Abstract
Medulloblastoma is the most common malignant brain tumor in children, and despite improvements in the overall survival rate, it still lacks an effective treatment. Src plays an important role in cancer, and recently high Src activity was documented in medulloblastoma. In this report, we examined the effects of novel pyrazolo-[3,4-d]-pyrimidine derivative Src inhibitors in medulloblastoma. By MTS assay, we showed that the pyrimidine derivatives indicated as S7, S29, and SI163 greatly reduce the growth rate of medulloblastoma cells by inhibiting Src phosphorylation, compared with HT22 non-neoplastic nerve cells. These compounds also halt cells in the G2/M phase, and this effect likely occurs through the regulation of cdc2 and CDC25C phosphorylation, as shown by Western blot. Moreover, the exposure to pyrimidine derivatives induces apoptosis, assayed by the supravital propidium iodide assay, through modulation of the apoptotic proteins Bax and Bcl2, and inhibits tumor growth in vivo in a mouse model. Notably, S7, S29, and SI163 show major inhibitory effects on medulloblastoma cell growth compared with the chemotherapeutic agents cisplatin and etoposide. In conclusion, our results suggest that S7, S29, and SI163 could be novel attractive candidates for the treatment of medulloblastoma or tumors characterized by high Src activity.—Rossi, A., Schenone, S., Angelucci, A., Cozzi, M., Caracciolo, V., Pentimalli, F., Puca, A., Pucci, B., La Montagna, R., Bologna, M., Botta, M., Giordano, A. New pyrazolo-[3,4-d]-pyrimidine derivative Src kinase inhibitors lead to cell cycle arrest and tumor growth reduction of human medulloblastoma cells.
Keywords: tyrosine kinase inhibitors, apoptosis, cancer therapy, brain tumors, cdc2
Medulloblastomas are the most common cerebellar tumors of the central nervous system in childhood (1). They are neuroepithelial tumors arising from the cerebellum and account for ∼20% of all intracranial tumors and for 40% of all posterior fossa tumors in children. Although the incidence of medulloblastoma peaks at 8 yr of age, ∼30% of tumors occurs in adults (2).
Therapeutic approaches for medulloblastoma have evolved slowly over the past 30 yr and currently are based on surgery, radiotherapy, and chemotherapy. Unfortunately, radiotherapy has devastating effects on intellectual function and on children’s growth as a result of side effects including growth-hormone deficiency, early puberty development, and compromised spinal growth (1, 3). Subsequently, the combination of radiotherapy and chemotherapeutic agents, including ifosfamide, cisplatin, and etoposide, improved the survival rate of 70–80% and also reduced the risk of radiotherapy-related cognitive and endocrine effects (1, 4,5,6).
Despite the improvements in the overall survival rate following the multimodality treatment, a small but substantial number of patients will have a recurrent or progressive disease. Unfortunately, attempts to further reduce the morbidity and mortality associated with medulloblastoma have been limited by the toxicity of conventional treatments and the low permeability of the blood-brain barrier (BBB), which restricts the entry of hydrophilic and large lipophilic compounds into the brain (1, 3). Therefore, it is important to develop innovative therapeutic drugs that can easily cross the BBB in order to achieve higher rates of disease control and fewer neurocognitive toxic effects.
Over the past decade, several discoveries have increased our understanding of medulloblastoma tumor formation, identifying a crucial role for different genes (such as various receptor tyrosine kinases and telomerase). Other crucial findings include alteration of different molecular pathways (such as the hedgehog, Notch and Wnt pathways; refs 1, 7, 8). Lately, Sikkema et al.(9) identified a distinguishing panel of tyrosine kinase profiles associated with pediatric brain tumors such as medulloblastoma, astrocytoma, and ependymoma. In particular, the researchers showed high Src family kinase activity in these tumors, as established by high levels of phosphorylation, with respect to the normal tissues, which suggests that Src could have a key role in the development of medulloblastoma (9).
The Src family kinases (SFKs) comprise a subclass of membrane-associated nonreceptor tyrosine kinases including Src, Yes, Fyn, Lyn, Hck, Blk, Fgr, and Yrk, which are activated in response to cellular signals that promote proliferation, survival, motility, and invasiveness (10, 11). Moreover, Src plays an important role in angiogenesis and metastasis (12). Deregulated Src activity has been implicated in the development and progression of several human cancers, including breast, brain, and leukemia (12, 13, 14). Given that Src has been known to be an important molecular target in cancer, highly specific pharmaceutical compounds are currently available, and a number of Src inhibitors are being investigated in different tumors (15, 16). New pyrazolo-[3,4-d]-pyrimidine derivative Src kinase inhibitors, binding the ATP pocket of the Src kinase, were recently synthesized and demonstrated to have antiproliferative and proapoptotic properties in a broad panel of carcinomas (17, 18, 19, 20, 21, 22).
In this study we examine the effects of novel Src selective tyrosine inhibitors in human medulloblastoma cells. These pyrimidine derivatives showed major inhibitory effects on cell proliferation in two medulloblastoma cell lines compared with non-neoplastic neuronal cells and the ability to inhibit tumor growth in vivo in a xenograft mouse model of medulloblastoma.
MATERIALS AND METHODS
Cell culture
The human medulloblastoma cell lines Daoy and D283-MED were obtained from American Type Culture Collection (ATCC; Manassas, VA, USA), and the HT22 neuronal cell line, derived from mouse hippocampus, was kindly provided by Prof. Khalili (Department of Neuroscience, Center for Neurovirology, Temple University School of Medicine, Philadelphia, PA, USA). These cell lines were cultured in DMEM (CellGro; Mediatech, Herndon, VA, USA) supplemented with 10% fetal bovine serum (Atlanta Biological, Norcross, GA, USA) at 37°C in a humidified atmosphere of 5% CO2 in air, according to the ATCC recommendations.
Cell treatment and MTS assay
The pyrazolo-[3,4-d]-pyrimidine derivative Src kinase inhibitors were synthesized and purified according to the procedure reported in Schenone et al.(23), where compounds were synthesized with methods similar to those used to make SI83, SI91, SI165, S7, S29, and SI163. These pyrimidine derivatives were then dissolved in DMSO to make a stock solution of 25 mM and then diluted in culture medium to final concentrations before use. Daoy cells were seeded in a 96-well tissue culture plate at the initial density of 12,500 cells/well. After 24 h, cells were exposed to 25 μM of compounds SI83, SI91, SI165, S7, S29, and SI163 or to DMSO (control) (Sigma; Sigma-Aldrich, Milan, Italy). An MTS assay (Promega, Milan, Italy) was performed after 48 h of treatment, following the procedures recommended by the manufacturer. The resulting absorbance of each individual well was read at dual wavelengths (540 and 630 nm).
To evaluate the IC50 for S7, S29, and SI163, HT22 cells (8000 cells/well), Daoy and D283-MED cells (25,000 cells/well) were tested with a dose range of the compounds (from 1 to 100 μM) or to DMSO (control), which was used at the concentration corresponding to the highest dose of assayed molecules, and an MTS test was conducted after 48 h of exposure. Furthermore, the medulloblastoma and HT22 cells were exposed to the chemotherapeutic agents cisplatin (Ebewe Pharma, Rome, Italy) and etoposide (Teva Pharma, Milan, Italy) or physiological solution (control) at the same experimental conditions used for the compounds.
Evaluation of combined drug effect
The cytotoxic effects of pyrimidine derivatives were investigated in combination with standard chemoterapeutic agents in medulloblastoma cell lines. Drug combination studies were based on concentration-effect curves generated by plotting the fraction of untreated cells (DMSO) vs. drug concentration, after 48 h of treatment. To evaluate the relative contribution of each drug to the synergism, 9 mixtures of pyrimidine derivatives/chemotherapeutic agents at different molar ratios were tested by MTS assay. Data were analyzed using the Chou-Talalay median-effect method (24). After fitting the combined dose-response curve to a Chou-Talalay line, combination indices (CIs) were calculated. CI = 1 indicates additivity; CI > 1 and CI < 1 indicate antagonism and synergy, respectively.
