Abstract
Children grow, but adults do not. The cessation of growth in multiple organs is the end result of a progressive decline in cell proliferation beginning in early life. The mechanisms responsible for this growth deceleration are largely unknown. Using expression microarray and real-time PCR, we identified a common program of gene expression in lung, kidney, and liver during growth deceleration in juvenile rats. Gene ontology analyses and siRNA-mediated knockdown in vitro indicated that many of the down-regulated genes are growth promoting. Down-regulated genes in the program showed declining histone H3K4 trimethylation with age, implicating underlying epigenetic mechanisms. To investigate the physiological processes driving the genetic program, a tryptophan-deficient diet was used to temporarily inhibit juvenile growth in newborn rats for 4 wk. Afterward, microarray analysis showed that the genetic program had been delayed, implying that it is driven by body growth itself rather than age. Taken together, the findings suggest that growth in early life induces progressive down-regulation of a large set of proliferation-stimulating genes, causing organ growth to slow and eventually cease.—Lui, J. C., Forcinito, P., Chang, M., Chen, W., Barnes, K. M., Baron, J. Coordinated postnatal down-regulation of multiple growth-promoting genes: evidence for a genetic program limiting organ growth.
Keywords: organ size, microarray, histone modification, proliferation
The human fetus grows at an enormous rate, increasing in mass >100-fold between the end of embryogenesis and birth (1). Somatic growth then slows progressively in postnatal life and ceases by the end of the second decade. The deceleration in body growth is due primarily to a progressive decline in cell proliferation (2, 3), but the underlying mechanisms are largely unknown.
One clue regarding the mechanism is that growth deceleration occurs coordinately in multiple organs in order to maintain body proportions, and yet this coordination does not appear to be orchestrated by a systemic mechanism. Hormones, such as growth hormone, thyroid hormone, and IGF-I, may modulate the growth rate and affect adult body size (4), but levels of these hormones do not decline with age and thus could not account for growth deceleration (5, 6). Furthermore, rapid proliferation continues in juvenile organs that are transplanted into adult recipients (7,8,9), which suggests that declining growth rate is an intrinsic property of the organs. We therefore hypothesized that postnatal growth deceleration results from a genetic program that is occurring simultaneously in multiple tissues. Consistent with this hypothesis, we previously identified an extensive program of gene expression that is occurring between 1 and 8 wk of age simultaneously in lung, kidney, and heart of mice (10). In the current study, we sought to determine whether this postnatal genetic program is in fact responsible for growth deceleration, and to explore the prior physiological and molecular mechanisms that drive this program.
MATERIALS AND METHODS
Animal procedures
All animal procedures were approved by the National Institute of Child Health and Human Development Animal Care and Use Committee. Sprague-Dawley rats were obtained from Charles River Laboratories (Raleigh, NC, USA) and provided with food and water ad libitum. Delay of postnatal growth in male rat pups was induced by giving a tryptophan-deficient diet (11, 12) (25 mg/100 g food; Harland Teklad, Madison, WI, USA) to the lactating mother from birth of the pups until they reached 4 wk of age. As a control, lactating mothers received a diet that was identical but with adequate tryptophan for normal nutritional needs (280 mg/100 g food). Both groups were switched to regular chow (Zeigler Bros, Gardners, PA, USA) at 4 wk of age. The control group was weaned at 3 wk and the tryptophan deficient group at 4.5 wk because of difference in pup size and maturity. The litter sizes in both groups were maintained at 10–12. Animal growth was assessed by anthropometric measures including body weight and tail length (weekly), organ weights, and tibia length (1, 2, 4, 5, 6, 8, 12, 16, and 20 wk).
RNA extraction and purification
Total RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA, USA) followed by RNeasy Mini Kit purification (Qiagen, Valencia, CA, USA). All RNA samples had a 260/280 nm ratio between 1.9 and 2.1. RNA integrity was determined using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA), and only high-quality RNA (28S/18S>1.8) was used for microarray analysis and real-time RT-PCR.
Expression microarray
Two micrograms of RNA per sample was processed and analyzed by the NIDDK Core Facility at the National Institutes of Health using Affymetrix Rat Genome 230 2.0 Array GeneChips (30,000 transcripts; Affymetrix, Santa Clara, CA, USA). Five chips were used for each organ (kidney and lung) at each of 2 time points in the control group (1 and 5 wk) and one time point in the tryptophan-deficient group (5 wk). Each microarray chip was hybridized to labeled RNA derived from a single animal. Raw data were deposited to the GEO database (accession number: GSE16792). Microarray signals were analyzed using the Affymetrix MAS5 algorithm. ANOVA was performed, and false discovery rate (FDR) reports were generated using Partek Pro software (Partek, St. Charles, MO, USA). Gene ontology analyses and pathway analyses were done using GeneGO (GeneGO, St. Joseph, MI, USA) and IPA Software 7.1 (Ingenuity Systems, Redwood City, CA, USA). Correlation analyses (Pearson’s pairwise comparison) were performed, and heat maps were generated using JMP 7 software (SAS Institute, Cary, NC, USA).
Quantitative real-time RT-PCR
Real-time PCR was used to assess specific mRNA levels in mice and rats. For mice, liver, kidney, lung, and heart at 1, 4, and 8 wk of age were dissected from C57BL/6 mice (n=5 animals/time point). In liver, 2 additional time points, embryonic day 16 (E16) and newborn, were added to compare prenatal and postnatal expression. Liver, kidney, and lung were dissected from Sprague Dawley rats at 1, 2, 4, 5, 6, and 8 wk of age for control group and 2, 4, 5, and 6 wk of age for the tryptophan-deficient group (n=5 animals/time point). Total RNA (100–200 ng) was reverse transcribed using SuperScript III Reverse Transcriptase (Invitrogen). Quantitative real-time PCR was performed for 18S, Mest, Plagl1, Peg3, Ezh2, Mdk, Gpc3, Mycn, Meis1, Gapdh, and Alb1 using commercially available FAM- or VIC-labeled Taqman assays, or custom-designed Taqman assays (Applied Biosystems, Foster City, CA, USA). All assays or primer pairs were intron-spanning to avoid amplification of genomic DNA. The assay ID or sequence information for different assays and primers are as follow: 18S, 4319413E; Gapdh (mouse), Mm99999915_g1; Alb1 (mouse), Mm00802090_m1; Mest (mouse), Mm00484993_m1; Mest (rat), Rn01500324_m1; Plagl1 (mouse), Mm00494250_m1; Plagl1 (rat), Rn00688897_m1; Peg3 (mouse), Mm01337379_m1; Peg3 (rat), custom designed taqman assay, forward primer 5′ AGACCGCTGGCCATACAC, reverse primer 5′ GTCTTGACATCACAGGGAGAGAAAG, probe 5′ CAGAAGCAGGATGCCTC; Ezh2 (mouse), Mm00468464_m1; Ezh2 (rat), custom designed taqman assay, forward primer 5′ GGGAGAGAACAACGATAAAGAAGAAGA, reverse primer 5′ GGCTTCATCTTTATTGGTGTTTGACA, probe 5′ TCCAGTTCCTCTGA AGCAA; Mdk (mouse), Mm00440279_m1; Mdk (rat), Rn00578324_m1; Gpc3 (mouse), Mm00516722_m1; Gpc3 (rat), Rn00516722_m1; Mycn (mouse), Mm00476449_m1; Mycn (rat), Rn01473353_m1; Meis1(mouse), Mm00487664_m1; Meis1 (rat), Rn00485207_m1. Reactions were performed in triplicate on cDNA derived from each animal using the ABI prism 7300 Sequence Detection System instrument (Applied Biosystems). The relative quantity of each mRNA was calculated using the formula Relative Expression = 2−ΔCt × 106, where Ct represents the threshold cycle and ΔCt = (Ct of gene of interest) − (Ct of 18S rRNA). Values were multiplied by 106 for convenience of comparison.
