Abstract
Deletion of PHE508 (ΔF508) from the first nucleotide-binding domain (NBD1) of CFTR, which causes most cystic fibrosis, disrupts the folding and assembly of the protein. Although the folding pathways and yield of isolated NBD1 are altered, its global structure is not, and details of the changes in the rest of the protein remain unclear. To gain further insight into how the whole mutant protein is altered, we have determined the influence of known second-site suppressor mutations in NBD1 on the conformation of this domain and key interfaces between domains. We found that the suppressors restored maturation of only those processing mutations located in NBD1, but not in other domains, including those in the C-terminal cytoplasmic loop of the second membrane-spanning domain, which forms an interface with the NBD1 surface. Nevertheless, the suppressors promoted the formation of this interface and others in the absence of F508. The suppressors restored maturation in a ΔF508 construct from which NBD2 was absent but to a lesser extent than in the full-length, indicating that ΔF508 disrupts interactions involving NBD2, as well as other domains. Rescue of ΔF508-CFTR by suppressors required the biosynthesis of the entire full-length protein in continuity, as it did not occur when N- and C-terminal “halves” were coexpressed. Simultaneous with these interdomain perturbations, ΔF508 resulted in suppressor reversed alterations in accessibility of residues both in the F508-containing NBD1 surface loop and in the Q loop within the domain core. Thus, in the context of the full-length protein, ΔF508 mutation causes detectable changes in NBD1 conformation, as well as interdomain interactions.—He, L., Aleksandrov, L. A., Cui, L., Jensen, T. J., Nesbitt, K. L., Riordan, J. R. Restoration of domain folding and interdomain assembly by second-site suppressors of the ΔF508 mutation in CFTR.
Keywords: cystic fibrosis, interdomain interactions, cysteine accessibility, cysteine cross-linking
Cystic fibrosis (CF) is an inherited disease caused by the impaired function or absence of the cystic fibrosis transmembrane conductance regulator (CFTR), an epithelial chloride channel. CFTR belongs to the ATP-binding cassette (ABC) transporter superfamily of proteins and consists of 2 membrane-spanning domains (MSD1 and MSD2), 2 nucleotide-binding domains (NBD1 and NBD2), and a unique R domain.
The deletion of phenylalanine 508 (ΔF508) in NBD1 is the most frequent CF-associated mutation, occurring in at least one allele in 90% of patients (http://www.genet.sickkids.on.ca/cftr/app). The ΔF508-CFTR protein is misfolded and targeted for proteosomal degradation at the endoplasmic reticulum, so that it fails to reach the plasma membrane (1). However, the exact mechanism of how the ΔF508 mutation causes the folding and functional defects is still unclear. Crystal structures of wild-type (WT)- and ΔF508-NBD1 revealed minor structural differences, with alterations only in mobile surface loop regions, including that containing F508, i.e., 509–511 (2, 3). Structural alignment of human and mouse NBD1 with different solubilizing mutations demonstrated that the protein conformation of each core subdomain is conserved. Despite the lack of major conformation changes in ΔF508-NBD1, molecular dynamics simulations suggested that the ΔF508 mutation alters the folding kinetics of NBD1 (4). Chemical denaturation experiments comparing isolated WT- and ΔF508-NBD1 show that the protein refolding yield is diminished in vitro by ΔF508, especially at higher temperature, even though there is no significant difference in their equilibrium folding properties (5).
Despite the apparent lack of effect of the ΔF508 mutation on the NBD1 core structure, this mutation prevents the proper folding and assembly of CFTR, leading to increased protease sensitivity of other domains (6, 7). As elaborated in a recent publication (8), maturation-compromising point mutations introduced in various other domains of CFTR also result in similar domain instability, as detected by their increased sensitivity to limited protease digestion. Another recent study coexpressing N- and C-terminal halves of CFTR showed that unlike the WT N-terminal half of CFTR (CFTR/1–837), ΔF508-CFTR/1–837 is unable to form a native association with the C-terminal half (CFTR/837–1480), or promote its complex glycosylation (9). These observations further support previous studies suggesting that the ΔF508 mutation disrupts interdomain associations (6, 7, 10).
The folding and assembly defect caused by ΔF508 mutation can be partially alleviated by growing CFTR-expressing cells at reduced temperature (11). However, the temperature-rescued ΔF508-CFTR has defective channel activity, even though the protein has trafficked to the cell surface (12, 13). Second-site mutations discovered using a chimeric construct of the yeast ABC exporter, STE6 containing NBD1 of CFTR, were found to compensate for the effects of the ΔF508 mutation (14,15,16). These suppressor mutations (I539T, G550E, R553M/Q, and R555K) promote ΔF508-CFTR maturation and trafficking to the cell surface, and also restore channel activity (16). The R555K mutation alone increases the channel activity of both WT- and ΔF508-CFTR by extending the open-channel burst duration (15). In addition to a similar effect, G550E also decreases the interburst interval of the ΔF508-CFTR channel, such that its open probability is higher than that of WT-CFTR (17).
The action of these suppressor mutations on channel activity may relate to the fact that they are located in or close to the LSGGQ signature sequence, which contributes to the composite ATP-binding site, and thus channel gating. However, the mechanism of how they rescue ΔF508-CFTR maturation is not clearly understood. Elucidation of this mechanism can provide a better understanding of the different effects of the ΔF508 mutation itself and what is required to overcome these by pharmaceutical means.
To do this, we first determined whether the rescue by suppressor mutations was restricted to point mutations in NBD1 or whether they could rescue other mutations at the NBD1-interacting interface with CL4. We also investigated whether suppressor mutations restored NBD/CL interfaces, recently shown to be crucial for the maturation and function of CFTR (18, 19). A requirement for the ordered assembly of domains was revealed by the inability of the suppressor mutations to exert their compensatory effects when ΔF508-CFTR was expressed as disjointed halves rather than as a single polypeptide. In addition, a cysteine-labeling assay enabled detection of ΔF508-caused changes in single-residue accessibility in the context of full-length CFTR. These results provide new insights into how the ΔF508 mutation impairs associations between CFTR domains and also provide new evidence that there are changes in the compactness of NBD1 itself within the whole CFTR protein, which were not evident from crystal structures of isolated WT- and ΔF508-NBD1. The suppressor mutations apparently act by reversing the changes at both levels.
MATERIALS AND METHODS
Antibodies
Mouse monoclonal CFTR antibodies to an N-terminal fragment (mAb13-4, IgG1κ), NBD1 (mAbL12B4, IgG2a; mAb660, IgG2b), and NBD2 (mAb596, IgG2b) were generated as described previously (7). Goat anti-mouse IgG-IR800 and IgG1-IR800 were from LiCor (Lincoln, NE, USA), and goat anti-mouse IgG2b-Alexa680 was from Invitrogen (Carlsbad, CA, USA).
