Abstract
Hyperhomocysteinemia is an independent risk factor for cardiovascular disease. Homocysteine (Hcy) metabolism involves multiple enzymes; however, tissue Hcy metabolism and its relevance to methylation remain unknown. Here, we established gene expression profiles of 8 Hcy metabolic and 12 methylation enzymes in 20 human and 19 mouse tissues through bioinformatic analysis using expression sequence tag clone counts in tissue cDNA libraries. We analyzed correlations between gene expression, Hcy, S-adenosylhomocysteine (SAH), and S-adenosylmethionine (SAM) levels, and SAM/SAH ratios in mouse tissues. Hcy metabolic and methylation enzymes were classified into two types. The expression of Type 1 enzymes positively correlated with tissue Hcy and SAH levels. These include cystathionine β-synthase, cystathionine-γ-lyase, paraxonase 1, 5,10-methylenetetrahydrofolate reductase, betaine:homocysteine methyltransferase, methionine adenosyltransferase, phosphatidylethanolamine N-methyltransferases and glycine N-methyltransferase. Type 2 enzyme expressions correlate with neither tissue Hcy nor SAH levels. These include SAH hydrolase, methionyl-tRNA synthase, 5-methyltetrahydrofolate:Hcy methyltransferase, S-adenosylmethionine decarboxylase, DNA methyltransferase 1/3a, isoprenylcysteine carboxyl methyltransferases, and histone-lysine N-methyltransferase. SAH is the only Hcy metabolite significantly correlated with Hcy levels and methylation enzyme expression. We established equations expressing combined effects of methylation enzymes on tissue SAH, SAM, and SAM/SAH ratios. Our study is the first to provide panoramic tissue gene expression profiles and mathematical models of tissue methylation regulation.—Chen, N. C., Yang, F., Capecci, L. M., Gu, Z., Schafer, A. I., Durante, W., Yang, X.-F., Wang, H. Regulation of homocysteine metabolism and methylation in human and mouse tissues.
Keywords: gene expression, homocysteine metabolism, methylation
Hyperhomocysteinemia (HHCY) has been identified as an independent risk factor for cardiovascular disease (CVD), diabetes, and Alzheimer’s disease. Numerous studies have established that homocysteine (Hcy) has profound biological effects, including accelerating atherosclerosis, impairing postinjury endothelial repair and endothelial function, dysregulating lipid metabolism, and inducing thrombosis. However, the biochemical basis by which HHcy contributes to CVD remains largely unknown. Tissue-specific distribution of Hcy metabolic enzymes, as well as the contribution of each enzyme to tissue Hcy metabolism and methylation has not been previously studied.
Hcy is a non-protein-forming, sulfur-containing amino acid that functions as a key intermediate in methionine (Met) metabolism. Met is first demethylated to form Hcy, which is then metabolized through two pathways: transsulfuration to cysteine (Cys) and remethylation to Met (Fig. 1A). Deficiency in Hcy metabolic enzymes could lead to abnormal Hcy levels in both humans and mice. It is known that cystathionine-β-synthase (CBS) and methylenetetrahydrofolate reductase (MTHFR) are key enzymes for Hcy metabolism (1, 2). Patients with CBS deficiency have plasma Hcy levels of 200–300 μM compared to ∼10 μM in healthy individuals (1). Similarly, CBS-deficient mice develop HHcy (plasma Hcy∼200 μM). In addition, polymorphisms of MTHFR are associated with HHcy in humans (2), and MTHFR-deficient mice develop moderate HHcy (plasma Hcy 33 μM) (3). Although the link between systemic deficiency of certain Hcy metabolic enzymes and global HHcy has been described, the contribution of tissue enzyme expression to tissue-specific Hcy metabolism remains to be explored.
Figure 1.
Hcy metabolism and study design A) Diagram of Hcy metabolism. Eight enzymes involved in Hcy metabolism were selected for database mining and expression profile analysis. 1) CBS, cystathioninine-beta-synthase; 2) CSE, cystathionine-γ-lyase; 3) PON1, paraoxonase 1; 4) BHMT, betaine:homocysteine methyltransferase; 5) MTHFR, 5,10-methylenetetrahydrofolate reductase; 6) AHCY, S-adenosylhomocysteine hydrolase or adenosylhomocysteinase; 7) MTR or MS, 5-methyltetrahydrofolate:homocysteine methyltransferase or methionine synthase; 8) MARS or AARS, methionyl-tRNA synthase. THF, tetrahydrofuran; CH2THF, 5-methylenetetrahydrofolate; CH3THF, 5-methyltetrahydrofolate; NADPH, reduced form of NADP+; NADP+, nicotinamide adenine dinucleotide phosphate; H2S, hydrogen sulfide; SAM, S-adenosylmethionine; SAH, S-adenosylhomocysteine. B) Strategies of database mining, analysis of gene expression profiles and regression analysis. Hcy metabolic and methylation enzymes were selected. Tissue gene mRNA levels (TMP) were retrieved from National Institutes of Health EST database. Generated relative mRNA expression units (REU) and tissue median adjusted mRNA expression units were evaluated. Confidence interval of housekeeping gene expression was determined. Finally, tissue gene expression profiles and relationship between gene expression and Hcy metabolites levels were established.
We have proposed hypomethylation as a specific biochemical mechanism by which Hcy induces vascular injury (4, 5). Hcy can form S-adenosyl-homocysteine (SAH), a potent inhibitor of cellular methylation. It has been reported that abnormal DNA methylation of genes such as PPARα (6), Pdx1 (7), and type 1B adrenal angiotensin receptor (8) contribute to the development of cardiovascular and metabolic diseases (9). We have demonstrated that Hcy arrests endothelial cell growth, possibly by increasing cellular SAH concentrations, consequently reducing DNA methyltransferase 1 (DNMT1) activity, and demethylating cyclin A promoter, which leads to cyclin A chromatin remodeling and transcriptional suppression (5, 10). We hypothesized that DNA hypomethylation is a key mechanism responsible for HHcy-related vascular disease (11, 12). Our hypomethylation hypothesis is supported by clinical studies demonstrating that elevated Hcy levels in patients are linked to increased SAH levels and impaired erythrocyte membrane protein methylation (13,14,15). Furthermore, animal studies corroborate our findings by showing that CBS-deficient mice have increased SAH levels and decreased DNA methylation (1, 16). The underlying molecular mechanism of SAM and SAH regulation in HHcy remains unknown.
It has been reported that the levels of Hcy, SAM, and SAH vary across different mouse tissues (17, 18) These data support the concept that circulating Hcy does not equilibrate Hcy levels across all tissues, and that Hcy metabolism and cellular methylation may be differentially regulated at the tissue level. We now postulate that a differential distribution of Hcy metabolic enzymes is responsible for tissue-specific Hcy metabolism, which contributes to distinct pathologies in HHcy. In this study, we examined gene expression of various Hcy-related metabolic and methylation enzymes using a bioinformatic approach and evaluated the relationship of enzyme expression and Hcy metabolism, as well as methylation status in human and mouse tissues.
MATERIALS AND METHODS
Hcy metabolic and methylation enzymes
We selected 8 key Hcy metabolic enzymes and 12 methylation enzymes for the assessment of tissue expression profiles and correlations (Table 1) based on their metabolic roles schematically described and numbered in Figs. 1A and 3A. Hcy metabolic enzymes include CBS, cystathionine-γ-lyase (CSE), paraoxonase 1 (PON1), betaine:homocysteine methyltransferase (BHMT), 1,5,10-methylenetetrahydrofolate reductase (MTHFR), S-adenosylhomocysteine hydrolase or adenosylhomocysteinase (AHCY), 5-methyltetrahydrofolate:homocysteine methyltransferase or methionine synthase (MS or MTR), and methionyl-tRNA synthetase (MARS). Methylation enzymes are glycine N-methyltransferase (GNMT), phosphatidylethanolamine N-methyltransferase (PEMT), methionine adenosyltransferase (MAT), BHMT, MTHFR, AHCY, MTR, S-adenosylmethionine decarboxylase (AMD), DNA methyltransferase I (DNMT1), DNA methyltransferase 3a (DNMT3a), isoprenylcysteine carboxyl methyltransferases (ICMT), and histone-lysine N-methyltransferase (HMT). Enzyme commission ID numbers and National Center for Biotechnology Information (NCBI)/UniGene ID numbers listed in Table 1 were obtained from the enzyme nomenclature database (http://www.expasy.ch/enzyme/) and the National Institutes of Health (NIH)/NCBI UniGene database (http://www.ncbi.nlm.nih.gov/sites/entrez?db=unigene). Four enzymes (enzymes 4 to 7: BHMT, MTHFR, AHCY, and MTR) fall under both Hcy metabolic and methylation enzyme groups, and are therefore listed with identical numbers in both sections of the table.
