Abstract
The autophagic/lysosomal system includes a variety of vesicular compartments that undergo dynamic fusion events. However, the characteristics and factors modulating these interactions remain, for the most part, unknown. To gain insights on the properties that govern lysosomal fusion events, we have established an in vitro fusion assay using different lysosomal/autophagic compartments isolated from mouse liver. We have found that autophagosome/lysosome fusion is a temperature-dependent process (fusion increment of 0.2±0.01%/°C), which requires ATP (1–3 mM), GTP (1–2 mM), Ca2+ (0.2–2 mM), and an acidic lysosomal pH (pH 5.2). Furthermore, changes in membrane lipid composition, induced either in vitro, by treatment with 25 mM methyl-β-cyclodextrin, or in vivo, by subjecting animals to a high-fat-diet challenge (60% kcal in fat) reduce autophagosome/lysosome fusion up to 70% of that observed in untreated fractions or from animals under a normal regular diet. These findings reveal a novel role for lipids in autophagic fusion and provide a mechanism for the reduced macroautophagic rates observed during exposure to a chronic lipid challenge. Changes in the intracellular lipid content (i.e., metabolic disorders) may thus have pronounced effects on the fusion step of macroautophagy and affect the overall activity of this intracellular proteolytic pathway.—Koga, H., Kaushik, S., Cuervo, A. M. Altered lipid content inhibits autophagic vesicular fusion.
Keywords: lysosomes, membrane fusion, proteolysis
Two major proteolytic systems, the ubiquitin/proteasome system and lysosomes, participate in continuous protein turnover and in the removal of altered, no-longer-functional proteins. The lysosomal system mainly degrades organelles and long-lived intracellular proteins via autophagy (1). Three different forms of autophagy have been described in mammals, depending on the mechanisms that mediate delivery of cargo to lysosomes: macroautophagy, microautophagy, and chaperone-mediated autophagy (CMA) (1, 2). Whereas in these two last autophagic pathways, cargo is delivered directly from the cytosol to lysosomes, either through invaginations in the lysosomal membrane (in microautophagy) or by directly crossing the membrane through a translocation complex (in CMA), the distinctive characteristic of macroautophagy is that cytosolic components are first sequestered in a double-membrane vesicle [autophagosome (APG)]. This double-membrane vesicle acquires the hydrolases required for cargo degradation upon fusion with lysosomes. Macroautophagic cargo can also reach the lysosomal lumen through fusion of APGs with early or late endosomes (to form amphisomes) (3).
Different molecular components of lysosomes and endosomes, such as Rab7 or AAA ATPase SKD, have been reported to be necessary for fusion of APGs with lysosomes or early/late endosomes in mammalian cells (4,5,6). In addition, APG/lysosome fusion can be inhibited by treatment with inhibitors of the lysosomal proton pump (bafilomycin A1) or weak bases (hydroxychloroquine), which neutralize the lysosomal acidic pH. This finding suggests that heterotypic fusion depends on luminal pH (7). However, the specific requirements for vesicular fusion in the autophagic pathway are poorly understood, mainly due to the difficulty of separating fusion itself from other cellular events directly related to this process, such as vesicular docking into the cytoskeleton network and directional vesicular trafficking.
A further level of complexity in the autophagic fusion arises from the fact that what have been classically cataloged as lysosomes include a heterogeneous pool of intracellular vesicles, possibly at different maturation stages and with different functions (8). In fact, we have reported previously the isolation of different lysosomal subgroups with different morphological and biochemical properties, a distinctive proteomic signature and with different roles in autophagy (8). Only one of these lysosomal subgroups is competent to perform CMA, as they contain the luminal chaperone required for lysosomal uptake of cytosolic proteins by this pathway (8). However, nothing is known about the participation of the different lysosomal subgroups in macroautophagy. Thus, whether different lysosomal types can undergo fusion with APGs or whether only a particular lysosomal subpopulation is dedicated to macroautophagy remains unknown.
Understanding the vesicular fusion events that take place between the different subcompartments involved in macroautophagy is important to further elucidate the basis of the crosstalk between autophagic and heterophagic pathways. In addition, alterations in vesicular fusion may be behind the increased number of autophagic vacuoles reported in human disorders of very different nature, including, among others, neurodegenerative diseases, myopathies, metabolic disorders, and some infectious diseases (9,10,11). Defects in degradation could arise from both impaired proteolytic activity of the lysosomal hydrolases or from alterations in fusion of APGs with the lytic compartments, either endosomes or lysosomes. Consequently, distinguishing whether improper fusion is a primary defect or an indirect consequence of alterations in vesicular traffic is essential to the design of future interventions aimed at repairing or improving vesicular fusion.
To analyze the characteristics of APGs/lysosome fusion independently of vesicular trafficking, we have developed a fusion assay using isolated APGs and different lysosomal subpopulations from both rodent tissues and cells in culture. Using this assay, we have characterized the basic requirements for heterotypic fusion of APGs with lysosomal compartments. Furthermore, we have identified that defective vesicular fusion is responsible, at least in part, for the decrease in autophagic activity that we have described previously to occur in mouse liver on exposure to a chronic lipid challenge such as a high-fat diet (HFD) (12). Our results support that changes in the intracellular lipid content in conditions such as metabolic disorders may have pronounced effects on the fusion step of macroautophagy and affect the overall activity of this intracellular proteolytic pathway.
MATERIALS AND METHODS
Antibodies and reagents
The antibody against light-chain 3 protein (LC3) was from MBL International Corp. (Woburn, MA, USA), against the lysosome-associated membrane protein type 1 (LAMP-1; clone 1D4B) and cation-dependent mannose-6-phosophate receptor (CD-M6PR) were from the Developmental Hybridoma Bank (University of Iowa, Iowa City, IA, USA), against cathepsin D and rab5 (clone D-11) were from Santa Cruz Biotechnology (Santa Cruz, CA, USA), against hsc70 (clone 13D3) from Novus Biotechnology Inc. (Littleton, CO, USA), and against hsp90 from Stressgen (Ann Arbor, MI, USA). LAMP-2A and B antibodies were developed in our laboratory (refs. 13, 14 and unpublished results). Reagents used in this work were as described before (8, 12, 13). Bafilomycin A1, methyl-β-cyclodextrin, and monodansylcadaverine were from Sigma (St. Louis, MO, USA), and LysoTracker Red DND 99 was from Molecular Probes (Eugene, OR, USA). The GFP-mcherry-LC3 construct was a generous gift from Dr. Terje Johansen (University of Trømso, Trømso, Norway).
Animals
Male C57BL/6 mice (6–8 wk old) were obtained from Jackson Laboratory (West Grove, PA, USA). Starved mice had free access to water. Animals were fed HFD (D12492, 60% kcal in fat; Research Diets, New Brunswick, NJ, USA) when they reached 3 wk of age, and this diet was continued for 16 wk. At the end of the treatment, some of the animals were starved for 6 h before isolation of APGs (15).
Cells and cell culture
Mouse fibroblasts (NIH3T3) were from the American Type Culture Collection (Manassas, VA, USA) and were cultured as described previously (16). The rat hepatocyte line RALA255-10G was cultured under nontransformed conditions as described previously (17). Oleic and palmitic acid were conjugated to albumin, as described previously (18), and cells were treated with oleate or palmitate for 24 h in high-glucose and serum-free DMEM. Acid compartments were labeled in cultured cells by adding 100 nM LysoTracker in serum-free DMEM for 20 min. Labeling with monodansylcadaverine (50 nM) was performed by incubation for 10 min.