Primary hepatocyte culture
Primary murine hepatocytes were isolated (13) and cultured (14) on collagen-coated 12-well plates as described previously. Fetal liver from C57BL/6 E15 embryos was pooled, briefly minced, and digested with buffer containing 0.5 mg/ml collagenase II (Life Technologies, Carlsbad, CA, USA), and 0.1 mg/ml DNase I (Invitrogen) followed by hemolysis with hypotonic buffer (ACK Lysis Buffer; Quality Biological, Gaithersburg, MD, USA). Dissociated cells were resuspended in culture media composed of William’s medium E (Invitrogen) supplemented with 10% FBS (Life Technologies), 2 mM l-glutamine (Life Technologies), 1% nonessential amino acid solution (Life Technologies), 0.5 mM ascorbic acid (Sigma-Aldrich, St. Louis, MO, USA), 1 μM insulin (Sigma-Aldrich), 1 μM dexamethasone (Sigma-Aldrich), 10 ng/ml murine epidermal growth factor (R&D Systems, Minneapolis, MN, USA), and 50 μg/ml gentamicin (Life Technologies). Cells were plated onto collagen-coated tissue culture plates (BD Biosciences, San Jose, CA, USA). Four hours after plating, unattached hematopoietic cells and cell debris were removed by extensive washing with PBS.
siRNA-mediated knockdown in cultured hepatocytes
Prior to transfection, cells plated on collagen-coated 12-well plates were washed with PBS twice to further remove hematopoietic cells and replenished with 500 μl of Opti-MEM (Invitrogen). Transfection of primary fetal murine hepatocytes was performed 2 d after isolation, at 30–50% confluence as described previously (15, 16). Lipofectamine 2000 (Invitrogen; 2 μl/600 μl of Opti-MEM, final concentration) and siRNA (25–150 nM final concentration) were added. After incubation at 37°C, 5% CO2 for 5 h, transfection reagents were removed, and cells were replenished with 1 ml of culture medium. Efficiency of transfection was generally 80% with no evidence of cytotoxicity, assessed by green fluorescence observed after transfecting with fluorescein amidite (FAM)-labeled negative control siRNA (Ambion, Austin, TX, USA) or pmaxGFP vector (Amaxa, Walkersville, MD, USA). Knockdown efficiencies were assessed by comparing the mRNA levels (by real-time PCR) of the target gene in cells transfected with specific siRNA compared to cells transfected with negative control siRNA. For each gene of interest, ≥2 different siRNAs that successfully inhibit target gene expression were used. To minimize the biological variation due to litter, each siRNA was used in 3–5 replicate experiments using hepatocytes from different litters. siRNA sequences and dosage: Mest siRNA 1, 5′-GGCCUACGCAUCUUCUACCtt-3′, 25 nM; Mest siRNA 2, 5′-GGCUUUCUCAUAUAACUCCtt-3′, 25 nM; Plagl1 siRNA 1, 5′-GCCUUCGUCUCCAAGUAUAtt-3′, 150 nM; Plagl1 siRNA 2, 5′-GGACCACCUGAAGAACCACtt-3′, 25 nM; Peg3 siRNA 1, 5′-GGAAGAAGCCUUUUGAG UGtt-3′, 50 nM; Peg3 siRNA 2, 5′-CCUUCAGUAGGAGUGCUGCtt-3′, 50 nM; Ezh2 siRNA 1, 5′-GCUUGCAUUCAUUUCAUACtt-3′, 50 nM; Ezh2 siRNA 2, 5′-GGUUCAGAAGAGCUGA UGAtt-3′, 25 nM; Mdk siRNA 1, 5′-GGAAUUUGGAGCCGACUGCtt-3′, 100 nM; Mdk siRNA 2, 5′-GGAAAAGGAAAGGACUAAGtt-3′, 100 nM; Gpc3 siRNA 1, 5′-GCAAGACGUGACCU GAAAGtt-3′, 50 nM; Gpc3 siRNA 2, 5′-CCCUGAAUCUCGGAAUUGAtt-3′, 25 nM; Mycn siRNA 1, 5′-GGCGGUAACCACUUUCACGtt-3′, 25 nM; Mycn siRNA 2, 5′-CCUAAGUACU GUAAGAAUAtt-3′, 25 nM; Meis1 siRNA 1, 5′-GGGAAAAUGCCUAUCGAUUtt-3′, 75 nM; Meis1 siRNA 2, 5′-GGUUUCAUCUGUUGGAAUUtt-3′, 75 nM; Gapdh, sequence not disclosed by manufacturer, 25 nM.
Proliferation assessment by 3H-thymidine uptake
Forty-eight hours after transfection, primary hepatocytes were incubated with 1 ml of fresh culture medium containing 2.5 μCi of 3H-thymidine (25 Ci/mmol, GE Healthcare UK, Little Chalfont, UK) at 37°C, 5% CO2 for 5 h, and then vigorously washed 3 times with PBS. Radioactivity was measured by liquid scintillation counting. At least 3 experiments were performed for each siRNA using hepatocytes isolated from different litters. We concurrently isolated mRNA for real-time PCR to verify the knockdown of target genes in the same experiment. All effects of siRNA on proliferation were compared to the negative control siRNA at equivalent dosage in the corresponding experiment.
5-Bromo-2-deoxyuridine (BrdU) immunohistochemistry
The proliferation rate in rat organs was determined by BrdU staining. BrdU was injected (0.1 mg/g body mass i.p.; Sigma-Aldrich) 2 h before the rats were killed. Tissues were fixed in 10% phosphate-buffered formalin for 24 h and transferred to 70% ethanol for storage. Tissues were embedded in paraffin, and 4 μm sections were mounted on Superfrost Plus slides. BrdU labeling was detected by immunohistochemistry using the BrdU In-Situ Detection Kit (BD Biosciences). Tissue slides were viewed by transmission light microscopy using an ×100 objective lens. In kidney, we assessed only tubular epithelial nuclei in the cortex. In liver, we assessed hepatocytes. In lung, we assessed pneumocytes. Five animals were used for each time point. For each animal, 5 fields were examined for BrdU-labeled nuclei. The total number of cells was determined from 2 electronic images of representative fields. The BrdU labeling index (BLI) is defined as BrdU labeled nuclei/total nuclei. The BLI is proportional to the fraction of cells in S phase and therefore reflects the proliferation rate.
Chromatin immunoprecipitation (ChIP)
Cell nuclei from animal tissues were isolated as described previously (17, 18). Briefly, liver, kidney, and lung (50–150 mg) of C57BL/6 mice (5 animals/time point, processed individually) at 1 and 4 wk of age were homogenized with a Blaessig glass homogenizer on ice and then cross-linked with 1% formaldehyde for 10 min at room temperature. Pellets of cell nuclei were resuspended in SDS lysis buffer from a commercial kit (Upstate ChIP Assay Kit; Millipore, Billerica, MA, USA), and chromatin was fragmented to ∼500 bp by sonication. Then 100 ng of DNA was used for each assay (Upstate ChIP Assay Kit; Millipore). Rabbit polyclonal antibody against acetyl-H3K9/K14 (Millipore; catalog no. 06-599, lot no. DAM1422332), trimethyl-H3K27 (Millipore; 07-449, lot DAM1514001), trimethyl-H3K4 (Abcam, Cambridge, MA, USA; ab8580, lots 533386, 598382), and nonspecific rabbit IgG (Millipore; PP64, lot LV1459875) were used for immunoprecipitation. After ChIP, the chromatin cross-linking was reversed, and DNA was recovered by phenol/chloroform extraction and ethanol precipitation. The DNA samples were reconstituted in deionized H2O. For each corresponding sample, a fraction of DNA without ChIP (input) was used to normalize the starting chromatin concentration. Histone modifications in different samples were quantified by real-time PCR using custom designed primers specific to regions close to the transcription start site.
