Abstract
Primary cilia are chemosensing and mechanosensing organelles that regulate remarkably diverse processes in a variety of cells. We previously showed that primary cilia play a role in mediating mechanosensing in bone cells through an unknown mechanism that does not involve extracellular Ca2+-dependent intracellular Ca2+ release, which has been implicated in all other cells that transduce mechanical signals via the cilium. Here, we identify a molecular mechanism linking primary cilia and bone cell mechanotransduction that involves adenylyl cyclase 6 (AC6) and cAMP. Intracellular cAMP was quantified in MLO-Y4 cells exposed to dynamic flow, and AC6 and primary cilia were inhibited using RNA interference. When exposed to flow, cells rapidly (<2 min) and transiently decreased cAMP production in a primary cilium-dependent manner. RT-PCR revealed differential expression of the membrane-bound isoforms of adenylyl cyclase, while immunostaining revealed one, AC6, preferentially localized to the cilium. Further studies showed that decreases in cAMP in response to flow were dependent on AC6 and Gd3+-sensitive channels but not intracellular Ca2+ release and that this response mediated flow-induced COX-2 gene expression. The signaling events identified provide important details of a novel early mechanosensing mechanism in bone and advances our understanding of how signal transduction occurs at the primary cilium.—Kwon, R. Y., Temiyasathit, S., Tummala, P., Quah, C. C., Jacobs, C. R. Primary cilium-dependent mechanosensing is mediated by adenylyl cyclase 6 and cyclic AMP in bone cells.
Keywords: mechanotransduction, fluid flow, osteocyte, osteoporosis
Mechanical loading is a critical regulatory factor in the development and maintenance of a number of important tissues and organs. However, in many cases, the molecular mechanisms utilized by cells to transduce mechanical loads into biochemical signals (mechanotransduction) are unknown. Understanding these mechanisms is central in understanding the etiology of diseases in which an abnormal response to mechanical loading contributes to their development, such as arthritis, osteoporosis, and atherosclerosis (1).
Bone is a mechanosensitive organ that alters its structure to suit its mechanical environment. Mechanical loading has been shown to generate pressure gradients that drive interstitial fluid through the lacunar-canalicular system, an interconnected network of voids (lacunae) in the mineralized tissue in which osteocytes reside and channels (canaliculi) that connect lacunae to one another (2, 3). A large body of evidence has implicated interstitial fluid flow as a potent regulator of bone metabolism. For example, numerous in vitro studies have shown that cultured osteoblasts and osteocytes respond to physiological flow profiles with both osteogenic (3,4,5,6) and antiresorptive responses (7, 8). In addition, enhanced interstitial fluid flow via externally generated intramedullary pressure (9, 10) has been shown to protect against disuse-induced bone loss in vivo. However, the molecular mechanisms by which bone cells transduce fluid flow into an intracellular biochemical signal remain largely unknown.
Primary cilia are solitary, microtubule-based organelles that project from the centrosome into the extracellular space. Their microtubule axoneme core is formed by a process known as intraflagellar transport (IFT). Once considered a vestigial organelle (11), it is now known that the primary cilium is critical for a variety of processes during development, and mutations in primary cilia genes produce a broad range of pathologies. For example, during embryonic development, the primary cilium is essential for establishment of left–right asymmetry (12, 13), neural tube closure (13), and skeletal patterning (14). In addition, dysfunction of the primary cilium has been linked to polycystic kidney disease (as well as growth of cystic lesions in the liver and pancreas) and has also been associated with hydrocephalus, mental impairment, blindness, obesity, and diabetes (15, 16).
Although the specific role of primary cilia in regulating these diverse processes and pathologies is not yet fully understood, their function as sensory organelles is clear. For example, they possess receptors for somatostatin (17) and PDGF (18), important regulators of cell proliferation and survival. They have also been shown to mediate sonic hedgehog signaling through reciprocal ciliary localization of Ptc1, the hedgehog receptor, and Smo, the regulatory target of Ptc1 (19). In addition to functioning as chemosensors, primary cilia have also been implicated in mediating sensation of physical stimuli such as tonicity (11) and temperature (20) through a mechanism involving the Ca2+-permeable channel TRPV4. Finally, primary cilia have been well documented to serve as mechanosensors of fluid flow in diverse cell types such as kidney epithelial cells (21, 22), bile duct cholangioctyes (23), vascular endothelial cells (24), and cells of the developmental node (25). These cells have been found to utilize signaling mechanisms consistent with a model whereby flow-induced bending of the primary cilium activates the mechanosensitive polycystin (PC) 1/2 ion channel complex, resulting in extracellular Ca2+-dependent intracellular Ca2+ release (21).
We previously showed that primary cilia regulate mechanotransduction of fluid flow in bone cells via an unidentified signaling mechanism that does not involve extracellular Ca2+-dependent intracellular Ca2+ release (3). Specifically, we demonstrated that bone cell primary cilia bend under subphysiological levels of flow, and inhibition of primary cilia eliminates dynamic fluid flow-induced osteogenic and antiresorptive gene transcription and protein release in osteoblasts and osteocytes. However, flow-induced increases in intracellular Ca2+ were not affected by inhibiting primary cilia, indicating that cilium-dependent mechanotransduction in bone cells utilizes an unidentified signaling mechanism that occurs independently of intracellular Ca2+ mobilization. Identification of this mechanism is central to advancing our understanding of ciliary mechanotransduction and may provide novel molecular targets for pharmacological therapies for bone loss. In addition, given recent experimental evidence suggesting that cilium-mediated sensation of distinct stimuli may involve common signaling components (20) and that the primary cilium may serve as a nexus where chemical and mechanical signals are integrated (23), elucidation of how cilium-mediated mechanotransduction occurs in bone has the potential to advance our understanding of how signal transduction occurs at the primary cilia of other tissues and in response to both mechanical and chemical stimuli.
In this study, we identify a molecular mechanism linking primary cilia and bone cell mechanotransduction that involves cyclic adenosine monophosphate (cAMP) and adenylyl cyclase 6 (AC6). Specifically, we show that osteocytes respond to dynamic flow with a rapid and transient decrease in intracellular levels of cAMP and that this response is dependent on the primary cilium. We identify the membrane-bound isoforms of adenylyl cyclase (the enzyme that catalyzes the generation of cAMP) expressed by these cells and show that one, AC6, preferentially localizes to the primary cilium. Finally, we show that flow-induced decreases in cAMP and subsequent increases in the osteogenic gene cyclo-oxygenase 2 (COX-2) are dependent on AC6 and primary cilia, and that this response depends on activation of Gd3+-inhibitable ion channels but not release of Ca2+ from intracellular stores. Identification of these flow-induced signaling events provide important details of a novel early mechanosensing mechanism in bone and advances our understanding of how signal transduction occurs at the primary cilium.
MATERIALS AND METHODS
Cell culture and fluid flow
MLO-Y4 osteocyte-like cells (passage 22–27) were cultured on tissue culture plates coated with type 1 collagen in growth medium (α-MEM with 5% FBS, 5% CS, and 1% penicillin/streptomycin) at 37°C and 5% CO2. Seventy-two hours prior to experimentation, cells were seeded on collagen-coated glass slides at 80,000 cells/slide. Cells were loaded into parallel plate flow chambers, and following a 30-min incubation period, exposed to oscillatory fluid flow at 1 Hz, resulting in 1-Pa peak shear stress on the slide surface (peak flow rate: 18.8 ml/min; chamber dimensions: 56×24×0.28 mm). For pharmacological studies, 50 nM thapsigargin (Sigma, St. Louis, MO, USA) or 10 μM GdCl3 (Sigma) was added to the flow medium. For cAMP studies, 0.5 mM of the phosphodiesterase (PDE) inhibitor 3-isobutyl-1-methylxanthine (IBMX) (Sigma) was added to the flow medium, unless otherwise noted. For all other studies, flow medium consisted of growth medium.
