Abstract
Choline is an essential nutrient, and deficiency causes liver and muscle dysfunction. Common genetic variations alter the risk of developing organ dysfunction when choline deficient, probably by causing metabolic inefficiencies that should be detectable even while ingesting a normal choline-adequate diet. We determined whether metabolomic profiling of plasma at baseline could predict whether humans will develop liver dysfunction when deprived of dietary choline. Fifty-three participants were fed a diet containing 550 mg choline/70 kg/d for 10 d and then fed <50 mg choline/70 kg/d for up to 42 d. Participants who developed organ dysfunction on this diet were repleted with a choline-adequate diet for ≥3 d. Plasma samples, obtained at baseline, end of depletion, and end of repletion, were used for targeted and nontargeted metabolomic profiling. Liver fat was assessed using magnetic resonance spectroscopy. Metabolomic profiling and targeted biochemical analyses were highly correlated for the analytes assessed by both procedures. In addition, we report relative concentration changes of other small molecules detected by the nontargeted metabolomic analysis after choline depletion. Finally, we show that metabolomic profiles of participants when they were consuming a control baseline diet could predict whether they would develop liver dysfunction when deprived of dietary choline.—Sha, W., da Costa, K., Fischer, L. M., Milburn, M. V., Lawton, K. A., Berger, A., Jia, W., Zeisel, S. H. Metabolomic profiling can predict which humans will develop liver dysfunction when deprived of dietary choline.
Keywords: fatty liver, metabolomics, plasma, PEMT
Choline is an essential nutrient for humans; ≥4 enzyme-catalyzed pathways utilize choline (1): it is oxidized to betaine aldehyde and then to betaine, a methyl donor (2); it is acetylated to form acetylcholine, the neurotransmitter (3); a base-exchange pathway involves substitution of choline for serine, inositol, or ethanolamine head groups on endogenous phospholipids (4); and phosphorylation of the hydroxyl group of choline, catalyzed by choline kinase, the first step in the pathway to the synthesis of phosphatidylcholine and sphingomyelin, important membrane phospholipids (1).
Choline, via its irreversible oxidation to betaine (2), methylates homocysteine to form methionine. This is the precursor for synthesis of S-adenosylmethionine (SAM), the universal methyl donor needed for methylation of DNA, RNA, and proteins. It is important to realize that choline, methionine, and folate metabolism are interrelated at the step that homocysteine is methylated to form methionine (3). Perturbing metabolism of one of the methyl donors results in compensatory changes in the other methyl donors due to the intermingling of these metabolic pathways (4,5,6). The only source of choline other than diet is from the de novo biosynthesis of phosphatidylcholine catalyzed by phosphatidylethanolamine-N-methyltransferase (PEMT) in liver. This enzyme uses SAM as a methyl donor and forms a new choline moiety (7).
Studies in humans show that dietary choline is required (reviewed in ref. 1 and discussed later). In 1998, the U.S. Institute of Medicine (Food and Nutrition Board) established an adequate intake (AI) and tolerable upper intake limit (UL) values for choline (8). The AI is 550 mg/70 kg body weight (kg bw), with upward adjustment in pregnant and lactating women (8). Using a clinical methodology for phenotyping individuals with respect to their susceptibility to developing organ dysfunction when fed a low-choline diet (9,10,11,12), significant individual variations in the susceptibility to developing organ dysfunction were identified (12). Eighty percent of men and postmenopausal women fed low-choline diets under controlled conditions developed reversible fatty liver (measured by magnetic resonance spectroscopy), as well as liver damage (elevated aspartate aminotransferase (AST) levels in plasma) and muscle damage [elevated creatine phosphokinase (CPK) levels in plasma], while only 44% of premenopausal women developed organ dysfunction (12). Fatty liver occurred because phosphatidylcholine is required for secretion of very low density lipoprotein (VLDL) from liver and, in its absence, triglyceride vectors to the cytoplasm and accumulates (13). Thus, choline is a lipotropic nutrient in that it promotes export of fat from the liver. Leakage of enzymes from liver and muscle occurred because choline deficiency induces apoptosis (10, 14, 15). Some of the variation in dietary requirement for choline can be explained by estrogen, which induces the gene (PEMT) that makes endogenous biosynthesis of choline possible (16). Thus, premenopausal women have an enhanced capacity for de novo biosynthesis of choline moiety and require less of this nutrient in their diet.
Though premenopausal women should be resistant to choline deficiency because of estrogen, a significant portion of them (44%) developed organ dysfunction when deprived of choline (12). Genetic variation likely underlies these differences in dietary requirements. As noted earlier, PEMT encodes for a protein responsible for endogenous formation of choline, and 78% of female carriers of the variant (C) allele in a SNP in the promoter region of the PEMT gene (rs12325817) developed organ dysfunction when fed a low-choline diet (odds ratio 21, P < 0.00005; P value recalculated from original publication data and additional subjects totaling 64 women studied) (17). The frequency of this variant allele was 0.70 in North Carolina, USA. The risk haplotype abrogated the induction of PEMT by estrogen, while the wild-type haplotype did not (Resseguie et al., unpublished results). Other, very common SNPs in genes of choline and folate metabolism also influence dietary requirements for choline (17, 18) by creating metabolic inefficiencies in these metabolic pathways.
Measurement of choline and choline metabolites is useful in estimating choline status, but the measure is not definitive. Fasting plasma choline concentrations vary from 7 to 15 μM, with most subjects having concentrations of 10 μM. Individuals that have starved for up to 7 d have diminished plasma choline, but levels never drop below 50% of normal, probably because tissue phospholipids are “cannibalized” to prevent concentrations of choline from falling further (19). Plasma phosphatidylcholine concentration also decreases in choline deficiency (20), but these values are also influenced by factors that change plasma lipoprotein levels. Thus, measurements of choline or phosphatidylcholine in blood may identify subjects with low dietary choline intake, but provide little help in differentiating the degree of deficiency.
A clinical measurement that could be used to estimate risk for developing choline deficiency is needed. The 2005 National Health and Nutrition Examination Survey (NHANES) data suggest that most people do not achieve the recommended AI for choline (21). In participants from the Framingham Offspring Study, the mean intake for total choline (energy adjusted) was 313 mg/d, which is well below the AI values (22). Women in California, eating diets low in choline, are at significantly increased risk for having a baby with birth defects (23, 24), and low dietary choline intake increases mortality in women with breast cancer (25). In addition, choline deficiency is a common side effect seen in patients treated with total parenteral nutrition, resulting in liver dysfunction that can limit the use of this therapy (26,27,28).
Fatty liver, sometimes associated with nonalcoholic steatohepatitis (NASH), is relatively common and of unknown etiology. While there are no studies relating dietary choline intake to NASH, a pilot study measuring plasma choline in NASH patients (n=29) found no correlation with severity of liver disease (29). However, there are several studies that found that individuals with a genetic variation (SNP) in PEMT are disproportionally represented among people who have fatty liver (30,31,32) (note that although another U.S. study concluded that the PEMT SNP did not occur more frequently in patients with fatty liver in Dallas, the variant allele was, indeed, more frequent in Caucasians with NASH; refs. 33, 34). Also, in experimental animals, choline deficiency is associated with fatty liver (35). A clinical measure of choline status and risk for developing choline deficiency would likely increase understanding of the mechanisms for many of the above human pathologies. If this risk is mediated by genetic variations that cause metabolic inefficiencies, these alterations might be detectable as changes in concentrations of small molecules in the blood. These changes might be detectable before frank organ dysfunction is apparent.