For data in Fig. 6, position of primers relative to the transcription start site of the gene and their sequences are listed in Supplemental Data.
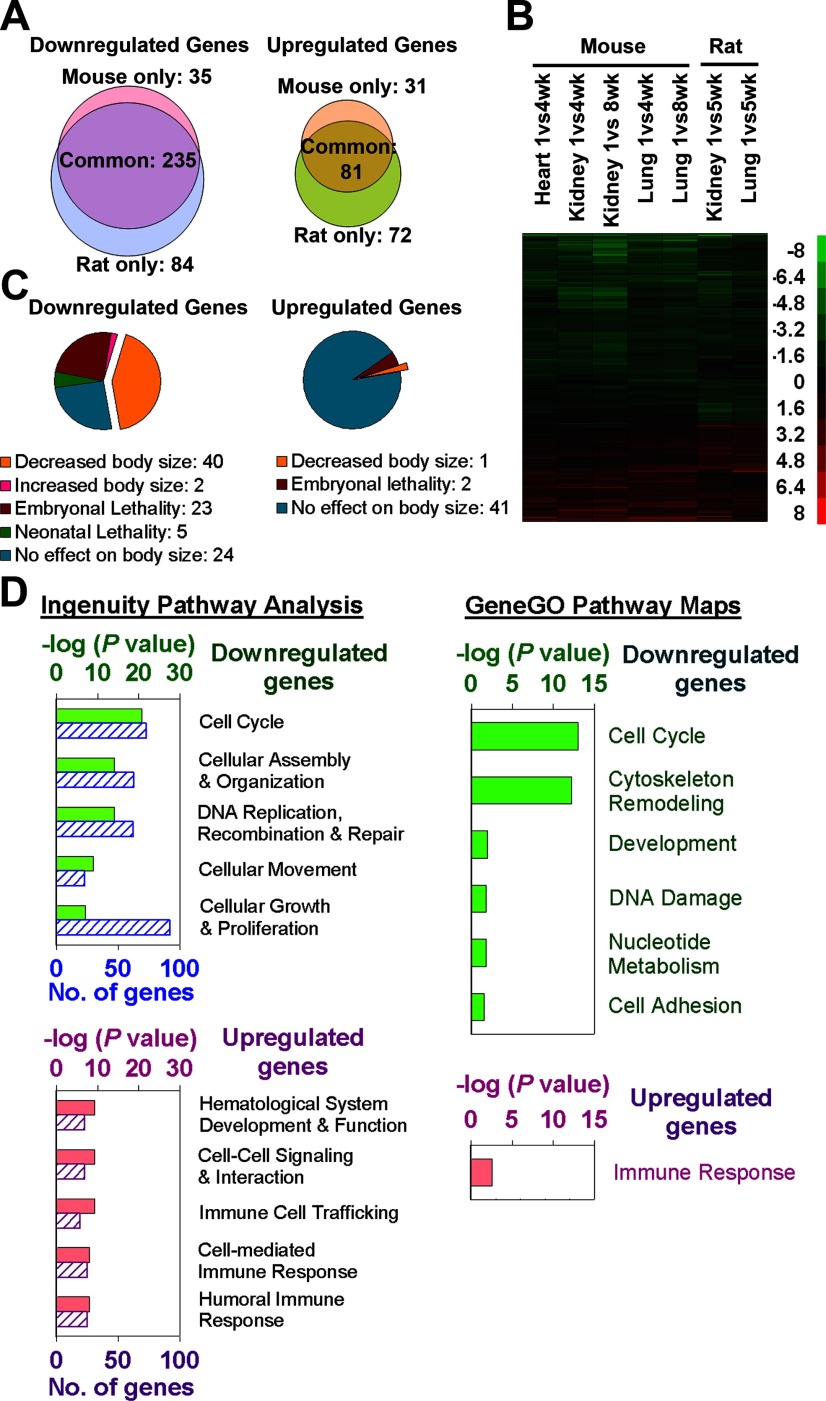
Figure 1.
A multiorgan postnatal genetic program common to mice and rats. A) Venn diagrams showing the number of genes significantly down-regulated and up-regulated with age by microarray in mouse vs. rat (5 microarray chips/time point, each representing a single animal). Analysis included 532 genes that showed age regulation (P<0.05; ≥2.0-fold) in all mouse organs studied (kidney, lung, heart) or both rat organs studied (kidney, lung). B) Heat maps based on microarray analysis of the same 532 genes. Each row corresponds to a gene, ranked by hierarchal clustering. Green, down-regulation with age; red, up-regulation. Scale values are log2(fold difference). C) Pie chart depicting knockout mouse phenotypes of 316 genes that showed uniform age regulation (≥2.0-fold, P<0.05) in all organs of both species (phenotype details in Supplemental Table 3). Genes that caused both embryonic lethality and decreased body size were categorized as having decreased body size for purposes of this graph. D) Gene ontology analyses of these 316 age-regulated genes. Up-regulated and down-regulated groups were separately analyzed, using Ingenuity Pathway Analysis (IPA) 7.1 and GeneGO. For IPA, the 5 most overrepresented molecular, cellular, or physiological functions are shown (solid bars, P value; striped bars, number of significant genes involved). For GeneGO, all significant (P<0.05) map folders are shown.
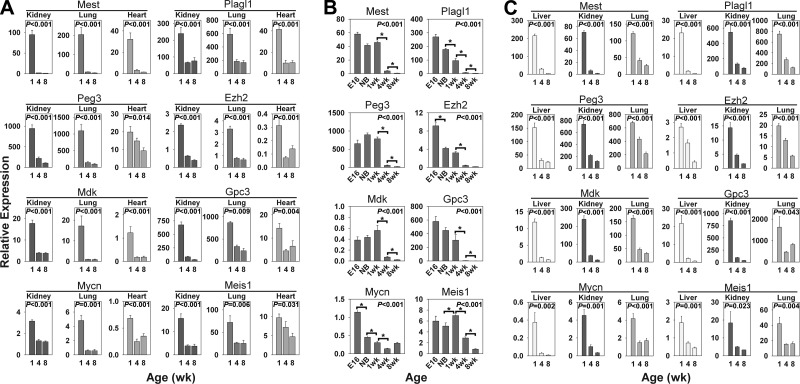
Figure 2.
Temporal changes in gene expression (mean±se) of Mest, Plagl1, Peg3, Ezh2, Mdk, Gpc3, Mycn, and Meis1 in multiple organs. Relative expression of each mRNA was measured by real-time PCR and normalized to 18S RNA in kidney, lung, and heart of 1-, 4-, and 8-wk-old mice (A); liver of E16, newborn (NB), and 1-, 4-, and 8-wk-old mice (B); and liver, kidney, and lung of 1-, 4-, and 8-wk-old rats (C); 5 animals/time point. P values (ANOVA) in each panel show overall change in expression with age. *P < 0.001 for pairwise comparisons. Postnatal time points for Mest, Plagl1, and Peg3 in mice have been previously reported (36) and are included for comparison.