RNA interference
Cells were transfected with 0.2 μM siRNA using Xtreme Gene (Roche Molecular Systems, Indianapolis, IN, USA) 48 h prior to experimentation. AC6 siRNA targeted 5′-CCTGCCACCTACAACAGCTCAATTA-3′. Sequence alignment using BLAST (26) revealed that AC2–5, AC7, and AC9 had low nucleotide sequence homology to the AC6 siRNA target sequence (9–15 bp matches over the entire 25-bp length of the siRNA), minimizing chances for nonspecific inhibition of these isoforms by AC6 siRNA treatment. Polaris siRNA targeted 5′-CCAGAAACAGATGAGGACGACCTTT-3′, as described previously (3). Controls consisted of cells transfected with 0.2-μM scrambled siRNA (Invitrogen, Carlsbad, CA, USA). We did not observe any gross effects on cellular morphology for all siRNA treatments.
Western blot analysis
Total cellular protein was isolated in RIPA lysis buffer (Santa Cruz Biotechnology, Santa Cruz, CA, USA), separated by electrophoresis in 4–12% NuPAGE Bis-Tris gels (Invitrogen), and transferred onto nitrocellulose membranes (Invitrogen), as in Malone et al. (3). Membranes were probed with rabbit anti-AC6 (1:400; Fabgennix, Frisco, TX, USA) or mouse anti-actin (clone AC-40, 1:2000; Abcam, Cambridge, MA, USA) antibodies. Chemiluminescent detection was performed using HRP-conjugated goat anti-rabbit IgG or goat anti-mouse IgG antibodies (1:2000 to 1:10,000; Millipore, Bedford, MA, USA).
Immunofluorescence
Cells were fixed in 10% formalin. Immunostaining was carried out using primary antibodies targeted against acetylated α-tubulin (clone 6-11B-1, 1:1000; Abcam) or AC2-7 and AC9 (AC3: Santa Cruz, ref. 27, 1:200; all others: 1:200; Fabgennix). Secondary antibodies were goat anti-mouse IgG Alexa Fluor 594 and rabbit anti-goat IgG Alexa Fluor 488 (1:200; Invitrogen). Nuclei were counterstained with DAPI. Cells were imaged on a laser-scanning confocal microscope (Nikon C-1; Nikon, Tokyo, Japan) using a 1.4 NA ×60 UV-corrected objective.
cAMP quantification
Slides were removed from the chambers immediately on cessation of flow, and ice-cold 0.1 M HCl with 0.5% Triton X-100 was added to the cells for 10 min. The slides were scraped, and the cell lysates were centrifuged. The supernatant was used to quantify cAMP using an enzyme immunoassay system (Assay Designs, Ann Arbor, MI, USA) normalized to total protein as quantified by a Bradford assay. All samples and standards were run in duplicate.
RT-PCR and real-time RT-PCR
Total RNA was extracted using TriReagent (Invitrogen), and cDNA was synthesized using TaqMan reverse transcriptase (Applied Biosystems, Foster City, CA, USA). PCR on cDNA samples was performed using primers targeted against mouse AC1-9 as given by Johnston et al. (28). For real-time analysis, PCR was performed on the resulting cDNA (ABI Prism 7900 sequence detection system), and relative gene levels between samples were quantified using the relative standard curve method as in Malone et al. (3). Primers and probes for GAPDH (4352339E), AC6 (Mm00475772_m1), and COX-2 (Mm00478374_m1) were obtained from Applied Biosystems. In preliminary experiments, we simultaneously quantified 18S and GAPDH levels in response to flow. Although both genes were relatively stable in response to flow, the stability of GAPDH was modestly better than 18S (data not shown), and thus GAPDH was used as an endogenous control for all experiments. All samples and standards were run in triplicate.
Analysis
For all flow experiments, experimental values were normalized to the mean static (no flow) control value. All data are expressed as means ± se and were analyzed using 1-way ANOVA followed by Fisher’s PLSD post hoc test. Values of P < 0.05 were considered statistically significant).
RESULTS
MLO-Y4 osteocyte-like cells respond to oscillatory flow with a rapid and transient decrease in cAMP levels
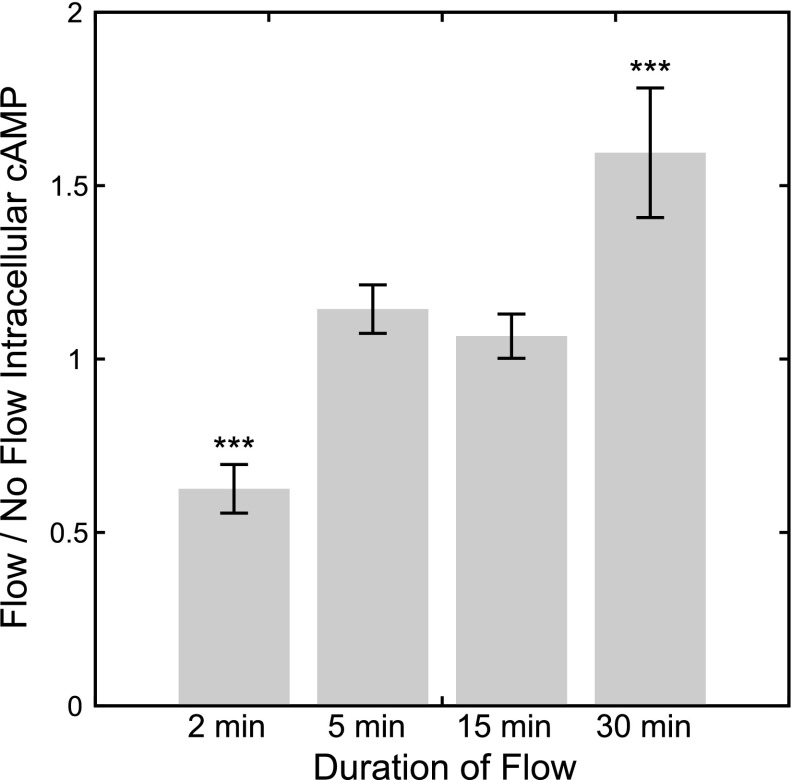
To determine whether dynamic fluid flow induces changes in intracellular cAMP levels in bone cells, we quantified cAMP levels in MLO-Y4 osteocyte-like cells immediately following exposure to 2, 5, 15, and 30 min of oscillatory fluid flow. We found that cells exposed to 2 min of oscillatory flow had significantly lower intracellular cAMP levels compared to static controls (no flow: 1.00±0.04; flow: 0.63±0.07; P<0.001, n=17; Fig. 1). This response was highly transient, as there was no significant difference in cAMP levels in cells following 5 min (no flow: 1.00±0.11; flow: 1.14±0.07; P=0.32, n=6; Fig. 1) or 15 min (no flow: 1.00±0.09; flow: 1.07±0.06; P=0.65, n=6; Fig. 1) of flow. Cells exposed to 30 min of flow had a significant increase in cAMP levels (no flow: 1.00±0.09; flow: 1.59±0.19; P<0.001, n=6; Fig. 1). Given the evidence for cAMP feedback mechanisms that can drive low-frequency cAMP oscillations in response to an initial stimulus (29, 30), we postulated that the increase in cAMP after 30 min of flow may be a compensatory response driven by the initial decrease in cAMP levels. Thus, our remaining experiments focused on investigating the mechanisms involved in the initial flow-induced decrease in cAMP, and the increase in cAMP after 30 min of flow was not investigated further.
Figure 1.
Flow/no flow intracellular cAMP levels in MLO-Y4 osteocyte-like cells immediately following 2, 5, 15, and 30 min of flow exposure. Flow/no flow ratio <1 or >1 indicates a decrease or increase of cAMP in response to flow, respectively. Oscillatory flow induced a rapid and transient decrease in cAMP levels after 2 min of exposure and an increase after 30 min of exposure. ***P < 0.001.
