Abstract
Objective
To review the literature on the epidemiology of Campylobacter associated ReA.
Methods
A Medline (PubMed) search identified studies from 1966–2006 that investigated the epidemiology of Campylobacter associated ReA. Search terms included: “reactive arthritis”, “spondyloarthropathy”, “Reiter’s syndrome”, “gastroenteritis”, “diarrhea”, “epidemiology”, “incidence”, “prevalence”, and “Campylobacter”.
Results
The literature available to date suggests that the incidence of Campylobacter reactive arthritis may occur in 1 to 5% of those infected. The annual incidence of ReA after Campylobacter or Shigella may be 4.3 and 1.3 respectively per 100,000. The duration of acute ReA varies considerably between reports, and the incidence and impact of chronic reactive arthritis from Campylobacter infection is virtually unknown.
Conclusions
Campylobacter associated ReA incidence and prevalence varies widely from reviews such as: case ascertainment differences, exposure differences, lack of diagnostic criteria for ReA and perhaps genetics and ages of exposed individuals. At the population level it may not be associated with HLA-B27 and inflammatory back involvement is uncommon. Follow up for long-term sequelae is largely unknown. Five percent of Campylobacter ReA may be chronic or relapsing (with respect to musculoskeletal symptoms).
Keywords: Campylobacter jejuni, Campylobacter coli, reactive arthritis, incidence, sequelae
Introduction
Campylobacter gastroenteritis is common
In both developed and developing countries, Campylobacter jejuni is the most common cause of human bacterial enteritis accounting for 5–14% of all diarrheal diseases worldwide [1]. Most cases are sporadic; however outbreaks do occur. For example Campylobacter accounts for 59% of water or food borne cases of diarrhea in Canada and its incidence is increasing [2]. Similarly in the USA, it is estimated that 2.1–2.4 million cases of human campylobacteriosis occur each year [3, 4]. The most common serotype of Campylobacter is C. jejuni, which accounts for 90–95% of positive stools. The second most common serotype C. coli only accounts for 5–10% of cases [2].
Clinical Presentation of Acute Reactive Arthritis (ReA) after Campylobacter and other Enteric Infections
ReA is one of the spondyloarthropathies, a group of diseases with a strong association with HLA-B27, absence of rheumatoid factor (RF), family aggregation and frequent extra-articular symptomatology [5]. Acute ReA is characterized by a sterile joint inflammation. It typically develops within four weeks following an intestinal or urogenital infection with obligate or facultative intracellular bacteria, such as Salmonella, Shigella, Yersinia and Campylobacter, and to a far lesser extent E. coli O157:H7 [6, 7]. The number of infectious agents associated with ReA is gradually increasing (Table 1). A detailed history is critical to establish the diagnosis - the triggering infection is mild and often overlooked as the inciting factor [8]. The symptomatology is predominantly joint and musculoskeletal (MSK) involvement, but skin and mucous membranes, gastrointestinal, ocular [9], and cardiac symptoms have also been described and should be specifically assessed [10, 11]. The joint symptoms vary from mild mono- or oligo-arthralgia to a severely disabling polyarthritis [12–15]. The arthritis has a predilection for joints of the lower extremity, particularly knees and ankles but not necessarily axial involvement. It can also involve small joints (swelling with or without erythematous discolouration of the joint) or it can present as tenosynovitis. ReA should be considered in the differential diagnosis of anyone presenting with a mono- or oligoarthritis of unknown aetiology.
Table 1.
Infectious agents associated with the development of reactive arthritis (Modified from [71])
|
Enteric bacteria |
|
|
Urinary bacteria |
|
Respiratory bacteria |
most common pathogens
Hypothesized causal agents
At present, there are no clear recommendations for testing to identify the causative bacterium of reactive arthritis in routine practice. For ReA severe enough to prompt the patient to seek medical attention, an acute phase reactants response and neutrophilia is usually seen. RF and antinuclear antibodies are negative. Microscopy along with a culture of synovial fluid should be undertaken to exclude septic arthritis and crystal-induced arthritis. Stool should also be cultured, as Salmonella, Yersinia and Campylobacter persists for some time after initial infection [16]. Additionally, serological techniques to identify Campylobacter exposure can be used; however their sensitivity and specificity appears lower than those for Salmonella and Yersinia [16]. High titres of IgG to Campylobacter are not necessarily diagnostic of a recent infection as the general population may commonly encounter this organism in food such as chicken and eggs. Rising IgG and/or persistently high IgA are therefore diagnostically preferable [16]. A major drawback in the diagnostic evaluation is that there are no uniform criteria for ReA.
Biological Pathogenesis of Acute ReA After Campylobacter and other Enteric Infections
This risk of developing post-enteric ReA seems to be slightly greater for infection with Salmonella and Yersinia than for Campylobacter, but further work is required to clarify this issue [17, 18]. The pathophysiology is largely unknown; however, it has been hypothesized that an interaction of host HLA-B27 and certain bacteria play a crucial role in the development of ReA. Population prevalence of HLA-B27 ranges from 0 to 50% [19]. In some, but not all studies, HLA-B27 positive individuals tend to have more severe disease with a higher tendency to develop chronic ReA compared to HLA-B27 negative individuals [14, 15, 20]. For example, in a review of reported Campylobacter-associated ReA cases in 1994, 56% of 29 subjects were HLA-B27 positive and the presence of the allele was associated with more severe disease [21]. However, a recent population-based study of Campylobacter-associated ReA showed no association with HLA-B27 [2]. There may be a referral bias to tertiary care centres in those with more severe or prolonged disease (HLA-B27+ as high as 70% in hospital-based reports) [11, 22–33]. This would select for a higher prevalence of HLA-B27, thus supporting that HLA-B27 is not necessary for ReA development, but it may increase disease severity and chronicity.
