL, Ashton T, McArdle A, Jackson MJ. Microdialysis studies of extracellular reactive oxygen species in skeletal muscle: factors influencing the reduction of cytochrome c and hydroxylation of salicylate. Free Radic Biol Med. 2005;39:1460–1467. doi: 10.1016/j.freeradbiomed.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 70.Coirault C, Guellich A, Barbry T, Samuel JL, Riou B, Lecarpentier Y. Oxidative stress of myosin contributes to skeletal muscle dysfunction in rats with chronic heart failure. Am J Physiol Heart Circ Physiol. 2007;292:H1009–H1017. doi: 10.1152/ajpheart.00438.2006. [DOI] [PubMed] [Google Scholar]
- 71.Commoner B, Townsend J, Pake GE. Free radicals in biological materials. Nature. 1954;174:689–691. doi: 10.1038/174689a0. [DOI] [PubMed] [Google Scholar]
- 72.Coombes JS, Powers SK, Hamilton KL, Demirel HA, Shanely RA, Zergeroglu MA, Sen CK, Packer L, Ji LL. Improved cardiac performance after ischemia in aged rats supplemented with vitamin E and alpha-lipoic acid. Am J Physiol Regul Integr Comp Physiol. 2000;279:R2149–R2155. doi: 10.1152/ajpregu.2000.279.6.R2149. [DOI] [PubMed] [Google Scholar]
- 73.Coombes JS, Powers SK, Rowell B, Hamilton KL, Dodd SL, Shanely RA, Sen CK, Packer L. Effects of vitamin E and alpha-lipoic acid on skeletal muscle contractile properties. J Appl Physiol. 2001;90:1424–1430. doi: 10.1152/jappl.2001.90.4.1424. [DOI] [PubMed] [Google Scholar]
- 74.Coombes JS, Rowell B, Dodd SL, Demirel HA, Naito H, Shanely RA, Powers SK. Effects of vitamin E deficiency on fatigue and muscle contractile properties. Eur J Appl Physiol. 2002;87:272–277. doi: 10.1007/s00421-002-0631-3. [DOI] [PubMed] [Google Scholar]
- 75.Cooper CE, Vollaard NB, Choueiri T, Wilson MT. Exercise, free radicals and oxidative stress. Biochem Soc Trans. 2002;30:280–285. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 76.Cotto JJ, Morimoto RI. Stress-induced activation of the heat-shock response: cell and molecular biology of heat-shock factors. Biochem Soc Symp. 1999;64:105–118. [PubMed] [Google Scholar]
- 77.Crespo CJ, Palmieri MR, Perdomo RP, McGee DL, Smit E, Sempos CT, Lee IM, Sorlie PD. The relationship of physical activity and body weight with all-cause mortality: results from the Puerto Rico Heart Health Program. Ann Epidemiol. 2002;12:543–552. doi: 10.1016/s1047-2797(01)00296-4. [DOI] [PubMed] [Google Scholar]
- 78.Criswell D, Powers S, Dodd S, Lawler J, Edwards W, Renshler K, Grinton S. High intensity training-induced changes in skeletal muscle antioxidant enzyme activity. Med Sci Sports Exerc. 1993;25:1135–1140. [PubMed] [Google Scholar]
- 79.Crowder MS, Cooke R. The effect of myosin sulphydryl modification on the mechanics of fibre contraction. J Muscle Res Cell Motil. 1984;5:131–146. doi: 10.1007/BF00712152. [DOI] [PubMed] [Google Scholar]
- 80.Csete M. Oxygen in the cultivation of stem cells. Ann NY Acad Sci. 2005;1049:1–8. doi: 10.1196/annals.1334.001. [DOI] [PubMed] [Google Scholar]
- 81.Csete M, Walikonis J, Slawny N, Wei Y, Korsnes S, Doyle JC, Wold B. Oxygen-mediated regulation of skeletal muscle satellite cell proliferation and adipogenesis in culture. J Cell Physiol. 2001;189:189–196. doi: 10.1002/jcp.10016. [DOI] [PubMed] [Google Scholar]
- 82.Culotta VC, Yang M, O’Halloran TV. Activation of superoxide dismutases: putting the metal to the pedal. Biochim Biophys Acta. 2006;1763:747–758. doi: 10.1016/j.bbamcr.2006.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Daiho T, Kanazawa T. Reduction of disulfide bonds in sarcoplasmic reticulum Ca2+-ATPase by dithiothreitol causes inhibition of phosphoenzyme isomerization in catalytic cycle. This reduction requires binding of both purine nucleotide and Ca2+ to enzyme. J Biol Chem. 1994;269:11060–11064. [PubMed] [Google Scholar]
- 84.Daneryd P, Aberg F, Dallner G, Ernster L, Schersten T, Soussi B. Coenzymes Q9 and Q10 in skeletal and cardiac muscle in tumour-bearing exercising rats. Eur J Cancer. 1995;31A:760–765. doi: 10.1016/0959-8049(95)00086-x. [DOI] [PubMed] [Google Scholar]
- 85.Davies KJ, Maguire JJ, Brooks GA, Dallman PR, Packer L. Muscle mitochondrial bioenergetics, oxygen supply, work capacity during dietary iron deficiency and repletion. Am J Physiol Endocrinol Metab. 1982;242:E418–E427. doi: 10.1152/ajpendo.1982.242.6.E418. [DOI] [PubMed] [Google Scholar]
- 86.Davies KJ, Quintanilha AT, Brooks GA, Packer L. Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun. 1982;107:1198–1205. doi: 10.1016/s0006-291x(82)80124-1. [DOI] [PubMed] [Google Scholar]
- 87.Davies KJ, Sevanian A, Muakkassah-Kelly SF, Hochstein P. Uric acid-iron ion complexes. A new aspect of the antioxidant functions of uric acid. Biochem J. 1986;235:747–754. doi: 10.1042/bj2350747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Davies MJ, Fu S, Wang H, Dean RT. Stable markers of oxidant damage to proteins and their application in the study of human disease. Free Radic Biol Med. 1999;27:1151–1163. doi: 10.1016/s0891-5849(99)00206-3. [DOI] [PubMed] [Google Scholar]
- 89.De Grey AD. A hypothesis for the minimal overall structure of the mammalian plasma membrane redox system. Protoplasma. 2003;221:3–9. doi: 10.1007/s00709-002-0061-4. [DOI] [PubMed] [Google Scholar]
- 90.De la Fuente M, Hernanz A, Vallejo MC. The immune system in the oxidative stress conditions of aging and hypertension: favorable effects of antioxidants and physical exercise. Antioxid Redox Signal. 2005;7:1356–1366. doi: 10.1089/ars.2005.7.1356. [DOI] [PubMed] [Google Scholar]
- 91.Dekhuijzen PN. Antioxidant properties of N-acetylcysteine: their relevance in relation to chronic obstructive pulmonary disease. Eur Respir J. 2004;23:629–636. doi: 10.1183/09031936.04.00016804. [DOI] [PubMed] [Google Scholar]
- 92.Dekkers JC, van Doornen LJ, Kemper HC. The role of antioxidant vitamins and enzymes in the prevention of exercise-induced muscle damage. Sports Med. 1996;21:213–238. doi: 10.2165/00007256-199621030-00005. [DOI] [PubMed] [Google Scholar]
- 93.Di Meo S, Venditti P. Mitochondria in exercise-induced oxidative stress. Biol Signals Recept. 2001;10:125–140. doi: 10.1159/000046880. [DOI] [PubMed] [Google Scholar]
- 94.Diaz PT, Brownstein E, Clanton TL. Effects of N-acetylcysteine on in vitro diaphragm function are temperature dependent. J Appl Physiol. 1994;77:2434–2439. doi: 10.1152/jappl.1994.77.5.2434. [DOI] [PubMed] [Google Scholar]
- 95.Diaz PT, Costanza MJ, Wright VP, Julian MW, Diaz JA, Clanton TL. Dithiothreitol improves recovery from in vitro diaphragm fatigue. Med Sci Sports Exerc. 1998;30:421–426. doi: 10.1097/00005768-199803000-00013. [DOI] [PubMed] [Google Scholar]
- 96.Diaz PT, She ZW, Davis WB, Clanton TL. Hydroxylation of salicylate by the in vitro diaphragm: evidence for hydroxyl radical production during fatigue. J Appl Physiol. 1993;75:540–545. doi: 10.1152/jappl.1993.75.2.540. [DOI] [PubMed] [Google Scholar]
- 97.Dickinson DA, Forman HJ. Glutathione in defense and signaling: lessons from a small thiol. Ann NY Acad Sci. 2002;973:488–504. doi: 10.1111/j.1749-6632.2002.tb04690.x. [DOI] [PubMed] [Google Scholar]
- 98.Dillard CJ, Litov RE, Savin WM, Dumelin EE, Tappel AL. Effects of exercise, vitamin E, ozone on pulmonary function and lipid peroxidation. J Appl Physiol. 1978;45:927–932. doi: 10.1152/jappl.1978.45.6.927. [DOI] [PubMed] [Google Scholar]
- 99.Diplock AT. Introduction: markers of oxidative damage and antioxidant modulation. Free Radic Res. 2000;33(Suppl):S21–S26. [PubMed] [Google Scholar]
- 100.Draper HH, McGirr LG, Hadley M. The metabolism of malondialdehyde. Lipids. 1986;21:305–307. doi: 10.1007/BF02536418. [DOI] [PubMed] [Google Scholar]
- 101.Drevet JR. The antioxidant glutathione peroxidase family and spermatozoa: a complex story. Mol Cell Endocrinol. 2006;250:70–79. doi: 10.1016/j.mce.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 102.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 103.Droge W. Redox regulation in anabolic and catabolic processes. Curr Opin Clin Nutr Metab Care. 2006;9:190–195. doi: 10.1097/01.mco.0000222098.98514.40. [DOI] [PubMed] [Google Scholar]
- 104.Du J, Wang X, Miereles C, Bailey JL, Debigare R, Zheng B, Price SR, Mitch WE. Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J Clin Invest. 2004;113:115–123. doi: 10.1172/JCI200418330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Duncan CJ. Mechanisms that produce rapid damage to myofilaments of amphibian skeletal muscle. Muscle Nerve. 1989;12:210–218. doi: 10.1002/mus.880120309. [DOI] [PubMed] [Google Scholar]
- 106.Duntas LH. Oxidants, antioxidants in physical exercise and relation to thyroid function. Horm Metab Res. 2005;37:572–576. doi: 10.1055/s-2005-870425. [DOI] [PubMed] [Google Scholar]
- 107.Duthie GG, Robertson JD, Maughan RJ, Morrice PC. Blood antioxidant status and erythrocyte lipid peroxidation following distance running. Arch Biochem Biophys. 1990;282:78–83. doi: 10.1016/0003-9861(90)90089-h. [DOI] [PubMed] [Google Scholar]
- 108.El-Agamey A, Lowe GM, McGarvey DJ, Mortensen A, Phillip DM, Truscott TG, Young AJ. Carotenoid radical chemistry and antioxidant/pro-oxidant properties. Arch Biochem Biophys. 2004;430:37–48. doi: 10.1016/j.abb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 109.Espinosa A, Leiva A, Pena M, Muller M, Debandi A, Hidalgo C, Carrasco MA, Jaimovich E. Myotube depolarization generates reactive oxygen species through NAD(P)H oxidase; ROS-elicited Ca2+ stimulates ERK, CREB, early genes. J Cell Physiol. 2006;209:379–388. doi: 10.1002/jcp.20745. [DOI] [PubMed] [Google Scholar]
- 110.Fabisiak JP, Ritov VB, Kagan VE. Reversible thiol-dependent activation of ryanodine-sensitive Ca2+ release channel by etoposide (VP-16) phenoxyl radical. Antioxid Redox Signal. 2000;2:73–82. doi: 10.1089/ars.2000.2.1-73. [DOI] [PubMed] [Google Scholar]
- 111.Fallon KE, Sivyer G, Sivyer K, Dare A. The biochemistry of runners in a 1600 km ultramarathon. Br J Sports Med. 1999;33:264–269. doi: 10.1136/bjsm.33.4.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fenster CP, Weinsier RL, Darley-Usmar VM, Patel RP. Obesity, aerobic exercise, vascular disease: the role of oxidant stress. Obes Res. 2002;10:964–968. doi: 10.1038/oby.2002.131. [DOI] [PubMed] [Google Scholar]
- 113.Flohe L, Budde H, Hofmann B. Peroxiredoxins in antioxidant defense and redox regulation. Biofactors. 2003;19:3–10. doi: 10.1002/biof.5520190102. [DOI] [PubMed] [Google Scholar]