Flow-induced decreases in cAMP levels are dependent on primary cilia
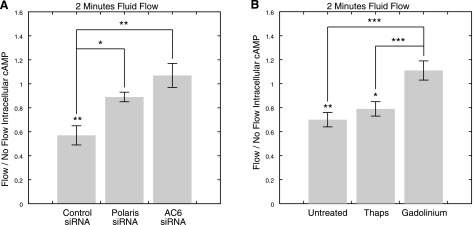
We next investigated whether primary cilia mediate the decrease in cAMP in response to flow. Using RNA interference, we inhibited primary cilia formation by depleting the ciliary component polaris. Polaris is a central component of IFT (31,32,33), and both conditional polaris deletion (34) and hypomorphic mutations in the polaris-encoding gene Tg737 (32, 33) have previously been shown to result in severe aberrations in ciliogenesis in vivo. We previously showed that polaris siRNA treatment reduces protein expression and significantly decreases the percentage of ciliated MLO-Y4 cells (3). This treatment allows the circumvention of nonspecific effects on cell physiology associated with pharmacological removal of primary cilia using chloral hydrate (3), which has been used to investigate the role of cilia in mechanosensing in the past (35). In our studies, polaris inhibition resulted in a significant decrease in the percentage of ciliated cells (control siRNA: 57.2±6.6%; polaris siRNA: 35.2±5.3%; P=0.02, n=10 slides, >100 cells/treatment counted). When cells treated with control siRNA were exposed to flow, there was a significant decrease in cAMP levels relative to static controls (no flow: 1.00±0.21; flow: 0.57±0.08; n=9, P=0.005; Fig. 5A). This response was lost in polaris-depleted cells (no flow: 1.00±0.05; flow: 0.89±0.04; n=12, P = 0.38; Fig. 5A). The difference in the flow/no flow fold change in cAMP levels between cells treated with control and polaris siRNA was statistically significant (P=0.02).
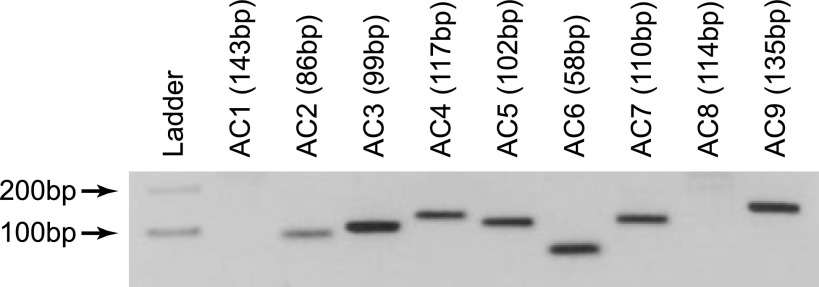
Figure 2.
RT-PCR targeting mouse AC1–9 reveals MLO-Y4 cells express AC2–7 and AC9.
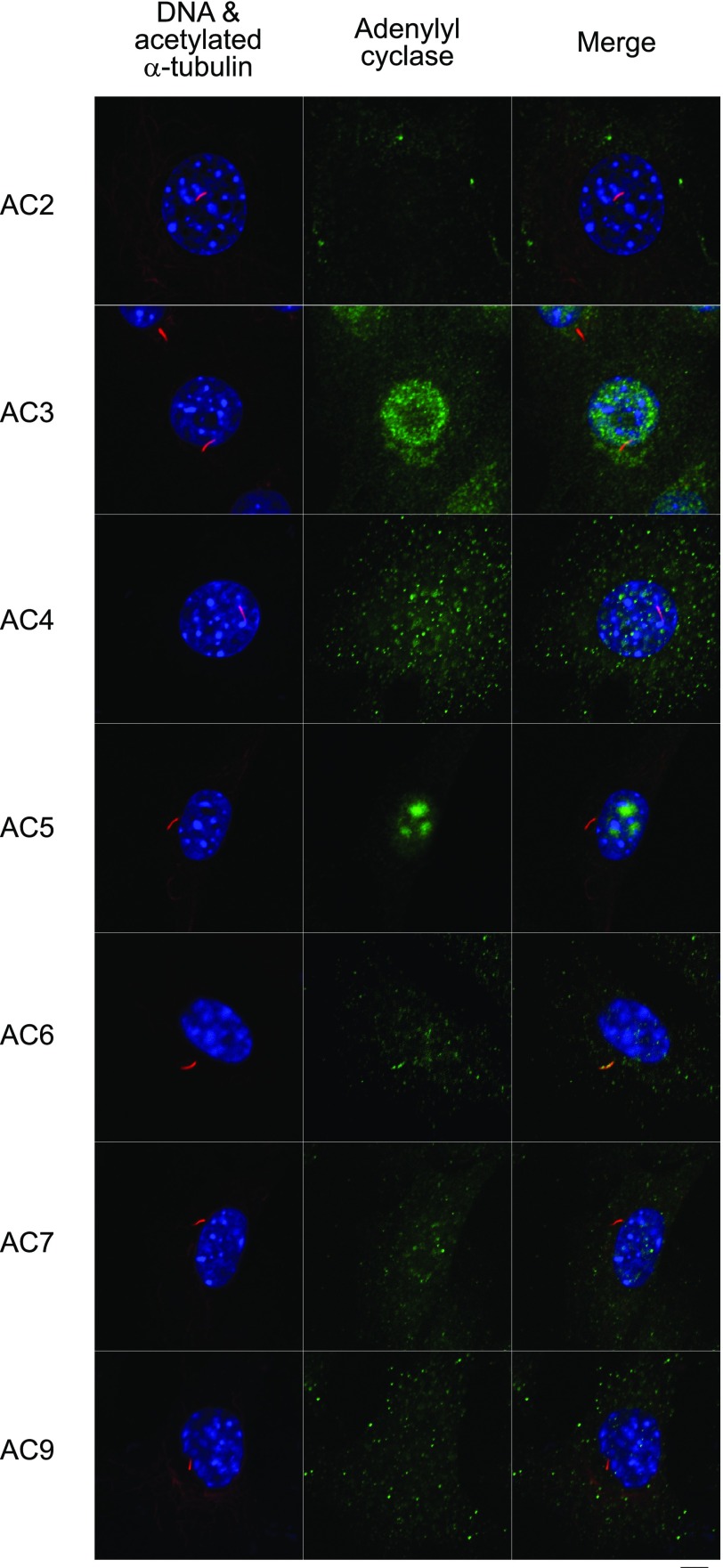
Figure 3.
Immunostaining for primary cilia, identified as linear structures enriched in acetylated α-tubulin, (red) and adenylyl cyclase (green). Nuclei are counterstained with DAPI (blue). Of the expressed isoforms (AC2–7 and AC9), only AC6 was found to preferentially localize to the primary cilium. Scale bar = 5 μm.
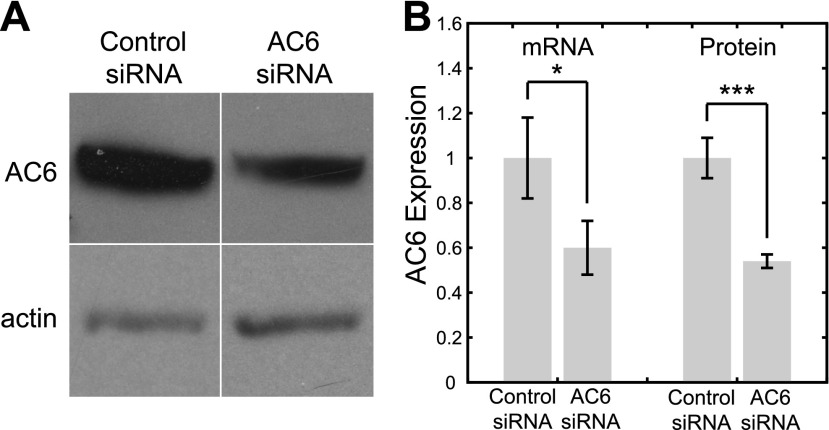
Figure 4.
