Abstract
Sphingosine-1-phosphate (S1P) is an important bioactive sphingolipid involved in angiogenesis and lymphangiogenesis, 2 important processes that influence the growth, survival, and spread of tumors. S1P acts as an extracellular mediator through binding to 5 highly specific S1P receptors, S1P1–5. Sphingosine kinase-1 (SK1), one of 2 known sphingosine kinase enzymes responsible for S1P production, appears to be overexpressed in many tumors. Although a role for S1P in angiogenesis and lymphangiogenesis has been established, it is unclear whether S1P secreted from cancer cells has a paracrine function in a tumor environment. Here we investigated whether modulation of cellular SK1 could initiate a paracrine angiogenic and lymphangiogenic switch. We found that SK1 overexpression in HEK cells or its down-regulation in glioma or breast cancer cells modulated extracellular S1P levels accordingly, which in turn increased or decreased both migration and tube formation in cocultured vascular or lymphatic endothelial cells. In contrast, down-regulation of sphingosine kinase 2 in both glioma and breast cancer cells had no appreciable effect on cellular or secreted S1P levels. In addition, vascular endothelial growth factors VEGF and VEGF-C down-regulation in cancer cells appeared insufficient to block the angiogenic and lymphangiogenic switch triggered by these cells. Moreover, S1P initiated endothelial cell sprouting in 3-dimensional collagen matrices, which is representative of a multistep angiogenic process. Our data collectively demonstrate for the first time that SK1 plays an essential role in regulating in vitro paracrine angiogenesis and lymphangiogenesis.—Anelli, V., Gault, C. R., Snider, A. J., Obeid, L. M. Role of sphingosine kinase-1 in paracrine/transcellular angiogenesis and lymphangiogenesis in vitro.
Sphingosine-1-phosphate (S1P) is involved in numerous biological processes, including cell growth, survival, migration, inflammation, and apoptosis (1). S1P can act either as an intracellular second messenger (2, 3) or as an extracellular mediator (1, 4,5,6) through its interaction with G-protein-coupled receptors S1P1–5. Platelets are one of the sources of extracellular S1P (7). In fact, blood platelets, which lack the S1P-metabolizing enzyme S1P lyase, store S1P abundantly and release this bioactive lipid into the extracellular milieu on stimulation. However, it is now clear that other cell types, including erythrocytes (8), endothelial cells (9), and cells from the nervous system (10) actively secrete S1P. Moreover, studies show that the S1P-generating enzyme sphingosine kinase 1 (SK1) is exported in certain physiological conditions, perhaps contributing to vascular S1P gradient establishment (11, 12).
S1P is formed by the action of 2 sphingosine kinases, SK1 and SK2. Although highly similar and conserved, these 2 enzymes have different subcellular localizations, tissue expression, substrate specificities, and functions. In particular, the highest expression of SK1 is found within the adult lung and spleen, followed, in decreasing order, by peripheral blood leukocytes, thymus, and kidney (13). SK1 is localized mainly in the cytosol but can also translocate to the plasma membrane on stimulation. Although SK1 can utilize d-erythro-dihydrosphingosine as a substrate, d-erythro-sphingosine is its preferred substrate (14). In contrast, mammalian SK2 mRNA is expressed mainly in the liver and heart (15). Therefore, its expression has a strikingly different tissue distribution from that of SK1, and it initially appears later in embryonic development. Many reports in the literature suggest that SK1 is up-regulated in human tumors (16, 17) and can act as an oncogene when overexpressed (18). Interestingly, higher SK1 expression correlates with a poor prognosis in both glioblastoma and gastric cancer (19, 20). Moreover, we recently reported that hypoxia, which occurs during tumor progression, increases SK1 expression with a subsequent elevation of S1P release (21).
In 1999, 2 papers revealed for the first time that S1P has a potential role in angiogenesis (22, 23). The authors reported that S1P stimulates DNA synthesis and chemotactic motility of human umbilical vein endothelial cells (HUVECs) in a dose-dependent manner. S1P was also shown to significantly induce tube formation of HUVECs in Matrigel. Moreover, Matrigel plug assay studies in mice revealed that S1P promotes angiogenesis in vivo(22). S1P1 was identified as the receptor involved in the angiogenic process and was shown to be strongly induced in tumor vessels. Chae et al.(24) reported that local injection of S1P1 siRNA into established tumors suppressed vascular stabilization and angiogenesis, dramatically suppressing tumor growth in vivo.
Also, a recent report suggests that S1P acts as a lymphangiogenic mediator, inducing migration, capillary-like tube formation, and intracellular Ca2+ mobilization, but not proliferation, in human lymphatic endothelial cells (HLECs) in vitro. Moreover, in a Matrigel plug assay, S1P promoted the outgrowth of new lymphatic vessels in vivo(25).
Many angiogenic and lymphangiogenic effects of S1P are receptor mediated; thus, S1P must be secreted into the extracellular environment. However, it is not clear what cell type in the tumor microenvironment is the source of extracellular S1P. In this study, we evaluated the roles of SK1 and SK2 in the generation of intra- and extracellular S1P in glioma and breast carcinoma cell lines, and the subsequent role of secreted S1P in regulating in vitro angiogenesis and lymphangiogenesis in cocultured endothelial or lymphatic endothelial cells. Our novel data suggest that only modulation of SK1 in HEK293, U87MG glioma, and MDA-MB-231 breast carcinoma cells modulates extracellular S1P and subsequently regulates in vitro angiogenesis and lymphangiogenesis in a paracrine manner.
MATERIALS AND METHODS
Materials and cell cultures
U87MG cells (human malignant glioma cell line), mouse embryonic fibroblasts (MEFs), and human embryonic kidney cell line HEK293 were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) in a 5% CO2 incubator at 37°C. MDA-MB-231 (human breast cancer cell line) was cultured under similar conditions in RPMI medium. Cell lines were obtained from American Type Culture Collection (Manassas, VA, USA). HUVECs were cultured in endothelial cell medium-2 (EBM-2) supplemented with 2% FCS and EGM-2 singleQuots (Lonza, Basel, Switzerland). Human dermal microvascular endothelial cells (HMVECs) were cultured in EBM-2 supplemented with 2% FCS and EGM-2 MV singleQuots. Both endothelial cell lines were obtained from Lonza and used for in vitro angiogenesis experiments between the 2nd and 8th passage. Wild-type (WT) and Sphk1−/− MEFs were obtained from 12-d pregnant WT and Sphk1−/− C57/Bl6 mice. After 2 passages, MEFs were immortalized using retroviral p53DN (gift from Carola Neumann, Medical University of South Carolina) and selected for with hygromycin.
Stable HEK cells overexpressing hSK1-GFP (construct made by K. R. Johnson and described in ref. 26) or empty vector pEGFP-C3 was obtained after transfection with oligofectamine (Invitrogen, Carlsbad, CA, USA). Transfectants were selected in the presence of geneticin and maintained in DMEM containing 10% FBS and 400 μg/ml of geneticin.
Tissue culture medium for tumor cell lines, heat-inactivated fetal bovine serum, phosphate-buffered saline, and oligofectamine were obtained from Invitrogen. Tissue culture medium for HUVECs and HMVECs was obtained from Lonza. S1P was obtained from Biomol (Plymouth Meeting, PA, USA), and deoxypyridoxine, β-glycerophosphate, sodium fluoride, fibrin, and aprotinin were obtained from Sigma (St. Louis, MO, USA). [3-3H]-d-erythro-sphingosine was from Perkin Elmer Life Sciences (Waltham, MA, USA). C17-d-erythro-sphingosine and VPC23019 were obtained from Avanti (Alabaster, AL, USA). Matrigel was from BD Biosciences (San Jose, CA, USA). CellTracker Green CMFDA was obtained from Molecular Probes (Invitrogen), and the Rac-1 activation assay kit was obtained from Upstate (Millipore, Billerica, MA, USA).