- 114.Freeman ML, Borrelli MJ, Syed K, Senisterra G, Stafford DM, Lepock JR. Characterization of a signal generated by oxidation of protein thiols that activates the heat shock transcription factor. J Cell Physiol. 1995;164:356–366. doi: 10.1002/jcp.1041640216. [DOI] [PubMed] [Google Scholar]
- 115.Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- 116.Fuchs J. Oxidative Injury in Dermatology. New York: Springer-Verlag; 1992. [Google Scholar]
- 117.Fuchs J, Milbradt R. Antioxidant inhibition of skin inflammation induced by reactive oxidants: evaluation of the redox couple dihydrolipoate/lipoate. Skin Pharmacol. 1994;7:278–284. doi: 10.1159/000211306. [DOI] [PubMed] [Google Scholar]
- 118.Fujii Y, Takahashi S, Toyooka H. Protection from diaphragmatic fatigue by nitric oxide synthase inhibitor in dogs. Anaesth Intensive Care. 1999;27:45–48. doi: 10.1177/0310057X9902700109. [DOI] [PubMed] [Google Scholar]
- 119.Gaeini AA, Rahnama N, Hamedinia MR. Effects of vitamin E supplementation on oxidative stress at rest and after exercise to exhaustion in athletic students. J Sports Med Phys Fitness. 2006;46:458–461. [PubMed] [Google Scholar]
- 120.Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- 121.Girotti AW. Mechanisms of lipid peroxidation. J Free Radic Biol Med. 1985;1:87–95. doi: 10.1016/0748-5514(85)90011-x. [DOI] [PubMed] [Google Scholar]
- 122.Gladyshev VN, Liu A, Novoselov SV, Krysan K, Sun QA, Kryukov VM, Kryukov GV, Lou MF. Identification and characterization of a new mammalian glutaredoxin (thioltransferase), Grx2. J Biol Chem. 2001;276:30374–30380. doi: 10.1074/jbc.M100020200. [DOI] [PubMed] [Google Scholar]
- 123.Glantzounis GK, Tsimoyiannis EC, Kappas AM, Galaris DA. Uric acid and oxidative stress. Curr Pharm Des. 2005;11:4145–4151. doi: 10.2174/138161205774913255. [DOI] [PubMed] [Google Scholar]
- 124.Go YM, Ziegler TR, Johnson JM, Gu L, Hansen JM, Jones DP. Selective protection of nuclear thioredoxin-1 and glutathione redox systems against oxidation during glucose and glutamine deficiency in human colonic epithelial cells. Free Radic Biol Med. 2007;42:363–370. doi: 10.1016/j.freeradbiomed.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gohil K, Rothfuss L, Lang J, Packer L. Effect of exercise training on tissue vitamin E and ubiquinone content. J Appl Physiol. 1987;63:1638–1641. doi: 10.1152/jappl.1987.63.4.1638. [DOI] [PubMed] [Google Scholar]
- 126.Goldfarb AH, McIntosh MK, Boyer BT, Fatouros J. Vitamin E effects on indexes of lipid peroxidation in muscle from DHEA-treated and exercised rats. J Appl Physiol. 1994;76:1630–1635. doi: 10.1152/jappl.1994.76.4.1630. [DOI] [PubMed] [Google Scholar]
- 127.Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 128.Gomez-Cabrera MC, Borras C, Pallardo FV, Sastre J, Ji LL, Vina J. Decreasing xanthine oxidase-mediated oxidative stress prevents useful cellular adaptations to exercise in rats. J Physiol. 2005;567:113–120. doi: 10.1113/jphysiol.2004.080564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gomez-Cabrera MC, Pallardo FV, Sastre J, Vina J, Garciadel-Moral L. Allopurinol and markers of muscle damage among participants in the Tour de France. JAMA. 2003;289:2503–2504. doi: 10.1001/jama.289.19.2503-b. [DOI] [PubMed] [Google Scholar]
- 130.Gong MC, Arbogast S, Guo Z, Mathenia J, Su W, Reid MB. Calcium-independent phospholipase A2 modulates cytosolic oxidant activity and contractile function in murine skeletal muscle cells. J Appl Physiol. 2006;100:399–405. doi: 10.1152/japplphysiol.00873.2005. [DOI] [PubMed] [Google Scholar]
- 131.Gross WL, Bak MI, Ingwall JS, Arstall MA, Smith TW, Balligand JL, Kelly RA. Nitric oxide inhibits creatine kinase and regulates rat heart contractile reserve. Proc Natl Acad Sci USA. 1996;93:5604–5609. doi: 10.1073/pnas.93.11.5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gutierrez-Martin Y, Martin-Romero FJ, Inesta-Vaquera FA, Gutierrez-Merino C, Henao F. Modulation of sarcoplasmic reticulum Ca2+-ATPase by chronic and acute exposure to peroxynitrite. Eur J Biochem. 2004;271:2647–2657. doi: 10.1111/j.1432-1033.2004.04193.x. [DOI] [PubMed] [Google Scholar]
- 133.Halliwell B. Free radicals and antioxidants: a personal view. Nutr Rev. 1994;52:253–265. doi: 10.1111/j.1753-4887.1994.tb01453.x. [DOI] [PubMed] [Google Scholar]
- 134.Halliwell B. How to characterize an antioxidant: an update. Biochem Soc Symp. 1995;61:73–101. doi: 10.1042/bss0610073. [DOI] [PubMed] [Google Scholar]
- 135.Halliwell B, Chirico S. Lipid peroxidation: its mechanism, measurement, significance. Am J Clin Nutr. 1993;57:715S–725S. doi: 10.1093/ajcn/57.5.715S. [DOI] [PubMed] [Google Scholar]
- 136.Halliwell B, Gutteridge J. Free Radicals in Biology and Medicine. Oxford, UK: Oxford Univ. Press; 2007. [Google Scholar]
- 137.Hammeren J, Powers S, Lawler J, Criswell D, Martin D, Lowenthal D, Pollock M. Exercise training-induced alterations in skeletal muscle oxidative and antioxidant enzyme activity in senescent rats. Int J Sports Med. 1992;13:412–416. doi: 10.1055/s-2007-1021290. [DOI] [PubMed] [Google Scholar]
- 138.Han D, Loukianoff S, McLaughlin L. Oxidative stress indices: analytical aspects and significance. In: Sen CK, Packer L, Hanninen O, editors. Handbook of Oxidants and Antioxidants in Exercise. Amsterdam: Elsevier; 2000. pp. 433–483. [Google Scholar]
- 139.Han SN, Adolfsson O, Lee CK, Prolla TA, Ordovas J, Meydani SN. Vitamin E and gene expression in immune cells. Ann NY Acad Sci. 2004;1031:96–101. doi: 10.1196/annals.1331.010. [DOI] [PubMed] [Google Scholar]
- 140.Hansen JM, Go YM, Jones DP. Nuclear and mitochondrial compartmentation of oxidative stress and redox signaling. Annu Rev Pharmacol Toxicol. 2006;46:215–234. doi: 10.1146/annurev.pharmtox.46.120604.141122. [DOI] [PubMed] [Google Scholar]
- 141.Hansen JM, Zhang H, Jones DP. Differential oxidation of thioredoxin-1, thioredoxin-2, glutathione by metal ions. Free Radic Biol Med. 2006;40:138–145. doi: 10.1016/j.freeradbiomed.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 142.Hart JD, Dulhunty AF. Nitric oxide activates or inhibits skeletal muscle ryanodine receptors depending on its concentration, membrane potential and ligand binding. J Membr Biol. 2000;173:227–236. doi: 10.1007/s002320001022. [DOI] [PubMed] [Google Scholar]
- 143.Haycock JW, Jones P, Harris JB, Mantle D. Differential susceptibility of human skeletal muscle proteins to free radical induced oxidative damage: a histochemical, immunocytochemical and electron microscopical study in vitro. Acta Neuropathol. 1996;92:331–340. doi: 10.1007/s004010050527. [DOI] [PubMed] [Google Scholar]
- 144.Hearn AS, Tu C, Nick HS, Silverman DN. Characterization of the product-inhibited complex in catalysis by human manganese superoxide dismutase. J Biol Chem. 1999;274:24457–24460. doi: 10.1074/jbc.274.35.24457. [DOI] [PubMed] [Google Scholar]
- 145.Hellsten Y, Apple FS, Sjodin B. Effect of sprint cycle training on activities of antioxidant enzymes in human skeletal muscle. J Appl Physiol. 1996;81:1484–1487. doi: 10.1152/jappl.1996.81.4.1484. [DOI] [PubMed] [Google Scholar]
- 146.Hellsten Y, Nielsen JJ, Lykkesfeldt J, Bruhn M, Silveira L, Pilegaard H, Bangsbo J. Antioxidant supplementation enhances the exercise-induced increase in mitochondrial uncoupling protein 3 and endothelial nitric oxide synthase mRNA content in human skeletal muscle. Free Radic Biol Med. 2007;43:353–361. doi: 10.1016/j.freeradbiomed.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 147.Hellsten Y, Svensson M, Sjodin B, Smith S, Christensen A, Richter EA, Bangsbo J. Allantoin formation and urate and glutathione exchange in human muscle during submaximal exercise. Free Radic Biol Med. 2001;31:1313–1322. doi: 10.1016/s0891-5849(01)00631-1. [DOI] [PubMed] [Google Scholar]
- 148.Hellsten Y, Tullson PC, Richter EA, Bangsbo J. Oxidation of urate in human skeletal muscle during exercise. Free Radic Biol Med. 1997;22:169–174. doi: 10.1016/s0891-5849(96)00286-9. [DOI] [PubMed] [Google Scholar]
- 149.Herrero A, Barja G. ADP-regulation of mitochondrial free radical production is different with complex I- or complex II-linked substrates: implications for the exercise paradox and brain hypermetabolism. J Bioenerg Biomembr. 1997;29:241–249. doi: 10.1023/a:1022458010266. [DOI] [PubMed] [Google Scholar]
- 150.Heunks LM, Cody MJ, Geiger PC, Dekhuijzen PN, Sieck GC. Nitric oxide impairs Ca2+ activation and slows cross-bridge cycling kinetics in skeletal muscle. J Appl Physiol. 2001;91:2233–2239. doi: 10.1152/jappl.2001.91.5.2233. [DOI] [PubMed] [Google Scholar]
- 151.Heunks LM, Machiels HA, de Abreu R, Zhu XP, van der Heijden HF, Dekhuijzen PN. Free radicals in hypoxic rat diaphragm contractility: no role for xanthine oxidase. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1402–L1412. doi: 10.1152/ajplung.2001.281.6.L1402. [DOI] [PubMed] [Google Scholar]
- 152.Heunks LM, Machiels HA, Dekhuijzen PN, Prakash YS, Sieck GC. Nitric oxide affects sarcoplasmic calcium release in skeletal myotubes. J Appl Physiol. 2001;91:2117–2124. doi: 10.1152/jappl.2001.91.5.2117. [DOI] [PubMed] [Google Scholar]
- 153.Hidalgo C, Aracena P, Sanchez G, Donoso P. Redox regulation of calcium release in skeletal and cardiac muscle. Biol Res. 2002;35:183–193. doi: 10.4067/s0716-97602002000200009. [DOI] [PubMed] [Google Scholar]
- 154.Hidalgo C, Sanchez G, Barrientos G, Aracena-Parks P. A transverse tubule NADPH oxidase activity stimulates calcium release from isolated triads via ryanodine receptor type 1 S-glutathionylation. J Biol Chem. 2006;281:26473–26482. doi: 10.1074/jbc.M600451200. [DOI] [PubMed] [Google Scholar]
- 155.Higuchi M, Cartier LJ, Chen M, Holloszy JO. Superoxide dismutase and catalase in skeletal muscle: adaptive response to exercise. J Gerontol. 1985;40:281–286. doi: 10.1093/geronj/40.3.281. [DOI] [PubMed] [Google Scholar]
- 156.Hirschfield W, Moody MR, O’Brien WE, Gregg AR, Bryan RM, Jr, Reid MB. Nitric oxide release and contractile properties of skeletal muscles from mice deficient in type III NOS. Am J Physiol Regul Integr Comp Physiol. 2000;278:R95–R100. doi: 10.1152/ajpregu.2000.278.1.R95. [DOI] [PubMed] [Google Scholar]
- 157.Hollander JM, Lin KM, Scott BT, Dillmann WH. Overexpression of PHGPx and HSP60/10 protects against ischemia/reoxygenation injury. Free Radic Biol Med. 2003;35:742–751. doi: 10.1016/s0891-5849(03)00400-3. [DOI] [PubMed] [Google Scholar]