Construction and expression of CFTR mutants
Point mutations and fragments of CFTR were generated from the WT or Cys-less CFTR constructs in the pcDNA3 vector using the Quick Exchange protocol (Stratagene, La Jolla, CA, USA) as described previously (18), and sequences were confirmed by automated DNA sequencing (University of North Carolina–Chapel Hill Genome Analysis Facility).
Human embryonic kidney (HEK293) cells and baby hamster kidney (BHK-21) cells were maintained in DMEM-F12 (Mediatech, Manassas, VA, USA) supplemented with 10% FBS (Atlanta Biologicals, Lawrenceville, GA, USA), 2 mM glutamax (Invitrogen, Carlsbad, CA, USA) and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin; Mediatech) at 37°C in an incubator with 5% CO2. Transfection was performed using jetPEI transfection reagent (Fermentas, Glen Burnie, MD, USA) according to the manufacturer’s instruction. Cells were harvested in RIPA buffer without SDS (50 mM Tris, 150 mM NaCl, 1% Triton X-100, and 1% deoxycholate, pH 7.4) plus protease inhibitor cocktail (1 μg/ml leupeptin, 2 μg/ml aprotinin, 3.57 μg/ml E64, 156.6 μg/ml benzamidine and 2 mM Pefablock), and equal amounts of proteins in SDS-PAGE sample buffer were subjected to 7.5% SDS-PAGE and Western blot analysis to determine CFTR expression and maturation. For stable expression, constructs were cotransfected with pNUT plasmid into BHK cells, which were selected and maintained in methotrexate containing media (20).
Metabolic pulse-chase labeling
BHK cells stably expressing WT- and mutant CFTR were pulsed labeled with [35S]-methionine for 20 min and chased for up to 4 h, as described previously (7). To determine the half-life of WT- and mutant CFTR, cells were labeled with [35S]-methionine for 8 h and chased for up to 32 h. Cell lysate was prepared in RIPA buffer, and CFTR was precipitated with mAb596. The amount of [35S] radioactivity in each band was quantified by electronic autoradiography (Packard Instant Imager; Packard Instruments, Meriden, CT, USA).
Cysteine cross-linking and detection
Disulfide cross-linking in cells with bifunctional methanethiosulfonate (MTS) cross-linkers was performed as described previously (18). Briefly, HEK293 cells transiently transfected with Cys-less CFTR variants were trypsinized and pelleted. Cells resuspended in PBS were incubated with 200 μM MTS reagent for 15 min at room temperature. The cross-linking reaction was stopped with Laemmli sample buffers with or without dithiothreitol (DTT). Proteins were resolved by 7.5% SDS-PAGE, and CFTR was detected with mAb596 and secondary goat anti-mouse IgG-IR800 using Odyssey infrared fluorescent imager (Licor Corp.).
Coexpression of CFTR halves and coimmunoprecipitation
For coexpression of N- and C-terminal halves of CFTR, the same amount of DNA with each half in pcDNA3 was transfected into HEK cells. The total amount of DNA was maintained the same with vector pcDNA3 when only the C half was expressed. The two halves were split between NBD1 and the R domain (1–634+634–1480), or between the R domain and MSD2 (1–830+837–1480). Forty-eight hours after transfection, HEK cells were harvested in an immunoprecipitation buffer (0.09% Nonidet P-40; 50 mM Tris, pH 7.4; and 150 mM NaCl) with protease inhibitor cocktail. In some experiments, 1% of n-dodecyl-β-d-maltoside was used to replace 0.09% Nonidet P-40. Lysate was cleared by centrifugation at 16,000 g for 15 min. Cleared lysate were incubated with L12B4- or mAB596-conjugated protein A agarose beads (Invitrogen) for 3 h, and the beads were washed 3 times with immunoprecipitation buffer. Bound proteins were eluted with 2× SDS-PAGE sample buffer without reducing agent to avoid the reduction of antibodies immobilized on the beads. Eluates were reduced with 50 mM DTT before subjecting to 7.5% SDS-PAGE and Western blot analysis. N and C halves of CFTR were detected simultaneously on the same blot with mAb13-4 (IgG1κ) and mAb596 (IgG2b), in combination with the isotype-specific secondary antibodies anti-IgG1-IR800 and anti-IgG2b-Alexa680.
Cysteine labeling and detection
Single Cys labeling was carried out in membrane vesicles prepared from BHK cells stably expressing CFTR with a single Cys introduced in NBD1. Membrane vesicles were prepared by differential centrifugation, as described previously (21). Membranes diluted to 0.5 μg proteins/μl in a Tris-saline buffer (50 mM Tris, 150 mM NaCl, and 2 mM MgCl2, pH 7.2) were incubated with 5 μM of DyLight800-Maleimide (Thermo Scientific, Rockford, IL, USA) for 5 min at room temperature. Labeling was stopped with 10 mM DTT, and membranes were resuspended in RIPA buffer (50 mM Tris, 150 mM NaCl, 1% Triton X-100, 1% deoxycholate, and 0.1% SDS, pH 7.4) before immunoprecipitation with protein A beads cross-linked with L12B4 antibody. Proteins were eluted with 2× SDS-PAGE sample buffer without DTT, and eluates were reduced with 50 mM DTT before subjecting to 7.5% SDS-PAGE and Western blot analysis with mAb596 and goat anti-mouse IgG2b-Alexa680 (Invitrogen). Cys-labeled CFTR and total CFTR were detected and quantified using the Odyssey infrared fluorescent imager at 800 nm and 700 nm, respectively. The relative Cys-labeling was calculated from the ratio of the intensity in the 800 channel to that in the 700 channel.
RESULTS
Suppressor mutations restore folding mutations in NBD1 but not elsewhere
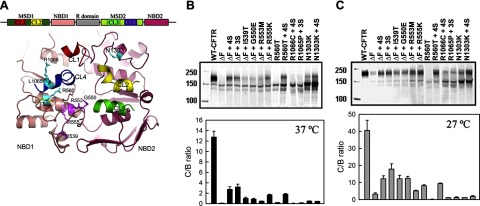
Four suppressor mutations (I539T, G550E, R553M, and R555K) were originally found to rescue ΔF508-CFTR maturation in a yeast mating screen using STE6/CFTR chimeras (14,15,16). We first assessed the relative effects of each of these second-site mutations individually in HEK293 cells, and the efficiency of rescue is represented by the mature/immature ratio (C/B ratio, Fig. 1). In HEK cells heterologously overexpressing CFTR, at 37°C, the majority of WT-CFTR was detected as the mature form, while a minimal amount of ΔF508-CFTR was mature, as previously shown. Among these four suppressor mutations, R555K had the largest effect in promoting ΔF508-CFTR maturation, while R553M had the least effect. The addition of G550E and R553M to R555K (3S) further increased its maturation, but no additional effect was detected by the addition of the fourth mutation I539T (4S) (Fig. 1A). The combination of the growth of cells at reduced temperature (27°C) with the suppressor mutations further increased the maturation of ΔF508-CFTR, suggesting that the suppressor mutations and low-temperature influence ΔF508-CFTR maturation by different mechanisms.