SiRNA down-regulation of SK1, SK2, vascular endothelial growth factor (VEGF)-A, VEGF-C, and S1P1
SK1 siRNA (5′-AAGGGCAAGGCCTTGCAGCTC-3′), SK2 siRNA (5′-AACGCTTTGCCCTCACCCTTA-3′), and scrambled siRNA (5′-AATTCTCCGAACGTGTCACGT-3′) were synthesized from Xeragon (Qiagen, Valencia, CA, USA). Predesigned VEGF-A siRNA (catalog no. AM16708, ID 4542) and silencer selected VEGF-C siRNA (4392420, ID S14783) were obtained from Ambion/Applied Biosystems (Foster City, CA, USA). S1P1 siRNA (sc-37086) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). All siRNA experiments were performed using 20 nM siRNA. In particular, cells were seeded in 100-mm dishes at a density of 5 × 105 for 24 h before down-regulation. Cells were transfected with oligofectamine reagent according to the manufacturer’s protocol. Briefly, 4 μl of 20 μM siRNA was resuspended in 800 μl of OptiMEM medium and mixed with 60 μl of oligofectamine-optiMEM medium complex (4:11 v/v). The mixture was then incubated for 20 min at room temperature and added to the cells (previously incubated for 20 min in 3.2 ml OptiMEM medium). After 4 h, 2 ml of 30% medium (DMEM or RPMI U87MG and MD-MB-231, respectively) or 2 ml of 6% medium (EBM-2 supplemented with EGM-2 or EGM-2 singleQuots) was added to the dishes, and the cells were further incubated for various times up to 72 h. The efficiency of the knockdown was determined for each experiment by quantitative real-time PCR for all down-regulated genes and immunoblotting with specific antibodies for SK1 and SK2.
Quantitative real-time PCR
Total RNA and proteins were isolated directly from fresh cultured cells using a PARIS kit (Ambion). Total RNA was treated with the Turbo RNase-free kit (Ambion) and measured using the Quant-iT RiboGreen RNA kit (Invitrogen). RNA (1 μg) was reverse transcribed into cDNA using the SuperScript First-Strand Synthesis System (Invitrogen). Quantitative real-time PCR was performed with an iCycler 1Q real-time detection system using the SYBR Green Supermix kit (Bio-Rad, Hercules, CA, USA). Primers used are shown in Table 1. Reaction conditions were as follows: 3 min at 95°C followed by 40 cycles with 45 s at 60°C, 1 min at 95°C, 1 min at 60°C, and 10 min at 4°C. Real-time PCR results were analyzed using Q-Gene software (Bio-Rad), which expresses data as mean normalized expression. All genes were normalized to expression of β-actin as an endogenous control.
TABLE 1.
Primer sequences used for real-time PCR
| RNA | Primer, 5′-3′ |
|
|---|---|---|
| Forward | Reverse | |
| SK1 | CTGGCAGCTTCCTTGAACCAT | TGTGCAGAGACAGCAGGTTCA |
| SK2 | CCAGTGTTGGAGAGCTGAAGGT | GTCCATTCATCTGCTGGTCCTC |
| S1P1 | CCGCAAGAACATTTCCAAGG | CGCTCAGGACGATAATTACGGT |
| S1P2 | GCCATTGTGGTGGAAAACCTT | CAGGTTGCCCAGAAACAGGTA |
| S1P3 | AGCGGCACTTGACAATGATCA | ACATCCCGATCAGGAGGAAGA |
| S1P5 | TGAAGGAGTAGTTCCCGAAGG | AAGCTTCTATGGCTCCCACCTC |
| VEGF-A | CCTGGTGGACATCTTCCAGGAGTACC | GAAGCTCATCTCTCCTATGTGCTGGC |
| VEGF-C | GACTCAACAGATGGATTCC | GGGCAGGTTCTTTTACAT |
| β-Actin | ATTGGCAATGAGCGGTTCC | GGTAGTTTCGTGGATGCCACA |
Mass spectrometry analysis
Cells were plated in 100-mm dishes and treated (or not treated, control) with different siRNA as described above and used at 70% confluence. At the moment of experimentation, medium was removed, and cells were incubated with DMEM (4 ml) or RPMI containing 20 mM HEPES-KOH (pH 7.4), 10 mM sodium glycerophosphate, 5 mM sodium fluoride, 0.5 mM deoxipyridoxine, and 0.1% BSA for 2 h for trapping S1P released from the cells (as previously reported by Lee et al., ref. 27, with some modifications). S1P mass from the cells and from the medium was quantified using positive mode electrospray ionization/tandem mass spectrometry analysis in the Medical University of South Carolina Lipidomics Core Facility, exactly as described previously (28).
In vivo assay for S1P formation and release
Cells, incubated for 2 h in trapping medium as described above, were pulsed for 10 min with C17-d-erythro-sphingosine to a final concentration of 1 μM, and then washed with PBS, harvested, and lysed by sonication in PBS. Protein content was determined, and lipids are then extracted with isopropanol/water/ethyl acetate (30:10:60, v/v). C17-S1P was measured in the cells and in the medium by mass spectrometry, as described previously (29).
Western blot
Western blot was used to analyze SK1. Total proteins were isolated directly from fresh cultured cells using a PARIS kit (Ambion) and kept on ice for 30 min. After brief sonication, protein concentration was measured with the BCA method, and protein (20 μg) was loaded in an SDS-10% polyacrylamide gel, transferred to a nitrocellulose membrane, and blotted with anti-hSK1 and actin. Immunoreactive bands were detected with horseradish peroxidase-conjugated anti-rabbit IgG (Jackson ImmunoResearch, West Grove, PA, USA) and an ECL detection kit (Pierce, Springfield, IL, USA). Intensity of the bands was evaluated with ImageJ (U.S. National Institutes of Health, Bethesda, MD, USA).
In vitro tube formation assay
An in vitro tube formation assay was performed to evaluate the properties of U87MG and MDA-MB-231 cells treated with different siRNAs, HEK-293-V cells, and HEK-293-SK1 cells on HUVEC and HMVEC tube formation. Cells (4×105) were seeded in 60-mm dishes and treated with scrambled or various siRNAs (20 nM) after 24 h. After 24 h incubation, cells were detached by trypsinization, and a volume of cells (2×105) was mixed with growth factor-reduced Matrigel matrix (100 μl; 450 μg/well of Matrigel diluted 1:1 with PBS; BD Biosciences) and seeded in a 24-well dish. Matrigel was allowed to polymerize 30 min in a 5% CO2 incubator at 37°C. Endothelial cells were seeded in 100-mm dishes (5×105) and used when they reached 80% confluence. After 48 h, endothelial cells were plated atop the Matrigel cell mixture. The fluorescent cellTracker Green CMFDA (for experiments with U87MG or MDA-MB-231 cells) or cellTracker Red CMTPX (for experiments with HEK-293-V and HEK-293-SK1 cells; both dyes from Molecular Probes, Eugene, OR, USA) were added to the endothelial cells (5 μM final concentration) and incubated for 45 min. Medium was then removed, and cells were incubated for another 4 h in EGM-2 medium (without FBS and singleQuots) containing 0.1% fatty-acid free BSA. After incubation, cells were trypsinized and seeded at 4 × 104/well atop the Matrigel cell mixture. Tube formation was observed using a laser-scanning confocal microscope (LSM 510 Meta; Carl Zeiss, Thornwood, NY, USA) after 4 h with the ×10 objective. Then a pixel analysis was performed of the tube formation area, and the image of the area was converted to black-scale and processed using NIH Image software to calculate the total number of pixels. Pixels were counted in 3 different areas, and the average was determined for each sample. The control sample was defined as 100% tube formation, and the percentage increase or decrease in tube formation relative to the control was calculated for each sample.
Fibrin gel bead assay
The fibrin gel bead assay was performed as described by Nakatsu et al.(30) with some modification. Specifically, 2500 cytodex 3 beads (Amersham Pharmacia, Piscataway, NJ, USA) were mixed in HUVECs (1×106) in EGM-2 medium (1.5 ml) and shaken gently every 20 min for 4 h at 37°C. After incubation, beads with cells were transferred to a 25-cm2 tissue culture flask and left 16 h in EGM-2 (5 ml) at 37°C and 5% CO2. The next day, beads were washed 3 times with EGM-2 medium and resuspended (final concentration 500 beads/ml) in 2.5 mg/ml fibrinogen (Sigma) containing 0.15 U/ml aprotinin. An aliquot (800 μl) was removed, and 625 U/ml thrombin was added to this aliquot. Then 250 μl of this solution was rapidly added to each well of a 24-well tissue culture plate and incubated at 37°C and 5% CO2 for 20 min. EGM-2 (1 ml, containing 0.1% BSA and 0.15 U/ml aprotinin) was added to each well in the presence or absence of 100 nM S1P. In some experiments, 2 × 104 MEFs were plated atop the clot in 1 ml of EGM-2 medium containing 2% FBS, and medium was changed every other day. Beads were monitored for 7 d.
