Abstract
d-Serine is thought to be a glia-derived transmitter that activates N-methyl d-aspartate receptors (NMDARs) in the brain. Here, we investigate the pathways for d-serine release using primary cultures, brain slices, and in vivo microdialysis. In contrast with the notion that d-serine is exclusively released from astrocytes, we found that d-serine is released by neuronal depolarization both in vitro and in vivo. Veratridine (50 μM) or depolarization by 40 mM KCl elicits a significant release of endogenous d-serine from primary neuronal cultures. Controls with astrocyte cultures indicate that glial cells are insensitive to veratridine, but release d-serine mainly by the opening of volume-regulated anion channels. In cortical slices perfused with veratridine, endogenous d-serine release is 10-fold higher than glutamate receptor-evoked release. Release of d-serine from slices does not require internal or external Ca2+, suggesting a nonvesicular release mechanism. To confirm the neuronal origin of d-serine, we selectively loaded neurons in cortical slices with d-[3H]serine or applied d-alanine, which specifically releases d-serine from neurons. Depolarization with veratridine promotes d-serine release in vivo monitored by high temporal resolution microdialysis of the striatum. Our data indicate that the neuronal pool of d-serine plays a major role in d-serine dynamics, with implications for the regulation of NMDAR transmission. Rosenberg, D., Kartvelishvily, E., Shleper, M., Klinker, C. M. C., Bowser, M. T., Wolosker, H. Neuronal release of d-serine: a physiological pathway controlling extracellular d-serine concentration.
Keywords: serine racemase, neurotransmission, microdialysis, NMDA receptor
N-methyl d-aspartate receptors (NMDARs) are key excitatory neurotransmitter receptors that play important roles in several brain functions, such as learning, memory, and behavior (1). Different from other neurotransmitter receptors, activation of NMDARs requires the binding of both an agonist (glutamate) and a coagonist (d-serine or glycine) (2, 3). The coagonist site is not saturated in vivo(4), suggesting that mechanisms regulating coagonist release/synthesis are likely to regulate NMDAR function as well.
Although previously considered an “unnatural” amino acid, d-serine is a key regulator of NMDAR activity by acting as a physiological ligand at the coagonist site. Thus, together with glutamate, endogenous d-serine mediates several NMDAR-dependent processes (5,6,7), including excitotoxicity (8, 9) and synaptic plasticity (10). d-Serine is synthesized from l-serine by serine racemase (SR), a brain-enriched enzyme (11, 12). Recent data with SR-knockout (KO) mice support the notion that d-serine is indeed a physiological endogenous coagonist of NMDARs. SR-KO mice exhibit decreased NMDAR responses, impaired long-term potentiation of synaptic transmission in the hippocampus (13), and resistance to NMDAR-elicited neurotoxicity and β-amyloid 1–42 peptide injury in vivo(14). Furthermore, SR-KO mice exhibit behavioral deficits that are relevant to schizophrenia, including impairments in prepulse inhibition, sociability, and spatial discrimination (15).
Despite the several roles attributed to d-serine, the mechanisms of d-serine release and the relative contributions of astrocytes vs. neurons in d-serine dynamics are not clear. d-Serine is present at high levels in brain astrocytes (12, 16), suggesting that they release d-serine to activate neuronal NMDARs. In astrocyte cultures, d-serine can be released by exocytosis on AMPA receptor stimulation (17). These data led to the proposal that d-serine is a gliotransmitter, while neurons are not thought to play a role in d-serine signaling (18). On the other hand, new data on the disposition of d-serine and SR suggest otherwise (3). Recent immunohistochemical studies employing new antibodies and SR-KO mice as negative controls demonstrate that SR is predominantly expressed in neurons throughout the brain (9, 19). Neuronal SR expression was also suggested by in situ hybridization studies (3, 20). Furthermore, immunoreactivity to d-serine is present in neurons of the cerebral cortex (9, 21), vestibular nuclei (22), and retina (23). Thus, the relative roles of glia vs. neurons in mediating d-serine release are yet to be elucidated.
Although neurons express the highest levels of SR, regulated endogenous d-serine release from neurons has not yet been demonstrated. To define the pathways and the roles of glia vs. neurons in mediating d-serine release, we now monitored endogenous d-serine release from cell cultures, acute brain slices, and in vivo microdialysis. We found robust endogenous d-serine release by neurons. Neuronal depolarization is a major pathway for d-serine release and is at least as important as glutamate receptor-evoked d-serine release. Our data indicate for the first time that neurons are a main source of releasable d-serine in the cerebral cortex, suggesting an important role for neuron-derived d-serine.
MATERIALS AND METHODS
Materials
l-Serine and d-serine were purchased from Bachem (Bubendorf, Switzerland). Bafilomycin A1, concanamycin A, kainic acid, α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA), 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS), flufenamic acid (FFA), 5-nitro-2-(3-phenylpropylamino)benzoic acid (NPPB), o-phthaldialdehyde, antiglial fibrilary acid protein (anti-GFAP), tetrodotoxin, and veratridine were obtained from Sigma (St. Louis, MO, USA). 2,3-Dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX) and 4-[(butyl-6,7-dichloro-2-cyclopentyl-2,3-dihydro-1-oxo-1H-iden-5-yl)oxy]butanoic acid (DCPIB) were purchased from Tocris (Ellisville, MO, USA). N-(tert-butyloxycarbonyl)-l-cysteine was obtained from Novabiochem (San Diego, CA, USA). Bapta-AM was purchased from Calbiochem. Tetanus toxin was purchased from Alomone (Jerusalem, Israel). Anti-NeuN was obtained from Chemicon (Temecula, CA, USA). d-[3H]Serine and d-[3H]alanine were obtained from American Radiolabeled Chemicals (St. Louis, MO, USA).
Neuronal cell cultures
Animals were killed by quick decapitation with the approval of the Committee for the Supervision of Animal Experiments (Technion-Israel Institute of Technology). Primary neuronal cultures from cerebral cortex were prepared from E18 Sprague-Dawley rat embryos as described previously (9). The culture medium consisted of Neurobasal medium with a lower concentration of glutamine (0.2 mM instead 0.5 mM) supplemented with B27, 1 × 105units/L penicillin and 100 mg/L streptomycin. Because of variation in commercial B27 batches, we produced our own B27 supplement, as described previously (24). Cultures were used 12–14 d after plating. The content of contaminant astrocytes was monitored with anti-GFAP, and accounted for <2%.
Astrocytic cell cultures
Primary astrocyte cultures were obtained from cerebral cortex of P0-P2 Sprague-Dawley rats, as described previously (9). Cultures were used 10–14 d after plating. The content of contaminant neurons was monitored with anti-NeuN, and accounted for <1%.
Endogenous d-serine release from cell cultures
Primary cells cultured in 96-well plates were washed twice with warm (37°C) HCSS (20 mM HEPES, 5.6 mM glucose, 5.4 mM KCl, 140 mM NaCl, 0.8 mM MgCl2, and 2 mM CaCl2). Subsequently, the cells were exposed for 3 min to HCSS (80 μl/well), supplemented with different drugs at room temperature. For HPLC analysis of released amino acids, the medium was collected and treated with purified glutaminase enzyme from Escherichia coli (grade VII; Sigma). For this, the pH was first adjusted to 4.9 by addition of Na acetate buffer (pH 4.5) to a final concentration of 100 mM. Then glutaminase was added to a final concentration of 0.6 U/ml, and the samples were incubated overnight at 37°C. Following deproteinization with 5% TCA, the samples were analyzed by HPLC as described previously (25), with some modifications. To identify the d-serine peak, sample aliquots were treated with purified d-serine deaminase enzyme (10–20 μg/ml), as described previously (8). To minimize interference by background peaks, the final concentration of derivatizing agents in the samples was decreased to 0.02 mg/ml of N-(tert-butyloxycarbonyl)-l-cysteine and 0.02 mg/ml o-phthaldialdehyde. Typically, no more than 7–10% d-serine was released during stimulation. In some experiments, the culture medium was supplemented with l-serine lacking contaminant d-serine (9) (2 mM final concentration).