ReA occurs after a urogenital or enteric infection, but the organism cannot always be identified [34–36]. In patients with ReA, bacterial antigens derived from the causative organism(s) seem to disseminate in the body, and bacterial antigens have been isolated in the synovial fluid and surrounding tissues, but only in the early stages of arthropathy [37–40]. Chlamydia DNA and RNA, and Yersinia DNA have been detected in the synovial fluid of joints of ReA patients; however Chlamydia nucleic acids were also present in the joints of asymptomatic controls [41–43]. Abnormalities in the production and levels, in addition to polymorphism, of proinflammatory cytokines seem to play an important role in the development and duration of ReA [44–47]. Molecular mimicry is thought to precipitate synovitis in ReA. Molecular mimicry is confusion of self-antigens with infectious antigens. There are many good review articles about lack of tolerance to self/molecular mimicry; however, this is beyond the scope of this review.
Gaston and Lillicrap recently proposed a hypothesis of the pathogenesis of ReA [48], in which bacteria persist in the epithelium, associated lymph nodes, liver and spleen, following invasion of the mucosa. Bacteria and/or their antigens disseminate into the joints, causing an inflammatory response leading to an arthritic process that is driven and probably also supported by CD4+ T-cells. Abnormal T helper responses may favour the bacteria and/or antigen persistence.
Methods
A Medline (PubMed) search was conducted to identify all studies from 1966–2006 that investigated the epidemiology of Campylobacter and other bacteria-associated reactive arthritis. Search terms included: “reactive arthritis”, “spondyloarthropathy”, “Reiter’s syndrome”, “gastroenteritis”, “diarrhea”, “epidemiology”, “incidence”, and “prevalence”, “Campylobacter”, “Shigella”, “E. coli”, “salmonella” and other individual know bacterial triggers of reactive arthritis. Various combinations of the search terms and connectors were used. Studies with relevance to the stated objective were included in this review.
Results
Epidemiology of Acute ReA after Campylobacter and E. coli
The prevalence of acute ReA has been estimated between 1–7%, where variability in the estimates may have been due to the criteria used for diagnosis and the setting in which the study was undertaken (hospital series vs. single-source outbreaks vs. community-based series) [9, 49–54]. The annual incidence of ReA after bowel or urogenital infection is 30–40/100,000 [55–59]; however, the true incidence is difficult to assess because of the varied clinical severity and milder cases that are frequently unreported.
Hannu et al. reported the annual incidence of ReA in Finland due to Campylobacter to be 4.3/100.000 [2], which is higher than the recently reported 1.3/100.00 incidence of Shigella-induced ReA [60]. As Campylobacter has become the most common cause of gastroenteritis in the western world, it can be predicted that the number of Campylobacter-induced ReA will increase [7, 9, 61].
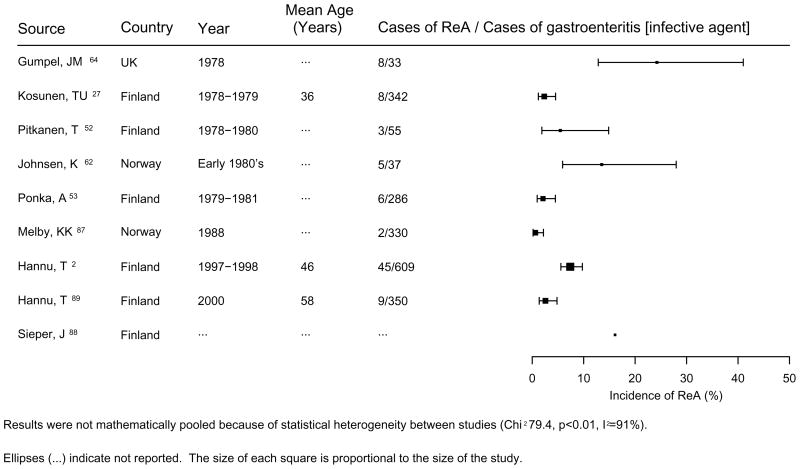
The most commonly affected age group is young adults with males and females being equally affected. ReA seems to be a rare complication of Campylobacter infection in children [24, 28, 62, 63], this is similar to the findings for ReA due to Salmonella [18] and Yersinia [14] infections. Sporadic cases of ReA have been described following Campylobacter infection [11, 22–33, 51, 62–66]. Only a few outbreaks have been followed from a rheumatologic perspective, and all were limited to short follow-up (Table 3). Following infection with Campylobacter species symptoms of probable chronic ReA, which include arthralgias to overt arthritis have been described in 0.7 and 24% of cases [2, 51, 59, 65, 67]. Similarly, symptoms of chronic ReA have developed in 6% of cases following an infection with E. coli [67] (Table 4). Hannu et al described a 7% incidence of ReA in a cohort of 870 post-Campylobacter enteritis patients, with the majority of cases (82%) associated with C. jejuni infection [2]. One study suggested that half of affected individuals had ReA symptoms lasting longer than one year [67]. Figure 1 summarizes the incidence of acute ReA following Campylobacter gastroenteritis reported from various studies since the late 1970s.
