Abstract
To study the role of WNT4 in the postnatal ovary, a mouse strain bearing a floxed Wnt4 allele was created and mated to the Amhr2tm3(cre)Bhr strain to target deletion of Wnt4 to granulosa cells. Wnt4flox/−;Amhr2tm3(cre)Bhr/+ mice had reduced ovary weights and produced smaller litters (P<0.05). Serial follicle counting demonstrated that Wnt4flox/−;Amhr2tm3(cre)Bhr/+ mice were born with a normal ovarian reserve and maintained normal numbers of small follicles until puberty but had only 25.2% of the normal number of healthy antral follicles. Some Wnt4flox/−;Amhr2tm3(cre)Bhr/+ mice had no antral follicles or corpora lutea and underwent premature follicle depletion. RT-PCR analyses of Wnt4flox/−;Amhr2tm3(cre)Bhr/+ granulosa cells and cultured granulosa cells that overexpress WNT4 demonstrated that WNT4 regulates the expression of Star, Cyp11a1, and Cyp19, steroidogenic genes previously identified as downstream targets of the WNT signaling effector CTNNB1. Decreased serum progesterone levels were found in immature, gonadotropin-treated Wnt4flox/−;Amhr2tm3(cre)Bhr/+ mice (P<0.05). WNT4- and CTNNB1-overexpressing cultured granulosa cells were analyzed by microarray for alterations in gene expression, which showed that WNT4 regulates additional genes involved in late follicle development via the WNT/CTNNB1 signaling pathway. Together, these data indicate that WNT4 is required for normal antral follicle development and may act by regulating granulosa cell functions including steroidogenesis.—Boyer, A., Lapointe, E., Zheng, X., Cowan, R. G., Li, H., Quirk, S. M., DeMayo, F. J., Richards, J. S., Boerboom, D. WNT4 is required for normal ovarian follicle development and female fertility.
Keywords: granulosa cell, conditional gene targeting, CTNNB1, microarray
The WNTs comprise a large family of local-acting, secreted glycoprotein signaling molecules that are known mostly for the numerous roles they play in embryonic development and cancer (1, 2). Several WNTs, their cognate receptors of the Frizzled (Fzd) family, and other components of the WNT signaling pathways are expressed in the postnatal ovary, but their roles in ovarian physiology remain poorly defined (3,4,5,6). The first indication of the importance of WNT signaling in the ovary came from a study by Vainio et al.(7), which reported that inactivation of the Wnt4 gene in mice results in the loss of a large fraction of the reserve of oocytes in the days before birth. Subsequent studies have shown that WNT4 expression is required during embryonic female gonadal development to suppress the formation of the male-specific coelomic blood vessel and that follistatin (FST) acts downstream of WNT4 to mediate this effect (8,9,10). In addition, WNT4 is required to prevent the migration of a steroidogenic cell population from the mesonephros into the female gonad (9, 11). It remains unclear, however, how (or whether) altered vascular development and the presence of ectopic steroidogenic cells in WNT4-knockout ovaries relate to the perinatal oocyte depletion phenotype. WNT4 expression in the mouse embryonic gonad is restricted to the somatic cells (7), and a descriptive study has shown that WNT4 is selectively expressed postnatally in follicular granulosa cells and corpora lutea (4). Interestingly, WNT4 expression increased markedly in response to an ovulatory dose of hCG in rat and mouse granulosa cells, suggesting that it could be important for some aspect of late follicle development, ovulation, and/or luteinization (4). Unfortunately, proof of this could not be obtained from the Wnt4-knockout mouse, which dies shortly after birth due to kidney defects (12), precluding analyses of postnatal ovarian follicular development.
Some insight into the roles of Wnt4 in granulosa cells may have been provided by a recent series of studies that examined the role of dysregulated WNT signaling in the pathogenesis of granulosa cell tumors (13,14,15,16). In these studies, a WNT signaling pathway known as the canonical or WNT/CTNNB1 pathway was constitutively activated in mouse granulosa cells by conditional gene targeting. The resulting Ctnnb1tm1Mmt/+;Amhr2tm3(cre)Bhr/+ mice were subfertile and developed unusual, follicle-like structures by 5–6 wk of age that consisted of nests of disorganized, pleomorphic granulosa cells that often formed a central, antrum-like cavity (13). However, the structures did not develop and regress in a manner similar to follicles, because they grew no larger than the size of antral follicles and persisted for the rest of the life of the animal. These structures were subsequently defined as premalignant lesions because they were found to develop into granulosa cell tumors in older mice or after inactivation of the antioncogene Pten(13, 15, 16). Furthermore, recent studies have shown that CTNNB1 can regulate the expression of steroidogenic enzymes such as STAR and CYP19 in granulosa cells, suggesting that the WNT/CTNNB1 pathway may be involved in regulating the biosynthesis of estrogen, a potent follicular growth factor (17,18,19). Based on these observations and the fact that WNT4 can signal via the WNT/CTNNB1 pathway in certain contexts (20,21,22), we hypothesized that WNT4 may regulate follicle development by controlling some aspects of granulosa cell proliferation, differentiation, and/or survival.
MATERIALS AND METHODS
Gene targeting
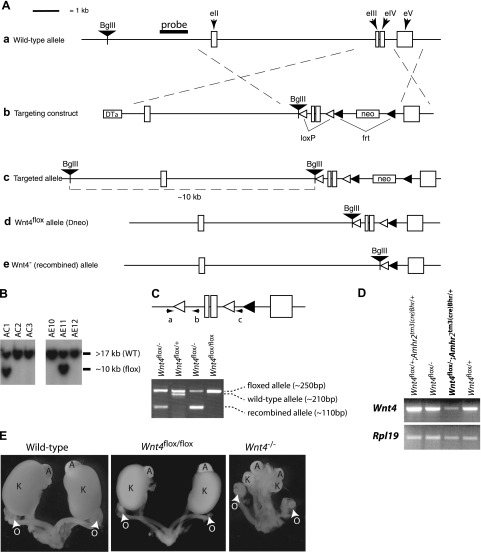
The Wnt4flox allele was created using standard gene targeting techniques. In brief, a targeting construct was built using the pKOII vector as a backbone (23), into which was inserted a 478-bp chromosomal DNA fragment flanked by loxP sites and encompassing the third and fourth exons and third intron of the Wnt4 gene, along with parts of the second and fourth introns (Fig. 1Ab). Homologous chromosomal sequences consisting of the 7000 bp upstream and 1518 bp downstream of the floxed region were then inserted into the vector to generate the construct illustrated in Fig. 1Ab. A BglII restriction site for use in Southern screening was also inserted before the upstream loxP site. All genomic sequences were isolated from R1 embryonic stem (ES) cell genomic DNA by PCR using the Expand Long Template PCR system (Roche Molecular Biochemicals, Laval, QC, Canada), and loxP and restriction sites were inserted within the oligonucleotide primers used to isolate the genomic DNA fragments. After linearization with NotI, targeting construct DNA was introduced into the R1 ES cell line (24) by electroporation, and recombinant colonies were selected by addition of 400 μg/ml Geneticin (Invitrogen, Burlington, ON, Canada) to the culture medium for 8–9 d. Selected colonies were cultured in triplicate, and duplicate sets were analyzed by Southern blotting to screen for proper homologous recombinants as described previously (25). Two of 384 colonies screened showed a second band consistent with the expected size for the targeted allele (Fig. 1B). Both cell lines were subsequently microinjected into blastocysts and transferred to pseudopregnant recipients according to standard protocols. Chimeric males derived from both cell lines sired pups heterozygous for the targeted allele (Fig 1Ac).
Figure 1.
Conditional targeting of Wnt4 in granulosa cells. A) Illustration of the strategies used to generate the Wnt4flox and Wnt4− alleles, as detailed in Materials and Methods. LoxP sites were inserted upstream of the third (eIII) and downstream of the fourth (eIV) exons. a) Placement of the genomic DNA probe used for Southern blotting. b) Targeting construct. c) Targeted allele. d) Wnt4flox allele. e) Wnt4− (i.e., Cre-recombined) allele. DTα, diphtheria toxin α chain; neo, neomycin resistance cassette. B) Generation of embryonic stem cell lines heterozygous for the targeted Wnt4 allele. Presence of the targeted allele was detected as a 10-kb BglII restriction fragment by Southern blotting (clones AC1 and AE11). WT, wild type. C) PCR genotyping analysis strategy (top panel) and typical results from tail biopsies (bottom panel). Oligonucleotides (a–c) were designed to generate PCR products of 250 bp for the Wnt4flox allele (a), 210 bp for the Wnt4+ allele (b), and 110 bp for the Wnt4− allele (c). D) RT-PCR analysis of Wnt4 expression in ovaries from mice of different genotypes. Results demonstrate markedly decreased Wnt4 expression in Wnt4flox/−;Amhr2tm3(cre)Bhr/+ mice relative to controls. E) Urogenital tracts from newborn mice of the indicated genotypes. Wnt4−/− mice generated by recombination of the Wnt4flox allele have developmental kidney defects identical to those previously reported in Wnt4-knockout mice, indicating that recombination of the Wnt4flox allele results in complete loss of function. O, ovary; A, adrenal; K, kidney.
Animal models
To eliminate the possibility of the neocassette interfering with WNT4 expression from the targeted allele, mice bearing the targeted allele were mated to the 129S4/SvJaeSor-Gt(ROSA)26Sortm1(FLP1)Dym/J strain (The Jackson Laboratory, Bar Harbor, ME, USA) to target Flp1-mediated excision of the frt-flanked neocassette (Fig. 1Ab) to the germline. The excision of the neocassette (Fig. 1Ad) was verified by PCR from tail biopsies from Wnt4flox/+;Gt(ROSA)26Sortm1(FLP1)Dym/+ mice, and the Flp1 transgene was eliminated in the following generation by mating to wild-type mice. To generate a null Wnt4 allele (Wnt4−, Fig. 1Ae), Wnt4flox/flox mice were mated to the B6.C-Tg(CMV-cre)1Cgn/J strain (The Jackson Laboratory), which expresses Cre in all tissues including the germline. After PCR verification of the Wnt4− allele (Fig. 1C), the Cre transgene was eliminated from Wnt4+/−;Gt(ROSA)26Sortm1Sor/+ mice in the following generation by mating to wild-type mice. Complex genotypes were then obtained by selective breeding of the Wnt4−, Wnt4flox, and previously described Amhr2tm3(cre)Bhr parental strains (26). Genotyping analyses for the Amhr2tm3(cre)Bhr allele were performed by PCR on DNA obtained from tail biopsies as described previously (27). Genotyping analyses for the Wnt4 alleles were performed using the oligonucleotide primers 5′-GCCAGGCTGTCTGCTGGCTCA-3′ (Fig 1Ca), 5′-GCATATGAGGCCTGCTGAATGCT-3′ (Fig 1Cb), and 5′-TAGGAACTTCAATTCCCCGCAAGA-3′ (Fig. 1Cc) and the same reagents and cycling parameters used for Amhr2tm3(cre)Bhr genotyping. This protocol results in PCR products of ∼250 bp for the wild-type Wnt4 allele, 210 bp for the floxed allele, and 110 bp for the knockout (i.e., Cre-recombined) allele (Fig. 1C). Ctnnb1tm1Mmt/tm1Mmt mice were genotyped as described previously (28). All animal procedures were approved by the institutional animal care and use committee and conformed to the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Mating experiment and ovary weights
The mating experiment was conducted using females that were 8 wk old at the outset. Adult males were placed in the cages for 6 mo, and the experiment terminated 22 d after their removal to allow for the final litter. Control animals were littermates of the Wnt4flox/−;Amhr2tm3(cre)Bhr/+ experimental animals and consisted of 2 Wnt4flox/− mice, 1 Wnt4flox/+;Amhr2tm3(cre)Bhr/+ mouse, and 1 Wnt4flox/+ mouse, all of which had comparable fertility.