Uptake and release of d-[3H]serine from cell cultures
Primary cells were cultured in 96-well plates and loaded with 5–10 μM d-[3H]serine (105 cpm/nmol d-serine) in HCSS for 20 min at 37°C. The uptake was terminated by washing the cells 3 times with ice-cold HCSS. After permeabilizing the cells with 0.2% Triton X-100, the radioactivity was monitored by scintillation counting (TRI-CARB 2100TR; Packard Instruments, Meriden, CT, USA). Blanks were carried out by incubating the cells in HCSS containing d-[3H]serine on ice and accounted for <10% of the total radioactivity. For d-[3H]serine release experiments, the cells were preloaded with d-[3H]serine for 20–40 min. Subsequently, the wells were washed twice with ice-cold HCSS and exposed for 3 min to HCSS supplemented with different drugs at room temperature. In some experiments, we included recombinant 10 μg/ml d-serine deaminase enzyme (DsdA; which converts any released d-[3H]serine into [3H]pyruvate) along with 4 mM unlabeled pyruvate to prevent reuptake of any released d-serine or pyruvate. The release medium was collected and the radioactivity was counted by liquid scintillation counting. The values were corrected for protein content in each well. Typically, no more than 7–10% d-serine was released during stimulation. To monitor the effect of hypoosmolarity in d-serine release, the osmolarity of the HCSS medium was monitored with a freezing point osmometer and corresponded to 286 mosmol. Hypoosmotic HCSS medium was prepared by a 5% dilution in H2O (269 mosmol) or by decreasing NaCl concentration to 50 mM (199 mosmol).
Endogenous d-serine release from slices
Two-month-old male Sprague-Dawley rats were killed by decapitation, and the cerebral cortices were dissected and chopped in two planes at right angles into 400- × 400-μm strips with a tissue chopper (26). After washing with oxygenated modified-Krebs-HEPES buffer (MKB; 127 mM NaCl, 1.3 mM NaH2PO4, 15 mM HEPES, 10 mM glucose, 5 mM KCl, and 2.5 mM CaCl2, pH 7.4 adjusted with NaOH), the slices were transferred to a Suprafusion 1000 (SF-6) apparatus (Brandel, Gaithersburg, MD, USA) and equilibrated in 0.3-ml chambers by superfusion with oxygenated MKB buffer for 20–30 min at a flow rate of 0.6 ml/min. After equilibration, samples were collected at 0.5- to 2-min intervals. To release all remaining endogenous d-serine and calculate the total d-serine content, the slices were incubated for 20 min with 0.2 M HCl. The endogenous d-serine peak was monitored by HPLC (8). In contrast with neuronal cell cultures, the identification of endogenous d-serine from slices did not require glutaminase treatment, since released glutamine was lower than that observed in neuronal cultures. In experiments involving preloading with BAPTA-AM, the slices were chopped into 300- × 300-μm strips and continuously perfused for 1 h with 50 μM BAPTA-AM in MKB buffer to ensure optimal penetration and chelation of intracellular calcium.
d-[3H]serine release from slices
Acute cortical slices were loaded with 5 μM d-[3H]serine by 20-min incubation in oxygenated MKB at 37°C. The slices were then washed 3 times with oxygenated MKB and transferred to the superfusion apparatus. d-[3H] serine release was monitored by liquid scintillation counting. Total d-[3H] serine loading was estimated as described above for endogenous d-serine release. In some experiments, the slices were preloaded with d-[3H]serine using a sodium-free MKB buffer (127 mM cholineCl, 1.3 mM KH2PO4, 15 mM HEPES, 10 mM glucose, 3.7 mM KCl, and 2.5 mM CaCl2, pH 7.4). To monitor subsequent d-[3H] serine release, regular MKB was returned during the equilibration step at the perfusion apparatus.
In vivo microdialysis
High-temporal-resolution online microdialysis-capillary electrophoresis (MD-CE) assays were carried out as described previously (27). Briefly, a microdialysis probe (200 μm outer diameter × 3 mm regenerated cellulose, 18,000 molecular weight cutoff, sampling region) was implanted into the left striatum (+0.2 mm AP, +3.0 mm ML, −6.5 mm DL from bregma) of an anesthetized (1.5% halothane in air) male Sprague-Dawley rat (250–350 g) using a stereotactic instrument. The probe was allowed to equilibrate for 45 min following implantation while being perfused with 25 μl/hr artificial cerebral spinal fluid (aCSF; 145 mM NaCl, 2.7 mM KCl, 1.0 mM MgSO4, and 1.2 mM CaCl2). After equilibration, primary and secondary amines in the dialysate were fluorescently labeled, as described previously (27). The derivatized dialysate was coupled to online capillary electrophoresis analysis using a flow gate interface. CE analysis of the dialysate was performed every 22 s. Fluorescently labeled amines were detected as they exited the separation capillary using laser-induced fluorescence in a sheath flow cuvette. The effect of veratridine on extracellular d- and d-serine concentrations was measured by switching aCSF flowing through the microdialysis probe to aCSF spiked with 100 μM veratridine for 3 min using a 4-port valve.
Statistical analysis
Statistical analysis was carried out by 1-way ANOVA followed by Tukey’s posttest or by paired t test.
RESULTS
d-Serine release by neurons
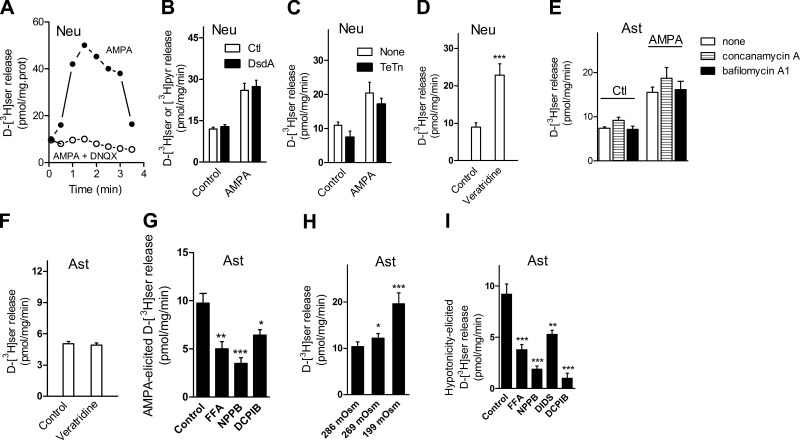
Primary neurons release preloaded d-[3H]serine on glutamate receptor stimulation (9), but endogenous d-serine release from cultured neurons has not been demonstrated. We now developed an HPLC technique that greatly improves the chromatographic separation of d-serine, allowing the injection of larger sample volumes with increased sensitivity. We observed that the small peak of released d-serine in the chromatogram is often obscured by the preceding large l-glutamine peak, which is normally supplemented to the culture medium (Fig. 1A). We found that l-glutamine removal allows easy detection of endogenous d-serine release in the samples (Fig. 1B). This is achieved by treating the samples with purified l-glutaminase enzyme prior to HPLC analysis (compare Fig. 1A, B). Endogenous d-serine peak is identified by its sensitivity to treatment with DsdA, which destroys d-serine (Fig. 1B). This technique has a linear response and detects as little as 0.2 pmol/ml released d-serine. We found that endogenous d-serine release from neurons is greatly enhanced by α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate receptor (AMPAR) stimulation (Fig. 1C). Supplementation of the culture medium with l-serine increases the amount of released d-serine, compatible with endogenous synthesis catalyzed by SR (Fig. 1C). Levels of endogenous d-serine released by neurons on AMPAR stimulation are similar to those observed with cultured astrocytes under the same conditions (data not shown).
Figure 1.
Endogenous d-serine release from neuronal cultures. A) Conventional HPLC analysis for d-serine does not reveal any endogenous d-serine release from neuronal cultures, as the d-serine peak is obscured by the large l-glutamine (l-gln) peak. B) Incubation of release medium with purified glutaminase that destroys all l-glutamine discloses a peak of d-serine that was previously fused to l-glutamine peak (arrow). Identity of d-serine peak was confirmed by its sensitivity to treatment with DsdA, denoted by an asterisk. Baseline of the sample treated with DsdA (noncontinuous trace) was offset for clarity. C) Addition of 100 μM AMPA and 50 μM cyclothiazide (to prevent AMPAR desensitization) increased the rate of endogenous d-serine release from neurons cultured in neurobasal/B27 medium (control). Endogenous d-serine release was augmented by supplementation of medium with 2 mM l-serine (+l-serine). Addition of 100 μM AMPA and 50 μM cyclothiazide, 40 mM KCl, or 50 μM veratridine further increased the rate of endogenous d-serine release from neuronal cultures maintained in medium supplemented with l-serine. D) Preincubation of the neurons with 1 μM bafilomycin A1 for 1 h had no effect on AMPA-elicited endogenous d-serine release, while 10 μM DNQX abolished the AMPA effect. Results represent average ± se of 5–7 experiments with different cultures done in quadruplicates. **P < 0.01, ***P < 0.001 vs. control.