VEGF-A and VEGF-C release measurement
Human VEGF and VEGF-C concentrations in cell culture medium were measured using a QuantiGlo chemiluminescent assay and quantikine immunoassay (for VEGF and VEGF-C, respectively; R&D Systems, Minneapolis, MN, USA) according to the protocol provided by the manufacturer.
Other methods
Total protein was assayed using the BCA method (Pierce) with BSA as a standard. Cell viability was assessed with the trypan blue exclusion test. Student’s t test was used for statistical analysis. Values of P < 0.05 were considered significant.
RESULTS
S1P induces the migration and tube formation of HUVECs and HMVECs through S1P receptors
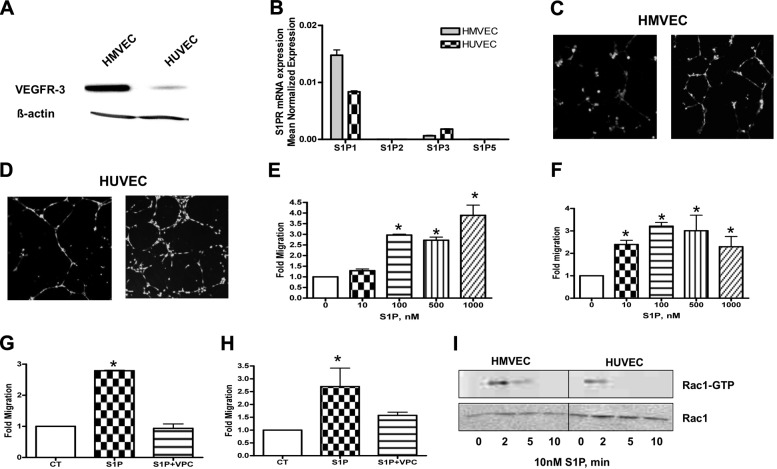
First, we characterized the expression of the S1P receptors 1–5 using real-time PCR in both HUVECs and HMVECs (the latter express the lymphatic endothelial cells marker VEGFR-3l; Fig. 1A). As shown in Fig. 1B, both of these endothelial cell lines express S1P1 and S1P3, although the ratio of the 2 receptors was found to be different. Next, it was important to confirm that both HUVECs and HMVECs respond to exogenous administration of S1P by increasing their capacity to migrate and form tubes. Both cell lines were tested for migration in response to increasing concentrations of S1P using a modified Boyden chamber transwell system. As shown in Fig. 1C, D, S1P induced capillary-like tube formation compared to control medium. Moreover, S1P significantly induced the migration of HUVECs and HMVECs in a dose-dependent manner (Fig. 1E, F). Next, we investigated whether this effect was due to the interaction of S1P with S1P1/S1P3 receptors using specific receptor antagonists. As shown in Fig. 1G, H, pretreatment with 1 μM VPC23019 (an S1P1/S1P3-receptor antagonist) completely inhibited S1P-induced cell migration.
Figure 1.
S1P induces migration and tube formation of HMVECs and HUVECs through the S1P1 receptor. A) HMVECs and HUVECs were collected for Western blot. Membranes were probed with the following antibodies: VEGFR3 (1:1000) and actin (1:10,000) as a loading control. B) Cells were subjected to mRNA extraction and real-time PCR analysis for S1P1–5 receptors. Real-time PCR data are expressed as mean normalized expression, using β-actin as a reference gene, and are presented as means ± sd of 3 independent experiments. C, D) HMVECs (C) and HUVECs (D) loaded with the fluorescent cell tracker CMFDA were seeded on Matrigel-coated 24-well dishes and incubated in EGM + 0.1% BSA (CT) or EGM + 0.1% BSA containing 100 nM S1P for 4 h. Photos are representative images of 3 independent experiments performed in quadruplicate. E, F) HMVECs (E) and HUVECs (F) loaded with the fluorescent cell tracker CMFDA were trypsinized and placed on the upper chamber of BD falcon Fluoroblok. Lower compartments were filled with increasing concentrations of S1P (0–1000 nM). After 4 h, the plate was read from the bottom in a fluorescent plate reader at excitation/emission wavelengths of 490/520 nm. Migratory ability was calculated as a percentage of control (no chemoattractant in bottom compartment). G, H) HMVECs (G) and HUVECs (H) loaded with the fluorescent cell tracker CMFDA were trypsinized and placed on the upper chamber of BD falcon Fluoroblok. Lower compartments were filled with EGM + 0.1% BSA (CT), EGM + 0.1% BSA + 100 nM S1P, or EGM + 0.1% BSA + 100 nM S1P + 1 μM VPC23019. I) Cells were incubated with 100 nM S1P for indicated times, and amounts of GTP-bound and total Rac1 were detected by immunoblotting. Representative images of 3 independent experiments are shown. *P < 0.05.
It is well known that the activated form of Rac1, a member of the Rho family of small GTPases, stimulates cell migration. We investigated whether S1P promotes activation of Rac1 in HUVECs and HMVECs. We found that S1P addition increased GTP-bound Rac1 in both endothelial cell types and that this activation peaked at 2 min and declined by 30 min. Of note, GTP-bound Rac1 was higher in HMVECs compared to HUVECs (Fig. 1I). These data indicate that S1P-induced Rac1 activation and concomitantly promotes cell migration and tube formation in both vascular and lymphatic endothelial cells.
Effect of SK1 overexpression on intracellular and extracellular S1P production
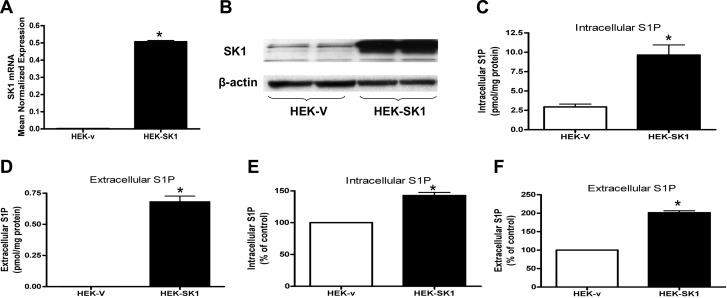
Because SK1 levels have been shown to be elevated in many tumors, we next evaluated whether SK1 overexpression could contribute to higher levels of intracellular and extracellular S1P. HEK293 cells were stably transfected with empty vector or hSK1 vector. The HEK293 cell line is an excellent model to study the role of SK1; it is characterized by very low SK1 mRNA, protein, and in vitro activity compared to other cell lines (data not shown). As shown in Fig. 2A, B, HEK293 cells stably transfected with hSK1 (HEK-SK1) had significantly increased SK1 mRNA and protein compared to cells transfected with empty vector (HEK-v). To evaluate whether overexpression of SK1 influences S1P levels, intracellular and extracellular S1P content was analyzed by mass spectrometry. Figure 2C, D shows that intracellular S1P increased >3 times in HEK-SK1 cells compared to HEK-v cells. Moreover, S1P was not detectable in the conditioned medium of HEK-v cells, but it became detectable in the conditioned medium collected from HEK-SK1 cells. To further demonstrate that the production and release of S1P was higher in HEK cells overexpressing hSK1, HEK-v and HEK-SK1 cells, incubated for 2 h in trapping medium, were pulsed for 10 min with C17-d-erythro-sphingosine to a final concentration of 1 μM. C17-S1P was measured in the cells and in the medium by mass spectrometry. The results (Fig. 2E, F) show that SK1 overexpression increased both intracellular and extracellular S1P levels by 143 and 201% respectively.
Figure 2.
SK1 overexpression causes intra- and extracellular increase of S1P in HEK293 cells. A) Stable HEK cells transfected with vector (HEK-V) or SK1 (HEK-SK1) were subjected to mRNA extraction and real-time PCR analysis for hSK1. Real-time PCR data are expressed as mean ± sd normalized expression of 3 independent mRNA extractions, using β-actin as reference gene. B) HEK-V and HEK-SK1 were collected for Western blot. Membranes were probed with the following antibodies: hSK1 (1:500) and actin (1:10,000). Blot is representative of 2 independent experiments performed in duplicate. C, D) HEK-V (C) and HEK-SK1 cells (D), incubated for 2 h in trapping medium (described in Materials and Methods), were collected and analyzed by mass spectrometry for intracellular and extracellular S1P content. Results are means ± sd (pmol/mg protein) of 3 independent experiments performed in duplicate. E, F) HEK-V (E) and HEK-SK1 cells (F), incubated for 2 h in trapping medium, were pulsed for 10 min with C17-d-erythro-sphingosine to a final concentration of 1 μM. C17-S1P was measured in the cells and in the medium by mass spectrometry. Results are mean ± sd percentage compared to control of 3 independent experiments performed in duplicate. *P < 0.05.
