Abstract
In atherogenesis, macrophage foam cell formation is modulated by pathways involving both the uptake and efflux of cholesterol. We recently showed that interleukin-10 (IL-10) modulates lipid metabolism by enhancing both uptake and efflux of cholesterol in macrophages. However, the mechanistic details of these properties in vivo have been unclear. Thus, the purpose of this study was to determine whether expression of IL-10 in macrophages would alter susceptibility to atherosclerosis and whether IL-10 exerts its antiatherosclerotic properties by modulating lipid metabolism in macrophages. We utilized a macrophage-specific retroviral vector that allows long-term in vivo expression of IL-10 in macrophages through transplantation of retrovirally transduced bone marrow cells (BMCs). IL-10 expressed by macrophages derived from transduced BMCs inhibited atherosclerosis in LDLR−/− mice by reducing cholesteryl ester accumulation in atherosclerotic sites. Experiments with primary macrophages indicated that macrophage source of IL-10 stimulated both the uptake (by up-regulating scavenger receptors) and efflux of cholesterol (by activating the PPARγ-LXR-ABCA1/ABCG1 pathway), thereby reducing inflammation and apoptosis in atherosclerosis. These findings indicate that BMC-transduced macrophage IL-10 production can act as a strong antiatherogenic agent, and they highlight a novel antiatherosclerotic therapy using a simple, yet effective, stem cell transduction system that facilitates long-term expression of IL-10 in macrophages.—Han, X., Kitamoto, S., Wang, H., Boisvert, W. A. Interleukin-10 overexpression in macrophages suppresses atherosclerosis in hyperlipidemic mice.
Keywords: macrophage-specific retroviral transduction, foam cell formation, cholesterol efflux, inflammation, apoptosis
Macrophages are an important cell type in atherosclerosis. Excessive uptake of atherogenic lipoproteins such as modified low-density lipoprotein (LDL) through scavenger receptors such as CD36 and scavenger receptor (SR) A, types I (SR-I) and II (SR-II), leads to macrophage foam cell formation (1) and eventually to the accumulation of these cells in the atherosclerosis-prone artery wall (2). During advanced stages of atherosclerosis, lipid-laden foam cells can die and cause local inflammation, as well as increased risk of rupture, which may ultimately lead to acute thrombotic events (3).
Conversely, macrophages can unload their excess cellular cholesterol stores via lipid efflux, a protective reverse cholesterol transport pathway. Molecules participating in this pathway, including peroxisome proliferator-activated receptor (PPAR) γ (4, 5), liver X receptor (LXR) α, ATP-binding cassette (ABC) A1 (5), and ABCG1 (6) are highly expressed in macrophages. PPARγ may promote cholesterol efflux by inducing LXRα expression (7), and activation of PPARγ/LXR pathway up-regulates the expression of genes involved in macrophage cholesterol efflux such as ABCA1 and ABCG1 (5, 8). ABCA1 mediates the efflux of cellular cholesterol to extracellular acceptors such as lipid-poor apoA1 (9), whereas ABCG1 facilitates macrophage cholesterol efflux to HDL (10). Disruption or overexpression of ABCA1 and ABCG1 in mice and humans profoundly affect homeostasis in cholesterol-loaded foam cells, as well as the development of atherosclerosis (10,11,12,13).
IL-10, a prototypical anti-inflammatory cytokine, is produced primarily by macrophages and lymphocytes. Its principal roles are to inhibit macrophage activation (by inhibiting NF-κB) and cell death, as well as to limit the production of matrix metalloproteinase (MMP) and proinflammatory cytokines (14). IL-10 has been demonstrated within human atherosclerotic plaques (15), primarily in macrophages and is implicated in atherogenesis (16). However, IL-10 produced by macrophages and its precise role in macrophage foam cell formation and the initiation and development of atherosclerosis has not been clearly elucidated. Although the anti-inflammatory and antiapoptotic effects of IL-10 are well established, our recent results show that IL-10 also has a powerful impact on macrophage cholesterol metabolism by stimulating both the uptake of cholesterol from modified lipoproteins, as well as the efflux of cholesterol from the cell (17). These findings led us to design and investigate whether sustained overexpression of IL-10 by the macrophages in the lesion environment utilizing a macrophage-specific retroviral vector that allows in vivo expression of genes in macrophages through transplantation of retrovirally transduced bone marrow cells (BMCs) would influence the local lipoprotein metabolism in the vasculature and ultimately alter susceptibility to atherosclerosis.
MATERIALS AND METHODS
Antibodies and reagents
Recombinant mouse interleukin-10, lipoprotein-deficient serum (human), and ApopTag Plus Peroxidase In Situ Apoptosis Detection Kit (cat. no. S7101) were purchased from Chemicon International (Temecula, CA, USA). Acetylated low-density lipoprotein (AcLDL), Acetylated low-density lipoprotein labeled with 1,1-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate(Dil-AcLDL), and apolipoprotein AI (apoAI) were purchased from Biomedical Technologies (Stoughton, MA, USA). Rabbit anti-human CD36 (100011) and cholesterol assay kit (10007640) were obtained from Cayman Chemical (Ann Arbor, MI). Rabbit antiactin (I-19) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Western Lightning Chemiluminescence Reagent Plus (NEL104) was purchased from PerkinElmer Life Science (Boston, MA, USA). Restore Western blot stripping buffer was from Pierce (Rockford, IL, USA). Superscript III reverse transcriptase, NuPAGE Novex 4–12% bis-Tris Gel, RNase Out recombinant ribonuclease inhibitor, Alex Fluor 546-conjugated secondary antibody, and BODIPY 493/503 (D-3922) were purchased from Invitrogen (San Diego, CA, USA). 1α,2α[N]-3H-cholesterol (C8794), AEC staining kit, and albumin from bovine serum (fatty acid free; FAFB) were purchased from Sigma-Aldrich (St. Louis, MO, USA). L-type TG H reagents for triglyceride measurement were from Wako Chemicals (Richmond, VA, USA). Recombinant murine interleukin-6 and interleukin-3 were purchased from ProSpec-Tany TechnoGene (Rehovot, Israel). Recombinant mouse M-CSF was from R&D Systems (Minneapolis, MN, USA). Mouse IL-10 ELISA kit was obtained from eBioscience (San Diego, CA, USA). Phoenix retroviral expression system was purchased from Orbigen (San Diego, CA, USA). Anti-mouse monocyte/macrophage monoclonal rat antibody (MOMA-2) was from Biosource International (Camarillo, CA, USA).
Retroviral vector generation
The construct of CD68S-based retroviruses expressing either HA-EGFP (CD68S-HA-EGFP, as control) or IL-10 (CD68S-IL-10) were generated by means of standard molecular biology techniques and were verified by DNA sequencing. The CD68S-HA-EGFP vector was constructed as described previously (18) and was a generous gift from Dr. E. Raines (University of Washington, Seattle, WA, USA). The vector (CD68-HA-EGFP) enables the direct macrophage-specific expression of an enhanced green fluorescent protein (EGFP) reporter gene and offers a promising new tool for analyzing macrophage function. Through the transplantation of transduced murine BMCs, it has been shown that levels of EGFP expression generated by this vector were greater than those generated by a standard Moloney murine leukemia retroviral vector and were stable for at least a year after transplantation of transduced BMCs (18). The cDNA encoding murine IL-10 was generated by PCR, using pc3.1 IL-10 vector from American Type Culture Collection (ATCC; Rockville, MD, USA) as a template. The full-length murine IL-10 cDNA was cloned into the CD68S-containing retroviral vector CD68S-HA-EGFP that has been digested with NotI to remove the HA-EGFP cDNA. High-titer retroviral supernatants were generated by selecting transfected Phoenix amphotropic and ecotropic packaging cell lines in medium containing 2 μg/ml puromycin. RAW264.7 murine macrophages and stem cells were transduced as described previously (19).