Neuronal depolarization with KCl elicits a 2- to 3-fold increase in endogenous d-serine release (Fig. 1C). Likewise, the addition of veratridine, which renders open voltage-dependent Na+ channels, elicits significant endogenous d-serine release from neurons (Fig. 1C). To verify whether the release is vesicular, we tested the effect of bafilomycin A1, an inhibitor of the vesicular V-ATPase required for the vesicular neurotransmitter filling. We found that bafilomycin A1 does not inhibit AMPAR-mediated neuronal d-serine release (Fig. 1D). As expected, the AMPAR antagonist DNQX blocks the AMPA effect (Fig. 1D). The data indicate that under our experimental conditions the large majority of d-serine release does not occur through synaptic vesicle exocytosis.
AMPAR-mediated d-serine release was confirmed by preloading the neurons with d-[3H]serine (Fig. 2A, B). Like that observed with endogenous d-serine, AMPA-mediated d-serine release is blocked by the antagonist DNQX (Fig. 2A). d-[3H]serine is extremely stable and is not degraded in neural cultures (28). In Fig. 2B, we included recombinant DsdA that converts any released d-[3H]serine into [3H]pyruvate to prevent reuptake of released d-serine. The magnitudes of AMPA-mediated release as measured for d-[3H]serine that is converted into [3H]pyruvate are the same relative to controls lacking DsdA (Fig. 2B), indicating the absence of significant reuptake of released d-serine during the experiment.
Figure 2.
Characteristics of d-[3H]serine release from primary cultures of neurons and astrocytes. A) d-[3H]serine release elicited by 100 μM AMPA and 50 μM cyclothiazide (solid circles) from neuronal cultures was blocked by 5 min preincubation with 20 μM DNQX (open circles). B) Addition of 10 μg/ml DsdA in release medium supplemented with 4 mM unlabeled pyruvate did not change the release elicited by 100 μM AMPA and 50 μM cyclothiazide. C) A 24-h preincubation with 2 μg/ml tetanus toxin (TeTn) failed to significantly inhibit AMPA/cyclothiazide-elicited d-[3H]serine release from neuronal cultures. D) Stimulation of d-[3H]serine release from neuronal cultures by 50 μM veratridine. E) A 1-h preincubation with 1 μM bafilomycin A1 or 1 μM concanamycin A had no effect on AMPA/cyclothiazide-elicited d-[3H]serine release from primary astrocyte cultures. F) Lack of effect of 50 μM veratridine on d-[3H]serine release from primary astrocyte cultures. G) Inhibition of AMPA/cyclothiazide-elicited d-[3H]serine release from primary astrocyte cultures by the VRAC blockers FFA (100 μM), NPPB (100 μM), and DCPIB (20 μM). H) Exposure to hypotonic medium increases rate of d-[3H]serine release from primary astrocyte cultures. I) inhibition of the hypotonicity-mediated d-[3H]serine release by FFA (100 μM), NPPB (100 μM), DIDS (100 μM), or DCPIB (20 μM) in primary astrocyte cultures. Hypotonicity-mediated release was calculated by subtracting release of d-serine observed in 286 mosmol from that in 199 mosmol in the absence and in the presence of drugs. Results are representative of 3 different experiments with different cultures (A) or represent average ± se of 4–9 experiments with different cultures done in quadruplicates (B–I). *P < 0.05, **P < 0.01, ***P < 0.001 vs. control. Neu, neurons; Ast, astrocytes.
Similar to that observed with endogenous d-serine, neuronal d-[3H]serine release occurs predominantly through a nonvesicular pathway. A 24-h preincubation with tetanus toxin (2 μg/ml) fails to significantly inhibit AMPAR-elicited d-[3H] serine release (Fig. 2C), confirming the nonvesicular nature of d-serine release by AMPA. Furthermore, like that observed with endogenous d-serine, veratridine more than doubles the rate of d-[3H] serine release (Fig. 2D). Altogether, the data suggest that d-[3H]serine faithfully labels the endogenous pools of d-serine, confirming the findings observed with endogenous d-serine.
d-Serine release from primary astrocyte cultures
Like neurons, release of preloaded d-[3H]serine from primary astrocyte cultures is augmented by AMPAR stimulation (Fig. 2E). However, we do not observe vesicular release of d-serine (17) under our experimental conditions. Thus, the effect of AMPA is not diminished by blocking the vesicular neurotransmitter filling with prolonged incubation with either bafilomycin A1 or concanamycin A (Fig. 2E). Unlike neurons, d-[3H]serine release from astrocytes is insensitive to veratridine (Fig. 2F).
Volume-regulated anion channels (VRACs) present in astrocytes are activated by cell volume changes and mediate the release of several amino acids and neuromodulators, including glutamate, taurine, and aspartate (29). Since AMPA/kainate receptor stimulation causes astrocytic swelling (30), we wondered whether AMPAR activation elicits d-serine release by opening VRACs, rather than by vesicular fusion. Accordingly, we found that a significant fraction of AMPAR-mediated d-[3H] serine release from astrocytes is inhibited by VRAC inhibitors, such as FFA, NPPB, and DCPIB (Fig. 2G). In contrast, AMPAR-mediated d-[3H] serine from neurons is largely insensitive to these VRAC inhibitors (data not shown). This is in agreement with the fact that astrocytes are the main cells that undergo VRAC activation under pathological and nonpathological conditions (31).
To confirm the possible involvement of VRACs, we directly stimulated their opening by a 6% (269 mosmol) and 30% hypoosmolarity (32). As expected, a decrease in osmolarity elicits substantial d-serine release from astrocyte cultures (Fig. 2H). Furthermore, the d-serine release elicited by 30% hypoosmolarity is diminished by different VRACs inhibitors (Fig. 2I). Therefore, the pharmacology of d-serine release from astrocytes differs from that observed in neurons, as astrocytes do not release d-serine on exposure to veratridine, but display a VRAC-dependent d-serine efflux on AMPAR stimulation.
d-Serine release from cortical slices
Although the experiments with primary cultures are useful in identifying new mechanisms for d-serine release, their physiological relevance cannot be inferred from in vitro cultures. Thus, we now monitored endogenous d-serine release from perfused acute cortical slices, which maintains the physiological neuronal/glia interactions and better reflects the in vivo situation.
We found that neuronal depolarization with veratridine induces a significant increase in the rate of endogenous d-serine release from perfused cortical slices (Fig. 3). The effect of veratridine is still observed after washout of the drug, which is compatible to the very slow dissociation of veratridine from Na+-channels (33). Neuronal depolarization with KCl also elicits a significant 50% increase in the rate of endogenous d-serine release from cortical slices (Fig. 3B, C). The addition of 150 μM AMPA plus 100 μM cyclothiazide (to block AMPAR desensitization) only marginally increases (by ∼20%) the rate of endogenous d-serine release from cortical slices (data not shown). A high concentration of AMPA (300 μM) promotes a 30% increase in the rate of endogenous d-serine release (Fig. 3C). Under the same conditions, veratridine almost tripled the rate of d-serine release (Fig. 3C).
Figure 3.
Depolarization-elicited endogenous d-serine release from cortical slices. A) HPLC analysis of perfusate from acute cortical slices demonstrating release of endogenous d-serine by a 2-min perfusion with 50 μM veratridine. Treatment of perfusate with 10 μg/ml DsdA confirmed the identity of the endogenous peak of d-serine (lower trace). Baselines were offset for clarity. B) Endogenous d-serine release from acute cortical slices evoked by a 3-min exposure to 50 μM veratridine (○), 40 μM KCl (•), or 300 μM AMPA plus 50 μM cyclothiazide (▵). C) Maximal stimulation of d-serine release from experiments as in B and expressed as percentage of basal release. Results are representative of 3 different experiments (A) or represent average ± se of 5–14 experiments with different slice preparations done in duplicates. ***P < 0.001 vs. control.