Classically, clinical nutrition studies performed targeted analyses of a modest number of molecules that the investigator predicted would be changed after a dietary intervention. Metabolomic profiling, the nontargeted measurement of the relative concentration of a large number of small molecules in a single biological sample, permits a broader examination of perturbations in metabolism (36). Metabolomic profiling is used in pharmaceutical discovery and development (37,38,39,40) and in biomarker discovery for disease diagnosis (41), but has not been as widely applied for nutrition studies.
In this study, an unbiased metabolomics analysis was carried out on plasma samples from healthy humans sequentially fed a choline-adequate diet, a choline-deficient diet, and then a choline-adequate diet. At the end of each diet period, plasma was analyzed using a classical targeted approach (clinical laboratory tests for measures of organ toxicity, choline and its metabolites, and homocysteine and its metabolites) and by metabolomic profiling. For overlapping tests, we determined whether the two approaches were correlated. In addition, we tested whether the biochemical profiling approach could detect changes due to the alterations in diet, and we report changes in relative concentrations of small molecules not examined in targeted assays but detected by the metabolomics analysis. As noted earlier, only a portion of the population develops liver dysfunction when they are deprived of choline. We determined whether the metabolomic profiles of our human participants, when they were consuming the choline-adequate baseline diet, could predict whether they would develop liver dysfunction when deprived of dietary choline.
MATERIALS AND METHODS
Study design
The following approved study design, which was conducted to determine the human requirements for the nutrient choline, has been previously reported in detail (12). Briefly, healthy males (n=31) and females (n=35) were recruited by advertising. They ranged in age from 18 to 70 yr and had body mass indices between 19 and 33. Informed consent was obtained from all participants after the nature and possible consequences of the study were explained; the criteria for subject selection and all details of the clinical protocol were approved by the Institutional Review Board of the University of North Carolina at Chapel Hill (UNC-CH). The ethnicity of the participants was Caucasian (65%), African-American (25%), Asian (5%), Native-American (3%), and other heritages (2%), reflecting the local population characteristics of the Raleigh-Durham-Chapel Hill area. Apart from one mother/daughter duo, there were no known relations between subjects. Inclusion was contingent on age-typical good state of health as determined by physical examination and standard clinical laboratory tests. Prior alcohol use or abuse was assessed as part of subject selection. Subjects were compensated for participating in the study. Of the originally recruited 66 participants, 61 completed at least the initial phase and the depletion phase. Of these 61, one subject was excluded due to a 9-kg weight loss during the study, 3 participants were excluded because they did not comply with diet restrictions, and 4 were excluded due to insufficient sample collection, leaving 53 participants (23 males and 30 females) included in the metabolomics analyses.
The participants were admitted to the UNC-CH General Clinical Research Center for the entire duration of the study and could leave only for brief periods under the supervision of study staff. The mean duration of their stay was 54.7 d, and the median was 56 d. The diets, which were composed of 0.8 g/kg high biological value protein, with 30% kcal coming from fat, and the remaining kilocalories from carbohydrate, were prepared in-house to protocol specifications and are described in detail in another publication (9). The dietary protein provided 17 ± 1 mg/kg/d Met + Cys which was above the 1989 RDA (13 mg/kg/d; this RDA was the current one at the time the study started). Subjects with unusual diets were excluded because their diets would interfere with the study. Initially, all participants received a diet of standard foods containing 550 mg choline/70 kg bw/d, the presumed “adequate intake” (8). After 10 d, the choline content of the diet was reduced to <50 mg/d, as confirmed by analysis of duplicate food portions (42). The diets provided adequate intakes of macronutrients and micronutrients. With the addition of a multivitamin and mineral supplement, subjects received 100–500 μg folate, 2–3 mg vitamin B6 and 6–8 μg vitamin B12 daily. The dietary B6, B12, folic acid, and methionine content were held constant on a per body weight basis, and subjects were required to eat all of the food provided to them. Total food intake during each diet period was designed to be isocaloric (i.e., the different diets contained roughly the same amount of calories per body weight per day) and were adjusted in any given individual to maintain constant body weight (we supplied energy levels ranging from 35 to 45 kcal/kg bw, based on individual requirements).
Periodic determinations of urinary choline and betaine concentrations during the different diet phases, measured by HPLC-MS (42), were used to confirm compliance with the dietary restrictions. Participants remained on this depletion diet until they developed organ dysfunction associated with choline deficiency or for 42 d if they did not. Humans were deemed to have organ dysfunction associated with choline deficiency if they had a >5-fold increase of serum creatine phosphokinase (CPK) activity, or a >1.5-fold increase in aspartate aminotransferase (AST) or alanine aminotransferase (ALT), or if they had an increase in liver fat content by >28%, while consuming the choline depletion diet, and if this increased CPK, AST, ALT, or increased liver fat resolved when choline was returned to the diet (12). After the depletion study, participants were repleted with a choline-adequate diet for ≥3 d (those who developed organ dysfunction were fed the repletion diet for 10 d or until dysfunction resolved, those who developed no dysfunction were fed the repletion diet for 3 d and then discharged; for comparison purposes, samples obtained at 3 d repletion were compared for all groups).
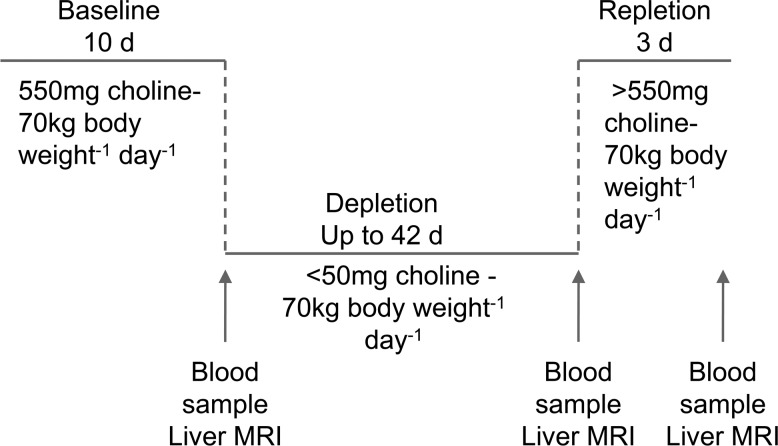
Liver fat was assessed at the end of the baseline choline-adequate diet, after 21 and 42 d of the choline-deficient diet, and at the end of the repletion with the choline-adequate diet (Fig. 1). Change in liver fat content was estimated by magnetic resonance spectroscopy with a Siemens Vision 41.5T clinical MR system (Siemens Medical Solutions, Malvern, PA, USA) using a modified in- and out-of-phase procedure (43). Software from Siemens Medical Solutions (Malvern, PA, USA) was used to estimate fat content using signal measurements across 3–5 liver slices/subject that were normalized as a ratio by the fat content of similarly measured slices of spleen (12), similar to a previously validated method (44). Data were collected by a single operator masked to the study phase to reduce bias. The 28% increase over baseline that we chose for depletion was an average grade of moderate steatosis from several studies (45).
Figure 1.
Research design. Fifty-three participants were fed a diet containing 550 mg choline/70 kg/d for 10 d and then fed <50 mg choline/70 kg/d until they developed organ dysfunction or for up to 42 d. Participants who developed organ dysfunction consuming this diet were repleted with a choline-adequate diet for ≥3 d. Plasma and serum samples, obtained at end of baseline, end of depletion and end of repletion, were used for targeted and nontargeted metabolomic profiling.