TABLE 1.
Enzyme identification
| Enzyme abbreviation and full name | Enzyme commission ID | NCBI/UniGene ID: human; mouse |
|---|---|---|
| Hcy metabolic enzymes | ||
| 1. CBS (cystathioninine-β-synthase) | EC4.2.1.22 | Hs.533013; Mm.206417 |
| 2. CSE (cystathionine-γ-lyase) | EC4.4.1.1 | Hs.19904; Mm.28301 |
| 3. PON1 (paraoxonase 1) | EC3.1.1.2 | Hs.370995; Mm.237657 |
| 4. BHMT (betaine:homocysteine methyltransferase) | EC2.1.1.5 | Hs.80756; Mm.423099 |
| 5. MTHFR (5,10-methylenetetrahydrofolate reductase) | EC1.1.99.15 | Hs.214142; Mm.89959 |
| 6. AHCY (S-adenosylhomocysteine hydrolase or adenosylhomocysteinase) | EC3.3.1.1 | Hs.388004; Mm.2203 |
| 7. MTR, MS (5-methyltetrahydrofolate:homocysteine methyltransferase or methionine synthase) | EC2.1.1.13 | Hs.498187; Mm.40335 |
| 8. MARS (mthionyl-tRNA synthetase) | EC6.1.1.10 | Hs.632707; Mm.19223 |
| Methylation enzymes | ||
| 1. GHMT (glycine N-methyltransferase) | EC2.1.1.20 | Hs.144914; Mm.29395 |
| 2. PEMT (phosphatidylethanolamine N-methyltransferase) | EC2.1.1.17 | Hs.287717; Mm.2731 |
| 3. MAT (methionine adenosyltransferase) | EC2.5.1.6 | Hs.282670; Mm.14064 |
| 4. BHMT (betaine:homocysteine methyltransferase) | EC2.1.1.5 | Hs.80756; Mm.423099 |
| 5. MTHFR (5,10-methylenetetrahydrofolate reductase) | EC1.1.99.15 | Hs.214142; Mm.89959 |
| 6. AHCY (S-adenosylhomocysteine hydrolase or adenosylhomocysteinase) | EC3.3.1.1 | Hs.388004; Mm.220328 |
| 7. MTR, MS (5-methyltetrahydrofolate:homocysteine methyltransferase or methionine synthase) | EC2.1.1.13 | Hs.498187; Mm.40335 |
| 8. AMD (S-adenosylmethionine decarboxylase) | EC4.1.1.50 | Hs.159118; Mm.253533 |
| 9. DNMT1 (DNA methyltransferase 1) | EC2.1.1.37 | Hs.202672; Mm.128580 |
| 10. DNMT3a (DNA methyltransferase 3a) | EC2.1.1.37 | Hs.515840; Mm.5001 |
| 11. ICMT (isoprenylcysteine carboxyl methyltransferases) | EC2.1.1.100 | Hs.515688; Mm.277464 |
| 12. HMT (histone-lysine N-methyltransferase) | EC2.1.1.43 | Hs.709218; Mm.35345 |
Enzyme identification, enzyme commission ID numbers, and NCBI/UniGene ID numbers are from the enzyme nomenclature database (http://www.expasy.ch/enzyme/) and the NIH/NCBI UniGene database (http://www.ncbi.nlm.nih.gov/sites/entrez?db = unigene).
Figure 2.
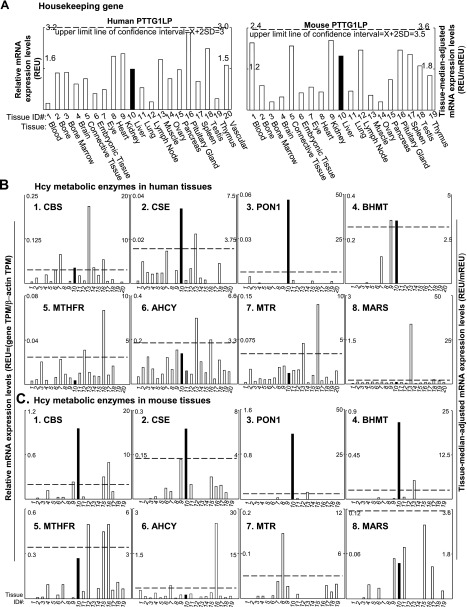
Tissue mRNA distribution profiles of Hcy metabolic enzymes. Twenty human and 19 mouse tissues were given tissue ID numbers and examined for mRNA expression by mining human and mouse EST databases in NCBI/UniGene site. Relative mRNA expression (REU) of the gene is obtained by normalizing gene transcripts per million (TPM) with that of β-actin. Tissue-median-adjusted mRNA expression levels (REU/mREU) were calculated for all genes. Confidence intervals of 3 housekeeping gene mRNA expression were established. Dashed lines are upper limits of the confidence intervals of the housekeeping gene. Left and right y axes describe REU and REU/mREU, respectively. Solid bars highlight
gene expressions in the liver. Enzyme abbreviations are listed in Table 1. A) Representative tissue mRNA distribution profile of housekeeping gene PTTG1LP in human and mouse. See Methods and Materials for details. B) mRNA distribution profiles of 8 Hcy metabolic enzymes in 20 human tissues. C) mRNA distribution profiles of 8 Hcy metabolic enzymes in 19 mouse tissues.
Figure 3.
Methylation enzymes and tissue expression profiles. A) Diagram of methylation-related genes in Hcy metabolism. Twelve methylation enzymes were selected for database mining and expression profile analysis. THF, tetrahydrofuran; CH2THF, 5-methylenetetrahydrofolate; CH3THF, 5-methyltetrahydrofolate; NADPH, reduced form of NADP+; NADP+, nicotinamide adenine dinucleotide phosphate; Met, methionine; Hcy, homocysteine; SAM, S-adenosylmethionine; SAH, S-adenosylhomocysteine; PE, phosphatidylethanolamine; PC, phosphatidylcholine; H3/H4, histone 3/histone 4. Enzyme abbreviations are listed in
Table 1. B) mRNA distribution profiles of methylation enzymes in human tissues. C) mRNA distribution profiles of methylation enzymes in mouse tissues. Expression profiles of 4 of 12 methylation enzymes listed in Table 1 (enzymes 4–7: BHMT, MTHFR, AHCY, and MTR) are omitted because they are included in the Hcy metabolic enzyme group and presented in Fig. 2B, C. Dashed lines are the upper limits of the confidence intervals of the housekeeping genes. Solid bars highlight gene expressions in the liver. Left and right y axes describe REU and REU/mREU, respectively. Enzyme abbreviations are listed in Table 1.