Treatment with AC6 siRNA results in significant decreases in AC6 expression at both gene and protein levels. A) Western blot of AC6 in cells treated with control and AC6 siRNA. Samples are normalized by actin. B) AC6 gene expression, as quantified by real time RT-PCR, and protein expression, as quantified by Western blot densitometry, in cells treated with control and AC6 siRNA. *P < 0.05, ***P < 0.001.
Figure 5.
Polaris and AC6 depletion inhibits flow-induced decreases in cAMP via a mechanism that involves Gd3+-sensitive ion channels but not release of Ca2+ from intracellular stores. A) Flow/no flow intracellular cAMP levels immediately following 2 min of flow exposure in cells treated with control, polaris, and AC6 siRNA. Flow-induced decreases in intracellular cAMP levels were significantly inhibited in polaris- and AC6-depleted cells. B) Flow/no flow intracellular cAMP levels following 2 min of flow in untreated cells and cells treated with thapsigargin or Gd3+. Intracellular cAMP levels significantly decreased in response to flow in untreated cells and cells treated with thapsigargin; however this response was lost in cells treated with Gd3+. *P < 0.05, **P < 0.01, ***P < 0.001.
AC6 localizes to the primary cilium and mediates flow-induced decreases in cAMP
Adenylyl cyclase (AC) is a GTP-dependent enzyme responsible for catalyzing the conversion of cAMP from ATP. Nine membrane-bound isoforms of AC have been identified, of which several have recently been found to localize to the primary cilium in other cell types: AC3 in mouse and rat neurons (27), AC5/6 in porcine renal epithelial cells (36) and AC4/6/8 in rat cholangiocytes (23, 37). Interestingly, Masyuk et al.(23) found that forskolin-stimulated increases in cAMP were rapidly suppressed in rat cholangiocytes by flow exposure in a primary cilium- and AC6-dependent manner. Thus, we hypothesized that one or more AC isoforms localized to the primary cilium of bone cells may be responsible for the primary cilium-dependent changes in cAMP levels in response to flow.
We first sought to determine which adenylyl cyclase isoforms are expressed in osteocytes and whether any of these isoforms localize to the primary cilium. Expression of AC1–9 is highly dependent on tissue type (38), and the isoforms that are expressed in bone have not been established. In our studies, RT-PCR using previously published primers against mouse AC1–9 (28) revealed bands corresponding to AC2–7 and AC9 (Fig. 2) in osteocytes. Bands corresponding to AC1 and AC8 were not detected, indicating that, similar to kidney, these two Ca2+/calmodulin-stimulable isoforms (38) are not expressed in bone. To determine whether any of the detected isoforms (AC2–7 and AC9) localize to the primary cilium, we coimmunostained the cells with antibodies against each of these isoforms and acetylated α-tubulin, which is enriched in the primary cilium. We were not able to detect any of the isoforms preferentially localized to the primary cilium except for AC6 (Fig. 3). It is unlikely that AC6 detection within the cilium was an artifact of colabeling with acetylated α-tubulin, since AC6 was never observed to preferentially localize with acetylated α-tubulin immunostaining outside of the cilium (data not shown). AC6 was also found to localize to the primary cilium in MC3T3-E1 osteoblastic cells (data not shown).
To investigate the role of AC6 in mediating flow-induced decreases in cAMP, we depleted AC6 using RNA interference. Gene expression levels, as quantified by real-time RT-PCR (control siRNA: 1.00±0.18; AC6 siRNA: 0.60±0.12; P=0.03, n=8) and protein expression levels, as assessed semiquantitatively by Western blot densitometry (control siRNA: 1.00±0.09; AC6 siRNA: 0.54±0.03; P<0.001, n=6), were both significantly reduced with AC6 siRNA treatment (Fig. 4). Gene expression levels of AC2–5, AC7, and AC9 did not appear to be inhibited as a consequence of nonspecific effects of AC6 siRNA treatment on other isoforms (as assessed by RT-PCR, data not shown). In the absence of flow, AC6 siRNA-treated cells exhibited only a slight decrease in cAMP levels that was not significantly different from controls (control siRNA: 1.00±0.14; AC6 siRNA: 0.85±0.17; P=0.51, n=8). However, when exposed to flow, cells with depleted AC6 lost their capacity to undergo flow-induced decreases in cAMP (no flow: 1.00±0.06; flow: 1.07±0.10; n=6, P=0.68; Fig. 5A). The difference in the flow/no flow fold change in cAMP levels between control and AC6 siRNA-treated cells was statistically significant (P=0.003), while there was no significant difference between cells treated with polaris and AC6 siRNA (P=0.24).
Decreases in cAMP with flow depend on Gd3+-sensitive ion channels but not release of Ca2+ from intracellular stores
AC6 is a Ca2+-inhibitable isoform of adenylyl cyclase. Thus, we next sought to investigate whether the AC6-mediated decrease in cAMP is dependent on flow-induced release of Ca2+ from intracellular stores and/or activation of plasma membrane Ca2+-permeable channels. Bone cells have previously been shown to increase intracellular Ca2+ levels in response to oscillatory flow (6) with peak concentrations (∼200 nM) in the range that yields maximal AC6 inhibition (39). This flow-induced Ca2+ mobilization is inhibited in the presence of 50 nM thapsigargin (6), an inhibitor of the sarco/endoplasmic reticulum ATP-dependent Ca2+ pump that, in turn, depletes intracellular Ca2+ stores. In addition, mechanotransduction in bone cells has been shown to be inhibited in the presence of 10 μM Gd3+(40,41,42), a blocker of several types of cation channels at this concentration, including stretch-activated cation channels, and L-type, T-type, and N-type Ca2+ channels (43). In our experiments, when cells treated with thapsigargin were exposed to flow, cAMP levels significantly decreased (no flow: 1.00±0.07; flow: 0.79±0.06; n=11–12, P=0.02; Fig. 5B) in a manner that was only slightly diminished when compared to untreated cells (no flow: 1.00±0.03; flow: 0.70±0.06; n=10–11, P=0.00; Fig. 5B). In contrast, this response was eliminated in cells treated with Gd3+ (no flow: 1.00±0.06; flow: 1.11±0.08; n=11–12, P=0.22; Fig. 5B). Treatment with Gd3+ resulted in significantly different flow/no flow fold changes in cAMP levels compared to untreated cells (P<0.001), whereas treatment with thapsigargin did not (P=0.36).