Subcellular fractionation
Isolation of subcellular fractions from mouse liver
APGs or lysosomes from mouse liver were isolated from a light mitochondrial-lysosomal fraction by centrifugation in metrizamide discontinuous gradients, as described previously. APGs were recovered in the 20–15% metrizamide interface, and lysosomes in the 26–24% metrizamide interface. Two lysosomal subpopulations with different CMA activity were isolated from mouse liver by the original protocol (19), modified as described previously (8). Purity of the lysosomal fractions was assessed by measuring total and specific enzymatic activities of mitochondrial, peroxisomal, and cytosolic enzymes (only possible contaminants in these fractions), and integrity of the lysosomal membrane was determined by measurement of leakage of lysosomal enzymes in the medium (20). Briefly, right after isolation or at the end of the incubation in the fusion buffer, lysosomes were subjected to centrifugation and the activity of the lysosomal hydrolase β-hexosaminidase was measured in both the pellet (lysosomes) and the supernatant. The enzyme present in the supernatant can originate only from lysosomal disruption, as secretion of this enzyme or permeation through the membrane are not possible. Consequently, the percentage of total β-hexosaminidase activity (pellet and supernatant) present outside lysosomes (supernatant) was used to monitor lysosomal breakage. We did not find differences in purity of the fractions or stability of the lysosomal membrane between fed and starved animals or with HFD treatment (for further purity information, please see refs. 12, 21). Endosomes were prepared from mouse livers 5 min after portal venous injection of Texas Red-labeled asialo-orosomucoid, the ligand of the hepatocyte-specific asialoglycoprotein receptor (22, 23). After homogenization and differential centrifugation, the postnuclear supernatant was subjected to chromatography on Sephacryl S200. Fluorescence-enriched peaks were collected and subjected to centrifugation (200,000 g for 135 min) on a sucrose step gradient of 1.4, 1.2, and 0.25 M sucrose. Vesicles were harvested from the 1.2/0.25 M sucrose.
Isolation of subcellular fractions from cells in culture
APGs and lysosomes were isolated from cultured cells using a modified protocol from Marzella et al. (24). Briefly, cells were disrupted by nitrogen cavitation, and a pellet enriched in APGs, lysosomes, and mitochondria was prepared by differential centrifugation.
In vitro vesicular fusion assay
Isolated APGs or lysosomes were incubated for 10 min at room temperature with primary antibodies, followed by incubation with fluorescence-conjugated secondary antibodies for an additional 10 min. Labeled vesicles were recovered by centrifugation and carefully resuspended in fusion buffer (10 mM HEPES, pH7; 10 mM KCl; 1.5 mM MgCl2; 1 mM DTT; 0.25 M sucrose; and protease inhibitors) and were mixed in the reaction buffer (3 mM ATP, 2 mM GTP, 2 mM CaCl2, 0.16 mg/ml creatine phosphokinase, 8 mM phosphocreatine, and protease inhibitors) and incubated at 37°C for 30 min. The mixture was spotted on a glass slide, fixed with 8% formaldehyde in 0.25M of sucrose for 15 min, and visualized under the fluorescence microscope. Images were acquired with an Axiovert 2000 fluorescence microscope (Carl Zeiss Ltd., Oberkochen, Germany) with a ×100 objective and 1.4 numerical aperture, subjected to deconvolution with the manufacturer’s software, and prepared using Adobe Photoshop 6.0 software (Adobe Systems Inc., San Jose, CA, USA). Quantification was performed using Image J software (U.S. National Institutes of Health, Bethesda, MD, USA) and colocalization was calculated by JACoP plugin. Where indicated, mixed vesicles before or after incubation were subjected to FACSAria (Becton Dickinson, Franklin Lakes, NJ, USA) high-speed cell-sorter flow cytometer. In all experiments, parallel reactions maintained at 4°C were set to discard any nonspecific clumping effect of the additions over the vesicles (a representative example in shown in Supplemental Fig. S7).
Intracellular protein turnover
To measure degradation of long-lived proteins in cultured hepatocytes, cells were labeled with [3H]leucine (2 mCi/ml) for 48 h at 37°C and then washed and maintained in serum-deprived medium containing an excess of unlabeled leucine (25). Aliquots of the medium taken at different times were precipitated with trichloroacetic acid, and proteolysis was measured as the percentage of the initial acid-insoluble radioactivity (protein) transformed into acid-soluble radioactivity (amino acids and small peptides) (25). Lysosomal-dependent degradation was inhibited by addition of 20 mM NH4Cl and macroautophagy-dependent degradation by addition of 10 mM 3-methyladenine.
Morphological procedures
Fluorescence microscopy
Cells were transiently transfected with a GFP-mcherry-LC3 construct and treated with lipids for 24 h. Cells were fixed with 3% formaldehyde and processed with mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI). Neutral lipids were stained with BODIPY 493/503 after fixation (12).
Electron microscopy
Isolated autophagic/lysosomal fractions were pelleted by centrifugation, fixed in 2.5% glutaraldehyde in 100 mM sodium cacodylate (pH 7.43) and 0.25 M sucrose, and osmium/uranyl stained as described before (8, 12). Ultrathin sections were uranyl acetate/lead citrate stained and viewed on a Jeol 1200EX transmission electron microscope (Jeol Ltd., Akishima, Japan)at 80 kV.
General methods
Protein concentration was determined by the Lowry method, using BSA as a standard (26). Oleic and palmitic acid were conjugated to albumin, as described previously (6), and cells were treated with oleate or palmitate for 24 h. For immunoblotting, the proteins recognized by the specific antibodies were visualized by chemiluminescence methods (Renaissance; NEN-Life Science, Boston, MA, USA). Quantification of intracellular cholesterol was performed by using the Amplex Red Cholesterol Assay kit (Molecular Probes) following manufacturer’s instructions.
RESULTS
A novel assay to monitor autophagosome/lysosome fusion
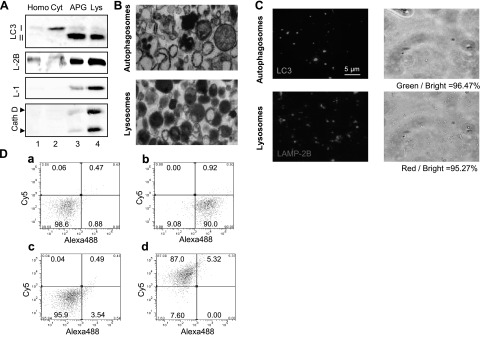
We have developed an image-based assay that allows tracking fusion between isolated vesicular compartments labeled with different fluorophores. We first isolated two different fractions from mouse liver highly enriched in APGs or in lysosomes using differential centrifugation and floatation in discontinuous density metrizamide gradients, as described previously (24). Biochemical analysis of both fractions revealed marked enrichment in the APG fraction of LC3-II, the lipid-conjugated form of the light-chain 3 protein (LC3) that decorates the cytosolic and luminal sides of the double membrane of APGs and the most commonly used marker for this compartment (Fig. 1A) (27). LC3-II could also be detected at lower levels in the lysosomal fraction because the luminal form of the protein is transferred from APGs to lysosomes after fusion (27). Hydrolases (cathepsin D shown here; Fig. 1A) and lysosome-associated membrane protein 1 (LAMP-1), detected in low levels in APGs, was highly enriched in the lysosomal fraction (Fig. 1A). Electron microscopy analysis of the isolated fractions revealed that the APG-enriched fraction contained primarily double-membrane vesicles with different types of cytosolic cargo in their lumen; whereas vesicles in the lysosome-enriched fraction were mainly single membrane and with more amorphous and denser content (Fig. 1B). The integrity of the isolated lysosomal fractions, essential to support vesicular fusion, was monitored by measuring the activity of the lysosomal hydrolase β-hexosaminidase in the incubation medium (which reflects enzyme released due to lysosomal leakage), and preparations with >5% of broken lysosomes or conditions resulting in lysosomal breakage higher than this value were discarded. The integrity of the autophagosomes on isolation and after incubation in the conditions of our assay was monitored by the release of autophagosomal cargo into the incubation medium (p62 and GAPDH, two well-characterized autophagosome cargo, are shown in Supplemental Fig. S1).
Figure 1.
Characterization of labeling of APG and lysosomal fractions. A) APGs and lysosomes (Lys) isolated from mouse liver were subjected to immunoblot for the indicated antibodies. Homo, homogenate; Cyt, cytosol. B) Ultrastructure of the isolated fractions. C) Fluorescence (left panels) and bright field images (right panels) of the same fractions labeled with antibodies against LC3 (APGs) and LAMP-2B (lysosomes), and the corresponding secondary antibodies conjugated with FITC or Cy5, respectively. Percentage of total particles labeled per field is indicated at bottom. D) Analysis of antibody labeling of APGs and lysosomes using FACS analysis. Unlabeled or antibody labeled fractions were subjected to FACS analysis. Particle sorting analysis and percentage of particles detected in each channel are shown for unlabeled APGs (a), LC3-Alexa488 labeled-APGs (b) and unlabeled lysosomes (c), and LAMP-2B-Cy5-labeled- lysosomes (d).