Data mining and gene expression profiles in human and mouse tissues
An experimental data mining strategy (Fig. 1B) was applied to establish the expression profiles of mRNA transcripts of the selected enzymes. The mRNA levels of selected enzymes across 20 human and 19 mouse tissues were examined (Fig. 2A) by mining human and mouse expression sequence tag (EST) databases deposited in the NIH UniGene database. The human and mouse tissues were given tissue ID numbers in Fig. 2A. The EST database is created via cDNA cloning from various tissue cDNA libraries followed by DNA sequencing. Gene mRNA levels are described as gene transcript units per million transcripts (TPM) (19). We generated the relative mRNA expression units (REU) of the gene by normalizing the TPM of the gene of interest with that of β-actin (left-side y axis in Figs. 2 and 3C). To fairly compare gene expression across selected tissues, we further adjusted by comparison to the median REU (mREU). The mREU was determined from REU in 20 human or 19 mouse tissues selected in this study. The ratio of REU/mREU is expressed as tissue median adjusted mRNA expression units and presented in Figs. 2B and 3B (right-side y axis). To establish a confidence interval of gene expression, we calculated the gene expression REUs and ratio of REU/mREU of 3 randomly selected housekeeping genes [pituitary tumor-transforming 1 interacting protein (PTTG1LP), pyruvate kinase muscle (PKM2), and heterogeneous nuclear ribonucleoprotein K (HNRNPK)] in 20 human or 19 mouse tissues. The mean ratio of REU/mREU of each housekeeping gene was then determined: x = Σ(REU1–20/median)/20 for human, and x = Σ(REU1–19/median)/19 for mouse. The 3 housekeeping gene mean ratios of REU/mREU were averaged: X = Σ(x1–3)/3. As we described previously (20), the confidence intervals expressing the general variation of housekeeping gene expression were established by calculating the averaged mean X ± 2sd of the ratio of REU/mREU of the 3 housekeeping genes (21). Tissue median adjusted mRNA expression levels that surpass the upper limit line of the confidence interval were recognized as high-level expression. Gene expression levels <1 copy of TPM for any given gene were considered as no expression.
Tissue Hcy, SAH, and SAM concentrations
The concentrations of Hcy, SAH, and SAM were determined previously by Ueland et al. (16) and Helland and Ueland (18, 22) in tissues from adult male mice under physiological condition. Tissue Hcy concentrations were measured using a radioactive method combined with high-pressure liquid chromatography (HPLC). Briefly, tissue extracts were prepared immediately after sacrificing the animals, deproteinized with perchloric acid, purified with dextran-coated charcoal, and incubated with 14C-adenosine and SAH hydrolase to convert Hcy to 14C-SAH, which is analyzed by HPLC on reversed-phase column (16). The SAM and SAH levels were measured in perchloric acid extracts by HPLC (22). SAM/SAH ratios were calculated for the current analysis based on Helland and Ueland’s (18, 22) results. Tissue Hcy, SAH, and SAM concentrations and SAM/SAH ratios were used for further comparison and regression analyses.
Linear regression analysis
Simple linear regression analyses were performed using Sigma Plot 9.0 (Systat Software, Inc, San Jose, CA, USA) by plotting mRNA levels (REU) of individual enzyme against concentrations of Hcy, SAH, SAM, and SAM/SAH ratios in 6 or 7 mouse tissues including the brain, kidney, liver, spleen, heart, lung, and thymus (see Table 2).
TABLE 2.
Hcy metabolite concentrations in mouse tissue
| Tissue | Metabolite concentration (nmol/g wet wt) |
|||
|---|---|---|---|---|
| Hcya | SAMb | SAHb | SAM:SAH | |
| Brain | 0.89 ± 0.09 | 35.8 ± 4.0 | 0.8 ± 0.1 | 47.1 ± 9.6 |
| Heart | 1.12 ± 0.10 | 58.5 ± 4.2 | 0.4 ± 0.3 | 142.7 ± 87.6 |
| Kidney | 1.29 ± 0.11 | 107.4 ± 5.5 | 4.2 ± 0.8 | 25.6 ± 5.0 |
| Liver | 3.63 ± 0.89 | 112.8 ± 12.4 | 25.5 ± 3.9 | 4.4 ± 0.8 |
| Lung | 1.02 ± 0.12 | 47.7 ± 3.6 | 5.5 ± 0.9 | 8.6 ± 1.5 |
| Spleen | 0.89 ± 0.09 | 65.2 ± 8.1 | 1.7 ± 0.4 | 38.4 ± 10.2 |
| Thymus | NA | 41.3 ± 11.5 | 1.2 ± 0.3 | 34.7 ± 13.6 |
Multivariable regression analyses
We performed multivariable regression analyses to evaluate the combined effect of methylation enzymes on tissue methylation regulation. Because tissue concentrations of SAH and SAM are at or below low micromole levels, we intend to predict SAH and SAM levels using gene transcription information. Therefore, methylation enzymes, whose expressions exhibit significant correlation with Hcy metabolites identified from linear regression analyses, were defined as the independent variables, whereas SAH and SAM concentrations were used as the dependent variables. Each variable contains 7 data points (7 mouse tissues). Multivariable regression analyses were performed between multiple independent variables, and each single dependent variable, using Sigma Plot 9.0 (Systat). Finally, significant variables were selected and used for further multivariable regression analyses to establish a finalized multivariable regression equation. The program was set to reject normality and constant variance tests if P ≥ 0.05.
RESULTS
Hcy metabolic enzymes are differentially expressed in human and mouse tissues
mRNA expression profiles of selected genes in human and mouse tissues are presented in Figs. 2B, C and 3B, C. Confidence intervals expressing the general variations of housekeeping gene mRNA levels were generated based on mRNA levels of 3 housekeeping genes (PTTG1IP, PKM2, and HNRNPK), which have relatively consistent mRNA levels across the selected tissues in both humans and mice. PTTG1IP expression profiles were presented in Fig. 2A as representative of housekeeping genes. The confidence intervals of the house gene expression were X ± 2sd = 1.31 ± 1.59 in human tissue and X ± 2sd = 1.34 ± 2.18 in mouse tissue, and not significantly different between species. Lines denoting an upper limit of the confidence interval were placed in all gene tissue expression profile bar graphs in Figs. 2 and 3 (X+2sd=3 in human tissue; and X+2sd=3.5 in mouse tissue) to determine the significance of gene expression. Gene mRNA expression levels higher than the upper limit line of the confidence interval are recognized as high-level expression. Distribution patterns of each individual enzyme appear to differ between human and mouse tissue. Hcy metabolic and methylation enzymes were more abundantly and evenly distributed in human tissue than in mouse tissue across the tissues examined, except for BHMT (Figs. 2B, C and 3B, C).
Among Hcy metabolic enzymes, CBS transcripts were expressed in most human tissues examined, except for adipose tissue (not shown), adrenal gland (not shown), bone marrow, heart, and thymus. In human tissues (Fig. 2B), CBS mRNA was dominantly expressed in muscle and highly expressed in eye, liver, muscle, nerve (not shown), ovary, pituitary gland, and prostate (not shown). In mouse tissues (Fig. 2C), on the other hand, CBS mRNA was dominantly expressed in liver, and highly expressed in pancreas and pituitary gland.
CSE mRNA was expressed in most tissues except heart, pituitary gland, spleen, thymus, and vascular tissue. It was dominant in the liver of both humans and mice, and high in human muscle. PON1 was dominantly expressed in the liver of humans and mice, high in mouse muscle, low in brain, liver, lung, pancreas, and testis of humans, as well as mouse eye and lung. BHMT was high in the liver and kidney of humans, but high in the liver and muscle of mice. MTHFR transcripts were expressed in the majority of tissues examined, except adipose tissue (not shown), adrenal gland (not shown), bladder (not shown), connective tissue, thymus, and vascular tissue. MTHFR was dominant in the pituitary gland and high in the lymph node of humans. High levels of MTHFR mRNAs were also found in the pituitary gland, lymph node, and pancreas of mice. AHCY transcripts were expressed in most human tissues except nerve (not shown). It was high in muscle and pituitary gland of humans, while high in pituitary gland and eye of mice. MTR was also high in muscle and pituitary gland of humans, but high in the heart tissue of mice. MARS transcripts were ubiquitously expressed at low levels in tissues examined. The higher expression levels of MARS were found only in muscle tissue in humans, and in the pancreas in mice.