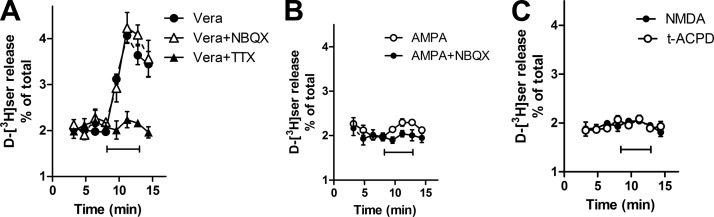
The same pattern is observed in cortical slices preloaded with d-[3H]serine, in which veratridine is much more effective than AMPA (200 vs. 20% stimulation of d-[3H]serine release) (Fig. 4A, B). Veratrine-mediated d-[3H]serine release is blocked by tetrodotoxin, a selective antagonist of veratridine-sensitive Na+-channels (Fig. 4A). The AMPAR antagonist NBQX has no effect on veratridine-induced d-serine efflux, indicating that the effect of veratridine does not involve secondary AMPAR stimulation due to local glutamate release (Fig. 4A). The addition of a high concentration of the metabotropic glutamate receptor agonist t-ACPD (0.5 mM), previously shown to elicit vesicular d-serine release from cultured astrocytes (17), does not elicit any d-serine release from cortical slices (Fig. 4C). NMDAR stimulation, previously shown to promote d-[3H]serine release from cultures (9) does not induce significant d-[3H]serine release from cortical slices either (Fig. 4C). To allow maximal NMDAR stimulation, we employed a perfusion medium lacking magnesium. In some experiments, we included glycine to saturate the NMDAR coagonist site, but addition of NMDA is still ineffective in eliciting d-serine release from slices (data not shown). The data suggest that acute cortical slices are much less responsive to glutamate receptor stimulation than primary cultures and that depolarization-mediated neuronal d-serine release seems to be the major pathway for d-serine release from acute brain cortical slices.
Figure 4.
Effect of depolarization and glutamate receptor agonists on d-[3H]serine release from acute cortical slices. A) d-[3H]serine release from acute cortical slices evoked by 50 μM veratridine (•) is unaffected by a 10-min preincubation with 20 μM NBQX (▵) but blocked by 1 μM tetrodotoxin (▴). B) d-[3H]serine release from acute cortical slices evoked by 300 μM AMPA plus 50 μM cyclothiazide (○) is blocked by 20 μM NBQX (•) added 10 min before stimulation. C) Lack of effect of perfusion with 0.5 mM NMDA (•) or 0.5 mM t-ACPD (○) on d-[3H]serine release from acute cortical slices. Results represent average ± SE of 5–10 experiments with different slice preparations done in duplicates.
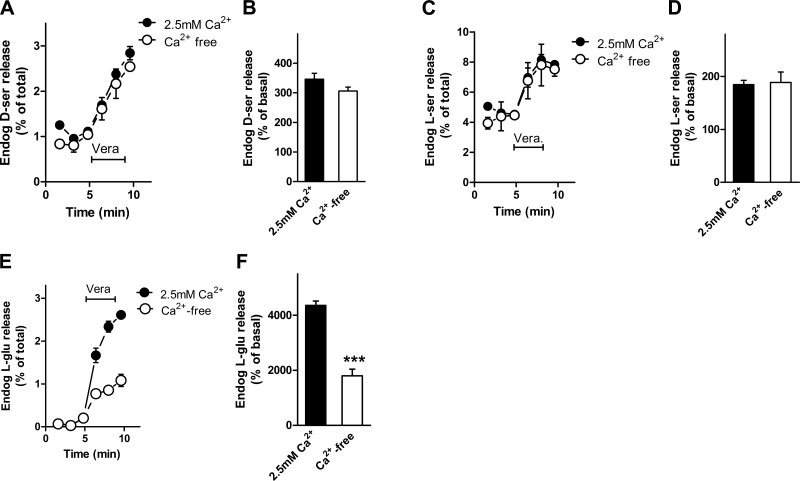
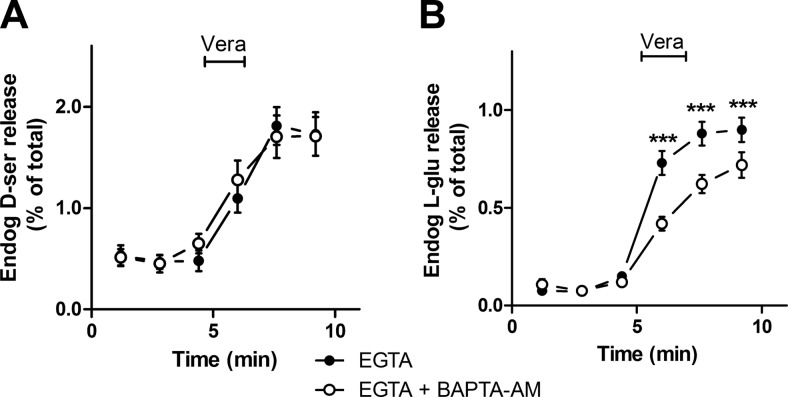
Neuronal depolarization by veratridine elicits exocytosis of synaptic vesicles by activating voltage-gated Ca2+ channels. To investigate the requirements for Ca2+, we compared the kinetics of the release of d-serine and glutamate from slices. Veratridine-elicited endogenous d-serine release from cortical slices does not depend on external Ca2+ (Fig. 5A, B). Veratridine also induces l-serine release in a Ca2+-independent fashion (Fig. 5C, D). As expected, glutamate release evoked by veratridine is diminished by chelating external Ca2+ (Fig. 5E, F) (34). Since astrocytic vesicular exocytosis can be observed on release of Ca2+ from intracellular stores, we investigated the effect of preloading the slices with BAPTA-AM in Ca2+-free medium. Chelation of both intracellular and extracellular Ca2+ does not affect d-serine release (Fig. 6A), while it inhibits the release of l-glutamate elicited by veratridine from cortical slices (Fig. 6B). Thus, in contrast with l-glutamate, depolarization-elicited d-serine release from cortical slices is not mediated by synaptic vesicle exocytosis.
Figure 5.
External calcium requirements for endogenous amino acid release from acute cortical slices. A) Release of endogenous d-serine from perfused cortical slices by 50 μM veratridine in the presence of 2.5 mM CaCl2 (•) or 0.2 mM EGTA instead of CaCl2 (Ca2+-free) (○). B) Maximal endogenous d-serine release promoted by veratridine in the presence and absence of Ca2+. C) Release of endogenous l-serine from cortical slices by 50 μM veratridine is independent of external Ca2+. D) Maximal endogenous l-serine release promoted by veratridine in the presence and absence of Ca2+. E) Release of endogenous l-glutamate from cortical slices by 50 μM veratridine is decreased by removal of external Ca2+. F) Maximal endogenous l-glutamate release promoted by veratridine in the presence and absence of Ca2+. Results represent average ± se of 5 experiments with different slice preparations done in triplicates. ***P < 0.001 vs. control.
Figure 6.
Internal calcium requirements for endogenous amino acid release from acute cortical slices. A) Release of endogenous d-serine by 100 μM veratridine in control slices (•) or slices preloaded with 50 μM BAPTA-AM (○). Release medium contained 0.2 mM EGTA to chelate external Ca2+. B) Endogenous l-glutamate release elicited by veratridine was inhibited by preincubation with BAPTA-AM. Results represent means ± se of 4 experiments with different slice preparations done in triplicates. ***P < 0.001 vs. control.
Selective loading of neurons with d-[3H]serine
Previous studies demonstrated that the Asc-1 transporter mediates high-affinity Na+-independent uptake of d-serine by neurons (35,36,37). This transporter is localized exclusively in neurons throughout the brain (38). Furthermore, the kinetic properties of Asc-1 indicate that this transporter operates in a Na+-independent manner (36, 37). Synaptosomes obtained from Asc-1-KO mice display drastically reduced accumulation of d-serine, indicating that the neuronal Asc-1 is the primary transporter that mediates d-serine uptake in the brain (35). These unique properties of the Asc-1 transporter allowed us to develop a strategy to specifically load neurons with d-[3H]serine in cortical slices.