Cell cycle analysis and apoptosis
To perform FACS analysis, Daoy cells were treated with DMSO or with S7, S29, and SI163 at dose range of 1–100 μM. After 24, 48, and 72 h of exposure, 1 × 106 cells were harvested and fixed in 70% ethanol after washes with cold phosphate-buffered saline (PBS), and then stored at 4°C until the analysis. After centrifugation, the resulting cell pellet was incubated in the dark, in 0.3 ml of freshly prepared PBS containing 0.02 mg/ml propidium iodide (PI) and 0.25 mg/ml ribonuclease A (Sigma). The samples were then analyzed for DNA content using a FACStar Plus flow-cytometer (Becton Dickinson, Franklin Lakes, NJ, USA) (10,000 events/sample).
Apoptosis induction was determined by supravital PI assay (25). Daoy and D283-MED cells were treated with different doses of pyrimidine derivatives and chemotherapeutic agents used either individually or in combination. Cells were incubated during the last 30 min of treatment in the dark with 50 mg/ml PI. The suspended cells were washed with PBS, to remove PI, and then analyzed by flow cytometry. Detached and adherent cells were rinsed twice with PBS and finally pooled and resuspended in PBS until analysis. Apoptotic and necrotic cells were detected as PIdim and PIbright clusters, respectively, by FACStar Plus flow-cytometer (Beckton-Dickinson) (10,000 events/sample).
Furthermore, apoptotic nuclei were visualized by 4,6-diamidino-2-phenylindole (DAPI) staining. After S7, S29, and SI163 treatments, Daoy cells were fixed in 3.7% (v/v) formaldehyde/PBS and permeabilized with 90% methanol/PBS (v/v). The samples were then mounted in Vectashield (Vector Laboratories, Burlingame, CA, USA) containing DAPI, and subsequently analyzed by fluorescence microscopy.
Western blot
Protein extracts were prepared from Daoy cells with or without S7 (15 μM), S29 and SI163 (7.5 μM) for 24, 48, and 72 h. Cells were lysed in ice-cold lysis buffer containing 50 mM Tris-HCL (pH 7.5), 150 mM NaCl, 0.1% SDS, 1% Nonidet P-40, the protease inhibitor cocktail without EDTA (Sigma), 1 mM NaF, 1 mM PMSF, and 1 mM Na3VO4. Proteins (30 μg) were resuspended in 4× Laemmli sample buffer (100 mM Tris-HCl, pH 6.8; 4% SDS; 20% glycerol; 200 mM DTT; and 0.01% bromphenol blue) at a 4:1 ratio, boiled for 5 min, and resolved by 12% SDS-PAGE. The proteins were blotted onto activated PVDF membranes and then probed overnight at 4°C with primary antibodies against β-actin, Bcl-2, Bax, CDC25C (Santa Cruz Biotechnology, Santa Cruz, CA, USA), Src, phospho-SrcY416, cyclin B1, cdc2, phospho-cdc2Y15, and phospho-CDC25C (Ser216; Cell Signaling, Danvers, MA, USA). After 1 h of incubation at room temperature with the corresponding horseradish peroxidase-conjugated secondary antibodies, immunoreactive bands were detected by enhanced chemilumiscence (Amersham Bioscience, Little Chalfont, UK). Equal protein loading was assessed through Ponceau Red (Sigma) staining (not shown) and through analysis of β-actin expression (see Figs. 3 and 4C).
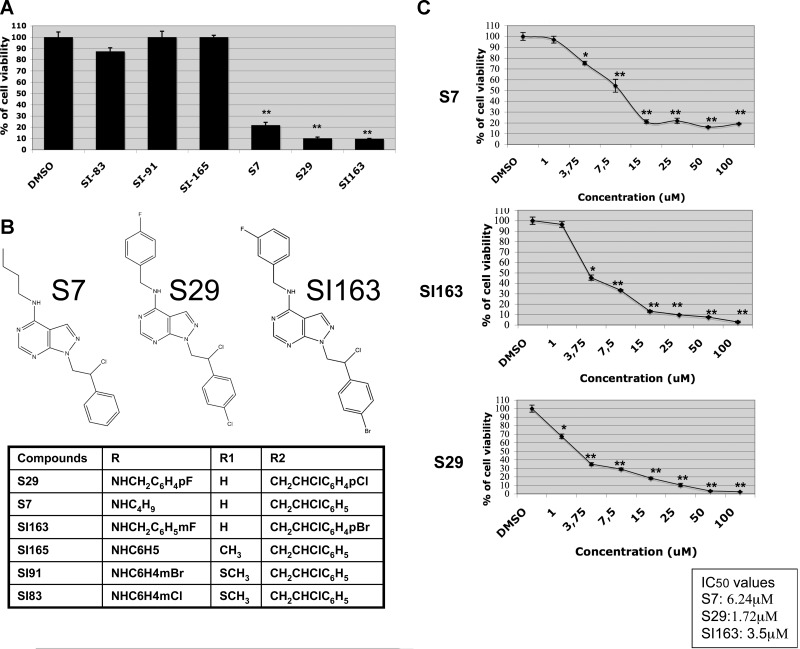
Figure 1.
A) Daoy cells were cultured with 25 μM of different pyrimidine derivatives or in DMSO for 48 h, and cell viability was measured by MTS assay. B) Molecular structure of S7, S29, and SI163. C) Antiproliferative effects of S7, S29, and SI163 were evaluated at a concentration range of 1–100 μM; data are expressed as percentage values compared to control cells. IC50 values were determined for each compound as reported in the graph. Data shown are representative of ≥2 independent experiments. Error bars = sd from mean values. *P < 0.05; **P < 0.01.
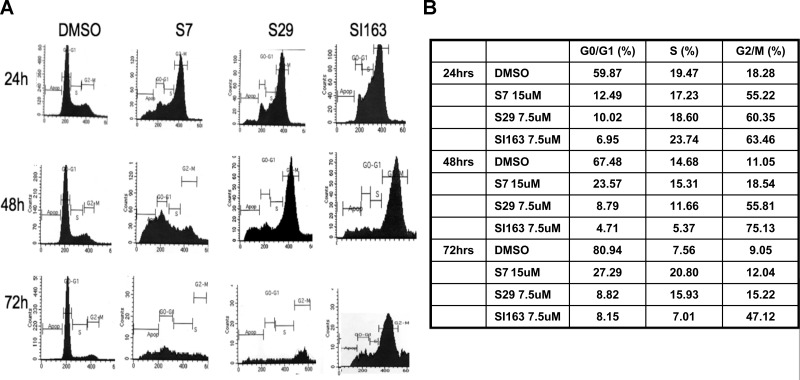
Figure 2.
A) Flow cytrometric analysis in Daoy cells before and after exposure to S7 (15 μM), S29 (7.5 μM) and SI163 (7.5 μM). Treatment induces cell arrest in G2/M phase of the cell cycle after 24 h and cell death at 48 h. B) Percentages of cells in different phases of cell cycle at each time point of treatment. Results are representative of 3 independent experiments.
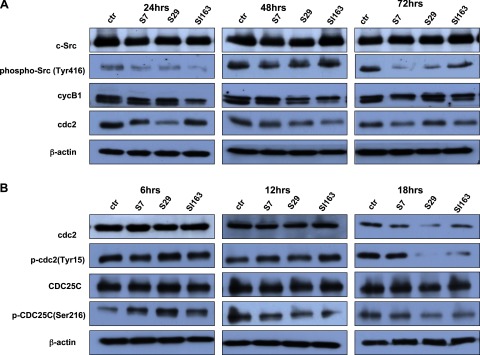
Figure 3.
A) Daoy cells were treated with S7, S29, and SI163, and protein levels of total and phosphorylated c-Src at Y416 were evaluated by Western blot. p-ScrY416 decreased starting from 24 h of treatment, with respect to control. Increased cyclinB1 phosphorylation and cdc2 down-regulation were evident after 24 h of exposure to the compounds. B) S7, S29, and SI163 treatment causes up-regulation of p-cdc2(Tyr15) via the inactivating phosphorylation of CDC25C (Ser216). Results are representative of 3 independent experiments.