Adult ovary weights were obtained from 6- to 10-mo-old Wnt4flox/−;Amhr2tm3(cre)Bhr/+ females. Control animals consisted of littermates of the experimental animals, genotypes were Wnt4flox/− (n=7), Wnt4flox/+;Amhr2tm3(cre)Bhr/+ (n=6), and Wnt4flox/+ (n=5), and weights did not differ by genotype among the control animals (P>0.05). Ovary weights from immature, gonadotropin-treated animals were obtained from 21- to 23-d-old Wnt4flox/−;Amhr2tm3(cre)Bhr/+ and Wnt4flox/− (control) mice treated with eCG (5 IU i.p., Folligon; Intervet, Whitby, ON, Canada) followed after 48 h by hCG (5 IU i.p., Chorulon; Intervet) and sacrificed 12 h later.
Cell isolation and culture, adenovirus infection, and microarray analyses
Granulosa cells were obtained from 20- to 26-d-old mice of various genotypes 48 h after eCG treatment, using the needle puncture method as described previously (29). For experiments involving cell culture, cells were suspended in DMEM/F-12 medium (Invitrogen, Burlington, ON, Canada) with 1% fetal bovine serum and allowed to attach overnight at a final density of ∼50%. Cells were then infected with adenoviruses to express eGFP or Cre or overexpress WNT4 (30) for 24 h in serum-free medium and subsequently were harvested for RNA extraction as described below. The Ad-WNT4 virus was a kind gift from Dr. Peter Hornsby (University of Texas, Austin, TX, USA); the Ad-Cre and Ad-eGFP viruses were obtained from the Baylor College of Medicine Vector Development Laboratory (Houston, TX, USA). Preliminary experiments demonstrated that an infection efficiency of >80% could be obtained at an MOI of ∼50 (as determined by analysis of fluorescent signal in Ad-eGFP-infected cells) and that the Ad-Cre and Ad-WNT4 viruses produced efficient recombination of the floxed Ctnnb1 alleles and robust WNT4 overexpression, respectively (not shown).
Microarray analyses were done using triplicate RNA samples from each adenovirus treatment described above using mouse expression set 430 microarrays (Affymetrix, Santa Clara, CA, USA). Granulosa cells from Ctnnb1tm1Mmt/tm1Mmt mice were used for all treatment groups to permit comparison of the Ad-WNT4 and Ad-Cre treatments on the same genetic background. All steps of RNA quality control, probe synthesis, hybridization, washing, and array scanning were done by the Microarray Core Facility of the Baylor College of Medicine (Houston, TX, USA). Microarray data were analyzed using FlexArray 1.3 software (Génome Québec, Montréal, QC, Canada). Ad-Wnt4, Ad-Cre, and control Ad-GFP data were processed using the robust multiarray average algorithm for normalization, background correction, and expression value calculation. Robustness of the data was further enhanced by the EB (Wright and Simon) algorithm and P value calculation. A P value threshold of 0.05 and 2-fold change cutoff values were used for identification of differentially expressed genes. Overlap between genes regulated by Ad-Wnt4 and Ad-Cre was also determined using this software. Array data were deposited in the Gene Expression Omnibus (GEO) database, with accession number GSE18704.
Semiquantitative and real-time RT-PCR
Semiquantitative RT-PCR to measure relative expression of Wnt4 and ribosomal protein L19 (Rpl19, control gene) was performed on 1 μg of ovarian RNA samples from mice of the genotypes indicated in Fig. 1D and purified with the RNeasy mini kit (Qiagen, Valencia, CA, USA). RT-PCR was done using the SuperScript One-Step RT-PCR kit (Invitrogen) as directed by the manufacturer. Oligonucleotides primers used for Wnt4 and Rpl19 are indicated in Table 1. Cycling conditions were 94°C for 1 min followed by 25 cycles (Rpl19) or 35 cycles (Wnt4) of 94°C for 30 s, 55°C for 1 min, and 72°C for 1 min. Preliminary experiments done for Wnt4 and Rpl19 ensured that the cycle numbers selected fell within the linear range of PCR amplification (data not shown). PCR products were separated by electrophoresis on 1.8% TAE-agarose gels containing ethidium bromide and photographed under UV light.
TABLE 1.
Oligonucleotides for RT-PCR analyses
| Primer name | Sequence (5′ to 3′) |
|---|---|
| Adamts1-S | GTGAAGCCAAAGGCATTGGCTACT |
| Adamts1-AS | TGGCTCCAGCAGGAATTGTGACA |
| Bmp2-S | AGACGTCCTCAGCGAATTTGAGT |
| Bmp2-AS | TCTGGAAGTTCCTCCAGCGCTT |
| Cdkn1a-S | TCCAATCCTGGTGATGTCCGACCT |
| Cdkn1a-AS | TCTTGCAGAAGACCAATCTGCGCT |
| Cyp11a1-S | GTGACCTTGCAGAGGTACACTGT |
| Cyp11a1-AS | GTGACTCCAGCCTTCAGTTCACA |
| Cyp19-S | CTGAACATCGGAAGAATGCACAG |
| Cyp19-AS | GAGTAGATAGGCCACACTTCTTCA |
| Ddit3-S | AACCTGGTCCACGTGCAGTCAT |
| Ddit3-AS | GGAATCTGGAGAGCGAGGGCTT |
| Fshr-S | GTCATGCTCATTGAGGCCAGCCT |
| Fshr-AS | TTGGCTTGTGGTCAGGACCACCA |
| Fst-S | CAAAGTCCTGTGAAGATATCCAG |
| Fst-AS | TAGGAAAGTTGTAGTCCTGGTCT |
| Herpud1-S | CACCACGTCGGGTGGTTTCCGT |
| Herpud1-AS | GCACAAGGGCCATCAGTTGGCT |
| Klf4-S | CCTGCCAGACCAGATGCAGTCA |
| Klf4-AS | GGTCACATCCACTACGTGGGAT |
| Lhcgr-S | GCTGGAGTCCATTCAGACGCTCA |
| Lhcgr-AS | AGCATCTGGTTCTGGAGTACATTG |
| Nt5e-S | AGTCCACCGGAGAGTTCCTGCA |
| Nt5e-AS | GTATTCAGAAACCACGCTGATATCT |
| Ptgs2-S | CCTGAAGCCGTACACATCATTTGA |
| Ptgs2-AS | AGGCACTTGCATTGATGGTGGCT |
| Rpl19-S | CTGAAGGTCAAAGGGAATGTG |
| Rpl19-AS | GGACACAGTCTTGATGATCTC |
| Star-S | GATTAAGGCACCAAGCTGTGCTG |
| Star-AS | CTGCTGGCTTTCCTTCTTCCAGC |
| Thbs1-S | GGAATGCACTGTGGCACACAGG |
| Thbs1-AS | CTCGACACTCGTATTTCATGTGTG |
| Trib3-S | CCCAGGCGGTGCTGGCACCTT |
| Trib3-AS | CCAGGTTCTCCAGCACCAGCTT |
| Vegfa-S | TGATCAAGTTCATGGATGTCTACCA |
| Vegfa-AS | CTGCATTCACATCTGCTGTGCTGT |
| Wnt4-S | AGCTGTCATCGGTGGGCAGCAT |
| Wnt4-AS | ACTGTCCGGTCACAGCCACACT |
Gene expression analyses in cultured or freshly isolated granulosa cells were done by real-time PCR using RNA prepared as described above. Reactions were formulated using the SuperScript III Platinum Two-Step RT-PCR kit with SYBR Green (Invitrogen) according to the manufacturer’s instructions and using the oligonucleotide primer pairs listed in Table 1. Thermal cycling and data capture were performed using a Rotor-Gene RG-3000 apparatus (Corbett Research, Mortlake, NSW, Australia) and the manufacturer’s recommended conditions. Relative gene expression was calculated using Rotor-Gene 6.0 software (Corbett Research) by comparing amplification curves to a series of standard curves obtained by amplification of serial dilutions of an Rpl19 cDNA fragment. All data were subsequently normalized by dividing expression levels of individual genes by corresponding Rpl19 values.
Follicle counting
Left ovaries from 5- and 42-d-old mice were collected, stored in Bouin’s fixative for 24 h, and rinsed in 70% ethanol before paraffin embedding. Ovaries were then serially sectioned at a thickness of 6 μm, and every fifth section was stained with hematoxylin and eosin [n=5–12 (5 d) or 14–30 (42 d) sections/ovary]. Stained sections were photographed at ×400 so as to obtain image sets representing the entire section. All follicles with a visible oocyte nucleus were subsequently classified by stage of development and scored as healthy or atretic by systematic analysis of the image sets. The follicle classification system was based on Pedersen’s system (31): oocyte surrounded by a layer of flat granulosa cells = primordial, Pedersen class 3 = primary, Pedersen classes 4–5 = secondary, and Pedersen classes 6–8 = antral. Atresia was determined for each follicle based on histological criteria using a weighted scoring system (based on refs. 32,33,34). The primary criteria of atresia scoring were 1) presence and degree of pyknosis (condensed granulosa cells or cells with condensed nuclei), 2) presence and degree of loss of granulosa cell attachment to oocytes or loss of cumulus cells, 3) presence of polymorphonuclear neutrophils or lymphocytes, and 4) presence of pyknotic oocytes (primary follicles only). Secondary criteria were granulosa cell vacuolation, sparse or missing granulosa cells, and integrity of the basement membrane.
Immunohistochemistry
Immunohistochemical analysis of activated caspase-3 was done on formalin-fixed, paraffin-embedded, 7-μm ovary sections using a Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA, USA) as directed by the manufacturer. Sections were probed with a primary antibody against activated caspase-3 (catalog no. 9661; Cell Signaling Technology, Inc., Danvers, MA, USA), and staining was done using a 3,3′-diaminobenzidine peroxidase substrate kit (Vector Laboratories). Sections were briefly counterstained with hematoxylin before mounting.
Steroid hormone radioimmunoassays
Estradiol (E2) and progesterone (P4) radioimmunoassays were performed as described previously (35, 36). Intra-assay coefficients of variation were 8.6% for P4 and 6.24% for E2.
Statistical analyses
Effects of genotype on litter sizes, ovary weights, gene expression levels, follicle numbers, and steroid hormone levels in adult mice were analyzed by unpaired, 2-tailed t tests. P < 0.05 was considered statistically significant. Effects of genotype and time after hCG on steroid hormone levels in immature mice were analyzed by 2-way ANOVA. Log transformation of data was performed before statistical analysis when unequal variances were detected using Bartlett’s test. Analyses were done using Prism 4.0a (GraphPad Software, Inc., San Diego, CA, USA) and JMP (SAS Institute, Inc., Cary, NC, USA) software.