Laboratory analyses
Fasting blood samples were taken every 3 or 4 d for blood clinical chemistries (including CPK, AST, and ALT), and samples for targeted and metabolomic profiling analyses were collected at 10 d of the baseline choline-adequate diet, at the end of the choline-deficient diet (depletion phase), and at the end of the choline-adequate repletion diet. Plasma or serum was separated and stored at −80°C until they were used for the targeted and nontargeted analyses.
Serum uric acid, triglycerides, CPK, AST, and ALT activity were determined using colorimetric methods by the McClendon Clinical Laboratories at University of North Carolina Hospitals, which is both Clinical Laboratory Improvement Act and College of American Pathologists accredited. Choline and its metabolites were analyzed and quantified directly by HPLC-MS [liquid chromatography/electrospray ionization–isotope dilution mass sprectrometry (LC/ESI-IDMS)] after the addition of internal standards labeled with stable isotopes that were used to correct for recovery (42). Methionine, homocysteine, sarcosine, dimethylglycine, cysteine, and cystathionine concentrations in serum were measured using a capillary gas chromatography/mass spectrometry (GC/MS) method previously described (24). Briefly, serum samples were extracted and metabolites were isolated using an anion-exchange column, derivatized with N-methyl-N-(tert-butyldimethylsilyl)-trifluoroacetamide, and analyzed by MS. Deuterated standards were used to correct for recovery. Samples analyzed by MS were assayed in duplicate, and the mean value was used.
Nontargeted metabolomics analysis
Samples were prepared and analyzed essentially as described previously (41, 46, 47). Proteins were precipitated from 100 μl of human plasma with methanol containing 4 standards, which permitted the monitoring of extraction efficiency, using an automated liquid handler (Hamilton LabStar, Salt Lake City, UT, USA). The resulting supernatant was split into 4 aliquots and dried under nitrogen and then stored in vacuo. For liquid chromatography/mass spectrometry (LC/MS) analysis, one aliquot was reconstituted in 50 μl 0.1% formic acid in water, and one aliquot was reconstituted in 50 μl 6.5 mM ammonium bicarbonate in water, pH 8. For GC/MS analysis, aliquots were derivatized using equal parts bistrimethyl-silyl-trifluoroacetamide and solvent mixture acetonitrile: dichloromethane:cyclohexane (v/v 5:4:1) with 5% triethylamine at 60°C for 1 h. All reconstitution solvents contained instrument internal standards to monitor instrument performance and for use as retention index markers. The remaining aliquot was dried and stored for no longer than 2 d at −80°C for rerun purposes, if necessary. All samples were run in duplicate.
To assess process variability throughout the data set, on all analytical platforms, technical replicates, referred to as QC matrix samples (MTRX), were prepared from pooled samples of plasma that were extracted 5 independent times per run day. In addition, 100 μl of water were extracted 5 independent times per run day to serve as process blanks. Every sample analyzed was spiked with standards to monitor and evaluate instrument and extraction performance and align chromatograms. The standards were carefully chosen so as not to interfere with the measurement of endogenous species.
The samples for LC/MS were analyzed on a Waters Acquity UPLC (Waters, Millford, MA, USA) using the method described by Evans et al.(48). Briefly, the extracts that were reconstituted in formic acid were gradient eluted at 350 μl/min using 1) 0.1% formic acid in water and 2) 0.1% formic acid in methanol (0 to 70% B in 4 min, 70 to 98% B in 0.5 min, 98% B for 0.9 min), while the extracts reconstituted in ammonium bicarbonate were gradient eluted at 350 μl/min using 1) 6.5 mM ammonium bicarbonate in water, pH 8, and 2) 6.5 mM ammonium bicarbonate in 95:5 methanol:water (same gradient profile as above). A 5-μl aliquot of sample was injected using 2 times overfill and analyzed using an LTQ mass spectrometer (Thermo Fisher Corp., Waltham, MA, USA) with ESI. The instrument scanned 99–1000 m/z and alternated between MS and MS/MS scans using dynamic exclusion with an exclusion time of 3 s. The acidic extracts were monitored for positive ions, and the basic extracts were monitored for negative ions in independent injections using separate acid/base dedicated 2.1 × 100 mm Waters BEH C18 1.7-μm particle columns heated to 40°C.
The derivatized samples for GC/MS were analyzed on a Thermo-Finnigan Trace DSQ fast-scanning single-quadrupole MS (Thermo Finnegan, San Jose, CA, USA) operated at unit mass resolving power. The GC column was 20 m × 0.18 mm with 0.18-μm film phase consisting of 5% phenyldimethyl silicone, initial oven temperature was 60°C ramped to 340°C in a 16-min period, and helium was the carrier gas. GC/MS was operated using electron impact ionization with a 50- to 750-amu scan range and was tuned and calibrated daily for mass resolution and mass accuracy.
Raw instrument data were processed by first converting retention times (RT) to retention indexes and aligning all samples based on RT markers present throughout the chromatogram (49). Next, ion features were detected and integrated based on a MS signal to noise threshold, raw MS area threshold, and peak shape criteria. Finally, individual ion features were grouped based on peak apex retention time for ease of viewing similarly retained ion features. To ensure data quality, including instrument performance, chromatography, mass calibration, and extraction efficiency, process coefficients of variation for all standards were checked each run day. Internal standard retention times and alignment were also checked and validated.
Compounds were identified by automated comparison to reference chemical library entries using software developed for creating library entries from known chemical entities and then automatically fitting those spectra to experimentally derived spectra (49). Peaks that elute from either the LC or GC method were compared to the library at a particular retention time and its associated spectra for that compound. Internal standards were primarily used in both the LC and GC methods to calibrate retention times of compounds across all of the samples in the study and for quality control of each instrument run. Identification of known chemical entities was based on comparison with Metabolon’s library entries of purified external standards.
All identifications were subjected to quality control to verify the quality of the identification. This final quality control step removed process artifacts, compounds with poor peak shape and therefore poor integration, and MS/MS rejected library identifications. Process artifacts were defined as biochemicals present in the biological samples at ≤3× the level in the water process blanks. In final compound tallies, redundant data were reduced by selecting one platform to represent the compound. That is, for compounds detected on both positive and negative channels of LC, one channel was chosen to represent the compound. For compounds detected on both LC and GC, one platform was chosen to represent the compound. Process relative sd were calculated for both internal standards and endogenous biochemicals that were detected in 100% of the technical replicate injections of the pooled human plasma MTRX sample.
Raw area counts for each compound in each sample were normalized to correct for variation resulting from instrument interday tuning differences. Raw area counts for a compound were divided by the median value, setting the medians equal for each day’s run. Missing values were assumed to result from areas being below the limits of detection. Missing values for a given compound were imputed with the observed minimum after the normalization step. Quantitative values were derived from integrated raw detector counts of the mass spectrometers. Importantly, while peak area comparisons between samples represent relative amounts of each ion detected, different compounds and ions have different ionization potentials. To preserve all of the variation, yet allow compounds of widely different raw peak areas to be compared directly on a similar graphical scale, the normalized intensities were scaled by their median values for each compound.