- 158.Holmgren A. Hydrogen donor system for Escherichia coli ribonucleoside-diphosphate reductase dependent upon glutathione. Proc Natl Acad Sci USA. 1976;73:2275–2279. doi: 10.1073/pnas.73.7.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Holmgren A. Thioredoxin. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- 160.Holmgren A. Thioredoxin and glutaredoxin systems. J Biol Chem. 1989;264:13963–13966. [PubMed] [Google Scholar]
- 161.Holmgren A, Johansson C, Berndt C, Lonn ME, Hudemann C, Lillig CH. Thiol redox control via thioredoxin and glutaredoxin systems. Biochem Soc Trans. 2005;33:1375–1377. doi: 10.1042/BST0331375. [DOI] [PubMed] [Google Scholar]
- 162.Howell RR, Wyngaarden JB. On the mechanism of peroxidation of uric acids by hemoproteins. J Biol Chem. 1960;235:3544–3550. [PubMed] [Google Scholar]
- 163.Hwang ES, Kim GH. Biomarkers for oxidative stress status of DNA, lipids, proteins in vitro and in vivo cancer research. Toxicology. 2007;229:1–10. doi: 10.1016/j.tox.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 164.Jackson MJ. Free radicals in skin and muscle: damaging agents or signals for adaptation? Proc Nutr Soc. 1999;58:673–676. doi: 10.1017/s0029665199000877. [DOI] [PubMed] [Google Scholar]
- 165.Jackson MJ, Edwards RH, Symons MC. Electron spin resonance studies of intact mammalian skeletal muscle. Biochim Biophys Acta. 1985;847:185–190. doi: 10.1016/0167-4889(85)90019-9. [DOI] [PubMed] [Google Scholar]
- 166.Jackson MJ, Jones DA, Edwards RH. Experimental skeletal muscle damage: the nature of the calcium-activated degenerative processes. Eur J Clin Invest. 1984;14:369–374. doi: 10.1111/j.1365-2362.1984.tb01197.x. [DOI] [PubMed] [Google Scholar]
- 167.Jackson MJ, Jones DA, Edwards RH. Vitamin E and skeletal muscle. Ciba Found Symp. 1983;101:224–239. doi: 10.1002/9780470720820.ch14. [DOI] [PubMed] [Google Scholar]
- 168.Jackson MJ, Khassaf M, Vasilaki A, McArdle F, McArdle A. Vitamin E and the oxidative stress of exercise. Ann NY Acad Sci. 2004;1031:158–168. doi: 10.1196/annals.1331.015. [DOI] [PubMed] [Google Scholar]
- 169.Jackson MJ, Papa S, Bolanos J, Bruckdorfer R, Carlsen H, Elliott RM, Flier J, Griffiths HR, Heales S, Holst B, Lorusso M, Lund E, Oivind Moskaug J, Moser U, Di Paola M, Polidori MC, Signorile A, Stahl W, Vina-Ribes J, Astley SB. Antioxidants, reactive oxygen and nitrogen species, gene induction and mitochondrial function. Mol Aspects Med. 2002;23:209–285. doi: 10.1016/s0098-2997(02)00018-3. [DOI] [PubMed] [Google Scholar]
- 170.Jackson MJ, Pye D, Palomero J. The production of reactive oxygen and nitrogen species by skeletal muscle. J Appl Physiol. 2007;102:1664–1670. doi: 10.1152/japplphysiol.01102.2006. [DOI] [PubMed] [Google Scholar]
- 171.Janero DR. Therapeutic potential of vitamin E in the pathogenesis of spontaneous atherosclerosis. Free Radic Biol Med. 1991;11:129–144. doi: 10.1016/0891-5849(91)90193-7. [DOI] [PubMed] [Google Scholar]
- 172.Javesghani D, Magder SA, Barreiro E, Quinn MT, Hussain SN. Molecular characterization of a superoxide-generating NAD(P)H oxidase in the ventilatory muscles. Am J Respir Crit Care Med. 2002;165:412–418. doi: 10.1164/ajrccm.165.3.2103028. [DOI] [PubMed] [Google Scholar]
- 173.Jenkins RR, Friedland R, Howald H. The relationship of oxygen uptake to superoxide dismutase and catalase activity in human skeletal muscle. Int J Sports Med. 1984;5:11–14. doi: 10.1055/s-2008-1025872. [DOI] [PubMed] [Google Scholar]
- 174.Jevtovic-Todorovic V, Guenthner TM. Depletion of a discrete nuclear glutathione pool by oxidative stress, but not by buthionine sulfoximine. Correlation with enhanced alkylating agent cytotoxicity to human melanoma cells in vitro. Biochem Pharmacol. 1992;44:1383–1393. doi: 10.1016/0006-2952(92)90540-y. [DOI] [PubMed] [Google Scholar]
- 175.Ji LL. Antioxidant enzyme response to exercise and aging. Med Sci Sports Exerc. 1993;25:225–231. [PubMed] [Google Scholar]
- 176.Ji LL. Antioxidant signaling in skeletal muscle: a brief review. Exp Gerontol. 2007;42:582–593. doi: 10.1016/j.exger.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 177.Ji LL. Antioxidants and oxidative stress in exercise. Proc Soc Exp Biol Med. 1999;222:283–292. doi: 10.1046/j.1525-1373.1999.d01-145.x. [DOI] [PubMed] [Google Scholar]
- 178.Ji LL. Exercise and oxidative stress: role of the cellular antioxidant systems. Exerc Sport Sci Rev. 1995;23:135–166. [PubMed] [Google Scholar]
- 179.Ji LL. Exercise at old age: does it increase or alleviate oxidative stress? Ann NY Acad Sci. 2001;928:236–247. doi: 10.1111/j.1749-6632.2001.tb05653.x. [DOI] [PubMed] [Google Scholar]
- 180.Ji LL, Fu R, Mitchell EW. Glutathione and antioxidant enzymes in skeletal muscle: effects of fiber type and exercise intensity. J Appl Physiol. 1992;73:1854–1859. doi: 10.1152/jappl.1992.73.5.1854. [DOI] [PubMed] [Google Scholar]
- 181.Ji LL, Gomez-Cabrera MC, Vina J. Exercise and hormesis: activation of cellular antioxidant signaling pathway. Ann NY Acad Sci. 2006;1067:425–435. doi: 10.1196/annals.1354.061. [DOI] [PubMed] [Google Scholar]
- 182.Ji LL, Stratman FW, Lardy HA. Antioxidant enzyme systems in rat liver and skeletal muscle. Influences of selenium deficiency, chronic training, acute exercise. Arch Biochem Biophys. 1988;263:150–160. doi: 10.1016/0003-9861(88)90623-6. [DOI] [PubMed] [Google Scholar]
- 183.Ji LL, Stratman FW, Lardy HA. Enzymatic down regulation with exercise in rat skeletal muscle. Arch Biochem Biophys. 1988;263:137–149. doi: 10.1016/0003-9861(88)90622-4. [DOI] [PubMed] [Google Scholar]
- 184.Ji L, La JH. Antioxidant defense: effects of aging and exercise. In: Radak Z, editor. Free Radicals in Exercise and Aging. Champaign, IL: Human Kinetics; 2000. pp. 35–72. [Google Scholar]
- 185.Jones DA, Jackson MJ, McPhail G, Edwards RH. Experimental mouse muscle damage: the importance of external calcium. Clin Sci. 1984;66:317–322. doi: 10.1042/cs0660317. [DOI] [PubMed] [Google Scholar]
- 186.Jones DP. Redefining oxidative stress. Antioxid Redox Signal. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 187.Jornot L, Petersen H, Junod AF. Modulation of the DNA binding activity of transcription factors CREP, NFkappaB and HSF by H2O2 and TNF alpha. Differences between in vivo and in vitro effects. FEBS Lett. 1997;416:381–386. doi: 10.1016/s0014-5793(97)01244-1. [DOI] [PubMed] [Google Scholar]
- 188.Judge AR, Dodd SL. Xanthine oxidase and activated neutrophils cause oxidative damage to skeletal muscle after contractile claudication. Am J Physiol Heart Circ Physiol. 2004;286:H252–H256. doi: 10.1152/ajpheart.00684.2003. [DOI] [PubMed] [Google Scholar]
- 189.Kagan VE, Shvedova A, Serbinova E, Khan S, Swanson C, Powell R, Packer L. Dihydrolipoic acid–a universal antioxidant both in the membrane and in the aqueous phase. Reduction of peroxyl, ascorbyl and chromanoxyl radicals. Biochem Pharmacol. 1992;44:1637–1649. doi: 10.1016/0006-2952(92)90482-x. [DOI] [PubMed] [Google Scholar]
- 190.Kaikkonen J, Kosonen L, Nyyssonen K, Porkkala-Sarataho E, Salonen R, Korpela H, Salonen JT. Effect of combined coenzyme Q10 and D-alpha-tocopheryl acetate supplementation on exercise-induced lipid peroxidation and muscular damage: a placebo-controlled double-blind study in marathon runners. Free Radic Res. 1998;29:85–92. doi: 10.1080/10715769800300101. [DOI] [PubMed] [Google Scholar]
- 191.Kaneko M, Suzuki H, Masuda H, Yuan G, Hayashi H, Kobayashi A, Yamazaki N. Effects of oxygen free radicals on Ca2+ binding to cardiac troponin. Jpn Circ J. 1992;56(Suppl 5):1288–1290. [PubMed] [Google Scholar]
- 192.Kang SW, Rhee SG, Chang TS, Jeong W, Choi MH. 2-Cys peroxiredoxin function in intracellular signal transduction: therapeutic implications. Trends Mol Med. 2005;11:571–578. doi: 10.1016/j.molmed.2005.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kanter MM. Free radicals, exercise, antioxidant supplementation. Int J Sport Nutr. 1994;4:205–220. doi: 10.1123/ijsn.4.3.205. [DOI] [PubMed] [Google Scholar]
- 194.Kanter MM, Hamlin RL, Unverferth DV, Davis HW, Merola AJ. Effect of exercise training on antioxidant enzymes and cardiotoxicity of doxorubicin. J Appl Physiol. 1985;59:1298–1303. doi: 10.1152/jappl.1985.59.4.1298. [DOI] [PubMed] [Google Scholar]
- 195.Kanter MM, Nolte LA, Holloszy JO. Effects of an antioxidant vitamin mixture on lipid peroxidation at rest and postexercise. J Appl Physiol. 1993;74:965–969. doi: 10.1152/jappl.1993.74.2.965. [DOI] [PubMed] [Google Scholar]
- 196.Kanter MM, Williams MH. Antioxidants, carnitine, choline as putative ergogenic aids. Int J Sport Nutr. 1995;5(Suppl):S120–S131. doi: 10.1123/ijsn.5.s1.s120. [DOI] [PubMed] [Google Scholar]
- 197.Karanth J, Kumar R, Jeevaratnam K. Response of antioxidant system in rats to dietary fat and physical activity. Indian J Physiol Pharmacol. 2004;48:446–452. [PubMed] [Google Scholar]
- 198.Kasai H. Analysis of a form of oxidative DNA damage, 8-hydroxy-2′-deoxyguanosine, as a marker of cellular oxidative stress during carcinogenesis. Mutat Res. 1997;387:147–163. doi: 10.1016/s1383-5742(97)00035-5. [DOI] [PubMed] [Google Scholar]
- 199.Kelly GS. Clinical applications of N-acetylcysteine. Altern Med Rev. 1998;3:114–127. [PubMed] [Google Scholar]
- 200.Khanna S, Atalay M, Lodge JK, Laaksonen DE, Roy S, Hanninen O, Packer L, Sen CK. Skeletal muscle and liver lipoyllysine content in response to exercise, training and dietary alpha-lipoic acid supplementation. Biochem Mol Biol Int. 1998;46:297–306. doi: 10.1080/15216549800203812. [DOI] [PubMed] [Google Scholar]
- 201.Khassaf M, Child RB, McArdle A, Brodie DA, Esanu C, Jackson MJ. Time course of responses of human skeletal muscle to oxidative stress induced by nondamaging exercise. J Appl Physiol. 2001;90:1031–1035. doi: 10.1152/jappl.2001.90.3.1031. [DOI] [PubMed] [Google Scholar]
- 202.Khassaf M, McArdle A, Esanu C, Vasilaki A, McArdle F, Griffiths RD, Brodie DA, Jackson MJ. Effect of vitamin C supplements on antioxidant defence and stress proteins in human lymphocytes and skeletal muscle. J Physiol. 2003;549:645–652. doi: 10.1113/jphysiol.2003.040303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Khawli FA, Reid MB. N-acetylcysteine depresses contractile function and inhibits fatigue of diaphragm in vitro. J Appl Physiol. 1994;77:317–324. doi: 10.1152/jappl.1994.77.1.317. [DOI] [PubMed] [Google Scholar]
- 204.Kim K, Kim IH, Lee KY, Rhee SG, Stadtman ER. The isolation and purification of a specific “protector” protein which inhibits enzyme inactivation by a thiol/Fe(III)/O2 mixed-function oxidation system. J Biol Chem. 1988;263:4704–4711. [PubMed] [Google Scholar]