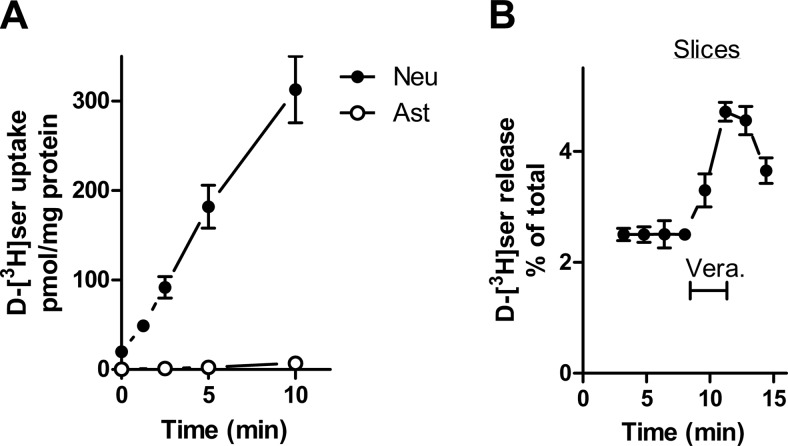
In agreement with the selective expression of Asc-1 (36, 38), we found that only neurons take up d-[3H]serine in the absence of Na+, while astrocytes display negligible d-[3H]serine uptake under the same conditions (Fig. 7A). To ensure selective loading of neurons with d-[3H]serine, acute cortical slices were first loaded with d-[3H]serine in a Na+-free medium prepared by substituting cholineCl for NaCl. Subsequently, d-[3H]serine release was monitored after sodium was returned during the perfusion of the slices. We found that veratridine effect is unchanged by removal of Na+ during d-[3H]serine loading, confirming that veratridine stimulates d-serine release from a neuronal pool of d-serine (Fig. 7B).
Figure 7.
Selective loading and release of d-[3H]serine from neuronal cells. A) d-[3H]serine uptake in neuronal (•) or astrocytic (○) primary cultures was assayed in a Na+-free in which cholineCl was substituted for NaCl (Na+-free HCSS). B) Release of d-[3H]serine from cortical slices preloaded with d-[3H]serine in the absence of Na+. Cortical slices were first loaded with d-[3H]serine in HMK containing cholineCl instead of NaCl. Then, the slices were perfused with NaCl-containing HMK, and d-[3H]serine release was elicited by veratridine. Results represent average ± se of 4 or 5 experiments with different preparations done in triplicates. Neu, neurons; Ast, astrocytes.
d-Alanine-elicited neuronal d-serine release
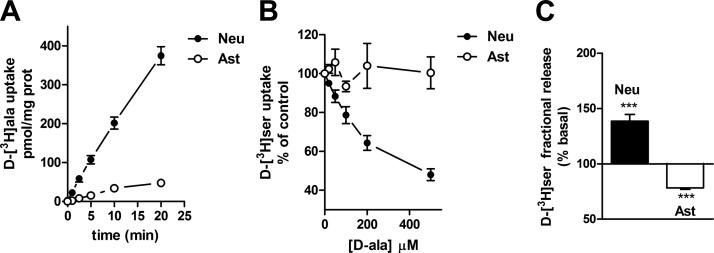
To confirm the neuronal origin of d-serine release, we further explored the unique d-amino acid selectivity of the neuronal Asc-1 transporter. Asc-1-KO mice are also deficient in d-alanine transport, indicating that Asc-1 is the primary transporter that uses d-alanine in the brain (35). These data raise the possibility that d-alanine is taken up selectively by neurons. In agreement, we found that d-[3H]alanine is taken up mainly by neuronal cultures with only a very small uptake in astrocyte cultures (Fig. 8A). Furthermore, d-alanine effectively inhibits the uptake of d-[3H]serine by neuronal cultures, but not by astrocytes, confirming its selective interaction with neuronal Asc-1 transporter (Fig. 8B).
Figure 8.
Selective neuronal uptake of d-alanine and d-alanine-induced d-serine release from neurons. A) d-alanine uptake in primary neuronal and astrocyte cultures monitored in HCSS medium supplemented with 10 μM d-[3H]alanine. B) Inhibition of d-[3H]serine uptake by d-alanine in primary neurons, but not astrocytes. C) d-alanine-elicits release of preloaded d-[3H]serine from primary neuronal cultures, whereas it decreases fractional d-[3H]serine release from primary astrocyte cultures. Results represent average ± se of 4 or 5 experiments with different preparations done in triplicates. ***P < 0.001 vs. control. Neu, neurons; Ast, astrocytes.
The Asc-1 transporter catalyzes amino acid heteroexchange (37), in which the uptake of d-alanine into the cells is coupled to the efflux of neutral amino acids (37). To investigate possible d-alanine/d-serine heteroexchange, we first loaded neuronal and astrocyte cultures with d-[3H]serine and monitored any d-serine efflux by adding d-alanine. In agreement, we found that d-alanine elicits significant release of d-[3H]serine from neuronal cultures (Fig. 8C). In contrast, d-[3H]serine release from astrocytes is even reduced by d-alanine (Fig. 8C). This indicates that d-alanine can specifically elicit d-serine release from neurons.
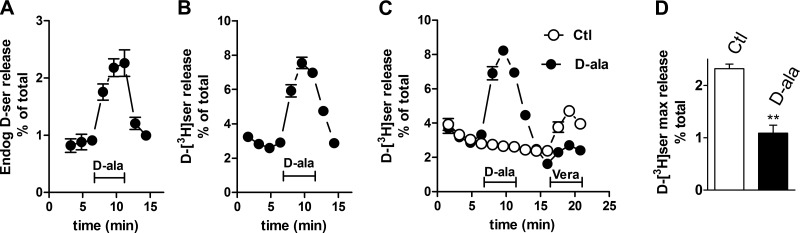
Perfusion of cortical slices with d-alanine induces a robust release of both endogenous and preloaded d-[3H]serine (Fig. 9A, B), supporting the existence of a substantial endogenous pool of neuronal d-serine. Interestingly, d-alanine displays significant selectivity for d-serine, as it promotes much smaller changes in the efflux of other neutral amino acids (Table 1). Thus, d-alanine does not promote any glutamate or aspartate release from cortical slices, which is in agreement with the selectivity of Asc-1 for neutral amino acids. Our results indicate the existence of a substantial d-alanine-responsive neuronal pool of d-serine.
Figure 9.
d-Alanine-induced d-serine release from slices. A) endogenous d-serine release from acute cortical slices promoted by perfusion with 0.5 mM d-alanine. B) d-[3H]serine release from acute cortical slices promoted by perfusion with 0.5 mM d-alanine. C) Effect of d-alanine perfusion on veratridine-elicited d-[3H]serine release. Slices preloaded with d-[3H]serine were first perfused with solution containing either none (○) or 0.5 mM d-alanine (•). After d-alanine washout, further d-[3H]serine release was induced by perfusion with 50 μM veratridine. D) Quantitative analysis of effect of veratridine in slices with and without previous perfusion with d-alanine as in C. ** P < 0.01 vs. control.
TABLE 1.
Effect of d-alanine on endogenous amino acid release
| Amino acid | Fractional release (%) |
% Control | |
|---|---|---|---|
| Control | d-Ala | ||
| d-Serine | 0.78 ± 0.10 | 2.33 ± 0.23 | 339 ± 47 |
| l-Serine | 4.06 ± 0.37 | 5.85 ± 0.29 | 146 ± 7 |
| l-Aspartate | 0.10 ± 0.01 | 0.08 ± 0.01 | 82 ± 5 |
| l-Glutamate | 0.04 ± 0.005 | 0.04 ± 0.01 | 97 ± 8 |
| l-Glutamine | 5.2 ± 0.24 | 6.01 ± 0.14 | 114 ± 3 |
| l-Asparagine | 4.84 ± 0.10 | 5.71 ± 0.31 | 117 ± 5 |
Cortical slices were perfused with 0.5 mM d-alanine for 3 min, and endogenous amino acid release was monitored by HPLC (25). Percentage control was calculated as d-ala × 100/control. Values represent average ± se of 5–11 experiments.