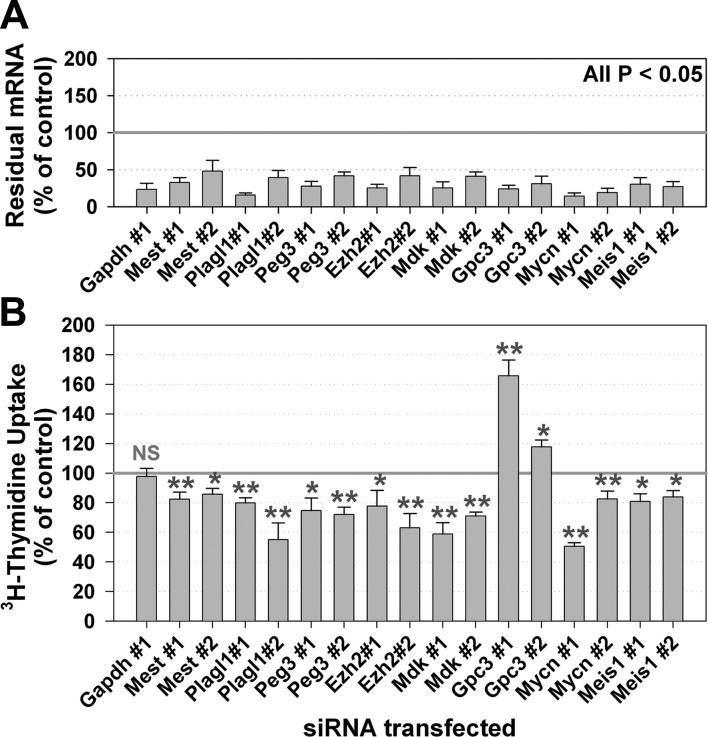
Figure 3.
siRNA-mediated knockdown of genes in the multiorgan postnatal program suppresses proliferation in primary murine hepatocytes. A) Residual target mRNA levels after transfection of siRNA for 8 different age-down-regulated genes and a housekeeping gene (Gapdh) into cultured hepatocytes isolated from E15 fetal mice. Two different siRNAs were used for each target gene. mRNA was measured by real-time PCR and normalized to results from control siRNA. B) Proliferation of hepatocytes was assessed by 3H-thymidine uptake 2 d after transfection and normalized to results from control siRNA. At least 3 experiments were performed for each siRNA. Each experiment used hepatocytes from a different litter and was analyzed in triplicate cell culture wells. Values are means ± se. NS, not significant. *P < 0.05, **P < 0.01; ANOVA.
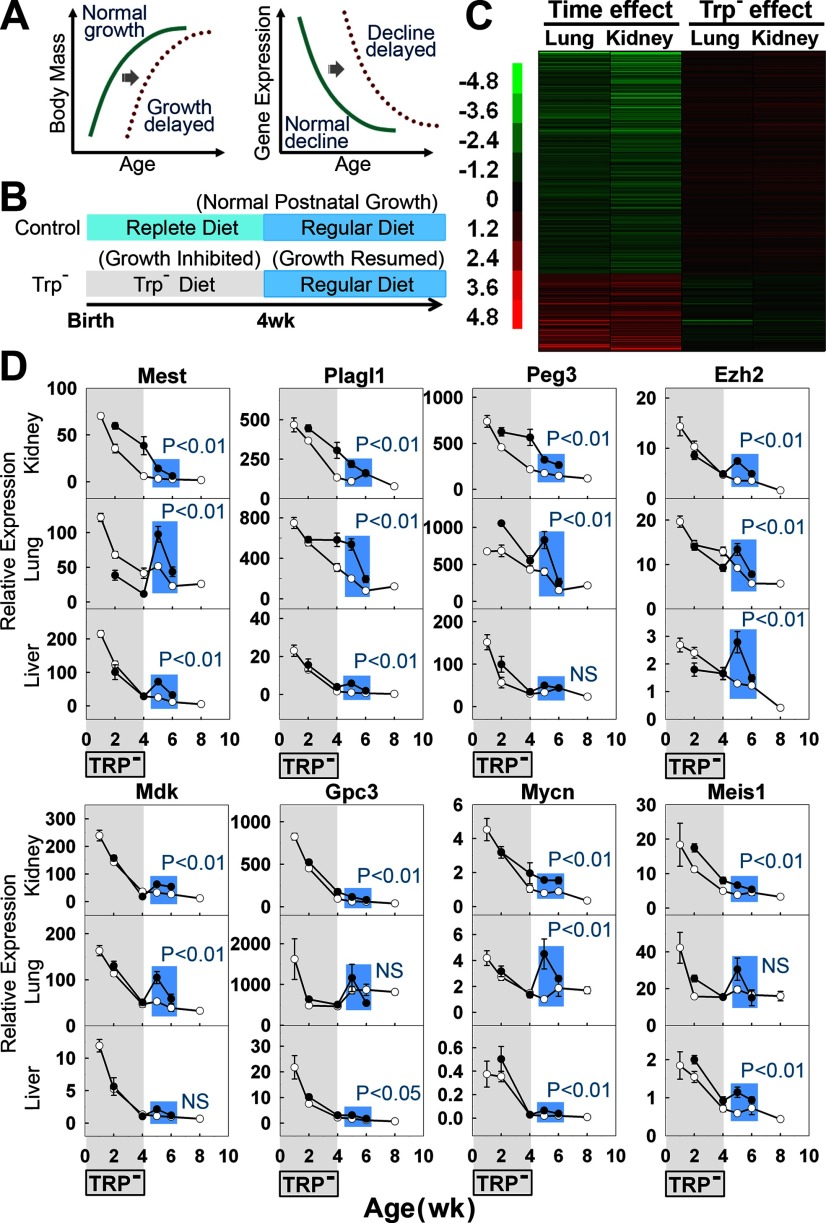
Figure 4.
Multiorgan postnatal genetic program does not depend simply on age per se, but on body growth. A) Schematic diagram depicting the hypothetical delay of gene down-regulation caused by growth delay, if the program is growth dependent. B) Feeding scheme for control and tryptophan deficient (Trp−) groups. C) Effect of transient tryptophan deficiency on the postnatal genetic program. Heat map based on microarray analysis of the 316 genes that change uniformly with age in both mouse and rat organs (5 microarray chips/group, each representing a single animal). In the first 2 columns, time effect was assessed by comparing expression of normal 5-wk-old rats to normal 1-wk-old rats. In the third and fourth columns, the effect of previous Trp− diet was assessed by comparing expression of 5-wk-old Trp− rats to 5-wk-old control rats. Green, down-regulation with age or treatment; red, up-regulation. Scale values are log2(fold difference). For genes that were down-regulated with age, expression at 5 wk was higher in rats that had previously received Trp− diet than in control rats, suggesting that a delay in growth leads to a delay in the postnatal genetic program. A similar delay was observed for up-regulated genes. D) Effect of transient tryptophan deficiency on mRNA expression (means±se) of selected genes by real-time PCR (5 animals/time point). Statistical analysis included only time points following the period of tryptophan deficiency (Trp−vs. control at 5–6 wk, comparison highlighted in blue) in order to isolate the effects of the prior growth history and to exclude the effects of concurrent malnutrition at earlier time points. Solid circles, Trp− group; open circles, control group. NS, not significant.
Figure 5.
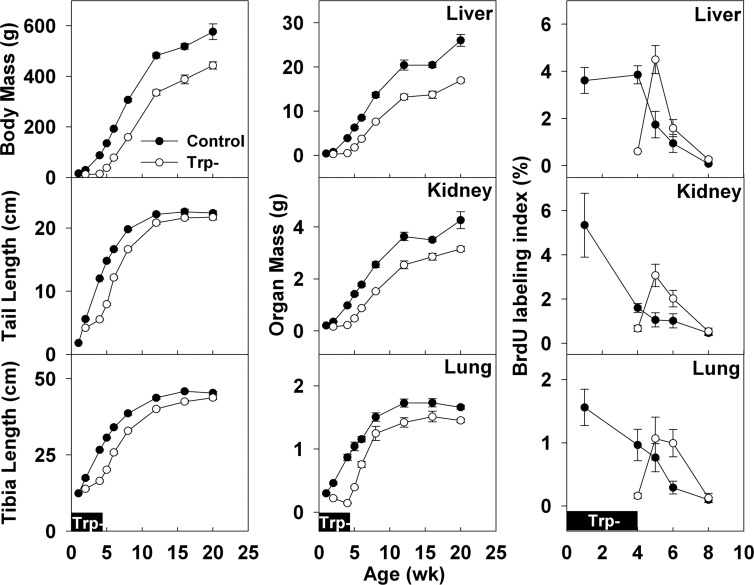
Growth (means±se) in body mass, tail length, tibia length, organ (liver, kidney, and lung) mass, and organ BrdU-labeling index during and after tryptophan deficiency in young rats. During tryptophan deficiency (0–4 wk, solid boxes, pertains to all graphs), growth was inhibited in the tryptophan-deficient group (25 mg tryptophan/100 g food, open symbols) but not in the control group (280 mg tryptophan/100 g food, solid symbols). After 4 wk, a regular diet was given to both groups, and growth was resumed in the tryptophan-deficient group. To assess proliferation rates, BrdU was injected 2 h before animals were killed and detected by immunohistochemistry. Labeled nuclei in renal cortical tubular epithelium, hepatocytes, and pneumocytes were counted and normalized to total number of nuclei. Each time point represents ≥6 animals.