Flow-induced COX-2 expression is dependent on primary cilia and AC6
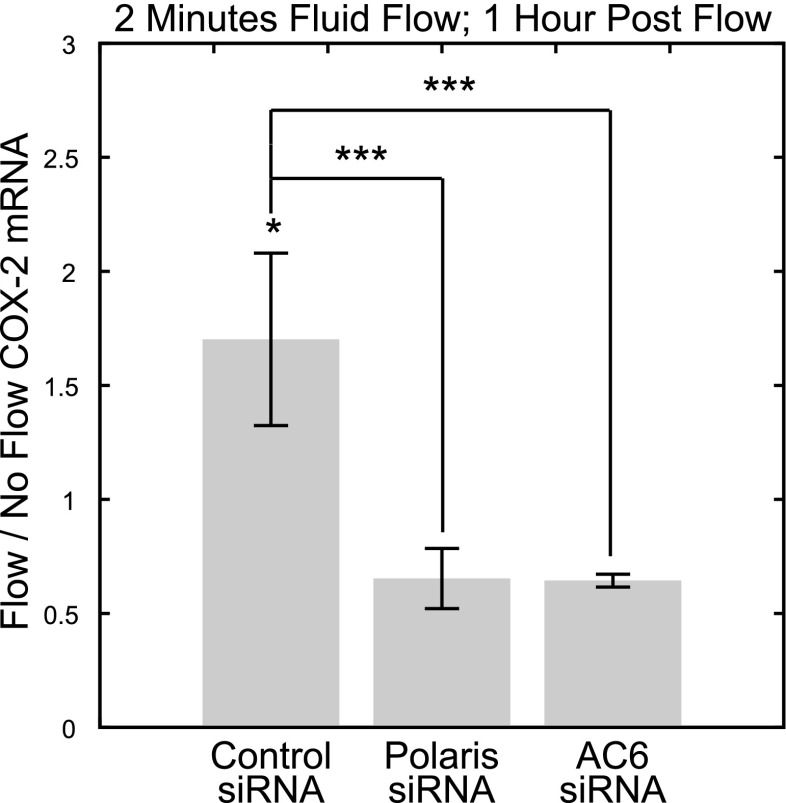
We next sought to determine the potential role of the rapid decrease in cAMP in response to flow as an early signaling response that results in alterations in gene expression downstream. We previously showed that flow-induced expression of COX-2, an inducible isozyme that catalyzes the synthesis of prostaglandins, is mediated by the primary cilium in osteocytes (3). To investigate the role of flow-induced decreases in cAMP in this response, we quantified COX-2 mRNA following 2 min of flow exposure and a 1-h postflow incubation period. This experimental regimen was distinct from the COX-2 mRNA quantification immediately following 1 h of flow exposure used by Malone et al.(3), and it allowed us to investigate whether the early flow-induced decrease in cAMP levels on flow exposure was sufficient to stimulate COX-2 gene expression downstream in the absence of further stimulation. When cells treated with control siRNA were exposed to flow, there was a significant increase in COX-2 mRNA levels compared to static controls (no flow: 1.00±0.07; flow: 1.70±0.38; n=8, P=0.02; Fig. 6). This response was lost in cells treated with polaris siRNA (no flow: 1.00±0.20; flow: 0.65±0.13; n=7–8, P=0.24; Fig. 6), indicating that primary cilia mediate expression of COX-2 via a pathway that is activated within 2 min of flow exposure. This response was also lost in cells treated with AC6 siRNA (no flow: 1.00±0.20; flow: 0.64±0.03; n=8, P = 0.21; Fig. 6). There were significant differences in the flow/no flow fold change in COX-2 mRNA levels between cells treated with control siRNA and cells treated with AC6 (P<0.001) and polaris siRNA (P<0.001). Although there appeared to be a trend of decreased COX-2 mRNA expression in response to flow in polaris and AC6 siRNA-treated cells, neither trend was statistically significant.
Figure 6.
Two minutes of fluid flow induces COX-2 gene expression in a primary cilium- and AC6-dependent manner. Flow/no flow COX-2 mRNA levels following 2 min of flow exposure and a 1-h postflow incubation period indicate that COX-2 expression was significantly increased in response to flow exposure in cells treated with control siRNA but not in cells treated with polaris and AC6 siRNA. *P < 0.05, ***P < 0.001.
DISCUSSION
Primary cilia mediate the capacity to sense and respond to fluid flow in diverse cell types such as kidney tubule epithelial cells (21, 22), vascular endothelial cells (24), bile duct cholangiocytes (23), cells of the embryonic node (25), and bone cells (osteoblasts/osteocytes) (3). With the exception of bone cells, these cell types have been found to utilize a signaling mechanism consistent with a model whereby flow-induced bending of the primary cilium activates the mechanosensitive PC1/2 ion channel complex, resulting in extracellular Ca2+-dependent intracellular Ca2+ release (21). We recently showed that primary cilia mediate osteogenic and antiresorptive responses to flow in bone cells such as PGE2 release, expression of OPN and COX-2, and increased OPG/RANKL mRNA ratio. Like kidney tubule epithelial cells, vascular endothelial cells, bile duct cholangiocytes, and nodal cells, bone cells rapidly respond to flow by releasing Ca2+ from intracellular stores (6, 44). However, unlike these cells, flow-induced Ca2+ release was found to be independent of the primary cilium, indicating that extracellular Ca2+-dependent intracellular Ca2+ release is not involved in ciliary mechanosensing in bone cells. In this study, we identified a novel early signaling mechanism in mechanotransduction of fluid flow in osteocytes that involves the primary cilium, AC6, and cAMP.
We have implicated, for the first time, flow-induced decreases in intracellular levels of cAMP as an important early signal in mechanotransduction in bone cells. This response is different from the rapid and sustained increases in cAMP in bone cells exposed to steady flow, which was observed by Reich et al.(45) at the same time point used in this study. This difference may have been due to the use of dynamic vs. steady flow profiles, which have previously been shown to induce distinct responses in bone cells (46, 47) and activate independent signaling pathways (48). Alternatively, this difference may have been due to differences in the concentrations of the PDE inhibitor IBMX (0.5 vs. 5 mM), which slows cAMP degradation, or differences in cell type (osteocytes vs. osteoblasts). To address this, we exposed MC3T3-E1 osteoblast-like cells to 2 min of oscillatory flow (1 Pa, 1 Hz) in the presence of 5 mM IBMX. We observed a significant decrease in intracellular cAMP in response to flow (no flow: 1.00±0.32; flow: 0.32±0.08; P=0.04, n=6–8), indicating that flow-induced decreases in cAMP can occur in multiple bone cell types, and that oscillatory flow may uniquely stimulate a decrease in cAMP.
We found that inhibition of primary cilia formation abrogated the rapid decrease in cAMP in response to flow, implicating cAMP as a previously unidentified second messenger molecule mediating ciliary mechanotransduction in bone cells. When compared to previous findings showing that primary cilia do not contribute to flow-induced intracellular Ca2+ mobilization in these cells (3), these data indicate that at least two distinct signaling mechanotransduction pathways exist in bone cells. Specifically, one is primary cilium-dependent, involves cAMP, and does not involve release of Ca2+ from intracellular stores, while another is cilium-independent and leads to intracellular Ca2+ mobilization. Interestingly, it has been shown that flow-induced expression of OPN is lost by emptying intracellular Ca2+ stores (6), as well as inhibiting primary cilia by treatment with chloral hydrate or polaris siRNA (3). This suggests that the “cilium-dependent/cAMP” and “cilium-independent/intracellular Ca2+ release” mechanotransduction pathways may coregulate the anabolic effect of dynamic flow stimulation. The dual involvement of cilium-dependent and cilium-independent signaling pathways within the same cell type may not be unique to bone cells. For example, Praetorius and Spring (22) demonstrated that in kidney cells, intracellular Ca2+ release due to micropipette manipulation of the primary cilium requires extracellular Ca2+, whereas Ca2+ release due to manipulation of the apical membrane does not. They also showed that Ca2+ mobilization due to manipulation of the apical membrane is not inhibited by removal of the primary cilium via treatment with chloral hydrate (35). Thus, it appears that kidney cells, like bone cells, possess cilium-dependent and cilium-independent mechanotransduction mechanisms.
There is a rapidly growing body of evidence that adenylyl cyclase is an important component of ciliary signaling. For example, in the past several years, 5 membrane-bound isoforms of adenylyl cyclase have been identified to localize to the primary cilium in various cell types: AC3, AC4, AC5, AC6, and AC8. In our studies, we found that four of these isoforms were expressed in osteocytes (AC3–6), but only AC6 appeared to preferentially localize to the primary cilium. When we exposed cells to flow, we found that depleting AC6 blocked decreases in cAMP in response to flow. The involvement of AC6 (which catalyzes the production of cAMP) in mediating flow-induced decreases in cAMP suggests that bone cells possess basal AC6 enzymatic activity and that this activity is decreased in response to flow. The similar effects of depleting polaris and AC6 on inhibiting flow-induced decreases in cAMP levels strongly suggest that AC6 localized to the cilium mediates this response. One interesting observation is that while AC6 inhibition diminished flow-induced decreases in cAMP, it only modestly decreased baseline levels of cAMP in the absence of flow. This trend is similar to those of Masyuk et al.(23), who found that depletion of AC6 moderately reduced increases in cAMP levels in response to forskolin, but substantially inhibited suppression of forskolin-stimulated cAMP levels in response to flow. This suggests there may be a feedback mechanism in response to AC6 depletion that results in increased activity of other AC isoforms. Indeed, differential effects of AC6 inhibition on cAMP levels in response to a stimulus but not on baseline levels in the absence of a stimulus has been previously observed, and has been postulated to be the result of such a compensatory mechanism (49).