To investigate whether veratridine and d-alanine act at the same neuronal d-serine pool, we tested the effects of prestimulation with d-alanine on veratridine-elicited d-serine release. We found that partial depletion of the neuronal pool of d-serine by d-alanine largely decreases the effect of veratridine in releasing d-serine (Fig. 9C, D). In contrast, veratridine-elicited glutamate release is unaffected by previous treatment with d-alanine (data not shown).
In vivo endogenous d-serine release
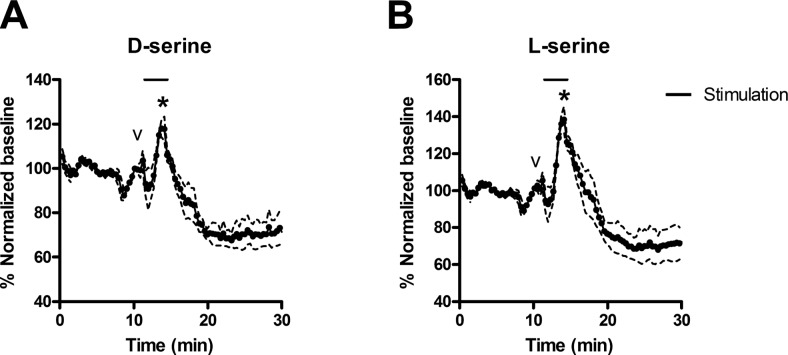
A reason for skepticism on the existence of a neuronal pool of d-serine comes from previous studies showing that veratridine does not increase d-serine release in vivo by microdialysis (39). Since conventional microdialysis analyzes samples at 20-min intervals, we raised the possibility that this technique may not be suitable to resolve faster release of amino acids. We now investigated the role of neurons in releasing d-serine in vivo by carrying out a brain microdialysis technique capable of accurately detecting d-serine release within seconds (40,41,42). In contrast to earlier reports (39), we found that veratridine elicits a significant increase in extracellular d-serine concentrations in the striatum (Fig. 10A), demonstrating that depolarization is a physiological pathway for d-serine release in vivo. Similar increases are seen with l-serine (Fig. 10B) that is also coreleased with d-serine in acute cortical slices (Fig. 5). Maximum increase in d- and l-serine during veratridine stimulation were 21 ± 3 and 40 ± 6%, respectively.
Figure 10.
Effect of veratridine on extracellular d- and l-serine concentrations in rat striatum. Online MD-CE was used to monitor d-serine (A) and l-serine (B) concentrations in rat striatum. CE analyses were performed every 22 s. Black bar denotes the 3-min period (corrected for time to travel to the detector) where aCSF perfusing the probe was changed to aCSF spiked with 100 μM veratridine. Smaller peak (indicated by v) is an artifact that occurs when the valve is switched to introduce the drug-containing aCSF into the line. Larger peak (indicated by asterisk) corresponds to veratridine-induced d-or l-serine release. Dashed lines mark se of 3 replicate measurements.
We also monitored the effect of veratridine after prolonged times. Interestingly, 25 min after veratridine stimulation, the baseline concentrations of d- and l-serine decreased by 29 ± 6 and 30 ± 9% (Fig. 10). This may correspond to the decrease in d-serine release promoted by veratridine, as detected in previous studies (39). This late decrease in extracellular d-serine levels suggests a possible down-regulation of d-serine synthesis/release following prolonged depolarization.
DISCUSSION
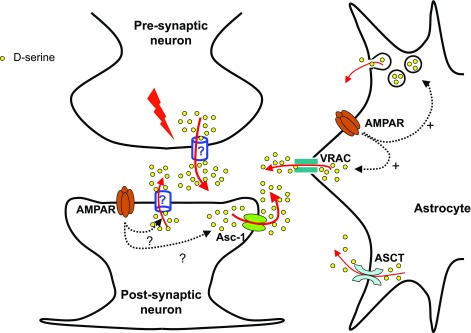
Our study provides the first evidence that neurons play a role in endogenous d-serine release. This was demonstrated by using different approaches in cell cultures, cortical slices and in vivo by high temporal resolution microdialysis. Our data also suggest that neuronal depolarization is an important pathway for endogenous d-serine release and more prominent than AMPAR-evoked d-serine release in physiological preparations. The new pathways for d-serine identified in the present study are depicted in Fig. 11.
Figure 11.
Pathways for d-serine release. We propose that neuronal d-serine release may occur following depolarization via an amino acid-permeable channel or through the Asc-1 d-serine transporter, which also catalyzes unidirectional efflux of amino acids. It is not clear whether neuronal d-serine release takes place at the presynaptic or postsynaptic regions and whether it is controlled by glutamate receptor activation. In astrocytes, d-serine can be released through the ASCT transporter (28), metabotropic or AMPAR-elicited vesicular release (17), or by the opening of VRACs following AMPA receptor activation (this study). In primary astrocyte cultures, our data suggest that VRAC opening accounts for a large fraction of AMPA-mediated d-serine release.
Previous reports suggest that astrocytes, rather than neurons, physiologically release d-serine (10, 16–18, 43). In contrast, we now demonstrate that endogenous d-serine is released by cultured neurons and that veratridine, a depolarizing agent, induces d-serine release from cultured neurons, but not astrocytes. This is in agreement with the lack of veratridine effect on neurotransmitter release from cortical astrocytes (44, 45). Neuronal depolarization by KCl is also effective in eliciting endogenous d-serine release from neuronal cultures. The relevance of the depolarization-mediated d-serine efflux pathway was confirmed in acute cortical slices. Surprisingly, glutamate receptor agonists, previously thought to be the primary regulators of d-serine release in glia (10, 16, 17), are much less effective than veratridine. Thus, even though AMPA-evoked d-serine release is prominent in neural cell cultures, its effect is much smaller than veratridine in releasing d-serine from acute cortical slices (20–30% vs. 200–300% increase in d-serine release). Metabotropic glutamate receptor activation was also ineffective in inducing d-serine release from slices. Our data suggest that depolarization-mediated neuronal d-serine release is the predominant pathway for d-serine release in physiological preparations.
To confirm the neuronal source of d-serine release, we explored the selective expression of the Asc-1 transporter by neurons in vitro and in vivo(36, 38) and its role as the primary transporter mediating Na+-independent d-serine and d-alanine uptake in the brain (35,36,37). Accordingly, neurons, but not astrocytes, take up d-serine in the absence of Na+ (Fig. 7). After preloading neuronal populations in slices with d-[3H]serine using a Na+-free medium, we found that veratridine robustly releases of d-[3H]serine. These data support a role for neuronal depolarization in evoking neuronal d-serine release in acute cortical slices.
Like d-serine, d-alanine is transported with high affinity by the neuronal Asc-1 transporter (35). We confirmed the selective interaction of d-alanine with neuronal Asc-1 transporter by showing that d-alanine is transported mainly by neurons; d-alanine inhibits d-serine transport in neurons, but not in astrocytes; and d-alanine elicits d-serine release from neurons but not astrocytes (presumably by d-amino acid heteroexchange). Most important, we demonstrated robust d-serine release evoked by d-alanine in cortical slices, strengthening the existence of a substantial neuronal pool of d-serine.
Remarkably, d-alanine displays little effect on the release of other amino acids, indicating that this d-amino acid could be used as a tool to specifically deplete the neuronal pool of d-serine. The high specificity of d-alanine toward d-serine release may be due to the fact that Asc-1 is the major transporter of d-serine in the brain (35). The extracellular concentration of other neutral amino acids that might undergo heteroexchange with d-alanine is probably regulated by many other transporters as well.
In contrast with initial studies that found that depolarization decreases the extracellular concentration of d-serine (39), we show that veratridine actually increases extracellular d-serine at short time scales (Fig. 9). The in vivo demonstration of depolarization-evoked d-serine release favors the notion that d-serine is a neuron-derived neuromodulator.