Figure 6.
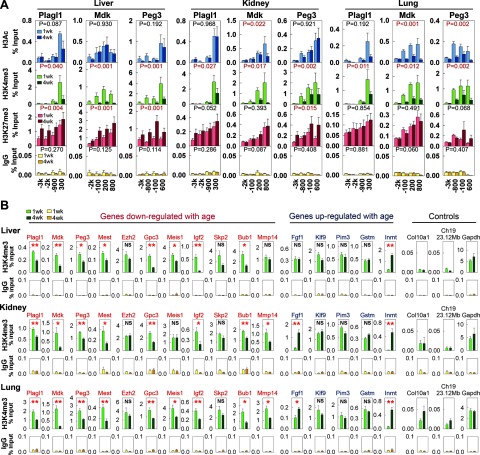
Histone H3K4 trimethylation decreases with age in the promoter regions of age-down-regulated genes. Histone modifications were studied by ChIP followed by real-time PCR using chromatin isolated from 1 and 4 wk murine organs (5 animals/time point). A) Primers specific for 4 different regions −3 kb to +1 kb from the transcription start sites of Plagl1, Mdk, and Peg3 were used. ChIP was performed with antibodies against acetyl-H3K9/K14 (H3Ac), trimethyl-H3K4 (H3K4me3) or trimethyl-H3K27 (H3K27me3), and IgG (negative control). B) Primers specific for the promoter regions of 11 age-down-regulated genes, 5 age-up-regulated genes, and 3 controls (including chromosome 19 23.12 Mb, a genomic region distant from any promoters) were used. ChIP was performed with an antibody against H3K4me3 different from that in panel A. Results (means±se) were normalized to input (DNA before ChIP). *P < 0.05, **P < 0.01; ANOVA.
The amount of histone modification at a particular position was quantified with a standard curve generated by serially diluted mouse genomic DNA. Chromatin enrichment was expressed as percentage input = 100 × (amount of target DNA by real-time PCR after ChIP/amount of target DNA by real-time PCR in input)/(volume of DNA solution used for ChIP/volume of DNA solution set aside as input).
Statistical analyses
Data are presented as means ± se. For microarray data sets, a 1-way ANOVA for the effect of age was performed. Pearson correlation coefficient was used for pairwise correlations between temporal changes of gene expression in different organs in the mouse and the rat. The probability of overlap between different data sets was determined using Pearson’s χ2 test. For the real-time PCR assessment of temporal changes in gene expression and histone modifications of individual genomic positions, ANOVA for the effect of age was performed. For the real-time PCR and proliferation data of hepatocyte culture, a 2-way ANOVA for the effects of litter and siRNA treatment was performed. For the tryptophan deficiency model, a 2-way ANOVA for the effects of age and treatment was performed for all growth and cell proliferation data. For assessment of the effect of growth delay on expression of age-regulated genes, a 2-way ANOVA for the effect of age and treatment was performed at 5 and 6 wk of age. For the real-time PCR study of histone modifications for multiple positions in the promoter region of a single gene, a 2-way ANOVA for the effect of age and positions was performed.
RESULTS
Multiorgan postnatal genetic program conserved in mice and rats
We recently identified a multiorgan genetic program that occurs in C57BL/6 mice during juvenile growth deceleration (10). To provide broad validation of the program, here we first performed an analogous study in Sprague-Dawley rats. Microarray was used to compare gene expression between rats at age 1 wk (when growth is rapid) and 5 wk (when growth is slowing) in kidney and lung. In kidney, 5042 genes were significantly (P<0.01, ANOVA) down-regulated and 5960 genes up-regulated with age. Similarly, in lung, 3927 genes were significantly (P<0.01) down-regulated, and 3973 genes were up-regulated with age. For both the kidney and lung, the FDR was <5%. Thus, for example, of the 5042 genes found to be up-regulated in kidney, fewer than 252 genes would be expected to be false positives. There was a striking similarity between the 2 organs: 2867 genes were commonly down-regulated and 2422 genes were commonly up-regulated in both organs, far more than expected by chance (P<0.0001, Pearson’s χ2 test, Supplemental Fig. 1A). We next compared the microarray data between the rat and mouse (10). For this analysis, we narrowed our focus to genes that showed a fold change ≥2 and P < 0.05 and thus were more likely to have a major physiological effect. Among genes that changed substantially in either the mouse (≥2.0-fold, P<0.05, the same direction in all 3 organs studied) or the rat (≥2.0-fold, P<0.05, the same direction in both organs studied), there was a strong correlation between the 2 species (n=532 genes; Pearson’s r=0.67, kidney; r=0.72, lung; P<0.0001, both organs). Moreover, in this list, 235 down-regulated genes and 81 up-regulated genes were changing significantly with age (defined by P<0.05) in the same direction in both species and in all organs studied (Fig. 1A, B), far more overlap than expected by chance (P<0.001, χ2 test, Supplemental Fig. 1B). Thus, the multiorgan, postnatal genetic program is highly conserved in mice and rats.
Bioinformatic analyses of the genetic program
To characterize the biological functions of this genetic program, the 316 genes that showed uniform regulation (≥2.0-fold, P<0.05) in all organs of both species (81 up-regulated and 235 down-regulated; Supplemental Table 1) were subjected to bioinformatic analyses (Fig. 1D). For the down-regulated genes, gene ontology analyses using Ingenuity Pathway Analysis (IPA) 7.1 and GeneGO indicated strong overrepresentation of genes implicated in the cell cycle (P=1.8×10−21, IPA, Supplemental Table 2) and cell growth/proliferation (P=9.0×10−8, IPA, Supplemental Table 2). This overrepresentation was not observed for up-regulated genes (Fig. 1D). These findings were further supported by tabulating the reported knockout phenotypes of the 316 genes (Supplemental Table 3). Of the 94 uniformly down-regulated genes for which a knockout mouse has been reported, 42 showed change in body size (40 decreased, 2 increased), 36 of which were not associated with any detected underlying disease (Fig. 1C; Supplemental Table 3). In contrast, of the 49 uniformly up-regulated genes that have a knockout model, only 1 showed a change in body size (Fig. 1C; Supplemental Table 3). Taken together, these findings suggest that the postnatal genetic program includes a large set of growth-promoting genes that are down-regulated with age simultaneously in multiple organs.
Effect of gene knockdown on proliferation
To verify that these uniformly down-regulated genes in the program regulate cell proliferation, we performed expression and functional studies on a subset of the 42 genes in the program that showed a growth-related phenotype in knockout mice. We chose to focus on genes that were more likely to play a regulatory role, such as transcription factors or paracrine growth factors, rather than genes that play an obligatory role in the cell cycle machinery (such as Aurka, which is involved in chromosome segregation). The declining expression of 8 genes (Ezh2, Gpc3, Mdk, Meis1, Mest, Mycn, Peg3, and Plagl1) with age was first corroborated by real-time PCR (Fig. 2A). We found that mRNA expression of all these genes was down-regulated from 1 to 8 wk in lung, kidney, and heart in mice. The same decline also occurred in liver, with the majority of the declines occurring postnatally (Fig. 2B). Similar declines in expression occurred in rat liver, lung, and kidney (Fig. 2C). Thus, the real-time PCR analysis confirmed that this genetic program is occurring concurrently in multiple organs with widely differing physiological functions and embryonic derivations.