Table 3.
Incidence of acute reactive arthritis (ReA) following Campylobacter gastroenteritis
| Source/Year of infection | Cases of ReA/Cases of gastroenteritis [infective agent] | Mean age (yrs) | Comments |
|---|---|---|---|
| Gumpel, JM [64] UK 1978 |
8/33 (24%) [serotypes not reported] |
NR |
|
| Kosunen, TU [27] Finland 1978–1979 |
8/342 (2.3%) [C. jejuni] |
36 |
|
| Pitkanen, T [52] Finland 1978–1980 |
3/55 (1.7%) [C. jejuni] |
NR |
|
| Johnsen, K [62] Norway ‡ Early 1980’s |
5/37 (13.5%) [C. jejuni] |
NR |
|
| Pönkä, A [53] Finland 1979–1981 |
6/283 (2.1%) [C. jejuni] |
NR |
|
| Pitkänen, T [56] Finland 1978 – 1981 |
9/188 (5%) [C. jejuni] |
NR |
|
| Melby, KK [87] Norway 1988 |
2/330 (0.6%) [serotypes not reported for ReA cases] |
NR |
|
| Hannu, T [2] Finland ‡ 1997–1998 |
45/609 (7%) [C. jejuni N=37 C. coli N=8] |
46 |
|
| Hannu, T [9] Finland 2000 |
9/350 (2.6%) [C. jejuni] |
58 |
|
| Sieper, J for Leirisalo-Repo [88] Finland |
Overall ReA frequency 16.1% | NR |
|
NR, not reported; RA, rheumatoid arthritis; Re, reactive; ReTEB, reactive tendonitis, enthesopathy or bursitis; Q, questionnaire
[67] At the population level, the frequency of ReA seems to be higher vs. post-outbreak Campylobacter ReA, have low association with HLA-B27 and arthritis seems to affect small joints.
Table 4.
Chronic Sequelae of Campylobacter and E. coli gastroenteritis
| Source/Year of infection | Cases of ReA/Cases of gastroenteritis | Mean age (yrs) | Chronic ReA | Comments |
|---|---|---|---|---|
| Eastmond [51] United Kingdom 1979 |
1/130 (0.8%) [C. jejuni] |
34 |
|
|
| Bremell, [65] Sweden 1981 |
5/86 (5.8%) [C. jejuni] |
27 |
Group A 1/35
|
|
|
Group B 4/31 Symptoms started 3–8 months after outbreak:
| ||||
Group C 0/20
| ||||
| Locht, [67] Denmark 1997–1999 |
27/173 (16%) ReA spectrum [C. jejuni, C. coli] 10/177 (6%) [ETEC] |
36 (median) 43 (median) |
5 pts symptomatic for > 1 year |
|
Q = questionnaire
Figure 1.
Incidence of acute ReA following Camplobacter gastroenteritis
At the time of arthritis, the symptoms of triggering infection have usually subsided and the stool cultures are usually negative. If cultures are negative, the causative agent has to be confirmed by serological methods, which may very between facilities. Table 3 summarizes the incidence data on the acute ReA due to Campylobacter infection.
Discussion
The Possibility of Chronic Arthritis and other Long-term Musculoskeletal Symptoms after Campylobacter or E. coli Infection
Data are lacking on the proportion of patients that develop chronic ReA. The duration of arthritis for more than 6 months has been arbitrarily regarded as a sign of chronic pathology. Very few cohort studies have investigated the incidence and chronicity of ReA after infection with Campylobacter species and/or enterotoxigenic Escherichia coli (ETEC) [2, 51, 65, 67, 68]. It was only in the late 1970’s that ReA was noted to be triggered by an infection with Campylobacter [64]. E. coli urinary infection or diarrhea has also been associated with ReA [40, 67, 69]. The typical duration of symptoms is less than 6 months; however, some individuals exhibit chronic symptoms for a longer period [40]. It has been reported that ReA recurred 7 years after the initial exposure in a HLA-B27 positive woman[65]. Acute gastroenteritis may lead to a chronic inflammatory state with elevated CRP and ESR [55]. The signs and intensity of ReA in relation to the initial gastrointestinal infection has not been completely determined. Locht et al observed that Campylobacter patients who reported joint pain had more severe gastrointestinal symptoms and a longer duration of diarrhea compared to those who did not report joint pain [67].
In contrast, Bremell et al found minimal association between acute gastroenteritis severity and the chronicity of ReA - 20% of patients with acute intestinal infection developed a self-limited ReA of less than one months duration, while 13% of patients with positive Campylobacter serology but no symptoms of gastroenteritis reported long-term rheumatic problems months following infection [67]. Table 4 summarizes data regarding the chronic sequelae of Campylobacter and E. coli ReA.
Depending on the infectious agent and follow-up time 18% of patients may suffer from chronic arthritis, up to 49% and 26% from sacroiliitis and ankylosing spondylitis, respectively [70]. The long-term prognosis for post-Campylobacter ReA is not defined in the current literature (Table 4). A 5-year follow-up of patients with acute ReA following Campylobacter enteritis reported chronic or relapsing rheumatic symptoms in 5% of the exposed population [65], but the numbers in the outbreak were small.