Methylation enzymes are differentially expressed in human and mouse tissues
In human tissues (Fig. 3B), both GNMT and MAT had preferential expression in the liver. DNMT1 exhibited ubiquitously low expression in most tissues, but was highly expressed in the lymph node. In comparison, DNMT3a was primarily expressed in embryonic tissue, eye, heart, and muscle. HMT exhibited high expression levels in the muscle. PEMT, ICMT, and AMD exhibited ubiquitous expression across most tissues, with comparatively low expression in the liver. In mouse tissues (Fig. 3C), GNMT, MAT, and PEMT had dominant expression in the liver. In addition, GNMT also had a high level of expression in the lymph node. HMT had high expression levels in the eye, lymph node, and pancreas. DNMT1 was primarily expressed in the eye and ovary. DNMT3a had significant expression in the brain, eye, pancreas, and pituitary gland. AMD was highly expressed in the lymph node, muscle, ovary, and pituitary gland. ICMT was high in the eye, lymph node, and pituitary gland.
Hcy is differentially regulated and positively correlated with SAH concentrations in mouse tissues
We summarized tissue concentrations of Hcy, SAH, and SAM in brain, heart, kidney, liver, lung, spleen, and thymus (SAM and SAH only), which were reported previously (16, 18). As demonstrated in Table 2, the concentrations of Hcy, SAH, and SAM varied across mouse tissues. Liver Hcy concentrations was 3.63 nmol/g, 4.1-fold greater than the concentrations found in the brain and spleen (0.89 nmol/g), where the lowest Hcy levels were observed. The Hcy concentration tissue gradient was liver > kidney > heart > lung > spleen/brain. SAH concentrations were more variable. The SAH level in the liver was 25.5 nmol/g, which was 63.7-fold greater than the concentration in the heart (0.4 nmol/g) .Tissue SAH levels also had a different concentration gradient in the order of liver > lung > kidney > spleen > thymus ≫ brain > heart. SAM was differentially distributed and had a tissue distribution pattern of liver > kidney > spleen > heart ≫ lung > thymus > brain. The liver SAM level was 112.8 nmol/g, 3.15-fold greater than the concentration in the brain (35.8 nmol/g). Furthermore, Hcy concentrations positively correlated with SAH levels in the tested mouse tissues (Fig. 4). Tissue Hcy and its metabolites have not been examined in normal human subjects and have only been tested in a very few rodent studies (17, 23, 24). Mouse tissue Hcy measured by Ueland et al.(16) corroborated that measured by Svardal et al.(17) in the same tissues from rats, with the exception of dissecting the brain into two parts (cerebrum Hcy 0.78 nmol/g and cerebellum Hcy 5.15 nmol/g). Mouse tissue SAM and SAH concentrations were also examined by the Choi group (23) and the James group (24) and exhibited identical concentration gradient, liver > kidney > brain, for both SAM and SAH. However, probably due to the technique variations, these studies (23, 24) presented concentration ranges for both SAM (19.1–112.8 nmol/g in liver, 13.51–107.4 nmol/g in kidney, and 8.7–34.9 nmol/g in brain), and SAH (20.6–25.5 nmol/g in liver, 5.0–24.5 nmol/g in kidney, and 0.2–15.1 nmol/g in brain). In this study, we used Hcy, SAH, and SAM data generated by Ueland’s group (16, 18, 22) for the consideration of methodology consistency. In addition, the radioactive HPLC method used by Ueland’s group provided the best sensitivity for measuring relatively low levels of Hcy in the tissue.
Figure 4.
Correlation of tissue Hcy levels with SAH and SAM concentrations and SAM/SAH ratios in mice. Note that tissue Hcy levels are positively correlated with SAH levels.
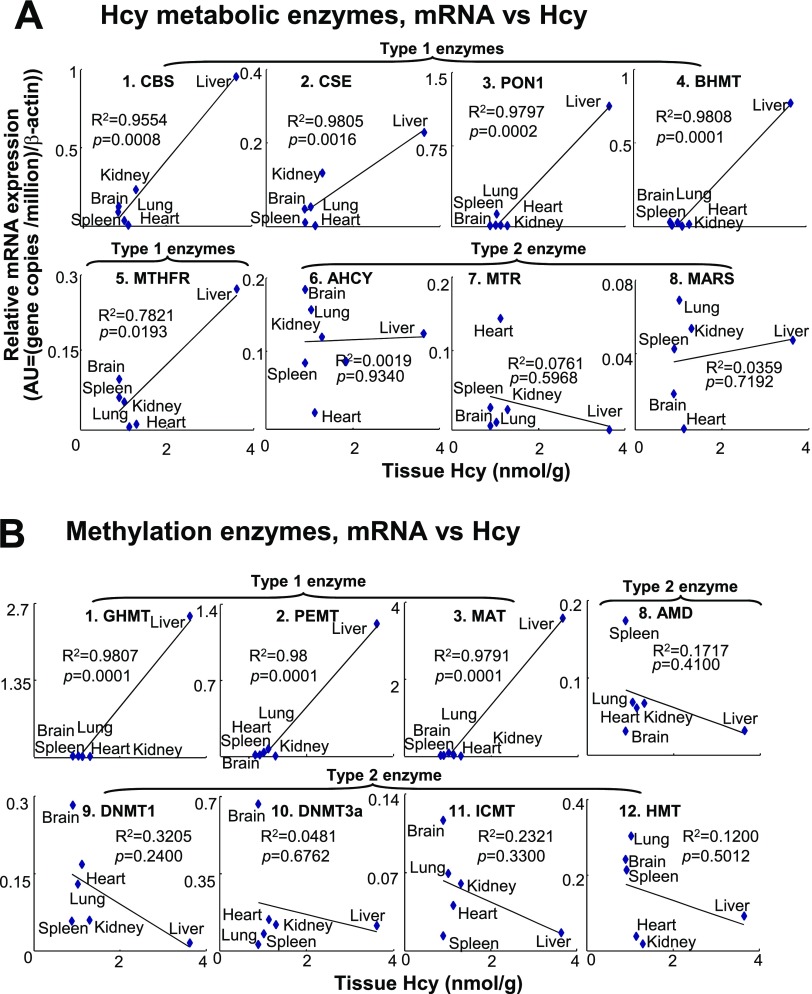
Transcripts of 5 Hcy metabolic enzymes (CBS, CSE, PON1, BHMT, and MTHFR), and 3 methylation enzymes (GNMT, PEMT, and MAT) are significantly correlated with tissue Hcy levels
We assessed the correlation between transcripts (REU) of Hcy metabolic/methylation enzymes and Hcy levels in 6 mouse tissues, including kidney, liver, brain, heart, lung, and spleen, to identify the relationship between enzyme expression and Hcy concentrations in these tissues. We found that the tissue mRNA levels of 5 Hcy metabolic enzymes, CBS, CSE, PON1, BHMT, and MTHFR, were positively correlated with tissue Hcy levels, with regression constants of 0.95, 0.98, 0.97, 0.98, and 0.78, respectively (Fig. 5A). Similarly, transcripts of the methylation enzymes GNMT, PEMT, and MAT were well correlated with tissue Hcy levels, with regression constants of 0.98, 0.98, and 0.97 (Fig. 5B). In contrast, transcripts of the Hcy metabolic enzymes AHCY, MARS, and MTR (Fig. 5A) and the methylation enzymes AMD, DNMT1, DNMT3a, ICMT, and HMT (Fig. 5B) were not correlated with tissue HCY levels. We classified those enzymes, whose transcripts positively correlated with tissue Hcy levels, as type 1 enzymes, and those that did not exhibit correlation as type 2 enzymes (Fig. 5).
Figure 5.
Correlations of Hcy metabolic enzyme expression with Hcy levels in tissues. Relative expression of selected enzymes was determined as described in Fig. 2 and expressed by relative mRNA expression levels (REU). A, B) Correlation between mRNA levels of Hcy metabolic enzymes (A) and methylation enzymes (B), and Hcy levels in mouse tissues. Tissue relative expressions of enzyme mRNA were plotted against tissue Hcy levels shown in Table 1. Linear regression analyses were performed using data points from 7 mouse tissues. Genes are classified into 2 types. Type 1 enzymes exhibited positive correlation between gene expression and Hcy levels in selected tissues. Type 2 enzymes do not correlate with tissue Hcy concentration. Enzymes 4–7 (BHMT, MTHFR, AHCY, and MTR) are members of both groups. Enzyme abbreviations are listed in Table 1.