Animal models, bone marrow transduction, and transplantation
LDLR−/− mice in this experiment were obtained from Jackson Laboratory (Bar Harbor, ME, USA). BMCs were harvested from male 6-wk-old LDLR−/− donor mice and were transduced with macrophage-specific retroviral vectors encoding HA-EGFP (CD68S-HA-EGFP) or IL-10 (CD68S–IL-10) as described before (18, 19). After a total of 48 h of transduction, adherent and nonadherent cells were harvested, washed twice in PBS, and 1 × 106 transduced cells were injected into lethally irradiated (10.5-Gy single dose) recipient LDLR−/− male mice at 6–8 wk of age via the retro-orbital plexus. After bone marrow transplantation (BMT), the mice were allowed to repopulate with the donor marrow for 4 wk and then given the atherogenic diet containing 15.8% (wt/wt) fat and 1.25% cholesterol (no cholate, diet 94059; Harlan Teklad, Madison, WI, USA) for 12 wk. Blood was taken every 4 wk for lipid analysis and IL-10 production in the circulation. The mice were maintained in the Harvard Medical School animal facilities. The Standing Committee on Animals at Harvard Medical School approved all protocols pertaining to experimentation with animals.
Analysis of atherosclerosis and other measurements in plasma
Mice were sacrificed 16 wk after transplantation and perfused via the left ventricle with 10 ml PBS containing 1 mM EDTA and 30 ml fixative (PBS, 4% paraformaldehyde and 5% sucrose) (n=8/group). The heart and aortic tree were dissected and fixed overnight. The atherosclerosis was analyzed in the heart aortic valve, as well as the entire length of the aorta as described previously (20). The sections were stained with Oil Red-O to reveal the lesions, and the neointima stained bright red was quantitated using a computer-assisted imaging system. The plasma collected from retro-orbital bleeding of the mice was used for several different assays. First, plasma total cholesterol and triglyceride levels were analyzed to see whether IL-10 and its receptor have an effect on these circulating lipids. Second, plasma concentrations of IL-10 were examined using an ELISA kit, according to the manufacturer’s recommendations.
IL-10 ELISA
Total circulating IL-10 was measured in the plasma of the mice at 0, 4, 8, and 12 wk on the atherogenic diet utilizing a Mouse IL-10 Quantikine Immunoassay kit (eBiosciences). Cell culture supernatant from either transduced RAW264.7 cells or transduced BMCs, which were then differentiated into macrophages, were analyzed to determine the secreted IL-10 concentration by ELISA. The mouse macrophage cell line RAW264.7 (ATCC) was transduced with either CD68-HA-EGFP or CD68-IL-10 retrovirus. The transduced cells were maintained in vitro in growth medium (DMEM containing 10% FBS, 10 mM l-glutamine, 10 mM HEPES, and 100 U/ml of penicillin/streptomycin) for indicated time and refreshed with fresh growth medium every 48 h. For BMCs, cells were isolated and transduced as described for transplantation (18, 19). Portion of the transduced cells were cultured ex vivo for IL-10 protein determination after a 24-h incubation. The transduced BMCs were incubated with growth medium containing 30% L929 culture supernatant (as a source of CSF-1),10% FBS, 10 mM l-glutamine, 10 mM HEPES, and 100 U/ml of penicillin/streptomycin for 4 d to differentiate into macrophages as described previously (21). BMCs from either control or IL-10 Tg mice were isolated at the time of sacrifice and differentiated into macrophages as described above. The differentiated macrophages were maintained in the same growth medium for indicated time and were refreshed every 48 h. Expression of IL-10 by either RAW264.7 cells or BMCs transduced with either medium alone, CD68-HA-EGFP (control) or CD68S–IL-10 retroviruses were assessed by ELISA from macrophages cultured either in vitro (for RAW264.7 cells) or ex vivo [for bone marrow-derived macrophages (BMDMs)] for 24 h.
RNA extraction and analysis
After transduction of bone marrow cells with retrovirus as described above, the cells were differentiated with macrophage DMEM medium containing 20% L929 culture supernatant plus 10% FCS plus antibiotics until the macrophages were confluent. The cells were then incubated with or without AcLDL (50 μg/ml) for 24 h in DMEM with 0.2% FAFB. Total cellular RNA was extracted from macrophages using RNABee (Tel-Test, Friendswood, TX, USA) and subjected to RNA purification using RNeasy mini kit (Qiagen). Real-time quantitative RT-PCR (q-PCR) was performed using the ABI Prism 7700 (Applied Biosystems, Foster City, CA, USA) SYBR Green analysis. The sequence-specified PCR primers for SR-I, SR-II, CD36, ABCA1, ABCG1, apoE, PPARγ, LXRα, LXRβ, MCP-1, TNF-α, MMP-9, TIMP-1, and GAPDH are described below. The gene products were quantified using SYBR Green assays (Applied Biosystems). GAPDH mRNA signal was used for normalization.
Sequence-specific PCR primers
SR-I (forward: 5′TTAAAGGTGATCGGGGACAAA3′; reverse: 5′-CAACCAGTCGAACTGTCTTAAG-3′); SR-II (forward: 5′-TTAAAGGTGATCGGGGACAAA-3′; reverse: 5′-AGCTGATCTTAAAAGGGTCTTG-3′); CD36 (forward: 5′-CAAGCTCCTTGGCATGGTAGA-3′; reverse: 5′-TGGATTTGCAAGCACAATATGAA-3′); PPARγ (forward: 5′-GCAGCTACTGCATGTGATCAAGA-3′; reverse: 5′-GTCAGCGGGTGGGACTTTC-3′); LXRα (forward: 5′- AGGAGTGTCGACTTCGCAAA-3′; reverse: 5′-CTCTTCTTGCCGCTTCAGTTT-3′); LXRβ (forward: 5′-AAGCAGGTGCCAGGGTTCT-3′; reverse: 5′-TGCATTCTGTCTCGTGGTTGT-3′); ABCA1 (forward: 5′-CCCAGAGCAAAAAGCGACTC-3′; reverse: 5′-GGTCATCATCACTTTGGTCCTTG-3′); ABCG1 (forward: 5′-CAAGACCCTTTTGAAAGGGATCTC-3′; reverse: 5′-GCCAGAATATTCATGAGTGTGGAC-3′); MCP-1 (forward: 5′-TCACTGAAGCCAGCTCTCTCT-3′; reverse: 5′-GTGGGGCGTTAACTGCAT-3′); TNF-α (forward: 5′-CTGTAGCCCACGTCGTAGC-3′; reverse: 5′-GGTTGTCTTTGAGATCCATGC-3′); MMP-9 (forward: 5′-TTCTGGCACACGCCTTTC-3′; reverse: 5′-CCATAGTAAGTGGGGATCACG-3′); TIMP-1 (forward: 5′-TACGCCTACACCCCAGTCAT-3′; reverse: 5′-ATGTGCAAATTTCCGTTCCT-3′); GAPDH (forward: 5′-GGCAAATTCAACGGCACAGT-3′; reverse: 5′-CGCTCCTGGAAGATGGTGAT-3′).
Immunohistochemistry
Immunohistochemical characterization of the lesions was performed using antibodies recognizing rat anti-mouse MOMA-2 monoclonal antibody for monocytes/macrophages, rabbit anti-mouse IL-10, and rabbit anti-mouse CD3. Primary antibodies were incubated for 1 h at 37°C, with the exception of anti-IL-10, which was incubated for 16 h at 4°C. As negative controls, adjacent sections were handled similarly but incubated with irrelevant primary IgG of the same species followed by the identical treatment. Immunohistochemistry was performed on 5-μm-thick cryostat sections, fixed for 5 min in acetone at 4°C. The tissues were incubated with an HRP-conjugated second antibody and visualized with DAB chromogen (Vector Laboratories, Burlingame, CA) as described previously (20).
Western blot analysis
Cells were lysed in SDS sample buffer, and 40 μg of protein was run on NuPAGE Novex 4–12% bis-Tris Gel and transferred to PVDF membrane. Blots were blocked in 5% nonfat dry milk in TBS containing 1% Tween-20 (TBS-T), and incubated with primary antibody (1:250 rabbit anti-human CD36) at 4°C overnight. Blots were washed in TBS-T, incubated with appropriate HRP-conjugated secondary antibody (1:10,000 dilution) for 45 min and developed with Western Lightning Chemiluminescence Reagent Plus. The membranes were stripped with Restore Western blot stripping buffer and were reprobed with an anti-actin antibody before being quantified by densitometry.
Analysis of circulating plasma cholesterol and triglyceride
The circulating cholesterol level was determined by the enzymatic, fluorometric method, using “cholesterol assay kit” from Cayman Chemical (Ann Arbor, MI, USA), as described in the instructions. An enzymatic assay was used to quantitate the triglyceride content in plasma as described by Tong et al.(22).