Campylobacter and to a lesser extent E. coli infection play a role in the development of acute ReA; however it is uncertain to what extent they induce chronic MSK symptomatology. Future studies are required to look at the health and economic impact of ReA, especially its chronic forms.
Table 2.
Reactive Arthritis Classification Criteria
|
Proposed Diagnostic Criteria for Reactive Arthritis [85] | |
| Exclusion criteria | |
|
Other known causes of mono/oligoarthritis, such as: |
Plus
|
|
|
Proposal for classification of reactive arthritis for patients entering clinical and experimental studies [86] | |
| Probable reactive arthritis |
|
| Definite reactive arthritis triggered by bacteria |
|
| Bacteria associated undifferentiated oligoarthritis or spondyloarthropathy | Classification which may be used in the absence of the above stated criteria |
Acknowledgments
This work was supported by a grant (#76289) from the Canadian Institutes of Health Research (CIHR).
Grant support: CIHR Grant #76289
Contributor Information
Janet E. Pope, Professor of Medicine and Epidemiology & Biostatistics, Division of Rheumatology, Department of Medicine, The University of Western Ontario, Canada.
Adriana Krizova, The University of Western Ontario, Canada.
Amit X. Garg, Director, London Kidney Research Unit; Assistant Professor of Medicine and Epidemiology & Biostatistics, Division of Nephrology, The University of Western Ontario and London Health Sciences Centre, London, Canada.
Heather Thiessen-Philbrook, London Kidney Research Unit, London Health Sciences Centre, London, Canada.
Janine M. Ouimet, Department of Medicine, The University of Western Ontario.
References
- 1.Rautelin H, Hanninen ML. Campylobacters: the most common bacterial enteropathogens in the Nordic countries. Ann Med. 2000;32(7):440–5. doi: 10.3109/07853890009002018. [DOI] [PubMed] [Google Scholar]
- 2.Hannu T, Mattila L, Rautelin H, Pelkonen P, Lahdenne P, Siitonen A, et al. Campylobacter-triggered reactive arthritis: a population-based study. Rheumatology (Oxford) 2002;41(3):312–8. doi: 10.1093/rheumatology/41.3.312. [DOI] [PubMed] [Google Scholar]
- 3.Altekruse SF, Stern NJ, Fields PI, Swerdlow DL. Campylobacter jejuni--an emerging foodborne pathogen. Emerg Infect Dis. 1999;5(1):28–35. doi: 10.3201/eid0501.990104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedman CR, Hoekstra RM, Samuel M, Marcus R, Bender J, Shiferaw B, et al. Risk factors for sporadic Campylobacter infection in the United States: A case-control study in FoodNet sites. Clin Infect Dis. 2004;38(Suppl 3):S285–96. doi: 10.1086/381598. [DOI] [PubMed] [Google Scholar]
- 5.Dougados M, van der Linden S, Juhlin R, Huitfeldt B, Amor B, Calin A, et al. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum. 1991;34(10):1218–27. doi: 10.1002/art.1780341003. [DOI] [PubMed] [Google Scholar]
- 6.Braun J, Kingsley G, van der Heijde D, Sieper J. On the difficulties of establishing a consensus on the definition of and diagnostic investigations for reactive arthritis. Results and discussion of a questionnaire prepared for the 4th International Workshop on Reactive Arthritis, Berlin, Germany, July 3–6, 1999. J Rheumatol. 2000;27(9):2185–92. [PubMed] [Google Scholar]
- 7.Rees JR, Pannier MA, McNees A, Shallow S, Angulo FJ, Vujia DJ. Persistent diarrhea, arthritis, and other complications of enteric infections: a pilot survey based on California FoodNet surveillance, 1998–1999. Clin Infect Dis. 2004;38(Suppl 3):S311–7. doi: 10.1086/381601. [DOI] [PubMed] [Google Scholar]
- 8.Kvien TK, Glennas A, Melby K, Granfors K, Andrup O, Karstensen B, et al. Reactive arthritis: incidence, triggering agents and clinical presentation. J Rheumatol. 1994;21(1):115–22. [PubMed] [Google Scholar]
- 9.Hannu T, Sihto-Kauppi K, Kotaniemi K, Kauppi M. Acute anterior uveitis in association with an outbreak of Campylobacter jejuni infection. Scand J Rheumatol. 2004;33(1):55–7. doi: 10.1080/03009740310004045. [DOI] [PubMed] [Google Scholar]
- 10.Hannu T, Mattila L, Rautelin H, Siitonen A, Leirisalo-Repo M. Three cases of cardiac complications associated with Campylobacter jejuni infection and review of the literature. Eur J Clin Microbiol Infect Dis. 2005;24(9):619–22. doi: 10.1007/s10096-005-0001-2. [DOI] [PubMed] [Google Scholar]
- 11.Ponka A, Pitkanen T, Pettersson T, Aittoniemi S, Kosunen TU. Carditis and arthritis associated with Campylobacter jejuni infection. Acta Med Scand. 1980;208(6):495–6. doi: 10.1111/j.0954-6820.1980.tb01237.x. [DOI] [PubMed] [Google Scholar]