Fusion and fission events in the endocytic pathway can be reproduced in vitro and monitored by immunofluorescence after labeling the compartments with antibodies (28). To adapt this assay to the study of APGs/lysosomal fusion, we first analyzed the efficiency of labeling in the isolated fractions with antibodies against different membrane proteins. Incubation of APGs with an antibody against LC3 and a FITC-conjugated secondary antibody labeled 96.5% of the particles in this fraction (Fig. 1C). Likewise, 95.3% of vesicles in the lysosomal fractions were labeled with an antibody against the cytosolic tail of LAMP-2B and a Cy5-conjugated secondary antibody (Fig. 1C). Analysis of the same fractions using fluorescence-activated cell sorting modified for small particle counting (FACSAria) revealed similar efficiency of labeling for both fractions (Fig. 1D). FACS analysis also allowed us to determine the size range of the different compartments present in the reaction and discard the presence of abnormally large vesicle “aggregates” that could be erroneously annotated as fusion in the image-based assay. Although LAMP-2B and LC3 can be detected to some extent in both fractions (Fig. 1A), we confirmed that they were in the same vesicle (Supplemental Fig. S2). In fact, cross-contamination between fractions was relatively small, as double-labeling with both antibodies of the APG fraction revealed that <5% of vesicles were positive for LAMP-2B but not LC3, and double-labeling of lysosomes revealed that <2% compartments were positive only for LC3 (Supplemental Fig. S2).
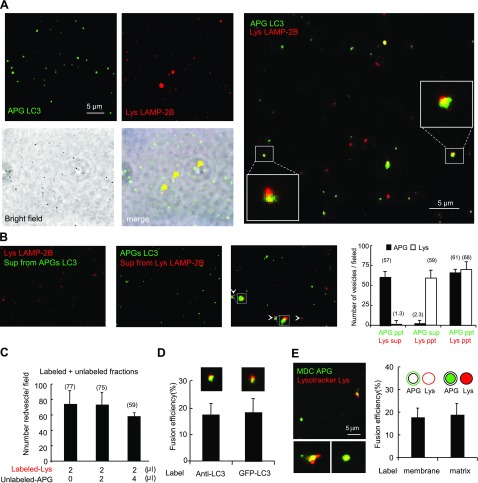
We then incubated both labeled fractions together in a modified fusion buffer (see Materials and Methods) (28) and observed that fusion events, calculated as the percentage of total number of vesicles positive for both fluorophores, were close to 20% in our conditions (Fig. 2A). We confirmed that the respective secondary antibodies did not cross-react with the other protein if they were to be released into the medium during the incubation, and that the release of primary antibody into the medium was negligible. As shown in Fig. 2B, vesicle labeling was not detectable on incubation of labeled APGs with the supernatant of labeled lysosomes or vice versa. Furthermore, incubation of labeled fractions with unlabeled counterparts did not result in higher number of labeled vesicles at the end of the incubation, as it would have been expected if the antibodies were released into the medium and reached the unlabeled compartment (Fig. 2C).
Figure 2.
Reconstitution of APG-lysosome fusion. A) Overlapping of bright field and merged fluorescent channels of mouse liver APGs and lysosomes (Lys) labeled as in Fig. 1C and incubated in fusion buffer. Right panel insets: higher-magnification images of fusion events. B) Negligible exchange of antibodies during the fusion reaction. Similarly labeled APG and lysosomes were incubated separately in fusion buffer, and after centrifugation their supernatants (sup) were collected and incubated with labeled lysosomes (left panel) and labeled APGs (left middle panel). Incubation in parallel with the originally labeled fractions is shown as a positive control of fusion (right middle panel). Graph (right panel) shows number of green or red vesicles per field in each of the three reactions. Values are also shown at top of respective bars. C) LAMP-2B-labeled lysosomes were incubated alone or with two different concentrations of unlabeled autophagosomes for 30 min at 37°C. Total number of red particles per field at the end of each of the incubations was quantified. D) Percentage of fusion events between lysosomes labeled with anti-LAMP-2B and APGs either labeled with anti-LC3 or isolated from GFP-LC3 transgenic mice. Values are expressed as percentage of fusion events relative to the total number of particles in the field. E) Fusion events between APGs and lysosomes isolated from cells previously incubated with MDC and with LysoTracker, respectively. Left panel: representative fluorescent image. Insets: examples of fusion events at higher magnification. Right panel: percentage of fusion events between vesicles labeled with antibodies against membrane proteins (as in A) or vesicles with fluorescent content. Values are expressed as in E. All values are means + se of 3 different experiments.
Early reports proposed a fusogenic role for LC3-II on the APG membrane (27). To determine whether labeling of APGs with antibodies against LC3 interfered with fusion, we isolated APGs from a transgenic mouse expressing GFP-LC3 (29), for which labeling with antibodies was no longer necessary. The similar rates of fusion observed with both types of labeled APGs (Fig. 2D) support that the labeling antibodies did not significantly interfere with fusion. Furthermore, we used labeling for other proteins previously described to localize on the surface of APGs (p62, HDAC6, and huntingtin) or in lysosomes (LAMP-2A, hsc70, and hsp90) and found that fusion of vesicles labeled with any of these antibodies was comparable to the one observed using LC3 and LAMP-2B labeling (Supplemental Fig. S3), supporting that these antibodies did not interfere with fusion events.
To determine whether the fusion events observed in our system resulted from tethering and hemileaflet fusion (only outer membrane) or complete fusion between both membranes, we monitored mixing of luminal components from both vesicular fractions both by fluorescent cargo mixing and by electron microscopy. We used monodansylcadaverine (MDC) and LysoTracker in different set of cells to label the lumen of APGs and acidic compartments, respectively. Although MDC and LysoTracker label multiple cellular compartments, our isolated fractions contained mainly labeled APGs or labeled lysosomes (Fig. 1A–C). Fusion assays with these labeled fractions revealed comparable colocalization frequencies to the ones observed with membrane-labeled vesicles, supporting mixing of the lumen content from both vesicular fractions (Fig. 2E). Furthermore, we subjected incubated and nonincubated vesicle mixtures to electron microscopy to determine the nature of the resulting compartments. In the incubated reactions, it was possible to detect the presence of compartments of different morphological characteristics than the starting vesicles, namely: reduced luminal density (compatible with degradation of cargo, possibly as result of lysosomal enzyme delivery); partial loss of the double membrane; higher fragmentation of the luminal cargo; and often continuity of the membrane of vesicles with very different density and morphologically compatible with the combination of APGs and lysosomes (Supplemental Fig. S4). These results support that complete fusion between the two compartments can be reproduced in our isolated system.
Basic requirements for heterotypic APG-lysosome fusion
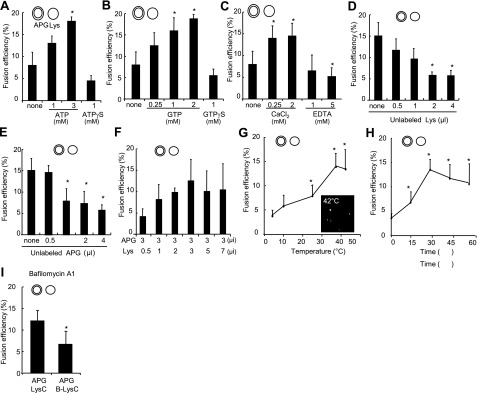
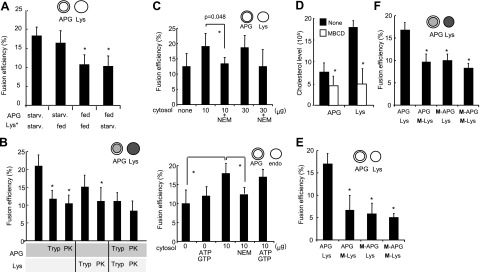
In vitro assays to reproduce fusion between different vesicular compartments have often used addition of cytosol to promote fusogenicity (28, 30). Because the identification afterward of the specific cytosolic components required for fusion is often difficult, we adopted instead a reverse approach in which individual components were added to the fusion reaction until the minimal requirements to reproduce vesicular fusion were identified. We found that addition of ATP or GTP increased fusion efficiency in a dose-dependent manner; whereas their nonhydrolyzable analogs (ATPγS and GTPγS) did not sustain fusion, either using surface labeling of the vesicles (Fig. 3A, B) or cargo mixing (Supplemental Fig. S5A, B). Macroautophagy is a calcium-dependent process (31, 32), but the steps in which the divalent cation is required are still under investigation. Our in vitro fusion studies revealed that concentrations of calcium chloride in the incubation buffer of ≥0.25 mM are required to sustain maximal APGs/lysosome fusion rates (Fig. 3C).