RESULTS
Generation of a floxed Wnt4 allele
To study the role of WNT4 in granulosa cells in vivo, a floxed Wnt4 allele was created to permit conditional targeting using the Cre-Lox system. A targeting construct designed to insert LoxP sites upstream of the third exon and downstream of the fourth exon of Wnt4 was built (Fig. 1A), and two properly targeted embryonic stem cell lines were obtained after transfection and antibiotic selection (Fig. 1B). Male chimeric mice obtained by injection of the targeted ES cells into blastocysts gave germline transmission of the floxed allele on mating to wild-type C57BL/6 females. Female Wnt4flox/flox mice were found to have morphologically normal genitourinary tracts at birth (Fig. 1E), developed normally through adulthood, and demonstrated fertility indistinguishable from that of C57BL/6 wild-type mice (data not shown), indicating an absence of functional hypomorphism of the Wnt4flox allele. Genetic recombination of the Wnt4flox allele was then obtained by mating Wnt4flox/flox mice to the B6.C-Tg(CMV-cre)1Cgn “Cre-deleter” strain, resulting in offspring bearing a presumptive null (Wnt4−) allele (Fig 1Ae). On backcross, the resulting Wnt4−/− mice were found to die within 48 h after birth, and this death was associated with severe developmental anomalies of the kidneys, reproductive tract, and gonads (Fig. 1E) that were essentially identical to those previously described in Wnt4-null mice (7, 12). These findings demonstrated the functionality of the Wnt4flox allele and that its recombination results in complete ablation of WNT4 activity.
Conditional targeting of Wnt4 in granulosa cells results in infertility and decreased ovary size
To target deletion of Wnt4 to granulosa cells, mice bearing the Wnt4flox allele were mated to the Amhr2tm3(cre)Bhr strain, which features the Cre transgene knocked-in to the Amhr2 locus (26). The Amhr2tm3(cre)Bhr allele was chosen because of its ability to drive Cre expression perinatally in granulosa cell precursors (16) and therefore to target Wnt4 from the early stages of follicle development. The Wnt4− locus, created as described above, was also used to generate mice with the Wnt4flox/−;Amhr2tm3(cre)Bhr/+ genotype, an experimental design meant to maximize the efficiency of Wnt4 ablation in granulosa cells. Semiquantitative RT-PCR performed on RNA extracted from adult ovaries demonstrated a substantial (>75%) reduction in Wnt4 mRNA in Wnt4flox/−;Amhr2tm3(cre)Bhr/+ animals compared with littermate controls bearing a wild-type Wnt4 allele or lacking the Amhr2tm3(cre)Bhr allele (Fig. 1D). Quantitative RT-PCR analysis of granulosa cells isolated from gonadotropin-treated immature mice also showed a 4- to 6-fold reduction in Wnt4 mRNA levels in Wnt4flox/−;Amhr2tm3(cre)Bhr/+ cells relative to Wnt4flox/− control cells (see Fig. 5B).
Figure 2.
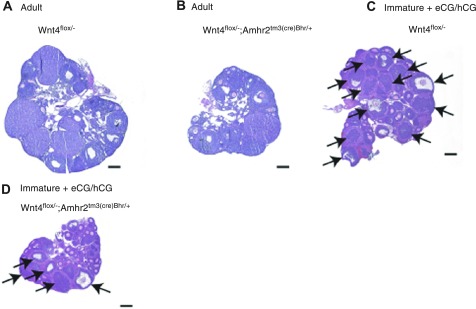
Wnt4flox/−;Amhr2tm3(cre)Bhr/+ mice have small ovaries and impaired follicle development. A, B) Sections from representative Wnt4flox/− (A, control) and Wnt4flox/−;Amhr2tm3(cre)Bhr/+ ovaries (B), demonstrating smaller ovary size and fewer large antral follicles and corpora lutea in Wnt4flox/−;Amhr2tm3(cre)Bhr/+ animals. Animals were 8-wk-old littermates. C, D) Immature Wnt4flox/− (C) and Wnt4flox/−;Amhr2tm3(cre)Bhr/+ mice (D) were treated with eCG (48 h), followed by an ovulatory dose of hCG, and were sacrificed 12 h later. Photomicrographs of representative ovaries show smaller ovary size in Wnt4flox/−;Amhr2tm3(cre)Bhr/+ animals and reduced numbers of preovulatory and ovulatory follicles (arrows). Scale bars = 200 μm.
Figure 3.
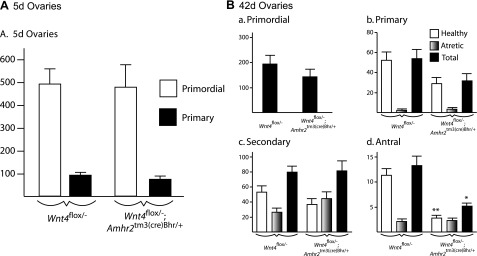
Quantitative analysis of ovarian follicles in newborn and pubertal mice. Ovaries from mice of the indicated ages and genotypes were serially sectioned, and all follicles from every fifth section were counted, categorized, and scored as healthy or atretic. One ovary (left) from each animal was evaluated. A) Ovaries of 5-d mice; n = 6/genotype. B) Ovaries of 42-d mice: primordial (a), primary (b), secondary (c), and antral follicles (d); n = 5 (Wnt4flox/−;Amhr2tm3(cre)Bhr/+), or n = 8 (Wnt4flox/−). Data represent raw follicle count numbers and were not adjusted to estimate the total ovarian follicle population. All data are expressed as means ± se (columns and error bars). *P < 0.05, **P < 0.01 vs. control.
Figure 4.
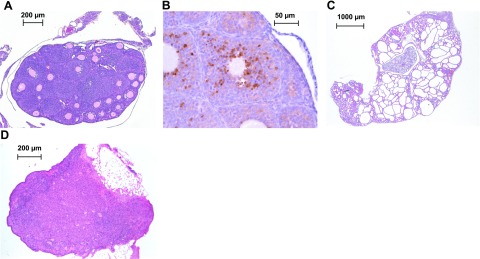
Rare Wnt4flox/−;Amhr2tm3(cre)Bhr/+ mice have very small ovaries and no antral follicles. A) Ovary from a 2-mo-old Wnt4flox/−;Amhr2tm3(cre)Bhr/+ mouse showing an absence of antral follicles or corpora lutea. B) Activated caspase-3 immunohistochemistry experiment showing an atretic secondary follicle from the ovary shown in A. C) Kidney from the animal shown in A, showing dramatic developmental anomalies and few glomeruli. D) Ovary from an 8-mo-old Wnt4flox/−;Amhr2tm3(cre)Bhr/+ mouse showing complete depletion of follicles.
Figure 5.
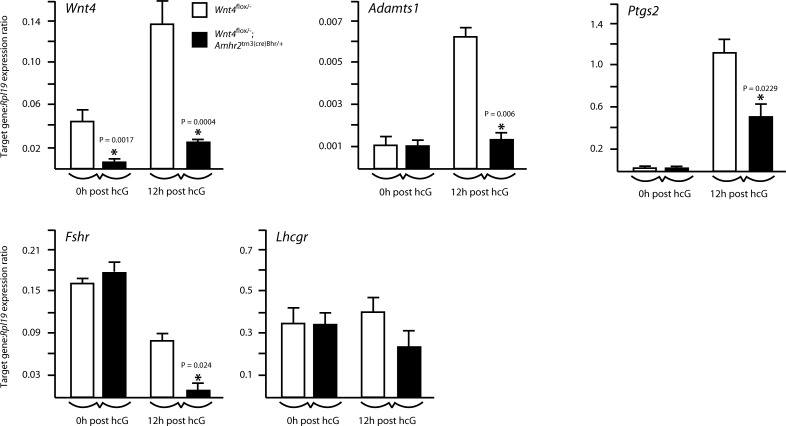
Expression of gonadotropin receptors and hCG-responsive genes in the Wnt4flox/−;Amhr2tm3(cre)Bhr/+ model. Quantitative RT-PCR analysis of the expression of the indicated genes in granulosa cells isolated from immature, eCG-treated mice of the indicated genotypes (n=4–5 animals/genotype/time point), either without (0h post hcG) or 12 h after the administration of an ovulatory dose of hCG (12h post hcG). All data were normalized to the housekeeping gene Rpl19 and are expressed as means ± se (columns and error bars). *P < 0.05 vs. control.
To study the effects of WNT4 loss on fertility, four Wnt4flox/−;Amhr2tm3(cre)Bhr/+ and four control females were placed with wild-type males for 6 mo. Although similar numbers of litters were obtained from both groups, the average litter size of Wnt4flox/−;Amhr2tm3(cre)Bhr/+ females was only 54% that of the control group (Table 2). Histopathological examination of the gonads and reproductive tracts of adult Wnt4flox/−;Amhr2tm3(cre)Bhr/+ females revealed no obvious anomalies, except that the ovaries appeared somewhat smaller in most animals examined (Fig. 2A, B). Analysis of ovarian weights confirmed the histological observations, showing that Wnt4flox/−;Amhr2tm3(cre)Bhr/+ ovaries were only 49% the size of those of littermate controls (Table 3). All stages of follicular development and corpora lutea could be identified in most adult Wnt4flox/−;Amhr2tm3(cre)Bhr/+ ovaries despite their reduced size, but large antral follicles and corpora lutea appeared less abundant. Likewise, immature (21–26 d old) Wnt4flox/−;Amhr2tm3(cre)Bhr/+ mice treated with eCG and hCG to stimulate follicle development and ovulation had ovaries that weighed only 51% of those of Wnt4flox/− control ovaries (Table 3). Histological analyses revealed that these Wnt4flox/−;Amhr2tm3(cre)Bhr/+ ovaries developed far fewer preovulatory and ovulatory follicles in response to gonadotropin treatment than control ovaries and often appeared to have fewer large secondary follicles as well (Fig. 2C, D).
TABLE 2.
Mating experiment
| Variable | Control | Wnt4flox/−;Amhr2tm3(cre)Bhr/− |
|---|---|---|
| Females | 4 | 4 |
| Total litters | 25 | 21 |
| Total pups | 179 | 81 |
| Litter size | 7.19 ± 0.64 | 3.86 ± 0.38* |
Litter size values are means ± se.
P < 0.05 vs. control.
TABLE 3.
Ovary weights
| Variable | Control | Wnt4flox/−; Amhr2tm3(cre)Bhr/+ |
|---|---|---|
| Adult ovaries | ||
| n | 36 | 18 |
| Weight (mg) | 5.25 ± 0.31 | 2.56 ± 0.25* |
| Immature + eCG/hCG ovaries | ||
| n | 12 | 6 |
| Weight (mg) | 6.16 ± 0.43 | 3.18 ± 0.27** |
Weight values are means ± se.
P < 0.05,
P < 0.01 vs. control.