- 12.Wright V, Moll J. In: Seronegative polyarthritis. Wright V, Moll J, editors. North Holland Publishing Company; Amsterdam: 1976. [Google Scholar]
- 13.Ahvonen P. Human yersiniosis in Finland. II. Clinical features. Ann Clin Res. 1972;4(1):39–48. [PubMed] [Google Scholar]
- 14.Leirisalo M, Skylv G, Kousa M, Voipio-Pulkki LM, Suoranta H, Nissila M, et al. Followup study on patients with Reiter’s disease and reactive arthritis, with special reference to HLA-B27. Arthritis Rheum. 1982;25(3):249–59. doi: 10.1002/art.1780250302. [DOI] [PubMed] [Google Scholar]
- 15.Lahesmaa-Rantala R, Toivanen A. Clinical spectrum of reactive arthritis. In: Toivanen A, Toivanen P, editors. Reactive arthritis. CRC Press; Boca Raton: 1988. pp. 1–13. [Google Scholar]
- 16.Fendler C, Laitko S, Sorensen H, Gripenberg-Lerche C, Groh A, Uksila J, et al. Frequency of triggering bacteria in patients with reactive arthritis and undifferentiated oligoarthritis and the relative importance of the tests used for diagnosis. Ann Rheum Dis. 2001;60(4):337–43. doi: 10.1136/ard.60.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill Gaston J, Lillicrap M. Arthritis associated with enteric infection. Best Practice & Res Clin Rheumatol. 2003;17(2):219–239. doi: 10.1016/s1521-6942(02)00104-3. [DOI] [PubMed] [Google Scholar]
- 18.Mattila L, Leirisalo-Repo M, Koskimies S, Granfors K, Siitonen A. Reactive arthritis following an outbreak of Salmonella infection in Finland. Br J Rheumatol. 1994;33(12):1136–41. doi: 10.1093/rheumatology/33.12.1136. [DOI] [PubMed] [Google Scholar]
- 19.Khan MA. HLA-B27 and its subtypes in world populations. Curr Opin Rheumatol. 1995;7(4):263–9. doi: 10.1097/00002281-199507000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Calin A, Fries J. An experimental epidemic of Reiter’s syndrome revisited. Follow-up evidence on genetic and environmental factors. Ann Intern Med. 1976;84:564–566. doi: 10.7326/0003-4819-84-5-564. [DOI] [PubMed] [Google Scholar]
- 21.Peterson MC. Rheumatic manifestations of Campylobacter jejuni and C. fetus infections in adults. Scand J Rheumatol. 1994;23(4):167–70. doi: 10.3109/03009749409103055. [DOI] [PubMed] [Google Scholar]
- 22.Berden JH, Muytjens HL, van de Putte LB. Reactive arthritis associated with Campylobacter jejuni enteritis. Br Med J. 1979;1(6160):380–1. doi: 10.1136/bmj.1.6160.380-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weir W, Keat A, Welsby P, Brear G. Reactive arthritis associated with Campylobacter infection of the bowel. J Infect. 1979;1:281–284. [Google Scholar]
- 24.Bekassy A, Enell H, Schalen C. Severe polyarthritis following Campylobacter jejuni enteritis. Arthritis Rheum. 1980;69:269–271. doi: 10.1111/j.1651-2227.1980.tb07075.x. [DOI] [PubMed] [Google Scholar]
- 25.Leung FY, Littlejohn GO, Bombardier C. Reiter’s syndrome after Campylobacter jejuni enteritis. Arthritis Rheum. 1980;23(8):948–50. doi: 10.1002/art.1780230813. [DOI] [PubMed] [Google Scholar]
- 26.van de Putte LB, Berden JH, Boerbooms MT, Muller WH, Rasker JJ, Reynvaan-Groendijk A, et al. Reactive arthritis after Campylobacter jejuni enteritis. J Rheumatol. 1980;7(4):531–5. [PubMed] [Google Scholar]
- 27.Kosunen TU, Ponka A, Kauranen O, Martio J, Pitkanen T, Hortling L, et al. Arthritis associated with Campylobacter jejuni enteritis. Scand J Rheumatol. 1981;10(2):77–80. doi: 10.3109/03009748109095276. [DOI] [PubMed] [Google Scholar]
- 28.Schaad UB. Reactive arthritis associated with Campylobacter enteritis. Pediatr Infect Dis. 1982;1(5):328–32. doi: 10.1097/00006454-198209000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Short CD, Klouda PT, Smith L. Campylobacter jejuni enteritis and reactive arthritis. Ann Rheum Dis. 1982;41(3):287–8. doi: 10.1136/ard.41.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Albert J, Spaeth PJ, Ott H, Buetler R. Symmetrical reactive oligoarthritis after Campylobacter jejuni enteritis--case report and study of the synovial complement. Z Rheumatol. 1983;42(3):104–6. [PubMed] [Google Scholar]
- 31.Bengtsson A, Lindstrom FD, Normann BE. Reactive arthritis after campylobacter jejuni enteritis. A case report. Scand J Rheumatol. 1983;12(2):181–2. doi: 10.3109/03009748309102908. [DOI] [PubMed] [Google Scholar]