Role of SK1 overexpression in HEK293 cells on in vitro HUVEC tube formation
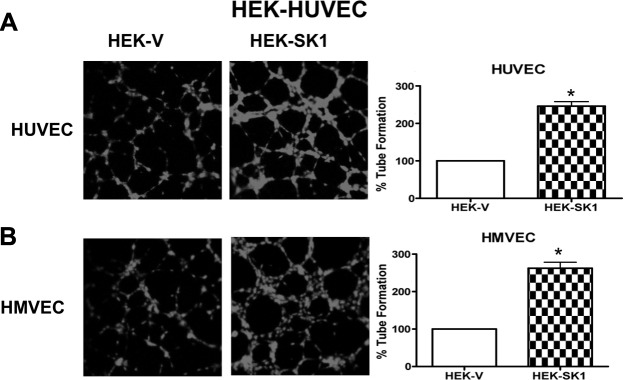
To investigate whether overexpression of SK1 in HEK293 cells could modulate the capacity of HUVECs and HMVECs to form tube-like structures in a paracrine fashion, HEK-v or HEK-SK1 cells were mixed with Matrigel and cultured for 4 d. HUVECs or HMVECs were then added atop the Matrigel-HEK cells and allowed to form tubes. S1P (100 nM) was used as positive control, and, as expected, it significantly enhanced tube formation (data not shown). As shown in Fig. 3, HEK-v cells allowed the formation of shorter and somewhat blunted tubes with both cocultured HUVECs and HMVECs. In contrast, HEK-SK1 cells induced the formation of a more complex and developed tube network with both cocultured endothelial cell types (Fig. 3A, B, respectively). These data support the notion that modulation of SK1 within tumor cells has an important role in neoangiogenesis.
Figure 3.
HEK cells overexpressing SK1 enhance HUVEC and HMVEC tube formation. HEK-V or HEK-SK1 cells were mixed with growth factor-reduced Matrigel and seeded in a 24-well dish. After 48 h, HUVECs (A) or HMVECs (B) loaded with cellTracker Red CMTPX were plated on the top of the mixture of Matrigel and cells, and tube formation was observed using a confocal microscope equipped with an ×10 objective after 4 h. Images are representative of 2 experiments performed in quadruplicate. Graphs show tube formation as a percentage of control value. *P < 0.05.
Role of SK1 and SK2 in S1P synthesis and release in glioma-derived U87MG cells
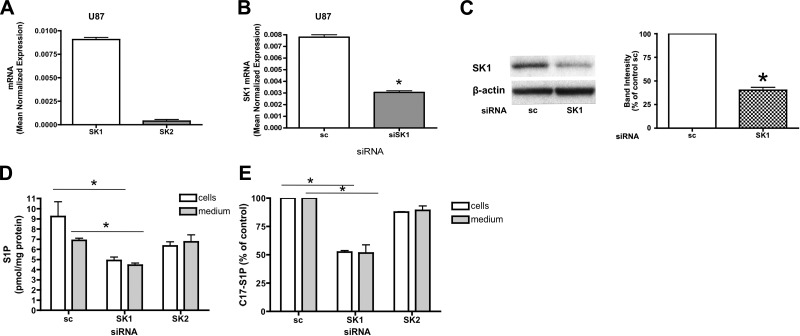
To establish the role of endogenous SK1 and SK2 in the production and extracellular release of S1P, we utilized U87MG glioma cells, a cell line characterized by high levels of SK1 and lower levels of SK2 (Fig. 4A). We initially knocked down SK1 by RNA interference and found that the SK1 message was efficiently down-regulated by treatment with 20 nM of siRNA after 72 h of silencing (Fig. 4B) with loss of SK1 protein confirmed by Western blot analysis (Fig. 4C). Next, we evaluated whether down-regulation of SK1, which is directly involved in the production of S1P, influences intra- and extracellular S1P levels. Figure 4D shows that intracellular and extracellular S1P (measured by mass spectrometry) were significantly decreased in U87MG cells treated with SK1 siRNA compared to U87MG cells treated with scrambled siRNA. In contrast, SK2 siRNA treatment led to ∼75% decrease in SK2 message (Supplemental Fig. 1A) but did not appreciably affect intracellular or extracellular S1P (Fig. 4D). To confirm the reduction in the production of S1P after SK1 down-regulation, we used an in-cell assay of S1P formation in which U87MG cells were treated with the indicated siRNA for 72 h, incubated for 2 h in trapping medium, and pulsed for 10 min with C17-d-erythro-sphingosine to a final concentration of 1 μM. C17-S1P was measured in the cells and in the medium by mass spectrometry. As shown in Fig. 4E, U87MG cells produced significantly less C17-S1P after SK1 silencing compared to control cells treated with scrambled siRNA. Moreover, analysis of the medium after the C17-Sph pulse revealed that C17-S1P was present in the extracellular milieu, and that it was significantly decreased after treatment with SK1 siRNA. In particular the intra- and extracellular levels were decreased by 50 and 52%, respectively. In contrast, treatment with SK2 siRNA did not affect intra- or extracellular C17-S1P levels (Fig. 4E). These data suggest that in U87MG glioma cells, SK1 but not SK2 has a crucial role in the regulation of intra- and extracellular S1P levels.
Figure 4.
SK1 down-regulation causes intra- and extracellular decrease of S1P in U87MG cells. A) U87MG cells were subjected to mRNA extraction and real-time PCR analysis for hSK1 and hSK2. Real-time PCR data are expressed as mean ± sd normalized expression of 3 independent experiments, using β-actin as a reference gene. B) U87MG cells treated with 20 nM scrambled (sc) or SK1 siRNA for 72 h were collected using PARIS kit lysis buffer and subjected to mRNA extraction and real-time PCR analysis for hSK1. Real-time PCR data are expressed as mean ± sd normalized expression of 3 independent mRNA extractions, using β-actin as a reference gene. C) Cell lysates were analyzed by Western blot. Membranes were probed with the following antibodies: hSK1 (1:500) and actin (1:10,000). Blot is representative of 3 separate experiments performed in duplicate. D) U87MG cells treated with sc or SK1 siRNA for 72 h were incubated for 2 h in trapping medium (described in Materials and Methods). At the end of incubation, cells were collected and analyzed by mass spectrometry for intracellular and extracellular S1P content. Results are means ± sd (pmol/mg protein) of 3 independent experiments performed in duplicate. E) U87MG cells treated with sc or SK1 siRNA as described above were incubated for 2 h in trapping medium and pulsed for 10 min with C17-d-erythro-sphingosine to a final concentration of 1 μM. C17-S1P was measured in the cells and in the medium by mass spectrometry. Results are mean ± sd percentage compared to control of 3 independent experiments performed in duplicate. *P < 0.05.
Role of SK1 and SK2 down-regulation in U87MG cells on in vitro HUVEC tube formation
Since modulation only of SK1 affected cellular S1P levels in glioma cells, we next evaluated a role for glioma SK1 on HUVEC neoangiogenesis. Glioma cells were treated with scrambled siRNA or SK1 siRNA, embedded in Matrigel, and cultured for 72 h. HUVECs were then plated atop the Matrigel-glioma cell mixture and allowed to form tubes for 4 h. HUVECs plated on Matrigel mixed with PBS were used as negative control and did not induce noticeable tube-like structures (data not shown). Incubation of HUVECs atop Matrigel mixed with glioma cells treated with scrambled siRNA formed tube-like structures (Fig. 5A), and this effect was attenuated when HUVECs were plated atop U87MG cells treated with SK1 siRNA. Parallel experiments using SK2 siRNA did not have any effect on modulation of tube-like formation (Supplemental Fig. 1B).