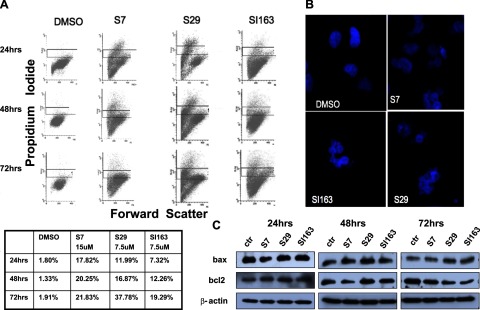
Figure 4.
A) Src inhibitors induce apoptosis in Daoy cells. PI supravital exposure was performed in untreated cells and treated with S7 (15 μM), S29 (7.5 μM), and SI163 (7.5 μM). Percentages of apoptotic cells are reported for each representative time point. B) Nuclear fragmentation, visualized by DAPI staining, was evident following treatment. All photomicrographs were taken with the same magnification. C) Western blot analysis showing bax and bcl2 protein levels in cells before and after exposure to the compounds. Results are representative of 3 independent experiments.
Daoy xenograft
CD1 nude mice (Charles River, Milan, Italy) were maintained under the guidelines established by the University of L’Aquila (Medical School and Science and Technology School Board Regulations, complying with the Italian government regulation n.116 January 27 1992 for the use of laboratory animals). Before tumor cell implantation, mice were anesthesized with a mixture of ketamine (25 mg/ml)/xylazine (5 mg/ml). Xenografts were obtained by injecting s.c. 1 × 106 Daoy cells in 100 μl of 12 mg/ml Matrigel (Becton Dickinson). On a daily basis, mice received the drug by per os administration at 100 mg/kg S29, starting from the first day the tumor was palpable (∼4 mm3). Mice in the control group were treated by per os administration of the drug vehicle (10% v/v 1:1 chremphor/ethanol in saline solution). Tumor growth was monitored daily by measuring the average tumor diameter (2 perpendicular axes of the tumor were measured by a caliper). The volume of the tumor was expressed in cubic millimeters according to the formula 4/3πr3.
Statistical analysis
Data are expressed as means ± sd of 3 independent experiments; statistical significance was evaluated using Student’s t test. For each statistical analysis, an associated value of P < 0.05 was considered significant.
RESULTS
Novel pyrazolo-[3,4-d]-pyrimidines reduce Daoy cell proliferation leading to the G2/M phase arrest
SI83, S7, S29, SI163, SI91, and SI165 are tyrosine kinase inhibitors with a pyrazolo-[3,4-d]-pyrimidine structure. Their selectivity for c-Src was determined by in vitro assays measuring [32P]ATP in peptide substrates, and Src activity inhibition was demonstrated for all compounds analyzed, at nanomolar concentrations (26).
To evaluate the antiproliferative effects of these novel pyrimidine derivatives in human medulloblastoma Daoy cell line, we treated cells with all the compounds at 25 μM dose, according to previous studies (22). Significant antiproliferative effects were demonstrated after S7, S29, and SI163 treatment, showing an inhibition of cell growth rate of 78, 89, and 90%, respectively, compared to vehicle DMSO alone; whereas no effects on cell growth were observed after exposure to SI91, SI83, and SI165 (Fig. 1A).
On the basis of these results, we focused our attention on the role of the most effective pyrimidine derivatives. Our MTS assay demonstrated a dose-dependent inhibitory effect on cell proliferation following 48 h of exposure to the dose range of 1–100 μM. S29 and SI163 were the most active molecules, as shown by the maximum growth rate inhibition of 97%, compared to 80% for S7, and by the IC50 values: 6.24 μM for S7, 1.72 μM for S29, and 3.5 μM for SI163. In particular, the kinetics of cell proliferation inhibition were different for the compounds, probably due to their different affinities for the target. S29 was significantly active starting from 1 μM, resulting in a reduction of 33% of cell proliferation, and its activity gradually increased up to the highest concentration; whereas SI163 was less active at low doses, and its activity increased rapidly at concentrations > 3.75 μM (Fig. 1B, C).
Src family kinases play an important role in regulating mitotic events. Src activity and localization change during mitosis suggesting a function in this cell process (27, 28). To verify if the growth-inhibitory effects of S7, S29, and SI163 were due to a phase-specific inhibition of cell cycle progression, we performed a cytometric analysis before and after exposure to the compounds. The pyrimidine derivatives caused a dramatic cell accumulation in the G2/M phase of the cell cycle (Fig. 2A). This effect was observed beginning from 24 h of treatment at IC50 doses of the compounds (data not shown), but it was more evident at higher doses: at the concentrations of 15 μM for S7 and 7.5 μM for both S29 and SI163, we observed a cell accumulation in the G2/M phase of 55.22, 60.35, and 63.46%, respectively, compared to 18.28% of the control cells (Fig. 2B). After 48 h, drug treatment also induced cell death, and this effect was even more evident after 72 h (Fig. 2A).
According to these results, we chose the concentrations of 15 μM for S7 and 7.5 μM for both S29 and SI163, to conduct the following experiments.
Pyrimidine derivatives inhibit Src-Y416 phosphorylation in Daoy cells
Src activity is thinly regulated by phosphorylation events. In particular, the phosphorylation of Y416 in the activation loop of the kinase domain up-regulates the enzyme activity. However, the phosphorylation of the carboxy-terminal tail by the C-terminal Src kinase (Csk) renders the enzyme less active (29). To verify whether S7, S29, and SI163 influenced Src activity in Daoy cells, we evaluated the phosphorylation state of Src by Western blot before and after treatment with pyrimidine derivatives.
The exposure to all 3 compounds caused a strong decrease of Src phosphorylation at Y416 beginning from 24 h, as reported in Fig. 3A.
Pyrimidine derivative treatment increases cdc2 phosphorylation via the modulation of CDC25C
In order to identify the functional targets of Src kinase at the G2/M phase transition, we analyzed the expression of the mitosis promoting factor (MPF), the cell cycle player regulating this checkpoint. MPF is made up of cdc2 and cyclinB1, which are synthesized in the cytoplasm and move to the nucleus where they can complex to exert their function. Mitosis occurs only when cdc2 and cyclinB1 are expressed and MPF complex is formed in the nucleus (30, 31). Four cyclinB1 phosphorylation sites, located in the cytoplasmic retention signal domain, were identified to regulate its translocation to the nucleus at the G2/M checkpoint (32). Western blot analysis in treated Daoy cells revealed the presence of cyclinB1, mainly in its phosphorylated form, suggesting that cyclinB1 can shuttle to the nucleus, where it can associate with cdc2. Interestingly, S7, S29, and SI163 treatment resulted in the decrease of cdc2 protein levels as early as 24 h (Fig. 3A). These findings suggest that the treatment might induce cell cycle arrest in G2/M phase through the down-regulation of cdc2. To determine whether the observed effect was due to the reduction of cdc2 mRNA transcription, we performed a real-time PCR. We did not detect any modification in cdc2 mRNA levels in treated cells compared to the control (data not shown), suggesting that the observed decrease in cdc2 protein levels, mediated by the drugs, occurred through post-transcriptional mechanisms. The critical step in activating cdc2 during progression into mitosis is its dephosphorylation of Tyr 15, which is done by CDC25C phosphatase (33, 34). Thus, we aimed to determine whether S7, S29, and SI163 treatment could also influence the cdc2 phosphorylation, and as a consequence, its activity. Given that we observed the down-regulation of cdc2 after 24 h of exposure to Src inhibitors, we analyzed its phosphorylation status at earlier time intervals. As reported in Fig. 3B, our results showed an increase of cdc2(Tyr15) phosphorylation after 12 h of exposure to S7 and SI163 or at 6 h for S29. This effect was followed, after 18 h, by the down-regulation of cdc2. The inhibitory phosphorylation of cdc2 on Tyr15, and the decrease in total cdc2 levels following the treatment, suggest that the pyrimidine derivatives can suppress cdc2 activity via its phosphorylation as well as its protein reduction. We then analyzed the phosphorylation of CDC25(Ser216), which is responsible for its nuclear export and the subsequent inhibition of nuclear cdc2 dephosphorylation (33, 34). Although total protein levels of CDC25C were not altered by the treatment, levels of p-CDC25C(Ser216) increased after 6 h. On the basis of these results, we postulated a 2-stage inhibition of mitosis: in the early phase, the increase of p-CDC25C(Ser216) may lead to the high level of cdc2(Tyr15) phosphorylation, thereby inactivating the cdc2/cyclinB1 complex. Even if the levels of p-CDC25C(Ser216) decreased at subsequent time intervals, in the late phase the mitosis could be blocked by cdc2 down-regulation (Fig. 3B).