Transcripts of methylation enzymes GNMT, PEMT, MAT, BHMT, and MTHFR are significantly correlated with tissue SAH levels
SAM and SAH are metabolites of Hcy. SAM is the substrate and SAH is the product of methyltransferase reactions. SAM and SAH levels are important metabolic indicators of cellular methylation status (25). To determine the regulatory effect of methylation enzymes on tissue SAM and SAH metabolism, as well as tissue methylation status, we performed regression analysis with mRNA expression levels (REU) of individual enzymes against SAH, SAM levels, and SAM/SAH ratios in 7 mouse tissues (Table 2) (18). The correlations between methylation enzyme mRNA levels and tissue SAH levels exhibited similar patterns as that between methylation enzymes and Hcy, as shown in Fig. 6A. Five methylation enzymes (GNMT, PEMT, MAT, BHMT, and MTHFR) exhibited positive correlations between tissue mRNA and SAH levels. In addition, BHMT and MTR mRNA levels were positively correlated with SAM concentrations and SAM/SAH ratios, respectively (Fig. 6B, C). AHCY expression did not vary with the change in tissue Hcy, SAH, and SAM concentrations. Enzymes that positively correlated with tissue SAH concentrations overlapped with those that correlated with Hcy concentrations described previously and were therefore classified as type 1 enzymes. Those methylation enzymes that did not correlate with SAH concentrations were classified as type 2 enzymes (Fig. 6A). The type 2 enzymes correlate with neither tissue Hcy nor tissue SAH concentrations. Interestingly, among all methylation enzymes, BHMT is the only one that positively correlated with SAM levels, and MTR is the only one that correlated with SAM/SAH ratios (Fig. 6B, C).
Figure 6.
Correlation between methylation enzyme transcripts and Hcy, SAM, and SAH concentrations and SAM/SAH ratio. Linear regression analyses were performed between enzyme mRNA levels (Fig. 3C) and Hcy, SAM, and SAH concentrations, as well as SAM/SAH ratios (Table 2) in 7 mouse tissues. A) Correlations between enzyme mRNAs and SAH levels. B) Correlations between enzyme mRNA and SAM levels. C) Correlations between enzyme mRNAs and SAM/SAH ratio. Genes are classified into 2 types. Expression of type 1 enzymes is positively correlated with tissue SAH levels; that of type 2 enzymes is not. Enzyme abbreviations are listed in Table 1.
MTHFR and MTR are negatively correlated with SAH, but GNMT and PEMT are positively correlated with SAH levels in mouse tissues
We selected 7 enzymes, of 12 analyzed, whose transcripts exhibited significant positive correlations with SAH, SAM, or SAM/SAH ratio, through linear regression analyses, and performed multivariable regression analyses to delineate the regulatory relationship between enzyme mRNA levels and SAH, SAM, or SAM/SAH ratios. Three multivariable regression equations were established (Fig. 7A). Significant negative correlations were identified for MTHFR and MTR (P=0.042 and P=0.033), and positive correlations were identified for GNMT, PEMT, and MAT (P=0.013, collinear and interchangeable) with SAH (Fig. 7A, Eq. 1). The coefficients, which indicate the strength of the correlation between enzymes and SAH, appeared to be the strongest for MTHFR (99.39), less so for MTR (67.73), and mild for GNMT (18.60). A significant positive correlation was found between MTR (P=0.024) and SAM/SAH ratio (Fig. 7A, Eq. 3). After eliminating nonsignificant variables based on P values, a working model of enzymatic methylation regulation was established and presented as a finalized multivariable regression equation (Fig. 7B): SAH = 6.869 − (59.267·MTHFR) − (57.087·MTR) + (28.318·GNMT). A schematic representation of this regulation is shown in Fig. 7C.
Figure 7.
Multivariable regression analysis of methylation enzymes. A) Multivariable regression equations. Multivariable regression analyses are performed between mRNA levels of 6 methylation enzymes (MTHFR, GNMT, BHMT, PEMT, MTR, MAT) and the SAH and SAM concentrations or SAM/SAH ratio. These enzymes were significantly correlated with tissue SAH and SAM concentrations or SAM/SAH ratio in Fig. 6. B) Finalized multivariable regression equation. Five methylation enzymes (MTHFR, GNMT, PEMT, MTR, and MAT) and SAH were selected for multivariable regression analysis based on significance identified in A to establish the finalized multivariable regression equation. C) Working model; enzymatic methylation regulation. Arrow line with positive sign indicates positive correlation between enzymes (MAT, GNMT, and PEMT) mRNA and SAH levels identified from multivariable regression equation in B. Arrow line with negative signs indicates reversed correlation between enzyme (MTR and MTHFR) mRNA and SAH levels. Abbreviations of enzymes and other molecules are shown in Fig. 3A legend.
DISCUSSION
Hcy metabolism involves multiple enzymes, and its metabolites SAM and SAH are key modulators of cellular methylation. It is well documented that enzymatic regulation determines Hcy metabolism, as >100 HHcy-related human disorders are associated with CBS or MTHFR mutations (2, 26, 27). Moreover, cofactors of CBS and MTR (vitamins B6, B12, and folate) lower Hcy levels in patients with HHcy (27, 28). We previously reported that Hcy causes cell-type-specific hypomethylation in endothelial cells (4, 5) and hypothesized that Hcy metabolism and methylation may be differentially regulated in tissues, resulting in tissue-specific pathology in HHcy. The goal of the present study was to establish tissue expression profiles of Hcy metabolic and methylation enzymes and to identify key enzymes that contribute to tissue-specific regulation of Hcy metabolism and methylation.
We now report 4 major findings from our study: 1) Hcy metabolic and methylation enzymes are differentially expressed in human and mouse tissues; 2) for all enzymes examined, the enzyme transcript tissue distribution profiles of mice exhibit different patterns than those of humans; 3) 5 Hcy metabolic and 5 methylation type I enzymes are positively correlated with tissue Hcy and SAH levels, respectively; and 4) Hcy-related methylation is delineated by the correlations between SAH concentration and the transcripts of MTHFR, MTR, GNMT, PEMT, and MAT, which can be expressed using a multivariable regression equation (Fig. 7B).
We have developed a database-mining approach combined with statistical analysis to evaluate the regulatory relationship between Hcy metabolic/methylation enzyme gene expression and its metabolites using existing data sets. The EST gene expression data and the metabolic data were derived from different animals under physiological conditions. Hypotheses generated from this study shall be tested by future experiments in humans and animals under physiological and pathological conditions and should provide important insights into tissue Hcy metabolic and methylation regulation.
Gene expression was assessed through a database-mining approach utilizing the NCBI/UniGene database. The EST database has been generated via the continued efforts of an international consortium over the past 20 yr, based on random selection of sequencing cDNA libraries (19). It is a highly recognized and reliable resource for gene mRNA expression in normal tissues. EST represents the averaged values of different data entries and describes gene mRNA levels as the copy number of EST cDNA clones per 106 clones sequenced. We believe that the EST database has significant advantages over traditional methods of evaluating gene mRNA expression levels (Northern blot, dot blot, reverse transcription-polymerase chain reaction (RT-PCR), and real-time PCR), which involve RNA/DNA binding to DNA probes/primers and may introduce nonspecificity and inaccuracy.