Wnt4flox/−;Amhr2tm3(cre)Bhr/+ mice have defects in follicle development
To further study ovarian follicle development in Wnt4flox/−;Amhr2tm3(cre)Bhr/+ mice, serial sections were prepared from ovaries of 5- and 42-d-old animals, and follicles were counted and categorized by phase of development and viability (i.e., healthy or atretic). Results from 5-d-old mice showed no significant differences (P>0.05) in numbers of primordial or primary follicles between Wnt4flox/−;Amhr2tm3(cre)Bhr/+ mice and Wnt4flox/− controls (Fig. 3A), indicating that Wnt4flox/−;Amhr2tm3(cre)Bhr/+ mice are born with normal numbers of oocytes and that early folliculogenesis occurs normally. Both primordial and primary follicle numbers in 42-d-old Wnt4flox/−;Amhr2tm3(cre)Bhr/+ ovaries appeared somewhat lower than those in control ovaries; however, statistical testing failed to show that these differences were significant (P>0.05), and total numbers of secondary follicles were essentially identical in both groups (Fig. 3B). However, Wnt4flox/−;Amhr2tm3(cre)Bhr/+ ovaries had far fewer healthy antral follicles, with only 25.2% the number found in Wnt4flox/− mice (P<0.05). Although comparable numbers of atretic antral follicles were found in Wnt4flox/−;Amhr2tm3(cre)Bhr/+and Wnt4flox/− mice, fully 46.2% of all antral follicles were classified as atretic in Wnt4flox/−;Amhr2tm3(cre)Bhr/+ ovaries vs. 16.0% in Wnt4flox/− control ovaries. An apparent increase in the proportion of atretic follicles was also noted at the secondary stage, as more than half of all secondary follicles in Wnt4flox/−;Amhr2tm3(cre)Bhr/+ ovaries were atretic vs. fewer than one-third in control ovaries (Fig. 3B), although statistical testing failed to show differences between groups (P>0.05). These data therefore linked the decreased ovary size and fertility observed in Wnt4flox/−;Amhr2tm3(cre)Bhr/+ mice to reduced numbers of antral follicles, possibly due in part to an increased rate of atresia.
A more severe phenotype was observed in a small subset (∼5%) of Wnt4flox/−;Amhr2tm3(cre)Bhr/+ mice. These mice had very small ovaries (<1 mg) that were devoid of antral follicles and corpora lutea at 8–12 wk of age (Fig. 4A). Many of the larger secondary follicles in these ovaries were atretic, as determined by histological analyses and activated caspase-3 immunohistochemical analysis (Fig. 4B). However, the rarity of these mice precluded quantitative analyses of follicle numbers and rates of atresia. The severe ovarian phenotypes were always accompanied by variable degrees of growth retardation, poor body condition, a disheveled appearance, and frequently premature death. These effects were attributed to the presence of kidney defects (Fig. 4C) presumably related to those previously reported in Wnt4-null mice (12). Wnt4flox/−;Amhr2tm3(cre)Bhr/+ mice with the severe ovarian/kidney phenotype that survived beyond the age of 6 mo showed complete depletion of their follicular reserves (Fig. 4D).
WNT4 regulates the expression of LH-responsive and steroidogenic genes
To determine the mechanism of WNT4 action, we first sought to determine whether impaired follicle development in Wnt4flox/−;Amhr2tm3(cre)Bhr/+ could be related to altered gonadotropin receptor expression. Quantitative RT-PCR was performed on granulosa cell RNA from immature, eCG-treated Wnt4flox/−;Amhr2tm3(cre)Bhr/+ or control mice, with or without administration of an ovulatory dose of hCG. Results showed that both Fshr and Lhcgr are expressed at normal levels in eCG-stimulated ovarian follicles from Wnt4flox/−;Amhr2tm3(cre)Bhr/+ mice (Fig. 5). After 12 h of hCG treatment, Lhcgr expression was not changed in Wnt4flox/−;Amhr2tm3(cre)Bhr/+ granulosa cells relative to that in control cells, whereas Fshr levels decreased more precipitously in Wnt4flox/−;Amhr2tm3(cre)Bhr/+ granulosa cells than in control cells (P<0.05). We next evaluated the expression of the LH/hCG-responsive genes Adamts1 and Ptgs2(37, 38) and found the expression of both to be lower in Wnt4flox/−;Amhr2tm3(cre)Bhr/+ granulosa cells than in control cells at 12 h after hCG (P<0.05) (Fig. 5). Together, these results suggest that WNT4 does not regulate follicle development mainly at the level of gonadotropin receptor expression but can act downstream of the LH-choriogonadotropin receptor to modulate the expression of LH/hCG target genes.
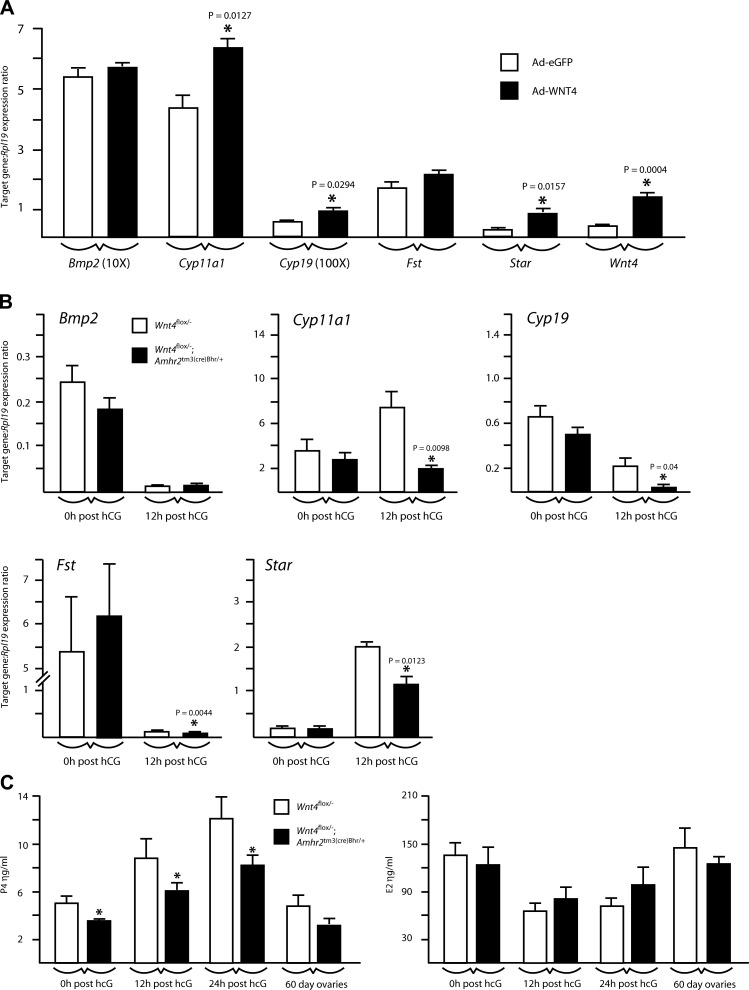
We next studied the effects of WNT4 on the expression of known ovarian WNT signaling target genes. Granulosa cells isolated from immature, eCG-treated mice were placed in culture and infected with adenoviruses to induce the expression of eGFP (control) or the overexpression of WNT4. WNT4 overexpression was found to increase the expression of the steroidogenic genes Star, Cyp11a1, and Cyp19 (Fig. 6A), which had previously been identified as potential downstream transcriptional targets of the canonical WNT signaling effector CTNNB1 (17, 19). To verify whether WNT4 regulates steroidogenic genes in vivo, RT-PCR was performed on granulosa cell RNA from gonadotropin-treated Wnt4flox/−;Amhr2tm3(cre)Bhr/+ mice. Although steroidogenic gene expression in granulosa cells from eCG-treated Wnt4flox/−;Amhr2tm3(cre)Bhr/+ mice was comparable to that of Wnt4flox/− controls, their expression was significantly lower in granulosa cells from Wnt4flox/−;Amhr2tm3(cre)Bhr/+ mice at 12-h after hCG (P<0.05)(Fig. 6B), the developmental stage at which WNT4 is normally most highly expressed (4). Conversely, the expression of Bmp2 and Fst, previously identified as downstream targets of WNT4 during embryonic ovarian development (8, 10), was not altered by changes in WNT4 expression in vitro or in vivo, except for Fst, which was decreased in Wnt4flox/−; Amhr2tm3(cre)Bhr/+ granulosa cells at 12 h after hCG (P<0.05) (Fig. 6B).
Figure 6.
Regulation of established ovarian WNT/CTNNB1 pathway target genes by WNT4. A) Quantitative RT-PCR analysis of the expression of the indicated genes in cultured granulosa cells treated with the Ad-eGFP or Ad-Wnt4 adenovirus; n = 4 samples/treatment, performed in triplicate. B) Quantitative RT-PCR analysis of the expression of the indicated genes in granulosa cells isolated from immature, eCG-treated mice of the indicated genotypes (n=4–5 animals/genotype/time point), either without (0h post hCG) or 12 h after the administration of an ovulatory dose of hCG (12h post hCG). All data were normalized to the housekeeping gene Rpl19. C) Serum P4 and E2 levels in mice of the indicated genotypes. Samples were taken from immature, eCG-treated mice either without or 12 or 24 h after the administration of an ovulatory dose of hCG. Samples from adult mice were taken at 8 wk of age from random-cycling females; n = 4–10 samples/genotype/time point. All data are expressed as means ± se (columns and error bars). *P < 0.05 vs. control.
To verify whether altered steroidogenic gene expression in Wnt4flox/−;Amhr2tm3(cre)Bhr/+ ovaries resulted in changes in ovarian steroidogenesis, serum P4 and E2 levels were evaluated in gonadotropin-treated immature mice and in random cycling adults. Results showed that P4 levels increased in response to hCG in eCG-treated immature Wnt4flox/−;Amhr2tm3(cre)Bhr/+ and control animals (P<0.05) and that P4 levels were lower in Wnt4flox/−;Amhr2tm3(cre)Bhr/+ animals relative to those of control animals at all time points examined (P<0.05) (Fig. 6C). Conversely, neither time nor genotype had significant effects on E2 levels, and neither E2 nor P4 levels differed between genotypes in adult mice. Together, these data indicate that WNT4 may act to regulate ovarian progesterone synthesis by modulating steroidogenic gene expression in granulosa cells.