Pyrimidine derivatives induce apoptosis via the modulation of Bcl2 and Bax proteins
Several studies have highlighted that the antiproliferative activity of Src inhibitors is frequently associated with their ability to induce apoptosis in different tumors (18, 19, 22). As cited above, our FACS analysis results revealed an accumulation of Daoy cells with sub-G1 DNA content, following exposure to the drugs. This finding suggests that cell death may occur as a consequence of the treatment (Fig. 2A). Therefore, we evaluated whether S7, S29, and SI163 induced proapoptotic effects in medulloblastoma cells. We performed a supravital PI assay, as described in Zamai et al.(25), which can distinguish necrotic and apoptotic cells. As reported in Fig. 4A, results revealed an increased apoptotic cell population after 24 h of treatment. Maximal effects were observed at 72 h, showing apoptosis of 37.78% for S29, 21.83% for S7, and 19.29% for SI163, compared to 1.91% for DMSO-treated cells. At lower doses, the compounds induced only a marginal increase of apoptotic cells (data not shown). Furthermore, the cells were stained with DAPI in order to visualize the nuclei. Compared to control cells with normal nuclear morphology, treated cells showed morphological changes typical of apoptosis, such as nuclear fragmentation, starting at 24 h of exposure (Fig. 4B).
Next, we investigated the molecular mechanism of the drug-induced apoptosis. The apoptotic program occurs through two major pathways (35). The extrinsic pathway involves the activation of cell surface death receptors, whereas the intrinsic pathway is mediated by caspase-9 through the mitochondrial death cascade (35, 36). It is well established that the Bcl2 protein family is one of the most important mediators of the mitochondrial cascade (37). Bcl2 is a membrane-associated antiapoptotic protein that can promote cell survival through interactions with other Bcl2 family members, such as the cell death suppressors Bcl-xl Mcl-1, Bcl-w, and A1, or with the death agonists, such as Bax, Bak, Bik, Bad, and BID. During apoptosis, proapoptotic Bax up-regulation, along with Bcl2 down-regulation, causes the loss of mitochondrial membrane potential, cytocrome c release and the subsequent caspase activation, which leads to cell death (37, 38). Bcl2 promotes cell survival through the stabilization of the mitochondrial membrane since it prevents the release of cytocrome c from mitochondria (37, 38). Bcl2 can also bind the C-terminal part of Apaf-1, inhibiting its association with caspase-9 and thus interfering with the activation of the cytocrome c/Apaf-1 pathway. Bax and Bak instead induce cell death via channel-forming activity, which promotes the mitochondrial permeability transition. Bax also forms heterodimers with Bcl2 and counteracts its death-repressor activity. Given that Bax and Bcl2 play an important role in apoptosis (39), we verified the time course effects of the compounds on their expression in Daoy cells. We observed a time-dependent increase in Bax protein levels starting from 48 h of treatment and, at the same time, a decrease of Bcl2 levels in S7- and SI163-treated cells. S29 could equally reduce Bcl2 expression, but only after 72 h of exposure (Fig. 4C). Thus, these data suggest that S7, S29, and SI163 treatment induces apoptosis in medulloblastoma cells via the mitochondria-mediated pathway.
Pyrimidine derivative treatment shows more toxic effects in Daoy and D283-MED cells than cisplatin and etoposide
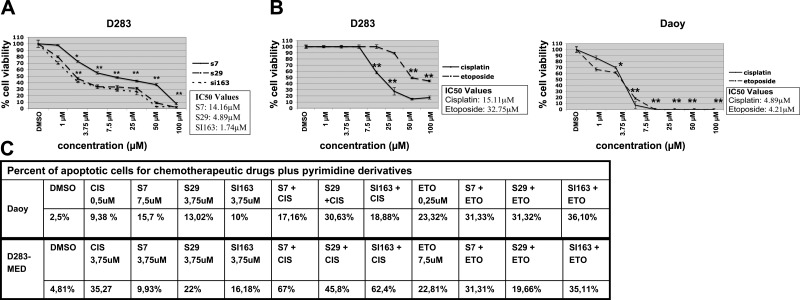
In addition to Daoy cells, we also verified the antiproliferative effects of these compounds in another medulloblastoma cell line, D283-MED, derived from a peritoneal metastasis. Our data also showed that D283-MED cells were sensitive to the pyrimidine derivatives, which reduced their growth rate in a dose-dependent manner. After 48 h of treatment, the IC50 values were calculated: 14.16 μM for S7, 4.89 μM for S29, and 1.74 μM for SI163. As shown in Daoy cells, S29 and SI163 treatment resulted in higher inhibitory effects with respect to S7 in D283-MED cells as well (Fig. 5A).
Figure 5.
A) Inhibition curves for S7, S29, SI163 in D283-MED cells. B) S7, S29, and SI163 show major effects in medulloblastoma cell proliferation compared to cisplatin and etoposide. Data are representative of ≥2 independent experiments. Error bars = sd from mean values. C) PI supravital exposure was performed in untreated cells and treated with pyrimidine derivatives alone and in combination with chemotherapeutic agents, for 48 h. Percentages of apoptotic cells are reported for each representative time point. *P < 0.05; **P < 0.01.
Current therapies for medulloblastoma are based on the combination of chemoterapeutic agents, such as cisplatin and etoposide, and radiotherapy (1). This finding led us to compare the antiproliferative potential of these pyrimidine derivatives to that of the conventional treatments. Thus, we performed the MTS assay in Daoy and D283-MED cells after exposure to cisplatin and etoposide, in the same experimental conditions used for S7, S29, and SI163. Interestingly, cisplatin and etoposide showed lower antiproliferative effects in medulloblastoma cells, with respect to Src inhibitors. In particular, the inhibition of D283-MED cell growth was evident at concentrations ≥15 μM for cisplatin and ≥25 μM for etoposide. In Daoy cells, the IC50 values calculated for cisplatin and etoposide were 4.89 and 4.21 μM, respectively, which are higher than the IC50 values for S29 (1.72 μM) and SI163 (3.5 μM); the chemotherapeutic drugs tested showed a higher effect only with respect to S7 (IC50: 6.24 μM) (Fig. 5B). These data are consistent with the minor potency of S7 with respect to S29 and SI163, as described above.
To determine the potential synergy of pyrimidine derivatives with each chemotherapeutic agent, we analyzed the inhibitory effects of drug combinations in Daoy and D283-MED cells. We selected 3 concentrations of each drug, based on their IC50 values, and cells were treated for 48 h. Exposure to a combination of S7, S29, and SI163 with cisplatin and etoposide resulted in a clear synergy in D283-MED cells, as analyzed according to the method of Chou-Talalay (24). In the Daoy cell line, etoposide showed synergistic effects in combination with all the compounds, whereas cisplatin seemed to exert these effects only when used in association with S29 and SI163. The results from the combination analysis are summarized in Tables 1 and 2.
TABLE 1.