- 32.Ebright JR, Ryan LM. Acute erosive reactive arthritis associated with Campylobacter jejuni-induced colitis. Am J Med. 1984;76(2):321–3. doi: 10.1016/0002-9343(84)90794-0. [DOI] [PubMed] [Google Scholar]
- 33.Pipalia DH, Plumber ST, Vora S, Mehta A, Vora IM, Naik SR. Campylobacter jejuni infection with acute self limiting colitis and polyarthritis. Indian J Gastroenterol. 1988;7(1):47–8. [PubMed] [Google Scholar]
- 34.Calin A, Taurog J. The spondylarthropathies. Oxford: Oxford University Press; 1998. [Google Scholar]
- 35.Hughes R, Keat A. Reactive arthritis: the role of bacterial antigens in inflammatory arthritis. Baillieres Clin Rheumatol. 1992;6(2):285–308. doi: 10.1016/s0950-3579(05)80175-x. [DOI] [PubMed] [Google Scholar]
- 36.Spencer Wells R, Parham P. In: HLA Class I genes: structure and diversity in HLA and MHC genes, molecules and function. Browning M, McMichael A, editors. BIOS Scientific Publisher; Oxford: 1996. pp. 77–96. [Google Scholar]
- 37.Granfors K, Merilahti-Palo R, Luukkainen R, Mottonen T, Lahesmaa R, Probst P, et al. Persistence of Yersinia antigens in peripheral blood cells from patients with Yersinia enterocolitica O:3 infection with or without reactive arthritis. Arthritis Rheum. 1998;41(5):855–62. doi: 10.1002/1529-0131(199805)41:5<855::AID-ART12>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 38.Nikkari S, Rantakokko K, Ekman P, Mottonen T, Leirisalo-Repo M, Virtala M, et al. Salmonella-triggered reactive arthritis: use of polymerase chain reaction, immunocytochemical staining, and gas chromatography-mass spectrometry in the detection of bacterial components from synovial fluid. Arthritis Rheum. 1999;42(1):84–9. doi: 10.1002/1529-0131(199901)42:1<84::AID-ANR11>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 39.Gaston JS, Cox C, Granfors K. Clinical and experimental evidence for persistent Yersinia infection in reactive arthritis. Arthritis Rheum. 1999;42(10):2239–42. doi: 10.1002/1529-0131(199910)42:10<2239::AID-ANR29>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 40.Flores D, Marquez J, Garza M, Espinoza LR. Reactive arthritis: newer developments. Rheum Dis Clin North Am. 2003;29(1):37–59. vi. doi: 10.1016/s0889-857x(02)00081-9. [DOI] [PubMed] [Google Scholar]
- 41.Bas S, Cunningham T, Kvien TK, Glennas A, Melby K, Vischer TL. Synovial fluid and serum antibodies against Chlamydia in different forms of arthritis: intra-articular IgA production in Chlamydia sexually acquired reactive arthritis. Br J Rheumatol. 1996;35(6):548–52. doi: 10.1093/rheumatology/35.6.548. [DOI] [PubMed] [Google Scholar]
- 42.Gerard HC, Branigan PJ, Schumacher HR, Jr, Hudson AP. Synovial Chlamydia trachomatis in patients with reactive arthritis/Reiter’s syndrome are viable but show aberrant gene expression. J Rheumatol. 1998;25(4):734–42. [PubMed] [Google Scholar]
- 43.Schumacher HR, Jr, Arayssi T, Crane M, Lee J, Gerard H, Hudson AP, et al. Chlamydia trachomatis nucleic acids can be found in the synovium of some asymptomatic subjects. Arthritis Rheum. 1999;42(6):1281–4. doi: 10.1002/1529-0131(199906)42:6<1281::AID-ANR27>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 44.Butrimiene I, Jarmalaite S, Ranceva J, Venalis A, Jasiuleviciute L, Zvirbliene A. Different cytokine profiles in patients with chronic and acute reactive arthritis. Rheumatology (Oxford) 2004;43(10):1300–4. doi: 10.1093/rheumatology/keh323. [DOI] [PubMed] [Google Scholar]
- 45.Appel H, Neure L, Kuhne M, Braun J, Rudwaleit M, Sieper J. An elevated level of IL-10- and TGFbeta-secreting T cells, B cells and macrophages in the synovial membrane of patients with reactive arthritis compared to rheumatoid arthritis. Clin Rheumatol. 2004;23(5):435–40. doi: 10.1007/s10067-004-0916-5. [DOI] [PubMed] [Google Scholar]
- 46.Braun J, Yin Z, Spiller I, Siegert S, Rudwaleit M, Liu L, et al. Low secretion of tumor necrosis factor alpha, but no other Th1 or Th2 cytokines, by peripheral blood mononuclear cells correlates with chronicity in reactive arthritis. Arthritis Rheum. 1999;42(10):2039–44. doi: 10.1002/1529-0131(199910)42:10<2039::AID-ANR3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 47.Kaluza W, Leirisalo-Repo M, Marker-Hermann E, Westman P, Reuss E, Hug R, et al. IL10.G microsatellites mark promoter haplotypes associated with protection against the development of reactive arthritis in Finnish patients. Arthritis Rheum. 2001;44(5):1209–14. doi: 10.1002/1529-0131(200105)44:5<1209::AID-ANR205>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 48.Hill Gaston JS, Lillicrap MS. Arthritis associated with enteric infection. Best Pract Res Clin Rheumatol. 2003;17(2):219–39. doi: 10.1016/s1521-6942(02)00104-3. [DOI] [PubMed] [Google Scholar]