WNT4 regulates the transcription of genes associated with late follicle development and the cellular stress response via the WNT/CTNNB1 pathway
To identify additional downstream targets of WNT4, granulosa cells from immature, eCG-treated Ctnnb1tm1Mmt/tm1Mmt mice were placed in culture and treated with adenoviruses to express either eGFP (Ad-eGFP, control) or WNT4 (Ad-Wnt4), and genes specifically regulated by Ad-Wnt4 treatment were identified by microarray analysis. This short-term culture-based strategy was designed to preferentially identify direct/early transcriptional targets of WNT4. Ad-WNT4 treatment increased the expression of 46 genes (53 probe sets) and decreased the expression of 27 genes (28 probe sets) by more than 3-fold over control (Table 4). Regulation of select genes by Ad-WNT4 was confirmed by real-time RT-PCR, using a distinct set of RNA samples from wild-type mouse granulosa cells (Table 5). Among the WNT4-regulated genes were a group of genes with known functions in follicle development, including Star, Klf4, Vegfa, Ptger4, Cdkn1a, Prss23, Adamts5, Thbs1, and Igfbp5. In addition, Ptgs2 was found to be regulated by WNT4 by real-time RT-PCR (Table 5), although it had not been identified as being significantly induced in the microarray analyses (P>0.05). Notably, the ovarian genes whose expression normally increases during the late phases of follicle development (Star, Vegfa, Ptger4, Cdkn1a, and Ptgs2) were induced by Ad-WNT4, whereas those whose expression normally decreases or is associated with atresia (Adamts5, Prss23, Thbs1, and Igfbp5) were repressed by Ad-WNT4 (38,39,40,41,42,43,44,45). This result suggests that WNT4 functions as a positive regulator of late follicle development. A second, larger group of genes identified in the array analysis consisted of genes associated with the cellular response to stressful stimuli (Table 4) (46,47,48,49,50,51,52,53,54,55,56,57). This group notably included 14 of 20 (70%) of the probe sets most strongly induced by Ad-WNT4.
TABLE 4.
Genes regulated by WNT4
Probe sets up-regulated by WNT4
| Probe set | Fold change | se | P | Gene symbol |
|---|---|---|---|---|
| Up-regulated by WNT4 | ||||
| 1428547_at | 10.391 | 0.235 | 0.000 | Nt5ea |
| 1436058_at | 8.228 | 0.829 | 0.004 | Rsad2* |
| 1417516_at | 6.264 | 0.565 | 0.003 | Ddit3a |
| 1418728_at | 6.024 | 0.386 | 0.001 | Star*,b |
| 1417394_at | 5.944 | 0.327 | 0.001 | Klf4*,c |
| 1426065_a_at | 5.795 | 0.546 | 0.003 | Trib3a |
| 1449133_at | 5.788 | 1.497 | 0.037 | Sprr1aa |
| 1418580_at | 5.775 | 0.603 | 0.005 | Rtp4 |
| 1423754_at | 5.273 | 0.361 | 0.001 | Ifitm3a |
| 1438040_a_at | 4.866 | 0.400 | 0.003 | Hsp90b1a |
| 1456174_x_at | 4.861 | 1.302 | 0.042 | Ndrg1a |
| 1448185_at | 4.822 | 0.379 | 0.002 | Herpud1*,a |
| 1419029_at | 4.790 | 0.792 | 0.016 | Ero1la |
| 1435626_a_at | 4.539 | 0.741 | 0.016 | Herpud1*,a |
| 1436994_a_at | 4.421 | 0.365 | 0.003 | Hist1h1c* |
| 1452754_at | 4.271 | 0.470 | 0.006 | Creld2a |
| 1420909_at | 4.219 | 0.714 | 0.018 | Vegfac |
| 1416101_a_at | 4.185 | 0.232 | 0.001 | Hist1h1c* |
| 1418206_at | 4.174 | 0.525 | 0.009 | Sdf2l1a |
| 1436200_at | 4.045 | 0.228 | 0.001 | Lonrf3 |
| 1421009_at | 4.041 | 0.630 | 0.016 | Rsad2* |
| 1449201_at | 4.003 | 0.435 | 0.007 | Star*,b |
| 1417961_a_at | 4.000 | 0.450 | 0.007 | Trim30 |
| 1451382_at | 3.876 | 0.717 | 0.024 | Chac1a |
| 1424208_at | 3.753 | 0.237 | 0.002 | Ptger4b |
| 1421679_a_at | 3.684 | 0.102 | 0.000 | Cdkn1ac |
| 1427258_at | 3.656 | 0.206 | 0.001 | Trim24 |
| 1417395_at | 3.615 | 0.415 | 0.009 | Klf4*,c |
| 1418536_at | 3.526 | 0.847 | 0.044 | H2-Q7 |
| 1419524_at | 3.512 | 0.805 | 0.041 | Tph1 |
| 1440559_at | 3.499 | 0.114 | 0.000 | Hmga2-ps1 |
| 1457275_at | 3.405 | 0.764 | 0.040 | Synm |
| 1459897_a_at | 3.401 | 0.107 | 0.000 | Sbsn* |
| 1418318_at | 3.361 | 0.092 | 0.000 | Rnf128 |
| 1416184_s_at | 3.300 | 0.120 | 0.000 | Hmga1a |
| 1422824_s_at | 3.279 | 0.731 | 0.042 | Eps8 |
| 1418392_a_at | 3.265 | 0.281 | 0.005 | Gbp3 |
| 1426276_at | 3.260 | 0.205 | 0.002 | Ifih1 |
| 1423584_at | 3.223 | 0.615 | 0.032 | Igfbp7a |
| 1460303_at | 3.212 | 0.232 | 0.003 | Nr3c1 |
| 1448380_at | 3.208 | 0.294 | 0.006 | Lgals3bp |
| 1450769_s_at | 3.176 | 0.309 | 0.007 | Stard5* |
| 1439630_x_at | 3.162 | 0.091 | 0.000 | Sbsn* |
| 1416897_at | 3.144 | 0.177 | 0.002 | Parp9 |
| 1418072_at | 3.141 | 0.130 | 0.001 | Hist1h2bb |
| 1450389_s_at | 3.131 | 0.122 | 0.001 | Pip5k1b |
| 1419162_s_at | 3.123 | 0.473 | 0.021 | Dnajc3a |
| 1435849_at | 3.067 | 0.195 | 0.002 | Jakmip3 |
| 1421322_a_at | 3.058 | 0.317 | 0.009 | Irf9 |
| 1425400_a_at | 3.052 | 0.506 | 0.026 | Cited4 |
| 1436212_at | 3.017 | 0.330 | 0.010 | Tmem71 |
| 1416188_at | 3.015 | 0.400 | 0.016 | Gm2a |
| 1422822_at | 3.013 | 0.214 | 0.003 | Stard5* |
| Down-regulated by WNT4 | ||||
| 1433919_at | 0.332 | 0.304 | 0.009 | Asb4 |
| 1438549_a_at | 0.331 | 0.156 | 0.001 | Srr |
| 1436309_at | 0.324 | 0.238 | 0.004 | Neto2 |
| 1455993_at | 0.324 | 0.240 | 0.004 | Odz4 |
| 1459589_at | 0.324 | 0.476 | 0.022 | Cryl1 |
| 1448342_at | 0.321 | 0.193 | 0.002 | Mapk10*,a |
| 1460578_at | 0.320 | 0.247 | 0.004 | Fgd5 |
| 1446560_at | 0.319 | 0.425 | 0.016 | Prss23b |
| 1434950_a_at | 0.315 | 0.128 | 0.001 | Armc8 |
| 1460302_at | 0.310 | 0.394 | 0.012 | Thbs1b |
| 1451718_at | 0.309 | 0.457 | 0.017 | Plp1 |
| 1447825_x_at | 0.297 | 0.178 | 0.001 | Pcdh8 |
| 1447625_at | 0.290 | 0.659 | 0.029 | E2f5 |
| 1437351_at | 0.288 | 0.129 | 0.000 | Cxxc4 |
| 1426284_at | 0.287 | 0.069 | 0.000 | Krt20 |
| 1456404_at | 0.280 | 0.463 | 0.012 | Adamts5b |
| 1460011_at | 0.279 | 0.556 | 0.018 | Cyp26b1 |
| 1418599_at | 0.279 | 0.729 | 0.031 | Col11a1 |
| 1443798_at | 0.278 | 0.505 | 0.014 | Pik3cd |
| 1434413_at | 0.278 | 0.147 | 0.000 | Igf1 |
| 1418745_at | 0.272 | 0.341 | 0.005 | Omd |
| 1449158_at | 0.271 | 0.338 | 0.005 | Kcnk2 |
| 1448788_at | 0.264 | 0.220 | 0.001 | Cd200 |
| 1451191_at | 0.253 | 0.191 | 0.001 | Crabp2 |
| 1437195_x_at | 0.238 | 0.295 | 0.002 | Mapk10*,a |
| 1433792_at | 0.231 | 0.432 | 0.005 | Nrip2 |
| 1452114_s_at | 0.228 | 0.280 | 0.001 | Igfbp5b |
| 1419663_at | 0.189 | 0.936 | 0.017 | Ogn |
Asterisks denote genes that appear more than once in the table.
Gene associated with the stress response.
Gene with known ovarian functions.
Gene involved in both stress response and ovarian functions.
TABLE 5.
Quantitative RT-PCR confirmation of array data
| Gene | Ad-WNT4 | Ad-GFP | Fold induction | P |
|---|---|---|---|---|
| Cdkn1a | 0.397 ± 0.063 | 0.130 ± 0.011 | 3.05 | 0.004 |
| Ddit3 | 0.283 ± 0.038 | 0.113 ± 0.022 | 2.59 | 0.008 |
| Herpud1 | 0.742 ± 0.059 | 0.340 ± 0.026 | 2.18 | 0.001 |
| Klf4 | 0.066 ± 0.008 | 0.028 ± 0.006 | 2.40 | 0.010 |
| Ptgs2 | 0.160 ± 0.011 | 0.073 ± 0.006 | 2.19 | 0.001 |
| Thbs1 | 0.710 ± 0.050 | 0.942 ± 0.043 | 0.75 | 0.017 |
| Trib3 | 0.255 ± 0.020 | 0.037 ± 0.002 | 6.90 | 0.000 |
Gene expression levels are ratios of target gene expression to housekeeping gene (Rpl19) expression; means ± se.
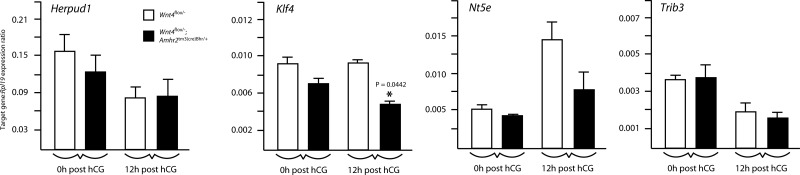
To determine whether the WNT4 target genes identified by microarray were also regulated by WNT4 in vivo, their expression was analyzed by quantitative RT-PCR in Wnt4flox/−;Amhr2tm3(cre)Bhr/+ and control granulosa cells from gonadotropin-treated immature mice. Results showed that the follicle development-associated gene Klf4 was expressed at significantly lower levels in Wnt4flox/−;Amhr2tm3(cre)Bhr/+ granulosa cells at 12 h after hCG (Fig. 7). However, the expression of stress response-associated genes Herpud1, Nt5e, Ddit3, and Trib3 was not significantly decreased in Wnt4flox/−;Amhr2tm3(cre)Bhr/+ mice (Fig. 7 and data not shown).
Figure 7.
In vivo expression of WNT4 target genes identified by microarray analyses. Quantitative RT-PCR analysis of the expression of the indicated genes in granulosa cells isolated from immature, eCG-treated mice of the indicated genotypes (n=4–5 animals/genotype/time point), either without (0h post hCG) or 12 h after the administration of an ovulatory dose of hCG (12h post hCG). All data were normalized to the housekeeping gene Rpl19 and are expressed as means ± se (columns and error bars). *P < 0.05) vs. control.