Figure 1.
Influence of suppressor mutations in NBD1 on maturation of ΔF508 and other processing mutants. A) Top: schema of CFTR primary structures containing 2 membrane-spanning domains, 2 nucleotide-binding domains, and a regulatory R domain. Bottom: Three-dimensional structure model of CFTR (see ref. 18), colored the same as the schema, with suppressor mutations magenta, F508 yellow, and other maturation-compromising mutations studied in this work cyan. For viewing clarity, the membrane-spanning domains are not shown, except for the 4 cytoplasmic loops (CL1–CL4). B, C) HEK293 cells were transiently transfected with CFTR variants with maturation-compromising mutations introduced in different domains, in the presence or absence of single or combined suppressor mutations (4S: I539T/G550E/R4553M/R555K; 3S: G550E/R553M/R555K). Cells were grown at 37°C and harvested 48 h after transfection (B), or 24 h after transfection, grown at 27°C for another 48 h before harvesting (C). Cell lysates with equal amounts of proteins were subjected to Western blot analysis with anti-CFTR antibody 660. Amount of mature (C band) and immature (B band) CFTR was quantified using the Licor Odyssey IR Fluorescence Imager; C/B ratio was calculated to assess steady-state level of CFTR (n=3–5).
Since crystal structures comparing ΔF508-NBD1 with and without suppressor mutations showed minimal conformational changes in the core of NBD1, while substantial changes were observed in the conformation of surface loops (22), we speculated that the suppressor mutations promote CFTR maturation partially by improving the interdomain interactions involving NBD1. If this were indeed the case, other mutations in NBD1, which compromise CFTR maturation and trafficking might also be rescued by the suppressor mutations. To test this hypothesis, we introduced suppressor mutations into another NBD1-processing mutant, R560T (17). According to a CFTR structural model (18), R560 is located at the NBD1/CL4 interface (Fig. 1A). As shown in Fig. 1B, R560T-CFTR, although not rescued by G550E alone (17), was very effectively rescued by the 4S combination. Interestingly, while lowering the temperature alone did not promote R560T-CFTR maturation, it augmented the extent of rescue by 4S (Fig. 1C).
F508 is at the NBD1/CL4 interface (18), and the ΔF508 mutation compromises this interfacial interaction (see below). One hypothesis is that the suppressor mutations reverse the ΔF508 defect by restoring this interface. Indeed, some substitutions on the CL4 side of this interface such as R1070W have been reported to improve ΔF508-CFTR maturation (23). These effects may be due to restoration of the CL4/NBD1 interface disrupted by the ΔF508 mutation. Therefore, we tested whether the suppressor mutations could also reverse the processing defects caused by disease-associated mutations (R1066C and L1065P; ref. 24) on the CL4 side of the NBD1/CL4 interface. Introduction of the 4S mutations into R1066C or the 3S mutations into L1065P did not promote CFTR maturation (Fig. 1B). Even though maturation of these two CL4 mutants was improved somewhat by lowering the temperature, the addition of the suppressor mutations did not further increase this level (Fig. 1C, data not shown). Thus, the suppressor mutations are unable to alleviate or compensate for the influence of CL4 mutations that prevent maturation.
In yet another variant harboring N1303K, which is the only known mutation in NBD2 that impairs CFTR maturation, the introduction of suppressor mutations did not increase the proportion of the mature form either. However, the suppressors did result in slightly increased amounts of both mature and immature N1303K-CFTR, and this effect was enhanced at reduced temperature (Fig. 1B, C). This may suggest that there is some stabilization of the immature form of this NBD2 mutant without any promotion of its maturation by the NBD1 suppressors. Overall, the findings indicate that these second-site suppressors are compensatory only for misprocessing mutations in NBD1 and not those in other parts of the protein.
Suppressor mutations restore ΔF508-CFTR maturation and stability
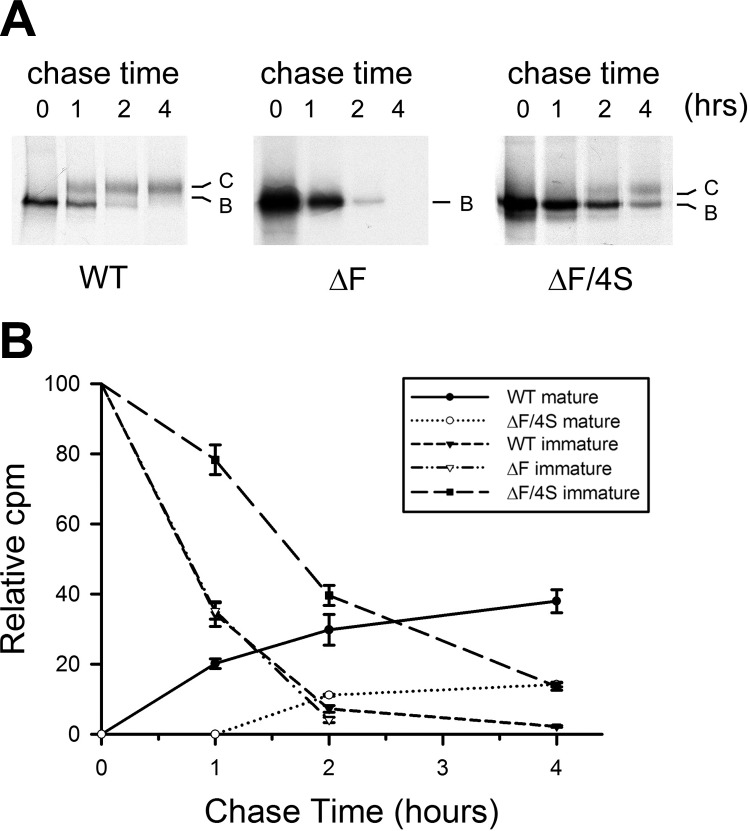
Figure 1 showed that the steady-state level of band C for ΔF508-CFTR was significantly enhanced by the combination of the 4 suppressors. To evaluate whether this was due to an increase in conversion of the nascent immature form to the mature product or in its life-time, pulse-chase experiments were performed. As previously shown, with WT-CFTR, close to one-third of the total pulse labeled immature CFTR was converted to the mature form during a 4-h chase, while none was detected with ΔF508-CFTR (Fig. 2). The addition of the 4 suppressors to ΔF508-CFTR increased this conversion efficiency to nearly 15%, or about one-third that of the WT-CFTR. Notably, this is similar to the estimates from the relative steady-state amounts (Fig. 1B). Even though the rate of conversion of the core-glycosylated precursor to the complex glycosylated mature form was slower than that of WT-CFTR, the half-life was similar at ∼12 h, in the range of those observed by others (25, 26), as measured in long-term chase experiments (Supplemental Fig. 1). These results indicate that the suppressor mutations increased the steady-state level of mature ΔF508-CFTR by apparently increasing the efficiency of maturation and by definitely stabilizing the mature form.