- 205.Kim K, Rhee SG, Stadtman ER. Nonenzymatic cleavage of proteins by reactive oxygen species generated by dithiothreitol and iron. J Biol Chem. 1985;260:15394–15397. [PubMed] [Google Scholar]
- 206.Kirkman HN, Gaetani GF. Mammalian catalase: a venerable enzyme with new mysteries. Trends Biochem Sci. 2007;32:44–50. doi: 10.1016/j.tibs.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 207.Kobzik L, Reid MB, Bredt DS, Stamler JS. Nitric oxide in skeletal muscle. Nature. 1994;372:546–548. doi: 10.1038/372546a0. [DOI] [PubMed] [Google Scholar]
- 208.Kobzik L, Stringer B, Balligand JL, Reid MB, Stamler JS. Endothelial type nitric oxide synthase in skeletal muscle fibers: mitochondrial relationships. Biochem Biophys Res Commun. 1995;211:375–381. doi: 10.1006/bbrc.1995.1824. [DOI] [PubMed] [Google Scholar]
- 209.Kochevar I. Basic Principles in Photomedicine and Photochemistry. New York: Dekker; 1993. [Google Scholar]
- 210.Koh TJ, Tidball JG. Nitric oxide inhibits calpain-mediated proteolysis of talin in skeletal muscle cells. Am J Physiol Cell Physiol. 2000;279:C806–C812. doi: 10.1152/ajpcell.2000.279.3.C806. [DOI] [PubMed] [Google Scholar]
- 211.Kondo H, Miura M, Itokawa Y. Antioxidant enzyme systems in skeletal muscle atrophied by immobilization. Pflügers Arch. 1993;422:404–406. doi: 10.1007/BF00374299. [DOI] [PubMed] [Google Scholar]
- 212.Kondo H, Miura M, Itokawa Y. Oxidative stress in skeletal muscle atrophied by immobilization. Acta Physiol Scand. 1991;142:527–528. doi: 10.1111/j.1748-1716.1991.tb09191.x. [DOI] [PubMed] [Google Scholar]
- 213.Kondo H, Miura M, Kodama J, Ahmed SM, Itokawa Y. Role of iron in oxidative stress in skeletal muscle atrophied by immobilization. Pflügers Arch. 1992;421:295–297. doi: 10.1007/BF00374844. [DOI] [PubMed] [Google Scholar]
- 214.Kondo H, Miura M, Nakagaki I, Sasaki S, Itokawa Y. Trace element movement and oxidative stress in skeletal muscle atrophied by immobilization. Am J Physiol Endocrinol Metab. 1992;262:E583–E590. doi: 10.1152/ajpendo.1992.262.5.E583. [DOI] [PubMed] [Google Scholar]
- 215.Kondo H, Nishino K, Itokawa Y. Hydroxyl radical generation in skeletal muscle atrophied by immobilization. FEBS Lett. 1994;349:169–172. doi: 10.1016/0014-5793(94)00641-5. [DOI] [PubMed] [Google Scholar]
- 216.Konig D, Wagner KH, Elmadfa I, Berg A. Exercise and oxidative stress: significance of antioxidants with reference to inflammatory, muscular, systemic stress. Exerc Immunol Rev. 2001;7:108–133. [PubMed] [Google Scholar]
- 217.Koren A, Sauber C, Sentjurc M, Schara M. Free radicals in tetanic activity of isolated skeletal muscle. Comp Biochem Physiol B Biochem Mol Biol. 1983;74:633–635. doi: 10.1016/0305-0491(83)90241-9. [DOI] [PubMed] [Google Scholar]
- 218.Kourie JI. Interaction of reactive oxygen species with ion transport mechanisms. Am J Physiol Cell Physiol. 1998;275:C1–C24. doi: 10.1152/ajpcell.1998.275.1.C1. [DOI] [PubMed] [Google Scholar]
- 219.Kozlov AV, Szalay L, Umar F, Kropik K, Staniek K, Niedermuller H, Bahrami S, Nohl H. Skeletal muscles, heart, lung are the main sources of oxygen radicals in old rats. Biochim Biophys Acta. 2005;1740:382–389. doi: 10.1016/j.bbadis.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 220.Kramer HF, Goodyear LJ. Exercise, MAPK, NF-kappaB signaling in skeletal muscle. J Appl Physiol. 2007;103:388–395. doi: 10.1152/japplphysiol.00085.2007. [DOI] [PubMed] [Google Scholar]
- 221.Kretzschmar M, Muller D. Aging, training and exercise. A review of effects on plasma glutathione and lipid peroxides. Sports Med. 1993;15:196–209. doi: 10.2165/00007256-199315030-00005. [DOI] [PubMed] [Google Scholar]
- 222.Krinsky NI. The antioxidant and biological properties of the carotenoids. Ann NY Acad Sci. 1998;854:443–447. doi: 10.1111/j.1749-6632.1998.tb09923.x. [DOI] [PubMed] [Google Scholar]
- 223.Kumar CT, Reddy VK, Prasad M, Thyagaraju K, Reddanna P. Dietary supplementation of vitamin E protects heart tissue from exercise-induced oxidant stress. Mol Cell Biochem. 1992;111:109–115. doi: 10.1007/BF00229581. [DOI] [PubMed] [Google Scholar]
- 224.Lambertucci RH, Levada-Pires AC, Rossoni LV, Curi R, Pithon-Curi TC. Effects of aerobic exercise training on antioxidant enzyme activities and mRNA levels in soleus muscle from young and aged rats. Mech Ageing Dev. 2007;128:267–275. doi: 10.1016/j.mad.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 225.Lander HM. An essential role for free radicals and derived species in signal transduction. FASEB J. 1997;11:118–124. [PubMed] [Google Scholar]
- 226.Lau KS, Grange RW, Chang WJ, Kamm KE, Sarelius I, Stull JT. Skeletal muscle contractions stimulate cGMP formation and attenuate vascular smooth muscle myosin phosphorylation via nitric oxide. FEBS Lett. 1998;431:71–74. doi: 10.1016/s0014-5793(98)00728-5. [DOI] [PubMed] [Google Scholar]
- 227.Laughlin MH, Simpson T, Sexton WL, Brown OR, Smith JK, Korthuis RJ. Skeletal muscle oxidative capacity, antioxidant enzymes, exercise training. J Appl Physiol. 1990;68:2337–2343. doi: 10.1152/jappl.1990.68.6.2337. [DOI] [PubMed] [Google Scholar]
- 228.Lawler JM, Demaree SR. Relationship between NADP-specific isocitrate dehydrogenase and glutathione peroxidase in aging rat skeletal muscle. Mech Ageing Dev. 2001;122:291–304. doi: 10.1016/s0047-6374(00)00235-9. [DOI] [PubMed] [Google Scholar]
- 229.Lawler JM, Kwak HB, Song W, Parker JL. Exercise training reverses downregulation of HSP70 and antioxidant enzymes in porcine skeletal muscle after chronic coronary artery occlusion. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1756–R1763. doi: 10.1152/ajpregu.00271.2006. [DOI] [PubMed] [Google Scholar]
- 230.Lawler JM, Powers SK, Criswell DS. Inducibility of NADP-specific isocitrate dehydrogenase with endurance training in skeletal muscle. Acta Physiol Scand. 1993;149:177–181. doi: 10.1111/j.1748-1716.1993.tb09610.x. [DOI] [PubMed] [Google Scholar]
- 231.Lawler JM, Powers SK, Van Dijk H, Visser T, Kordus MJ, Ji LL. Metabolic and antioxidant enzyme activities in the diaphragm: effects of acute exercise. Respir Physiol. 1994;96:139–149. doi: 10.1016/0034-5687(94)90122-8. [DOI] [PubMed] [Google Scholar]
- 232.Lawler JM, Powers SK, Visser T, Van Dijk H, Kordus MJ, Ji LL. Acute exercise and skeletal muscle antioxidant and metabolic enzymes: effects of fiber type and age. Am J Physiol Regul Integr Comp Physiol. 1993;265:R1344–R1350. doi: 10.1152/ajpregu.1993.265.6.R1344. [DOI] [PubMed] [Google Scholar]
- 233.Lawson JA, Rokach J, FitzGerald GA. Isoprostanes: formation, analysis and use as indices of lipid peroxidation in vivo. J Biol Chem. 1999;274:24441–24444. doi: 10.1074/jbc.274.35.24441. [DOI] [PubMed] [Google Scholar]
- 234.Leeuwenburgh C, Fiebig R, Chandwaney R, Ji LL. Aging and exercise training in skeletal muscle: responses of glutathione and antioxidant enzyme systems. Am J Physiol Regul Integr Comp Physiol. 1994;267:R439–R445. doi: 10.1152/ajpregu.1994.267.2.R439. [DOI] [PubMed] [Google Scholar]
- 235.Leeuwenburgh C, Hollander J, Leichtweis S, Griffiths M, Gore M, Ji LL. Adaptations of glutathione antioxidant system to endurance training are tissue and muscle fiber specific. Am J Physiol Regul Integr Comp Physiol. 1997;272:R363–R369. doi: 10.1152/ajpregu.1997.272.1.R363. [DOI] [PubMed] [Google Scholar]
- 236.Li JM, Shah AM. Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1014–R1030. doi: 10.1152/ajpregu.00124.2004. [DOI] [PubMed] [Google Scholar]
- 237.Liu DF, Wang D, Stracher A. The accessibility of the thiol groups on G- and F-actin of rabbit muscle. Biochem J. 1990;266:453–459. doi: 10.1042/bj2660453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Liu H, Colavitti R, Rovira II, Finkel T. Redox-dependent transcriptional regulation. Circ Res. 2005;97:967–974. doi: 10.1161/01.RES.0000188210.72062.10. [DOI] [PubMed] [Google Scholar]
- 239.Loschen G, Azzi A, Richter C, Flohe L. Superoxide radicals as precursors of mitochondrial hydrogen peroxide. FEBS Lett. 1974;42:68–72. doi: 10.1016/0014-5793(74)80281-4. [DOI] [PubMed] [Google Scholar]
- 240.Lundberg M, Johansson C, Chandra J, Enoksson M, Jacobsson G, Ljung J, Johansson M, Holmgren A. Cloning and expression of a novel human glutaredoxin (Grx2) with mitochondrial and nuclear isoforms. J Biol Chem. 2001;276:26269–26275. doi: 10.1074/jbc.M011605200. [DOI] [PubMed] [Google Scholar]
- 241.Maes K, Testelmans D, Powers S, Decramer M, Gayan-Ramirez G. Leupeptin inhibits ventilator-induced diaphragm dysfunction in rats. Am J Respir Crit Care Med. 2007;175:1134–1138. doi: 10.1164/rccm.200609-1342OC. [DOI] [PubMed] [Google Scholar]
- 242.Malech HL, Gallin JI. Current concepts: immunology. Neutrophils in human diseases. N Engl J Med. 1987;317:687–694. doi: 10.1056/NEJM198709103171107. [DOI] [PubMed] [Google Scholar]
- 243.Malpica R, Sandoval GR, Rodriguez C, Franco B, Georgellis D. Signaling by the arc two-component system provides a link between the redox state of the quinone pool and gene expression. Antioxid Redox Signal. 2006;8:781–795. doi: 10.1089/ars.2006.8.781. [DOI] [PubMed] [Google Scholar]
- 244.Marechal G, Beckers-Bleukx G. Effect of nitric oxide on the maximal velocity of shortening of a mouse skeletal muscle. Pflügers Arch. 1998;436:906–913. doi: 10.1007/s004240050722. [DOI] [PubMed] [Google Scholar]
- 245.Marechal G, Gailly P. Effects of nitric oxide on the contraction of skeletal muscle. Cell Mol Life Sci. 1999;55:1088–1102. doi: 10.1007/s000180050359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Marin E, Kretzschmar M, Arokoski J, Hanninen O, Klinger W. Enzymes of glutathione synthesis in dog skeletal muscles and their response to training. Acta Physiol Scand. 1993;147:369–373. doi: 10.1111/j.1748-1716.1993.tb09513.x. [DOI] [PubMed] [Google Scholar]
- 247.Marinov BS, Olojo RO, Xia R, Abramson JJ. Non-thiol reagents regulate ryanodine receptor function by redox interactions that modify reactive thiols. Antioxid Redox Signal. 2007;9:609–621. doi: 10.1089/ars.2006.1426. [DOI] [PubMed] [Google Scholar]
- 248.Martinez-Galisteo E, Padilla CA, Holmgren A, Barcena JA. Characterization of mammalian thioredoxin reductase, thioredoxin and glutaredoxin by immunochemical methods. Comp Biochem Physiol B Biochem Mol Biol. 1995;111:17–25. doi: 10.1016/0305-0491(94)00235-m. [DOI] [PubMed] [Google Scholar]
- 249.Matuszczak Y, Arbogast S, Reid MB. Allopurinol mitigates muscle contractile dysfunction caused by hindlimb unloading in mice. Aviat Space Environ Med. 2004;75:581–588. [PubMed] [Google Scholar]