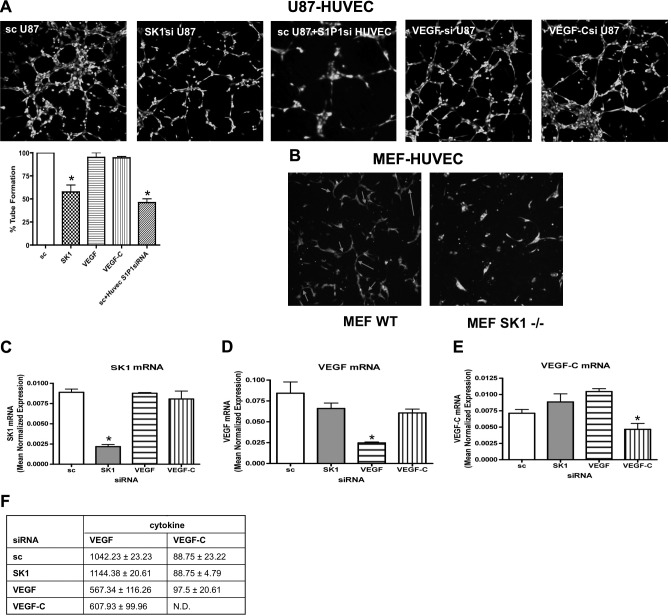
Figure 5.
SK1 down-regulation in U87MG cells decreases in vitro angiogenesis. A) U87MG cells treated with 20 nM sc, SK1, VEGF, or VEGF-C for 24 h were trypsinized, mixed with growth factor-reduced Matrigel, and seeded in a 24-well dish. After 48 h, HUVECs loaded with fluorescent cellTracker Green CMFDA were plated on top of the mixture of Matrigel and cells, and tube formation was observed using a confocal microscope. In one case, HUVECs were pretreated with 10 nM S1P1 siRNA for 48 h and then plated on top of U87MG cells treated with 20 nM sc. Graphs show tube formation as mean ± sd percentage compared to control of 3 independent experiments performed in triplicate. B) HUVECs loaded with fluorescent cellTracker CMFDA were plated on top of a confluent layer of MEF WT or MEF SK1−/− cells. Cells were cocultivated for 48 h. Cell differentiation was observed using a confocal microscope. Photos are representative of 2 independent experiments performed in quadruplicate. C–E) U87MG cells treated with 20 nM scrambled, SK1, VEGF, and VEGF-C siRNA were collected and subjected to mRNA extraction and real-time PCR analysis for SK1 (C), VEGF (D), and VEGF-C (E). Real-Time PCR data are expressed as mean± sd normalized expression of 3 independent mRNA extractions, using β-actin as a reference gene. F) U87MG cells treated with different siRNAs for 72 h were incubated for 2 h in EGM + 0.1% BSA, and the conditioned medium was used to test the presence of VEGF and VEGF-C using an ELISA kit. Data are means ± sd, normalized for protein content, of 2 independent experiments performed in quadruplicate. *P < 0.05.
To further confirm the role of SK1 in tube formation, we obtained MEFs from both WT and Sphk1−/− mice. Sphk1−/− MEFs had less intracellular and extracellular S1P compared to WT MEFs when measured by mass spectrometry (data not shown). WT and Sphk1−/− MEFs were mixed with Matrigel for 4 d, and HUVECs were plated atop the Matrigel-MEF mixture and allowed to form tubes for 4 h. As shown in Fig. 5B, HUVECs plated atop Sphk1−/− MEFs were unable to form complete tube structures in comparison to WT MEFs.
Collectively, these data suggest that SK1 in tumor cells may be sufficient to induce an HUVEC angiogenic response. In contrast, VEGF and VEGF-C down-regulation was not sufficient to block the angiogenic switch triggered by the tumor cells (Fig. 5A). Moreover, HUVECs treated with 20 nM S1P1 siRNA for 48 h and plated on top of U87 cells failed to form tube-like structures (Fig. 5A), indicating that the S1P1 receptor likely mediates the signal necessary to differentiate endothelial cells on S1P binding. The specificity of each gene down-regulation was assessed by real-time PCR (Fig. 5C–E). Moreover, cytokines (VEGF and VEGF-C) released from a glioma after each treatment did not change (Fig. 5F).
Role of SK1 in HUVEC sprouting
Next, we evaluated the role of SK1 in HUVEC cell alignment using a fibrin bead assay. As shown in Fig. 6 (top panel), endothelial cells were mixed with dextran-coated beads and resuspended in fibrin gel, on top of which WT or Sphk1−/− MEFs were plated. In some experiments, HUVEC-coated beads were incubated in the presence or absence of S1P for 48 h. As shown in Fig. 6 (bottom panel), S1P initiated endothelial cell sprouting. However, after 4 d of S1P administration, S1P alone was not sufficient for the formation of mature capillaries because the vessels tended to be thin and developed only slit-like intracellular lumens. Moreover, many cells migrated away from the beads. This suggests that S1P could initiate the sprouting of endothelial cells, but could not form complete vessels. As shown by Nakatsu et al. (31), when fibroblasts were plated atop fibrin gel, HUVEC-coated beads formed complete vessels due to different growth factors released by fibroblasts. With this evidence, we evaluated whether MEFs derived from Sphk1−/− mice could induce HUVEC sprouting compared to MEFs derived from WT mice. As shown in Fig. 6, HUVECs were unable to start sprouting in the presence of Sphk1−/− MEFs. This further confirms the hypothesis that S1P is critical in the initial steps of angiogenesis.
Figure 6.
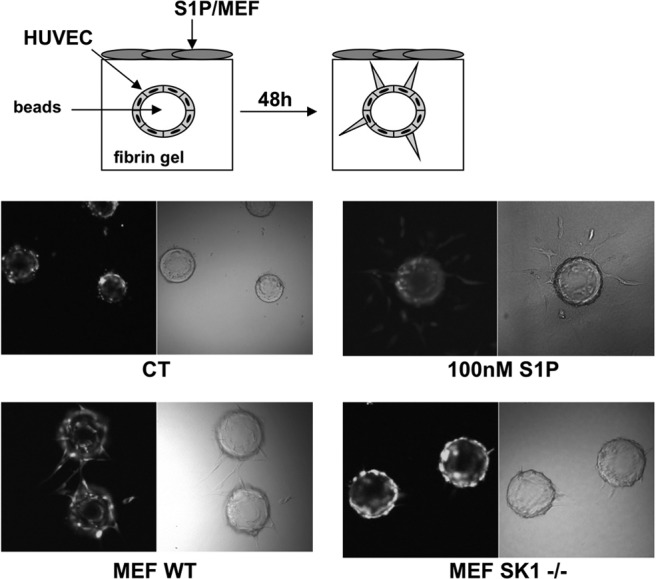
S1P is involved in the initial step of angiogenesis. HUVECs loaded with cellTracker CMFDA were cultured on microcarrier beads and embedded in fibrin gel for 48 h in the absence (ct) or presence of 100 nM S1P. In another set of experiments. beads with HUVECs were cocultivated with MEF WT or MEF SK1−/− cells for 48 h. Cell differentiation was observed using a confocal microscope. Photos are representative of 2 independent experiments performed in quadruplicate. *P < 0.05.
Role of SK1 and SK2 in S1P synthesis and release in breast cancer-derived MDA-MB-231 cells
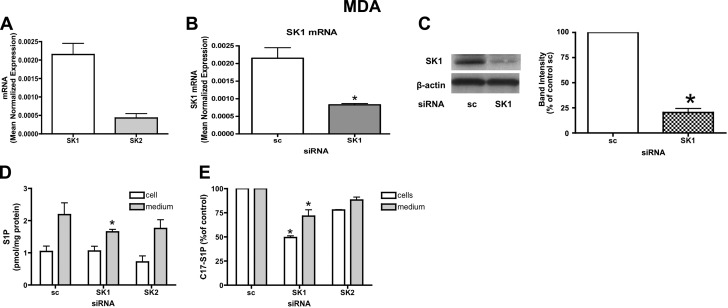
Next, we investigated whether endogenous SK1 and SK2 are involved in vitro lymphangiogenesis; the latter process is thought to be an important step in the metastasis of advanced breast cancer. This concept was supported by recent work indicating that S1P was involved in regulating lymphangiogenesis (25). We wished to evaluate whether SK within tumor cells could influence in vitro lymphangiogenesis. To test this, we chose MDA-MB-231 breast cancer cells and evaluated levels of SK1 and SK2 expression. MDA-MB-231 breast cancer cells were found to have high levels of SK1 and low levels of SK2 expression (Fig. 7A). Next, we evaluated whether SK1 and SK2 siRNA treatment could decrease S1P production and extracellular S1P release. SK1 and SK2 message were ∼75% down-regulated after 72 h of silencing (Fig. 7B and Supplemental Fig. 1A, respectively); moreover SK1 protein levels were also significantly decreased by SK1 siRNA (Fig. 7C). Intra- and extracellular levels of S1P were then measured by mass spectrometry. Unexpectedly, intracellular S1P was not changed after SK1 down-regulation, but extracellular S1P levels were significantly reduced (Fig. 7D). In contrast, SK2 siRNA treatment had no effect on intracellular or extracellular S1P. The lack of change in intracellular steady state levels of S1P on knockdown of each SK in this cell line may be due to compensatory regulation of the other SK- or of S1P-metabolizing enzymes (lyase, phosphatase). Next, to test the rate of S1P production and release in response to the knockdown of SK1 and SK2, MDA-MB-231 cells were pretreated with the indicated siRNA for 72 h, incubated for 2 h in trapping medium, and pulsed for 10 min with C17-d-erythro-sphingosine to a final concentration of 1 μM. C17-S1P was measured in the cells and in the medium by mass spectrometry. The results (Fig. 7E) show that SK1 RNAi treatment caused a significant decrease in both the intracellular and extracellular levels of C17-S1P, 48 and 25%, respectively. Also in this assay, SK2 siRNA treatment did not influence intra- or extracellular C17-S1P levels. These data indicate that in these cells, knockdown of SK1 but not of SK2 likely affects newly synthesized and released S1P and may more accurately reflect acute changes in S1P levels.