Figure 3.
Requirements for heterotypic APG-lysosome fusion. APGs and lysosomes (Lys) isolated from 6-h-starved mouse livers were labeled with antibodies against LC3 (+FITC) and LAMP-2B (+Cy5), respectively. A–C) Fractions were diluted to the same final protein concentration (0.01 mg/ml), and fusion efficiency was calculated after incubation at 37°C for 30 min in the presence of different concentrations of ATP and its nonhydrolyzable analog ATPγS (A), GTP and its nonhydrolyzable analog GTPγS (B), or calcium chloride and the calcium chelator EDTA (C). D, E) Fusion efficiency of equal concentrations of lysosomes and APGs incubated with increasing amounts of unlabeled lysosomes (D) or APGs (E). F) APGs and lysosomes were diluted to the same concentration of particles and labeled, and a fixed amount of APGs was incubated with increasing concentrations of lysosomes. G, H) Fusion efficiency of equal concentrations of lysosomes and APGs incubated in fusion medium for 30 min at indicated temperatures (G) or at 37°C for indicated periods of time (H). I) Fusion efficiency of APGs labeled as above and incubated in fusion buffer with untreated lysosomes or lysosomes pretreated with Bafilomycin A1 (B-Lys). Values are expressed as in Fig. 1D and are means + se of 3–5 different experiments. *P < 0.05.
To further confirm that the fusion observed in our assay did not result from random interactions among vesicles or mere formation of vesicular agglomerates, we analyzed fusion rates of APGs and lysosomes incubated with increasing concentrations of unlabeled lysosomes. The number of fusion events decreased with the increase in the amount of unlabeled lysosomes (Fig. 3D), and the same is true when increasing amounts of unlabeled APGs were added to the fusion mixture (Fig. 3E). This competition was not a consequence of advantage of the unlabeled fraction, because a similar tendency to saturation was observed when labeled APGs were incubated with increasing concentration of labeled lysosomes (Fig. 3F). These results support that fusion is a saturable process, proportional to the number of vesicles available and not an artifact from nonspecific vesicular overlapping. Fusion is time and temperature dependent (Fig. 3G, H and Supplemental Fig. S5C, D shows similar assays by cargo mixing and Fig. S6 by FACS analysis), although the increase in lysosomal breakage observed at temperatures beyond 37°C or when vesicles were incubated for more than 35 min made us limit the incubation reactions to 30 min at 37°C. The low fusion efficiency observed at low temperatures allowed us to use 4°C incubations as a systematic control in all reactions to discard nonspecific “clumping” effects of any of the reagents or fractions added to the reaction (Supplemental Fig. S7).
Previous studies have reported that bafilomycin A1, a specific inhibitor of the vacuolar type H+-ATPase, blocks fusion of APGs with lysosomes (7). Pretreatment of our isolated lysosomes with bafilomycin A1 reduced APG/lysosome fusion efficiency up to 50% (Fig. 3I), whereas pretreatment of the APGs with the inhibitor showed no significant changes (data not shown). These results confirm that acidification of lysosomal compartments is required for heterotypic fusion. The dependence of the heterotypic fusion on Ca2+, GTP, and ATP hydrolysis and an acidic luminal pH, all of them previously proposed to modulate macroautophagy in vivo, validates our assay as a good system to analyze possible changes in the fusion step of macroautophagy under physiological and pathological conditions.
APG fusion to different lysosome subpopulations
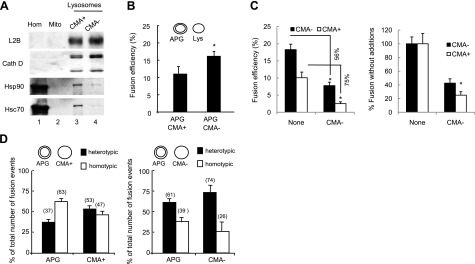
We have previously optimized a method to separate two different types of secondary lysosomes (8). They are morphologically similar and contain comparable levels of most lysosome-associated membrane proteins (Fig. 4A; LAMP-2B shown here) and lytic enzymes (Fig. 4A; cathepsin D shown here). However, they differ in their enrichment in the chaperone (hsc70) required for the selective uptake of cytosolic proteins in lysosomes via CMA (Fig. 4A). Lysosomes enriched in hsc70 are competent for CMA (CMA+), whereas the group of lysosomes with negligible amounts of this chaperone cannot perform CMA (CMA−) (8). The involvement of each of these lysosomal subgroups in macroautophagy has not been analyzed. Using the fusion assay, we examined the ability of APG to fuse with these two separate subpopulations of lysosomes and found that APGs fuse moderately more efficiently (30%) with CMA− lysosomes than with CMA+ lysosomes (Fig. 4B). In fact, using competition assays with unlabeled CMA− lysosomes, we found a higher inhibitory effect for the same amount of CMA− lysosomes over the fusion of APGs with CMA+ lysosomes than with CMA− lysosomes (Fig. 4C), further supporting the moderate but consistent higher fusogenic capability of CMA− lysosomes with APGs.
Figure 4.
Fusion events between APGs and different lysosomal subpopulations. A) Homogenate (Homo), mitochondria (Mito), and lysosomes with high (CMA+) and low (CMA−) CMA activity isolated from starved mice were subjected to immunoblots for the indicated antibodies. B) APGs CMA+ and CMA− lysosomes isolated from livers of starved mice were labeled with antibodies against LC3, hsc70, and LAMP-2B, respectively, and the corresponding fluorophore-conjugated secondary antibodies. Fractions were incubated for 30 min at 37°C, and fusion events were analyzed. Values are expressed as percentage of fusion events relative to the total number of particles in the field and are means + se of 3–5 different experiments. C) APGs and CMA+ and CMA− lysosomes labeled as in B were incubated alone or in the presence of unlabeled CMA− lysosomes, and fusion events were analyzed. Values are expressed as percentage of fusion events relative to the total number of particles in the field (left panel; percentage of decrease in the presence of unlabeled lysosomes is shown) or as percentage of the fusion efficiency in the absence of unlabeled CMA− lysosomes (right panel). Values are means + se of 3 different experiments. D) Homotypic and heterotypic fusion events in fusion reactions like those described in B for APGs with CMA+ lysosomes (left panel) or CMA− lysosomes (right panel). Values are expressed as percentage of total fusion reactions and are means + se of 3 different experiments. *P < 0.05.
Fusion in between the same compartments (homotypic fusion) is a common feature of the endocytic system, but it has not been analyzed in detail for the autophagic system. To determine whether the differences in fusogenic ability of CMA+ and CMA− lysosomes with APGs could be a consequence of differences in their homotypic fusion ability, we calculated the number of vesicles involved in homotypic and heterotypic fusion in each fusion assay. We counted the number of individual vesicles from each type (APGs and lysosomes) before and after incubation in the fusion reaction and calculated the percentage of homotypic fusion events as the decrease in the number of vesicles of each compartment (since heterotypically fused compartments still maintain the fluorescence of each of the merged compartments). Both homotypic and heterotypic events were occurring at the same time, but that the percentage of heterotypic fusion of APGs with CMA− lysosomes (61±6.4%) was almost double than with CMA+ lysosomes (37±4.5%) (Fig. 4D). These results support a higher tendency of the CMA− lysosomes to fuse with APGs than with themselves. Interestingly, the lower rates of fusion of APGs with CMA+ lysosomes seem to favor homotypic fusion of APGs (63% compared to 39% in the presence of CMA− lysosomes). Our results support that although both groups of lysosomes can fuse with APGs, the observed differences in their fusion efficiency may determine their main function inside cells.