Because WNT4 appears to be able to signal via both canonical and noncanonical WNT signaling pathways, depending on the cell type and physiological context (58, 59), an additional microarray experiment was conducted to determine the extent of overlap between the transcriptional targets of WNT4 and CTNNB1. Cultured Ctnnb1tm1Mmt/tm1Mmt granulosa cells were infected with an adenovirus to express Cre (Ad-Cre), which acts to stabilize CTNNB1 and therefore constitutively activates the WNT/CTNNB1 pathway in the Ctnnb1tm1Mmt model (28). These samples were subjected to microarray analysis, which showed an increase in the expression of 21 genes (23 probe sets) and a decrease in the expression of 6 genes (7 probe sets) by more than 2-fold over control (Table 6). Of the 30 regulated probe sets, 22 (73.3%) were also regulated >2-fold by Ad-WNT4 in the array experiment described above. This finding suggests that there is a substantial overlap between the transcriptional targets of WNT4 and those of the WNT/CTNNB1 pathway and therefore that WNT4 signals via this pathway in granulosa cells. Interestingly, Wnt4 and the WNT receptor Fzd1 were both induced by Ad-Cre (Table 6), suggesting a potential CTNNB1-WNT4-FZD1 positive autoregulatory loop.
TABLE 6.
Genes regulated by CTNNB1
Probe sets up-regulated by CTNNB1
| Probe set | Fold change | se | P | Gene symbol |
|---|---|---|---|---|
| Up-regulated by CTNNB1 | ||||
| 1428547_at | 4.260 | 0.155 | 0.000 | Nt5ea |
| 1436994_a_at | 2.840 | 0.408 | 0.023 | Hist1h1ca |
| 1423754_at | 2.782 | 0.135 | 0.002 | Ifitm3a |
| 1436845_at | 2.665 | 0.167 | 0.004 | Axin2 |
| 1449152_at | 2.632 | 0.293 | 0.016 | Cdkn2ba |
| 1418318_at | 2.552 | 0.048 | 0.000 | Rnf128*,a |
| 1436200_at | 2.506 | 0.317 | 0.023 | Lonrf3a |
| 1437284_at | 2.498 | 0.111 | 0.002 | Fzd1 |
| 1434802_s_at | 2.488 | 0.283 | 0.019 | Ntf3a |
| 1436212_at | 2.468 | 0.384 | 0.036 | Tmem71a |
| 1441687_at | 2.459 | 0.257 | 0.016 | Wnt4* |
| 1417394_at | 2.431 | 0.149 | 0.005 | Klf4a |
| 1423753_at | 2.429 | 0.178 | 0.007 | Bambia |
| 1449036_at | 2.376 | 0.101 | 0.002 | Rnf128*,a |
| 1451612_at | 2.373 | 0.132 | 0.004 | Mt1 |
| 1450782_at | 2.341 | 0.154 | 0.006 | Wnt4* |
| 1435697_a_at | 2.259 | 0.364 | 0.048 | Cytip |
| 1420664_s_at | 2.252 | 0.221 | 0.018 | Procra |
| 1434628_a_at | 2.231 | 0.262 | 0.028 | Rhpn2 |
| 1426663_s_at | 2.128 | 0.153 | 0.011 | Slc45a3a |
| 1450922_a_at | 2.066 | 0.174 | 0.018 | Tgfb2 |
| 1425162_at | 2.055 | 0.053 | 0.001 | Rorba |
| 1417409_at | 2.011 | 0.199 | 0.027 | Juna |
| Down-regulated by CTNNB1 | ||||
| 1445539_at | 0.460 | 0.206 | 0.019 | Pde7ba |
| 1426284_at | 0.456 | 0.033 | 0.000 | Krt20a |
| 1455993_at | 0.444 | 0.325 | 0.040 | Odz4a |
| 1449158_at | 0.427 | 0.292 | 0.027 | Kcnk2a |
| 1437195_x_at | 0.406 | 0.453 | 0.050 | Mapk10*,a |
| 1448342_at | 0.391 | 0.329 | 0.023 | Mapk10*,a |
| 1460578_at | 0.359 | 0.277 | 0.011 | Fgd5a |
Asterisks denote genes that appear more than once in the table.
Gene that was also regulated ≥2-fold by WNT4.
DISCUSSION
Whereas the roles of WNTs in embryonic development and cancer are well established, their roles in adult tissues have only recently become an area of intense investigation. WNT4 in particular has well-established roles in sex determination and embryonic gonadal development (7, 9, 10), but it is also expressed postnatally in ovarian follicles and corpora lutea (4). The fact that WNT4 expression increases in response to gonadotropin during late follicle development (4) was particularly suggestive to us of its importance for ovarian function. In this study we report for the first time that WNT4 expression in the adult ovary is required for normal female fertility. Our analyses of Wnt4flox/−;Amhr2tm3(cre)Bhr/+ ovaries revealed that these animals are born with a normal complement of ovarian follicles and that these follicles subsequently develop normally through the secondary stage, but the number of healthy antral follicles present at puberty was substantially decreased. To gain insight into the mechanisms by which WNT4 may affect follicle development, we analyzed granulosa cell gene expression in response to WNT4 overexpression in vitro and to WNT4 loss in vivo in the Wnt4flox/−;Amhr2tm3(cre)Bhr/+ model. Our results showed that WNT4 acts to regulate the expression of genes that are involved in a number of crucial follicular processes, including steroidogenesis (Star, Cyp11a1, Cyp19, and Klf4), prostaglandin biosynthesis and action (Ptger4 and Ptgs2), tissue remodeling (Prss23, Adamts1, and Adamts5), and angiogenesis (Vegfa and Thbs1) (38, 39, 41, 43, 45, 60, 61). Together, these results suggest that WNT4 serves as an important regulator of late follicle development, acting to coordinate various granulosa cell functions during the final steps of follicle maturation and ovulation/luteinization.
Whether the reduced numbers of antral follicles present in Wnt4flox/−;Amhr2tm3(cre)Bhr/+ ovaries result from a developmental blockage at the secondary-to-antral transition, an increased rate of atresia, or a combination of both remains unclear. However, we could not find any evidence for a buildup of secondary follicles, and some Wnt4flox/−;Amhr2tm3(cre)Bhr/+ mice showed clear evidence of depletion of their ovarian follicle reserve, supporting the notion that increased atresia is involved. Although the gene regulation and steroid hormone biosynthesis data presented herein clearly demonstrate the importance of WNT4 in late follicle developmental processes (i.e., those occurring downstream of LH/hCG), exactly how these data relate to the antral follicle developmental defect observed in Wnt4flox/−;Amhr2tm3(cre)Bhr/+ mice remains to be resolved. Our identification of Cyp19 as a WNT4 transcriptional target provides a possible mechanistic explanation for this phenotype. Estradiol functions to promote granulosa cell proliferation and differentiation, as well as to inhibit apoptosis, and Cyp19-null animals present an antral follicle developmental blockage/atresia phenotype not unlike what we observe in Wnt4flox/−;Amhr2tm3(cre)Bhr/+ ovaries, albeit more severe (62). It is therefore plausible that reduced Cyp19 expression and estradiol synthesis in follicles around the transition to the antral stage in Wnt4flox/−;Amhr2tm3(cre)Bhr/+ mice increased the proportion that underwent atresia, resulting in fewer follicles continuing development to the preovulatory stage. Why this did not result in reduced serum estradiol levels in adult or immature, gonadotropin-treated Wnt4flox/−;Amhr2tm3(cre)Bhr/+ animals is unclear, but subtle differences in intrafollicular estradiol levels in early antral follicles may not have an appreciable effect on circulating estradiol levels. Alternatively, the follicle development phenotype in Wnt4flox/−;Amhr2tm3(cre)Bhr/+ mice may be entirely unrelated to steroidogenesis defects and could be related to distinct roles of WNT4 acting downstream of (or in coordination with) FSH. For example, the WNT4 target gene Vegfa (which encodes vascular endothelial growth factor A) is known to function to suppress apoptosis in granulosa cells (63, 64), indicating another mechanism by which WNT4 may direct follicles to avoid atresia. A potentially important consideration is that the gene regulation analyses detailed in the present study were conducted using granulosa cells isolated from immature mice treated with eCG, which promotes follicle development to the preovulatory stage. Because of this, the WNT4 downstream target genes that we have identified probably relate mostly to its roles in ovulation and luteinization, and its target genes in secondary and early antral follicles may be partly (or entirely) distinct. Additional studies analyzing gene expression in granulosa cells from earlier follicle developmental stages will therefore be required to better define the potential roles of WNT4 in promoting follicle survival/development around the secondary-to-antral transition.
Our microarray analysis of WNT4 downstream target genes detected a large number of genes associated with the cellular stress response. The main role of the cellular stress response is to favor cell survival by inducing the expression of genes required for processes such as DNA repair, metabolic adaptations, and antiapoptotic signaling (65,66,67). This may therefore represent another mechanism by which WNT4 acts to promote follicle survival. On the other hand, we were not able to consistently demonstrate a loss of stress response gene expression in Wnt4flox/−;Amhr2tm3(cre)Bhr/+ granulosa cells, perhaps indicating that mediation of the stress response is not a major function of WNT4 in vivo. Indeed, the microarray analysis was performed on cultured granulosa cells forced to overexpress WNT4 or a dominant-stable CTNNB1 mutant, which may have resulted in a cellular stress response indirectly caused by supraphysiological activation of the canonical WNT signaling pathway, rather than the transcriptional activation of stress response genes that are bona fide direct target genes of the WNT4/CTNNB1 pathway. Further experiments will be required to determine whether WNT4 signaling can indeed suppress apoptosis caused by environmental stressors and whether this is a physiologically relevant or necessary follicle survival mechanism.
WNTs can signal via at least three distinct pathways, often referred to as the canonical (WNT/CTNNB1), WNT/calcium, and planar cell polarity pathways (59). WNT4 seems capable of functioning alternatively as a canonical or as a noncanonical WNT, depending on the exact cell type and conditions (58, 59). In this report, we offer the first evidence that WNT4 can signal via the WNT/CTNNB1 pathway in granulosa cells. Although our data do not exclude the possibility that WNT4 can also signal via noncanonical mechanisms, the fact that WNT4 overexpression in granulosa cells induced most of the genes that were induced by stabilization of CTNNB1 argues that the WNT/CTNNB1 pathway is probably a major transducer of the WNT4 signal. The mechanisms of WNT4 signaling during sex determination and the embryonic development of the ovary have not yet been completely resolved and could involve CTNNB1 as well as noncanonical effectors (59). How WNTs such as WNT4 can signal via distinct pathways according to cell type or developmental stage remains unclear, although some evidence suggests that the precise composition of the cell surface receptor complex determines the downstream signaling mechanisms that a given WNT can activate (59). The identity of the physiological receptors for WNT4 remains unknown, but their differential expression during ovarian development and later follicle development could conceivably explain differences between the embryonic and adult functions of WNT4 in the ovary. Consistent with this hypothesis, Bmp2 and other WNT4 target genes that have been identified in embryonic ovaries (8, 10) are not similarly regulated by WNT4 in cultured granulosa cells, with the possible exception of Fst. Indeed, we report herein a set of adult granulosa cell WNT4-responsive genes that seems to be almost entirely distinct from its embryonic counterpart, reflecting the distinct physiological roles of WNT4 in the adult and embryonic gonad.