Figure 2.
Metabolic labeling of CFTR. Stable BHK cells overexpressing WT-CFTR and ΔF508-CFTR with and without 4 suppressor mutations (I539T/G550E/R553M/R555K, ΔF/4S) were pulse labeled with 100 μCi/ml [35S] methionine for 20 min, followed by 0, 1, 2, and 4 h chase. CFTR was immunoprecipitated with mAb596 conjugated to protein A beads and separated with 6% SDS-PAGE. Gels were fixed and dried before exposed to film (A) and quantified by electronic autoradiography (B). Data are presented as percentage of band B and C at a given time point during the chase relative to total labeled B band at time 0 (n=3).
Suppressor mutations restore the NBD/CL interfaces disrupted by ΔF508
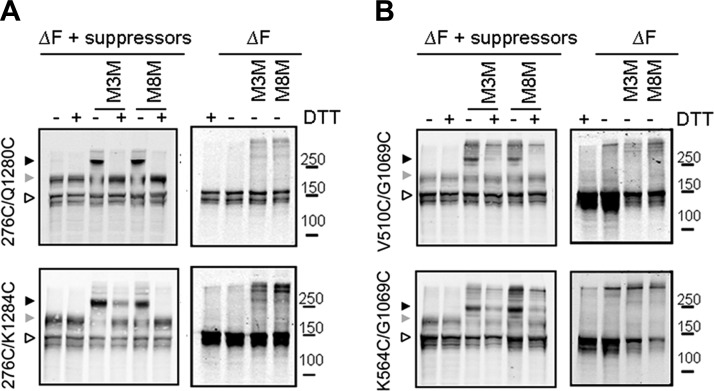
Several experimental observations using limited trypsin digestion or coexpression of different fragments of CFTR suggest that some cooperative interdomain interactions in CFTR occur post-translationally, after the core-glycosylation of CFTR (6,7,8,9). Cysteine cross-linking experiments show that all of the NBD/CL interfaces are formed only in the mature complex-glycosylated CFTR, but not in the immature core-glycosylated counterpart (18, 19). Since ΔF508-CFTR exists only in the immature core-glycosylated form, we expected that these cytoplasmic-membrane domain associations may have not yet occurred. To test this, the ΔF508 mutation was introduced into Cys-less CFTR constructs with Cys pairs at the NBD2/CL2 and NBD1/CL4 interfaces (18). As shown in Fig. 3A, when ΔF508 was introduced into Cys-less CFTR containing pairs 276C/Q1280C or 276C/K1284C at the NBD2/CL2 interface, no mature CFTR was detected, and neither M3M nor M8M (200 μM) caused cross-linking of the immature band. Cys-pair cross-linking also was not observed with Cys pairs at V510C/G1069C and K564C/G1069C in the NBD1/CL4 interface of ΔF508-CFTR (Fig. 3B).
Figure 3.
Restoration of MSD/NBD interfaces by suppressor mutations. HEK293 cells were transiently transfected with Cys-less ΔF508-CFTR in the presence or absence of suppressor mutations I539T/G550E/R553M/R555K, with Cys pairs introduced at CL2/NBD2 (A) or CL4/NBD1 (B) interfaces. At 72 h after transfection, cells were incubated with 200 μM M3M or M8M, and cell lysates in SDS-PAGE sample buffer with or without DTT as indicated were subjected to Western blot analysis, as described in Materials and Methods. Solid arrowheads indicate cross-linked CFTR; shaded arrowheads indicate mature complex-glycosylated CFTR; open arrowheads indicate immature core-glycosylated CFTR.
To test whether these NBD/CL interfaces not formed in ΔF508-CFTR could be restored by the suppressor mutations, the 4 combined suppressor mutations, I539T/G550E/R553M/R555K (4S) were introduced into the ΔF508-CFTR constructs with the Cys pairs at the potential interfaces. As shown in Fig. 3A, ΔF508-CFTR maturation was restored as was the cross-linking of the Cys pairs 276C/Q1280C and 276C/K1284C at NBD2/CL2, as indicated by the slower migration rate of the mature bands in SDS-PAGE in samples treated with M3M and M8M (18). In addition, Cys pairs V510C/G1069C and K564C/G1069C at the NBD1/CL4 interface also were able to be cross-linked by M3M and M8M (Fig. 3B). Thus, the suppressors restored not only the interface between NBD1 and CL4, in which F508 normally participates but also that between NBD2 and CL2, indicating both short- and long-range conformational effects of the NBD1 second-site substitutions.
The rescue of ΔF508 by suppressor mutations is diminished in the absence of NBD2
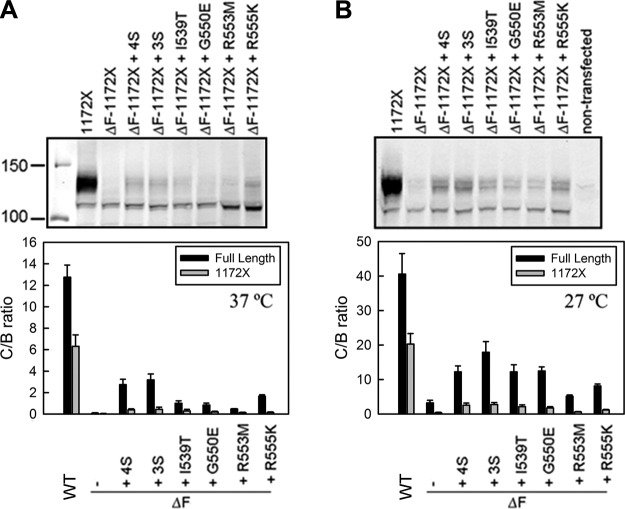
According to a structural model of CFTR (18), some of the suppressor mutations (I539T and G550E) are located at the NBD1/NBD2 interface (Fig. 1A), and ΔF508 is known to destabilize NBD2 (6, 7). Therefore, it would be reasonable to think that one action of the suppressors might be to stabilize the NBD1/NBD2 interface. However, disruption of this interface cannot be entirely responsible for the failure of maturation caused by ΔF508 because that also is observed in constructs containing only the N-terminal 4 core domains of CFTR in the entire absence of NBD2 (7, 27). To determine the extent to which NBD2 is or is not involved in the rescue of ΔF508 by the suppressors, we compared their influence in the presence and absence of NBD2.