- 250.Matuszczak Y, Farid M, Jones J, Lansdowne S, Smith MA, Taylor AA, Reid MB. Effects of N-acetylcysteine on glutathione oxidation and fatigue during handgrip exercise. Muscle Nerve. 2005;32:633–638. doi: 10.1002/mus.20385. [DOI] [PubMed] [Google Scholar]
- 251.McArdle A, Dillmann WH, Mestril R, Faulkner JA, Jackson MJ. Overexpression of HSP70 in mouse skeletal muscle protects against muscle damage and age-related muscle dysfunction. FASEB J. 2004;18:355–357. doi: 10.1096/fj.03-0395fje. [DOI] [PubMed] [Google Scholar]
- 252.McArdle A, Jackson MJ. Exercise, oxidative stress and ageing. J Anat. 2000;197:539–541. doi: 10.1046/j.1469-7580.2000.19740539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.McArdle A, Pattwell D, Vasilaki A, Griffiths RD, Jackson MJ. Contractile activity-induced oxidative stress: cellular origin and adaptive responses. Am J Physiol Cell Physiol. 2001;280:C621–C627. doi: 10.1152/ajpcell.2001.280.3.C621. [DOI] [PubMed] [Google Scholar]
- 254.McArdle F, Spiers S, Aldemir H, Vasilaki A, Beaver A, Iwanejko L, McArdle A, Jackson MJ. Preconditioning of skeletal muscle against contraction-induced damage: the role of adaptations to oxidants in mice. J Physiol. 2004;561:233–244. doi: 10.1113/jphysiol.2004.069914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.McClung JM, Kavazis AN, DeRuisseau KC, Falk DJ, Deering MA, Lee Y, Sugiura T, Powers SK. Caspase-3 regulation of diaphragm myonuclear domain during mechanical ventilation-induced atrophy. Am J Respir Crit Care Med. 2007;175:150–159. doi: 10.1164/rccm.200601-142OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 257.McKenna MJ, Medved I, Goodman CA, Brown MJ, Bjorksten AR, Murphy KT, Petersen AC, Sostaric S, Gong X. N-acetyl-cysteine attenuates the decline in muscle Na+, K+-pump activity and delays fatigue during prolonged exercise in humans. J Physiol. 2006;576:279–288. doi: 10.1113/jphysiol.2006.115352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.McKenzie EC, Jose-Cunilleras E, Hinchcliff KW, Holbrook TC, Royer C, Payton ME, Williamson K, Nelson S, Willard MD, Davis MS. Serum chemistry alterations in Alaskan sled dogs during five successive days of prolonged endurance exercise. J Am Vet Med Assoc. 2007;230:1486–1492. doi: 10.2460/javma.230.10.1486. [DOI] [PubMed] [Google Scholar]
- 259.Medved I, Brown MJ, Bjorksten AR, Leppik JA, Sostaric S, McKenna MJ. N-acetylcysteine infusion alters blood redox status but not time to fatigue during intense exercise in humans. J Appl Physiol. 2003;94:1572–1582. doi: 10.1152/japplphysiol.00884.2002. [DOI] [PubMed] [Google Scholar]
- 260.Medved I, Brown MJ, Bjorksten AR, McKenna MJ. Effects of intravenous N-acetylcysteine infusion on time to fatigue and potassium regulation during prolonged cycling exercise. J Appl Physiol. 2004;96:211–217. doi: 10.1152/japplphysiol.00458.2003. [DOI] [PubMed] [Google Scholar]
- 261.Medved I, Brown MJ, Bjorksten AR, Murphy KT, Petersen AC, Sostaric S, Gong X, McKenna MJ. N-acetylcysteine enhances muscle cysteine and glutathione availability and attenuates fatigue during prolonged exercise in endurance-trained individuals. J Appl Physiol. 2004;97:1477–1485. doi: 10.1152/japplphysiol.00371.2004. [DOI] [PubMed] [Google Scholar]
- 262.Meijer AE. The histochemical localization of reduced glutathione in skeletal muscle under different pathophysiological conditions. Acta Histochem. 1991;90:147–154. doi: 10.1016/s0065-1281(11)80053-x. [DOI] [PubMed] [Google Scholar]
- 263.Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 264.Mizuno M, Quistorff B, Theorell H, Theorell M, Chance B. Effects of oral supplementation of coenzyme Q10 on 31P-NMR detected skeletal muscle energy metabolism in middle-aged post-polio subjects and normal volunteers. Mol Aspects Med. 1997;18(Suppl):S291–S298. doi: 10.1016/s0098-2997(97)00001-0. [DOI] [PubMed] [Google Scholar]
- 265.Mohr S, Stamler JS, Brune B. Posttranslational modification of glyceraldehyde-3-phosphate dehydrogenase by S-nitrosylation and subsequent NADH attachment. J Biol Chem. 1996;271:4209–4214. doi: 10.1074/jbc.271.8.4209. [DOI] [PubMed] [Google Scholar]
- 266.Molina y Vedia L, McDonald B, Reep B, Brune B, Di Silvio M, Billiar TR, Lapetina EG. Nitric oxide-induced S-nitrosylation of glyceraldehyde-3-phosphate dehydrogenase inhibits enzymatic activity and increases endogenous ADP-ribosylation. J Biol Chem. 1992;267:24929–24932. [PubMed] [Google Scholar]
- 267.Moopanar TR, Allen DG. Reactive oxygen species reduce myofibrillar Ca2+ sensitivity in fatiguing mouse skeletal muscle at 37 degrees C. J Physiol. 2005;564:189–199. doi: 10.1113/jphysiol.2005.083519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Moran LK, Gutteridge JM, Quinlan GJ. Thiols in cellular redox signalling and control. Curr Med Chem. 2001;8:763–772. doi: 10.2174/0929867013372904. [DOI] [PubMed] [Google Scholar]
- 269.Morre DJ. Quinone oxidoreductases of the plasma membrane. Methods Enzymol. 2004;378:179–199. doi: 10.1016/S0076-6879(04)78015-5. [DOI] [PubMed] [Google Scholar]
- 270.Morrison SJ, Csete M, Groves AK, Melega W, Wold B, Anderson DJ. Culture in reduced levels of oxygen promotes clonogenic sympathoadrenal differentiation by isolated neural crest stem cells. J Neurosci. 2000;20:7370–7376. doi: 10.1523/JNEUROSCI.20-19-07370.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Moskaug JO, Carlsen H, Myhrstad M, Blomhoff R. Molecular imaging of the biological effects of quercetin and quercetin-rich foods. Mech Ageing Dev. 2004;125:315–324. doi: 10.1016/j.mad.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 272.Muller FL, Liu Y, Van Remmen H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J Biol Chem. 2004;279:49064–49073. doi: 10.1074/jbc.M407715200. [DOI] [PubMed] [Google Scholar]
- 273.Muralikrishna Adibhatla R, Hatcher JF. Phospholipase A2, reactive oxygen species, lipid peroxidation in cerebral ischemia. Free Radic Biol Med. 2006;40:376–387. doi: 10.1016/j.freeradbiomed.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 274.Murphy MP, Smith RA. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu Rev Pharmacol Toxicol. 2007;47:629–656. doi: 10.1146/annurev.pharmtox.47.120505.105110. [DOI] [PubMed] [Google Scholar]
- 275.Negash S, Chen LT, Bigelow DJ, Squier TC. Phosphorylation of phospholamban by cAMP-dependent protein kinase enhances interactions between Ca-ATPase polypeptide chains in cardiac sarcoplasmic reticulum membranes. Biochemistry. 1996;35:11247–11259. doi: 10.1021/bi960864q. [DOI] [PubMed] [Google Scholar]
- 276.Nethery D, Callahan LA, Stofan D, Mattera R, DiMarco A, Supinski G. PLA2 dependence of diaphragm mitochondrial formation of reactive oxygen species. J Appl Physiol. 2000;89:72–80. doi: 10.1152/jappl.2000.89.1.72. [DOI] [PubMed] [Google Scholar]
- 277.Nethery D, Stofan D, Callahan L, DiMarco A, Supinski G. Formation of reactive oxygen species by the contracting diaphragm is PLA2 dependent. J Appl Physiol. 1999;87:792–800. doi: 10.1152/jappl.1999.87.2.792. [DOI] [PubMed] [Google Scholar]
- 278.Niess AM, Simon P. Response and adaptation of skeletal muscle to exercise–the role of reactive oxygen species. Front Biosci. 2007;12:4826–4838. doi: 10.2741/2431. [DOI] [PubMed] [Google Scholar]
- 279.Novelli GP, Bracciotti G, Falsini S. Spin-trappers and vitamin E prolong endurance to muscle fatigue in mice. Free Radic Biol Med. 1990;8:9–13. doi: 10.1016/0891-5849(90)90138-9. [DOI] [PubMed] [Google Scholar]
- 280.O’Neill CA, Stebbins CL, Bonigut S, Halliwell B, Longhurst JC. Production of hydroxyl radicals in contracting skeletal muscle of cats. J Appl Physiol. 1996;81:1197–1206. doi: 10.1152/jappl.1996.81.3.1197. [DOI] [PubMed] [Google Scholar]
- 281.Oba T, Ishikawa T, Murayama T, Ogawa Y, Yamaguchi M. H2O2 and ethanol act synergistically to gate ryanodine receptor/calcium-release channel. Am J Physiol Cell Physiol. 2000;279:C1366–C1374. doi: 10.1152/ajpcell.2000.279.5.C1366. [DOI] [PubMed] [Google Scholar]
- 282.Oba T, Kurono C, Nakajima R, Takaishi T, Ishida K, Fuller GA, Klomkleaw W, Yamaguchi M. H2O2 activates ryanodine receptor but has little effect on recovery of releasable Ca2+ content after fatigue. J Appl Physiol. 2002;93:1999–2008. doi: 10.1152/japplphysiol.00097.2002. [DOI] [PubMed] [Google Scholar]
- 283.Oguma Y, Sesso HD, Paffenbarger RS, Jr, Lee IM. Physical activity and all cause mortality in women: a review of the evidence. Br J Sports Med. 2002;36:162–172. doi: 10.1136/bjsm.36.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Ohishi S, Kizaki T, Nagasawa J, Izawa T, Komabayashi T, Nagata N, Suzuki K, Taniguchi N, Ohno H. Effects of endurance training on superoxide dismutase activity, content and mRNA expression in rat muscle. Clin Exp Pharmacol Physiol. 1997;24:326–332. doi: 10.1111/j.1440-1681.1997.tb01196.x. [DOI] [PubMed] [Google Scholar]
- 285.Ohkuwa T, Sato Y, Naoi M. Glutathione status and reactive oxygen generation in tissues of young and old exercised rats. Acta Physiol Scand. 1997;159:237–244. doi: 10.1046/j.1365-201X.1997.576351000.x. [DOI] [PubMed] [Google Scholar]
- 286.Ong SH, Steiner AL. Localization of cyclic GMP and cyclic AMP in cardiac and skeletal muscle: immunocytochemical demonstration. Science. 1977;195:183–185. doi: 10.1126/science.188135. [DOI] [PubMed] [Google Scholar]
- 287.Oyanagui Y. Reevaluation of assay methods and establishment of kit for superoxide dismutase activity. Anal Biochem. 1984;142:290–296. doi: 10.1016/0003-2697(84)90467-6. [DOI] [PubMed] [Google Scholar]
- 288.Packer JE, Slater TF, Willson RL. Direct observation of a free radical interaction between vitamin E and vitamin C. Nature. 1979;278:737–738. doi: 10.1038/278737a0. [DOI] [PubMed] [Google Scholar]
- 289.Packer L. Antioxidant properties of lipoic acid and its therapeutic effects in prevention of diabetes complications and cataracts. Ann NY Acad Sci. 1994;738:257–264. doi: 10.1111/j.1749-6632.1994.tb21811.x. [DOI] [PubMed] [Google Scholar]
- 290.Packer L. Protective role of vitamin E in biological systems. Am J Clin Nutr. 1991;53:1050S–1055S. doi: 10.1093/ajcn/53.4.1050S. [DOI] [PubMed] [Google Scholar]
- 291.Packer L, Witt EH, Tritschler HJ. alpha-Lipoic acid as a biological antioxidant. Free Radic Biol Med. 1995;19:227–250. doi: 10.1016/0891-5849(95)00017-r. [DOI] [PubMed] [Google Scholar]