In summary, we report for the first time that WNT4 is required for normal female fertility. Our data demonstrate that WNT4 is required for antral follicle development and acts to regulate genes involved in several aspects of granulosa cell function via the WNT/CTNNB1 signaling pathway. Taken together, these results reveal the essential role of a novel pathway in follicular development and begin to define the mechanism of WNT4 action in granulosa cells.
Acknowledgments
The authors thank Dr. Lisa White, Dr. John Lydon, Dr. Marilène Paquet, Dr. Bruce D. Murphy, Dr. Alan K. Goff, Céline Forget, Mayra Tsoi, Janet DeMayo, Mira Dobias-Goff, and Aron Cory for expertise and technical assistance with microarray analyses, targeting vector design, embryonic stem cell procedures, histopathological analyses, mouse colony management, steroid hormone radioimmunoassays, statistical analyses, dissections, and follicle counting. Amhr2tm3(cre)Bhr and Ctnnb1tm1Mmt mice were kindly provided by Dr. Richard R. Behringer (University of Texas, Houston, TX, USA) and Dr. Makoto M. Taketo (Kyoto University, Kyoto, Japan), respectively. The R1 embryonic stem cell line was provided by Dr. Andras Nagy, Reka Nagy, Dr. Janet Rossant, and Dr. Wanda Abramow-Newerly (University of Toronto, Toronto, ON, Canada). This work was supported by a Canadian Institutes of Health Research operating grant and the Canada Research Chair in Ovarian Molecular Biology and Functional Genomics (D.B.) and U.S. National Institutes of Health grants HD16272 and HD07495 (J.S.R.).
References
- Logan C. Y., Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Lustig B., Behrens J. The Wnt signaling pathway and its role in tumor development. J Cancer Res Clin Oncol. 2003;129:199–221. doi: 10.1007/s00432-003-0431-0. [DOI] [PubMed] [Google Scholar]
- Hsieh M., Boerboom D., Shimada M., Lo Y., Parlow A. F., Luhmann U. F., Berger W., Richards J. S. Mice null for Frizzled4 (Fzd4-/−) are infertile and exhibit impaired corpora lutea formation and function. Biol Reprod. 2005;73:1135–4116. doi: 10.1095/biolreprod.105.042739. [DOI] [PubMed] [Google Scholar]
- Hsieh M., Johnson M. A., Greenberg N. M., Richards J. S. Regulated expression of Wnts and Frizzleds at specific stages of follicular development in the rodent ovary. Endocrinology. 2002;143:898–908. doi: 10.1210/endo.143.3.8684. [DOI] [PubMed] [Google Scholar]
- Hsieh M., Mulders S. M., Friis R. R., Dharmarajan A., Richards J. S. Expression and localization of secreted frizzled-related protein-4 in the rodent ovary: evidence for selective up-regulation in luteinized granulosa cells. Endocrinology. 2003;144:4597–4606. doi: 10.1210/en.2003-0048. [DOI] [PubMed] [Google Scholar]
- Ricken A., Lochhead P., Kontogiannea M., Farookhi R. Wnt signaling in the ovary: identification and compartmentalized expression of wnt-2, wnt-2b, and frizzled-4 mRNAs. Endocrinology. 2002;143:2741–2749. doi: 10.1210/endo.143.7.8908. [DOI] [PubMed] [Google Scholar]
- Vainio S., Heikkila M., Kispert A., Chin N., McMahon A. P. Female development in mammals is regulated by Wnt-4 signalling. Nature. 1999;397:405–409. doi: 10.1038/17068. [DOI] [PubMed] [Google Scholar]
- Heikkila M., Peltoketo H., Leppaluoto J., Ilves M., Vuolteenaho O., Vainio S. Wnt-4 deficiency alters mouse adrenal cortex function, reducing aldosterone production. Endocrinology. 2002;143:4358–4365. doi: 10.1210/en.2002-220275. [DOI] [PubMed] [Google Scholar]
- Coveney D., Ross A. J., Slone J. D., Capel B. A microarray analysis of the XX Wnt4 mutant gonad targeted at the identification of genes involved in testis vascular differentiation. Gene Expr Patterns. 2008;8:529–537. doi: 10.1016/j.gep.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Yao H. H., Matzuk M. M., Jorgez C. J., Menke D. B., Page D. C., Swain A., Capel B. Follistatin operates downstream of Wnt4 in mammalian ovary organogenesis. Dev Dyn. 2004;230:210–215. doi: 10.1002/dvdy.20042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeays-Ward K., Hoyle C., Brennan J., Dandonneau M., Alldus G., Capel B., Swain A. Endothelial and steroidogenic cell migration are regulated by WNT4 in the developing mammalian gonad. Development. 2003;130:3663–3670. doi: 10.1242/dev.00591. [DOI] [PubMed] [Google Scholar]
- Stark K., Vainio S., Vassileva G., McMahon A. P. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature. 1994;372:679–683. doi: 10.1038/372679a0. [DOI] [PubMed] [Google Scholar]
- Boerboom D., Paquet M., Hsieh M., Liu J., Jamin S. P., Behringer R. R., Sirois J., Taketo M. M., Richards J. S. Misregulated Wnt/β-catenin signaling leads to ovarian granulosa cell tumor development. Cancer Res. 2005;65:9206–9215. doi: 10.1158/0008-5472.CAN-05-1024. [DOI] [PubMed] [Google Scholar]
- Boerboom D., White L. D., Dalle S., Courty J., Richards J. S. Dominant-stable β-catenin expression causes cell fate alterations and Wnt signaling antagonist expression in a murine granulosa cell tumor model. Cancer Res. 2006;66:1964–1973. doi: 10.1158/0008-5472.CAN-05-3493. [DOI] [PubMed] [Google Scholar]
- Boyer A., Paquet M., Laguë M. N., Hermo L., Boerboom D. Dysregulation of WNT/CTNNB1 and PI3K/AKT signaling in testicular stromal cells causes granulosa cell tumor of the testis. Carcinogenesis. 2009;30:869–878. doi: 10.1093/carcin/bgp051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laguë M. N., Paquet M., Fan H. Y., Kaartinen M. J., Chu S., Jamin S. P., Behringer R. R., Fuller P. J., Mitchell A., Doré M., Huneault L. M., Richards J. S., Boerboom D. Synergistic effects of Pten loss and WNT/CTNNB1 signaling pathway activation in ovarian granulosa cell tumor development and progression. Carcinogenesis. 2008;29:2062–2072. doi: 10.1093/carcin/bgn186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy L., McDonald C. A., Jiang C., Maroni D., Zeleznik A. J., Wyatt T. A., Hou X., Davis J. S. Convergence of 3′,5′-cyclic adenosine 5′-monophosphate/protein kinase A and glycogen synthase kinase-3β/β-catenin signaling in corpus luteum progesterone synthesis. Endocrinology. 2009;150:5036–5045. doi: 10.1210/en.2009-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez Gifford J. A., Hunzicker-Dunn M. E., Nilson J. H. Conditional deletion of β-catenin mediated by Amhr2cre in mice causes female infertility. Biol Reprod. 2009;80:1282–1292. doi: 10.1095/biolreprod.108.072280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parakh T. N., Hernandez J. A., Grammer J. C., Weck J., Hunzicker-Dunn M., Zeleznik A. J., Nilson J. H. Follicle-stimulating hormone/cAMP regulation of aromatase gene expression requires β-catenin. Proc Natl Acad Sci U S A. 2006;103:12435–12440. doi: 10.1073/pnas.0603006103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biason-Lauber A., Konrad D., Navratil F., Schoenle E. J. A WNT4 mutation associated with Müllerian-duct regression and virilization in a 46,XX woman. N Engl J Med. 2004;351:792–798. doi: 10.1056/NEJMoa040533. [DOI] [PubMed] [Google Scholar]
- Lyons J. P., Mueller U. W., Ji H., Everett C., Fang X., Hsieh J. C., Barth A. M., McCrea P. D. Wnt-4 activates the canonical β-catenin-mediated Wnt pathway and binds Frizzled-6 CRD: functional implications of Wnt/β-catenin activity in kidney epithelial cells. Exp Cell Res. 2004;298:369–387. doi: 10.1016/j.yexcr.2004.04.036. [DOI] [PubMed] [Google Scholar]
- Terada Y., Tanaka H., Okado T., Shimamura H., Inoshita S., Kuwahara M., Sasaki S. Expression and function of the developmental gene Wnt-4 during experimental acute renal failure in rats. J Am Soc Nephrol. 2003;14:1223–1233. doi: 10.1097/01.asn.0000060577.94532.06. [DOI] [PubMed] [Google Scholar]
- Bardeesy N., Sinha M., Hezel A. F., Signoretti S., Hathaway N. A., Sharpless N. E., Loda M., Carrasco D. R., DePinho R. A. Loss of the Lkb1 tumour suppressor provokes intestinal polyposis but resistance to transformation. Nature. 2002;419:162–167. doi: 10.1038/nature01045. [DOI] [PubMed] [Google Scholar]
- Nagy A., Rossant J., Nagy R., Abramow-Newerly W., Roder J. C. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci U S A. 1993;90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambook J., Fritsch E. F., Maniatis T. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: Molecular Cloning: A Laboratory Manual. (2nd ed.) 1989 [Google Scholar]
- Jamin S. P., Arango N. A., Mishina Y., Hanks M. C., Behringer R. R. Requirement of Bmpr1a for Müllerian duct regression during male sexual development. Nat Genet. 2002;32:408–410. doi: 10.1038/ng1003. [DOI] [PubMed] [Google Scholar]
- Jorgez C. J., Klysik M., Jamin S. P., Behringer R. R., Matzuk M. M. Granulosa cell-specific inactivation of follistatin causes female fertility defects. Mol Endocrinol. 2004;18:953–967. doi: 10.1210/me.2003-0301. [DOI] [PubMed] [Google Scholar]
- Harada N., Tamai Y., Ishikawa T., Sauer B., Takaku K., Oshima M., Taketo M. M. Intestinal polyposis in mice with a dominant stable mutation of the β-catenin gene. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeleznik A. J., Midgley A. R., Jr, Reichert L. E., Jr Granulosa cell maturation in the rat: increased binding of human chorionic gonadotropin following treatment with follicle-stimulating hormone in vivo. Endocrinology. 1974;95:818–825. doi: 10.1210/endo-95-3-818. [DOI] [PubMed] [Google Scholar]
- Chen M., Hornsby P. J. Adenovirus-delivered DKK3/WNT4 and steroidogenesis in primary cultures of adrenocortical cells. Horm Metab Res. 2006;38:549–555. doi: 10.1055/s-2006-950500. [DOI] [PubMed] [Google Scholar]
- Pedersen T., Peters H. J. Proposal for a classification of oocytes and follicles in the mouse ovary. Reprod Fertil. 1968;17:555–557. doi: 10.1530/jrf.0.0170555. [DOI] [PubMed] [Google Scholar]