Of all the tissues examined, liver plays an important role in the systemic regulation of Hcy metabolism (29,30,31). Interestingly, we found that hepatic enzyme expression differs between humans and mice, suggesting that the two species rely on systemic Hcy control to different degrees. Only CSE, PON, GNMT, and MAT exhibit liver-dominant expression patterns in humans. In comparison, more enzymes, including CBS, CSE, PON1, BHMT, GNMT, PEMT, and MAT exhibit liver-dominant expression patterns in mice (Figs. 2B and 3A). This pattern suggests that humans possess a higher level of tissue-specific regulation of Hcy metabolism, whereas mouse Hcy metabolism is dominantly controlled by the liver. This hypothesis is supported by tissue profiles of CBS, the primary Hcy clearance enzyme. We identified more tissues expressing CBS, the primary Hcy clearance enzyme, than previously reported (27) for both mice and humans. Among human tissues, CBS mRNA levels are higher in the eye, muscle, and pituitary gland than in the liver. This suggests that unlike mice, in which CBS is predominantly expressed in the liver, human Hcy metabolism requires tissue-specific regulation. It also may be of clinical relevance that homocystinuria, a multisystem disease caused by CBS deficiency, predominantly manifests in serious eye anomalies, including lens dislocation, myopia, glaucoma, and optic atrophy (32). Similarly, MTHFR and MTR, both of which are folate cycle enzymes, do not have a liver-dominant expression pattern in either mice or humans (Fig. 2B, C). These results suggest that folic acid metabolism is highly regulated at the tissue level for both species. Congruent with previous reports (33), however, we found that the expression of BHMT, a crucial enzyme that catalyzes the transformation of Hcy to methionine, is limited to liver, kidney, and eye in humans and to liver and muscle in mice (Figs. 2B, C). This aspect of Hcy metabolism is highly liver dependent for both humans and mice.
On the basis of linear regression analysis findings presented in Fig. 6, we classified Hcy metabolic and methylation enzymes into two types. Type 1 enzymes include Hcy metabolic enzymes CBS, CSE, PON1, BHMT, and MTHFR, as well as methylation enzymes GNMT, PEMT, and MAT, whose transcripts display positive correlations with tissue Hcy levels. Type 2 enzymes include Hcy metabolic enzymes AHCY, MTR, and MARS, and methylation enzymes AMD, DNMT1, DNMT3a, ICMT, and HMT, which do not correlate with tissue Hcy levels. Data suggest that the type 1 enzymes are sensitive to changes in Hcy concentrations and play important regulatory roles in tissue Hcy metabolism. Furthermore, among the 5 type 1 enzymes, spontaneous HHcy has been associated with homozygous gene deficiency of CBS (plasma Hcy ∼200 μM) (34), MTHFR (plasma Hcy 33 μM) (3), and CSE (plasma Hcy 18 μM) (35), as well as inhibition of BHMT activity (plasma Hcy 18.5 μM) (36) in mouse models but has not been observed in mice deficient in PON1 (37). (As a point of reference, the plasma Hcy level in the control wild-type mouse is 3–5 μM). Hcy levels are regulated by enzyme expression levels. These data suggest that the expressions of type 1 enzymes all influence plasma Hcy levels and further support our hypothesis that Hcy metabolism is coordinated by multiple enzymes in a tissue-specific manner.
Moreover, we identified significant positive correlations between 5 methylation enzymes (GNMT, PEMT, MAT, BHMT, and MTHFR) and SAH, between BHMT and SAM, as well as between MTR and SAM/SAH ratios (Fig. 6). The positive correlation patterns between the 5 methylation enzyme transcripts and SAH levels (Fig. 6A) are consistent with their correlation with Hcy concentrations (Fig. 5B). We classified these 5 enzymes as type 1 enzymes, which have significant positive correlations with tissue Hcy and SAH production. More interestingly, we found that tissue Hcy levels positively correlated with SAH levels, but not with SAM concentrations or SAM/SAH ratios (Fig. 4). Taken together, our data indicate that Hcy metabolic product SAH functions as the primary predictor of tissue-specific methylation. This observation is consistent with previous report on SAH as the principal indicator of cellular methylation status (25). In a cohort of young healthy women, the plasma Hcy correlated strongly with plasma SAH level, but not with either SAM level or SAM/SAH ratio. In the same patient group, plasma SAH and lymphocyte DNA hypomethylation exhibited significant correlation (38). Furthermore, in CBS−/+ mice fed a methyl-deficient diet for 24 wk, an increase in SAH alone affected tissue DNA methylation in liver, kidney, brain, and testes, while SAM level did not correlate significantly with tissue DNA methylation status (24).
The multivariable regression analyses evaluated correlation of multiple enzyme mRNA expression with Hcy metabolites. This method simulated the biological environment, in which all examined methylation enzymes are present simultaneously. We found that the tissue expressions of MTHFR and MTR were negatively correlated with tissue SAH, but GNMT, PEMT, and MAT were positively correlated with tissue SAH levels (Fig. 7B). This multivariable equation suggests a dual directional regulatory relationship between gene expression and Hcy metabolites. Changes in enzyme mRNAs alter tissue SAH levels, and vice versa. This equation underscores the biochemical functions of the enzymes. MAT, GNMT, and PEMT promote the production of tissue SAH, while MTHFR and MTR facilitate the removal of tissue SAH. Consistent with this hypothesis, a positive relationship between SAH and GNMT was observed in GNMT−/− mice (39). Similarly, MAT expression and SAH concentration were both increased in the liver of rats after partial hepatectomy (40). PEMT activity positively correlated with plasma SAH in mice (41). On the other hand, multiple experimental data also demonstrated a direct effect from SAH on the expression and activities of methyltransferases. SAH inhibited DNA methylation of rat liver nuclei (42), suppressed DNMT1 activities in mouse erythroleukemia cell extracts (43), inhibited PEMT activities in rat and guinea pig liver microsomes (44) and in rabbit adrenal gland extracts (45), and reduced GNMT activities in rabbit liver extracts (46). Consistent with the negative correlation of MTHFR with SAH suggested by our equation, increased SAH levels were observed in MTHFR−/+ mice (47). Moreover, elevated GNMT activities/expression and reduced MTHFR activities were accompanied with elevation in plasma Hcy in diabetic mice (48,49,50), as well as diabetic patients (51). It has been suggested that GNMT elevation and MTHFR reduction may exacerbate HHcy among diabetic patients (52).
In general, enzyme function is described as enzyme activities and mostly determined by protein levels. Changes in mRNA expression may not be the directly indicative of changes in protein and enzyme activity. However, the significant correlations between MTHFR, MTR, GNMT, PEMT, MAT, and SAH identified by both linear and multivariable regression analyses in Figs. 6A and 7B suggest that at least for these 5 genes, transcriptional regulation most likely determines their function and directly modulates SAH production. For the similar consideration, the type 1 enzymes significantly correlated with tissue Hcy levels identified by linear regression analyses in Fig. 5A may be regulated at the transcriptional levels as well.
In summary, we demonstrated, for the first time, a panoramic tissue gene expression profile of 8 Hcy metabolic and 12 methylation enzymes in 20 human and 19 mouse tissues. We identified 5 Hcy metabolic and 5 methylation enzymes as the type 1 enzymes, whose transcription displays significant correlation with tissue Hcy and SAH concentrations. We also established a multivariable regression equation depicting the relationship between methylation enzyme mRNA and tissue SAH levels. The gene expression profiles and the mathematical equation presented here should provide important insights for future metabolic and mechanistic studies to identify molecular targets for treating HHcy and hypomethylation-related human disease. The significant correlation between folate cycle enzymes and tissue SAH levels support the hypothesis that folate cycle modulation is a valid therapeutic strategy for treating HHcy and hypomethylation-related pathology. This is consistent with numerous findings from Hcy-lowering clinical trials, which demonstrated that folate therapy is the most effective or the only effective Hcy-lowering treatment (27, 53,54,55). Furthermore, the tissue-specific nature of folate cycle metabolism suggests that folate supplementation targeting on isolated pathological sites could enhance the efficacy and eliminate the associated systemic side effects.
Acknowledgments
This work was supported, in part, by NIH grants HL67033, HL82774, and HL77288 (H.W.), HL094451 (X.F.Y.), and HL74966 (W.D.).