CI values for chemotherapeutic agents plus pyrimidine derivatives in Daoy cell line
| Agent | S7 |
||
|---|---|---|---|
| 3.75 μM | 6.24 μM | 25 μM | |
| Etoposide | |||
| 1 μM | 0.544 ± 0.003 | 0.425 ± 0.07 | 1.029 ± 0.02 |
| 4.21 μM | 0.644 ± 0.04 | 0.557 ± 0.022 | 0.501 ± 0.007 |
| 6.5 μM | 0.526 ± 0.0098 | 0.511 ± 0.004 | 0.510 ± 0.028 |
| Cisplatin | |||
| 1.25 μM | 1.152 ± 0.09 | 0.958 ± 0.020 | 1.229 ± 0.013 |
| 4.89 μM | 1.126 ± 0.0063 | 1.177 ± 0.016 | 1.634 ± 0.05 |
| 7.5 μM | 1.027 ± 0.026 | 0.766 ± 0.004 | 1.914 ± 0.005 |
Values are means ± sd of ≥3 independent experiments done in quadruplicate. CI values were calculated according to Chou-Talalay by isobologram analysis done on CalcuSyn software.
TABLE 1.
(continued)
| S29 |
SI163 |
||||
|---|---|---|---|---|---|
| 0.86 μM | 1.72 μM | 15 μM | 1.75 μM | 3.5 μM | 8.5 μM |
| 1.500 ± 0.022 | 0.613 ± 0.0127 | 0.797 ± 0.036 | 0.786 ± 0.035 | 0.868 ± 0.086 | 0.783 ± 0.013 |
| 0.985 ± 0.018 | 0.553 ± 0.005 | 0.844 ± 0.0021 | 0.641 ± 0.038 | 0.720 ± 0.047 | 0.630 ± 0.006 |
| 0.970 ± 0.29 | 0.354 ± 0.096 | 0.585 ± 0.068 | 0.588 ± 0.27 | 0.561 ± 0.001 | 0.424 ± 0.001 |
| 0.798 ± 0.079 | 0.765 ± 0.02 | 1.591 ± 0.16 | 0.645 ± 0.06 | 0.728 ± 0.02 | 0.868 ± 0.19 |
| 1.194 ± 0.084 | 0.827 ± 0.010 | 0.970 ± 0.010 | 1.254 ± 0.008 | 1.086 ± 0.009 | 1.035 ± 0.007 |
| 0.670 ± 0.039 | 1.564 ± 0.013 | 0.560 ± 0.015 | 0.825 ± 0.002 | 0.906 ± 0.002 | 1.239 ± 0.04 |
TABLE 2.
CI values for chemotherapeutic agents plus pyrimidine-derivatives in D283-MED cell line
| Agent | S7 |
||
|---|---|---|---|
| 3.75 μM | 7.5 μM | 25 μM | |
| Etoposide | |||
| 15 μM | 0.286 ± 0.003 | 0.315 ± 0.007 | 0.514 ± 0.007 |
| 25 μM | 0.162 ± 0.001 | 0.231 ± 0.005 | 0.408 ± 0.01 |
| 50 μM | 0.118 ± 0.007 | 0.096 ± 0.01 | 0.225 ± 0.07 |
| Cisplatin | |||
| 7.5 μM | 0.504 ± 0.002 | 0.669 ± 0.01 | 1.293 ± 0.002 |
| 15 μM | 0.690 ± 0.0008 | 0.429 ± 0.007 | 0.744 ± 0.009 |
| 25 μM | 0.692 ± 0 | 0.633 ± 0.007 | 0.758 ± 0.007 |
Values are means ± sd of ≥3 independent experiments done in quadruplicate. CI values were calculated according to Chou-Talalay by isobologram analysis done on CalcuSyn software.
TABLE 2.
(continued)
| S29 |
SI163 |
||||
|---|---|---|---|---|---|
| 1 μM | 3.75 μM | 7.5 μM | 1 μM | 3.75 μM | 7.5 μM |
| 0.247 ± 0.007 | 0.275 ± 0.009 | 0.210 ± 0.003 | 0.399 ± 0.009 | 0.274 ± 0.01 | 0.197 ± 0.002 |
| 0.162 ± 0.004 | 0.241 ± 0.01 | 0.290 ± 0.01 | 0.141 ± 0.005 | 0.214 ± 0.005 | 0.350 ± 0.002 |
| 0.121 ± 0.006 | 0.204 ± 0.007 | 0.233 ± 0.0007 | 0.112 ± 0.006 | 0.184 ± 0.003 | 0.169 ± 0.006 |
| 1.194 ± 0.01 | 0.794 ± 0.008 | 0.742 ± 0.0007 | 1.408 ± 0.008 | 0.625 ± 0.01 | 0.490 ± 0.01 |
| 0.498 ± 0.002 | 0.684 ± 0.01 | 0.596 ± 0.01 | 0.869 ± 0.007 | 0.578 ± 0.01 | 0.739 ± 0.01 |
| 0.594 ± 0.01 | 0.735 ± 0.01 | 0.302 ± 0.001 | 0.468 ± 0.1 | 0.484 ± 0.005 | 0.120 ± 0.01 |
The next step was to test whether the synergy between pyrimidine derivatives and chemotherapeutic agents was due to an increase in apoptosis induction. For this purpose, we tested different doses of drugs individually and in combination, and apoptosis was determined after 48 h. Although each agent individually induced cell death in medulloblastoma cells, the combination of S7, SI163, and S29 with cisplatin and etoposide strongly increased such effect in Daoy cells, as shown in Fig. 5C. In D283-MED, the compounds increased the apoptotic cell percentage when combined with the chemotherapeutic agents, whereas no effect was observed after the association of S29 with etoposide (Fig. 5C).
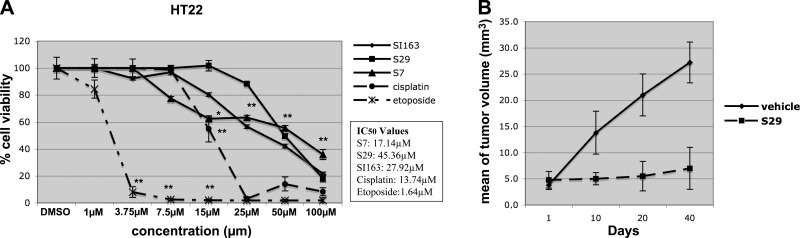
Pyrimidine derivatives show less cytotoxic effects on the neuronal cell line HT22. In order to evaluate the possible cytotoxic effects of pyrimidine derivatives on normal cells, we used a neuronal cell line, HT22, from mouse hippocampus. As reported in Fig. 6A, lower inhibitory effects on cell proliferation were observed in HT22 cells following exposure to the compounds with respect to cells treated with cisplatin and etoposide, used at the same experimental conditions tested in medulloblastoma cells. In fact, the resulting IC50 values for SI163, S29, and S7 (27.92, 45.36, and 17.14 μM, respectively) were higher than those for cisplatin and etoposide (13.74 and 1.64 μM, respectively).
Figure 6.
A) Effects of S7, S29, and SI163 in comparison to cisplatin and etoposide on HT22 neuronal cell line. Data are representative of ≥2 independent experiments. Error bars = sd from mean values. B) Inhibition of tumor progression in Daoy xenograft in nude mice. Mean tumor volume increment over days is shown. *P < 0.05; **P < 0.01.
S29 inhibits tumor growth in a medulloblastoma xenograft mouse model
Based on our promising in vitro results showing that pyrimidine derivatives inhibit medulloblastoma cell growth by inducing G2/M phase arrest, we verified their ability to reduce tumor formation and progression in vivo. To this aim, we injected Daoy cells into nude mice and, when the tumor reached the volume of ∼4 mm3, mice were treated with the compound administered per os. We chose the highly effective S29 molecule as reference compound for our class of Src inhibitors, to test the in vivo effects. Tumor volumes were measured after 10, 20, and 40 d, starting from the first day of treatment (Fig. 6B). Our results showed that the administration of 100 mg/kg S29 could significantly reduce tumor growth in Daoy xenograft. After 40 d of treatment, a ∼25% reduction of the tumor burden was observed in the treated group respect to the control group (P=0.04).