Binding and uptake of AcLDL
To determine the receptor-specific binding and uptake of modified LDL, fluorescence-labeled acetylated LDL (Dil-AcLDL) was used as described previously (23). BMCs were transduced with either control or IL-10 virus and were then differentiated into BMDMs as described above. BMDMs seeded on chamber slide (Nalge Nunc International, Rochester, NY, USA) were incubated with 10 μg/ml Dil-AcLDL in medium for 2 h at 37°C. The medium containing Dil-AcLDL was removed from culture, and the cells were washed twice with probe-free medium. The cells were visualized using a standard rhodamine excitation:emission filter set. Fluorescence intensity was photographed using Nikon C1 Confocal System (Nikon, Tokyo, Japan) and quantified using ImageJ software (U.S. National Institutes of Health, Bethesda, MD, USA).
Detection of lipid accumulation in atherosclerotic lesion
For detecting lipid accumulation in lesions, Oil Red-O staining and BODIPY staining techniques were employed as described previously (24),(25). The frozen sections from either control or IL-10 Tg mice were fixed with paraformaldehyde (PFA) (4% PFA in PBS) for 30 min at room temperature. The Oil Red-O-stained lipids were morphologically evaluated by microscopy. Accumulated lipid was also identified and visualized by BODIPY staining. Intimal lipid accumulation in aortic lesion was assessed by comparing the BODIPY staining patterns. For BODIPY fluorescence staining, slides with sections were rinsed twice with PBS followed by incubation with 1.5 mg/ml glycine/ml PBS for 10 min at room temperature to quench the PFA. The slides were stained with BODIPY 493/503 (10 μg/ml in PBS) working solution for 2 h at room temperature and then rinsed twice with PBS and mounted on the slides. Confocal imaging was recorded in a LSM 510 META (Carl Zeiss Microimaging, Jena, Germany) using an excitation wavelength of 488 nm and an emission wavelength of 505 nm for BODIPY.
Cholesterol efflux
BMDMs were seeded in 24-well plates for incubation overnight and then labeled with loading medium [DMEM/glutamine/p/s/0.2% FAFB, AcLDL (50 μg/ml), 1 μCi/μl of [3H] cholesterol] with or without IL-10 (10 ng/ml) for 36 h. The cells were washed twice with PBS after which DMEM/glutamine/0.2% FAFB ± IL-10 (10 ng/ml) was added into each well and incubated for 1–2 h. For apoAI-independent cholesterol efflux assay, efflux medium [DMEM/glutamine/0.2% FAFB, ± IL-10 (10 ng/ml)] without apoAI was added to one set of wells. For apoAI-dependent cholesterol efflux assay, efflux medium mentioned above with 20 μg/ml of apoAI was added to another set of wells. After incubation for 8 h, 100 μl of medium was removed and transferred to a 1.5 ml Eppendorf tube. Cell debris was spun down at full speed for 5 min, and the supernatant was gently transferred to 5-ml counting fluid to measure radioactivity as effluxed cholesterol (5 min program). At the end of the experiment, 0.5 ml of 0.1 N NaOH was added for 5 h at room temperature to lyse the cells. 100 μl of lysed cell material was transferred to 5 ml counting fluid to measure radioactivity. apoAI-induced [3H]cholesterol efflux was measured as the fraction of total radiolabeled cholesterol appearing in the medium in the presence of apoAI after subtracting the values for apoAI-free medium. ApoAI-mediated efflux was determined using the following formulas: 1) % efflux = efflux/total labeled cholesterol; 2) apoAI specific efflux = % efflux (with apoAI) − % efflux (without apoAI); 3) % increase in apoAI-mediated efflux = %(apoAI specific efflux)/% efflux (without apoAI).
TUNEL assay
DNA fragmentation in apoptotic cells on aortic sinus tissue sections was assessed by the TUNEL assay. Then TUNEL reactions were performed using ApopTag Plus Peroxidase In Situ Apoptosis Detection Kit (S7101; Chemicon International), according to the manufacturer’s instructions. TUNEL-positive macrophages were identified using a fluorescence microscope.
Statistics
Statistical comparisons between groups were performed with Prism software (GraphPad, San Diego, CA, USA). Data are presented as means ± se. The statistical significance of differences between groups was analyzed by Student’s t test in all cases except aortic sinus lesions, which were analyzed using the Mann-Whitney U-test. Differences were considered significant at a value of P < 0.05.
RESULTS
Retroviral vectors can drive macrophage-specific IL-10 expression in macrophages
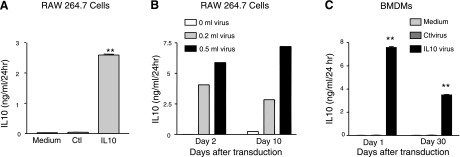
Because the IL-10 transgene in our murine construct is under the control of the CD68 promoter, the expression of transgene-derived IL-10 is restricted to differentiated macrophages (18, 19). To assess the level of IL-10 expression generated by retroviral vectors encoding IL-10, we performed IL-10 ELISA. IL-10 protein expression was detected in supernatant from either transduced RAW264.7 cells (Fig. 1A, B) or BMDMs (Fig. 1C) cultured ex vivo for 24 h. In a pilot experiment, the transduced RAW264.7 cells generated a high yield of IL-10 protein in a retrovirus titration-dependent manner (Fig. 1B). Transduced BMCs differentiated into macrophages produced considerable amounts of IL-10 even 30 d after the transduction (Fig. 1C).
Figure 1.
CD68S–IL-10 vector-driven expression of IL-10 in macrophages. A) IL-10 expression in RAW264.7 cells transduced with either medium alone, CD68-HA-EGFP (control) or CD68S–IL-10 retroviruses was assessed by ELISA from macrophages cultured in vitro for 24 h. B) IL-10 expression in RAW264.7 cells transduced with various concentrations of CD68S–IL-10 retrovirus stocks was assessed by ELISA from macrophages cultured in vitro for 24 h. Supernatants from the 2nd and 10th day from the time of transduction were collected for IL-10 ELISA. C) IL-10 expression in BMDMs. BMCs from LDLR−/− mice were transduced with either medium alone, CD68-HA-EGFP (control) or CD68S–IL-10 retroviruses stocks and differentiated into macrophages as described in Materials and Methods. IL-10 protein in supernatant was assessed by ELISA from macrophages cultured ex vivo for 24 h. Supernatants from d 1 and d 30 after infection were collected for IL-10 ELISA assay. **P < 0.01 vs. control group.
Macrophage-specific expression of IL-10 reduces atherosclerosis
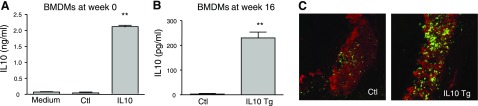
Bone marrow transduction and transplantation were performed as described in Materials and Methods. A portion of the BMCs acquired for transplantation was cultured and differentiated into macrophages for another 48 h to determine the extent to which IL-10 remains highly expressed in macrophages. The medium from differentiated macrophages collected for IL-10 measurement after culture for 24 h showed that considerable amount of IL-10 protein was produced by differentiated macrophages (Fig. 2A). Although there was abundant IL-10 production in the freshly transduced BMCs cultured for 48 h, we questioned whether the expression remained high even several months after transplantation. Thus, stem cells from sacrificed mice were isolated and differentiated into macrophages to test whether high level of IL-10 expression persisted even 16 wk after transplantation. Expression of IL-10 protein at wk 16, although lower than at wk 0, was 90-fold higher in macrophages from mice receiving BMCs transduced with CD68S-HA-IL-10 compared to macrophages from CD68-HA-EGFP group (Fig. 2B).
Figure 2.
Macrophage-specific expression of IL-10. A, B) BMCs were transduced with either CD68-HA-EGFP (control) or CD68S–IL-10 retroviruses. Before transplantation, a portion of the cells was cultured and differentiated into macrophages (A). At 16 wk after transplantation into donor mice, BMCs were isolated and differentiated into macrophages (B). IL-10 levels in supernatant were assessed by ELISA from macrophages cultured ex vivo for 24 h. **P < 0.01 vs. control groups. C) Analysis of IL-10 expression in atherosclerotic lesions. Immunohistochemistry using anti-MOMA-2 and anti-IL-10 antibodies revealed a more intense IL-10 staining (green fluorescence) in MOMA-2-positive regions (red fluorescence) of aortic sinus from IL-10 Tg mice than from control mice.