The capacity for submicromolar concentrations of Ca2+ to decrease AC6 basal enzymatic activity has been well documented (39, 50, 51). Interestingly, AC6 has previously been shown to be selectively inhibited by extracellular Ca2+ entry over release from intracellular stores (51, 52). In our studies, we found that emptying intracellular stores did not block flow-induced decreases in cAMP, while inhibiting Gd3+-sensitive channels eliminated this response. This suggests that activation of Gd3+-sensitive ion channels may be causing decreased AC6 activity and cAMP production in response to flow. For example, activation of Gd3+-sensitive ion channels, influx of extracellular Ca2+, and subsequent inhibition of basal AC6 activity at the primary cilium may be a potential mechanism by which bone cells decrease cAMP production in response to flow. Interestingly, two Gd3+-sensitive ion channels have previously been shown to localize to the primary cilium and to mediate flow sensing in kidney epithelial cells: PC2 and TRPV4 (20, 21, 53). Masyuk et al.(23) postulated that suppression of forskolin-stimulated cAMP levels in cholangiocytes exposed to flow was caused by axoneme bending-induced activation of PC2 at the cilium, influx of extracellular Ca2+, and subsequent inactivation of ciliary AC6. The involvement of PC2, TRPV4, and/or other Gd3+-sensitive channels in transducing ciliary bending into decreased cAMP production in bone cells would be consistent with the findings here, although more investigation is necessary in this regard.
Our results suggest that the rapid mechanically stimulated decrease in cAMP, although transient, may be an important early intracellular signal in regulating osteogenic behavior. When we exposed cells to only 2 min of fluid flow, we found that COX-2 gene expression was significantly increased. Further, we found that this response was dependent on both primary cilia and AC6. We previously demonstrated that primary cilium-dependent transcriptional changes in COX-2 in osteocytes occur after exposure to 1 h of fluid flow (3). However, the increased expression following only 2 min of fluid flow (and a 1-h incubation period) observed in this study implies that the decrease in cAMP may be sufficient to induce altered gene expression and that further cellular mechanical stimulation is not necessary to continue the propagation of this signaling pathway. Interestingly, previous studies have demonstrated that flow-induced COX-2 expression in bone cells is lost when protein kinase A (PKA) activity, the main effector molecule of cAMP, is inhibited (54). This suggests that the initial decrease in cAMP observed in our study may lead to a subsequent increase in cAMP accumulation and PKA activity that is important for transcriptional changes in COX-2 to occur. Such a mechanism may be similar to the increased levels of cAMP that occurs in cells exposed to 30 min of flow, which we postulate to be a compensatory response to the initial decrease.
Our findings add to the growing body of evidence that ciliary mechanotransduction of fluid flow can involve different combinations of molecular mechanisms that are shared across multiple and diverse cell types. For example, like bone cells, AC6 localizes to the primary cilium in cholangiocytes, although the function appears different. Unlike bone cells, baseline cAMP levels were not found to decrease with flow exposure in cholangiocytes, although forskolin-stimulated increases in cAMP did decrease in response to flow in a primary cilium- and AC6-dependent manner (23). Further, in bone cells, the increase in intracellular Ca2+ with flow occurs independent of primary cilia, while in cholangiocytes, this increase is dependent on primary cilia, PC1/2, and extracellular Ca2+ in a manner similar to kidney cells (23). Contrasting these signaling pathways raises the question of whether flow-induced decreases in cAMP occur in kidney cells. Indeed, several animal models of polycystic kidney disease exhibit high intracellular levels of cAMP, which has been attributed to hyperactivity of Ca2+-inhibitable ACs due to low levels of Ca2+(55).
There are important limitations that need to be considered when interpreting our findings. First, the efficacy of polaris knockdown in inhibiting primary cilia was assessed by quantifying the percentage of ciliated cells. However, it is important to note that polaris knockdown may have decreased the delivery of ciliary components, while allowing for axoneme growth in some cells (3). This can result in cells with nonfunctional primary cilia. For example, RNAi knockdown of the IFT component IFT20 results in axonemes that appear to be normal while eliminating ciliary localization of PC2 (56). Thus, it is possible that the efficacy of RNAi knockdown of polaris in inhibiting primary cilia functionality was greater than the reduction in cilium formation would indicate (3). This may explain how a relatively modest decrease in the percentage of ciliated cells could result in a relatively large reduction in flow-induced changes in cAMP and COX-2 expression. Second, it is unknown whether AC6 is expressed in osteocytes in vivo, and if so, whether mechanical loading results in primary cilium- and AC6- dependent decreases in cAMP in bone in vivo. Interestingly, however, Rodan et al.(57) showed that isolated tibiae from chick embryos subjected to hydrostatic pressure exhibit a rapid and significant decrease in cAMP levels. More recently, Tang et al.(50) developed an AC6-null mouse, and found that tibial lengths were not significantly different compared to wild-type controls. While quantification of finer morphometric indices of bone structure were not performed, a mechanosensory role for AC6 may only be revealed by mechanically challenging these mice, (e.g., tibial/ulnar loading or hind limb suspension), as previous studies have demonstrated that deletion of proteins that impair skeletal mechanosensitivity does not always result in an obvious skeletal phenotype (58, 59).
In summary, we have identified a molecular mechanism linking primary cilia and bone cell mechanotransduction that may lead to new molecular targets for pharmacological therapies for bone loss. Given the likelihood that signal transduction at the primary cilium involves common signaling components in diverse cell types, the early flow-induced AC6- and primary cilium-dependent signaling events elucidated by this study not only advance our understanding of how primary cilia regulate mechanotransduction in bone, but suggest further investigation of the roles of AC6 and cAMP in cilium-mediated signal transduction in other tissues as well.
Acknowledgments
The authors thank Drs. Tim Stearns and Charlie Anderson for helpful discussions. This study was supported by the Department of Veterans Affairs, the National Institutes of Health (grants AR045989 and AR054156 to C.R.J.), a National Science Foundation Graduate Fellowship (to R.Y.K.), and a Department of Veterans Affairs Pre-Doctoral Associated Health Rehabilitation Research Fellowship (to S.T.).