Statistical analysis
Paired t test with permutation in MeV (http://www.tm4.org/mev) was used to compare baseline data with depletion data to find metabolites whose concentrations were significantly changed [false discovery rate (FDR) adjusted value of P<0.05] by choline depletion. Similarly, paired t test with permutation was used to compare depletion profile with repletion profile to examine whether metabolites changed by choline depletion were recovered by repletion. Kruskal-Wallis test in MeV was used to select metabolites that have different concentrations among the 3 organ dysfunction groups (fatty liver, muscle dysfunction, and no organ dysfunction) at baseline. FDR adjustments for multiple comparisons were made using Q-VALUE (50) (http://genomics.princeton.edu/storeylab/qvalue/). Principal component analysis (PCA; SIMCA-P 11.5 software package; Umetrics, Umeå, Sweden) was used to classify baseline, depletion, and repletion samples with reduced feature space dimensions.
Wilcoxon, Mann-Whitney U test and uncorrelated shrunken centroid (USC) (51) in MeV were used to select markers for predicting which individuals would develop fatty liver by using the baseline metabolomics profile. USC removes highly correlated features (in this case, metabolites), leaving a relative small list of independent predictive metabolites while retaining good accuracy of prediction (51). The 9 subjects who developed organ dysfunction while on the baseline diet were not included in this analysis. We also excluded the 5 subjects who developed muscle dysfunction because this group was too small for the predictive analysis. The remaining 39 baseline samples were divided into 8 groups, and an 8-round cross-validation was applied to prevent overfitting. During each cross-validation, 7 groups were selected as training samples, and one group was left out as testing samples. Wilcoxon, Mann-Whitney U test was first used to identify candidate metabolites that were different (P<0.05) between fatty liver group and no organ dysfunction group at baseline in the training data. USC then further selected metabolites based on the accuracy during inner cross-validation within the training data. Using the selected metabolites, USC then predicted the clinical outcomes of the testing samples by searching for the nearest shrunken centroid. This analysis was repeated 8 times, so that each group has a chance to be left out and be predicted once. The accuracy of prediction was defined as the percentage of correct classifications on the testing samples. Metabolites that were selected ≥7 times during the 8 analyses were considered important markers for predicting the development of fatty liver.
Another prediction analysis method, projection to latent structure discriminant analysis (PLS-DA; SIMCA-P 11.5), was used to classify participants who developed fatty liver and participants who developed no organ dysfunction, and to detect baseline metabolites that could best separate the two groups. The default 7-round cross-validation in the SIMCA-P software package was applied with 1/7 of the samples being left out from the mathematical model in each round. The rank of each metabolite was calculated from a variable influence on projection (VIP) score in the PLS-DA model. Metabolites with rank ≤ 30 were considered the most important metabolites responsible for the differentiation of no organ dysfunction and fatty liver groups.
RESULTS
Targeted laboratory data and nontargeted metabolomic data were correlated
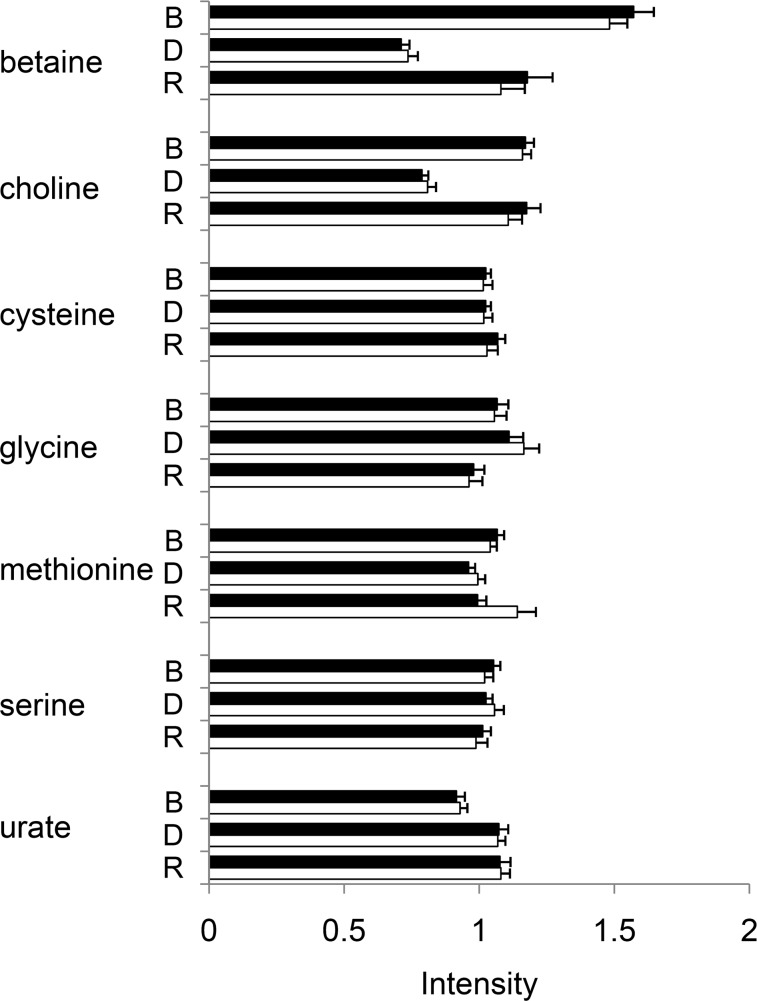
Metabolites that are known to be involved in choline metabolism or hypothesized to be affected by choline depletion were measured in the targeted laboratory analysis. A complete list of metabolites measured in the targeted analysis is in Supplemental Table 1. Among these metabolites, 7 were also measured in the nontargeted metabolomic analysis. We compared the intensities of each individual sample from targeted and nontargeted analyses for the commonly identified metabolites and found the average correlation coefficient between the two analyses was 0.68. Because the averaged intensities across samples at each stage of the experiment are what were to be compared and studied, we then asked whether the averaged intensities across samples were consistent between targeted and nontargeted analyses (Fig. 2). The correlation coefficient of the averaged intensities was 0.95. Thus, for the purpose of comparing groups of samples, data from targeted and nontargeted analyses were highly correlated (Fig. 2). Further, the two analytical approaches complement each other, as 12 of the metabolites measured in the targeted analysis were not measured in the nontargeted analysis, and the nontargeted metabolomics analysis provided additional metabolites that were not determined in targeted analyses but that were affected by choline depletion and potentially predictive of organ dysfunction.
Figure 2.
Targeted laboratory analysis and nontargeted metabolomics analysis were highly correlated. Fifty-three participants were treated as described in Fig. 1. Shown are the 7 metabolites that were measured using both the targeted and the nontargeted analyses. Averaged intensities from targeted analysis (solid column) and nontargeted analysis (open column) of each metabolite at the end of baseline (B), depletion (D), and repletion (R) were highly correlated (r=0.95) between the two analyses. Error bar = 1 se.
Metabolites that are significantly changed by choline depletion
Among the 53 participants, 9 (17%) developed fatty liver (n=4) or muscle dysfunction (n=5) at the end of baseline, suggesting that, in these subjects, the diet containing the adequate intake amount of choline was not sufficient to prevent organ dysfunction. These 9 individuals were not advanced to the choline depletion phase. Data from these individuals was not used in PCA and paired t test analyses presented below.