Changes in fusogenic activity of lysosomal and autophagic compartments under conditions with different autophagic activity
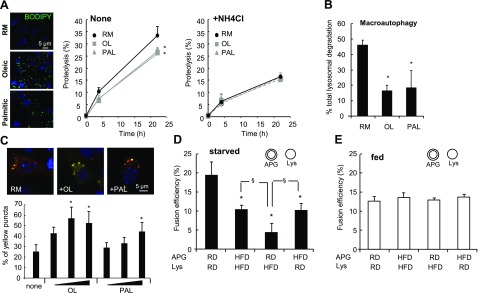
Starvation is one of the best characterized stimuli of macroautophagy in liver (33), where macroautophagy is maximally up-regulated 4–6 h into nutrient deprivation due to enhanced APG formation (34). To determine whether possible changes in the fusogenic ability of the autophagic/lysosomal compartments could also contribute to higher macroautophagy-dependent degradation under these conditions, we analyzed APG/lysosome fusion independently of other steps in macroautophagy using fractions isolated from livers of mice fed or starved for 6 h. As shown in Fig. 5A, fusion between APGs and lysosomes isolated from starved animals was significantly higher than between the same fractions isolated from fed mice. The higher rates of fusion in the fractions from starved animals was mainly due to higher fusogenic ability of the APGs during starvation, as fusion was comparable when these APGs were presented to lysosomes from fed animals, whereas fusion decreased in the reactions that APGs were contributed from fed animals (Fig. 5A). These findings support that primary changes in fusogenic ability contribute to the enhanced rates of macroautophagy during starvation, that and intrinsic properties of the autophagic compartments are solely responsible for the observed changes, since they can be detected in in vitro.
Figure 5.
Effect of changes in autophagic activity and in the membrane components of the autophagic-lysosomal fusion. A) APGs and lysosomes (Lys) isolated from livers of mice fed or starved for 6 h before isolation were labeled with anti-LC3 (+FITC) or anti-LAMP-2B (+Cy5), respectively. Values represent percentage of fusion events relative to the total number of particles in the field and are means + se of 4 different experiments. B) APGs and Lys isolated from cells previously incubated with MDC and LysoTracker, respectively, were treated with 10 mg/ml of trypsin at room temperature for 15 min or with 10 mg/ml of proteinase K (PK) at 4°C for 15 min. Samples were collected by centrifugation; further action of the proteases was inhibited at the end of the incubation by addition of a cocktail of protease inhibitors. Efficiency of the protease treatment and of the protease inhibitor cocktail was tested by immunoblot as shown in Supplemental Fig. S9. Treated fractions were subjected to in vitro fusion assay. Fusion efficiency is expressed as percentage of fusion events relative to total number of particles in field; values are means + se of 3 different experiments. C) Starved mouse APGs labeled as in A were incubated with LAMP-2B-labeled lysosomes (left panel) or Texas-red asialoglycoprotein labeled endosomes (right panel) in fusion buffer alone (none) or supplemented with indicated amounts of cytosol from the same animals. Where indicated, 1 mM ATP, GTP, or NEM was added to the cytosolic fraction. Fusion efficiency was expressed as in A. Values are means + se of 3 different experiments. D, E) APGs and Lys isolated from livers of mice starved for 6 h were subjected to treatment with methyl-β-cyclodextrin (M). Levels of cholesterol in treated and untreated fractions were measured (D). Fractions were labeled as in A, and fusion events were analyzed (E). Values are means + se of 3 different experiments. F) Fusion events between monodansylcadaverine-labeled APGs and LysoTracker-labeled Lys isolated from NIH-3T3 cells and subjected to the same treatments as in E. Values are means + se of 3 different experiments are shown. *P < 0.05.
This last observation prompted us to analyze the contribution of different components—proteins and lipids—of the APG membranes to their fusogenic abilities. As shown in Fig. 5B, treatment of isolated APG or lysosomes with either trypsin or proteinase K to remove peripherally associated proteins and cytosolic portions of membrane proteins, reduced APGs/lysosome fusion ∼50%, supporting the participation of proteins on the surface of both APG and lysosomes in the fusion process. To determine further the requirement for cytosolic proteins, we analyzed the effect of adding increasing concentrations of cytosol from the same animals into the fusion reaction. In contrast to other vesicular fusion events (i.e., lysosome-phagosome, endosome-endosome, or even endosome-APG), where cytosolic components are necessary for the fusion reaction, addition of cytosol resulted in a relatively discrete (∼25%) increase in fusion efficiency (Fig. 5C, left), in contrast to the 50% increase observed in endosome-APG fusion (Fig. 5C, right). In both cases, this effect was completely abolished when the cytosolic fraction was treated with N-ethylmaleimide (NEM), a well-characterized inhibitor of the cytosolic chaperone (NSF) required for SNARE-mediated vesicular fusion (Fig. 5C). These results support that, as described before for endosome-APG fusion (28, 30), the fusion of APG to lysosomes is also only partially dependent on SNAREs.
We then analyzed whether changes in levels of lipids, the other major membrane constituent, could also affect APGs/lysosome fusion efficiency. We focused on cholesterol levels since it is often the main component of functional membrane microdomains that serve as sites for specific protein-lipid interactions (35). Preincubation of APGs and lysosomes with the cholesterol-extracting agent methyl-β-cyclodextrin (MBCD) reduced their cholesterol levels by 25 and 70%, respectively (Fig. 5D). Despite the relatively discrete changes in cholesterol levels in APGs this treatment reduced fusion efficiency by 40–50% both in the surface labeling assay (Fig. 5E) and in the cargo mixing (Fig. 5F). Similar reduction was observed with MBCD-treated lysosomes (Fig. 5E, F), suggesting that optimal lipid composition in both APGs and lysosomes is required to support vesicular fusion.
Altered vesicular fusion is behind the impairment of autophagy induced by lipid challenges
We have recently found that macroautophagy contributes to lipid metabolism by mediating the mobilization and lipolysis of intracellular lipid stores (mainly constituted by triglycerides and cholesterol). Most cells respond to a moderate lipid stimulus by increasing macroautophagic flow to accommodate to the influx of lipids without considerably altering the size of intracellular lipid stores (12). However, we found that acute exposure to high concentration of lipids (such as oleate or palmitate) or to a chronic lipid stimuli (animals on HFD) reduced autophagy of lipids (12). Formation of APGs is preserved under those conditions, because the subset of cellular proteins that initiate macroautophagy remained unchanged. The fact that changes in the lipid composition of the autophagosomal or lysosomal membranes could modify their fusogenic abilities, and thus contribute to impaired APG clearance, prompted us to investigate further the changes in autophagy in response to lipid stimuli.
We first determined whether the inhibitory effect on macroautophagy of acute lipid stimulation was restricted to degradation of lipid or whether it also affects protein degradation. Treatment of cultured hepatocytes with both oleic or palmitic acid, which increased intracellular content of lipid droplets (Fig. 6A, left), significantly decreased the degradation of long-lived proteins (typical autophagic cargo) (Fig. 6A, middle). Differences between treated and untreated cells were no longer evident when the lysosomal proteolysis was inhibited (Fig. 6A, right), supporting that they were indeed due to impaired functioning of the lysosomal system. The decrease in protein degradation in presence of the lipid stimuli was mainly due to reduced macroautophagy, calculated as the percentage of lysosomal degradation (sensitive to NH4Cl) that was inhibited by 3-methyladenine (an inhibitor of phosphatidylinositol 3-kinase type I broadly used to inhibit macroautophagy) (Fig. 6B).
Figure 6.