To examine whether expression of IL-10 by macrophages had any effect on circulating IL-10 levels, plasma from control and IL-10 Tg mice were collected at 0, 4, 8, and 12 wk on the atherogenic diet to measure IL-10 levels by ELISA. There was no detectable IL-10 in plasma of either group at any of the time points (data not shown), suggesting that circulating monocytes did not produce any measurable IL-10 in the IL-10 Tg mice and that IL-10 was produced locally by differentiated macrophages, including those in the lesion. We also examined whether IL-10 expression was increased locally in macrophages within atherosclerotic lesions. Immunofluorescence analysis of IL-10 was performed in aortic lesions from both control and IL-10 Tg mice, predominantly in macrophages (Fig. 2C). Because IL-10 is known to be expressed in atherosclerotic lesions (15), it was not surprising that IL-10 was detected in aortic lesions from both control and IL-10 Tg mice (Fig. 2C). However, IL-10 staining, which largely colocalized with macrophage staining, was much more intense in lesions from IL-10 Tg mice than from control mice (Fig. 2C). This suggests that retroviral vectors can drive macrophage-specific IL-10 expression in atherosclerotic lesions for long periods of time.
Because the T lymphocyte is another important source for IL-10 (14), we wondered if T lymphocytes would also contribute to the IL-10 production locally in aortic lesions. Immunohistochemical analysis using various antibodies against T cells failed to detect any T cells in the aortic lesions from control and IL-10 Tg mice, although T cells in spleen sections were clearly detected using the same technique (data not shown). This suggests that T lymphocytes did not contribute to the IL-10 production in the lesion. Although T lymphocytes are present in significant numbers early in lesion development in LDL-R−/− mice, the presence of intralesional T lymphocytes has been reported to decrease as lesions progress over time (26). Another possible explanation for the lack of T cells in our model is that overexpression of IL-10 by the macrophages may have altered the distribution of T cells in the lesion area. Our previous study indicates that overexpression of IL-10 by T cells inhibits advanced atherosclerotic lesions in mice by slowing activation of monocytes and marked shift to Th2 phenotype (20). In the current study, the possibility exists that IL-10 expressed by local macrophages in lesions may also have influenced T-cell chemotaxis, as well as its function.
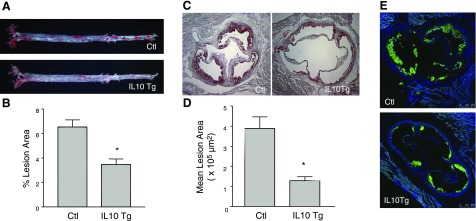
Atherosclerosis was assessed in mice fed the atherogenic diet for 12 wk. The percent area of aorta covered with atherosclerotic lesions was determined by en face analysis. Figure 3A shows representative images of aortae obtained from control LDLR−/− and IL-10 Tg/LDLR−/− mice. A marked decrease in atherosclerotic lesion area was observed in the entire length of the aorta from IL-10 Tg/LDLR−/− mice (Fig. 3A). Quantification of the surface area occupied by atherosclerotic lesions (Fig. 3B) showed that, compared with control mice, transgenic expression of IL-10 in IL-10 Tg/LDLR−/− mice led to a significant decrease in atherosclerotic lesions in the aortae (6.54±1.29 vs. 3.46±0.93%, P<0.05).
Figure 3.
IL-10 Tg mice have reduced atherosclerosis compared with control mice. Aortae (aortic arch to the iliac bifurcation) from control and IL-10 Tg mice fed an atherogenic diet for 12 wk were opened and stained en face with Oil Red-O. A) Representative images of the aortae showing the surface plaque. B) Quantification of surface area occupied by lesions in the entire length of the aortae. Data (% area occupied by lesions) are expressed as means ± se for each group. *P < 0.05. C, D) Aortic sinus atherosclerosis in control and IL-10 Tg mice. Atherosclerotic lesion area in the aortic sinus was quantified from serial sections stained with Oil Red-O and counterstained with hematoxylin. C) Representative Oil Red-O-stained sections of aortic valves from control and IL-10 Tg mice 16 wk after BMT. Cryosections were stained with Oil Red-O to reveal the neutral lipids in the lesions (×100). D) Aortic sinus lesion area of control and IL-10 Tg mice. *P < 0.05. E) Macrophage distribution in aortic sinus lesions. Sections were stained with MOMA-2 antibody and Alexa 488-labeled secondary antibody and observed under fluorescence microscope. Sections were counterstained with DAPI to locate nuclei of cells.
Assessment of atherosclerosis in the aortic sinus showed similar results. Figure 3C displays representative images of aortic sinus obtained from control LDLR−/− and IL-10 Tg/LDLR−/− mice. Quantification of the Oil Red-O-stained lesion area revealed a significant reduction in atherosclerotic lesion development in the IL-10 Tg/LDLR−/− mice (Fig. 3D), compared with control LDLR−/− mice (393,899±57,361 vs. 128,974±4839 μm2; P<0.05). Measurement of circulating plasma lipid levels before (0 wk) and after BMT (4, 8, 12 wk) revealed that there were no significant differences in either plasma cholesterol or triglyceride between the two groups at any of the time points (data not shown). Thus, circulating cholesterol and triglyceride levels did not account for the decrease in atherosclerotic lesion surface area in IL-10 Tg mice in this study.
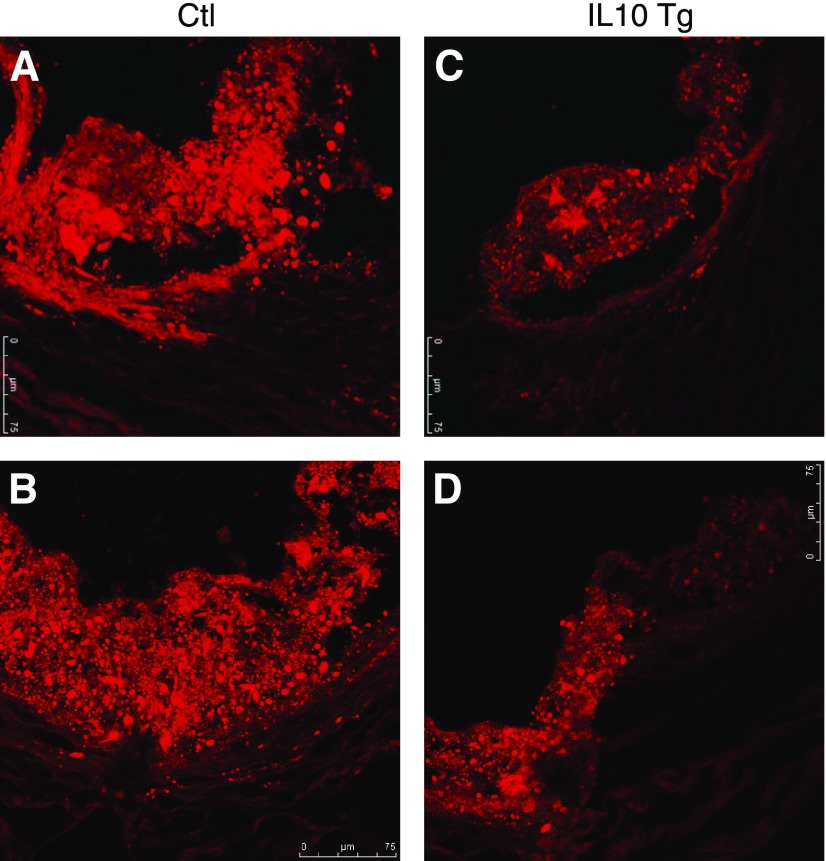
Immunohistochemical detection of lesion macrophages indicated that macrophage-rich areas were smaller in the IL-10 Tg/ LDLR−/− mice in proportion to the lesion area (Fig. 3E). Analysis of the aortic sinus using Oil Red-O and fluorescent BODIPY to stain neutral lipids, including esterified cholesterol (27) showed a considerably reduced concentration of lipid accumulation in the IL-10 Tg mice compared with control mice (Fig. 4), suggesting a protective role of IL-10 in macrophages in modulation of lipid metabolism within the vessel wall. These findings indicate that macrophage-specific IL-10 expression attenuated diet-induced atherosclerosis in the LDLR−/− mice.
Figure 4.
IL-10 overexpression in macrophages decreases neutral lipid accumulation in frozen sections of aortic sinus. Neutral lipid in aortic lesions was stained with BODIPY (view ×250). BODIPY staining in aortic lesions from either control (A, B) or IL-10 Tg mice (C, D) shows that there was much less neutral lipid accumulation in the lesions of IL-10 Tg mice.