Figure 7.
SK1 down-regulation causes intra- and extracellular decrease of S1P in MDA-MB-231 cells. A) MDA-MB-231 cells were subjected to mRNA extraction and real-time PCR analysis for hSK1 and hSK2. Real-time PCR data are expressed as mean ± sd normalized expression of 3 independent experiments, using β-actin as a reference gene. B) MDA-MB-231 cells treated with 20 nM scrambled (sc) or SK1 siRNA for 72 h were collected using PARIS kit lysis buffer and submitted to mRNA extraction and real-time PCR analysis for hSK1. Real-time PCR data are expressed as mean ± sd normalized expression of 3 independent experiments, using β-actin as a reference gene. C) Cell lysates were analyzed by Western blot. Membranes were probed with the following antibodies: hSK1 (1:500) and actin (1:10,000). Blot is representative of 3 separate experiments performed in duplicate. D) MDA-MB-231 cells treated with sc or SK1 siRNA for 72 h were incubated for 2 h in trapping medium (described in Materials and Methods). At the end of incubation, cells were collected and analyzed by mass spectrometry for intra- and extracellular S1P content. Results are means ± sd (pmol/mg protein) of 3 independent experiments performed in duplicate. E) MDA-MB-231 cells treated with sc or SK1 siRNA as described above were incubated for 2 h in trapping medium and pulsed for 10 min with C17-d-erythro-sphingosine to a final concentration of 1 μM. C17-S1P was measured in cells and medium by mass spectrometry. Results are mean ± sd percentage compared to control of 3 independent experiments performed in duplicate. *P < 0.05.
Role of SK1 and SK2 down-regulation in MDA-MB-231 on tube formation in cocultured HMVECs
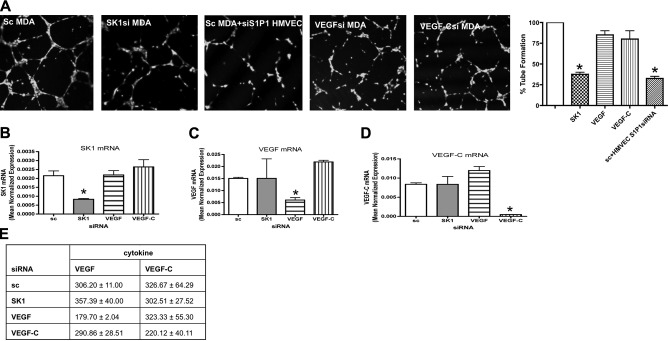
Since down-regulation of SK1 but not SK2 affected extracellular S1P levels, we next set out to evaluate the role of SK1 in MDA-MB-231-induced in vitro lymphangiogenesis. First, MDA cells treated with scrambled siRNA or SK1 siRNA were embedded in Matrigel and cultured for 72 h. HMVECs were then plated atop the Matrigel-MDA cell mixture and allowed to form tubes for 4 h. Incubation of HMVECs atop Matrigel mixed with MDA cells treated with scrambled siRNA formed tube-like structures (Fig. 8A); this effect was attenuated when HMVECs were plated atop MDA-MB231 treated with SK1 siRNA. Parallel experiments using SK2 siRNA did not have any effect on modulation of tube-like formation (Supplemental Fig. 1C). These data suggest that SK1 in tumor cells may be sufficient to induce the HMVEC lymphangiogenic response. In contrast, VEGF-A and VEGF-C down-regulation was insufficient to block the lymphangiogenic switch triggered by tumor cells. Moreover, when HMVECs were treated for 2 d with 10 nM S1P1 siRNA (the S1P receptor predominantly expressed in this cell line) and then plated on top of MDA cells, there was a significant reduction in their capacity to form tube-like structures. The specificity of each gene down-regulation was assessed by real-time PCR (Fig. 8B–D). Moreover, cytokines release from MDA-MB-231 cells (VEGF and VEGF-C) after each treatment with siRNA did not change (Fig. 8E).
Figure 8.
SK1 down-regulation in MDA decreases in vitro lymphangiogenesis. A) MD-MB-231 cells treated with 20 nM sc, SK1, VEGF, or VEGF-C for 24 h were trypsinized, mixed with growth factor-reduced Matrigel, and seeded in a 24-well dish. After 48 h, HMVECs loaded with fluorescent cellTracker Green CMFDA were plated on the top of the mixture of Matrigel and cells, and tube formation was observed using a confocal microscope. In one experiment, HMVECs were pretreated with 10 nM S1P1 siRNA for 48 h and then plated on top of MDA cells treated with 20 nM sc. Graphs show tube formation as a percentage of control value. Cell differentiation was observed using a confocal microscope. Photos are representative of 2 independent experiments performed in quadruplicate. B–D) MDA treated with 20 nM scrambled, SK1, VEGF, and VEGF-C siRNA were collected and subjected to mRNA extraction and real-time PCR analysis for SK1 (B), VEGF (C), and VEGF-C (D). Real-time PCR data are expressed as mean ± sd normalized expression of 3 independent mRNA extractions, using β-actin as reference gene. E) MDA treated with different siRNAs for 72 h were incubated for 2 h in EGM + 0.1% BSA, and conditioned medium was used to test the presence of VEGF and VEGF-C using an ELISA kit. Data are means ± sd, normalized for the protein content, of 2 independent experiments performed in quadruplicate. *P < 0.05.
DISCUSSION
S1P is an important sphingolipid metabolite involved in tumor development and progression. Although a role for S1P in angiogenesis and lymphangiogenesis has been established, it is unclear if S1P is generated by aberrant cancer cells, tumor-associated stroma, adjacent endothelial cells, or immune cell infiltrate. SK1 has been shown to be increased in many tumors; therefore, in this study we investigated whether SK1 modulation in a tumor cells could initiate an angiogenic and lymphangiogenic switch in a paracrine manner. To test this hypothesis, we overexpressed SK1 in HEK cells, which already express very low levels of endogenous SK1, and observed an increase in vitro angiogenesis and lymphangiogenesis. In addition, we down-regulated SK1 and SK2 in 2 cancer cell lines that have high levels of SK1 (but lower levels of SK2) expression and activity: U87MG, a glioma-derived cell line, and MDA-MB-231, a breast cancer-derived cell line. Our data show, for the first time, that SK1 overexpression or down-regulation in cells modulates extracellular S1P levels, which in turn regulates in vitro angiogenesis and lymphangiogenesis. In contrast, down-regulation of SK2 did not appear to affect intra or extracellular S1P levels nor in vitro angiogenesis and lymphangiogenesis in these cells. Interestingly, we found that down-regulation of VEGF-A or VEGF-C alone was insufficient to block the angiogenic and lymphangiogenic switch triggered by these cancer cells. Moreover, S1P alone could initiate endothelial cell sprouting in a 3-dimensional collagen gel, which is representative of a multistep angiogenic process.