Lipid stimuli inhibit macroautophagy by reducing APG-lysosome fusion. A) Right panels: degradation of long-lived proteins in hepatocytes cultured in regular medium (RM) or in medium supplemented with 0.25 mM oleate (OL) or palmitate (PAL) without additions (None, left graph) or in the presence of 20 mM ammonium chloride (NH4Cl, right graph). Values are expressed as percentage of proteolysis and are means + se of 4 different experiments. Left panel: BODIPY 493/503 staining of the cultured cells to highlight the cellular content of lipid droplets. Nuclei are labeled with DAPI. B) Macroautophagy activity in the same cells was calculated as the percentage of lysosomal degradation (inhibited by NH4Cl) sensitive to 3-methyladenine. Both treatments were added at the beginning of the chase period to avoid any effect of the treatments on protein synthesis. Values are means + se of 4 different experiments. C) NIH-3T3 cells were transiently transfected with the GFP-mcherry-LC3 reporter, and the percentage of APGs (yellow) and autophagolysosomes (red) present in untreated cells and cells treated with oleate (0.125, 0.25, and 0.5 mM) or palmitate (0.25, 0.5, and 0.75 mM) for 24 h was determined. Values are means + se of 3 different experiments. Representative images are shown; full size fields are shown in Supplemental Fig. S8. D, E) APGs and lysosomes (Lys) isolated from livers of mice fed a regular diet (RD) or an HFD for 16 wk and starved (D) or not (E) for 6 h before isolation were labeled with anti-LC3 (+FITC) or anti-LAMP-2B (+Cy5), respectively. Incubations of same fractions at 4°C did not reveal significant differences in background fusion between these compartments. Values are expressed as percentage of fusion events relative to total number of particles in the field and are means + se of 4 different experiments. *,§P < 0.05.
We then used a previously published pH-sensitive reporter (GFP-mcherry-LC3) (36) that allows visualization in intact cells of APGs before lysosomal fusion as yellow puncta (since both fluorophores fluoresce in the same molecule) and autophagolysosomes (APGL; postlysosomal fusion) as red puncta (because green fluorescent proteins are sensitive to the low pH). Quantification of the fluorescence images revealed higher number of APGs and a lower number of APGLs in cells exposed to lipid stimuli (Fig. 6C and Supplemental Fig. S8), pointing toward problems in clearance of APGs in these cells. However, the studies in intact cells cannot discriminate whether decreased clearance originates from a direct problem in fusion between APGs and lysosomes, or is a consequence of reduced vesicular traffic, altered lysosomal acidification, or impaired protease activity.
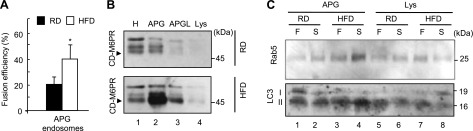
To test directly possible changes in vesicular fusion, we used APGs and lysosomes isolated from livers of mice maintained on a regular diet (RD) or an HFD for 16 wk, both starved for 6 h to maximally activate macroautophagy. As described before, we observed a reduction of 30 and 60% in the levels of cholesterol in the membrane and lumen, respectively, of lysosomes isolated from the HFD mice. Fusion rates between the fractions isolated from the HFD group were up to 40% lower than in those isolated from the RD mice (Fig. 6D). In contrast, fusion was comparable in both groups when fractions were isolated under fed conditions (basal macroautophagy) (Fig. 6E). Four-way cross assays revealed that the defect in fusion was already evident when APGs from HFD were presented to RD lysosomes, supporting that alterations in the APG membrane are enough to compromise fusion. Surprisingly, combination of APGs from RD with lysosomes from the HFD mice resulted in a more pronounced decrease (80%) than when both organelles were from the HFD group (Fig. 6D). A plausible explanation for this finding could be that the APGs from HFD may have developed compensatory mechanisms to overcome their limited fusion with lysosomes.
Previous reports have shown increased fusion of APG with endosomes (to form amphisomes) when fusion with lysosomes is impaired (16, 37). To test this possibility, we analyzed fusion between APGs isolated from RD- or HFD-fed mice and endosomes labeled by injection of RD mice with Texas Red-labeled asialo-orosomucoid (38). As shown in Fig. 7A, APGs from HFD mouse fuse with endosomes more frequently (45%) than APGs from RD mouse (22%). It is thus possible that the APGs in these mice have acquired fusion factors through previous fusion with endosomes, and this could explain why they fuse better to lysosomes from HFD than regular APGs (Fig. 6D). In fact, immunoblot analysis revealed a marked increase of endosomal markers (CD-M6PR and Rab5) in the APGs from HFD mice compared with APGs from RD mice (Fig. 7B, C).
Figure 7.
Enhanced interaction of APG with the endocytic compartment during a lipid stimulus. A) APGs isolated from livers of mice fed a regular diet (RD) or an HFD for 16 wk and labeled as in Fig. 6D were incubated with endosomes labeled with Texas-red asialoglycoprotein, and fusion was quantified. Values are expressed as percentage of fusion events relative to total number of particles in field and are means + se of 4 different experiments. *P < 0.05. B, C) Homogenates (H), APGs, autophagolysosomes (APGL), and lysosomes (Lys) isolated from 6-h-starved (B and S in C) or fed (F) mice maintained on an RD or HFD were subjected to immunoblots for the indicated proteins. Arrow indicates CD-M6PR; other bands result from nonspecific cross-reaction.
Our results support that maintained lipid stimuli results in a primary defect in APG/lysosome fusion, which could explain the decrease in macroautophagic degradation under those conditions (Fig. 7A, B; ref. 12) and that reinforces the importance of changes in intracellular lipid content on autophagic vesicular fusion.
DISCUSSION
The complexity of vesicular fusion events that take place in macroautophagy and the close interrelation of membrane fusion with other steps of this pathway, such as vesicular docking on microtubules and directional trafficking, which makes it difficult to study primary vesicular fusion changes in intact cells. To overcome these limitations, we have developed an assay that allows recapitulating in vitro vesicle membrane fusion independently of vesicular traffic. Using this assay, we have identified a previously unknown role of lipids in APG/lysosome fusion and a primary defect in APG/lysosome fusion in cells exposed to pathological levels of lipids. This finding provides a possible mechanistic explanation for the impairment in macroautophagy observed as result of an HFD.
The in vitro fusion assay allows us to directly analyze membrane fusion and to isolate fusion events from any other autophagic steps. Although it is difficult to determine how the frequency of fusion events in the in vitro assay compares to in vivo fusion, the relative comparison of fusion efficiency between different vesicular populations and under different conditions is a useful way to start dissecting preferences in fusion (between which compartments) as well as minimal requirements of this process.
The requirement for Ca2+ in APG/lysosome fusion is shared with vesicular fusion in other systems where it has been proposed to localize at the docking site of fusing membranes (39). Similar to endosomes, APGs also require acidification of the lysosomal lumen to fuse to this compartment (Fig. 3I). How the acidity of the lysosomal lumen contributes to fusion remains unclear. Although, it has been proposed that optimal activity of lysosome luminal proteases may be required to activate and/or mediate fusion, and hence the need for a low pH; the fact that we can reproduce fusion and a complete mix of cargo in the presence of protease inhibitors argues against this possibility. It is plausible that the acidic lumen is required to stabilize the proteins involved in membrane fusion. The discrete effect of NEM treatment supports that both fusion of APGs to endosomes and to lysosomes can occur for the most part independent of SNAREs or that the SNAREs involved in this process do not require priming by the chaperone. The fact that most proteomic studies have failed to identify SNARE proteins in APGs makes us favor the first possibility and that the effect of NEM likely reflects inhibition of homotypic lysosomal or endosomal fusion. Despite these similarities between the fusion of APGs to endosomes and lysosomes, our study has defined unique characteristics of each of them, such as the different requirements of nucleotides. As previously reported (28, 30), fusion of APGs to endosomes did not require nucleotides; whereas hydrolyzable forms of ATP and GTP were necessary for APG-lysosome fusion (Fig. 5C).
Our studies support that homotypic fusion occurs not only between APGs but also between lysosomes. This latter event could set the basis for the multifunctionality of different lysosomal subpopulations and the possibility of recruiting a particular subset of lysosomes for a given autophagic pathway when needed, by merging lysosomes usually involved in different autophagic pathways. Thus, the preferential fusion of APGs with lysosomes usually inactive for CMA (lacking the luminal hsc70) supports the existence of a lysosomal subgroup usually dedicated to CMA and a second subset more prone to macroautophagy.