To determine whether these genes affect proliferation in growing tissues and to simulate the decline in expression that occurs with age, we knocked down expression by transfecting specific siRNA into hepatocytes isolated from fetal mouse liver. A 50–80% reduction in mRNA levels was achieved for each target gene using each of 2 different siRNAs (Fig. 3A). Knocking down expression of 7 of the 8 target genes induced a significant (P<0.05, ANOVA) decrease in hepatocyte proliferation (by 3H-thymidine uptake) compared to negative control siRNA (Fig. 3B). Unlike the other genes of interest, Gpc3 knockdown stimulated hepatocyte proliferation, which corresponds to the increased body size reported for knockout of this gene in vivo. The effects on proliferation were observed using 2 independent siRNAs for each gene and thus were unlikely to be due to off-target effects. In contrast, there was no effect on proliferation after knockdown of Gapdh, which served as an additional negative control. The siRNA-induced decrease in proliferation did not appear to be caused by cell death, as cells remained attached and morphologically normal. Furthermore, real-time PCR showed that expression of Alb1, which encodes a major liver-specific secretary product, albumin, either remained unchanged or increased after transfection (Supplemental Fig. 2), also suggesting that the hepatocytes remained viable. It is still possible that knockdown of these genes induced subtle cellular dysfunction, which indirectly hindered proliferation. Nonetheless, our findings support the hypothesis that some of the genes down-regulated in multiple organs are required for rapid proliferation in embryonic cells and that their subsequent down-regulation contributes to growth deceleration.
Some of the siRNAs induced changes in expression of genes other than their immediate target gene (Supplemental Fig. 2). These effects could be related to off-target effects of the siRNA or could be indirect effects, that is, altered expression of genes that are regulated by the siRNA target gene (Supplemental Fig. 2). Whether such indirect effects contribute to the observed decreases in proliferation remains to be determined.
Genetic program depends on growth, not simply age
Next, we investigated the physiological process that drives this multiorgan postnatal genetic program, asking whether the program is dependent on age per se or on body growth. We reasoned that, if the program were dependent on body growth, then delaying growth (by inhibiting growth for a period of time and subsequently removing the inhibition to resume growth) would also delay this genetic program (Fig. 4A). Alternatively, if the genetic program were dependent on the organism’s age per se, then the changes in gene expression would not be affected by growth delay.
To delay growth in newborn male rats, we provided a tryptophan-deficient (Trp−) diet to the lactating mother from birth of the pups until 4 wk of age (Fig. 4B). The Trp− diet strongly attenuated somatic growth, compared to control rats receiving a replete diet (Fig. 5). At 4 wk of age, a regular diet was resumed. To evaluate the effects of prior growth inhibition without the confounding effects of concurrent tryptophan deficiency, we studied rats at 5 wk of age, when growth had recovered, as indicated by increased body mass, tibial and tail length, and proliferation in various organs (Fig. 5). At 5 wk, the rats that had previously experienced growth inhibition showed a delay in the postnatal genetic program, as assessed by microarray analysis (Fig. 4C). Of the 235 genes that were uniformly down-regulated with age, 159 (68%) were delayed significantly (P<0.05) by previous tryptophan deficiency (Fig. 4C). Of the 81 genes uniformly up-regulated with age, 34 (42%) were delayed significantly (P<0.05) by previous tryptophan deficiency (Fig. 4C). Among these 316 age-regulated genes there was a strong negative correlation between change with age and the effect of prior tryptophan deficiency (Pearson’s r=−0.73, kidney; r=−0.77, lung; P<0.0001, both organs).
Next, we verified the effect of prior growth inhibition on expression of the 8 genes selected for more detailed study, using real-time PCR. In the 3 organs studied (kidney, liver, and lung), the expression of these 8 genes was generally higher in Trp− rats than in control rats after normal diet was resumed (Fig. 4D, Trp−vs. control at 5–6 wk, P<0.05 in 20 of 24 comparisons), again consistent with a delayed decline in expression. The analysis included only the 5 and 6 wk time points because these occur after recovery from tryptophan deficiency. Consequently, at these time points, the experimental group and control group are comparable in that they are both well nourished and thriving, but differ in that the experimental group has undergone less prior growth. At 2 and 4 wk of age the experimental group differs from the control group not only in the amount of prior growth undergone but also in the current nutritional state, which could have direct, confounding effects on gene expression. Indeed, at these time points occurring within the treatment period, the effects on gene expression were quite variable. In general, the 2 groups differ more at the 5 wk time point than at the 6 wk time point. However, this effect would be expected for a right-shift of a decreasing, concave-up (flattening) curve.
We also considered the possibility that the observed changes in gene expression might be related to weaning, which occurs at ∼3 wk of age. However, the onset of the decline in expression consistently preceded the time of weaning (Fig. 4D). Thus, the data suggest that many changes in gene expression that occur in early postnatal life in multiple organs depend on body growth.
Declining H3K4 trimethylation of down-regulated genes
We then investigated the molecular mechanisms that orchestrate the down-regulation of this large set of genes as the body grows. Because chromatin state can serve as a cellular memory (19, 20) and broadly regulate transcription (21), we reasoned that epigenetic mechanisms might mediate the effects of prior growth on gene expression postnatally. We therefore compared histone modifications in mice of 1 and 4 wk of age by ChIP with antibodies against acetyl-H3K9/K14 (H3Ac), trimethyl-H3K4 (H3K4me3), or trimethyl-H3K27 (H3K27me3), followed by real-time PCR using primers specific for the promoter regions of 3 genes that are down-regulated with age: Mdk, Peg3, and Plagl1. H3Ac and H3K4me3 are associated with actively transcribing genes (22,23,24), whereas H3K27me3 is associated with gene silencing (24,25,26). We did not find consistent temporal changes of local H3Ac in different organs. H3K27me3 increased significantly from 1 to 4 wk in the liver, but not consistently in kidney and lung (Fig. 6A). In contrast, H3K4me3 significantly decreased from 1 to 4 wk in all 3 organs, in the promoter regions of all 3 genes (Fig. 6A). To verify and extend this finding, we used a different antibody against H3K4me3 and studied a wider variety of genomic positions, including promoters of 11 age-down-regulated genes (Mdk, Peg3, Plagl1, Mest, Ezh2, Gpc3, Meis1, Igf2, Skp2, Bub1, Mmp14), 5 age-upregulated genes (Fgf1, Klf9, Pim3, Gstm, Inmt), a gene not expressed in these organs (Col10a1), a housekeeping gene (Gapdh), and a genomic region distant from any promoters (mouse chromosome 19, 23.12 Mb) (Fig. 6B). H3K4me3 signals remained low at Col10a1 promoter and the nonpromoter genomic region in 1 and 4 wk, and remained high at the Gapdh promoter, which agreed with the notion that H3K4me3 is associated with actively transcribed genes. For Mdk, Peg3, and Plagl1, H3K4me3 declined with age, confirming our previous findings. Of 11 age-down-regulated genes (including Mdk, Peg3, and Plagl1), 9 showed consistent declines in H3K4me3 in their promoters (except Ezh2 and Skp2), whereas only 1 out of 5 age-up-regulated genes (Inmt) showed changes. Because H3K4me3 is associated with transcriptional activation, our findings suggest that this decline in H3K4me3 may be involved in the coordinated down-regulation of multiple growth-regulating genes with age.
DISCUSSION
In this study, we investigated a program of gene expression that occurs concurrently in kidney, lung, heart, and liver, as body growth slows. We found that this multiorgan, postnatal genetic program has been highly conserved in the ∼20 million yr since mice and rats diverged (27), providing strong confirmation of the validity and biological importance of the program.