Based on information from loss- and gain-of-function studies, we know that key signaling pathways in endothelial cell activation involve VEGF-A and the Notch signaling pathway. Subsequently, slit, ephrins, cadherins, wnts and angiopoetins, transforming growth factor β, and integrins participate in postactivation stages to guide, remodel, stabilize, and differentiate the newly formed vessels (32). VEGF-A binds 2 receptor-type tyrosine kinases, VEGFR1 and VEGFR2, and transduces major signals for angiogenesis and vascular permeability. VEGF-A expression is also induced by hypoxia and regulates not only physiological but also pathological angiogenesis, such as tumor angiogenesis (33, 34). VEGF-C primarily acts through VEGFR3, and this system is the first known to directly regulate lymphangiogenesis (35). It is also well established that S1P is key in the angiogenic process. Our data show that S1P released by cancer cells can initiate the angiogenic switch alone. Indeed, we have previously shown in our laboratory (21) that hypoxia stimulates S1P release, an event that occurs in the initial phases of tumor formation. Therefore, it is possible that S1P release could occur before or concurrently with VEGF activation and release. However, we noticed that even though S1P can initiate endothelial cell sprouting, it was insufficient for formation of mature capillaries alone.
S1P is emerging as a new and highly potent proangiogenic factor, which acts on endothelial cells through activation of specific G-coupled receptors S1P1–5(36). In particular, it has been shown that S1P is sufficient for the induction of HUVEC tube formation within a Matrigel matrix in vitro and in vivo, as assessed by a Matrigel plug assay (22). Interestingly, a recent study revealed that the addition of an anti-S1P antibody into several mouse xenograft models inhibited in vivo angiogenesis and tumor growth (37). Moreover, several reports support the idea that S1P has a potent migratory effect on vascular endothelial cells (22, 23, 38, 39). Recently, S1P was described as being able mediate lymphangiogenesis (25); the authors showed that S1P induced migration and capillary-like tube formation in human lymphatic endothelial cells in vitro. Lymphangiogenesis is a process that occurs in the context of tissue repair, inflammation, and tumor growth, and increasing evidence suggests that tumors can actively induce lymphangiogenesis and that tumor-associated lymphangiogenesis actively promotes cancer metastasis (40). In our study we observed that S1P can induce in vitro endothelial tube formation and cell migration in endothelial cell lines derived from vascular and lymphatic systems at nanomolar concentrations. Furthermore, we also demonstrated that S1P induces cell migration in both the vascular and lymphatic endothelial cell type through the activation of Rac1. Most important, we showed that modulation of intracellular SK1 but not SK2 in tumor cells leads to modulation of intracellular and extracellular S1P and thus may regulate S1P in tumors. SIP secretion can cause directional endothelial cell migration to the tumor mass.
It has been established that different kinds of solid tumor are characterized by high expression of SK1 when compared to the adjacent tissue (16, 17, 19). This evidence prompted us to investigate whether the up- and down-regulation of SK1 could modulate the intracellular and extracellular S1P, which may be a direct effector involved in tumor progression. In our system, we found that up-regulation or down-regulation of SK1 increased or decreased S1P released from cells manipulated with insertion of a plasmid containing human SK1 or siRNA technology. In contrast down-regulation of SK2 by siRNA did not affect intra- or extracellular levels of S1P in either cell line. These data do not preclude a role for SK2 in regulating S1P levels, but because SK2 levels were already low in these cells, likely indicate a less prominent role for SK2 in regulating S1P levels in these cells.
We also found that S1P derived from tumor cells can increase or decrease the ability of endothelial cells to form tube-like structures, and we confirmed these results in MEFs obtained from WT and Sphk1−/− mice. Notably, our results suggest that the modulation of VEGF-A and VEGF-C is insufficient to stop the organization of endothelial cells into tubular structures, suggesting that these growth factors could act in concert with or secondarily to S1P in this process. These observations are in agreement with Visentin et al. (37), who demonstrated that S1P induces substantial increases in VEGF release from tumor cell lines in vitro. Moreover, they demonstrated that MDA MB-231 xenografted animals treated with anti-S1P antibody had significantly reduced circulating proangiogenic cytokines, compared to control xenografted animals. Both in vitro and in vivo data revealed that one of the effects of S1P on angiogenesis might be its ability to induce the release of a variety of other proangiogenic compounds.
In summary, our data indicate for the first time that S1P could originate from tumor cells overexpressing SK1 and could initiate an in vitro angiogenic and lymphangiogenic switch. Moreover, we found that S1P is sufficient to initiate endothelial cell sprouting in a 3-dimensional collagen matrix, which is representative of a multistep angiogenic process. Altogether our data suggest that tumor cells, which are characterized by high levels of SK1 expression, could release S1P into the extracellular space, which in turn could lead to endothelial cell migration and organization into tubular structures.
Supplementary Material
Acknowledgments
The authors thank Kathy Wiita-Fisk for administrative assistance. The authors also thank the Lipidomics Core at the Medical University of South Carolina. Grant support came from National Institutes of Health grants NIH/NIGMS GM062887 (L.M.O.), NIH/NCI CA097132 (L.M.O.), NIH/P20 RR17677 for the Centers of Biomedical Research Excellence (COBRE) Lipidomics Core, and Hollings Cancer Center core grant P30-CA-56036 for the Microscopy Core.
References
- Spiegel S., Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- Olivera A., Spiegel S. Sphingosine-1-phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature. 1993;365:557–560. doi: 10.1038/365557a0. [DOI] [PubMed] [Google Scholar]
- Zhang H., Desai N. N., Olivera A., Seki T., Brooker G., Spiegel S. Sphingosine-1-phosphate, a novel lipid, involved in cellular proliferation. J Cell Biol. 1991;114:155–167. doi: 10.1083/jcb.114.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodemote K. A., Mattie M. E., Berger A., Spiegel S. Involvement of a pertussis toxin-sensitive G protein in the mitogenic signaling pathways of sphingosine 1-phosphate. J Biol Chem. 1995;270:10272–10277. doi: 10.1074/jbc.270.17.10272. [DOI] [PubMed] [Google Scholar]
- Lee M. J., Van Brocklyn J. R., Thangada S., Liu C. H., Hand A. R., Menzeleev R., Spiegel S., Hla T. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science. 1998;279:1552–1555. doi: 10.1126/science.279.5356.1552. [DOI] [PubMed] [Google Scholar]
- Alvarez S. E., Milstien S., Spiegel S. Autocrine and paracrine roles of sphingosine-1-phosphate. Trends Endocrinol Metab. 2007;18:300–307. doi: 10.1016/j.tem.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Yatomi Y., Ozaki Y., Ohmori T., Igarashi Y. Sphingosine 1-phosphate: synthesis and release. Prostaglandins. 2001;64:107–122. doi: 10.1016/s0090-6980(01)00103-4. [DOI] [PubMed] [Google Scholar]
- Hanel P., Andreani P., Graler M. H. Erythrocytes store and release sphingosine 1-phosphate in blood. FASEB J. 2007;21:1202–1209. doi: 10.1096/fj.06-7433com. [DOI] [PubMed] [Google Scholar]
- Venkataraman K., Lee Y. M., Michaud J., Thangada S., Ai Y., Bonkovsky H. L., Parikh N. S., Habrukowich C., Hla T. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ Res. 2008;102:669–676. doi: 10.1161/CIRCRESAHA.107.165845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anelli V., Bassi R., Tettamanti G., Viani P., Riboni L. Extracellular release of newly synthesized sphingosine-1-phosphate by cerebellar granule cells and astrocytes. J Neurochem. 2005;92:1204–1215. doi: 10.1111/j.1471-4159.2004.02955.x. [DOI] [PubMed] [Google Scholar]