As we had found previously (7), WT-CFTR truncated after MSD2 at residue 1172 matures at only a slightly lower level than the full-length protein, and this maturation is completely prevented by ΔF508 just as in the full-length (Fig. 4A). Introduction of each of the four individual suppressor mutations or the combinations of the 3 or 4 suppressors slightly increased ΔF508–1172X maturation, but the efficiency of the rescue of ΔF508–1172X maturation was drastically decreased compared to that of full-length ΔF508-CFTR. These results are consistent with the suppressors in NBD1 acting to restore ΔF508-disrupted interactions that involve NBD2, as well ones that do not. Interestingly, whereas growth at 27°C results in substantial maturation of full-length ΔF508-CFTR, as is well established, when NBD2 is absent there is little response to reduced temperature (compare lanes 2 in Figs. 4A, B). Nevertheless, the suppressors augment maturation to a slightly greater extent at 27°C than at 37°C.
Figure 4.
Reduced suppressor rescue of ΔF508-CFTR in the absence of NBD2. HEK293 cells were transiently transfected with 1172X-CFTR or ΔF508–1172X-CFTR in the presence or absence of single or combined suppressor mutations (4S: I539T/G550E/R4553M/R555K; 3S: G550E/R553M/R555K). Cells were grown at 37°C and harvested 48 h after transfection (A), or 24 h after transfection, grown at 27°C for another 48 h before harvesting (B). Cell lysates with equal amount of proteins were subjected to Western blot analysis employing anti-CFTR antibody 660. Amount of mature (C′ band) and immature (B′ band) 1172X-CFTR was quantified using the Licor Odyssey imager system; C/B ratio was calculated to assess the steady-state level of 1172X-CFTR (n=3–5). Data for full-length CFTR are from Fig. 1.
A continuous full-length CFTR polypeptide rather than coexpressed halves is required for suppressor rescue of ΔF508 maturation
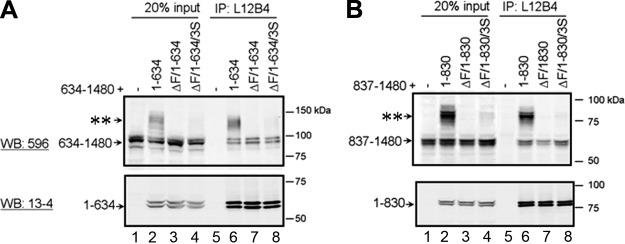
To further investigate whether suppressor mutations rescue ΔF508 by simply reversing the conformation changes in NBD1 or by restoring interdomain interactions, or both, we coexpressed split halves of CFTR, with the N half carrying the ΔF508 mutation in the absence or presence of suppressor mutations. Coexpression of N-half and C-half CFTR has been shown to promote complex glycosylation of the latter, but not when the N half carries the ΔF508 mutation (8, 9). If suppressor mutations rescue ΔF508-CFTR by merely rescuing the NBD1 folding defect, then it is expected that coexpression of N-half ΔF508-CFTR with suppressor mutations will also promote the complex glycosylation of C-half CFTR. When N-half CFTR (1–634) was coexpressed with C-half CFTR (634–1480), it promoted the complex glycosylation of the C half (Fig. 5A, top blot; compare lanes 1 and 2), but not when the N half carried the ΔF508 mutation (Fig. 5A, lane 3), as has been shown previously (8, 9). However, when suppressor mutations (3S: G550E/R553M/R555K) were introduced into the N-half ΔF508-CFTR, they did not promote complex glycosylation of the C half (Fig. 5A, lane 4), as they did in full-length CFTR (Fig. 1). Similar results were observed when the N and C halves were expressed as 1–830 and 837–1480, which were separated between the R domain and MSD2 (Fig. 5B, lanes 1–4). These results suggest that for suppressor mutations to rescue ΔF508, ordered interdomain interactions involving NBD1 must be restored so that other domains C-terminal of the R domain can be cooperatively folded.
Figure 5.
Maturation of coexpressed CFTR halves containing ΔF508 is not rescued by suppressor mutations. N and C halves of CFTR were coexpressed in HEK293 cells. N halves were either WT or carried the ΔF508 mutation, in the absence or presence of suppressor mutations (3S: G550E/R553M/R555K). The two halves were split either between NBD1 and the R domain (1–634+634–1480) (A), or between the R domain and MSD2 (1–830+837–1480) (B). At 48 h after transfection, cells were solubilized, and lysates were immunoprecipitated with L12B4 conjugated to protein A agarose beads. Bound proteins were eluted with 2× SDS-PAGE sample buffer, and eluates were reduced and subjected to Western blot analysis using anti-CFTR antibodies mAb13-4 and mAb596, to detect the N- and C-terminal halves, respectively. Goat anti-mouse IgG1-IR800 and IgG2b-Alexa680 were used to detect both halves of CFTR simultaneously in the same blot. Arrows with asterisks indicate complex glycosylated forms of the C halves (n=3).
Interestingly, the same amount of N-half CFTR was expressed irrespective of the ΔF508 mutation or suppressor mutations (Figs. 5A, B, bottom blots, lanes 2–4). These results further indicate that the effects of the ΔF508 mutation on NBD1 and the N-terminal half of the protein may be relatively subtle with the more pronounced impact on interdomain interactions involving the C-terminal half.
To verify whether the lack of promotion of C-half complex glycosylation by N-half ΔF508-CFTR was due to the lack of interaction between these halves, we determined the interaction of the 2 halves by coimmunoprecipitation. As shown in Fig. 5A, L12B4, which binds to an epitope in NBD1, pulled down the C half of CFTR (634–1480), as well as the N half (1–634), irrespective of whether the N half carried ΔF508, and it pulled down both mature and immature C halves (lanes 6–8). The lack of C-half pulldown when no N half was coexpressed indicates a specific interaction between the N and C halves (lane 5). Similar results were found when N and C halves were expressed as 1–830 and 837–1480 segments (Fig. 5B, lanes 5–8), or when cells were lyzed using a different detergent (1% n-dodecyl-β-d-maltoside instead of 0.09% Nonidet P-40; Supplemental Fig. 2A), or when C-half antibody 596 was used for coimmunoprecipitation (Supplemental Fig. 2B). These results demonstrate that not all interdomain interactions were disrupted by the ΔF508 mutation. Most significantly, the failure of the suppressor mutations to restore maturation of ΔF508-CFTR during coexpression of disjointed halves suggests a requirement for not only ordered but also properly timed events in associations between domains during assembly.
NBD1 conformation is altered by ΔF508 in full-length CFTR
Although isolated WT- and ΔF508-NBD1 are similar in their core structures (22), it is unknown whether this holds true in the context of full-length CFTR. We have addressed this question using a technique that enables us to explore conformational change at the single-residue level, by tracking changes in the relative accessibility to a sulfhydryl reagent of individual cysteines introduced into Cys-less CFTR (18) at the location of interest. Although this Cys-less construct matures somewhat less well than WT-CFTR, pulse-chase experiment showed it does mature over the same time course as WT-CFTR (Supplemental Fig. 3), and previous experiments show that its protease susceptibility is not grossly altered (28). Membranes prepared from BHK cells stably expressing these CFTR variants were incubated with a hydrophilic fluorescent maleimide reagent (DyLight800-Maleimide). CFTR was immunoprecipitated from detergent-solubilized membranes and resolved by SDS-PAGE. Amounts of total and labeled CFTR were determined from Western blots with simultaneous detection of a fluorescently labeled second antibody at 700 nm and Dylight800 fluorescence at 800 nm in a LiCor Odyssey IR Imager. Comparison of the ratios of labeled to total CFTR revealed changes in cysteine accessibility at specific residue positions due to the ΔF508 and suppressor mutations.