Multiple lines of evidence suggest that at least part of this program contributes to body growth deceleration. For the down-regulated genes in the program, gene ontology analyses indicated strong overrepresentation of genes implicated in cell growth/proliferation. Furthermore, of the age-down-regulated genes that have a reported knockout phenotype, >1/3 showed a decrease in body size without any detected underlying disease, implying that these age-down-regulated genes in the program promote somatic growth. This concept was further supported by in vitro studies using siRNA-mediated knockdown of gene expression, which demonstrated that a subset of age-down-regulated genes in the program are required for rapid proliferation of cultured fetal hepatocytes. The observed effects on cell proliferation in vitro were concordant with effects on body size reported in mice with targeted ablation of these genes and thus suggest that the previously observed altered body size represents a direct effect on cell proliferation, rather than only indirect effects such as systemic disease, placental dysfunction, or impaired prior organogenesis. Taken together, the findings imply that the multiorgan postnatal genetic program involves the down-regulation of many genes, some of which are required for rapid proliferation in early life, supporting the hypothesis that part of this program contributes to growth deceleration. The finding that this program occurs concurrently in kidney, lung, heart, and liver provides a potential explanation for how growth slows coordinately in these organs, thus maintaining body proportions. However, growth of different organs may also be modulated by tissue-specific mechanisms, thus explaining, for example, why proliferation in liver is open to modulation after cell loss (28) or in response to metabolic needs (29), whereas pancreatic growth appears to be less responsive (28). Whether this genetic program also controls growth of other tissues remains to be determined.
Next, we investigated the physiological mechanisms that drive this multiorgan postnatal genetic program. We showed that delaying growth by inducing tryptophan deficiency in juvenile rats caused a striking delay in the genetic program, suggesting that the multiorgan postnatal genetic program is not simply driven by a biological timing mechanism, but instead depends on growth. Thus, the program could, for example, be driven by increasing cumulative number of cell divisions undergone (a cell division counter) or by increasing tissue mass (30). The dependence of the program on growth is also supported by preliminary observations in another model of growth inhibition, hypothyroidism (10). It will be interesting to test this hypothesis further using other interventions that inhibit and stimulate growth.
We also investigated the molecular mechanisms driving the growth-limiting genetic program and found that H3K4me3 declined with age in multiple age-down-regulated genes and multiple organs. Because H3K4me3 is a signature of permissive chromatin (31), the observed declining H3K4me3 may reflect the conversion of chromatin into a nonpermissive state with body growth, which may thus orchestrate the observed down-regulation of multiple genes. Recent studies have shown that transcription factors that are capable of recruiting histone methyltransferases (HMTs) can activate a broad range of downstream target genes by altering their H3K4 trimethylation (32,33,34). Our data therefore also raise the possibility that HMT-interacting transcription factors may potentially act as a master switch for the growth-limiting genetic program.
Taken together, our findings support the following model to explain limitation of organ and body size in mammals. Somatic growth deceleration results from a multiorgan postnatal genetic program, primarily involving down-regulation of a large set of growth-promoting genes. Because this growth-limiting genetic program is occurring simultaneously in multiple tissues, the decline in growth rate of various organs occurs in a concerted fashion, which serves to maintain body proportions. This growth-limiting program depends not simply on age but on somatic growth itself and may be orchestrated by epigenetic mechanisms including declining H3K4me3. Therefore, growth leads to progression of this program with down-regulation of many growth-promoting genes, which in turn causes growth of these organs to slow and eventually cease, thus setting a fundamental limit on adult organ size.
Although this model is consistent with the findings in the current study, additional studies will be needed to test the model further. Our in vitro proliferation study was restricted to 8 genes; large-scale functional manipulation of gene expression using high-throughput methodology will help to determine which other down-regulated genes in the program are growth-promoting in normal cells. Ideally, to confirm that this program is responsible for physiological somatic growth deceleration, it would be important to demonstrate that positive and negative genetic manipulation of the program in vivo leads to corresponding changes in growth deceleration. In addition, the current findings only demonstrate a temporal association between declining H3K4me3 and down-regulation of expression with age in a subset of genes in the program. Study on a genome-wide basis and functional manipulation of histone methylation is needed to establish the causal relationships.
The findings in this study shed light on one of the major unsolved mysteries of biology: how organs and organisms know when to stop growing (35). Understanding the fundamental mechanisms that control mammalian body size is likely to yield insight into the many human genetic disorders that cause childhood growth failure and overgrowth. In addition, further investigation of the identified growth-limiting genetic program may lead to broader medical applications, because disruption of this program might contribute to oncogenesis, and conversely transient therapeutic suspension of growth-limiting mechanisms in adult cells might be used to achieve tissue regeneration.
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
References
- Brenner W. E., Edelman D. A., Hendricks C. H. A standard of fetal growth for the United States of America. Am J Obstet Gynecol. 1976;126:555–564. doi: 10.1016/0002-9378(76)90748-1. [DOI] [PubMed] [Google Scholar]
- Chang M., Parker E. A., Muller T. J., Haenen C., Mistry M., Finkielstain G. P., Murphy-Ryan M., Barnes K. M., Sundaram R., Baron J. Changes in cell-cycle kinetics responsible for limiting somatic growth in mice. Pediatr Res. 2008;64:240–245. doi: 10.1203/PDR.0b013e318180e47a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winick M., Noble A. Quantitative changes in DNA, RNA, and protein during prenatal and postnatal growth in the rat. Dev Biol. 1965;12:451–466. doi: 10.1016/0012-1606(65)90009-6. [DOI] [PubMed] [Google Scholar]
- Sutter N. B., Bustamante C. D., Chase K., Gray M. M., Zhao K., Zhu L., Padhukasahasram B., Karlins E., Davis S., Jones P. G., Quignon P., Johnson G. S., Parker H. G., Fretwell N., Mosher D. S., Lawler D. F., Satyaraj E., Nordborg M., Lark K. G., Wayne R. K., Ostrander E. A. A single IGF1 allele is a major determinant of small size in dogs. Science. 2007;316:112–115. doi: 10.1126/science.1137045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapf J., Walter H., Froesch E. R. Radioimmunological determination of insulinlike growth factors I and II in normal subjects and in patients with growth disorders and extrapancreatic tumor hypoglycemia. J Clin Invest. 1981;68:1321–1330. doi: 10.1172/JCI110379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose S. R., Municchi G., Barnes K. M., Kamp G. A., Uriarte M. M., Ross J. L., Cassorla F., Cutler G. B., Jr Spontaneous growth hormone secretion increases during puberty in normal girls and boys. J Clin Endocrinol Metab. 1991;73:428–435. doi: 10.1210/jcem-73-2-428. [DOI] [PubMed] [Google Scholar]