- Byskov A. G. Cell kinetic studies of follicular atresia in the mouse ovary. J Reprod Fertil. 1974;37:277–285. doi: 10.1530/jrf.0.0370277. [DOI] [PubMed] [Google Scholar]
- Devine P. J., Payne C. M., McCuskey M. K., Hoyer P. B. Ultrastructural evaluation of oocytes during atresia in rat ovarian follicles. Biol Reprod. 2000;63:1245–1252. doi: 10.1095/biolreprod63.5.1245. [DOI] [PubMed] [Google Scholar]
- Peluso J. J., England-Charlesworth C., Bolender D. L., Steger R. W. Ultrastructural alterations associated with the initiation of follicular atresia. Cell Tissue Res. 1980;211:105–115. doi: 10.1007/BF00233727. [DOI] [PubMed] [Google Scholar]
- Gévry N. Y., Lopes F. L., Ledoux S., Murphy B. D. Aberrant intracellular cholesterol transport disrupts pituitary and ovarian function. Mol Endocrinol. 2004;18:1778–1786. doi: 10.1210/me.2003-0323. [DOI] [PubMed] [Google Scholar]
- Douglas D. A., Song J. H., Moreau G. M., Murphy B. D. Differentiation of the corpus luteum of the mink (Mustela vison): mitogenic and steroidogenic potential of luteal cells from embryonic diapause and postimplantation gestation. Biol Reprod. 1998;58:1163–1169. doi: 10.1095/biolreprod58.5.1163. [DOI] [PubMed] [Google Scholar]
- Robker R. L., Russell D. L., Espey L. L., Lydon J. P., O'Malley B. W., Richards J. S. Progesterone-regulated genes in the ovulation process: ADAMTS-1 and cathepsin L proteases. Proc Natl Acad Sci U S A. 2000;97:4689–4694. doi: 10.1073/pnas.080073497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segi E., Haraguchi K., Sugimoto Y., Tsuji M., Tsunekawa H., Tamba S., Tsuboi K., Tanaka S., Ichikawa A. Expression of messenger RNA for prostaglandin E receptor subtypes EP4/EP2 and cyclooxygenase isozymes in mouse periovulatory follicles and oviducts during superovulation. Biol Reprod. 2003;68:804–811. doi: 10.1095/biolreprod.102.003590. [DOI] [PubMed] [Google Scholar]
- Greenaway J., Gentry P. A., Feige J. J., LaMarre J., Petrik J. J. Thrombospondin and vascular endothelial growth factor are cyclically expressed in an inverse pattern during bovine ovarian follicle development. Biol Reprod. 2005;72:1071–1078. doi: 10.1095/biolreprod.104.031120. [DOI] [PubMed] [Google Scholar]
- Jirawatnotai S., Moons D. S., Stocco C. O., Franks R., Hales D. B., Gibori G., Kiyokawa H. The cyclin-dependent kinase inhibitors p27Kip1 and p21Cip1 cooperate to restrict proliferative life span in differentiating ovarian cells. J Biol Chem. 2003;278:17021–17027. doi: 10.1074/jbc.M301206200. [DOI] [PubMed] [Google Scholar]
- Joyce I. M., Pendola F. L., O'Brien M., Eppig J. J. Regulation of prostaglandin-endoperoxide synthase 2 messenger ribonucleic acid expression in mouse granulosa cells during ovulation. Endocrinology. 2001;142:3187–3197. doi: 10.1210/endo.142.7.8268. [DOI] [PubMed] [Google Scholar]
- Putowski L. T., Choi D., Mordacq J., Scherzer W. J., Mayo K. E., Adashi E. Y., Rohan R. M. In vivo hormonal regulation of insulin-like growth factor binding protein-5 mRNA in the immature rat ovary. J Soc Gynecol Investig. 1995;2:735–742. [PubMed] [Google Scholar]
- Richards J. S., Hernandez-Gonzalez I., Gonzalez-Robayna I., Teuling E., Lo Y., Boerboom D., Falender A. E., Doyle K. H., LeBaron R. G., Thompson V., Sandy J. D. Regulated expression of ADAMTS family members in follicles and cumulus oocyte complexes: evidence for specific and redundant patterns during ovulation. Biol Reprod. 2005;72:1241–1255. doi: 10.1095/biolreprod.104.038083. [DOI] [PubMed] [Google Scholar]
- Ronen-Fuhrmann T., Timberg R., King S. R., Hales K. H., Hales D. B., Stocco D. M., Orly J. Spatio-temporal expression patterns of steroidogenic acute regulatory protein (StAR) during follicular development in the rat ovary. Endocrinology. 1998;139:303–315. doi: 10.1210/endo.139.1.5694. [DOI] [PubMed] [Google Scholar]
- Wahlberg P., Nylander A., Ahlskog N., Liu K., Ny T. Expression and localization of the serine proteases high-temperature requirement factor A1, serine protease 23, and serine protease 35 in the mouse ovary. Endocrinology. 2008;149:5070–5077. doi: 10.1210/en.2007-1736. [DOI] [PubMed] [Google Scholar]
- Chen B., Nelson D. M., Sadovsky Y. N-myc down-regulated gene 1 modulates the response of term human trophoblasts to hypoxic injury. J Biol Chem. 2006;28:1:2764–2772. doi: 10.1074/jbc.M507330200. [DOI] [PubMed] [Google Scholar]
- Colgan S. P., Eltzschig H. K., Eckle T., Thompson L. F. Physiological roles for ecto-5′-nucleotidase (CD73) Purinergic Signal. 2006;2:351–360. doi: 10.1007/s11302-005-5302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer B., Künzel W., Kleinstein J., Gips H. Oxygen tension in follicular fluid falls with follicle maturation. Eur J Obstet Gynecol Reprod Biol. 1992;43:39–43. doi: 10.1016/0028-2243(92)90241-p. [DOI] [PubMed] [Google Scholar]
- Fukuda S., Sumii M., Masuda Y., Takahashi M., Koike N., Teishima J., Yasumoto H., Itamoto T., Asahara T., Dohi K., Kamiya K. Murine and human SDF2L1 is an endoplasmic reticulum stress-inducible gene and encodes a new member of the Pmt/rt protein family. Biochem Biophys Res Commun. 2001;280:407–414. doi: 10.1006/bbrc.2000.4111. [DOI] [PubMed] [Google Scholar]
- Guyton K. Z., Xu Q., Holbrook N. J. Induction of the mammalian stress response gene GADD153 by oxidative stress: role of AP-1 element. Biochem J. 1996;314:547–554. doi: 10.1042/bj3140547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokame K., Agarwala K. L., Kato H., Miyata T. Herp, a new ubiquitin-like membrane protein induced by endoplasmic reticulum stress. J Biol Chem. 2000;275:32846–32853. doi: 10.1074/jbc.M002063200. [DOI] [PubMed] [Google Scholar]
- Li Z., Srivastava P. Heat-shock proteins. Wiley; Hoboken, NJ, USA: Current Protocols in Immunology (Coico, R, ed) Suppl 58, App 1T. 2004 doi: 10.1002/0471142735.ima01ts58. [DOI] [PubMed] [Google Scholar]
- Liu Y., Wang J., Yi Y., Zhang H., Liu J., Liu M., Yuan C., Tang D., Benjamin I. J., Xiao X. Induction of KLF4 in response to heat stress. Cell Stress Chaperones. 2006;11:379–389. doi: 10.1379/CSC-210.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld G., Cohen T., Gengrinovitch S., Poltorak V. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- Oh-Hashi K., Koga H., Ikeda S., Shimada K., Hirata Y., Kiuchi K. CRELD2 is a novel endoplasmic reticulum stress-inducible gene. Biochem Biophys Res Commun. 2009;387:504–510. doi: 10.1016/j.bbrc.2009.07.047. [DOI] [PubMed] [Google Scholar]
- Ohoka N., Yoshii S., Hattori T., Onozaki K., Hayashi H. TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death. EMBO J. 2005;24:1243–1255. doi: 10.1038/sj.emboj.7600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradervand S., Yasukawa H., Muller O. G., Kjekshus H., Nakamura T., St. Amand T. R., Yajima T., Matsumura K., Duplain H., Iwatate M., Woodard S., Pedrazzini T., Ross J., Firsov D., Rossier B. C., Hoshijima M., Chien K. R. Small proline-rich protein 1A is a gp130 pathway- and stress-inducible cardioprotective protein. EMBO J. 2004;23:4517–4525. doi: 10.1038/sj.emboj.7600454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard P., Harley V. R. Wnt4 action in gonadal development and sex determination. Int J Biochem Cell Biol. 2007;39:31–43. doi: 10.1016/j.biocel.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Tevosian S. G., Manuylov N. L. To β or not to β: Canonical β-catenin signaling pathway and ovarian development. Dev Dyn. 2008;237:3672–3680. doi: 10.1002/dvdy.21784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamnongjit M., Hammes S. R. Ovarian steroids: the good, the bad, and the signals that raise them. Cell Cycle. 2006;5:1178–1183. doi: 10.4161/cc.5.11.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natesampillai S., Kerkvliet J., Leung P. C., Veldhuis J. D. Regulation of Kruppel-like factor 4, 9, and 13 genes and the steroidogenic genes LDLR, StAR, and CYP11A in ovarian granulosa cells. Am J Physiol Endocrinol Metab. 2008;294:E385–E391. doi: 10.1152/ajpendo.00480.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt K. L., Drummond A. E., Dyson M., Wreford N. G., Jones M. E., Simpson E. R., Findlay J. K. The ovarian phenotype of the aromatase knockout (ArKO) mouse. J Steroid Biochem Mol Biol. 2001;79:181–185. doi: 10.1016/s0960-0760(01)00158-3. [DOI] [PubMed] [Google Scholar]
- Greenaway J., Connor K., Pedersen H. G., Coomber B. L., LaMarre J., Petrik J. Vascular endothelial growth factor and its receptor, Flk-1/KDR, are cytoprotective in the extravascular compartment of the ovarian follicle. Endocrinology. 2004;145:2896–2905. doi: 10.1210/en.2003-1620. [DOI] [PubMed] [Google Scholar]
- Abramovich D., Irusta G., Parborell F., Tesone M. Intrabursal injection of vascular endothelial growth factor trap in eCG-treated prepubertal rats inhibits proliferation and increases apoptosis of follicular cells involving the PI3K/AKT signaling pathway. Fertil Steril. 2009;93:1369–1377. doi: 10.1016/j.fertnstert.2009.01.127. [DOI] [PubMed] [Google Scholar]
- Das S., Boswell S. A., Aaronson S. A., Lee S. W. P53 promoter selection: choosing between life and death. Cell Cycle. 2008;7:154–157. doi: 10.4161/cc.7.2.5236. [DOI] [PubMed] [Google Scholar]
- Kültz D. Molecular and evolutionary basis of the cellular stress response. Annu Rev Physiol. 2005;67:225–257. doi: 10.1146/annurev.physiol.67.040403.103635. [DOI] [PubMed] [Google Scholar]
- Sedding D. G. FoxO transcription factors in oxidative stress response and ageing—a new fork on the way to longevity? Biol Chem. 2008;389:279–283. doi: 10.1515/BC.2008.033. [DOI] [PubMed] [Google Scholar]