DISCUSSION
Medulloblastoma is the most common malignant brain tumor occurring in the childhood. Although in the past few years treatment regimens including surgery, chemotherapy, and radiotherapy have substantially improved survival, to date medulloblastoma is incurable in about one-third of patients, and current treatments cause neurocognitive sequelae (1). Substantial progress has been made in understanding the molecular features underlying medulloblastoma tumorigenesis and in offering new targets for the development of more effective and specific therapies. One of these new therapeutic targets may be represented by Src, which is highly expressed in medulloblastoma (9). In this study, we showed that novel pyrazolo-[3,4-d]-pyrimidine derivative Src inhibitors, designated S7, S29, and SI163, reduce proliferation in human medulloblastoma cells Daoy and D283-MED. To date, many small molecules are designed to target Src, and some of them, like PP2, demonstrate high target selectivity. Interestingly, our pyrimidine derivatives were found to be more active than PP2 in inhibiting cell proliferation and in inducing apoptosis in different carcinomas (22, 40, 41). Therefore, these new compounds may represent powerful tools in designing novel pharmacological therapies for medulloblastoma.
Our data indicate that the antiproliferative activity of S7, S29, and SI163 is mediated by their specific cell cycle inhibitory effects. All compounds, in fact, cause the arrest in G2/M phase of the cell cycle in Daoy cells. These results are in accordance with other studies showing that Src changes its activity during the G2/M checkpoint (42). Mizenina et al.(43) identified a subset of Src inhibitors that can inhibit tumor cell growth through cell cycle arrest either in S phase or in mitosis.
Besides cell cycle arrest, the pyrimidine derivatives tested here also induced apoptosis in Daoy cells. This effect was mediated by modulation of bax and bcl2 protein levels. Consistently, PD173955, a well-known Src inhibitor, also showed the ability to arrest cell cycle progression leading to cell accumulation in the M phase and apoptotic cell death (44). It was shown, in fact, that Src oncogenic signaling could prevent cell death via Bax inhibition. In particular, Src phosphorylates Bif-1, and this impairs its interaction with Bax, resulting in Bax inhibition and repression of apoptosis (45). According to another study, garcinol from the fruit rind of Garcinia indica induces apoptosis through the down-regulation of Src, and this is associated with change in the ratio of apoptotic proteins Bcl2 and Bax (46).
Consistent with the central role of the MPF complex, constituted by cdc2 and cyclinB1, in the control of G2/M checkpoint, treatment with the pyrimidine derivatives caused the down-regulation of cdc2. Besides protein expression levels, the activity of cdc2 is controlled tightly at several levels, including cyclinB1 binding and Tyr15 activating dephosphorylation, which occurs via CDC25C phosphatase (33, 34). We also found increased levels of p-cdc2(Tyr15) following drug treatment that were associated with the inactivating phosphorylation of CDC25C(Ser216). Mizenina et al.(43) showed that the inhibition of Src leads to aberrant activation of cdc2 by modulating its phosphorylation. Another study reported that genistein, the most abundant isoflavonoid in soybeans, induces the G2/M phase arrest in tumor prostatic cells via both cdc2 down-regulation and its induced phosphorylation. This effect was also associated with the increase of p-CDC25C(Ser216) (47). Taken together, our results suggest that pyrimidine derivatives block the cells at the G2/M phase through the modulation of cdc2 and CDC25C and subsequently induce apoptosis. Moreover, our findings show that blocking the Src pathway not only affects cdc2 phosphorylation, as documented, but also its protein levels by post-transcriptional mechanisms. However, extensive studies to determine the molecules regulating the cdc2 down-regulation, as well as CDC25C(Ser216) phosphorylation and G2/M phase arrest, are needed to elucidate the molecular mechanisms of S7, S29, and SI163. Such studies are currently under investigation in our laboratory.
Medulloblastoma is a radiosensitive tumor, and it was shown that ionizing radiation leads to direct apoptotic events or induces arrest at the G2/M checkpoint in the tumor cells (48, 49). Therefore, the findings that Src inhibitors, here tested, can block cells in the G2/M phase and induce apoptosis suggest that their combination with radiotherapy could have synergistic effects. Moreover, it is noteworthy that S7, S29, and SI163 showed higher cytotoxic effects in medulloblastoma cells compared to the alkylating agent cisplatin and the topoisomerase inhibitor etoposide, currently used in therapy for this tumor. Thus, the use of S7, S29, and SI163 in association with radiotherapy could allow the reduction of radiation doses and, consequently, the avoidance of radiotherapy-related cognitive and endocrine toxic effects. The findings that S7, S29, and SI163 also showed synergistic effects when combined with cisplatin and etoposide suggest their possible use in association with chemotherapy.
Another key issue when treating primary and metastatic brain tumors is the low permeability of the BBB (50). Src inhibitors, currently used in clinical trial for the treatment of chronic myeloid leukemia, showed low efficacy in the treatment of metastasis to the brain. For instance, imatinib does not appear to prevent CNS relapse due to its poor penetration of the BBB (51). Although other studies suggest that dasatinib has the ability to pass through the BBB and eliminate tumor cells from within the neuraxis, the concentrations of the drug delivered beyond the barrier are not known (52, 53). Another advantage of using the pyrimidine derivatives, here tested, in the treatment of medulloblastoma is the fact that these compounds are more lipophilic than dasatinib and therefore can cross the BBB more easily, as confirmed by our studies (unpublished results).
Besides the possible use of S7, S29, and SI163 in the treatment of medulloblastoma, these compounds could be useful to develop new pharmacologic inhibitors to study the physiological and oncogenic functions of Src. For instance, the use of the Src inhibitor PD173955 has permitted further insight about the role of Src in mitosis (44).
Finally, we evaluated the ability of the pyrimidine compounds to inhibit tumor progression in a medulloblastoma xenograft model and our promising results showed a reduction of tumor growth rate. These findings, combined with the low toxic effects observed on normal neuronal cells, render the small molecules S7, S29, and SI163 attractive pharmaceutical compounds for the treatment of medulloblastoma or other tumors characterized by high Src activity.
Acknowledgments
This study was supported by U.S. National Institutes of Health grants and by the Sbarro Health Research Organization (http://www.shro.org), the Human Health Foundation Onlus Spoleto, Italy (http://www.hhfonlus.org), and the Teresa and Luigi de Beaumont Bonelli Foundation (to A.G.). The authors also thank Dr. A. Cuppone for assistance with flow cytometry analysis.