References
- Wang L. Q., Chen X. L., Tang B. Q., Hua X., Klein-Szanto A., Kruger W. D. Expression of mutant human cystathionine beta-synthase rescues neonatal lethality but not homocystinuria in a mouse model. Hum Mol Genet. 2005;14:2201–2208. doi: 10.1093/hmg/ddi224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Z., Hoffman G., Kurtycz D., Yu J. Prevalence of the C677T substitution of the methylenetetrahydrofolate reductase (MTHFR) gene in Wisconsin. Genet Med. 2003;5:458–459. doi: 10.1097/01.gim.0000095001.12570.a8. [DOI] [PubMed] [Google Scholar]
- Chen Z., Karaplis A. C., Ackerman S. L., Pogribny I. P., Melnyk S., Lussier-Cacan S., Chen M. F., Pai A., John S. W., Smith R. S., Bottiglieri T., Bagley P., Selhub J., Rudnicki M. A., James S. J., Rozen R. Mice deficient in methylenetetrahydrofolate reductase exhibit hyperhomocysteinemia and decreased methylation capacity, with neuropathology and aortic lipid deposition. Hum Mol Genet. 2001;10:433–443. doi: 10.1093/hmg/10.5.433. [DOI] [PubMed] [Google Scholar]
- Wang H., Yoshizumi M., Lai K., Tsai J. C., Perrella M. A., Haber E., Lee M. E. Inhibition of growth and p21ras methylation in vascular endothelial cells by homocysteine but not cysteine. J Biol Chem. 1997;272:25380–25385. doi: 10.1074/jbc.272.40.25380. [DOI] [PubMed] [Google Scholar]
- Jamaluddin M. D., Chen I., Yang F., Jiang X., Jan M., Liu X., Schafer A. I., Durante W., Yang X., Wang H. Homocysteine inhibits endothelial cell growth via DNA hypomethylation of the cyclin A gene. Blood. 2007;110:3648–3655. doi: 10.1182/blood-2007-06-096701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillycrop K. A., Slater-Jefferies J. L., Hanson M. A., Godfrey K. M., Jackson A. A., Burdge G. C. Induction of altered epigenetic regulation of the hepatic glucocorticoid receptor in the offspring of rats fed a protein-restricted diet during pregnancy suggests that reduced DNA methyltransferase-1 expression is involved in impaired DNA methylation and changes in histone modifications. Brit J Nutr. 2007;97:1064–1073. doi: 10.1017/S000711450769196X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. H., Stoffers D. A., Nicholls R. D., Simmons R. A. Development of type 2 diabetes following intrauterine growth retardation in rats is associated with progressive epigenetic silencing of Pdx1. J Clin Invest. 2008;118:2316–2324. doi: 10.1172/JCI33655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdarina I., Welham S., King P. J., Burns S. P., Clark A. J. L. Epigenetic modification of the renin-angiotensin system in the fetal programming of hypertension. Circ Res. 2007;100:520–526. doi: 10.1161/01.RES.0000258855.60637.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman P. D., Hanson M. A., Buklijas T., Low F. M., Beedle A. S. Epigenetic mechanisms that underpin metabolic and cardiovascular diseases. Nat Rev Endocrinol. 2009;5:401–408. doi: 10.1038/nrendo.2009.102. [DOI] [PubMed] [Google Scholar]
- Wang H., Jiang X., Yang F., Chapman G. B., Durante W., Sibinga N. E. S., Schafer A. I. Cyclin A transcriptional suppression is the major mechanism mediating homocysteine-induced endothelial cell growth inhibition. Blood. 2002;99:939–945. [PMC free article] [PubMed] [Google Scholar]
- Lee M. E., Wang H. Homocysteine and hypomethylation A novel link to vascular disease. Trends Cardiovasc Med. 1999;9:49–54. doi: 10.1016/s1050-1738(99)00002-x. [DOI] [PubMed] [Google Scholar]
- Jamaluddin M. S., Yang X., Wang H. Hyperhomocysteinemia, DNA methylation and vascular disease. Clin Chem Lab Med. 2007;45:1660–1666. doi: 10.1515/CCLM.2007.350. [DOI] [PubMed] [Google Scholar]
- Perna A. F., Ingrosso D., Zappia V., Galletti P., Capasso G., De Santo N. G. Enzymatic methyl esterification of erythrocyte membrane proteins is impaired in chronic renal failure Evidence for high levels of the natural inhibitor S-adenosylhomocysteine. J Clin Invest. 1993;91:2497–2503. doi: 10.1172/JCI116485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perna A. F., Ingrosso D., De Santo N. G., Galletti P., Zappia V. Mechanism of erythrocyte accumulation of methylation inhibitor S-adenosylhomocysteine in uremia. Kidney Int. 1995;47:247–253. doi: 10.1038/ki.1995.31. [DOI] [PubMed] [Google Scholar]
- Perna A. F., Ingrosso D., De Santo N. G., Galletti P., Brunone M., Zappia V. Metabolic consequences of folate-induced reduction of hyperhomocysteinemia in uremia. J Am Soc Nephrol. 1997;8:1899–1905. doi: 10.1681/ASN.V8121899. [DOI] [PubMed] [Google Scholar]
- Ueland P. M., Helland S., Broch O. J., Schanche J. S. Homocysteine in tissues of the mouse and rat. J Biol Chem. 1984;259:2360–2364. [PubMed] [Google Scholar]
- Svardal A., Refsum H., Ueland P. M. Determination of in vivo protein binding of homocysteine and its relation to free homocysteine in the liver and other tissues of the rat. J Biol Chem. 1986;261:3156–3163. [PubMed] [Google Scholar]
- Helland S., Ueland P. M. Effect of 2′-deoxycoformycin infusion on S-adenosylhomocysteine hydrolase and the amount of S-adenosylhomocysteine and related compounds in tissues of mice. Cancer Res. 1983;43:4142–4147. [PubMed] [Google Scholar]
- Pontius J., Wagner L., Schuler G. D. UniGene: a unified view of the transcriptome. National Center for Biotechnology Information; Bethesda, MD, USA: The NCBI Handbook, Chap 21. 2003:pp. 1–13. [Google Scholar]
- Yin Y., Yan Y., Jiang X., Mai J., Chen N. C., Wang H., Yang X. F. Inflammasomes are differentially expressed in cardiovascular and other tissues. Int J Immunopathol Pharmacol. 2009;22:311–322. doi: 10.1177/039463200902200208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Z., Yan Y., Song J., Fang P., Yin Y., Yang Y., Cowan A., Wang H., Yang X. F. Expression of TCTP antisense in CD25(high) regulatory T cells aggravates cuff-injured vascular inflammation. Atherosclerosis. 2009;203:401–408. doi: 10.1016/j.atherosclerosis.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helland S., Ueland P. M. S-adenosylhomocysteine and S-adenosylhomocysteine hydrolase in various tissues of mice given injection of 9-beta-D-arabinofuranosyladenine. Cancer Res. 1983;43:1847–1850. [PubMed] [Google Scholar]
- Choumenkovitch S. F., Selhub J., Bagley P. J., Maeda N., Nadeau M. R., Smith D. E., Choi S.-W. In the cystathionine β-synthase knockout mouse, elevations in total plasma homocysteine increase tissue S-adenosylhomocysteine, but responses of S-adenosylmethionine and DNA methylation are tissue specific. J Nutr. 2002;132:2157–2160. doi: 10.1093/jn/132.8.2157. [DOI] [PubMed] [Google Scholar]
- Caudill M. A., Wang J. C., Melnyk S., Pogribny I. P., Jernigan S., Collins M. D., Santos-Guzman J., Swendseid M. E., Cogger E. A., James S. J. Intracellular S-adenosylhomocysteine concentrations predict global DNA hypomethylation in tissues of methyl-deficient cystathionine β-synthase heterozygous mice. J Nutr. 2001;131:2811–2818. doi: 10.1093/jn/131.11.2811. [DOI] [PubMed] [Google Scholar]
- James S. J., Melnyk S., Pogribna M., Pogribny I. P., Caudill M. A. Elevation in S-adenosylhomocysteine and DNA hypomethylation: potential epigenetic mechanism for homocysteine-related pathology. J Nutr. 2002;132:2361S–2366S. doi: 10.1093/jn/132.8.2361S. [DOI] [PubMed] [Google Scholar]