Our study also contributes to ascertain the relevance of the different d-serine release pathways. Cultured astrocytes mediate vesicular d-serine release after stimulation of AMPA or metabotropic glutamate receptors (17). Although our study does not directly investigate vesicular release from astrocytes, our present results suggest that vesicular exocytosis is not the primary pathway for d-serine release in astrocytic cultures. First, we do not observe significant inhibition of astrocytic d-serine release by bafilomycin A1 or concanamycin A. Second, we found that a significant fraction of the AMPAR-mediated d-serine release from cultured astrocytes is inhibited by volume-regulated anion channel (VRAC) inhibitors. VRACs are activated by cell swelling and mediate the release of several organic osmolytes and amino acids (29, 46). AMPAR stimulation induces prominent cell swelling in glia (30), suggesting that AMPAR stimulation activates VRACs and promotes nonvesicular d-serine release through these channels.
It is conceivable that our methodology to determine d-serine release from cultured astrocytes does not allow the detection of a small vesicular release component from astrocyte cultures or slices. Thus, our results do not exclude that under certain conditions d-serine works as a gliotransmitter. Nevertheless, our data showing that endogenous d-serine release from slices is Ca2+ independent, while glutamate release is clearly Ca2+ dependent, indicate that vesicular release of d-serine is not the main release pathway and, if present, it should account for a small fraction of total release.
The molecular mechanism for neuronal d-serine release that we identified requires further investigation. The Asc-1 transporter also promotes unidirectional amino acid efflux (37), making it a natural candidate to mediate d-serine release. It is conceivable that depolarization facilitates d-serine release by the neuronal amino acid transporter Asc-1. Opening of channels permeable to d-serine may also be involved in neuronal d-serine release. In this context, selective blockers of d-serine release may be useful to prevent NMDAR overactivation and neurotoxicity.
In summary, we demonstrate a new pathway for d-serine release that is mediated by neurons in primary cultures, acute slices and in vivo. Furthermore, a large fraction of AMPA-mediated d-serine release observed in primary astrocyte cultures seems to be mediated by VRAC opening. Our results suggest that previous conclusions on the exclusive role of astrocytic d-serine in mediating NMDAR responses ought to be reexamined in light of the significant endogenous d-serine release from neurons.
Acknowledgments
This work was supported by grants from the Israel Science Foundation, the Rappaport Institute of Medical Research, NARSAD, The Sternheim Research Fund, The L. Aronberg Research Fund in Neurology, the ISF-Legacy Heritage Fund, and the Ministry of Health to H.W.; and from the U.S. National Institutes of Health (GM 063533) to M.T.B.
References
- Wolosker H., Dumin E., Balan L., Foltyn V. N. D-amino acids in the brain: d-serine in neurotransmission and neurodegeneration. FEBS J. 2008;275:3514–3526. doi: 10.1111/j.1742-4658.2008.06515.x. [DOI] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Wolosker H. NMDA receptor regulation by d-serine: new findings and perspectives. Mol Neurobiol. 2007;36:152–164. doi: 10.1007/s12035-007-0038-6. [DOI] [PubMed] [Google Scholar]
- Danysz W., Parsons A. C. Glycine and N-methyl-d-aspartate receptors: physiological significance and possible therapeutic applications. Pharmacol Rev. 1998;50:597–664. [PubMed] [Google Scholar]
- Mothet J. P., Parent A. T., Wolosker H., Brady R. O., Jr, Linden D. J., Ferris C. D., Rogawski M. A., Snyder S. H. d-serine is an endogenous ligand for the glycine site of the N-methyl-d- aspartate receptor. Proc Natl Acad Sci U S A. 2000;97:4926–4931. doi: 10.1073/pnas.97.9.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens E. R., Esguerra M., Kim P. M., Newman E. A., Snyder S. H., Zahs K. R., Miller R. F. d-serine and serine racemase are present in the vertebrate retina and contribute to the physiological activation of NMDA receptors. Proc Natl Acad Sci U S A. 2003;100:6789–6794. doi: 10.1073/pnas.1237052100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson E. C., Stevens E. R., Wolosker H., Miller R. F. Endogenous d-serine contributes to NMDA-receptor-mediated light-evoked responses in the vertebrate retina. J Neurophysiol. 2007;98:122–130. doi: 10.1152/jn.00057.2006. [DOI] [PubMed] [Google Scholar]
- Shleper M., Kartvelishvily E., Wolosker H. d-serine is the dominant endogenous coagonist for NMDA receptor neurotoxicity in organotypic hippocampal slices. J Neurosci. 2005;25:9413–9417. doi: 10.1523/JNEUROSCI.3190-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartvelishvily E., Shleper M., Balan L., Dumin E., Wolosker H. Neuron-derived d-serine release provides a novel means to activate N-methyl-d-aspartate receptors. J Biol Chem. 2006;281:14151–14162. doi: 10.1074/jbc.M512927200. [DOI] [PubMed] [Google Scholar]
- Panatier A., Theodosis D. T., Mothet J. P., Touquet B., Pollegioni L., Poulain D. A., Oliet S. H. Glia-derived d-serine controls NMDA receptor activity and synaptic memory. Cell. 2006;125:775–784. doi: 10.1016/j.cell.2006.02.051. [DOI] [PubMed] [Google Scholar]
- De Miranda J., Panizzutti R., Foltyn V. N., Wolosker H. Cofactors of serine racemase that physiologically stimulate the synthesis of the N-methyl-d-aspartate (NMDA) receptor coagonist d-serine. Proc Natl Acad Sci U S A. 2002;99:14542–14547. doi: 10.1073/pnas.222421299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosker H., Blackshaw S., Snyder S. H. Serine racemase: a glial enzyme synthesizing d-serine to regulate glutamate-N-methyl-d-aspartate neurotransmission. Proc Natl Acad Sci U S A. 1999;96:13409–13414. doi: 10.1073/pnas.96.23.13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A. C., Tsai G. E., Ma C. L., Ehmsen J. T., Mustafa A. K., Han L., Jiang Z. I., Benneyworth M. A., Froimowitz M. P., Lange N., Snyder S. H., Bergeron R., Coyle J. T. Targeted disruption of serine racemase affects glutamatergic neurotransmission and behavior. Mol Psychiatry. 2009;14:719–727. doi: 10.1038/mp.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R., Hashimoto K., Harai T., Mori H. NMDA- and β-amyloid1–42-induced neurotoxicity is attenuated in serine racemase knock-out mice. J Neurosci. 2008;28:14486–14491. doi: 10.1523/JNEUROSCI.5034-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrie V., Fukumura R., Rastogi A., Fick L. J., Wang W., Boutros P. C., Kennedy J. L., Semeralul M. O., Lee F. H., Baker G. B., Belsham D. D., Barger S. W., Gondo Y., Wong A. H., Roder J. C. Serine racemase is associated with schizophrenia susceptibility in humans and in a mouse model. Hum Mol Genet. 2009;18:3227–3243. doi: 10.1093/hmg/ddp261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell M. J., Molliver M. E., Snyder S. H. d-serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release. Proc Natl Acad Sci U S A. 1995;92:3948–3952. doi: 10.1073/pnas.92.9.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothet J. P., Pollegioni L., Ouanounou G., Martineau M., Fossier P., Baux G. Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the gliotransmitter d-serine. Proc Natl Acad Sci U S A. 2005;102:5606–5611. doi: 10.1073/pnas.0408483102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliet S. H., Mothet J. P. Regulation of N-methyl-d-aspartate receptors by astrocytic d-serine. Neuroscience. 2009;158:275–283. doi: 10.1016/j.neuroscience.2008.01.071. [DOI] [PubMed] [Google Scholar]