Among the remaining 44 participants, 23 participants developed hepatic steatosis (or fatty liver), 5 developed muscle damage (elevated CPK), and 16 did not develop any organ dysfunction by the end of the depletion period. Only 1 subject had both elevated liver enzymes and hepatic steatosis at the end of the depletion period. No other organ dysfunction was observed. Participants developed organ dysfunction after 10–52 d in the study (12). When muscle damage occurred, plasma CPK concentrations rose rapidly (over days) and returned to normal within a week after choline repletion. Fatty liver was assessed discontinuously; therefore, we do not have good information about the timing of fat accumulation. Metabolite concentrations were measured at the end of the baseline, depletion, and repletion phases, so intermediate data between these times were not available. Participants who were obese (BMI≥30, n=4) did not have significantly more liver fat at baseline than did the other subjects (L/S ratio 2.2±0.1 vs. 1.6±0.1), nor did they have a significantly greater increment in liver fat after choline depletion (60±10 vs. 30±6%, mean± se), although at both times, obese subjects trended higher.
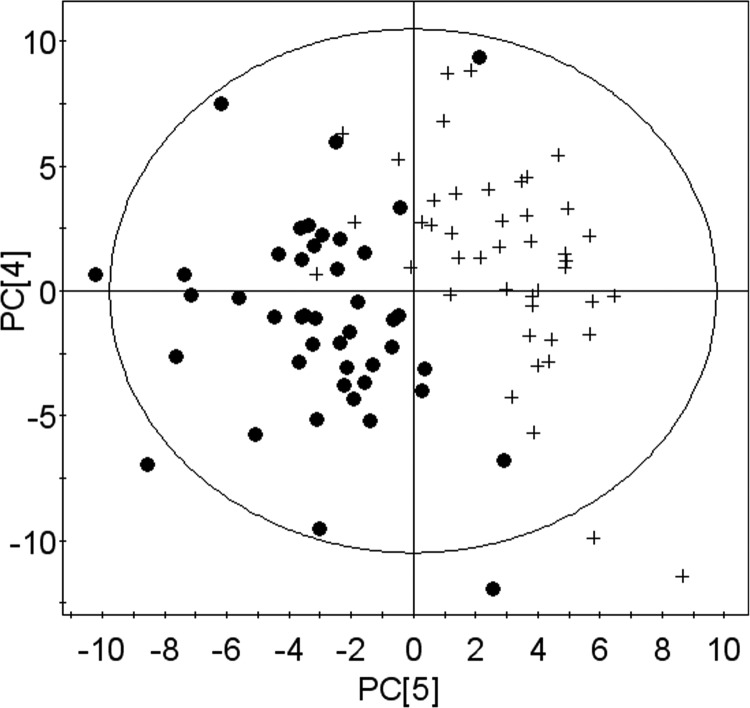
We first used PCA to visualize the pattern of the metabolomic profile at baseline, depletion, and repletion. PCA provided a good separation between baseline and depletion (Fig. 3), but the data points from the end of the repletion phase were not separated from either group (data not shown), presumably because participants’ metabolomic profile had moved to a state between the baseline and depletion profiles after 3 d of choline repletion.
Figure 3.
PCA of metabolomic profiles separates values at end of depletion period from values at baseline. Samples were obtained and analyzed as described in Fig. 1. PCA results in a good separation of baseline values (solid circles) and depletion values (plus signs).
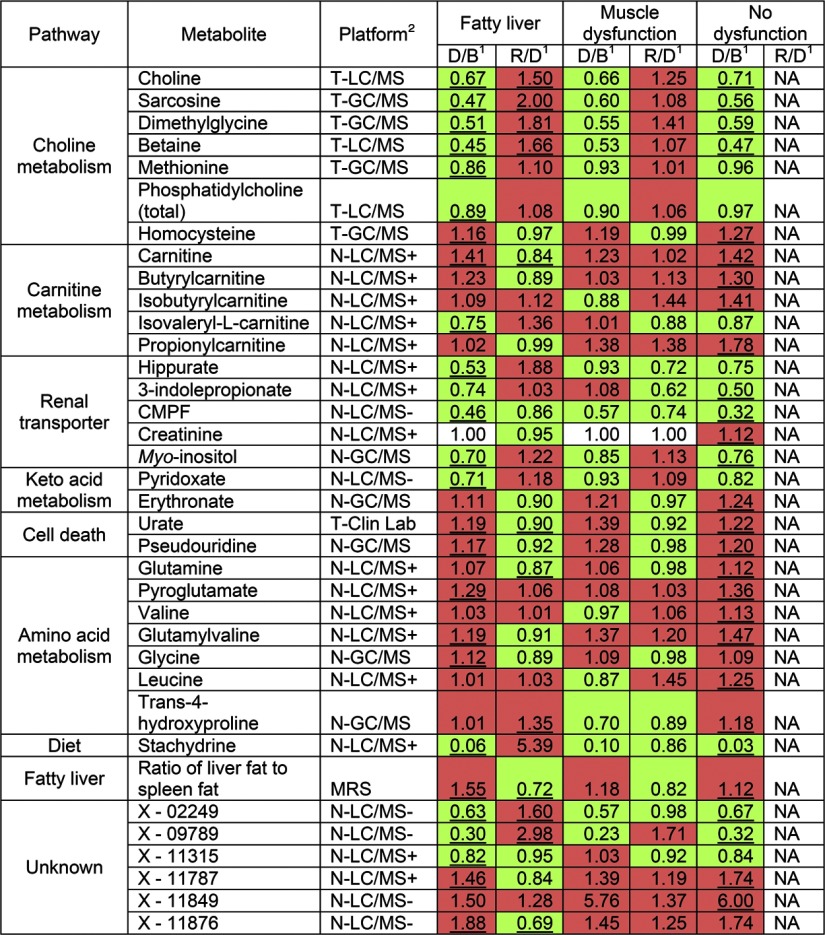
Using paired t tests with permutation, we compared the metabolomic profiles at the end of depletion with those at the end of baseline. Because some participants developed liver dysfunction, while others developed muscle dysfunction, and some developed no organ dysfunction, we analyzed each group separately. We detected 63 metabolites, including 30 named and 33 unknown metabolites, significantly different (FDR adjusted P<0.05) between baseline and depletion in at least one organ dysfunction group (Fig. 4). For the unknowns, only the most significantly (FDR adjusted P<0.001) changed unknowns are shown in Fig. 4. For metabolites determined on both targeted and nontargeted assays, we present only the targeted analysis result. Results calculated using data from the nontargeted method were essentially the same (data not shown).
Figure 4.
Metabolites that significantly changed in intensity after choline depletion in ≥1 organ dysfunction group. Human subjects were fed diets with choline, then deprived of choline, and then repleted with choline, as described in Fig. 1. Some of the subjects developed organ dysfunction (liver or muscle) when deprived of choline. Plasma samples were collected at the end of each diet period and analyzed by targeted and nontargeted biochemical assays. All named metabolites that changed significantly with FDR adjusted P < 0.05 in ≥1 organ dysfunction group are shown. Only the most significantly changed (FDR adjusted P<0.001) unknown metabolites (not identified with an authentic standard) are shown. Spectra information of these unknown metabolites is provided in Table 2. 1Fold change between depletion and baseline (D/B) and between repletion and depletion (R/D) are shown, with significant changes (FDR adjusted P<0.05) underscored. Fold change that indicates an increase by choline depletion or repletion is shaded red; change that indicates a decrease is shaded green. NA, not performed. 2Analytical platforms used for analyses are indicated by the following abbreviations: T-GC/MS, targeted GC/MS; T-LC/MS, targeted LC/MS; T-Clin Lab, targeted clinical laboratory; N-LC/MS+, nontargeted LC/MS-positive ionization; N-LC/MS−, nontargeted LC/MS negative ionization; N-GC/MS, nontargeted GC/MS; MRS, magnetic resonance spectroscopy.