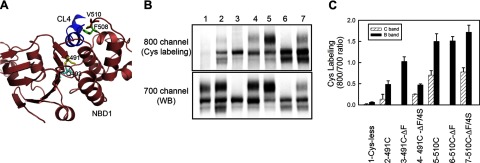
Using this assay, we compared the accessibility of 491C and V510C in NBD1 of full-length CFTR in the absence or presence of ΔF508. These two residues were chosen because according to the NBD1 structure, C491 located in the Q loop, is “buried” within the domain interior, while V510 is exposed in the F508 containing surface loop (Fig. 6A). As shown in Fig. 6B, C, there was no nonspecific background labeling of either the mature or immature forms of Cys-less CFTR (lane 1). There was very little labeling of the mature form of the protein containing only the single native cysteine at position 491, reflecting its inaccessibility in the fully folded protein. However, it was strongly labeled in the core-glycosylated immature form, indicating that NBD1 is still relatively unfolded at this stage (lane 2). Nevertheless, labeling of 491C immature band was further increased 2-fold by the ΔF508 mutation, indicating an even lesser degree of folding in the mutant (lane 3). The suppressor mutations promoted ΔF508-CFTR maturation, as expected, and 491C in the mature C band became buried again, as indicated by the decrease of Cys-labeling compared to B band labeling in ΔF508-CFTR (lane 4). In addition, the labeling of immature band B was also substantially reduced by the suppressors. Overall, these observations clearly demonstrate that ΔF508 does cause a conformational change within NBD1, which is partially reversed by the suppressor mutations. To determine whether suppressors have the same effect on WT-CFTR, we compared Cys-labeling of 491C in constructs with and without suppressors. As shown in Supplemental Fig. 4, the introduction of suppressors increased the maturation of Cys-less CFTR, but the labeling of 491C in both the mature and immature band was not affected. This result suggests that suppressors do not directly alter 491C accessibility, at least not to a significant level that can be detected by Cys-labeling.
Figure 6.
Influence of ΔF508 and suppressor mutations on single-residue Cys-accessibility in NBD1 of full-length CFTR. A) Membrane vesicles prepared from BHK overexpressing Cys-less CFTR with single Cys introduced at residue 491 or 510, as shown in the scheme, were incubated with 10 μM of DyLight 800-Maleimide for 5 min at room temperature. Cys labeling was stopped with 50 mM DTT. Membranes were then solubilized with RIPA buffer before immunoprecipitation with anti-CFTR antibody L12B4 cross-linked to protein A agarose beads. Eluted proteins were reduced and subjected to 7.5% SDS-PAGE and Western blot analysis with mAb 596 in combination with secondary antibody conjugated with Alexa-680. B) Representative blots showing Cys-labeled CFTR and total CFTR visualized with the Odyssey Fluorescent Imager at 800- and 700-nm channels, respectively. C) Amounts of labeled mature CFTR (C band) and immature CFTR (B band) were quantified and normalized to total mature and immature CFTR protein by calculating the ratio of intensity at 800 channel to that at 700 channel. No data for labeling of mature ΔF508-CFTR, as no mature band was detected by Western blot analysis (n=3).
In contrast to the buried 491C, V510C was clearly labeled in both immature and mature forms of WT-CFTR (lane 5). However, the extent of labeling of the immature form was twice that of the mature form. Thus, even the V510-containing surface loop on NBD1 becomes relatively less accessible during conformational maturation of the entire protein. This may reflect the involvement of this loop in the NBD1/CL4 interface, as shown with the cross-linking of V510C/G1069C in the mature but not in the immature form (18). In contrast to the more buried 491C, introduction of ΔF508 did not make V510C more accessible to labeling compared to the immature WT-CFTR (lane 6). Introduction of the suppressor mutations did not appear to reduce exposure of V510C in the immature form, as it had the more buried 491C. However, the accessibility of V510C in the mature form, which was restored by the suppressors, was similar to that in the WT-CFTR (lane 7). Thus, in the context of the full-length CFTR protein, both the ΔF508 mutation itself and the second-site mutations clearly influence not only domain interactions but also the local conformations on and within NBD1. However, we cannot exclude the possibility that the conformational change in NBD1 is a secondary effect caused by altered interdomain interactions.
DISCUSSION
The primary aim of this study was to determine whether the ΔF508 mutation in CFTR, responsible for most cystic fibrosis, acts by perturbing the NBD1 domain, in which F508 is located, or by preventing normal interactions between domains in the whole protein, or both. This question has remained unresolved because the 3-D structure of isolated NBD1 is virtually unaltered by the mutation, even though other regions of the multidomain protein exhibit significant alterations in susceptibility to limited proteolysis. Not long after the discovery of the common ΔF508 mutation and its impact, Teem and coworkers (14,15,16) uncovered a set of intragenic suppressor mutations, all mapping to NBD1 where F508 normally resides. It is logical to assume that they act by restoring a more native conformation to NBD1, which might either directly promote its recognition as more normal by elements of cellular quality control systems or favorably influence the tertiary structure of the rest of the molecule. However a complete understanding of the molecular impact of ΔF508 and its alleviation by the suppressors has remained elusive. Here, we have focused primarily on changes in interdomain associations but also on small changes in NBD1 itself at the single-residue level. The increase in the amount of mature ΔF508-CFTR caused by the presence of the combined suppressor substitutions was found to reflect a stabilization of the lifetime of the mature form of the protein (Fig. 2 and Supplemental Fig. 1).
We determined whether CFTR processing mutants other than ΔF508 could be rescued by the suppressors. Basically, only those in NBD1 were compensated for but not others, including those on the other side of the NBD1/CL4 interface, in which F508 participates (Fig. 1). This is consistent with the idea that suppressors act to restore the conformation of the domain in which they are situated and in so doing enable reestablishment of its interactions with other regions of the molecule, including CL4. Mutations in CL4 that disrupt the NBD1/CL4 interface are not compensated for as might be expected.
Direct evidence of restoration by the suppressors of not only the association of NBD1 with CL4 but also of the corresponding interaction between NBD2 and CL2 came from the ability of Cys pairs to be cross-linked across these interfaces (Fig. 3). This indication that interactions involving NBD2 were among those disrupted by ΔF508 and restored by the suppressors was substantiated by the finding that C-terminally truncated ΔF508-CFTR not containing NBD2 was far less effectively rescued by the suppressors than when NBD2 was present (Fig. 4).