References
- Rossi A., Caracciolo V., Russo G., Reiss K., Giordano A. Medulloblastoma: from molecular pathology to therapy. Clin Cancer Res. 2008;14:971–976. doi: 10.1158/1078-0432.CCR-07-2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D. L., Keene D., Bartels U., Carret A. S., Crooks B., Eisenstat D. D., Fryer C., Lafay-Cousin L., Larouche V., Moghrabi A., Wilson B., Zelcer S., Silva M., Brossard J., Bouffet E. Medulloblastoma in children under the age of three years: a retrospective Canadian review. J Neurooncol. 2009;94:51–56. doi: 10.1007/s11060-009-9799-2. [DOI] [PubMed] [Google Scholar]
- Nieder C., Mehta M. P., Jalali R. Combined radio- and chemotherapy of brain tumours in adult patients. Clin Oncol (R Coll Radiol) 2009;21:515–524. doi: 10.1016/j.clon.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Packer R. J., Gajjar A., Vezina G., Rorke-Adams L., Burger P. C., Robertson P. L., Bayer L., LaFond D., Donahue B. R., Marymont M. H., Muraszko K., Langston J., Sposto R. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24:4202–4208. doi: 10.1200/JCO.2006.06.4980. [DOI] [PubMed] [Google Scholar]
- Gajjar A., Chintagumpala M., Ashley D., Kellie S., Kun L. E., Merchant T. E., Woo S., Wheeler G., Ahern V., Krasin M. J., Fouladi M., Broniscer A., Krance R., Hale G. A., Stewart C. F., Dauser R., Sanford R. A., Fuller C., Lau C., Boyett J. M., Wallace D., Gilbertson R. J. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7:813–820. doi: 10.1016/S1470-2045(06)70867-1. [DOI] [PubMed] [Google Scholar]
- Taylor R. E., Bailey C. C., Robinson K., Weston C. L., Ellison D., Ironside J., Lucraft H., Gilbertson R., Tait D. M., Walker D. A., Pizer B. L., Imeson J., Lashford L. S. Results of a randomized study of preradiation chemotherapy versus radiotherapy alone for nonmetastatic medulloblastoma: the International Society of Paediatric Oncology/United Kingdom Children’s Cancer Study Group PNET-3 Study. J Clin Oncol. 2003;21:1581–1591. doi: 10.1200/JCO.2003.05.116. [DOI] [PubMed] [Google Scholar]
- Rossi A., Russo G., Puca A., La Montagna R., Caputo M., Mattioli E., Lopez M., Giordano A., Pentimalli F. The antiretroviral nucleoside analogue Abacavir reduces cell growth and promotes differentiation of human medulloblastoma cells. Int J Cancer. 2009;125:235–243. doi: 10.1002/ijc.24331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guessous F., Li Y., Abounader R. Signaling pathways in medulloblastoma. J Cell Physiol. 2008;217:577–583. doi: 10.1002/jcp.21542. [DOI] [PubMed] [Google Scholar]
- Sikkema A. H., Diks S. H., den Dunnen W. F., ter Elst A., Scherpen F. J., Hoving E. W., Ruijtenbeek R., Boender P. J., de Wijn R., Kamps W. A., Peppelenbosch M. P., de Bont E. S. Kinome profiling in pediatric brain tumors as a new approach for target discovery. Cancer Res. 2009;69:5987–5995. doi: 10.1158/0008-5472.CAN-08-3660. [DOI] [PubMed] [Google Scholar]
- Courtneidge S. A. Role of Src in signal transduction pathways The Jubilee Lecture. Biochem Soc Trans. 2002;30:11–17. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- Abu-Ghazaleh R., Kabir J., Jia H., Lobo M., Zachary I. Src mediates stimulation by vascular endothelial growth factor of the phosphorylation of focal adhesion kinase at tyrosine 861, and migration and anti-apoptosis in endothelial cells. Biochem J. 2001;360:255–264. doi: 10.1042/0264-6021:3600255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame M. C. Src in cancer: deregulation and consequences for cell behaviour. Biochim Biophys Acta. 2002;1602:114–130. doi: 10.1016/s0304-419x(02)00040-9. [DOI] [PubMed] [Google Scholar]
- Du J., Bernasconi P., Clauser K. R., Mani D. R., Finn S. P., Beroukhim R., Burns M., Julian B., Peng X. P., Hieronymus H., Maglathlin R. L., Lewis T. A., Liau L. M., Nghiemphu P., Mellinghoff I. K., Louis D. N., Loda M., Carr S. A., Kung A. L., Golub T. R. Bead-based profiling of tyrosine kinase phosphorylation identifies SRC as a potential target for glioblastoma therapy. Nat Biotechnol. 2009;27:77–83. doi: 10.1038/nbt.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summy J. M., Gallick G. E. Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev. 2003;22:337–358. doi: 10.1023/a:1023772912750. [DOI] [PubMed] [Google Scholar]
- Ma W. W., Adjei A. A. Novel agents on the horizon for cancer therapy. CA Cancer J Clin. 2009;59:111–137. doi: 10.3322/caac.20003. [DOI] [PubMed] [Google Scholar]
- Haura E. B., Turkson J., Jove R. Mechanisms of disease: Insights into the emerging role of signal transducers and activators of transcription in cancer. Nat Clin Pract Oncol. 2005;2:315–324. doi: 10.1038/ncponc0195. [DOI] [PubMed] [Google Scholar]
- Schenone S., Bruno O., Bondavalli F., Ranise A., Mosti L., Menozzi G., Fossa P., Donnini S., Santoro A., Ziche M., Manetti F., Botta M. Antiproliferative activity of new 1-aryl-4-amino-1H-pyrazolo[3,4-d]pyrimidine derivatives toward the human epidermoid carcinoma A431 cell line. Eur J Med Chem. 2004;39:939–946. doi: 10.1016/j.ejmech.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Carraro F., Pucci A., Naldini A., Schenone S., Bruno O., Ranise A., Bondavalli F., Brullo C., Fossa P., Menozzi G., Mosti L., Manetti F., Botta M. Pyrazolo [3,4-d]pyrimidines endowed with antiproliferative activity on ductal infiltrating carcinoma cells. J Med Chem. 2004;47:1595–1598. doi: 10.1021/jm034257u. [DOI] [PubMed] [Google Scholar]
- Angelucci A., Schenone S., Gravina G. L., Muzi P., Festuccia C., Vicentini C., Botta M., Bologna M. Pyrazolo[3,4-d]pyrimidines c-Src inhibitors reduce epidermal growth factor-induced migration in prostate cancer cells. Eur J Cancer. 2006;42:2838–2845. doi: 10.1016/j.ejca.2006.06.024. [DOI] [PubMed] [Google Scholar]
- Donnini S., Monti M., Castagnini C., Solito R., Botta M., Schenone S., Giachetti A., Ziche M. Pyrazolo-pyrimidine-derived c-Src inhibitor reduces angiogenesis and survival of squamous carcinoma cells by suppressing vascular endothelial growth factor production and signaling. Int J Cancer. 2007;120:995–1004. doi: 10.1002/ijc.22410. [DOI] [PubMed] [Google Scholar]
- Manetti F., Pucci A., Magnani M., Locatelli G. A., Brullo C., Naldini A., Schenone S., Maga G., Carraro F., Botta M. Inhibition of Bcr-Abl phosphorylation and induction of apoptosis by pyrazolo[3,4-d]pyrimidines in human leukemia cells. Chem Med Chem. 2007;2:343–353. doi: 10.1002/cmdc.200600214. [DOI] [PubMed] [Google Scholar]
- Spreafico A., Schenone S., Serchi T., Orlandini M., Angelucci A., Magrini D., Bernardini G., Collodel G., Di Stefano A., Tintori C., Bologna M., Manetti F., Botta M., Santucci A. Antiproliferative and proapoptotic activities of new pyrazolo[3,4-d]pyrimidine derivative Src kinase inhibitors in human osteosarcoma cells. FASEB J. 2008;22:1560–1571. doi: 10.1096/fj.07-9873com. [DOI] [PubMed] [Google Scholar]
- Schenone S., Brullo C., Bruno O., Bondavalli F., Mosti L., Maga G., Crespan E., Carraro F., Manetti F., Tintori C., Botta M. Synthesis, biological evaluation and docking studies of 4-amino substituted 1H-pyrazolo[3,4-d]pyrimidines. Eur J Med Chem. 2008;43:2665–2676. doi: 10.1016/j.ejmech.2008.01.034. [DOI] [PubMed] [Google Scholar]
- Bennett J. J., Adusumilli P., Petrowsky H., Burt B. M., Roberts G., Delman K. A., Zager J. S., Chou T. C., Fong Y. Up-regulation of GADD34 mediates the synergistic anticancer activity of mitomycin C and a gamma1345 deleted oncolytic herpes virus (G207) FASEB J. 2004;18:1001–1003. doi: 10.1096/fj.02-1080fje. [DOI] [PubMed] [Google Scholar]