- 49.Hakansson U, Low B, Eitram R, Winblad S. HL-A27 and reactive arthritis in an outbreak of salmonellosis. Tissue Antigens. 1975;6(5):366–7. [PubMed] [Google Scholar]
- 50.Simon DG, Kaslow RA, Rosenbaum J, Kaye RL, Calin A. Reiter’s syndrome following epidemic shigellosis. J Rheumatol. 1981;8(6):969–73. [PubMed] [Google Scholar]
- 51.Eastmond CJ, Rennie JA, Reid TM. An outbreak of Campylobacter enteritis--a rheumatological followup survey. J Rheumatol. 1983;10(1):107–8. [PubMed] [Google Scholar]
- 52.Pitkanen T, Pettersson T, Ponka A, Kosunen TU. Clinical and serological studies in patients with Campylobacter fetus ssp. jejuni infection: I. Clinical findings. Infection. 1981;9(6):274–8. doi: 10.1007/BF01640990. [DOI] [PubMed] [Google Scholar]
- 53.Ponka A, Pitkanen T, Sarna S, Kosunen T. Infection due to Campylobacter jejuni: a report of 524 outpatients. Infection. 1984;12(3):175–178. doi: 10.1007/BF01640893. [DOI] [PubMed] [Google Scholar]
- 54.Peterson MC. Clinical aspects of Campylobacter jejuni infections in adults. West J Med. 1994;161(2):148–52. [PMC free article] [PubMed] [Google Scholar]
- 55.Aho K. Bowel infection predisposing to reactive arthritis. Baillieres Clin Rheumatol. 1989;3(2):303–19. doi: 10.1016/s0950-3579(89)80023-8. [DOI] [PubMed] [Google Scholar]
- 56.Isomaki H, Raunio J, von Essen R, Hameenkorpi R. Incidence of inflammatory rheumatic diseases in Finland. Scand J Rheumatol. 1978;7(3):188–92. doi: 10.3109/03009747809095652. [DOI] [PubMed] [Google Scholar]
- 57.Hammer HB, Kvien TK, Glennas A, Melby K. A longitudinal study of calprotectin as an inflammatory marker in patients with reactive arthritis. Clin Exp Rheumatol. 1995;13(1):59–64. [PubMed] [Google Scholar]
- 58.Savolainen E, Kaipiainen-Seppanen O, Kroger L, Luosujarvi R. Total incidence and distribution of inflammatory joint diseases in a defined population: results from the Kuopio 2000 arthritis survey. J Rheumatol. 2003;30(11):2460–8. [PubMed] [Google Scholar]
- 59.Soderlin MK, et al. Annual incidence of inflammatory joint diseases in a population based study in southern Sweden. Ann Rheum Dis. 2002;61(10):911–5. doi: 10.1136/ard.61.10.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hannu T, et al. Reactive arthritis attributable to Shigella infection: a clinical and epidemiological nationwide study. Ann Rheum Dis. 2005;64(4):594–8. doi: 10.1136/ard.2004.027524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Soderlin MK, Kautiainen H, Puolakkainen M, Hedman K, Soderlund-Venermo M, Skogh T, et al. Infections preceding early arthritis in southern Sweden: a prospective population-based study. J Rheumatol. 2003;30(3):459–64. [PubMed] [Google Scholar]
- 62.Johnsen K, Ostensen M, Melbye AC, Melby K. HLA-B27-negative arthritis related to Campylobacter jejuni enteritis in three children and two adults. Acta Med Scand. 1983;214(2):165–8. doi: 10.1111/j.0954-6820.1983.tb08589.x. [DOI] [PubMed] [Google Scholar]
- 63.Policastro AM. Index of suspicion. Case 3. Campylobacter gastroenteritis with joint pain. Pediatr Rev. 1994;15(3):117, 119. [PubMed] [Google Scholar]
- 64.Gumpel JM, Martin C, Sanderson PJ. Reactive arthritis associated with campylobacter enteritis. Ann Rheum Dis. 1981;40(1):64–5. doi: 10.1136/ard.40.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bremell T, Bjelle A, Svedhem A. Rheumatic symptoms following an outbreak of campylobacter enteritis: a five year follow up. Ann Rheum Dis. 1991;50(12):934–8. doi: 10.1136/ard.50.12.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goudswaard J, Sabbe L, te Winkel W. Reactive arthritis as a complication of Campylobacter lari enteritis. J Infect. 1995;31(2):171. doi: 10.1016/s0163-4453(95)92385-3. [DOI] [PubMed] [Google Scholar]
- 67.Locht H, Krogfelt KA. Comparison of rheumatological and gastrointestinal symptoms after infection with Campylobacter jejuni/coli and enterotoxigenic Escherichia coli. Ann Rheum Dis. 2002;61(5):448–52. doi: 10.1136/ard.61.5.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Glennas A, Kvien TK, Melby K, Overboo A, Andrup O, Karstensen B, et al. Reactive arthritis: a favorable 2 year course and outcome, independent of triggering agent and HLA-B27. J Rheumatol. 1994;21(12):2274–80. [PubMed] [Google Scholar]