References
- Ingber D. E. Mechanobiology and diseases of mechanotransduction. Ann Med. 2003;35:1–14. doi: 10.1080/07853890310016333. [DOI] [PubMed] [Google Scholar]
- Knothe Tate M. L. “Whither flows the fluid in bone?” An osteocyte’s perspective. J Biomech. 2003;36:1409–1424. doi: 10.1016/s0021-9290(03)00123-4. [DOI] [PubMed] [Google Scholar]
- Malone A. M., Anderson C. T., Tummala P., Kwon R. Y., Johnston T. R., Stearns T., Jacobs C. R. Primary cilia mediate mechanosensing in bone cells by a calcium-independent pathway. Proc Natl Acad Sci U S A. 2007;104:13325–13330. doi: 10.1073/pnas.0700636104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Nulend J., Semeins C. M., Ajubi N. E., Nijweide P. J., Burger E. H. Pulsating fluid flow increases nitric oxide (NO) synthesis by osteocytes but not periosteal fibroblasts—correlation with prostaglandin upregulation. Biochem Biophys Res Commun. 1995;217:640–648. doi: 10.1006/bbrc.1995.2822. [DOI] [PubMed] [Google Scholar]
- Vezeridis P. S., Semeins C. M., Chen Q., Klein-Nulend J. Osteocytes subjected to pulsating fluid flow regulate osteoblast proliferation and differentiation. Biochem Biophys Res Commun. 2006;348:1082–1088. doi: 10.1016/j.bbrc.2006.07.146. [DOI] [PubMed] [Google Scholar]
- You J., Reilly G. C., Zhen X., Yellowley C. E., Chen Q., Donahue H. J., Jacobs C. R. Osteopontin gene regulation by oscillatory fluid flow via intracellular calcium mobilization and activation of mitogen-activated protein kinase in MC3T3–E1 osteoblasts. J Biol Chem. 2001;276:13365–13371. doi: 10.1074/jbc.M009846200. [DOI] [PubMed] [Google Scholar]
- You L., Temiyasathit S., Lee P., Kim C. H., Tummala P., Yao W., Kingery W., Malone A. M., Kwon R. Y., Jacobs C. R. Osteocytes as mechanosensors in the inhibition of bone resorption due to mechanical loading. Bone. 2008;42:172–179. doi: 10.1016/j.bone.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S. D., de Vries T. J., Kuijpers-Jagtman A. M., Semeins C. M., Everts V., Klein-Nulend J. Osteocytes subjected to fluid flow inhibit osteoclast formation and bone resorption. Bone. 2007;41:745–751. doi: 10.1016/j.bone.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Qin Y. X., Kaplan T., Saldanha A., Rubin C. Fluid pressure gradients, arising from oscillations in intramedullary pressure, is correlated with the formation of bone and inhibition of intracortical porosity. J Biomech. 2003;36:1427–1437. doi: 10.1016/s0021-9290(03)00127-1. [DOI] [PubMed] [Google Scholar]
- Kwon R. Y., Meays D. R., Tang W. J., Frangos J. A. Microfluidic enhancement of intramedullary pressure increases interstitial fluid flow and inhibits bone loss in hindlimb suspended mice [E-pub ahead of print] J Bone Miner Res. 2010 doi: 10.1002/jbmr.74. doi: 10.1002/jbmr.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradilone S. A., Masyuk A. I., Splinter P. L., Banales J. M., Huang B. Q., Tietz P. S., Masyuk T. V., LaRusso N. F. Cholangiocyte cilia express TRPV4 and detect changes in luminal tonicity inducing bicarbonate secretion. Proc Natl Acad Sci U S A. 2007;104:19138–19143. doi: 10.1073/pnas.0705964104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka S., Yoshiba S., Watanabe D., Ikeuchi S., Goto T., Marshall W. F., Hamada H. De novo formation of left-right assymetry by posterior tilt of nodal cilia. Public Library Sci Biol. 2005;3:e268. doi: 10.1371/journal.pbio.0030268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murcia N. S., Richards W. G., Yoder B. K., Mucenski M. L., Dunlap J. R., Woychik R. P. The Oak Ridge Polycystic Kidney (orpk) disease gene is required for left-right axis determination. Development. 2000;127:2347–2355. doi: 10.1242/dev.127.11.2347. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Murcia N. S., Chittenden L. R., Richards W. G., Michaud E. J., Woychik R. P., Yoder B. K. Loss of the Tg737 protein results in skeletal patterning defects. Dev Dyn. 2003;227:78–90. doi: 10.1002/dvdy.10289. [DOI] [PubMed] [Google Scholar]
- Davenport J. R., Yoder B. K. An incredible decade for the primary cilium: a look at a once-forgotten organelle. Am J Physiol Renal Physiol. 2005;289:F1159–F1169. doi: 10.1152/ajprenal.00118.2005. [DOI] [PubMed] [Google Scholar]
- Yoder B. K. Role of primary cilia in the pathogenesis of polycystic kidney disease. J Am Soc Nephrol. 2007;18:1381–1388. doi: 10.1681/ASN.2006111215. [DOI] [PubMed] [Google Scholar]
- Händel M., Schulz S., Stanarius A., Schreff M., Erdtmann-Vourliotis M., Schmidt H., Wolf G., Höllt V. Selective targeting of somatostatin receptor 3 to neuronal cilia. Neuroscience. 1999;89:909–926. doi: 10.1016/s0306-4522(98)00354-6. [DOI] [PubMed] [Google Scholar]
- Schneider L., Clement C. A., Teilmann S. C., Pazour G. J., Hoffmann E. K., Satir P., Christensen S. T. PDGFRaa signaling is regulated through the primary cilium in fibroblasts. Curr Biol. 2005;15:1861–1866. doi: 10.1016/j.cub.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Rohatgi R., Milenkovic L., Scott M. P. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- Köttgen M., Buchholz B., Garcia-Gonzalez M. A., Kotsis F., Fu X., Doerken M., Boehlke C., Steffl D., Tauber R., Wegierski T., Nitschke R., Suzuki M., Kramer-Zucker A., Germino G. G., Watnick T., Prenen J., Nilius B., Kuehn E. W., Walz G. TRPP2 and TRPV4 form a polymodal sensory channel complex. J Cell Biol. 2008;182:437–447. doi: 10.1083/jcb.200805124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauli S. M., Alenghat F. J., Luo Y., Williams E., Vassilev P., Li X., Elia A. E., Lu W., Brown E. M., Quinn S. J., Ingber D. E., Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- Praetorius H. A., Spring K. R. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol. 2001;184:71–79. doi: 10.1007/s00232-001-0075-4. [DOI] [PubMed] [Google Scholar]
- Masyuk A. I., Masyuk T. V., Splinter P. L., Huang B. Q., Stroope A. J., LaRusso N. F. Cholangiocyte cilia detect changes in luminal fluid flow and transmit them into intracellular Ca2+ and cAMP signaling. Gastroenterology. 2006;131:911–920. doi: 10.1053/j.gastro.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauli S. M., Kawanabe Y., Kaminski J. J., Pearce W. J., Ingber D. E., Zhou J. Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation. 2008;117:1161–1171. doi: 10.1161/CIRCULATIONAHA.107.710111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J., Somlo S., Makova S., Tian X., Brueckner M. Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell. 2003;114:61–73. doi: 10.1016/s0092-8674(03)00511-7. [DOI] [PubMed] [Google Scholar]
- Altschul S. F., Madden T. L., Schäffer A. A., Zhang J., Zhang Z., Miller W., Lipman D. J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop G. A., Berbari N. F., Lewis J., Myktyn K. Type III adenylyl cyclase localizes to primary cilia throughout the adult mouse brain. J Comp Neurol. 2007;505:562–571. doi: 10.1002/cne.21510. [DOI] [PubMed] [Google Scholar]
- Johnston C. A., Beazely M. A., Bilodeau M. L., Andrisani O., Watts V. J. Differentiation-induced alterations in cyclic AMP signaling in the Catha differentiated (CAD) neuronal cell line. J Neurochem. 2004;88:1497–1508. doi: 10.1046/j.1471-4159.2004.02285.x. [DOI] [PubMed] [Google Scholar]
- Gorbunova Y. V., Spitzer N. C. Dynamic interactions of cyclic AMP transients and spontaneous Ca2+ spikes. Nature. 2002;418:93–96. doi: 10.1038/nature00835. [DOI] [PubMed] [Google Scholar]