A particularly interesting finding with respect to the influence of the suppressor mutations on the ΔF508 protein was that their impact could only be exerted during the folding of the continuous full-length protein. Even though coexpression of disjointed halves of the WT sequence are known to assemble and function, and this is prevented by ΔF508, the presence of the suppressor substitutions in the N-terminal half does not restore maturation of the coexpressed C halves (Fig. 5). This may point to a requirement for the correct timing of the associations between folding domains as CFTR is assembled. When the two halves are coexpressed, their synthesis occurs more or less simultaneously on separate ribosomes, in contrast to the sequential formation and assembly of the domains to form the full-length molecule. As emphasized by Du and Lukacs (8), CFTR domains are strongly dependent on each other. This interdependence appears to be highly coordinated temporally as well as spatially.
A recent study by Loo et al.(29) showed that the N- and C-half association was disrupted when the N half included the ΔF508 mutation. However, in our coimmunoprecipitation experiments, the two halves associated with each other, irrespective of the ΔF508 mutation, although no maturation occurred in the latter case. The same results were obtained employing the same detergent as in their study, and when we reversed the order of antibodies used in the immunoprecipitation and blotting experiments to more closely mimic their protocols (Supplemental Fig. 2). It is also not possible to tell whether their primary pulldown of the C-terminal half with Ni-NTA beads was as effective when it was coexpressed with the ΔF508 N-terminal half instead of the wild type, as these controls were not shown. In our experience with purification of C-terminally His-tagged CFTR, pull down of the ΔF508 variant with Ni-NTA or Co-NTA beads is ≥4-fold less efficient than that from a comparable pool of WT-CFTR.
Although there is now quite strong evidence that ΔF508 perturbs CFTR domain assembly, the question of whether the mutation alters the structure of NBD1, other than in the local surface patch normally containing F508, has been difficult to resolve definitively. Here we found, from cysteine labeling, evidence of at least a minor change in the interior of the domain, as well as on its surface (Fig. 6). This is in agreement with observations of modest differences in protease sensitivity of NBD1 in the full-length mutant protein (6, 7, 30). However, neither the cysteine labeling experiments nor limited proteolysis assays have been applied to the isolated WT and mutant NBD1 domains used in the X-ray structure determinations. Such experiments should distinguish whether the small NBD1 structural changes detected biochemically in full-length CFTR are, in fact, indirect consequences of perturbed assembly of the whole protein, or due to a direct effect of the mutation within NBD1.
In summary, our study shows that suppressor mutations restore CL/NBD interfaces that are disrupted by the ΔF508 mutation. Coexpression of the ΔF508-N-half CFTR does not rescue C-half complex glycosylation, indicating that the cooperative folding of domains post-R domain is essential for suppressors to rescue ΔF508. This is supported by the decreased compensatory efficacy of the suppressor on ΔF508–1172X compared to full-length CFTR. ΔF508 alters NBD1 core structure in full-length CFTR, but not in isolated NBD1, indicating that the difference may result from altered interdomain interactions, although a direct effect of ΔF508 on NBD1 is not excluded. Therefore, therapeutic strategies designed to rescue ΔF508-CFTR should aim to restore these interdomain interactions.
Supplementary Material
Acknowledgments
This study is supported by grants from the U.S. National Institutes of Health (DK051870) and the CFF. The authors also thank Drs. Tamas Hegedus and Adrian W. R. Serohijos for reading the manuscript.
References
- Riordan J. R. CFTR function and prospects for therapy. Annu Rev Biochem. 2008;77:701–726. doi: 10.1146/annurev.biochem.75.103004.142532. [DOI] [PubMed] [Google Scholar]
- Lewis H. A., Buchanan S. G., Burley S. K., Conners K., Dickey M., Dorwart M., Fowler R., Gao X., Guggino W. B., Hendrickson W. A., Hunt J. F., Kearins M. C., Lorimer D., Maloney P. C., Post K. W., Rajashankar K. R., Rutter M. E., Sauder J. M., Shriver S., Thibodeau P. H., Thomas P. J., Zhang M., Zhao X., Emtage S. Structure of nucleotide-binding domain 1 of the cystic fibrosis transmembrane conductance regulator. EMBO J. 2004;23:282–293. doi: 10.1038/sj.emboj.7600040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis H. A., Wang C., Zhao X., Hamuro Y., Conners K., Kearins M. C., Lu F., Sauder J. M., Molnar K. S., Coales S. J., Maloney P. C., Guggino W. B., Wetmore D. R., Weber P. C., Hunt J. F. Structure and dynamics of NBD1 from CFTR characterized using crystallography and hydrogen/deuterium exchange mass spectrometry. J Mol Biol. 2010;396:406–430. doi: 10.1016/j.jmb.2009.11.051. [DOI] [PubMed] [Google Scholar]
- Serohijos A. W., Hegedus T., Riordan J. R., Dokholyan N. V. Diminished self-chaperoning activity of the ΔF508 mutant of CFTR results in protein misfolding. PLoS Comput Biol. 2008;4:e1000008. doi: 10.1371/journal.pcbi.1000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu B. H., Thomas P. J. Alteration of the cystic fibrosis transmembrane conductance regulator folding pathway. J Biol Chem. 1996;271:7261–7264. doi: 10.1074/jbc.271.13.7261. [DOI] [PubMed] [Google Scholar]
- Du K., Sharma M., Lukacs G. L. The DeltaF508 cystic fibrosis mutation impairs domain-domain interactions and arrests post-translational folding of CFTR. Nat Struct Mol Biol. 2005;12:17–25. doi: 10.1038/nsmb882. [DOI] [PubMed] [Google Scholar]
- Cui L., Aleksandrov L., Chang X. B., Hou Y. X., He L., Hegedus T., Gentzsch M., Aleksandrov A., Balch W. E., Riordan J. R. Domain interdependence in the biosynthetic assembly of CFTR. J Mol Biol. 2007;365:981–994. doi: 10.1016/j.jmb.2006.10.086. [DOI] [PubMed] [Google Scholar]