- 292.Pattwell DM, McArdle A, Morgan JE, Patridge TA, Jackson MJ. Release of reactive oxygen and nitrogen species from contracting skeletal muscle cells. Free Radic Biol Med. 2004;37:1064–1072. doi: 10.1016/j.freeradbiomed.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 293.Pereira B, Costa Rosa LF, Safi DA, Medeiros MH, Curi R, Bechara EJ. Superoxide dismutase, catalase, glutathione peroxidase activities in muscle and lymphoid organs of sedentary and exercise-trained rats. Physiol Behav. 1994;56:1095–1099. doi: 10.1016/0031-9384(94)90349-2. [DOI] [PubMed] [Google Scholar]
- 294.Perkins WJ, Han YS, Sieck GC. Skeletal muscle force and acto-myosin ATPase activity reduced by nitric oxide donor. J Appl Physiol. 1997;83:1326–1332. doi: 10.1152/jappl.1997.83.4.1326. [DOI] [PubMed] [Google Scholar]
- 295.Pessah IN, Feng W. Functional role of hyperreactive sulfhydryl moieties within the ryanodine receptor complex. Antioxid Redox Signal. 2000;2:17–25. doi: 10.1089/ars.2000.2.1-17. [DOI] [PubMed] [Google Scholar]
- 296.Plant DR, Lynch GS, Williams DA. Hydrogen peroxide modulates Ca2+-activation of single permeabilized fibres from fast- and slow-twitch skeletal muscles of rats. J Muscle Res Cell Motil. 2000;21:747–752. doi: 10.1023/a:1010344008224. [DOI] [PubMed] [Google Scholar]
- 297.Posterino GS, Cellini MA, Lamb GD. Effects of oxidation and cytosolic redox conditions on excitation-contraction coupling in rat skeletal muscle. J Physiol. 2003;547:807–823. doi: 10.1113/jphysiol.2002.035204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Powers S, Sen CK. Physiological antioxidants and exercise training. In: Sen CK, Packer l, Hanninen O, editors. Handbook of Oxidants and Antioxidants in Exercise. Amsterdam: Elsevier; 2000. pp. 221–242. [Google Scholar]
- 299.Powers SK, Criswell D, Lawler J, Ji LL, Martin D, Herb RA, Dudley G. Influence of exercise and fiber type on antioxidant enzyme activity in rat skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 1994;266:R375–R380. doi: 10.1152/ajpregu.1994.266.2.R375. [DOI] [PubMed] [Google Scholar]
- 300.Powers SK, Criswell D, Lawler J, Martin D, Ji LL, Herb RA, Dudley G. Regional training-induced alterations in diaphragmatic oxidative and antioxidant enzymes. Respir Physiol. 1994;95:227–237. doi: 10.1016/0034-5687(94)90118-x. [DOI] [PubMed] [Google Scholar]
- 301.Powers SK, Criswell D, Lawler J, Martin D, Lieu FK, Ji LL, Herb RA. Rigorous exercise training increases superoxide dismutase activity in ventricular myocardium. Am J Physiol Heart Circ Physiol. 1993;265:H2094–H2098. doi: 10.1152/ajpheart.1993.265.6.H2094. [DOI] [PubMed] [Google Scholar]
- 302.Powers SK, DeRuisseau KC, Quindry J, Hamilton KL. Dietary antioxidants and exercise. J Sports Sci. 2004;22:81–94. doi: 10.1080/0264041031000140563. [DOI] [PubMed] [Google Scholar]
- 303.Powers SK, Hamilton K. Antioxidants and exercise. Clin Sports Med. 1999;18:525–536. doi: 10.1016/s0278-5919(05)70166-6. [DOI] [PubMed] [Google Scholar]
- 304.Powers SK, Ji LL, Leeuwenburgh C. Exercise training-induced alterations in skeletal muscle antioxidant capacity: a brief review. Med Sci Sports Exerc. 1999;31:987–997. doi: 10.1097/00005768-199907000-00011. [DOI] [PubMed] [Google Scholar]
- 305.Powers SK, Kavazis AN, DeRuisseau KC. Mechanisms of disuse muscle atrophy: role of oxidative stress. Am J Physiol Regul Integr Comp Physiol. 2005;288:R337–R344. doi: 10.1152/ajpregu.00469.2004. [DOI] [PubMed] [Google Scholar]
- 306.Powers SK, Kavazis AN, McClung JM. Oxidative stress and disuse muscle atrophy. J Appl Physiol. 2007;102:2389–2397. doi: 10.1152/japplphysiol.01202.2006. [DOI] [PubMed] [Google Scholar]
- 307.Powers SK, Lawler J, Criswell D, Lieu FK, Martin D. Aging and respiratory muscle metabolic plasticity: effects of endurance training. J Appl Physiol. 1992;72:1068–1073. doi: 10.1152/jappl.1992.72.3.1068. [DOI] [PubMed] [Google Scholar]
- 308.Prochniewicz E, Lowe DA, Spakowicz DJ, Higgins L, O’Conor K, Thompson LV, Ferrington DA, Thomas DD. Functional, structural, chemical changes in myosin associated with hydrogen peroxide treatment of skeletal muscle fibers. Am J Physiol Cell Physiol. 2008;294:C613–C626. doi: 10.1152/ajpcell.00232.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Purintrapiban J, Wang MC, Forsberg NE. Degradation of sarcomeric and cytoskeletal proteins in cultured skeletal muscle cells. Comp Biochem Physiol B Biochem Mol Biol. 2003;136:393–401. doi: 10.1016/s1096-4959(03)00201-x. [DOI] [PubMed] [Google Scholar]
- 310.Pye D, Palomero J, Kabayo T, Jackson MJ. Real-time measurement of nitric oxide in single mature mouse skeletal muscle fibres during contractions. J Physiol. 2007;581:309–318. doi: 10.1113/jphysiol.2006.125930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Quintanilha AT. Effects of physical exercise and/or vitamin E on tissue oxidative metabolism. Biochem Soc Trans. 1984;12:403–404. doi: 10.1042/bst0120403. [DOI] [PubMed] [Google Scholar]
- 312.Quintanilha AT, Packer L. Vitamin E, physical exercise and tissue oxidative damage. Ciba Found Symp. 1983;101:56–69. doi: 10.1002/9780470720820.ch5. [DOI] [PubMed] [Google Scholar]
- 313.Quintanilha AT, Packer L, Davies JM, Racanelli TL, Davies KJ. Membrane effects of vitamin E deficiency: bioenergetic and surface charge density studies of skeletal muscle and liver mitochondria. Ann NY Acad Sci. 1982;393:32–47. doi: 10.1111/j.1749-6632.1982.tb31230.x. [DOI] [PubMed] [Google Scholar]
- 314.Radak Z, Taylor AW, Ohno H, Goto S. Adaptation to exercise-induced oxidative stress: from muscle to brain. Exerc Immunol Rev. 2001;7:90–107. [PubMed] [Google Scholar]
- 315.Rapola JM, Virtamo J, Ripatti S, Huttunen JK, Albanes D, Taylor PR, Heinonen OP. Randomised trial of alpha-tocopherol and beta-carotene supplements on incidence of major coronary events in men with previous myocardial infarction. Lancet. 1997;349:1715–1720. doi: 10.1016/S0140-6736(97)01234-8. [DOI] [PubMed] [Google Scholar]
- 316.Reid MB. Free radicals and muscle fatigue: of ROS, canaries, the IOC. Free Radic Biol Med. 2008;44:169–179. doi: 10.1016/j.freeradbiomed.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 317.Reid MB. Invited Review: redox modulation of skeletal muscle contraction: what we know and what we don’t. J Appl Physiol. 2001;90:724–731. doi: 10.1152/jappl.2001.90.2.724. [DOI] [PubMed] [Google Scholar]
- 318.Reid MB. Nitric oxide, reactive oxygen species, skeletal muscle contraction. Med Sci Sports Exerc. 2001;33:371–376. doi: 10.1097/00005768-200103000-00006. [DOI] [PubMed] [Google Scholar]
- 319.Reid MB. Role of nitric oxide in skeletal muscle: synthesis, distribution and functional importance. Acta Physiol Scand. 1998;162:401–409. doi: 10.1046/j.1365-201X.1998.0303f.x. [DOI] [PubMed] [Google Scholar]
- 320.Reid MB, Andrade FH, Balke CW, Esser KA. Redox mechanisms of muscle dysfunction in inflammatory disease. Phys Med Rehabil Clin N Am. 2005;16:925–949. doi: 10.1016/j.pmr.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 321.Reid MB, Haack KE, Franchek KM, Valberg PA, Kobzik L, West MS. Reactive oxygen in skeletal muscle. I. Intracellular oxidant kinetics and fatigue in vitro. J Appl Physiol. 1992;73:1797–1804. doi: 10.1152/jappl.1992.73.5.1797. [DOI] [PubMed] [Google Scholar]
- 322.Reid MB, Khawli FA, Moody MR. Reactive oxygen in skeletal muscle. III. Contractility of unfatigued muscle. J Appl Physiol. 1993;75:1081–1087. doi: 10.1152/jappl.1993.75.3.1081. [DOI] [PubMed] [Google Scholar]
- 323.Reid MB, Kobzik L, Bredt DS, Stamler JS. Nitric oxide modulates excitation-contraction coupling in the diaphragm. Comp Biochem Physiol A Mol Integr Physiol. 1998;119:211–218. doi: 10.1016/s1095-6433(97)00417-0. [DOI] [PubMed] [Google Scholar]
- 324.Reid MB, Moody MR. Dimethyl sulfoxide depresses skeletal muscle contractility. J Appl Physiol. 1994;76:2186–2190. doi: 10.1152/jappl.1994.76.5.2186. [DOI] [PubMed] [Google Scholar]
- 325.Reid MB, Shoji T, Moody MR, Entman ML. Reactive oxygen in skeletal muscle. II. Extracellular release of free radicals. J Appl Physiol. 1992;73:1805–1809. doi: 10.1152/jappl.1992.73.5.1805. [DOI] [PubMed] [Google Scholar]
- 326.Reid MB, Stokic DS, Koch SM, Khawli FA, Leis AA. N-acetyl-cysteine inhibits muscle fatigue in humans. J Clin Invest. 1994;94:2468–2474. doi: 10.1172/JCI117615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Rhee SG, Chae HZ, Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med. 2005;38:1543–1552. doi: 10.1016/j.freeradbiomed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 328.Rhee SG, Kang SW, Jeong W, Chang TS, Yang KS, Woo HA. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr Opin Cell Biol. 2005;17:183–189. doi: 10.1016/j.ceb.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 329.Rhee SG, Yang KS, Kang SW, Woo HA, Chang TS. Controlled elimination of intracellular H2O2: regulation of peroxiredoxin, catalase, glutathione peroxidase via post-translational modification. Antioxid Redox Signal. 2005;7:619–626. doi: 10.1089/ars.2005.7.619. [DOI] [PubMed] [Google Scholar]
- 330.Richmonds CR, Kaminski HJ. Nitric oxide synthase expression and effects of nitric oxide modulation on contractility of rat extraocular muscle. FASEB J. 2001;15:1764–1770. doi: 10.1096/fj.00-0899com. [DOI] [PubMed] [Google Scholar]
- 331.Roberts LJ, Morrow JD. Measurement of F(2)-isoprostanes as an index of oxidative stress in vivo. Free Radic Biol Med. 2000;28:505–513. doi: 10.1016/s0891-5849(99)00264-6. [DOI] [PubMed] [Google Scholar]
- 332.Robinson KM, Janes MS, Pehar M, Monette JS, Ross MF, Hagen TM, Murphy MP, Beckman JS. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc Natl Acad Sci USA. 2006;103:15038–15043. doi: 10.1073/pnas.0601945103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Rokitzki L, Logemann E, Huber G, Keck E, Keul J. α-Tocopherol supplementation in racing cyclists during extreme endurance training. Int J Sport Nutr. 1994;4:253–264. doi: 10.1123/ijsn.4.3.253. [DOI] [PubMed] [Google Scholar]
- 334.Rosenfeldt F, Hilton D, Pepe S, Krum H. Systematic review of effect of coenzyme Q10 in physical exercise, hypertension and heart failure. Biofactors. 2003;18:91–100. doi: 10.1002/biof.5520180211. [DOI] [PubMed] [Google Scholar]
- 335.Roy S, Packer L. Redox regulation of cell functions by alpha-lipoate: biochemical and molecular aspects. Biofactors. 1998;8:17–21. doi: 10.1002/biof.5520080104. [DOI] [PubMed] [Google Scholar]
- 336.Rudnick J, Puttmann B, Tesch PA, Alkner B, Schoser BG, Salanova M, Kirsch K, Gunga HC, Schiffl G, Luck G, Blottner D. Differential expression of nitric oxide synthases (NOS 1–3) in human skeletal muscle following exercise countermeasure during 12 wk of bed rest. FASEB J. 2004;18:1228–1230. doi: 10.1096/fj.03-0792fje. [DOI] [PubMed] [Google Scholar]