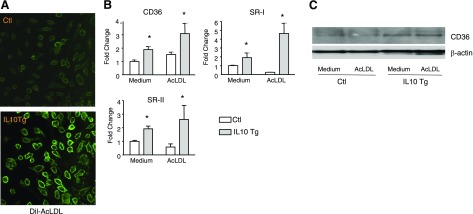
IL-10 up-regulates scavenger receptor expression and increases the uptake of modified LDL in macrophages
To examine the role of IL-10 in macrophage cholesterol uptake, Dil-labeled acetylated LDL (AcLDL) uptake was performed in BMDM infected with either control or IL-10 retrovirus. IL-10 expression significantly increased the Dil-AcLDL uptake by BMDM (Fig. 5A). In accordance with our recent data indicating that IL-10 promotes the uptake of modified LDL through up-regulation of both SRA and CD36 scavenger receptor expression in macrophages (17), real-time PCR analysis in the present study showed that IL-10 expression stimulated the expression of SR-I, SR-II, and CD36, the scavenger receptors that mediate the uptake of modified LDL (Fig. 5B). We also verified that CD36 protein levels were induced by IL-10 in these cells (Fig. 5C). These results suggested that the IL-10-stimulated expression of scavenger receptors likely accounted for the enhanced uptake of modified LDL in macrophages.
Figure 5.
IL-10 increases Dil-AcLDL uptake and up-regulates expression of scavenger receptors. A) BMDMs infected with either control or IL-10 retrovirus were loaded with AcLDL for 24 h and viewed under confocal microscopy to determine the extent of Dil-AcLDL uptake. B) Transcripts for scavenger receptors were detected by real-time PCR. BMDMs were infected with either EGFP or IL-10 retroviruses and differentiated into macrophages. BMDMs were then treated with either medium alone (blank column) or AcLDL (gray column) for 24 h. Results are representative of 3 independent experiments; values are expressed as mean ± se fold change in abundance. *P < 0.05 vs. control. C) CD36 protein was detected by Western blot analysis. Cells were infected and treated as in B. CD36 protein was detected using an anti-CD36 antibody as described in Materials and Methods.
IL-10 induces cholesterol efflux through PPARγ-LXR-ABCA1/ABCG1 activation
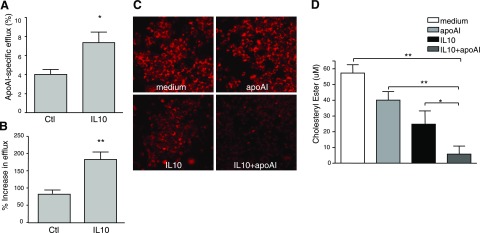
Next, we determined whether IL-10 plays a role in cholesterol efflux in the presence of a cholesterol acceptor such as lipid-free apoAI. We incubated macrophages with AcLDL for 36 h to induce cholesteryl ester accumulation. These cholesterol-laden cells were then exposed to lipid-free apoAI to induce cholesterol efflux in the presence or absence of IL-10. Treatment with IL-10 significantly increased apoAI-mediated cholesterol efflux from cholesterol-loaded macrophages (Fig. 6).
Figure 6.
A, B) IL-10 increases lipid-free apoAI-mediated reverse cholesterol transport. BMDMs were labeled with [3H]cholesterol for 36 h in the presence or absence of 10 ng/ml IL-10. ApoAI-dependent or -independent cholesterol efflux was measured by incubating [3H]cholesterol-labeled macrophages with or without lipid-free apoAI (20 μg/ml) for the indicated times. Radioactivity in the medium was determined as a percentage of total radioactivity in the cells and medium (n=3, mean±se). A) apoAI-specific efflux at 8 h. B) Increase in apoAI-mediated efflux by IL-10 at 8 h.. *P < 0.05, **P < 0.01 vs. control. C, D) IL-10 decreases lipid accumulation in the presence of apoAI. To obtain foam cells, RAW264.7 cells were incubated with AcLDL (50 μg/ml) for 36 h. Lipid-laden foam cells were then treated with either medium, apoAI (20 μg/ml), IL-10 (10 ng/ml), or IL-10 plus apoAI for another 24 h. Intracellular lipid accumulation was evaluated by either BODIPY staining (C) or measurement of intracellular cholesterol ester content (D) as described in Materials and Methods.
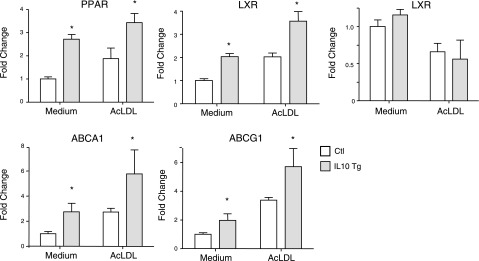
Because PPARγ, LXR, ABCA1, and ABCG1 are well-established participants in cholesterol efflux, we sought to determine whether the expression of these genes was influenced by IL-10 expression in BMDMs. Real-time PCR results demonstrated that PPARγ, LXRα (but not LXRβ), ABCA1, and ABCG1 mRNA levels in cells treated with either medium or AcLDL were significantly up-regulated in IL-10-overexpressing macrophages compared with control macrophages (Fig. 7). This indicates that the enhanced cholesterol efflux is likely due to increased PPARγ-LXRα activation leading to a more robust expression of the cholesterol transporters, ABCA1 and ABCG1.
Figure 7.
IL-10 activates PPARγ-LXR-ABCA1/ABCG1 pathway. BMDMs infected with either control or IL-10 retrovirus were incubated with either medium or AcLDL for 24 h before RNA isolation. Transcripts for PPARγ, LXRα, LXRβ, ABCA1, and ABCG1 were quantitated by real-time PCR. *P < 0.05 vs. control.
IL-10 expression in macrophages leads to reduced apoptosis in lesion
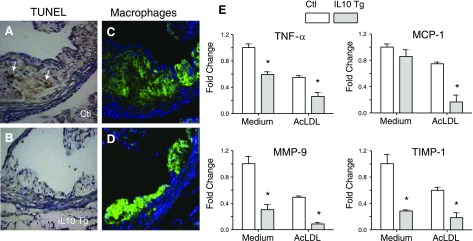
Microscopic analysis showed that the lesions of control mice were considerably more advanced, with enhanced presence of lipid-laden cells, fibrous caps and necrotic cores within the atherosclerotic lesions (Fig. 8A, C). In contrast, lesions in IL-10 Tg group were less developed and devoid of necrotic cores (Fig. 8B, D). To examine the extent of apoptosis in these lesions, we performed TUNEL staining in the aortic sinus lesions. There were occasional TUNEL-positive cells detected in the MOMA-2-positive lesion areas of the control group; however, lesions of the IL-10 Tg group displayed almost no TUNEL-positive cells (Fig. 8A–D). These data demonstrate that IL-10 expression in macrophages helped to prevent cell death and necrosis of lesion-associated foam cells.
Figure 8.
IL-10 decreases apoptosis and suppresses expression of proinflammatory genes. A–D) Decreased necrosis/apoptosis of macrophages in lesions from IL-10 Tg (B, D) compared with control mice (A, C). A, B) Serial sections were subjected to TUNEL assay as described in Materials and Methods. Arrows indicate TUNEL-positive nuclei. C, D) A second set of serial sections was immunostained with antibody to macrophage marker MOMA-2 (green fluorescence). Sections were counterstained with DAPI to mark nuclei of cells; images were merged to show colocalization. E) IL-10 suppresses expression of proinflammatory genes. Transcripts for TNF-α, MCP-1, MMP-9, and TIMP-1 were detected by real-time PCR. BMDMs were infected with either control or IL-10 retrovirus, followed by incubation with medium (open column) or AcLDL (gray column) for 24 h prior to RNA isolation. The results are representative of at least 3 independent experiments, and values are expressed as fold change in abundance (±se). *P < 0.05 vs. control.
IL-10 inhibits expression of proinflammatory molecules in macrophages
An important antiatherogenic mechanism of IL-10 is its ability to inhibit the expression of inflammatory mediators. To investigate whether the protective role of IL-10 involves regulation of proinflammatory molecules in IL-10-overexpressing macrophages, the expression patterns of TNF-α, MCP-1, MMP-9, and TIMP-1, all of which have been implicated in the pathogenesis of atherosclerosis (2), were examined using real-time PCR (Fig. 8E). There was a significant suppression of all four inflammatory molecules by IL-10 whether AcLDL was added or not, suggesting that IL-10, despite its role in promoting cholesterol uptake (which can have proinflammatory consequences), is effective in inhibiting the transcription of proinflammatory molecules in macrophages.