- Dunn T. A., Wang C. C. T., Colicos M. A., Zaccolo M., DiPilato L. M., Zhang J., Tsien R. Y., Feller M. B. Imaging of cAMP levels and protein kinase A activity reveals that retinal waves drive oscillations in second-messenger cascades. J Neurosci. 2006;26:12807–12815. doi: 10.1523/JNEUROSCI.3238-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole D. G., Diener D. R., Himelblau A. L., Beech P. L., Fuster J. C., Rosenbaum J. L. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J Cell Biol. 1998;141:993–1008. doi: 10.1083/jcb.141.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taulman P. D., Haycraft C. J., Balkovetz D. F., Yoder B. K. Polaris, a protein involved in left-right axis patterning, localizes to basal bodies and cilia. Mol Biol Cell. 2001;12:589–599. doi: 10.1091/mbc.12.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour G. J., Dickert B. L., Vucica Y., Seeley E. S., Rosenbaum J. L., Witman G. B., Cole D. G. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene Tg737, are required for assembly of cilia and flagella. J Cell Biol. 2000;151:709–718. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycraft C. J., Zhang Q., Song B., Jackson W. S., Detloff P. J., Serra R., Yoder B. K. Intraflagellar transport is essential for endochondral bone formation. Development. 2007;134:307–316. doi: 10.1242/dev.02732. [DOI] [PubMed] [Google Scholar]
- Praetorius H. A., Spring K. R. Removal of the MDCK cell primary cilium abolishes flow sensing. J Membr Biol. 2003;191:69–76. doi: 10.1007/s00232-002-1042-4. [DOI] [PubMed] [Google Scholar]
- Raychowdhury M. K., Ramos A. J., Zhang P., McLaughin M., Dai X. Q., Chen X. Z., Montalbetti N., Del Rocio Cantero M., Ausiello D. A., Cantiello H. F. Vasopressin receptor-mediated functional signaling pathway in primary cilia of renal epithelial cells. Am J Physiol Renal Physiol. 2009;296:F87–F97. doi: 10.1152/ajprenal.90509.2008. [DOI] [PubMed] [Google Scholar]
- Masyuk A. I., Gradilone S. A., Banales J. M., Huang B. Q., Masyuk T. V., Lee S. O., Splinter P. L., Stroope A. J., Larusso N. F. Cholangiocyte primary cilia are chemosensory organelles that detect biliary nucleotides via P2Y12 purinergic receptors. Am J Physiol Gastrointest Liver Physiol. 2008;295:G725–G734. doi: 10.1152/ajpgi.90265.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defer N., Best-Belpomme M., Hanoune J. Tissue specificity and physiological relevance of various isoforms of adenylyl cyclase. Am J Physiol Renal Physiol. 2000;279:F400–F416. doi: 10.1152/ajprenal.2000.279.3.F400. [DOI] [PubMed] [Google Scholar]
- Yoshimura M., Cooper D. M. Cloning and expression of a Ca2+-inhibitable adenylyl cyclase from NCB-20 cells. Proc Natl Acad Sci U S A. 1992;89:6716–6720. doi: 10.1073/pnas.89.15.6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Ryder K. D., Bethel J. A., Ramirez R., Duncan R. L. PTH-induced actin depolymerization increases mechanosensitive channel activity to enhance mechanically stimulated Ca2+ signaling in osteoblasts. J Bone Miner Res. 2006;21:1729–1737. doi: 10.1359/jbmr.060722. [DOI] [PubMed] [Google Scholar]
- Ryder K. D., Duncan R. L. Parathyroid hormone enhances fluid shear-induced [Ca2+](i) signaling in osteoblastic cells through activation of mechanosensitive and voltage-sensitive Ca2+ channels. J Bone Miner Res. 2001;16:240–248. doi: 10.1359/jbmr.2001.16.2.240. [DOI] [PubMed] [Google Scholar]
- Liu D., Genetos D. C., Shao Y., Geist D. J., Li J., Ke H. Z., Turner C. H., Duncan R. L. Activation of extracellular-signal regulated kinase (ERK1/2) by fluid shear is Ca2+- and ATP-dependent in MC3T3–E1 osteoblasts. Bone. 2008;42:644–652. doi: 10.1016/j.bone.2007.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell R. A., Clemo H. F., Baumgarten C. M. Using gadolinium to identify stretch-activated channels: technical considerations. Am J Physiol Cell Physiol. 1998;275:C619–C621. doi: 10.1152/ajpcell.1998.275.2.C619. [DOI] [PubMed] [Google Scholar]
- You J., Yellowley C. E., Donahue H. J., Zhang Y., Chen Q., Jacobs C. R. Substrate deformation levels associated with routine physical activity are less stimulatory to bone cells relative to loading-induced oscillatory fluid flow. J Biomech Eng. 2000;122:387–393. doi: 10.1115/1.1287161. [DOI] [PubMed] [Google Scholar]
- Reich K. M., Gay C. V., Frangos J. A. Fluid shear stress as a mediator of osteoblast cyclic adenosine monophosphate production. J Cell Physiol. 1990;143:100–104. doi: 10.1002/jcp.1041430113. [DOI] [PubMed] [Google Scholar]
- Jacobs C. R., Yellowley C. E., Davis B. R., Zhou Z., Cimbala J. M., Donahue H. J. Differential effect of steady versus oscillating flow on bone cells. J Biomech. 1998;31:969–976. doi: 10.1016/s0021-9290(98)00114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponik S. M., Triplett J. W., Pavalko F. M. Osteoblasts and osteocytes respond differently to oscillatory and unidirectional fluid flow profiles. J Cell Biochem. 2006;100:794–807. doi: 10.1002/jcb.21089. [DOI] [PubMed] [Google Scholar]
- McAllister T. N., Frangos J. A. Steady and transient fluid shear stress stimulate NO release in osteoblasts through distinct biochemical pathways. J Bone Miner Res. 1999;14:930–936. doi: 10.1359/jbmr.1999.14.6.930. [DOI] [PubMed] [Google Scholar]
- Tang T., Gao M. H., Lai N. C., Firth A. L., Takahashi T., Guo T., Yuan J. X., Roth D. M., Hammond H. K. Adenylyl cyclase type 6 deletion decreases left ventricular function via impaired calcium handling. Circulation. 2008;117:61–69. doi: 10.1161/CIRCULATIONAHA.107.730069. [DOI] [PubMed] [Google Scholar]
- Chabardès D., Imbert-Teboul M., Elalouf J.-M. Functional properties of Ca2+-inhibitable type 5 and type 6 adenylyl cyclases and role of Ca2+ increase in the inhibition of intracellular cAMP content. Cell Signal. 1999;11:651–663. doi: 10.1016/s0898-6568(99)00031-5. [DOI] [PubMed] [Google Scholar]
- Willoughby D., Cooper D. M. Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol Rev. 2007;87:965–1010. doi: 10.1152/physrev.00049.2006. [DOI] [PubMed] [Google Scholar]
- Chiono M., Mahey R., Tate G., Cooper D. M. Capacitive Ca2+ entry exclusively inhibits cAMP synthesis in C6–2B glioma cells. J Biol Chem. 1995;270:1149–1155. doi: 10.1074/jbc.270.3.1149. [DOI] [PubMed] [Google Scholar]
- Yoder B. K., Hou X., Guay-Woodford L. M. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol. 2002;13:2508–2516. doi: 10.1097/01.asn.0000029587.47950.25. [DOI] [PubMed] [Google Scholar]
- Wadhwa S., Choudhary S., Voznesensky M., Epstein M., Raisz L., Pilbeam C. Fluid flow induces COX-2 expression in MC3T3–E1 osteoblasts via a PKA signaling pathway. Biochem Biophys Res Commun. 2002;297:46–51. doi: 10.1016/s0006-291x(02)02124-1. [DOI] [PubMed] [Google Scholar]
- Gattone V. H., Wang X., Harris P. C., Torres V. E. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med. 2003;9:1323–1326. doi: 10.1038/nm935. [DOI] [PubMed] [Google Scholar]
- Follit J. A., Tuft R. A., Fogarty K. E., Pazour G. J. The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol Biol Cell. 2006;17:3781–3792. doi: 10.1091/mbc.E06-02-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodan G. A., Bourret L. A., Harvey A., Mensi T. Cyclic AMP and cyclic GMP: mediators of the mechanical effects on bone remodeling. Science. 1975;189:467–469. doi: 10.1126/science.168639. [DOI] [PubMed] [Google Scholar]
- Ishijima M., Tsuji K., Rittling S. R., Yamashita T., Kurosawa H., Denhardt D. T., Nifuji A., Noda M. Resistance to unloading-induced three-dimensional bone loss in osteopontin-deficient mice. J Bone Miner Res. 2002;17:661–667. doi: 10.1359/jbmr.2002.17.4.661. [DOI] [PubMed] [Google Scholar]
- Phillips J. A., Almeida E. A., Hill E. L., Aguirre J. I., Rivera M. F., Nachbandi I., Wronski T. J., van der Meulen M. C., Globus R. K. Role for beta1 integrins in cortical osteocytes during acute musculoskeletal disuse. Matrix Biol. 2008;27:609–618. doi: 10.1016/j.matbio.2008.05.003. [DOI] [PubMed] [Google Scholar]