Particularly interesting is the fact that intrinsic properties of the membrane, including both protein and lipid composition, modulate APG/lysosome fusion. Several proteomic studies have provided information on the protein composition of APGs and lysosomes (40), but no information is available on the specific subset of these proteins located on the cytosolic side of the membrane in these compartments. Identification of the fusogenic machinery was beyond the purpose of this study, but we analyzed the possible involvement of APG associated proteins for which participation in macroautophagy has been already reported. Using specific antibodies against some of these proteins, we have discarded the need in heterotypic fusion for p62, HDAC6, or huntingtin at the APG membrane. Interestingly, studies in LAMP-2-deficient cells have revealed defective fusion of lysosomes with phagosomes (41) and with APGs (42), but to date it was not clear whether fusion itself or vesicular motility were affected in these cells. Our studies with specific antibodies against the cytosolic tail of the LAMP-2 variants that localize in lysosomes (LAMP-2A and LAMP-2B) provide evidence against direct involvement of these proteins in the fusion step and favor the recently proposed role of LAMP-2 in vesicular trafficking (41).
The previously unknown effect of changes in the lipid composition of APGs and lysosomal membranes—in particular cholesterol—on their fusogenic capability, underscores the impact that changes in lipid metabolism and intracellular lipid content can have on macroautophagy. We have found in this work that reduced levels of cholesterol in the membrane of either APGs or lysosomes results in a dramatic reduction in fusion, supporting the participation of cholesterol from both vesicles in membrane fusion. The fact that similar defects in fusion were detected on exposure of cells to high levels of lipids or a chronic lipid challenge in whole animals could be misinterpreted as contradictory (low levels of cholesterol in vitro and high levels of lipids in vivo have a similar outcome in fusion). However, our previous studies have revealed that lysosomes and autophagic vacuoles from cells and animals exposed to high lipid concentrations have indeed lower levels of lipids (triglycerides and cholesterol) than the same organelles from control conditions (12). Consequently, the two experimental paradigms used in this study support that reduced levels of cholesterol in the membrane of APGs and lysosomes lower their fusogenic capability, and this could explain why rates of macroautophagy are reduced in these models despite a proper formation of APGs (12).
The molecular mechanism of how cholesterol content contributes to vesicular fusion requires further investigation. It is possible that cholesterol molecules participate directly in tethering and hemileaflet fusion, or that changes in cholesterol content modify properties of the membrane, such as rigidity or permeability, that could be important in the fusion process (43). Increased temperature and reduced cholesterol content, conditions that increased APGs-lysosome fusion in our assay, are both known to increase membrane fluidity, which has been shown to be important for plasma membrane fusion in very different systems (i.e., protoplasts, myoblasts, etc., reviewed in refs. 44, 45). The fact that increased fluidity facilitates divalent cation-induced fusion, even in between protein-free unilamellar lipid vesicles (46), supports a possible direct regulatory effect of changes in lipid composition of APGs on their fusogenic capabilities by triggering physical changes in the membrane bilayer required for the fusion process. It is also plausible that membrane cholesterol content determines the dynamics of the proteins involved in the fusion process. In fact, we have previously identified the presence of discrete lipid microdomains at the lysosomal membrane, which play a critical role in the dynamics and multimeric organization of the protein receptor for CMA (47). Likewise, assembling of the fusogenic complex on the surface of APGs could also be regulated through lipid microdomains.
Interestingly, our results show that APGs from HFD mice preferably fuse with endosomes despite the lower fusion efficiency between APGs and lysosomes (Fig. 6B), suggesting that fusion between APGs/lysosomes and APGs/endosomes may be governed differently. It is predicted that the lower acidification of the amphisomes (formed by fusion of endosomes with APGs) compared to autophagolysosomes will make hydrolysis in amphisomes less efficient and explain the lower protein degradation rates via macroautophagy observed under these conditions.
In summary, the possibility of studying fusion between compartments of the autophagic system independently of other interrelated steps of this process has allowed us to unveil a previously unknown effect of changes in the cholesterol composition of the membranes of these compartments in their fusogenic ability. Decreased fusion efficiency between APGs and lysosomes can explain the inhibitory effect of lipid stimuli, such as that induced by a HFD, on macroautophagy.
Supplementary Material
Acknowledgments
The authors are grateful to Samantha J. Orenstein and Dr. Fernando Macian for their insights and critical revision of this manuscript, and to Drs. Mark J. Czaja, Allan W. Wolkoff, and John W. Murray (Albert Einstein College of Medicine, Bronx, NY, USA) for providing us with valuable reagents. This work was supported by National Institutes of Health grants AG031782, AG021904, and DK041918. S.K. is supported by NIN/NIA T32AG023475.
References
- Mizushima N., Levine B., Cuervo A. M., Klionsky D. J. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo A. M. Autophagy: in sickness and in health. Trends Cell Biol. 2004;14:70–77. doi: 10.1016/j.tcb.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Liou W., Geuze H. J., Geelen M. J., Slot J. W. The autophagic and endocytic pathways converge at the nascent autophagic vacuoles. J Cell Biol. 1997;136:61–70. doi: 10.1083/jcb.136.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager S., Bucci C., Tanida I., Ueno T., Kominami E., Saftig P., Eskelinen E. L. Role for Rab7 in maturation of late autophagic vacuoles. J Cell Sci. 2004;117:4837–4848. doi: 10.1242/jcs.01370. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Polo R. A., Boya P., Pauleau A. L., Jalil A., Larochette N., Souquere S., Eskelinen E. L., Pierron G., Saftig P., Kroemer G. The apoptosis/autophagy paradox: autophagic vacuolization before apoptotic death. J Cell Sci. 2005;118:3091–3102. doi: 10.1242/jcs.02447. [DOI] [PubMed] [Google Scholar]
- Nara A., Mizushima N., Yamamoto A., Kabeya Y., Ohsumi Y., Yoshimori T. SKD1 AAA ATPase-dependent endosomal transport is involved in autolysosome formation. Cell Struct Funct. 2002;27:29–37. doi: 10.1247/csf.27.29. [DOI] [PubMed] [Google Scholar]
- Kawai A., Uchiyama H., Takano S., Nakamura N., Ohkuma S. Autophagosome-lysosome fusion depends on the pH in acidic compartments in CHO cells. Autophagy. 2007;3:154–157. doi: 10.4161/auto.3634. [DOI] [PubMed] [Google Scholar]
- Cuervo A. M., Dice J. F., Knecht E. A population of rat liver lysosomes responsible for the selective uptake and degradation of cytosolic proteins. J Biol Chem. 1997;272:5606–5615. doi: 10.1074/jbc.272.9.5606. [DOI] [PubMed] [Google Scholar]
- Yu W. H., Cuervo A. M., Kumar A., Peterhoff C. M., Schmidt S. D., Lee J. H., Mohan P. S., Mercken M., Farmery M. R., Tjernberg L. O., Jiang Y., Duff K., Uchiyama Y., Naslund J., Mathews P. M., Cataldo A. M., Nixon R. A. Macroautophagy–a novel Beta-amyloid peptide-generating pathway activated in Alzheimer’s disease. J Cell Biol. 2005;171:87–98. doi: 10.1083/jcb.200505082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino I., Fu J., Tanji K., Yamada T., Shimojo S., Koori T., Mora M., Riggs J., Oh S., Koga Y., Sue C., Yamamoto A., Murakami N., Shanske S., Byrne E., Bonilla E., Nonaka I., Dimauro S., Hirano M. Primary LAMP-2 deficiency causes X-linked vacuolar cardiomyopathy and myopathy (Danon disease) Nature. 2000;406:906–910. doi: 10.1038/35022604. [DOI] [PubMed] [Google Scholar]