- 337.Sabine B, Willenbrock R, Haase H, Karczewski P, Wallukat G, Dietz R, Krause EG. Cyclic GMP-mediated phospholamban phosphorylation in intact cardiomyocytes. Biochem Biophys Res Commun. 1995;214:75–80. doi: 10.1006/bbrc.1995.2258. [DOI] [PubMed] [Google Scholar]
- 338.Sachdev S, Davies KJ. Production, detection, adaptive responses to free radicals in exercise. Free Radic Biol Med. 2008;44:215–223. doi: 10.1016/j.freeradbiomed.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 339.Sahlin K, Ekberg K, Cizinsky S. Changes in plasma hypoxanthine and free radical markers during exercise in man. Acta Physiol Scand. 1991;142:275–281. doi: 10.1111/j.1748-1716.1991.tb09157.x. [DOI] [PubMed] [Google Scholar]
- 340.Salama G, Abramson JJ, Pike GK. Sulphydryl reagents trigger Ca2+ release from the sarcoplasmic reticulum of skinned rabbit psoas fibres. J Physiol. 1992;454:389–420. doi: 10.1113/jphysiol.1992.sp019270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Salama G, Menshikova EV, Abramson JJ. Molecular interaction between nitric oxide and ryanodine receptors of skeletal and cardiac sarcoplasmic reticulum. Antioxid Redox Signal. 2000;2:5–16. doi: 10.1089/ars.2000.2.1-5. [DOI] [PubMed] [Google Scholar]
- 342.Sale DG. Influence of exercise and training on motor unit activation. Exerc Sport Sci Rev. 1987;15:95–151. [PubMed] [Google Scholar]
- 343.Salminen A, Vihko V. Endurance training reduces the susceptibility of mouse skeletal muscle to lipid peroxidation in vitro. Acta Physiol Scand. 1983;117:109–113. doi: 10.1111/j.1748-1716.1983.tb07184.x. [DOI] [PubMed] [Google Scholar]
- 344.Salvador A, Sousa J, Pinto RE. Hydroperoxyl, superoxide and pH gradients in the mitochondrial matrix: a theoretical assessment. Free Radic Biol Med. 2001;31:1208–1215. doi: 10.1016/s0891-5849(01)00707-9. [DOI] [PubMed] [Google Scholar]
- 345.Sastre J, Asensi M, Gasco E, Pallardo FV, Ferrero JA, Furukawa T, Vina J. Exhaustive physical exercise causes oxidation of glutathione status in blood: prevention by antioxidant administration. Am J Physiol Regul Integr Comp Physiol. 1992;263:R992–R995. doi: 10.1152/ajpregu.1992.263.5.R992. [DOI] [PubMed] [Google Scholar]
- 346.Scarlett DJ, Herst PM, Berridge MV. Multiple proteins with single activities or a single protein with multiple activities: the conundrum of cell surface NADH oxidoreductases. Biochim Biophys Acta. 2005;1708:108–119. doi: 10.1016/j.bbabio.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 347.Schaffer S, Muller WE, Eckert GP. Tocotrienols: constitutional effects in aging and disease. J Nutr. 2005;135:151–154. doi: 10.1093/jn/135.2.151. [DOI] [PubMed] [Google Scholar]
- 348.Scherer NM, Deamer DW. Oxidative stress impairs the function of sarcoplasmic reticulum by oxidation of sulfhydryl groups in the Ca2+-ATPase. Arch Biochem Biophys. 1986;246:589–601. doi: 10.1016/0003-9861(86)90314-0. [DOI] [PubMed] [Google Scholar]
- 349.Schulte I, Bektas H, Klempnauer J, Borlak J. Vitamin E in heart transplantation: effects on cardiac gene expression. Transplantation. 2006;81:736–745. doi: 10.1097/01.tp.0000191661.90834.b5. [DOI] [PubMed] [Google Scholar]
- 350.Sen CK. Update on thiol status and supplements in physical exercise. Can J Appl Physiol. 2001;26(Suppl):S4–S12. doi: 10.1139/h2001-037. [DOI] [PubMed] [Google Scholar]
- 351.Sen CK, Atalay M, Hanninen O. Exercise-induced oxidative stress: glutathione supplementation and deficiency. J Appl Physiol. 1994;77:2177–2187. doi: 10.1152/jappl.1994.77.5.2177. [DOI] [PubMed] [Google Scholar]
- 352.Sen CK, Marin E, Kretzschmar M, Hanninen O. Skeletal muscle and liver glutathione homeostasis in response to training, exercise, immobilization. J Appl Physiol. 1992;73:1265–1272. doi: 10.1152/jappl.1992.73.4.1265. [DOI] [PubMed] [Google Scholar]
- 353.Sen CK, Packer L. Antioxidant and redox regulation of gene transcription. FASEB J. 1996;10:709–720. doi: 10.1096/fasebj.10.7.8635688. [DOI] [PubMed] [Google Scholar]
- 354.Sen CK, Packer L, Hanninen O. Handbook of Oxidants and Antioxidants in Exercise. Amsterdam: Elsevier; 2000. p. 1207. [Google Scholar]
- 355.Sen CK, Rankinen T, Vaisanen S, Rauramaa R. Oxidative stress after human exercise: effect of N-acetylcysteine supplementation. J Appl Physiol. 1994;76:2570–2577. doi: 10.1152/jappl.1994.76.6.2570. [DOI] [PubMed] [Google Scholar]
- 356.Sevanian A, Davies KJ, Hochstein P. Conservation of vitamin C by uric acid in blood. J Free Radic Biol Med. 1985;1:117–124. doi: 10.1016/0748-5514(85)90015-7. [DOI] [PubMed] [Google Scholar]
- 357.Shanely RA, Zergeroglu MA, Lennon SL, Sugiura T, Yimlamai T, Enns D, Belcastro A, Powers SK. Mechanical ventilation-induced diaphragmatic atrophy is associated with oxidative injury and increased proteolytic activity. Am J Respir Crit Care Med. 2002;166:1369–1374. doi: 10.1164/rccm.200202-088OC. [DOI] [PubMed] [Google Scholar]
- 358.Sharpe P. Oxidative stress and exercise: need for antioxidant supplementation? Br J Sports Med. 1999;33:298–299. [PubMed] [Google Scholar]
- 359.Shimomura Y, Suzuki M, Sugiyama S, Hanaki Y, Ozawa T. Protective effect of coenzyme Q10 on exercise-induced muscular injury. Biochem Biophys Res Commun. 1991;176:349–355. doi: 10.1016/0006-291x(91)90931-v. [DOI] [PubMed] [Google Scholar]
- 360.Shindoh C, Dimarco A, Nethery D, Supinski G. Effect of PEG-superoxide dismutase on the diaphragmatic response to endotoxin. Am Rev Respir Dis. 1992;145:1350–1354. doi: 10.1164/ajrccm/145.6.1350. [DOI] [PubMed] [Google Scholar]
- 361.Shindoh C, DiMarco A, Thomas A, Manubay P, Supinski G. Effect of N-acetylcysteine on diaphragm fatigue. J Appl Physiol. 1990;68:2107–2113. doi: 10.1152/jappl.1990.68.5.2107. [DOI] [PubMed] [Google Scholar]
- 362.Siems W, Capuozzo E, Lucano A, Salerno C, Crifo C. High sensitivity of plasma membrane ion transport ATPases from human neutrophils towards 4-hydroxy-2,3-trans-nonenal. Life Sci. 2003;73:2583–2590. doi: 10.1016/s0024-3205(03)00661-1. [DOI] [PubMed] [Google Scholar]
- 363.Sies H. Oxidative Stress. London: Academic; 1985. [Google Scholar]
- 364.Sies H, Cadenas E. Oxidative stress: damage to intact cells and organs. Philos Trans R Soc Lond B Biol Sci. 1985;311:617–631. doi: 10.1098/rstb.1985.0168. [DOI] [PubMed] [Google Scholar]
- 365.Simic M, Jovanociv S. Antioxidantion mechanisms of uric acid. J Am Chem Soc. 1989;111:5778. [Google Scholar]
- 366.Smith CV, Jones DP, Guenthner TM, Lash LH, Lauterburg BH. Compartmentation of glutathione: implications for the study of toxicity and disease. Toxicol Appl Pharmacol. 1996;140:1–12. doi: 10.1006/taap.1996.0191. [DOI] [PubMed] [Google Scholar]
- 367.Smith LM, Golub AS, Pittman RN. Interstitial PO2 determination by phosphorescence quenching microscopy. Microcirculation. 2002;9:389–395. doi: 10.1038/sj.mn.7800147. [DOI] [PubMed] [Google Scholar]
- 368.Smith MA, Reid MB. Redox modulation of contractile function in respiratory and limb skeletal muscle. Respir Physiol Neurobiol. 2006;151:229–241. doi: 10.1016/j.resp.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 369.Smith RA, Kelso GF, Blaikie FH, Porteous CM, Ledgerwood EC, Hughes G, James AM, Ross MF, Asin-Cayuela J, Cocheme HM, Filipovska A, Murphy MP. Using mitochondria-targeted molecules to study mitochondrial radical production and its consequences. Biochem Soc Trans. 2003;31:1295–1299. doi: 10.1042/bst0311295. [DOI] [PubMed] [Google Scholar]
- 370.Snider IP, Bazzarre TL, Murdoch SD, Goldfarb A. Effects of coenzyme athletic performance system as an ergogenic aid on endurance performance to exhaustion. Int J Sport Nutr. 1992;2:272–286. doi: 10.1123/ijsn.2.3.272. [DOI] [PubMed] [Google Scholar]
- 371.St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem. 2002;277:44784–44790. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- 372.Stahl W, Sies H. Antioxidant activity of carotenoids. Mol Aspects Med. 2003;24:345–351. doi: 10.1016/s0098-2997(03)00030-x. [DOI] [PubMed] [Google Scholar]
- 373.Stamler JS, Meissner G. Physiology of nitric oxide in skeletal muscle. Physiol Rev. 2001;81:209–237. doi: 10.1152/physrev.2001.81.1.209. [DOI] [PubMed] [Google Scholar]
- 374.Starke DW, Chen Y, Bapna CP, Lesnefsky EJ, Mieyal JJ. Sensitivity of protein sulfhydryl repair enzymes to oxidative stress. Free Radic Biol Med. 1997;23:373–384. doi: 10.1016/s0891-5849(97)00009-9. [DOI] [PubMed] [Google Scholar]
- 375.Starnes JW, Cantu G, Farrar RP, Kehrer JP. Skeletal muscle lipid peroxidation in exercised and food-restricted rats during aging. J Appl Physiol. 1989;67:69–75. doi: 10.1152/jappl.1989.67.1.69. [DOI] [PubMed] [Google Scholar]
- 376.Stocker R. Antioxidant activities of bile pigments. Antioxid Redox Signal. 2004;6:841–849. doi: 10.1089/ars.2004.6.841. [DOI] [PubMed] [Google Scholar]
- 377.Stocker R, Glazer AN, Ames BN. Antioxidant activity of albumin-bound bilirubin. Proc Natl Acad Sci USA. 1987;84:5918–5922. doi: 10.1073/pnas.84.16.5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 379.Sugiura T, Ito N, Goto K, Naito H, Yoshioka T, Powers SK. Estrogen administration attenuates immobilization-induced skeletal muscle atrophy in male rats. J Physiol Sci. 2006;56:393–399. doi: 10.2170/physiolsci.RP006906. [DOI] [PubMed] [Google Scholar]
- 380.Sun J, Xu L, Eu JP, Stamler JS, Meissner G. Classes of thiols that influence the activity of the skeletal muscle calcium release channel. J Biol Chem. 2001;276:15625–15630. doi: 10.1074/jbc.M100083200. [DOI] [PubMed] [Google Scholar]
- 381.Supinski G. Free radical induced respiratory muscle dysfunction. Mol Cell Biochem. 1998;179:99–110. doi: 10.1023/a:1006859920875. [DOI] [PubMed] [Google Scholar]
- 382.Supinski G, Nethery D, DiMarco A. Effect of free radical scavengers on endotoxin-induced respiratory muscle dysfunction. Am Rev Respir Dis. 1993;148:1318–1324. doi: 10.1164/ajrccm/148.5.1318. [DOI] [PubMed] [Google Scholar]
- 383.Supinski GS, Callahan LA. Free radical-mediated skeletal muscle dysfunction in inflammatory conditions. J Appl Physiol. 2007;102:2056–2063. doi: 10.1152/japplphysiol.01138.2006. [DOI] [PubMed] [Google Scholar]
- 384.Supinski GS, Stofan D, Ciufo R, DiMarco A. N-acetylcysteine administration alters the response to inspiratory loading in oxygen-supplemented rats. J Appl Physiol. 1997;82:1119–1125. doi: 10.1152/jappl.1997.82.4.1119. [DOI] [PubMed] [Google Scholar]
- 385.Suzuki K, Ohno H, Ohishi S, Kizaki T, Ookawara T, Fukii J, Radak A, Taniguchi N. Superoxide dismutases in exercise and disease. In: Sen CK, Packer L, Hanninen O, editors. Handbook of Oxidants and Antioxidants and Exercise. Amsterdam: Elsevier; 2000. pp. 243–295. [Google Scholar]