To examine whether the IL-10 expressed by macrophages functions in an autocrine or paracrine fashion, we treated macrophages with conditioned medium from either control or IL-10 retrovirus-infected macrophages and measured the expression of scavenger receptors and proinflammatory mediators, as well as cholesterol uptake. Our data show that conditioned medium from IL-10 retrovirus-infected macrophages stimulated the expression of scavenger receptors, while suppressing the expression of proinflammatory mediators (Supplemental Fig. 1) and enhancing the uptake of Dil-acLDL by the macrophages (Supplemental Fig. 2). These results suggest that IL-10 expressed by macrophages is capable of acting on the neighboring cells and acting in a paracrine fashion.
DISCUSSION
Despite previous reports by us and others highlighting the antiatherogenic properties of IL-10 (16, 28,29,30), the precise role of IL-10 in an in vivo setting has not been clearly defined. Because macrophages play a pivotal role during all stages of atherosclerosis, and because IL-10 is known to have many anti-inflammatory effects on the macrophage (14), our studies have focused on how IL-10 modulates macrophage function pertaining to atherosclerosis, such as cholesterol metabolism, production of inflammatory mediators, and the propensity of these cells to undergo apoptosis. Using a macrophage-specific system of long-term IL-10 overexpression, we sought to determine whether expression of this cytokine by the macrophages in the lesion area could lead to attenuation of atherosclerosis. Our results show that IL-10 overexpression by macrophages inhibits atherogenesis. This is likely due to IL-10’s ability to enhance lipid metabolism in macrophage foam cells, as well as dampen inflammation and apoptosis.
Apart from our current study, most other strategies of enhancing IL-10 in transgenic mice had systemic effects on multiple cells, including T cells, monocytes, and endothelial cells, resulting from an increase of IL-10 in the circulation (16, 20, 28, 29, 31, 32). For example, overexpression of IL-10 in activated T lymphocytes caused a decrease in activation of monocyte and a shift to either Th2 phenotype in some cases (20) or Th1 phenotype in other cases (33). IL-10 has diverse effects on most hematopoietic cell types. It inhibits activation and effector function of T cells, monocytes, and macrophages, as well as regulates growth and/or differentiation of B cells, NK cells, cytotoxic and helper T cells, mast cells, granulocytes, dendritic cells, keratinocytes, and endothelial cells (14). Thus, circulating IL-10 can influence the function of several immune cells and may affect the development of atherosclerosis. Importantly, an increase in circulating IL-10 can also influence plasma cholesterol (16, 29, 32), which can, in turn, affect atherogenesis. Thus, participation of other immune cells influenced by circulating IL-10, as well as lowering of plasma cholesterol are important confounding factors to consider when evaluating the role of IL-10 in atherosclerosis in these studies. In contrast, our approach of macrophage-specific expression of IL-10 driven by CD68 promoter allows the local expression of IL-10 by macrophages in the lesion area without affecting circulating levels of IL-10. This makes our technique ideal to evaluate the role of macrophage-produced IL-10 in local lesion areas. Our results indicate that local IL-10 overexpression can suppress the development of atherosclerosis despite ongoing hyperlipidemia.
An interesting finding in our study is that IL-10 increased the expression of scavenger receptors and led to enhanced cholesterol uptake by the macrophages. In addition, we have recently found that exogenous IL-10 also promotes the uptake of modified LDL through up-regulation of both SRA and CD36 scavenger receptor expression in macrophages (17). Scavenger receptors such as SRA and CD36 mediate the uptake of lipoproteins that result in foam cell formation. Although this is traditionally considered to be proatherogenic, there are lines of evidence to suggest that foam cell formation mediated by uptake of cholesterol from modified lipoproteins via scavenger receptor may not play a causal role in the atherosclerotic pathogenesis. For example, comparisons of atherosclerosis-prone and -resistant rabbit models have shown that SRA is increased in macrophages of rabbits with low atherosclerotic response (34). Overexpression of human SRA in either the LDLR−/− or apoE−/− mouse model can attenuate atherosclerotic lesion development (35, 36). Furthermore, SRA deficiency on apoE3-Leiden background results in similar or even more severe atherosclerosis (37). A recent finding suggests that loss of lipid uptake via CD36 may increase extracellular lipid accumulation and/or contribute to apoptosis of macrophage foam cells, leading to the formation of necrotic core in atherosclerotic lesion areas (38). This suggests that lipid uptake by SRs in these mice may protect against atherosclerosis. In fact, the deposition and retention of serum lipoproteins in the artery wall, where they are susceptible to modification through oxidization and enzymatic action, are believed to activate an immune response that initiates the chronic inflammatory cascade that characterizes atherosclerosis (39). Our studies suggest that IL-10-induced scavenger receptor activity and uptake of modified lipoprotein by macrophages may be beneficial for retarding early atherosclerotic lesion development by removing the proinflammatory modified LDLs from the artery wall.
Cholesterol homeostasis within macrophages is maintained by modulating both the uptake of modified lipid via the scavenger receptors and cholesterol efflux via the reverse cholesterol transport pathway. As an important mechanism that acts to protect cells from toxic effects of free cholesterol, cholesterol loading in macrophage results in up-regulation of ABCA1 and ABCG1 expression by activation of nuclear receptor LXR and/or PPARγ (5, 8, 10). Both ABCA1 (40) and ABCG1 (10) play an important role in the removal of lipid from cholesterol-loaded macrophages of the coronary plaque in the arterial wall. The physiological importance of ABCA1 in cholesterol efflux has been established from studies of patients who suffer from Tangier’s Disease due to loss-of-function mutations in the ABCA1 gene (11). The disruption of either ABCA1 (41) or ABCG1 (10) causes a massive lipid accumulation in macrophages and leads to more severe atherosclerosis (13, 41).
Our results indicate that, in the presence of cholesterol acceptors, such as apoAI, IL-10 enhances the efflux of cellular cholesterol, leading to a decrease in excess lipid accumulation within foam cells. Because activation of PPARγ-LXR-ABCA1/ABCG1 pathway inhibits the formation of atherosclerotic lesions by stimulating cholesterol efflux, and because IL-10 stimulates reverse cholesterol transport by up-regulating ABCA1 and ABCG1 in macrophages (42), it is likely that the activation of this pathway in response to IL-10 promoted the efflux of cholesterol from the arterial wall macrophage foam cells and contributed to less severe atherosclerosis in our IL-10 Tg mice. In fact, recent data from our laboratory indicate that IL-10 promotes not only the uptake of modified LDL via CD36 and SRA, but also the cholesterol efflux via PPARγ-ABCA1 pathway using a physiologically relevant model of lipoprotein-induced foam cells (17). The notion that IL-10 can mediate both the uptake of cholesterol from modified lipoproteins and the efflux of stored cholesterol suggests a protective role of IL-10 in effective removal of both harmful modified lipoprotein particles from the vessel wall and cytotoxic free cholesterol accumulation within lipid-laden foam cells.
Increased foam cell apoptosis induces the formation of lipid-laden necrotic cores during atherogenesis, possibly contributing to plaque destabilization (3). Increased local levels of IL-10 have been associated with decreased apoptotic activity within diseased arteries (15, 20) and in modified lipoprotein-stimulated macrophages (24). In agreement with these studies, our results suggest that IL-10 expression in macrophages can indeed curb the propensity of the lesion cells to undergo apoptosis and form necrotic cores, as seen in the atherosclerotic lesions of our IL-10 Tg mice.
Another way in which macrophages play a central role in atherogenesis is through the accumulation of modified LDL and the production of various inflammatory mediators, such as cytokines and extracellular matrix (ECM)-degrading enzymes (2). Because excessive inflammation within the arterial wall is a major risk factor for atherogenesis (2), the ability of IL-10 to limit inflammation in this setting would be beneficial in reducing the progression of atherosclerosis. Additionally, both matrix metalloproteinases (MMPs) such as MMP-9 and its endogenous inhibitor TIMP-1 are highly expressed in vulnerable regions of human atherosclerotic plaques, promoting destabilization and complication of atherosclerotic plaques (43). Several studies have suggested that MMP-9 plays a causative role in plaque rupture (19, 44). Given that IL-10 can modulate ECM by regulating the expression of MMPs, as well as TIMP (45), it may be that the antiatherogenic effect of IL-10 in macrophages was at least partially mediated by the inhibition of MMPs and TIMP in the atherosclerotic plaques.