- Miya K., Inoue R., Takata Y., Abe M., Natsume R., Sakimura K., Hongou K., Miyawaki T., Mori H. Serine racemase is predominantly localized in neurons in mouse brain. J Comp Neurol. 2008;510:641–654. doi: 10.1002/cne.21822. [DOI] [PubMed] [Google Scholar]
- Yoshikawa M., Takayasu N., Hashimoto A., Sato Y., Tamaki R., Tsukamoto H., Kobayashi H., Noda S. The serine racemase mRNA is predominantly expressed in rat brain neurons. Arch Histol Cytol. 2007;70:127–134. doi: 10.1679/aohc.70.127. [DOI] [PubMed] [Google Scholar]
- Yasuda E., Ma N., Semba R. Immunohistochemical evidences for localization and production of d-serine in some neurons in the rat brain. Neurosci Lett. 2001;299:162–164. doi: 10.1016/s0304-3940(01)01502-6. [DOI] [PubMed] [Google Scholar]
- Puyal J., Martineau M., Mothet J. P., Nicolas M. T., Raymond J. Changes in d-serine levels and localization during postnatal development of the rat vestibular nuclei. J Comp Neurol. 2006;497:610–621. doi: 10.1002/cne.21016. [DOI] [PubMed] [Google Scholar]
- Dun Y., Duplantier J., Roon P., Martin P. M., Ganapathy V., Smith S. B. Serine racemase expression and d-serine content are developmentally regulated in neuronal ganglion cells of the retina. J Neurochem. 2007;104:970–978. doi: 10.1111/j.1471-4159.2007.05015.x. [DOI] [PubMed] [Google Scholar]
- Price P. J., Brewer G. J. Serum-free media for neural cell cultures. Humana Press; Totowa, NJ, USA: Protocols for Neural Cell Culture. 2001:pp. 255–264. [Google Scholar]
- Hashimoto A., Nishikawa T., Oka T., Takahashi K., Hayashi T. Determination of free amino acid enantiomers in rat brain and serum by high-performance liquid chromatography after derivatization with N-tert-butyloxycarbonyl-L-cysteine and o-phthaldialdehyde. J Chromatogr. 1992;582:41–48. doi: 10.1016/0378-4347(92)80300-f. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Alvear G. M., Werling L. L. Regulation of [3H]dopamine release from rat striatal slices by sigma receptor ligands. J Pharmacol Exp Ther. 1994;271:212–219. [PubMed] [Google Scholar]
- Klinker C. C., Bowser M. T. 4-Fluoro-7-nitro-2,1,3-benzoxadiazole as a fluorogenic labeling reagent for the in vivo analysis of amino acid neurotransmitters using online microdialysis-capillary electrophoresis. Anal Chem. 2007;79:8747–8754. doi: 10.1021/ac071433o. [DOI] [PubMed] [Google Scholar]
- Ribeiro C. S., Reis M., Panizzutti R., de Miranda J., Wolosker H. Glial transport of the neuromodulator d-serine. Brain Res. 2002;929:202–209. doi: 10.1016/s0006-8993(01)03390-x. [DOI] [PubMed] [Google Scholar]
- Takano T., Kang J., Jaiswal J. K., Simon S. M., Lin J. H., Yu Y., Li Y., Yang J., Dienel G., Zielke H. R., Nedergaard M. Receptor-mediated glutamate release from volume sensitive channels in astrocytes. Proc Natl Acad Sci U S A. 2005;102:16466–16471. doi: 10.1073/pnas.0506382102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas J., Erdo S. L. Quisqualate-induced excitotoxic death of glial cells: transient vulnerability of cultured astrocytes. Glia. 1991;4:111–114. doi: 10.1002/glia.440040113. [DOI] [PubMed] [Google Scholar]
- Mulligan S. J., MacVicar B. A. VRACs CARVe a path for novel mechanisms of communication in the CNS. Sci STKE. 2006;2006:pe42. doi: 10.1126/stke.3572006pe42. [DOI] [PubMed] [Google Scholar]
- Mongin A. A., Kimelberg H. K. ATP regulates anion channel-mediated organic osmolyte release from cultured rat astrocytes via multiple Ca2+-sensitive mechanisms. Am J Physiol Cell Physiol. 2005;288:C204–C213. doi: 10.1152/ajpcell.00330.2004. [DOI] [PubMed] [Google Scholar]
- Kirpekar S. M., Prat J. C. Release of catecholamines from perfused cat adrenal gland by veratridine. Proc Natl Acad Sci U S A. 1979;76:2081–2083. doi: 10.1073/pnas.76.4.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughman R. W., Gilbert C. D. Aspartate and glutamate as possible neurotransmitters in the visual cortex. J Neurosci. 1981;1:427–439. doi: 10.1523/JNEUROSCI.01-04-00427.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter A. R., Fradley R. L., Garrett E. M., Chapman K. L., Lawrence J. M., Rosahl T. W., Patel S. Evidence from gene knockout studies implicates Asc-1 as the primary transporter mediating d-serine reuptake in the mouse CNS. Eur J Neurosci. 2007;25:1757–1766. doi: 10.1111/j.1460-9568.2007.05446.x. [DOI] [PubMed] [Google Scholar]
- Helboe L., Egebjerg J., Moller M., Thomsen C. Distribution and pharmacology of alanine-serine-cysteine transporter 1 (asc-1) in rodent brain. Eur J Neurosci. 2003;18:2227–2238. doi: 10.1046/j.1460-9568.2003.02966.x. [DOI] [PubMed] [Google Scholar]
- Fukasawa Y., Segawa H., Kim J. Y., Chairoungdua A., Kim D. K., Matsuo H., Cha S. H., Endou H., Kanai Y. Identification and characterization of a Na+-independent neutral amino acid transporter that associates with the 4F2 heavy chain and exhibits substrate selectivity for small neutral D- and L-amino acids. J Biol Chem. 2000;275:9690–9698. doi: 10.1074/jbc.275.13.9690. [DOI] [PubMed] [Google Scholar]
- Matsuo H., Kanai Y., Tokunaga M., Nakata T., Chairoungdua A., Ishimine H., Tsukada S., Ooigawa H., Nawashiro H., Kobayashi Y., Fukuda J., Endou H. High affinity D- and L-serine transporter Asc-1: cloning and dendritic localization in the rat cerebral and cerebellar cortices. Neurosci Lett. 2004;358:123–126. doi: 10.1016/j.neulet.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Hashimoto A., Kanda J., Oka T. Effects of N-methyl-d-aspartate, kainate or veratridine on extracellular concentrations of free d-serine and l-glutamate in rat striatum: an in vivo microdialysis study. Brain Res Bull. 2000;53:347–351. doi: 10.1016/s0361-9230(00)00357-9. [DOI] [PubMed] [Google Scholar]
- Ciriacks C. M., Bowser M. T. Monitoring d-serine dynamics in the rat brain using online microdialysis-capillary electrophoresis. Anal Chem. 2004;76:6582–6587. doi: 10.1021/ac0490651. [DOI] [PubMed] [Google Scholar]
- O'Brien K. B., Bowser M. T. Measuring d-serine efflux from mouse cortical brain slices using online microdialysis-capillary electrophoresis. Electrophoresis. 2006;27:1949–1956. doi: 10.1002/elps.200500770. [DOI] [PubMed] [Google Scholar]
- Ciriacks C. M., Bowser M. T. Measuring the effect of glutamate receptor agonists on extracellular d-serine concentrations in the rat striatum using online microdialysis-capillary electrophoresis. Neurosci Lett. 2006;393:200–205. doi: 10.1016/j.neulet.2005.09.080. [DOI] [PubMed] [Google Scholar]
- Henneberger C., Papouin T., Oliet S. H., Rusakov D. A. Long-term potentiation depends on release of d-serine from astrocytes. Nature. 463:232–236. doi: 10.1038/nature08673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi G., Patrizio M. Astrocyte heterogeneity: endogenous amino acid levels and release evoked by non-N-methyl-d-aspartate receptor agonists and by potassium-induced swelling in type-1 and type-2 astrocytes. J Neurochem. 1992;58:1943–1952. doi: 10.1111/j.1471-4159.1992.tb10073.x. [DOI] [PubMed] [Google Scholar]
- Neal M. J., Bowery N. G. Differential effects of veratridine and potassium depolarization on neuronal and glial GABA release. Brain Res. 1979;167:337–343. doi: 10.1016/0006-8993(79)90827-8. [DOI] [PubMed] [Google Scholar]
- Abdullaev I. F., Rudkouskaya A., Schools G. P., Kimelberg H. K., Mongin A. A. Pharmacological comparison of swelling-activated excitatory amino acid release and Cl− currents in cultured rat astrocytes. J Physiol. 2006;572:677–689. doi: 10.1113/jphysiol.2005.103820. [DOI] [PMC free article] [PubMed] [Google Scholar]