- 386.Svensson M, Malm C, Tonkonogi M, Ekblom B, Sjodin B, Sahlin K. Effect of Q10 supplementation on tissue Q10 levels and adenine nucleotide catabolism during high-intensity exercise. Int J Sport Nutr. 1999;9:166–180. doi: 10.1123/ijsn.9.2.166. [DOI] [PubMed] [Google Scholar]
- 387.Testelmans D, Maes K, Wouters P, Powers SK, Decramer M, Gayan-Ramirez G. Infusions of rocuronium and cisatracurium exert different effects on rat diaphragm function. Intensive Care Med. 2007;33:872–879. doi: 10.1007/s00134-007-0584-4. [DOI] [PubMed] [Google Scholar]
- 388.Tidball JG, Lavergne E, Lau KS, Spencer MJ, Stull JT, Wehling M. Mechanical loading regulates NOS expression and activity in developing and adult skeletal muscle. Am J Physiol Cell Physiol. 1998;275:C260–C266. doi: 10.1152/ajpcell.1998.275.1.C260. [DOI] [PubMed] [Google Scholar]
- 389.Tiidus PM, Houston ME. Vitamin E status and response to exercise training. Sports Med. 1995;20:12–23. doi: 10.2165/00007256-199520010-00002. [DOI] [PubMed] [Google Scholar]
- 390.Tiidus PM, Pushkarenko J, Houston ME. Lack of antioxidant adaptation to short-term aerobic training in human muscle. Am J Physiol Regul Integr Comp Physiol. 1996;271:R832–R836. doi: 10.1152/ajpregu.1996.271.4.R832. [DOI] [PubMed] [Google Scholar]
- 391.Travaline JM, Sudarshan S, Roy BG, Cordova F, Leyenson V, Criner GJ. Effect of N-acetylcysteine on human diaphragm strength and fatigability. Am J Respir Crit Care Med. 1997;156:1567–1571. doi: 10.1164/ajrccm.156.5.96-09133. [DOI] [PubMed] [Google Scholar]
- 392.Urso ML, Clarkson PM. Oxidative stress, exercise, antioxidant supplementation. Toxicology. 2003;189:41–54. doi: 10.1016/s0300-483x(03)00151-3. [DOI] [PubMed] [Google Scholar]
- 393.Valenzuela A. The biological significance of malondialdehyde determination in the assessment of tissue oxidative stress. Life Sci. 1991;48:301–309. doi: 10.1016/0024-3205(91)90550-u. [DOI] [PubMed] [Google Scholar]
- 394.Van Remmen H, Hamilton ML, Richardson A. Oxidative damage to DNA and aging. Exerc Sport Sci Rev. 2003;31:149–153. doi: 10.1097/00003677-200307000-00009. [DOI] [PubMed] [Google Scholar]
- 395.Vasilaki A, Mansouri A, Remmen H, van der Meulen JH, Larkin L, Richardson AG, McArdle A, Faulkner JA, Jackson MJ. Free radical generation by skeletal muscle of adult and old mice: effect of contractile activity. Aging Cell. 2006;5:109–117. doi: 10.1111/j.1474-9726.2006.00198.x. [DOI] [PubMed] [Google Scholar]
- 396.Vasilaki A, McArdle F, Iwanejko LM, McArdle A. Adaptive responses of mouse skeletal muscle to contractile activity: the effect of age. Mech Ageing Dev. 2006;127:830–839. doi: 10.1016/j.mad.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 397.Venditti P, Di Meo S. Antioxidants, tissue damage, endurance in trained and untrained young male rats. Arch Biochem Biophys. 1996;331:63–68. doi: 10.1006/abbi.1996.0283. [DOI] [PubMed] [Google Scholar]
- 398.Venditti P, Di Meo S. Effect of training on antioxidant capacity, tissue damage, endurance of adult male rats. Int J Sports Med. 1997;18:497–502. doi: 10.1055/s-2007-972671. [DOI] [PubMed] [Google Scholar]
- 399.Vincent HK, Powers SK, Demirel HA, Coombes JS, Naito H. Exercise training protects against contraction-induced lipid peroxidation in the diaphragm. Eur J Appl Physiol Occup Physiol. 1999;79:268–273. doi: 10.1007/s004210050505. [DOI] [PubMed] [Google Scholar]
- 400.Vincent HK, Powers SK, Stewart DJ, Demirel HA, Shanely RA, Naito H. Short-term exercise training improves diaphragm antioxidant capacity and endurance. Eur J Appl Physiol. 2000;81:67–74. doi: 10.1007/PL00013799. [DOI] [PubMed] [Google Scholar]
- 401.Viner RI, Krainev AG, Williams TD, Schoneich C, Bigelow DJ. Identification of oxidation-sensitive peptides within the cytoplasmic domain of the sarcoplasmic reticulum Ca2+-ATPase. Biochemistry. 1997;36:7706–7716. doi: 10.1021/bi970058z. [DOI] [PubMed] [Google Scholar]
- 402.Viner RI, Williams TD, Schoneich C. Nitric oxide-dependent modification of the sarcoplasmic reticulum Ca-ATPase: localization of cysteine target sites. Free Radic Biol Med. 2000;29:489–496. doi: 10.1016/s0891-5849(00)00325-7. [DOI] [PubMed] [Google Scholar]
- 403.Watson WH, Pohl J, Montfort WR, Stuchlik O, Reed MS, Powis G, Jones DP. Redox potential of human thioredoxin 1 and identification of a second dithiol/disulfide motif. J Biol Chem. 2003;278:33408–33415. doi: 10.1074/jbc.M211107200. [DOI] [PubMed] [Google Scholar]
- 404.Westerblad H, Allen DG, Lannergren J. Muscle fatigue: lactic acid or inorganic phosphate the major cause? News Physiol Sci. 2002;17:17–21. doi: 10.1152/physiologyonline.2002.17.1.17. [DOI] [PubMed] [Google Scholar]
- 405.Wierzba TH, Olek RA, Fedeli D, Falcioni G. Lymphocyte DNA damage in rats challenged with a single bout of strenuous exercise. J Physiol Pharmacol. 2006;57(Suppl 10):115–131. [PubMed] [Google Scholar]
- 406.Williams DL, Jr, Swenson CA. Disulfide bridges in tropomyosin Effect on ATPase activity of actomyosin. Eur J Biochem. 1982;127:495–499. [PubMed] [Google Scholar]
- 407.Wingert RA, Galloway JL, Barut B, Foott H, Fraenkel P, Axe JL, Weber GJ, Dooley K, Davidson AJ, Schmid B, Paw BH, Shaw GC, Kingsley P, Palis J, Schubert H, Chen O, Kaplan J, Zon LI. Deficiency of glutaredoxin 5 reveals Fe-S clusters are required for vertebrate haem synthesis. Nature. 2005;436:1035–1039. doi: 10.1038/nature03887. [DOI] [PubMed] [Google Scholar]
- 408.Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134:489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- 409.Xia R, Webb JA, Gnall LL, Cutler K, Abramson JJ. Skeletal muscle sarcoplasmic reticulum contains a NADH-dependent oxidase that generates superoxide. Am J Physiol Cell Physiol. 2003;285:C215–C221. doi: 10.1152/ajpcell.00034.2002. [DOI] [PubMed] [Google Scholar]
- 410.Xu KY, Zweier JL, Becker LC. Hydroxyl radical inhibits sarcoplasmic reticulum Ca2+-ATPase function by direct attack on the ATP binding site. Circ Res. 1997;80:76–81. doi: 10.1161/01.res.80.1.76. [DOI] [PubMed] [Google Scholar]
- 411.Yamada T, Mishima T, Sakamoto M, Sugiyama M, Matsunaga S, Wada M. Oxidation of myosin heavy chain and reduction in force production in hyperthyroid rat soleus. J Appl Physiol. 2006;100:1520–1526. doi: 10.1152/japplphysiol.01456.2005. [DOI] [PubMed] [Google Scholar]
- 412.Yi X, Maeda N. α-Lipoic acid prevents the increase in atherosclerosis induced by diabetes in apolipoprotein E-deficient mice fed high-fat/low-cholesterol diet. Diabetes. 2006;55:2238–2244. doi: 10.2337/db06-0251. [DOI] [PubMed] [Google Scholar]
- 413.Yoshioka J, Schreiter ER, Lee RT. Role of thioredoxin in cell growth through interactions with signaling molecules. Antioxid Redox Signal. 2006;8:2143–2151. doi: 10.1089/ars.2006.8.2143. [DOI] [PubMed] [Google Scholar]
- 414.Yu BP. Cellular defenses against damage from reactive oxygen species. Physiol Rev. 1994;74:139–162. doi: 10.1152/physrev.1994.74.1.139. [DOI] [PubMed] [Google Scholar]
- 415.Zable AC, Favero TG, Abramson JJ. Glutathione modulates ryanodine receptor from skeletal muscle sarcoplasmic reticulum. Evidence for redox regulation of the Ca2+ release mechanism. J Biol Chem. 1997;272:7069–7077. doi: 10.1074/jbc.272.11.7069. [DOI] [PubMed] [Google Scholar]
- 416.Zamocky M, Koller F. Understanding the structure and function of catalases: clues from molecular evolution and in vitro mutagenesis. Prog Biophys Mol Biol. 1999;72:19–66. doi: 10.1016/s0079-6107(98)00058-3. [DOI] [PubMed] [Google Scholar]
- 417.Zerba E, Komorowski TE, Faulkner JA. Free radical injury to skeletal muscles of young, adult, old mice. Am J Physiol Cell Physiol. 1990;258:C429–C435. doi: 10.1152/ajpcell.1990.258.3.C429. [DOI] [PubMed] [Google Scholar]
- 418.Zhang JZ, Wu Y, Williams BY, Rodney G, Mandel F, Strasburg GM, Hamilton SL. Oxidation of the skeletal muscle Ca2+ release channel alters calmodulin binding. Am J Physiol Cell Physiol. 1999;276:C46–C53. doi: 10.1152/ajpcell.1999.276.1.c46. [DOI] [PubMed] [Google Scholar]
- 419.Zhang Q, Scholz PM, He Y, Tse J, Weiss HR. Cyclic GMP signaling and regulation of SERCA activity during cardiac myocyte contraction. Cell Calcium. 2005;37:259–266. doi: 10.1016/j.ceca.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 420.Zhang YH, Zhang MH, Sears CE, Emanuel K, Redwood C, El-Armouche A, Kranias EG, Casadei B. Reduced phospholamban phosphorylation is associated with impaired relaxation in left ventricular myocytes from neuronal NO synthase deficient mice. Circ Res. 2008;102:242–249. doi: 10.1161/CIRCRESAHA.107.164798. [DOI] [PubMed] [Google Scholar]
- 421.Zhao X, Bey EA, Wientjes FB, Cathcart MK. Cytosolic phospholipase A2 (cPLA2) regulation of human monocyte NADPH oxidase activity. cPLA2 affects translocation but not phosphorylation of p67(phox) and p47(phox) J Biol Chem. 2002;277:25385–25392. doi: 10.1074/jbc.M203630200. [DOI] [PubMed] [Google Scholar]
- 422.Zhou LZ, Johnson AP, Rando TA. NFκB and AP-1 mediate transcriptional responses to oxidative stress in skeletal muscle cells. Free Radic Biol Med. 2001;31:1405–1416. doi: 10.1016/s0891-5849(01)00719-5. [DOI] [PubMed] [Google Scholar]
- 423.Ziegler D, Hanefeld M, Ruhnau KJ, Meissner HP, Lobisch M, Schutte K, Gries FA. Treatment of symptomatic diabetic peripheral neuropathy with the antioxidant alpha-lipoic acid. A 3-week multicentre randomized controlled trial (ALADIN study) Diabetologia. 1995;38:1425–1433. doi: 10.1007/BF00400603. [DOI] [PubMed] [Google Scholar]
- 424.Zima AV, Blatter LA. Redox regulation of cardiac calcium channels and transporters. Cardiovasc Res. 2006;71:310–321. doi: 10.1016/j.cardiores.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 425.Zissimopoulos S, Docrat N, Lai FA. Redox sensitivity of the ryanodine receptor interaction with FK506-binding protein. J Biol Chem. 2007;282:6976–6983. doi: 10.1074/jbc.M607590200. [DOI] [PubMed] [Google Scholar]
- 426.Zuo L, Christofi FL, Wright VP, Bao S, Clanton TL. Lipoxygenase-dependent superoxide release in skeletal muscle. J Appl Physiol. 2004;97:661–668. doi: 10.1152/japplphysiol.00096.2004. [DOI] [PubMed] [Google Scholar]
- 427.Zuo L, Christofi FL, Wright VP, Liu CY, Merola AJ, Berliner LJ, Clanton TL. Intra- and extracellular measurement of reactive oxygen species produced during heat stress in diaphragm muscle. Am J Physiol Cell Physiol. 2000;279:C1058–C1066. doi: 10.1152/ajpcell.2000.279.4.C1058. [DOI] [PubMed] [Google Scholar]