Among metabolites in pathways of choline or methionine metabolism, concentrations of choline, betaine, sarcosine, dimethylglycine, methionine, and phosphatidylcholine were significantly decreased, and homocysteine was significantly increased during choline depletion across the 3 phenotypes (Fig. 4). These changes resolved after the repletion phase of the study. Vitamin B12 is an important cofactor for methionine synthase, and depletion of this vitamin could result in high homocysteine. The participants received 6 μg/d vitamin B12 from a dietary supplement (RDA is 2.4 μg/d). For the 53 participants, methylmalonic acid (MMA; increases in B12 deficiency) concentrations were 163 ± 8 nM (baseline), 167 ± 9 nM (depletion) and 173 ± 10 nM (repletion) and were not statistically different (FDR adjusted P=0.5330). Normal MMA is <300 nM. We had 3 participants with MMA > 300 nM. The depletion and repletion diets delivered similar amounts of dietary betaine; thus, the increase in plasma betaine concentrations seen after repletion (Fig. 4) was presumably due to additional choline being converted to betaine.
A number of metabolites that accumulate in people with diminished renal function, including 3-carboxy-4-methyl-5-propyl-2-furanpropanoate (CMPF), hippurate and 3-indolepropionate were significantly decreased by choline depletion. Carnitine and several acylcarnitines’ concentrations were increased in choline deficiency. A number of amino acids and their metabolites increased (glutamine, pyroglutamate, glycine, valine, glutamyl-valine, and leucine). Finally, a number of metabolites released from dying cells (urate and pseudouridine) were elevated in choline deficiency. The alterations associated with choline depletion in most metabolites were at least partially corrected by choline repletion (Fig. 4).
As noted earlier, we identified three phenotypes in participants fed the choline-deficient diets; some developed fatty liver, some developed muscle damage, and some did not develop any organ dysfunction during 42 d of choline depletion. When comparing the three phenotypes, we found that the list of changed metabolites during choline deficiency and the directions of their changes were almost identical, although P values and fold-changes varied (Fig. 4). The participants who developed muscle damage (elevated CPK) often did so within a few days on the choline-deficient diet, and most of the metabolites that were significantly changed in the other two groups also changed in the same directions in muscle dysfunction group. However, none of these metabolites were significantly changed (FDR adjusted P<0.05) in the muscle dysfunction group after correction for multiple comparisons. The smallest FDR calculated from the muscle dysfunction group was 0.27. The small sample size for the muscle dysfunction group (n=5) made achieving significant differences more difficult.
Differences in metabolomic profiles among the three phenotypes at baseline
Of the 53 participants, 5 developed muscle damage and 4 developed fatty liver while eating the purported adequate choline baseline diet. We compared the baseline metabolomics profiles of participants who had muscle damage (n=5), fatty liver (n=4), and no organ dysfunction (n=44) while eating the baseline diet containing adequate choline (early depleters). Interestingly, we did not find any metabolites significantly different (FDR adjusted P<0.05) among early muscle damage, early fatty liver, and other participants at baseline. The small sample size of the early depleters could make it difficult to detect significantly different metabolites.
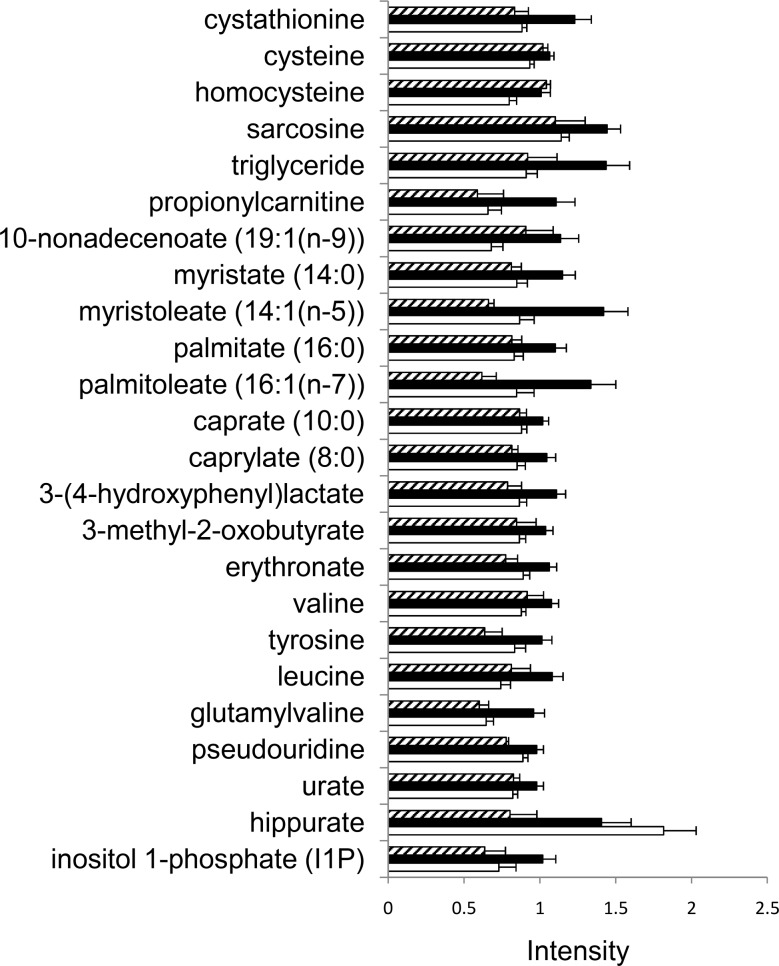
For the 44 participants that did not develop organ dysfunction while consuming the baseline diet, Kruskal-Wallis test was used to compare metabolomic profiles of participants at the end of the baseline period, grouped by their phenotype at the end of the depletion period (fatty liver, muscle damage, or no organ dysfunction). Twenty-four metabolites were found to be the most different, with P < 0.05 (Fig. 5, FDR 22%). Of these metabolites, several (choline metabolites, fatty acids, and amino acids) were most different in the group that would develop liver dysfunction when they were later deprived of choline.
Figure 5.
Metabolites that differ among the 3 outcome phenotypes while consuming the baseline choline-adequate diet. Subjects were fed diets as described in Fig. 1, and some developed organ dysfunction (fatty liver or muscle damage) when fed a choline-deficient diet while others did not. Metabolites in plasma samples were analyzed as described in Materials and Methods. Samples were obtained at the end of the baseline choline-adequate diet period and analyzed as described in Materials and Methods. Metabolites that were different (P<0.05, equivalent to FDR 22%) among the 3 groups when they were fed the baseline choline-adequate diet are shown. Of these metabolites, several (choline metabolites, fatty acids, and amino acids) were most different in the group that would develop liver dysfunction when they were later deprived of choline. Open bar denotes no organ dysfunction group; solid bar denotes group that will develop fatty liver when fed a choline-deficient diet; hatched bar denotes group that will develop muscle dysfunction when fed a choline-deficient diet. Error bar = 1 se.