- Zamai L., Canonico B., Luchetti F., Ferri P., Melloni E., Guidotti L., Cappellini A., Cutroneo G., Vitale M., Papa S. Supravital exposure to propidium iodide identifies apoptosis on adherent cells. Cytometry. 2001;44:57–64. [PubMed] [Google Scholar]
- Bondavalli F., Botta M., Bruno O., Ciacci A., Corelli F., Fossa P., Lucacchini A., Manetti F., Martini C., Menozzi G., Mosti L., Ranise A., Schenone S., Tafi A., Trincavellic M. L. Synthesis, molecular modeling studies, and pharmacological activity of selective A(1) receptor antagonists. J Med Chem. 2002;45:4875–4887. doi: 10.1021/jm0209580. [DOI] [PubMed] [Google Scholar]
- Chackalaparampil I., Shalloway D. Altered phosphorylation and activation of pp60c-src during fibroblast mitosis. Cell. 1988;52:801–810. doi: 10.1016/0092-8674(88)90422-9. [DOI] [PubMed] [Google Scholar]
- David-Pfeuty T., Nouvian-Dooghe Y. Immunolocalization of the cellular src protein in interphase and mitotic NIH c-src overexpresser cells. J Cell Biol. 1990;111:3097–3116. doi: 10.1083/jcb.111.6.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banavali N. K., Roux B. Flexibility and charge asymmetry in the activation loop of Src tyrosine kinases. Proteins. 2009;74:378–389. doi: 10.1002/prot.22153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu N., Chang D. C. Different thresholds of MPF inactivation are responsible for controlling different mitotic events in mammalian cell division. Cell Cycle. 2007;6:1639–1645. doi: 10.4161/cc.6.13.4385. [DOI] [PubMed] [Google Scholar]
- Lindqvist A., Rodriguez-Bravo V., Medema R. H. The decision to enter mitosis: feedback and redundancy in the mitotic entry network. J Cell Biol. 2009;185:193–202. doi: 10.1083/jcb.200812045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Meyer A. N., Donoghue D. J. Nuclear localization of cyclin B1 mediates its biological activity and is regulated by phosphorylation. Proc Natl Acad Sci U S A. 1997;94:502–507. doi: 10.1073/pnas.94.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier J., Solomon M. J., Booher R. N., Bazan J. F., Kirschner M. W. cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell. 1991;67:197–211. doi: 10.1016/0092-8674(91)90583-k. [DOI] [PubMed] [Google Scholar]
- Peng C. Y., Graves P. R., Thoma R. S., Wu Z., Shaw A. S., Piwnica-Worms H. Mitotic and G2 checkpoint control: regulation of 14–3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science. 1997;277:1501–1505. doi: 10.1126/science.277.5331.1501. [DOI] [PubMed] [Google Scholar]
- Khan N., Afaq F., Mukhtar H. Apoptosis by dietary factors: the suicide solution for delaying cancer growth. Carcinogenesis. 2007;28:233–239. doi: 10.1093/carcin/bgl243. [DOI] [PubMed] [Google Scholar]
- Thornberry N. A., Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- Green D. R., Reed J. C. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- Gross A., McDonnell J. M., Korsmeyer S. J. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- Mazoochi T., Salehnia M., Pourbeiranvand S., Forouzandeh M., Mowla S. J., Hajizadeh E. Analysis of apoptosis and expression of genes related to apoptosis in cultures of follicles derived from vitrified and non-vitrified ovaries. Mol Hum Reprod. 2009;15:155–164. doi: 10.1093/molehr/gap002. [DOI] [PubMed] [Google Scholar]
- Carraro F., Naldini A., Pucci A., Locatelli G. A., Maga G., Schenone S., Bruno O., Ranise A., Bondavalli F., Brullo C., Fossa P., Menozzi G., Mosti L., Modugno M., Tintori C., Manetti F., Botta M. Pyrazolo[3,4-d]pyrimidines as potent antiproliferative and proapoptotic agents toward A431 and 8701-BC cells in culture via inhibition of c-Src phosphorylation. J Med Chem. 2006;49:1549–1561. doi: 10.1021/jm050603r. [DOI] [PubMed] [Google Scholar]
- Schenone S., Bruno O., Ranise A., Bondavalli F., Brullo C., Fossa P., Mosti L., Menozzi G., Carraro F., Naldini A., Bernini C., Manetti F., Botta M. New pyrazolo [3,4-d]pyrimidines endowed with A431 antiproliferative activity and inhibitory properties of Src phosphorylation. Bioorg Med Chem Lett. 2004;14:2511–2517. doi: 10.1016/j.bmcl.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Roche S., Fumagalli S., Courtneidge S. A. Requirement for Src family protein tyrosine kinases in G2 for fibroblast cell division. Science. 1995;269:1567–1569. doi: 10.1126/science.7545311. [DOI] [PubMed] [Google Scholar]
- Mizenina O. A., Moasser M. M. S-phase inhibition of cell cycle progression by a novel class of pyridopyrimidine tyrosine kinase inhibitors. Cell Cycle. 2004;3:796–803. [PubMed] [Google Scholar]
- Moasser M. M., Srethapakdi M., Sachar K. S., Kraker A. J., Rosen N. Inhibition of Src kinases by a selective tyrosine kinase inhibitor causes mitotic arrest. Cancer Res. 1999;59:6145–6152. [PubMed] [Google Scholar]
- Yamaguchi H., Woods N. T., Dorsey J. F., Takahashi Y., Gjertsen N. R., Yeatman T., Wu J., Wang H. G. SRC directly phosphorylates Bif-1 and prevents its interaction with Bax and the initiation of anoikis. J Biol Chem. 2008;283:19112–19118. doi: 10.1074/jbc.M709882200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C. H., Sang S., Ho C. T., Lin J. K. Garcinol modulates tyrosine phosphorylation of FAK and subsequently induces apoptosis through down-regulation of Src, ERK, and Akt survival signaling in human colon cancer cells. J Cell Biochem. 2005;96:155–169. doi: 10.1002/jcb.20540. [DOI] [PubMed] [Google Scholar]
- Touny L. H., Banerjee P. P. Identification of both Myt-1 and Wee-1 as necessary mediators of the p21-independent inactivation of the cdc-2/cyclin B1 complex and growth inhibition of TRAMP cancer cells by genistein. Prostate. 2006;66:1542–1555. doi: 10.1002/pros.20495. [DOI] [PubMed] [Google Scholar]
- Chetty C., Bhoopathi P., Rao J. S., Lakka S. S. Inhibition of matrix metalloproteinase-2 enhances radiosensitivity by abrogating radiation-induced FoxM1-mediated G2/M arrest in A549 lung cancer cells. Int J Cancer. 2009;124:2468–2477. doi: 10.1002/ijc.24209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leskov K. S., Criswell T., Antonio S., Li J., Yang C. R., Kinsella T. J., Boothman D. A. When X-ray-inducible proteins meet DNA double strand break repair. Semin Radiat Oncol. 2001;11:352–372. doi: 10.1053/srao.2001.26912. [DOI] [PubMed] [Google Scholar]
- Warren K., Jakacki R., Widemann B., Aikin A., Libucha M., Packer R., Vezina G., Reaman G., Shaw D., Krailo M., Osborne C., Cehelsky J., Caldwell D., Stanwood J., Steinberg S. M., Balis F. M. Phase II trial of intravenous lobradimil and carboplatin in childhood brain tumors: a report from the Children’s Oncology Group. Cancer Chemother Pharmacol. 2006;58:343–347. doi: 10.1007/s00280-005-0172-7. [DOI] [PubMed] [Google Scholar]
- Decleves X., Amiel A., Delattre J. Y., Scherrmann J. M. Role of ABC transporters in the chemoresistance of human gliomas. Curr Cancer Drug Targets. 2006;6:433–445. doi: 10.2174/156800906777723930. [DOI] [PubMed] [Google Scholar]
- Mahon F. X., Hayette S., Lagarde V., Belloc F., Turcq B., Nicolini F., Belanger C., Manley P. W., Leroy C., Etienne G., Roche S., Pasquet J. M. Evidence that resistance to nilotinib may be due to BCR-ABL, Pgp, or Src kinase overexpression. Cancer Res. 2008;68:9809–9816. doi: 10.1158/0008-5472.CAN-08-1008. [DOI] [PubMed] [Google Scholar]
- De Groot J., Milano V. Improving the prognosis for patients with glioblastoma: the rationale for targeting Src. J Neurooncol. 2009;95:151–163. doi: 10.1007/s11060-009-9916-2. [DOI] [PubMed] [Google Scholar]