Our use of CD68S-based retroviral vectors to overexpress IL-10 at high levels by macrophages in atherosclerotic lesions has greatly facilitated the elucidation of the role of IL-10 expression in atherogenesis. The present studies have shown that the technique of transplanting retrovirally transduced BMCs into LDLR−/− mice is a valuable approach for studying IL-10 function specifically in atherosclerotic lesions. These results, coupled with previous studies, suggest that in the future, clinical use of these high-efficiency, macrophage-specific retroviral vectors may offer genetic therapies for atherosclerosis.
In summary, IL-10 expression in macrophages enhanced not only the uptake of proinflammatory modified LDL, but also activated the PPARγ-LXR-ABCA1/ABCG1 pathway to transport excess cellular cholesterol from tissue macrophages. The net result of IL-10’s action in macrophages may be efficient removal of the harmful modified lipoproteins from the artery wall and disposal of cytotoxic free cholesterol, thereby decreasing inflammation and apoptosis in the lesion. The delivery of IL-10 by stem cell transplantation may provide a novel treatment for atherosclerosis given its apparent effectiveness in attenuating the progression of the disease.
Acknowledgments
The authors thank E. Raines (University of Washington, Seattle, WA, USA) for providing the CD68S-containing retroviral vector, as well as for valuable comments. The authors are grateful to D. Ahl for assistance with BMT. This study was supported by National Institutes of Health grant HL075677 (to W.A.B.).
References
- Kunjathoor V. V., Febbraio M., Podrez E. A., Moore K. J., Andersson L., Koehn S., Rhee J. S., Silverstein R., Hoff H. F., Freeman M. W. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J Biol Chem. 2002;277:49982–49988. doi: 10.1074/jbc.M209649200. [DOI] [PubMed] [Google Scholar]
- Glass C. K., Witztum J. L. Atherosclerosis the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- Tabas I. Consequences of cellular cholesterol accumulation: basic concepts and physiological implications. J Clin Invest. 2002;110:905–911. doi: 10.1172/JCI16452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klappacher G. W., Glass C. K. Roles of peroxisome proliferator-activated receptor gamma in lipid homeostasis and inflammatory responses of macrophages. Curr Opin Lipidol. 2002;13:305–312. doi: 10.1097/00041433-200206000-00011. [DOI] [PubMed] [Google Scholar]
- Chawla A., Boisvert W. A., Lee C. H., Laffitte B. A., Barak Y., Joseph S. B., Liao D., Nagy L., Edwards P. A., Curtiss L. K., Evans R. M., Tontonoz P. A PPAR γ-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol Cell. 2001;7:161–171. doi: 10.1016/s1097-2765(01)00164-2. [DOI] [PubMed] [Google Scholar]
- Wang N., Lan D., Chen W., Matsuura F., Tall A. R. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc Natl Acad Sci U S A. 2004;101:9774–9779. doi: 10.1073/pnas.0403506101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A. C., Glass C. K. The macrophage foam cell as a target for therapeutic intervention. Nat Med. 2002;8:1235–1242. doi: 10.1038/nm1102-1235. [DOI] [PubMed] [Google Scholar]
- Li A. C., Binder C. J., Gutierrez A., Brown K. K., Plotkin C. R., Pattison J. W., Valledor A. F., Davis R. A., Willson T. M., Witztum J. L., Palinski W., Glass C. K. Differential inhibition of macrophage foam-cell formation and atherosclerosis in mice by PPARα, β/δ, and γ. J Clin Invest. 2004;114:1564–1576. doi: 10.1172/JCI18730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Bortnick A. E., Nickel M., Dhanasekaran P., Subbaiah P. V., Lund-Katz S., Rothblat G. H., Phillips M. C. Effects of apolipoprotein A-I on ATP-binding cassette transporter A1-mediated efflux of macrophage phospholipid and cholesterol: formation of nascent high-density lipoprotein particles. J Biol Chem. 2003;278:42976–42984. doi: 10.1074/jbc.M308420200. [DOI] [PubMed] [Google Scholar]
- Kennedy M. A., Barrera G. C., Nakamura K., Baldan A., Tarr P., Fishbein M. C., Frank J., Francone O. L., Edwards P. A. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005;1:121–131. doi: 10.1016/j.cmet.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Rust S., Rosier M., Funke H., Real J., Amoura Z., Piette J. C., Deleuze J. F., Brewer H. B., Duverger N., Denefle P., Assmann G. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat Genet. 1999;22:352–355. doi: 10.1038/11921. [DOI] [PubMed] [Google Scholar]
- Singaraja R. R., Fievet C., Castro G., James E. R., Hennuyer N., Clee S. M., Bissada N., Choy J. C., Fruchart J. C., McManus B. M., Staels B., Hayden M. R. Increased ABCA1 activity protects against atherosclerosis. J Clin Invest. 2002;110:35–42. doi: 10.1172/JCI15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eck M., Bos I. S., Kaminski W. E., Orso E., Rothe G., Twisk J., Bottcher A., Van Amersfoort E. S., Christiansen-Weber T. A., Fung-Leung W. P., Van Berkel T. J., Schmitz G. Leukocyte ABCA1 controls susceptibility to atherosclerosis and macrophage recruitment into tissues. Proc Natl Acad Sci U S A. 2002;99:6298–6303. doi: 10.1073/pnas.092327399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K. W., de Waal Malefyt R., Coffman R. L., O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Mallat Z., Heymes C., Ohan J., Faggin E., Leseche G., Tedgui A. Expression of interleukin-10 in advanced human atherosclerotic plaques: relation to inducible nitric oxide synthase expression and cell death. Arterioscler Thromb Vasc Biol. 1999;19:611–616. doi: 10.1161/01.atv.19.3.611. [DOI] [PubMed] [Google Scholar]
- Von Der Thusen J. H., Kuiper J., Fekkes M. L., De Vos P., Van Berkel T. J., Biessen E. A. Attenuation of atherogenesis by systemic and local adenovirus-mediated gene transfer of interleukin-10 in LDLr-/- mice. FASEB J. 2001;15:2730–2732. doi: 10.1096/fj.01-0483fje. [DOI] [PubMed] [Google Scholar]
- Han X., Kitamoto S., Lian Q., Boisvert W. A. Interleukin-10 facilitates both cholesterol uptake and efflux in macrophages. J Biol Chem. 2009;284:32950–32958. doi: 10.1074/jbc.M109.040899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough P. J., Raines E. W. Gene therapy of apolipoprotein E-deficient mice using a novel macrophage-specific retroviral vector. Blood. 2003;101:485–491. doi: 10.1182/blood-2002-07-2131. [DOI] [PubMed] [Google Scholar]
- Gough P. J., Gomez I. G., Wille P. T., Raines E. W. Macrophage expression of active MMP-9 induces acute plaque disruption in apoE-deficient mice. J Clin Invest. 2006;116:59–69. doi: 10.1172/JCI25074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinderski L. J., Fischbein M. P., Subbanagounder G., Fishbein M. C., Kubo N., Cheroutre H., Curtiss L. K., Berliner J. A., Boisvert W. A. Overexpression of interleukin-10 by activated T lymphocytes inhibits atherosclerosis in LDL receptor-deficient mice by altering lymphocyte and macrophage phenotypes. Circ Res. 2002;90:1064–1071. doi: 10.1161/01.res.0000018941.10726.fa. [DOI] [PubMed] [Google Scholar]
- Boltz-Nitulescu G., Wiltschke C., Holzinger C., Fellinger A., Scheiner O., Gessl A., Forster O. Differentiation of rat bone marrow cells into macrophages under the influence of mouse L929 cell supernatant. J Leukoc Biol. 1987;41:83–91. doi: 10.1002/jlb.41.1.83. [DOI] [PubMed] [Google Scholar]