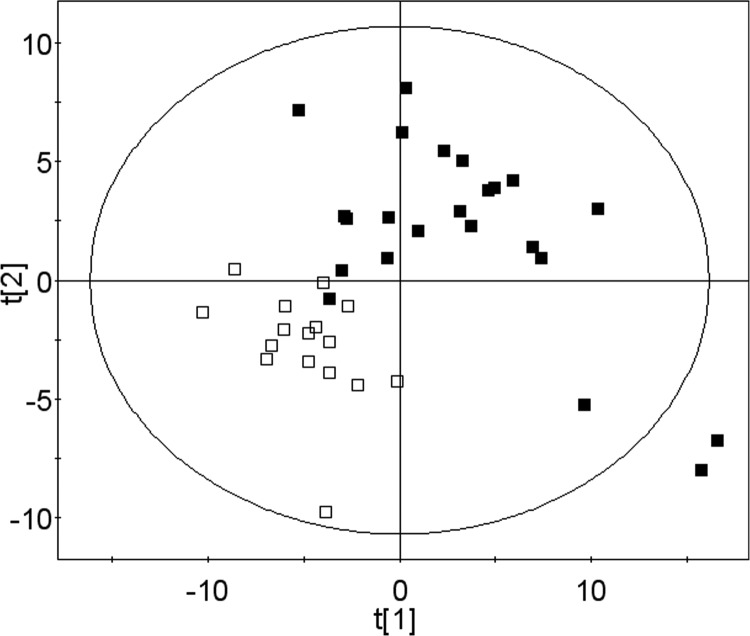
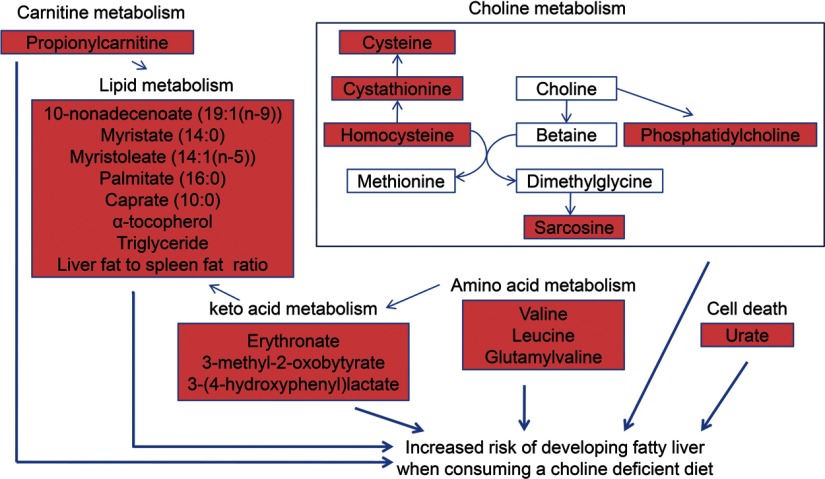
We then asked whether we could predict who will develop fatty liver when deprived of choline, based on the baseline metabolomics profile obtained when participants were consuming a choline-adequate diet. The baseline data from 39 participants, including 23 who developed fatty liver and 16 who developed no organ dysfunction by choline depletion, were used in the prediction analysis. Because of the small number of individuals who developed muscle dysfunction, these subjects were not included in the analysis. Two statistical methods (USC and PLS-DA) were used independently to predict who will develop fatty liver, and the predictive markers selected by the two methods were compared (Tables 1 and 2). When samples from all ethnic groups were used for prediction, the overall accuracy of prediction from cross-validation in USC was 69%. When only Caucasian samples were used, the accuracy increased to 80%. The markers selected by PLS-DA provided an excellent separation of the two groups at baseline (Fig. 6). The list of predictive markers selected by PLS-DA is consistent with the list of markers selected by USC (Table 1). Twenty-six metabolites were selected ≥7 times during the 8 cross-validations in USC. We compared this list of metabolites with the top 30 metabolites selected by PLS-DA and found that they share 22 metabolites in common. Fifteen metabolites were selected by all 8 cross-validations in USC, and among them, 11 were also on the top 15 list in PLS-DA. Both methods identified metabolites in choline metabolism, carnitine metabolism, lipid metabolism, and amino acid metabolism as markers for predicting who will develop fatty liver when fed a choline deficient diet (Table 1 and Fig. 7). These analyses do not attempt to predict the degree of severity of fatty liver when consuming a choline-deficient diet. For that information, we would have to further divide the FL group into mild, moderate and severe FL groups, and then reanalyze the data to identify a list of metabolites that could best predict those classes. However, to do so, we would need a much bigger sample size than we had. This is true for the group with elevated CPK as well.
TABLE 1.
Metabolites (measured at baseline when fed a choline-adequate diet) that were selected as markers to predict the risk for developing fatty liver when fed a choline-deficient diet
| Pathway | Metabolite | Platform | FL/ND | FL/ND 95% CI | Frequency of selection in USC | Rank in PLS-DA |
|---|---|---|---|---|---|---|
| Choline metabolism | Cysteine* | T-GC/MS | 1.14 | 1.05 to 1.24 | 8/8 | 4 |
| Homocysteine | T-GC/MS | 1.27 | 1.07 to 1.51 | 8/8 | 23 | |
| Sarcosine | T-GC/MS | 1.27 | 1.08 to 1.48 | 8/8 | 17 | |
| Cystathionine | T-GC/MS | 1.40 | 1.14 to 1.67 | 8/8 | 18 | |
| Phosphatidylcholine (total) | T-LC/MS | 1.17 | 1.05 to 1.31 | 5/8 | 11 | |
| Carnitine metabolism | Propionylcarnitine | N-LC/MS+ | 1.68 | 1.18 to 2.46 | 7/8 | 13 |
| Lipid metabolism | 10-Nonadecenoate [19:1(n-9)]* | N-LC/MS− | 1.68 | 1.22 to 2.31 | 8/8 | 7 |
| Myristate (14:0) | N-LC/MS− | 1.36 | 1.09 to 1.71 | 7/8 | 19 | |
| Myristoleate [14:1(n-5)] | N-LC/MS− | 1.64 | 1.19 to 2.27 | 6/8 | 14 | |
| Palmitate (16:0) | N-LC/MS− | 1.33 | 1.09 to 1.62 | 7/8 | 12 | |
| Caprate (10:0) | N-LC/MS− | 1.16 | 1.04 to 1.29 | 6/8 | 24 | |
| Ratio of liver fat to spleen fat | MRS | 1.36 | 1.08 to 1.71 | 7/8 | 30 | |
| Triglyceride | T-Clin Lab | 1.65 | 1.18 to 2.29 | 7/8 | 25 | |
| α-Tocopherol | N-GC/MS | 1.33 | 1.09 to 1.60 | 3/8 | 26 | |
| Keto acid metabolism | Erythronate | N-GC/MS | 1.20 | 1.05 to 1.37 | 7/8 | 20 |
| 3-Methyl-2-oxobutyrate | N-LC/MS− | 1.20 | 1.05 to 1.38 | 7/8 | 21 | |
| 3-(4-Hydroxyphenyl)lactate* | N-LC/MS− | 1.28 | 1.10 to 1.50 | 8/8 | 6 | |
| Amino acid metabolism | Valine* | N-LC/MS+ | 1.23 | 1.10 to 1.37 | 8/8 | 5 |
| Glutamylvaline* | N-LC/MS+ | 1.49 | 1.20 to 1.84 | 8/8 | 3 | |
| Leucine* | N-LC/MS+ | 1.46 | 1.18 to 1.84 | 8/8 | 2 | |
| Cell death | Urate | N-LC/MS− | 1.19 | 1.06 to 1.34 | 7/8 | 16 |
| Renal transporter | 3-Indolepropionate | N-LC/MS+ | 0.54 | 0.35 to 0.93 | 3/8 | 27 |
| Unknown | X-03056* | N-LC/MS+ | 1.34 | 1.10 to 1.62 | 8/8 | 10 |
| X-11319* | N-LC/MS− | 1.47 | 1.15 to 1.89 | 8/8 | 9 | |
| X-11550* | N-LC/MS− | 1.12 | 1.06 to 1.18 | 8/8 | 1 | |
| X-11908* | N-LC/MS− | 1.48 | 1.15 to 1.89 | 8/8 | 8 | |
| X-11478* | N-LC/MS− | 0.70 | 0.53 to 0.91 | 8/8 | 15 |
Plasma samples from subjects eating the control baseline diet (choline adequate) were analyzed to determine which metabolites best predicted development of fatty liver after the subjects were later fed a choline-deficient diet (as described in Fig. 1 legend). Two statistical approaches (USC and PLS-DA) generated consistent lists of metabolites for predicting development of fatty liver when consuming a choline-deficient diet. Twenty-six metabolites were selected ≥7 times during the 8 cross-validations in USC; among them, 22 (underscored) are on the top 30 list in PLS-DA. Fifteen metabolites were selected by all 8 cross-validations in USC; among them, 11 (indicated by an asterisk) are also on the top 15 list in PLS-DA. All of the named metabolites that were selected ≥7 times in USC, or that have a VIP rank ≤30 in PLS-DA, are listed. For unknown metabolites, only the most predictive ones (on top 15 list in both USC and PLS-DA, indicated by an asterisk) are shown. Spectra information of these unknown metabolites is provided in Table 2. FL/ND, fold change of metabolite concentrations at baseline between participants who developed fatty liver compared to participants who developed no organ dysfunction. Analytical platforms used for analyses: T-GC/MS, targeted GC/MS; T-LC/MS, targeted LC/MS; T-Clin Lab, targeted clinical laboratory; N-LC/MS+, nontargeted LC/MS positive ionization; N-LC/MS−, nontargeted LC/MS negative ionization; N-GC/MS, nontargeted GC/MS; MRS, magnetic resonance spectroscopy.