- 69.Laasila K, Leirisalo-Repo M. Recurrent reactive arthritis associated with urinary tract infection by Escherichia coli. J Rheumatol. 1999;26(10):2277–9. [PubMed] [Google Scholar]
- 70.Leirisalo-Repo M. Prognosis, course of disease, and treatment of the spondyloarthropathies. Rheum Dis Clin North Am. 1998;24(4):737–51. viii. doi: 10.1016/s0889-857x(05)70039-9. [DOI] [PubMed] [Google Scholar]
- 71.Leirisalo-Repo M. Reactive arthritis. Scand J Rheumatol. 2005;34(4):251–9. doi: 10.1080/03009740500202540. [DOI] [PubMed] [Google Scholar]
- 72.Hannu T. Medicine, Division of Rheumatology. Helsinki University Central Hospital; Helsinki: 2002. Reactive arthritis: aspects of aetiology, arthritogenicity, occurence, clinical picture, antimicrobial treatment and prognosis. [Google Scholar]
- 73.Urman JD, Zurier RB, Rothfield NF. Reiter’s syndrome associated with champylobacter fetus infection. Ann Intern Med. 1977;86(4):444–5. doi: 10.7326/0003-4819-86-4-444. [DOI] [PubMed] [Google Scholar]
- 74.Jacobs A, Barnard K, Fishel R, Gradon JD. Extracolonic manifestations of Clostridium difficile infections. Presentation of 2 cases and review of the literature. Medicine (Baltimore) 2001;80(2):88–101. doi: 10.1097/00005792-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 75.Tupchong M, Simor A, Dewar C. Beaver fever--a rare cause of reactive arthritis. J Rheumatol. 1999;26(12):2701–2. [PubMed] [Google Scholar]
- 76.Shaw RA, Stevens MB. The reactive arthritis of giardiasis. A case report. Jama. 1987;258(19):2734–5. [PubMed] [Google Scholar]
- 77.Barton JJ, Burke JP, Casey EB. Reactive arthritis--Giardia lamblia, another new pathogen? Ir Med J. 1986;79(8):223. [PubMed] [Google Scholar]
- 78.Durand DV, Lecomte C, Cathebras P, Rousset H, Godeau P. Whipple disease. Clinical review of 52 cases. The SNFMI Research Group on Whipple Disease. Societe Nationale Francaise de Medecine Interne. Medicine (Baltimore) 1997;76(3):170–84. doi: 10.1097/00005792-199705000-00003. [DOI] [PubMed] [Google Scholar]
- 79.Galadari I, Galadari H. Nonspecific urethritis and reactive arthritis. Clin Dermatol. 2004;22(6):469–75. doi: 10.1016/j.clindermatol.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 80.Thomas DG, Roberton DM. Reiter’s syndrome in an adolescent girl. Acta Paediatr. 1994;83(3):339–40. doi: 10.1111/j.1651-2227.1994.tb18110.x. [DOI] [PubMed] [Google Scholar]
- 81.Mackie SL, Keat A. Poststreptococcal reactive arthritis: what is it and how do we know? Rheumatology (Oxford) 2004;43(8):949–54. doi: 10.1093/rheumatology/keh225. [DOI] [PubMed] [Google Scholar]
- 82.Ferrieri P. Proceedings of the Jones Criteria workshop. Circulation. 2002;106(19):2521–3. doi: 10.1161/01.cir.0000037745.65929.fa. [DOI] [PubMed] [Google Scholar]
- 83.Braun J, Laitko S, Treharne J, Eggens U, Wu P, Distler A, et al. Chlamydia pneumoniae--a new causative agent of reactive arthritis and undifferentiated oligoarthritis. Ann Rheum Dis. 1994;53(2):100–5. doi: 10.1136/ard.53.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hannu T, Puolakkainen M, Leirisalo-Repo M. Chlamydia pneumoniae as a triggering infection in reactive arthritis. Rheumatology (Oxford) 1999;38(5):411–4. doi: 10.1093/rheumatology/38.5.411. [DOI] [PubMed] [Google Scholar]
- 85.Kingsley G, Sieper J. Third International Workshop on Reactive Arthritis. 23–26 September 1995, Berlin, Germany. Report and abstracts. Ann Rheum Dis. 1996;55(8):564–84. doi: 10.1136/ard.55.8.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pacheco-Tena C, Burgos-Vargas R, Vazquez-Mellado J, Cazarin J, Perez-Diaz JA. A proposal for the classification of patients for clinical and experimental studies on reactive arthritis. J Rheumatol. 1999;26(6):1338–46. [PubMed] [Google Scholar]
- 87.Melby KK, Svendby JG, Eggebo T, Holmen LA, Andersen BM, Lind L, et al. Outbreak of Campylobacter infection in a subartic community. Eur J Clin Microbiol Infect Dis. 2000;19(7):542–4. doi: 10.1007/s100960000316. [DOI] [PubMed] [Google Scholar]
- 88.Sieper J, Braun J, Kingsley GH. Report on the Fourth International Workshop on Reactive Arthritis. Arthritis Rheum. 2000;43(4):720–34. doi: 10.1002/1529-0131(200004)43:4<720::AID-ANR2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 89.Hannu T, Kauppi M, Tuomala M, Laaksonen I, Klemets P, Kuusi M. Reactive arthritis following an outbreak of Campylobacter jejuni infection. J Rheumatol. 2004;31(3):528–30. [PubMed] [Google Scholar]