- Pape L., Hoppe J., Becker T., Ehrich J. H., Neipp M., Ahlenstiel T., Offner G. Superior long-term graft function and better growth of grafts in children receiving kidneys from paediatric compared with adult donors. Nephrol Dial Transplant. 2006;21:2596–2600. doi: 10.1093/ndt/gfl119. [DOI] [PubMed] [Google Scholar]
- Cooke P. S., Yonemura C. U., Russell S. M., Nicoll C. S. Growth and differentiation of fetal rat intestine transplants: dependence on insulin and growth hormone. Biol Neonate. 1986;49:211–218. doi: 10.1159/000242533. [DOI] [PubMed] [Google Scholar]
- Schafer R., Knauf U., Zweyer M., Hogemeier O., de Guarrini F., Liu X., Eichhorn H. J., Koch F. W., Mundegar R. R., Erzen I., Wernig A. Age dependence of the human skeletal muscle stem cell in forming muscle tissue. Artif Organs. 2006;30:130–140. doi: 10.1111/j.1525-1594.2006.00199.x. [DOI] [PubMed] [Google Scholar]
- Finkielstain G. P., Forcinito P., Lui J. C., Barnes K. M., Marino R., Makaroun S., Nguyen V., Lazarus J. E., Nilsson O., Baron J. An extensive genetic program occurring during postnatal growth in multiple tissues. Endocrinology. 2009;150:1791–1800. doi: 10.1210/en.2008-0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segall P. E., Timiras P. S. Patho-physiologic findings after chronic tryptophan deficiency in rats: a model for delayed growth and aging. Mech Ageing Dev. 1976;5:109–124. doi: 10.1016/0047-6374(76)90012-9. [DOI] [PubMed] [Google Scholar]
- Orozco-Suarez S., Del Angel A. R., Beas-Zarate C., Manjarrrez G., Feria-Velasco A. Corn feeding during development induces changes in the number of serotonergic neurons in the raphe nuclei. Int J Dev Neurosci. 2003;21:13–22. doi: 10.1016/s0736-5748(02)00124-7. [DOI] [PubMed] [Google Scholar]
- El-Khattabi I., Gregoire F., Remacle C., Reusens B. Isocaloric maternal low-protein diet alters IGF-I, IGFBPs, and hepatocyte proliferation in the fetal rat. Am J Physiol Endocrinol Metab. 2003;285:E991–E1000. doi: 10.1152/ajpendo.00037.2003. [DOI] [PubMed] [Google Scholar]
- Sakai Y., Jiang J., Kojima N., Kinoshita T., Miyajima A. Enhanced in vitro maturation of fetal mouse liver cells with oncostatin M, nicotinamide, and dimethyl sulfoxide. Cell Transplant. 2002;11:435–441. [PubMed] [Google Scholar]
- Watanabe N., Tanaka M., Suzuki K., Kumanogoh A., Kikutani H., Miyajima A. Tim2 is expressed in mouse fetal hepatocytes and regulates their differentiation. Hepatology. 2007;45:1240–1249. doi: 10.1002/hep.21539. [DOI] [PubMed] [Google Scholar]
- Barreyro F. J., Kobayashi S., Bronk S. F., Werneburg N. W., Malhi H., Gores G. J. Transcriptional regulation of Bim by FoxO3A mediates hepatocyte lipoapoptosis. J Biol Chem. 2007;282:27141–27154. doi: 10.1074/jbc.M704391200. [DOI] [PubMed] [Google Scholar]
- O'Malley K. L., Jong Y. J., Gonchar Y., Burkhalter A., Romano C. Activation of metabotropic glutamate receptor mGlu5 on nuclear membranes mediates intranuclear Ca2+ changes in heterologous cell types and neurons. J Biol Chem. 2003;278:28210–28219. doi: 10.1074/jbc.M300792200. [DOI] [PubMed] [Google Scholar]
- Yao M., Stenzel-Poore M., Denver R. J. Structural and functional conservation of vertebrate corticotropin-releasing factor genes: evidence for a critical role for a conserved cyclic AMP response element. Endocrinology. 2007;148:2518–2531. doi: 10.1210/en.2006-1413. [DOI] [PubMed] [Google Scholar]
- Ringrose L., Paro R. Epigenetic regulation of cellular memory by the polycomb and trithorax group proteins. Annu Rev Genet. 2004;38:413–443. doi: 10.1146/annurev.genet.38.072902.091907. [DOI] [PubMed] [Google Scholar]
- Bantignies F., Cavalli G. Cellular memory and dynamic regulation of polycomb group proteins. Curr Opin Cell Biol. 2006;18:275–283. doi: 10.1016/j.ceb.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Sproul D., Gilbert N., Bickmore W. A. The role of chromatin structure in regulating the expression of clustered genes. Nat Rev Genet. 2005;6:775–781. doi: 10.1038/nrg1688. [DOI] [PubMed] [Google Scholar]
- Koch C. M., Andrews R. M., Flicek P., Dillon S. C., Karaoz U., Clelland G. K., Wilcox S., Beare D. M., Fowler J. C., Couttet P., James K. D., Lefebvre G. C., Bruce A. W., Dovey O. M., Ellis P. D., Dhami P., Langford C. F., Weng Z., Birney E., Carter N. P., Vetrie D., Dunham I. The landscape of histone modifications across 1% of the human genome in five human cell lines. Genome Res. 2007;17:691–707. doi: 10.1101/gr.5704207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintzman N. D., Stuart R. K., Hon G., Fu Y., Ching C. W., Hawkins R. D., Barrera L. O., Van C. S., Qu C., Ching K. A., Wang W., Weng Z., Green R. D., Crawford G. E., Ren B. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat Genet. 2007;39:311–318. doi: 10.1038/ng1966. [DOI] [PubMed] [Google Scholar]
- Barski A., Cuddapah S., Cui K., Roh T. Y., Schones D. E., Wang Z., Wei G., Chepelev I., Zhao K. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Schuettengruber B., Chourrout D., Vervoort M., Leblanc B., Cavalli G. Genome regulation by polycomb and trithorax proteins. Cell. 2007;128:735–745. doi: 10.1016/j.cell.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Ringrose L., Paro R. Polycomb/trithorax response elements and epigenetic memory of cell identity. Development. 2007;134:223–232. doi: 10.1242/dev.02723. [DOI] [PubMed] [Google Scholar]
- Springer M. S., Murphy W. J., Eizirik E., O'Brien S. J. Placental mammal diversification and the Cretaceous-Tertiary boundary. Proc Natl Acad Sci U S A. 2003;100:1056–1061. doi: 10.1073/pnas.0334222100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanger B. Z., Tanaka A. J., Melton D. A. Organ size is limited by the number of embryonic progenitor cells in the pancreas but not the liver. Nature. 2007;445:886–891. doi: 10.1038/nature05537. [DOI] [PubMed] [Google Scholar]
- Kam I., Lynch S., Svanas G., Todo S., Polimeno L., Francavilla A., Penkrot R. J., Takaya S., Ericzon B. G., Starzl T. E. Evidence that host size determines liver size: studies in dogs receiving orthotopic liver transplants. Hepatology. 1987;7:362–366. doi: 10.1002/hep.1840070225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomer R. H. Not being the wrong size. Nat Rev Mol Cell Biol. 2001;2:48–54. doi: 10.1038/35048058. [DOI] [PubMed] [Google Scholar]
- Yan C., Boyd D. D. Histone H3 acetylation and H3 K4 methylation define distinct chromatin regions permissive for transgene expression. Mol Cell Biol. 2006;26:6357–6371. doi: 10.1128/MCB.00311-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnell I. W., Ishibashi J., Le G. F., Punch V. G., Addicks G. C., Greenblatt J. F., Dilworth F. J., Rudnicki M. A. Pax7 activates myogenic genes by recruitment of a histone methyltransferase complex. Nat Cell Biol. 2008;10:77–84. doi: 10.1038/ncb1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Lee J., Lee S. K., Lee J. W. Activating signal cointegrator-2 is an essential adaptor to recruit histone H3 lysine 4 methyltransferases MLL3 and MLL4 to the liver X receptors. Mol Endocrinol. 2008;22:1312–1319. doi: 10.1210/me.2008-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan A., Ruyechan W. T., Kristie T. M. The coactivator host cell factor-1 mediates Set1 and MLL1 H3K4 trimethylation at herpesvirus immediate early promoters for initiation of infection. Proc Natl Acad Sci U S A. 2007;104:10835–10840. doi: 10.1073/pnas.0704351104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy D., Norman C. What don’t we know? Science. 2005;309:75. doi: 10.1126/science.309.5731.75. [DOI] [PubMed] [Google Scholar]
- Lui J. C., Finkielstain G. P., Barnes K. M., Baron J. An imprinted gene network that controls mammalian somatic growth is down-regulated during postnatal growth deceleration in multiple organs. Am J Physiol. 2008;295:R189–R196. doi: 10.1152/ajpregu.00182.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.