- Deretic V. Autophagy, an immunologic magic bullet: Mycobacterium tuberculosis phagosome maturation block and how to bypass it. Future Microbiol. 2008;3:517–524. doi: 10.2217/17460913.3.5.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R., Kaushik S., Wang Y., Xiang Y., Novak I., Komatsu M., Tanaka K., Cuervo A. M., Czaja M. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo A. M., Dice J. F. A receptor for the selective uptake and degradation of proteins by lysosomes. Science. 1996;273:501–503. doi: 10.1126/science.273.5274.501. [DOI] [PubMed] [Google Scholar]
- Zhang C., Cuervo A. M. Restoration of chaperone-mediated autophagy in aging liver improves cellular maintenance and hepatic function. Nat Med. 2008;14:959–965. doi: 10.1038/nm.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R., Wang Y., Xiang Y., Tanaka K. E., Gaarde W. A., Czaja M. J. Differential effects of JNK1 and JNK2 inhibition on murine steatohepatitis and insulin resistance. Hepatology. 2009;49:87–96. doi: 10.1002/hep.22578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey A. C., Follenzi A., Kiffin R., Zhang C., Cuervo A. M. Early cellular changes after blockage of chaperone-mediated autophagy. Autophagy. 2008;4:151–175. doi: 10.4161/auto.5654. [DOI] [PubMed] [Google Scholar]
- Wang Y., Schattenberg J. M., Rigoli R. M., Storz P., Czaja M. J. Hepatocyte resistance to oxidative stress is dependent on protein kinase C-mediated down-regulation of c-Jun/AP-1. J Biol Chem. 2004;279:31089–31097. doi: 10.1074/jbc.M404170200. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Basu S. K., Brown M. S. Receptor-mediated endocytosis flow-density lipoprotein in cultured cells. Methods Enzymol. 1983;98:241–260. doi: 10.1016/0076-6879(83)98152-1. [DOI] [PubMed] [Google Scholar]
- Wattiaux R., Wattiaux-De Coninck S., Ronveaux-Dupal M., Dubois F. Isolation of rat liver lysosomes by isopycnic centrifugation in a metrizamide gradient. J Cell Biol. 1978;78:349–368. doi: 10.1083/jcb.78.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storrie B., Madden E. Isolation of subcellular organelles. Methods Enzymol. 1990;182:203–225. doi: 10.1016/0076-6879(90)82018-w. [DOI] [PubMed] [Google Scholar]
- Cuervo A. M., Palmer A., Rivett A. J., Knecht E. Degradation of proteasomes by lysosomes in rat liver. Eur J Biochem. 1995;227:792–800. doi: 10.1111/j.1432-1033.1995.tb20203.x. [DOI] [PubMed] [Google Scholar]
- Murray J. W., Wolkoff A. W. In vitro motility system to study the role of motor proteins in receptor-ligand sorting. Methods Mol Biol. 2007;392:143–158. doi: 10.1007/978-1-59745-490-2_10. [DOI] [PubMed] [Google Scholar]
- Murray J. W., Bananis E., Wolkoff A. W. Reconstitution of ATP-dependent movement of endocytic vesicles along microtubules in vitro: an oscillatory bidirectional process. Mol Biol Cell. 2000;11:419–433. doi: 10.1091/mbc.11.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzella L., Ahlberg J., Glaumann H. Isolation of autophagic vacuoles from rat liver: morphological and biochemical characterization. J Cell Biol. 1982;93:144–154. doi: 10.1083/jcb.93.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auteri J., Okada A., Bochaki V., Dice J. Regulation of intracellular protein degradation in IMR-90 human diploid fibroblasts. J Cell Physiol. 1983;115:159–166. doi: 10.1002/jcp.1041150210. [DOI] [PubMed] [Google Scholar]
- Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Kabeya Y., Mizushima N., Ueno T., Yamamoto A., Kirisako T., Noda T., Kominami E., Ohsumi Y., Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bananis E., Murray J. W., Stockert R. J., Satir P., Wolkoff A. W. Regulation of early endocytic vesicle motility and fission in a reconstituted system. J Cell Sci. 2003;116:2749–2761. doi: 10.1242/jcs.00478. [DOI] [PubMed] [Google Scholar]
- Mizushima N., Yamamoto A., Matsui M., Yoshimori T., Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004;15:1101–1111. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morvan J., Kochl R., Watson R., Collinson L. M., Jefferies H. B., Tooze S. A. In vitro reconstitution of fusion between immature autophagosomes and endosomes. Autophagy. 2009;5:676–689. doi: 10.4161/auto.5.5.8378. [DOI] [PubMed] [Google Scholar]
- Gao W., Ding W. X., Stolz D. B., Yin X. M. Induction of macroautophagy by exogenously introduced calcium. Autophagy. 2008;4:754–761. doi: 10.4161/auto.6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon P. B., Holen I., Fosse M., Rotnes J. S., Seglen P. O. Dependence of hepatocytic autophagy on intracellularly sequestered calcium. J Biol Chem. 1993;268:26107–26112. [PubMed] [Google Scholar]
- Mortimore G. E., Poso A. R., Lardeux B. R. Mechanism and regulation of protein degradation in liver. Diabetes Metab Rev. 1989;5:49–70. doi: 10.1002/dmr.5610050105. [DOI] [PubMed] [Google Scholar]
- Kanazawa T., Taneike I., Akaishi R, Yoshizawa F, Furuya N, Fujimura S, Kadowaki M. Amino acids and insulin control autophagic proteolysis through different signaling pathways in relation to mTOR in isolated rat hepatocytes. J Biol Chem. 2004;279:8452–8459. doi: 10.1074/jbc.M306337200. [DOI] [PubMed] [Google Scholar]
- Lucero H. A., Robbins P. W. Lipid rafts-protein association and the regulation of protein activity. Arch Biochem Biophys. 2004;426:208–224. doi: 10.1016/j.abb.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Iwai-Kanai E., Yuan H., Huang C., Sayen M. R., Perry-Garza C. N., Kim L., Gottlieb R. A. A method to measure cardiac autophagic flux in vivo. Autophagy. 2008;4:322–329. doi: 10.4161/auto.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskelinen E. L., Illert A. L., Tanaka Y., Schwarzmann G., Blanz J., Von Figura K., Saftig P. Role of LAMP-2 in lysosome biogenesis and autophagy. Mol Biol Cell. 2002;13:3355–3368. doi: 10.1091/mbc.E02-02-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J. W., Wolkoff A. W. Roles of the cytoskeleton and motor proteins in endocytic sorting. Adv Drug Deliv Rev. 2003;55:1385–1403. doi: 10.1016/j.addr.2003.07.008. [DOI] [PubMed] [Google Scholar]
- Baker L., Piddington R., Goldman A., Egler J., Moehring J. Myo-inositol and prostaglandins reverse the glucose inhibition of neural tube fusion in cultured mouse embryos. Diabetologia. 1990;33:593–596. doi: 10.1007/BF00400202. [DOI] [PubMed] [Google Scholar]
- Overbye A., Fengsrud M., Seglen P. O. Proteomic analysis of membrane-associated proteins from rat liver autophagosomes. Autophagy. 2007;3:300–322. doi: 10.4161/auto.3910. [DOI] [PubMed] [Google Scholar]
- Huynh K. K., Eskelinen E. L., Scott C. C., Malevanets A., Saftig P., Grinstein S. LAMP proteins are required for fusion of lysosomes with phagosomes. EMBO J. 2007;26:313–324. doi: 10.1038/sj.emboj.7601511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Guhde G., Suter A., Eskelinen E.-L., Hartmann D., Lullmann-Rauch R., Janssen P., Blanz J., von Figura K., Saftig P. Accumulation of autophagic vacuoles and cardiomyopathy in Lamp-2-deficient mice. Nature. 2000;406:902–906. doi: 10.1038/35022595. [DOI] [PubMed] [Google Scholar]
- Deng D., Jiang N., Hao S. J., Sun H., Zhang G. J. Loss of membrane cholesterol influences lysosomal permeability to potassium ions and protons. Biochim Biophys Acta. 2009;1788:470–476. doi: 10.1016/j.bbamem.2008.11.018. [DOI] [PubMed] [Google Scholar]
- Lindblom G., Oradd G. Lipid lateral diffusion and membrane heterogeneity. Biochim Biophys Acta. 2009;1788:234–244. doi: 10.1016/j.bbamem.2008.08.016. [DOI] [PubMed] [Google Scholar]
- Mouritsen O. G., Zuckermann M. J. What’s so special about cholesterol? Lipids. 2004;39:1101–1113. doi: 10.1007/s11745-004-1336-x. [DOI] [PubMed] [Google Scholar]
- Wilschut J., Duzgunes N., Hoekstra D., Papahadjopoulos D. Modulation of membrane fusion by membrane fluidity: temperature dependence of divalent cation induced fusion of phosphatidylserine vesicles. Biochemistry. 1985;24:8–14. doi: 10.1021/bi00322a002. [DOI] [PubMed] [Google Scholar]
- Kaushik S., Massey A. C., Cuervo A. M. Lysosome membrane lipid microdomains: novel regulators of chaperone-mediated autophagy. EMBO J. 2006;25:3921–3933. doi: 10.1038/sj.emboj.7601283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.