- Matthews R. G., Elmore C. L. Defects in homocysteine metabolism: diversity among hyperhomocyst(e)inemias. Clin Chem Lab Med. 2007;45:1700–1703. doi: 10.1515/CCLM.2007.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maron B. A., Loscalzo J. The treatment of hyperhomocysteinemia. Annu Rev Med. 2009;60:39–54. doi: 10.1146/annurev.med.60.041807.123308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen D. W. Homocysteine and vitamins in cardiovascular disease. Clin Chem. 1998;44:1833–1843. [PubMed] [Google Scholar]
- Kharbanda K. K. Alcoholic liver disease and methionine metabolism. Semin Liver Dis. 2009;29:155–165. doi: 10.1055/s-0029-1214371. [DOI] [PubMed] [Google Scholar]
- Barak A. J., Beckenhauer H. C., Tuma D. J. Betaine, ethanol, and the liver: a review. Alcohol. 1996;13:395–398. doi: 10.1016/0741-8329(96)00030-4. [DOI] [PubMed] [Google Scholar]
- Stead L. M., Brosnan M. E., Brosnan J. T. Characterization of homocysteine metabolism in the rat liver. Biochem J. 2000;350:685–692. [PMC free article] [PubMed] [Google Scholar]
- De Franchis R., Sperandeo M. P., Sebastio G., Andria G. Clinical aspects of cystathionine beta-synthase deficiency: how wide is the spectrum? The Italian Collaborative Study Group on Homocystinuria. Eur J Pediatr Suppl. 1998;2:S67–S70. doi: 10.1007/pl00014309. [DOI] [PubMed] [Google Scholar]
- McKeever M. P., Weir D. G., Molloy A., Scott J. M. Betaine-homocysteine methyltransferase: organ distribution in man, pig and rat and subcellular distribution in the rat. Clin Sci (Lond) 1991;81:551–556. doi: 10.1042/cs0810551. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Osada J., Aratani Y., Kluckman K., Reddick R., Malinow M. R., Maeda N. Mice deficient in cystathionine beta-synthase: animal models for mild and severe homocyst(e)inemia. Proc Natl Acad Sci U S A. 1995;92:1585–1589. doi: 10.1073/pnas.92.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G., Wu L., Jiang B., Yang W., Qi J., Cao K., Meng Q., Mustafa A. K., Mu W., Zhang S., Snyder S. H., Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinsova M., Strakova J., Jiracek J., Garrow T. A. Inhibition of betaine-homocysteine S-methyltransferase causes hyperhomocysteinemia in mice. J Nutr. 2006;136:1493–1497. doi: 10.1093/jn/136.6.1493. [DOI] [PubMed] [Google Scholar]
- Shih D. M., Gu L., Xia Y. R., Navab M., Li W. F., Hama S., Castellani L. W., Furlong C. E., Costa L. G., Fogelman A. M., Lusis A. J. Mice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosis. Nature. 1998;394:284–287. doi: 10.1038/28406. [DOI] [PubMed] [Google Scholar]
- Yi P., Melnyk S., Pogribna M., Pogribny I. P., Hine R. J., James S. J. Increase in plasma homocysteine associated with parallel increases in plasma S-adenosylhomocysteine and lymphocyte DNA hypomethylation. J Biol Chem. 2000;275:29318–29323. doi: 10.1074/jbc.M002725200. [DOI] [PubMed] [Google Scholar]
- Luka Z., Capdevila A., Mato J. M., Wagner C. A glycine N-methyltransferase knockout mouse model for humans with deficiency of this enzyme. Transgen Res. 2006;15:393–397. doi: 10.1007/s11248-006-0008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z.-Z., Mao Z., Cai J., Lu S. C. Changes in methionine adenosyltransferase during liver regeneration in the rat. Am J Physiol Gastrointest Liver Physiol. 1998;275:G14–G21. doi: 10.1152/ajpgi.1998.275.1.G14. [DOI] [PubMed] [Google Scholar]
- Igolnikov A. C., Green R. M. Mice heterozygous for the Mdr2 gene demonstrate decreased PEMT activity and diminished steatohepatitis on the MCD diet. J Hepatol. 2006;44:586–592. doi: 10.1016/j.jhep.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Cox R., Prescott C., Irving C. C. The effect of S-adenosylhomocysteine on DNA methylation in isolated rat liver nuclei. Biochim Biophys Acta. 1977;474:493–499. doi: 10.1016/0005-2787(77)90070-3. [DOI] [PubMed] [Google Scholar]
- Flynn J., Reich N. Murine DNA (Cytosine-5-)-methyltransferase: Steady-state and substrate trapping analyses of the kinetic mechanism. Biochemistry. 1998;37:15162–15169. doi: 10.1021/bi9810609. [DOI] [PubMed] [Google Scholar]
- Hoffman D. R., Haning J. A., Cornatzer W.E. Microsomal phosphatidylethanolamine methyltransferase: Inhibition by S-adenosylhomocysteine. Lipids. 1980;16:561–567. doi: 10.1007/BF02534900. [DOI] [PubMed] [Google Scholar]
- Deguchi T., Barchas J. Inhibition of transmethylations of biogenic amines by S-adenosylhomocysteine. J Biol Chem. 1971;246:3175–3181. [PubMed] [Google Scholar]
- Kerr S. J. Competing methyltransferase systems. J Biol Chem. 1972;247:4248–4252. [PubMed] [Google Scholar]
- Devlin A. M., Arning E., Bottiglieri T., Faraci F. M., Rozen R., Lentz S. R. Effect of Mthfr genotype on diet-induced hyperhomocysteinemia and vascular function in mice. Blood. 2004;103:2624–2629. doi: 10.1182/blood-2003-09-3078. [DOI] [PubMed] [Google Scholar]
- Nieman K. M., Rowling M. J., Garrow T. A., Schalinske K. L. Modulation of methyl group metabolism by streptozotocin-induced diabetes and all-trans-retinoic acid. J Biol Chem. 2004;279:45708–45712. doi: 10.1074/jbc.M408664200. [DOI] [PubMed] [Google Scholar]
- Yeo E. J., Wagner C. Tissue distribution of glycine N-methyltransferase, a major folate-binding protein of liver. Proc Natl Acad Sci U S A. 1994;91:210–214. doi: 10.1073/pnas.91.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs R. L., Stead L. M., Brosnan M. E., Brosnan J. T. Hyperglucagonemia in rats results in decreased plasma homocysteine and increased flux through the transsulfuration pathway in liver. J Biol Chem. 2001;276:43740–43747. doi: 10.1074/jbc.M107553200. [DOI] [PubMed] [Google Scholar]
- Tessari P., Coracina A., Kiwanuka E., Vedovato M., Vettore M., Valerio A., Zaramella M., Garibotto G. Effects of insulin on methionine and homocysteine kinetics in type 2 diabetes with nephropathy. Diabetes. 2005;54:2968–2976. doi: 10.2337/diabetes.54.10.2968. [DOI] [PubMed] [Google Scholar]
- Meigs J. B., Jacques P. F., Selhub J., Singer D. E., Nathan D. M., Rifai N., D'Agostino R. B., Sr, Wilson P. W. Fasting plasma homocysteine levels in the insulin resistance syndrome: the Framingham offspring study. Diabetes Care. 2001;24:1403–1410. doi: 10.2337/diacare.24.8.1403. [DOI] [PubMed] [Google Scholar]
- Mager A., Orvin K., Koren-Morag N., Lev I. E., Assali A., Kornowski R., Shohat M., Battler A., Hasdai D. Impact of homocysteine-lowering vitamin therapy on long-term outcome of patients with coronary artery disease. Am J Cardiol. 2009;104:745–749. doi: 10.1016/j.amjcard.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Bazzano L. A. Folic acid supplementation and cardiovascular disease: the state of the art. Am J Med Sci. 2009;338:48–49. doi: 10.1097/MAJ.0b013e3181aaefd6. [DOI] [PubMed] [Google Scholar]
- Song Y., Cook N. R., Albert C. M., Van Denburgh M., Manson J. E. Effect of homocysteine-lowering treatment with folic Acid and B vitamins on risk of type 2 diabetes in women: a randomized, controlled trial. Diabetes. 2009;58:1921–1928. doi: 10.2337/db09-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]