- Du K., Lukacs G. L. Cooperative assembly and misfolding of CFTR domains in vivo. Mol Biol Cell. 2009;20:1903–1915. doi: 10.1091/mbc.E08-09-0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosser M. F., Grove D. E., Chen L., Cyr D. M. Assembly and misassembly of cystic fibrosis transmembrane conductance regulator: folding defects caused by deletion of F508 occur before and after the calnexin-dependent association of membrane-spanning domain (MSD) 1 and MSD2. Mol Biol Cell. 2008;19:4570–4579. doi: 10.1091/mbc.E08-04-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. Y., Bartlett M. C., Loo T. W., Clarke D. M. The DeltaF508 mutation disrupts packing of the transmembrane segments of the cystic fibrosis transmembrane conductance regulator. J Biol Chem. 2004;279:39620–39627. doi: 10.1074/jbc.M407887200. [DOI] [PubMed] [Google Scholar]
- Denning G. M., Anderson M. P., Amara J. F., Marshall J., Smith A. E., Welsh M. J. Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive. Nature. 1992;358:761–764. doi: 10.1038/358761a0. [DOI] [PubMed] [Google Scholar]
- Dalemans W., Barbry P., Champigny G., Jallat S., Dott K., Dreyer D., Crystal R. G., Pavirani A., Lecocq J. P., Lazdunski M. Altered chloride ion channel kinetics associated with the delta F508 cystic fibrosis mutation. Nature. 1991;354:526–528. doi: 10.1038/354526a0. [DOI] [PubMed] [Google Scholar]
- Hegedus T., Aleksandrov A., Cui L., Gentzsch M., Chang X. B., Riordan J. R. F508del CFTR with two altered RXR motifs escapes from ER quality control but its channel activity is thermally sensitive. Biochim Biophys Acta. 2006;1758:565–572. doi: 10.1016/j.bbamem.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Teem J. L., Berger H. A., Ostedgaard L. S., Rich D. P., Tsui L.-C., Welsh M. J. Identification of revertants for the cystic fibrosis ΔF508 mutation using STE6-CFTR chimeras in yeast. Cell. 1993;73:335–346. doi: 10.1016/0092-8674(93)90233-g. [DOI] [PubMed] [Google Scholar]
- Teem J. L., Carson M. R., Welsh M. J. Mutation of R555 in CFTR-delta F508 enhances function and partially corrects defective processing. Receptors Channels. 1996;4:63–72. [PubMed] [Google Scholar]
- DeCarvalho A. C., Gansheroff L. J., Teem J. L. Mutations in the nucleotide binding domain 1 signature motif region rescue processing and functional defects of cystic fibrosis transmembrane conductance regulator delta F508. J Biol Chem. 2002;277:35896–35905. doi: 10.1074/jbc.M205644200. [DOI] [PubMed] [Google Scholar]
- Roxo-Rosa M., Xu Z., Schmidt A., Neto M., Cai Z., Soares C. M., Sheppard D. N., Amaral M. D. Revertant mutants G550E and 4RK rescue cystic fibrosis mutants in the first nucleotide-binding domain of CFTR by different mechanisms. Proc Natl Acad Sci U S A. 2006;103:17891–17896. doi: 10.1073/pnas.0608312103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serohijos A. W., Hegedus T., Aleksandrov A. A., He L., Cui L., Dokholyan N. V., Riordan J. R. Phenylalanine-508 mediates a cytoplasmic-membrane domain contact in the CFTR 3D structure crucial to assembly and channel function. Proc Natl Acad Sci U S A. 2008;105:3256–3261. doi: 10.1073/pnas.0800254105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L., Aleksandrov A. A., Serohijos A. W., Hegedus T., Aleksandrov L. A., Cui L., Dokholyan N. V., Riordan J. R. Multiple membrane-cytoplasmic domain contacts in the cystic fibrosis transmembrane conductance regulator (CFTR) mediate regulation of channel gating. J Biol Chem. 2008;283:26383–26390. doi: 10.1074/jbc.M803894200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang X.-B., Tabcharani J. A., Hou Y.-X., Jensen T. J., Kartner N., Alon N., Hanrahan J. W., Riordan J. R. Protein kinase A (PKA) still activates CFTR chloride channel after mutagenesis of all ten PKA consensus phosphorylation sites. J Biol Chem. 1993;268:11304–11311. [PubMed] [Google Scholar]
- Aleksandrov L., Mengos A., Chang X., Aleksandrov A., Riordan J. R. Differential interactions of nucleotides at the two nucleotide binding domains of the cystic fibrosis transmembrane conductance regulator. J Biol Chem. 2001;276:12918–12923. doi: 10.1074/jbc.M100515200. [DOI] [PubMed] [Google Scholar]
- Lewis H. A., Zhao X., Wang C., Sauder J. M., Rooney I., Noland B. W., Lorimer D., Kearins M. C., Conners K., Condon B., Maloney P. C., Guggino W. B., Hunt J. F., Emtage S. Impact of the deltaF508 mutation in first nucleotide-binding domain of human cystic fibrosis transmembrane conductance regulator on domain folding and structure. J Biol Chem. 2005;280:1346–1353. doi: 10.1074/jbc.M410968200. [DOI] [PubMed] [Google Scholar]
- Thomas P. J., Thibodeau P., Millen L., Karamysehv A., Patrick A., Richardson J. Altered protein folding as a basis of cystic fibrosis. Federation of European Biochemical Societies; Innsbruck, Austria: 2nd FEBS Special Meeting on ABC Proteins: from Multidrug Resistance to Genetic Diseases. 2008:p. 69. [Google Scholar]
- Seibert F. S., Linsdell P., Loo T. W., Hanrahan J. W., Clarke D. M., Riordan J. R. Disease-associated mutations in the fourth cytoplasmic loop of cystic fibrosis transmembrane conductance regulator compromise biosynthetic processing and chloride channel activity. J Biol Chem. 1996;271:15139–15145. doi: 10.1074/jbc.271.25.15139. [DOI] [PubMed] [Google Scholar]
- Kopito R. R. Biosynthesis and degradation of CFTR. Physiol Rev. 1999;79:S167–S173. doi: 10.1152/physrev.1999.79.1.S167. [DOI] [PubMed] [Google Scholar]
- Lukacs G. L., Mohamed A., Kartner N., Chang X.-B., Riordan J. R., Grinstein S. Confirmational maturation of CFTR but not its mutant counterpart (ΔF508) occurs in the endoplasmic reticulum and requires ATP. EMBO J. 1994;13:6076–6086. doi: 10.1002/j.1460-2075.1994.tb06954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younger J. M., Chen L., Ren H. Y., Rosser M. F., Turnbull E. L., Fan C. Y., Patterson C., Cyr D. M. Sequential quality-control checkpoints triage misfolded cystic fibrosis transmembrane conductance regulator. Cell. 2006;126:571–582. doi: 10.1016/j.cell.2006.06.041. [DOI] [PubMed] [Google Scholar]
- Cui L., Aleksandrov L., Hou Y. X., Gentzsch M., Chen J. H., Riordan J. R., Aleksandrov A. A. The role of cystic fibrosis transmembrane conductance regulator phenylalanine 508 side chain in ion channel gating. J Physiol. 2006;572:347–358. doi: 10.1113/jphysiol.2005.099457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo T. W., Bartlett M. C., Clarke D. M. Correctors enhance maturation of ΔF508 CFTR by promoting interactions between the two halves of the molecule. Biochemistry. 2009;48:9882–9890. doi: 10.1021/bi9004842. [DOI] [PubMed] [Google Scholar]
- Wang X., Roy G., Saxena A., Chalfin E. M. Dynamic chaperone interactions mediate CFTR conformational maturation. Pediat Pulmonol Suppl. 2008;31:121. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.