- Tong H., Knapp H. R., VanRollins M. A low-temperature flotation method to rapidly isolate lipoproteins from plasma. J Lipid Res. 1998;39:1696–1704. [PubMed] [Google Scholar]
- Ricci R., Sumara G., Sumara I., Rozenberg I., Kurrer M., Akhmedov A., Hersberger M., Eriksson U., Eberli F. R., Becher B., Boren J., Chen M., Cybulsky M. I., Moore K. J., Freeman M. W., Wagner E. F., Matter C. M., Luscher T. F. Requirement of JNK2 for scavenger receptor A-mediated foam cell formation in atherogenesis. Science. 2004;306:1558–1561. doi: 10.1126/science.1101909. [DOI] [PubMed] [Google Scholar]
- Halvorsen B., Waehre T., Scholz H., Clausen O. P., von der Thusen J. H., Muller F., Heimli H., Tonstad S., Hall C., Froland S. S., Biessen E. A., Damas J. K., Aukrust P. Interleukin-10 enhances the oxidized LDL-induced foam cell formation of macrophages by antiapoptotic mechanisms. J Lipid Res. 2005;46:211–219. doi: 10.1194/jlr.M400324-JLR200. [DOI] [PubMed] [Google Scholar]
- Gocze P. M., Freeman D. A. Factors underlying the variability of lipid droplet fluorescence in MA-10 Leydig tumor cells. Cytometry. 1994;17:151–158. doi: 10.1002/cyto.990170207. [DOI] [PubMed] [Google Scholar]
- Roselaar S. E., Kakkanathu P. X., Daugherty A. Lymphocyte populations in atherosclerotic lesions of apoE−/− and LDL receptor−/− mice Decreasing density with disease progression. Arterioscler Thromb Vasc Biol. 1996;16:1013–1018. doi: 10.1161/01.atv.16.8.1013. [DOI] [PubMed] [Google Scholar]
- Kruth H. S. Localization of unesterified cholesterol in human atherosclerotic lesions Demonstration of filipin-positive, Oil-Red-O-negative particles. Am J Pathol. 1984;114:201–208. [PMC free article] [PubMed] [Google Scholar]
- Pinderski Oslund L. J., Hedrick C. C., Olvera T., Hagenbaugh A., Territo M., Berliner J. A., Fyfe A. I. Interleukin-10 blocks atherosclerotic events in vitro and in vivo. Arterioscler Thromb Vasc Biol. 1999;19:2847–2853. doi: 10.1161/01.atv.19.12.2847. [DOI] [PubMed] [Google Scholar]
- Yoshioka T., Okada T., Maeda Y., Ikeda U., Shimpo M., Nomoto T., Takeuchi K., Nonaka-Sarukawa M., Ito T., Takahashi M., Matsushita T., Mizukami H., Hanazono Y., Kume A., Ookawara S., Kawano M., Ishibashi S., Shimada K., Ozawa K. Adeno-associated virus vector-mediated interleukin-10 gene transfer inhibits atherosclerosis in apolipoprotein E-deficient mice. Gene Ther. 2004;11:1772–1779. doi: 10.1038/sj.gt.3302348. [DOI] [PubMed] [Google Scholar]
- Caligiuri G., Rudling M., Ollivier V., Jacob M. P., Michel J. B., Hansson G. K., Nicoletti A. Interleukin-10 deficiency increases atherosclerosis, thrombosis, and low-density lipoproteins in apolipoprotein E knockout mice. Mol Med. 2003;9:10–17. [PMC free article] [PubMed] [Google Scholar]
- Mallat Z., Besnard S., Duriez M., Deleuze V., Emmanuel F., Bureau M. F., Soubrier F., Esposito B., Duez H., Fievet C., Staels B., Duverger N., Scherman D., Tedgui A. Protective role of interleukin-10 in atherosclerosis. Circ Res. 1999;85:e17–e24. doi: 10.1161/01.res.85.8.e17. [DOI] [PubMed] [Google Scholar]
- Liu Y., Li D., Chen J., Xie J., Bandyopadhyay S., Zhang D., Nemarkommula A. R., Liu H., Mehta J. L., Hermonat P. L. Inhibition of atherogenesis in LDLR knockout mice by systemic delivery of adeno-associated virus type 2-hIL-10. Atherosclerosis. 2006;188:19–27. doi: 10.1016/j.atherosclerosis.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Zhou X., Paulsson G., Stemme S., Hansson G. K. Hypercholesterolemia is associated with a T helper (Th) 1/Th2 switch of the autoimmune response in atherosclerotic apo E-knockout mice. J Clin Invest. 1998;101:1717–1725. doi: 10.1172/JCI1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teupser D., Stein O., Burkhardt R., Nebendahl K., Stein Y., Thiery J. Scavenger receptor activity is increased in macrophages from rabbits with low atherosclerotic response: studies in normocholesterolemic high and low atherosclerotic response rabbits. Arterioscler Thromb Vasc Biol. 1999;19:1299–1305. doi: 10.1161/01.atv.19.5.1299. [DOI] [PubMed] [Google Scholar]
- Van Eck M., De Winther M. P., Herijgers N., Havekes L. M., Hofker M. H., Groot P. H., Van Berkel T. J. Effect of human scavenger receptor class A overexpression in bone marrow-derived cells on cholesterol levels and atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2000;20:2600–2606. doi: 10.1161/01.atv.20.12.2600. [DOI] [PubMed] [Google Scholar]
- Herijgers N., de Winther M. P., Van Eck M., Havekes L. M., Hofker M. H., Hoogerbrugge P. M., Van Berkel T. J. Effect of human scavenger receptor class A overexpression in bone marrow-derived cells on lipoprotein metabolism and atherosclerosis in low-density lipoprotein receptor knockout mice. J Lipid Res. 2000;41:1402–1409. [PubMed] [Google Scholar]
- De Winther M. P., Gijbels M. J., van Dijk K. W., van Gorp P. J., suzuki H., Kodama T., Frants R. R., Havekes L. M., Hofker M. H. Scavenger receptor deficiency leads to more complex atherosclerotic lesions in APOE3Leiden transgenic mice. Atherosclerosis. 1999;144:315–321. doi: 10.1016/s0021-9150(98)00332-3. [DOI] [PubMed] [Google Scholar]
- Moore K. J., Kunjathoor V. V., Koehn S. L., Manning J. J., Tseng A. A., Silver J. M., McKee M., Freeman M. W. Loss of receptor-mediated lipid uptake via scavenger receptor A or CD36 pathways does not ameliorate atherosclerosis in hyperlipidemic mice. J Clin Invest. 2005;115:2192–2201. doi: 10.1172/JCI24061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K. J., Freeman M. W. Scavenger receptors in atherosclerosis: beyond lipid uptake. Arterioscler Thromb Vasc Biol. 2006;26:1702–1711. doi: 10.1161/01.ATV.0000229218.97976.43. [DOI] [PubMed] [Google Scholar]
- Vaisman B. L., Lambert G., Amar M., Joyce C., Ito T., Shamburek R. D., Cain W. J., Fruchart-Najib J., Neufeld E. D., Remaley A. T., Brewer H. B., Jr, Santamarina-Fojo S. ABCA1 overexpression leads to hyperalphalipoproteinemia and increased biliary cholesterol excretion in transgenic mice. J Clin Invest. 2001;108:303–309. doi: 10.1172/JCI12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello R. J., Brees D., Bourassa P. A., Royer L., Lindsey S., Coskran T., Haghpassand M., Francone O. L. Increased atherosclerosis in hyperlipidemic mice with inactivation of ABCA1 in macrophages. Arterioscler Thromb Vasc Biol. 2002;22:630–637. doi: 10.1161/01.atv.0000014804.35824.da. [DOI] [PubMed] [Google Scholar]
- Rubic T., Lorenz R. L. Downregulated CD36 and oxLDL uptake and stimulated ABCA1/G1 and cholesterol efflux as anti-atherosclerotic mechanisms of interleukin-10. Cardiovasc Res. 2006;69:527–535. doi: 10.1016/j.cardiores.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Galis Z. S., Sukhova G. K., Lark M. W., Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994;94:2493–2503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenberg S., Rupprecht H. J., Poirier O., Bickel C., Smieja M., Hafner G., Meyer J., Cambien F., Tiret L. Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease. Circulation. 2003;107:1579–1585. doi: 10.1161/01.CIR.0000058700.41738.12. [DOI] [PubMed] [Google Scholar]
- Lacraz S., Nicod L. P., Chicheportiche R., Welgus H. G., Dayer J. M. IL-10 inhibits metalloproteinase and stimulates TIMP-1 production in human mononuclear phagocytes. J Clin Invest. 1995;96:2304–2310. doi: 10.1172/JCI118286. [DOI] [PMC free article] [PubMed] [Google Scholar]