- Ancellin N., Colmont C., Su J., Li Q., Mittereder N., Chae S. S., Stefansson S., Liau G., Hla T. Extracellular export of sphingosine kinase-1 enzyme: sphingosine 1-phosphate generation and the induction of angiogenic vascular maturation. J Biol Chem. 2002;277:6667–6675. doi: 10.1074/jbc.M102841200. [DOI] [PubMed] [Google Scholar]
- Venkataraman K., Thangada S., Michaud J., Oo M. L., Ai Y., Lee Y. M., Wu M., Parikh N. S., Khan F., Proia R. L., Hla T. Extracellular export of sphingosine kinase-1a contributes to the vascular S1P gradient. Biochem J. 2006;397:461–471. doi: 10.1042/BJ20060251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitson S. M., D'Andrea R., J., Vandeleur L., Moretti P. A., Xia P., Gamble J. R., Vadas M. A., Wattenberg B. W. Human sphingosine kinase: purification, molecular cloning and characterization of the native and recombinant enzymes. Biochem J. 2000;350:429–441. [PMC free article] [PubMed] [Google Scholar]
- Kohama T., Olivera A., Edsall L., Nagiec M. M., Dickson R., Spiegel S. Molecular cloning and functional characterization of murine sphingosine kinase. J Biol Chem. 1998;273:23722–23728. doi: 10.1074/jbc.273.37.23722. [DOI] [PubMed] [Google Scholar]
- Liu H., Sugiura M., Nava V. E., Edsall L. C., Kono K., Poulton S., Milstien S., Kohama T., Spiegel S. Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J Biol Chem. 2000;275:19513–19520. doi: 10.1074/jbc.M002759200. [DOI] [PubMed] [Google Scholar]
- Johnson K. R., Johnson K. Y., Crellin H. G., Ogretmen B., Boylan A. M., Harley R. A., Obeid L. M. Immunohistochemical distribution of sphingosine kinase 1 in normal and tumor lung tissue. J Histochem Cytochem. 2005;53:1159–1166. doi: 10.1369/jhc.4A6606.2005. [DOI] [PubMed] [Google Scholar]
- Kawamori T., Osta W., Johnson K. R., Pettus B. J., Bielawski J., Tanaka T., Wargovich M. J., Reddy B. S., Hannun Y. A., Obeid L. M., Zhou D. Sphingosine kinase 1 is up-regulated in colon carcinogenesis. FASEB J. 2006;20:386–388. doi: 10.1096/fj.05-4331fje. [DOI] [PubMed] [Google Scholar]
- Vadas M., Xia P., McCaughan G., Gamble J. The role of sphingosine kinase 1 in cancer: oncogene or non-oncogene addiction? Biochim Biophys Acta. 2008;1781:442–447. doi: 10.1016/j.bbalip.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Van Brocklyn J. R., Jackson C. A., Pearl D. K., Kotur M. S., Snyder P. J., Prior T. W. Sphingosine kinase-1 expression correlates with poor survival of patients with glioblastoma multiforme: roles of sphingosine kinase isoforms in growth of glioblastoma cell lines. J Neuropathol Exp Neurol. 2005;64:695–705. doi: 10.1097/01.jnen.0000175329.59092.2c. [DOI] [PubMed] [Google Scholar]
- Li W., Yu C. P., Xia J. T., Zhang L., Weng G. X., Zheng H. Q., Kong Q. L., Hu L. J., Zeng M. S., Zeng Y. X., Li M., Li J., Song L. B. Sphingosine kinase 1 is associated with gastric cancer progression and poor survival of patients. Clin Cancer Res. 2009;15:1393–1399. doi: 10.1158/1078-0432.CCR-08-1158. [DOI] [PubMed] [Google Scholar]
- Anelli V., Gault C. R., Cheng A. B., Obeid L. M. Sphingosine kinase 1 is up-regulated during hypoxia in U87MG glioma cells: role of hypoxia-inducible factors 1 and 2. J Biol Chem. 2008;283:3365–3375. doi: 10.1074/jbc.M708241200. [DOI] [PubMed] [Google Scholar]
- Lee O. H., Kim Y. M., Lee Y. M., Moon E. J., Lee D. J., Kim J. H., Kim K. W., Kwon Y. G. Sphingosine 1-phosphate induces angiogenesis: its angiogenic action and signaling mechanism in human umbilical vein endothelial cells. Biochem Biophys Res Commun. 1999;264:743–750. doi: 10.1006/bbrc.1999.1586. [DOI] [PubMed] [Google Scholar]
- English D., Kovala A. T., Welch Z., Harvey K. A., Siddiqui R. A., Brindley D. N., Garcia J. G. Induction of endothelial cell chemotaxis by sphingosine 1-phosphate and stabilization of endothelial monolayer barrier function by lysophosphatidic acid, potential mediators of hematopoietic angiogenesis. J Hematother Stem Cell Res. 1999;8:627–634. doi: 10.1089/152581699319795. [DOI] [PubMed] [Google Scholar]
- Chae S. S., Paik J. H., Furneaux H., Hla T. Requirement for sphingosine 1-phosphate receptor-1 in tumor angiogenesis demonstrated by in vivo RNA interference. J Clin Invest. 2004;114:1082–1089. doi: 10.1172/JCI22716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon C. M., Hong B. S., Moon H. G., Lim S., Suh P. G., Kim Y. K., Chae C. B., Gho Y. S. Sphingosine-1-phosphate promotes lymphangiogenesis by stimulating S1P1/Gi/PLC/Ca2+ signaling pathways. Blood. 2008;112:1129–1138. doi: 10.1182/blood-2007-11-125203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. R., Becker K. P., Facchinetti M. M., Hannun Y. A., Obeid L. M. PKC-dependent activation of sphingosine kinase 1 and translocation to the plasma membrane. J Biol Chem. 2002;277:35257–35262. doi: 10.1074/jbc.M203033200. [DOI] [PubMed] [Google Scholar]
- Lee Y. M., Venkataraman K., Hwang S. I., Han D. K., Hla T. A novel method to quantify sphingosine 1-phosphate by immobilized metal affinity chromatography (IMAC) Prostaglandins Other Lipid Mediat. 2007;84:154–162. doi: 10.1016/j.prostaglandins.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielawski J., Szulc Z. M., Hannun Y. A., Bielawska A. Simultaneous quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods. 2006;39:82–91. doi: 10.1016/j.ymeth.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Spassieva S., Bielawski J., Anelli V., Obeid L. M. Combination of C(17) sphingoid base homologues and mass spectrometry analysis as a new approach to study sphingolipid metabolism. Methods Enzymol. 2007;434:233–241. doi: 10.1016/S0076-6879(07)34012-3. [DOI] [PubMed] [Google Scholar]
- Nakatsu M. N., Davis J., Hughes C. C. Optimized fibrin gel bead assay for the study of angiogenesis. J Vis Exp. 2007;3:186. doi: 10.3791/186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsu M. N., Sainson R. C., Aoto J. N., Taylor K. L., Aitkenhead M., Perez-del-Pulgar S., Carpenter P. M., Hughes C. C. Angiogenic sprouting and capillary lumen formation modeled by human umbilical vein endothelial cells (HUVEC) in fibrin gels: the role of fibroblasts and angiopoietin-1. Microvasc Res. 2003;66:102–112. doi: 10.1016/s0026-2862(03)00045-1. [DOI] [PubMed] [Google Scholar]
- Rossant J., Howard L. Signaling pathways in vascular development. Annu Rev Cell Dev Biol. 2002;18:541–573. doi: 10.1146/annurev.cellbio.18.012502.105825. [DOI] [PubMed] [Google Scholar]
- Ferrara N., Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- Shibuya M., Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2006;312:549–560. doi: 10.1016/j.yexcr.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Joukov V., Pajusola K., Kaipainen A., Chilov D., Lahtinen I., Kukk E., Saksela O., Kalkkinen N., Alitalo K. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996;15:290–298. [PMC free article] [PubMed] [Google Scholar]
- Sanchez T., Hla T. Structural and functional characteristics of S1P receptors. J Cell Biochem. 2004;92:913–922. doi: 10.1002/jcb.20127. [DOI] [PubMed] [Google Scholar]
- Visentin B., Vekich J. A., Sibbald B. J., Cavalli A. L., Moreno K. M., Matteo R. G., Garland W. A., Lu Y., Yu S., Hall H. S., Kundra V., Mills G. B., Sabbadini R. A. Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell. 2006;9:225–238. doi: 10.1016/j.ccr.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Wang F., Van Brocklyn J. R., Hobson J. P., Movafagh S., Zukowska-Grojec Z., Milstien S., Spiegel S. Sphingosine 1-phosphate stimulates cell migration through a G (i) -coupled cell surface receptor Potential involvement in angiogenesis. J Biol Chem. 1999;274:35343–35350. doi: 10.1074/jbc.274.50.35343. [DOI] [PubMed] [Google Scholar]
- Kimura T., Watanabe T., Sato K., Kon J., Tomura H., Tamama K., Kuwabara A., Kanda T., Kobayashi I., Ohta H., Ui M., Okajima F. Sphingosine 1-phosphate stimulates proliferation and migration of human endothelial cells possibly through the lipid receptors, Edg-1 and Edg-3. Biochem J. 2000;348:71–76. [PMC free article] [PubMed] [Google Scholar]
- Skobe M., Hawighorst T., Jackson D. G., Prevo R., Janes L., Velasco P., Riccardi L., Alitalo K., Claffey K., Detmar M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.