TABLE 2.
| Metabolite | Retention time | Retention index | Mass | Platform | Polarity |
|---|---|---|---|---|---|
| X-02249 | 4.03 | 4025.0 | 267.20000 | LC-MS | − |
| X-09789 | 2.62 | 2613.0 | 153.10000 | LC-MS | − |
| X-11315 | 1.19 | 1210.0 | 130.20000 | LC-MS | + |
| X-11787 | 1.13 | 1126.0 | 148.10000 | LC-MS | + |
| X-11849 | 3.20 | 3229.0 | 266.20000 | LC-MS | − |
| X-11876 | 5.30 | 5257.0 | 635.30000 | LC-MS | − |
| X-03056 | 2.21 | 2264.0 | 185.20000 | LC-MS | + |
| X-11319 | 5.81 | 5700.0 | 269.40000 | LC-MS | − |
| X-11550 | 5.15 | 5130.0 | 250.20000 | LC-MS | − |
| X-11908 | 5.27 | 5244.0 | 429.40000 | LC-MS | − |
| X-11478 | 4.3 | 4286.0 | 166.20000 | LC-MS | − |
Figure 6.
Projection to latent structure discriminant analysis (PLS-DA) of metabolomic profiles while fed the baseline choline-adequate diet separates participants who will develop fatty liver from participants who will not develop organ dysfunction when fed a choline-deficient diet. Samples were obtained at the end of the baseline choline-adequate diet period and analyzed as described in Fig. 1. Using baseline data, PLS-DA provides a good separation of the group that will develop fatty liver (solid squares) from those who will not develop organ dysfunction (open squares) when fed a choline-deficient diet. Metabolites used for this separation are shown in Table 1.
Figure 7.
Molecules identified by metabolomic profiling at baseline that predicted the risk of developing fatty liver when fed a choline-deficient diet. Samples were obtained and analyzed as described in Fig. 1. Results at baseline that predicted the risk of developing fatty liver when fed a choline-deficient diet are summarized by grouping metabolites into shared metabolic pathways. Metabolites whose levels increased (red) or did not change (white) are shown.
DISCUSSION
In our study, metabolomic profiling detected 324 small molecules in plasma, and 152 of these were positively identified chemically (a complete list of positively identified molecules is provided in Supplemental Table 2). Some metabolites were derived from food constituents, for example, stachydrine (also called proline betaine), a constituent of orange juice (52) that was included in the baseline but not in the deficient diets (note that stachydrine almost disappears in plasma during the depletion period; Fig. 4). Some of the unknown metabolites are likely to be products of gut flora metabolism. Because different compounds and ions have different ionization potentials, metabolomic profiling provides relative concentrations, rather than absolute values. These changes in relative concentrations of small molecules that were not examined in targeted assays provided a much broader picture of changes in metabolism that occurred with the dietary intervention.
Metabolites that change when humans are fed a choline-deficient diet
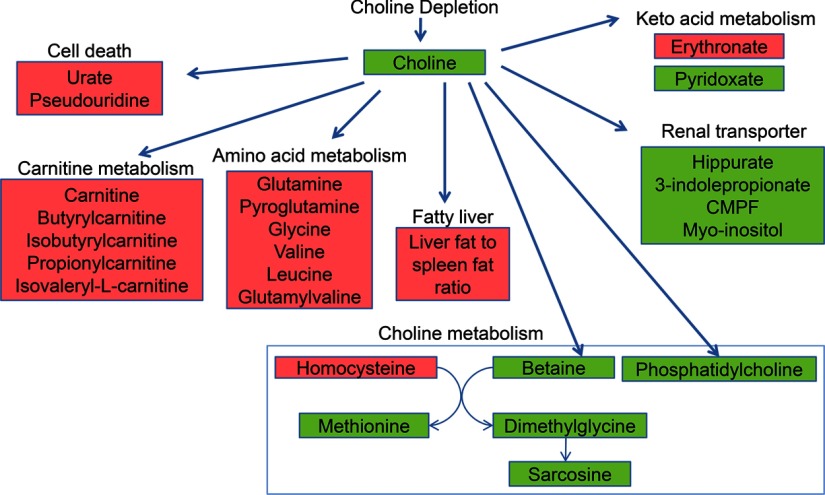
Metabolomic profiling complemented targeted analyses, and identified plasma metabolites that significantly changed when participants were fed the choline-deficient diet. These molecules could be grouped into several categories: metabolites in pathways of choline or methionine metabolism, metabolites that accumulate in uremia, metabolites related to carnitine metabolism, metabolites related to amino acid metabolism, and metabolites that are released when cells die (Fig. 8).
Figure 8.
Molecules identified by metabolomic profiling that were significantly changed at the end of choline depletion as compared to baseline values. Samples were obtained and analyzed as described in Fig. 1. Results that were significantly changed at the end of choline depletion as compared to baseline values are summarized by grouping metabolites into shared metabolic pathways. Metabolites whose levels increased (red) or decreased (green) are shown.
The changes observed in choline metabolites are consistent with decreased availability of choline for production of betaine, resulting in decreased methylation of homocysteine to form methionine, and decreased production of dimethylglycine and sarcosine (Fig. 8). While folate status is an important determinant of homocysteine, we previously showed that these subjects have a significant rise in homocysteine when fed a choline-deficient diet, whether they receive 100 μg or >500 μg folic acid daily, and this increase was not associated with their folate status (12). In addition, the change in homocysteine was not due to vitamin B12 deficiency, as dietary intake of B12 was adequate and MMA concentrations remained low. It is possible that PEMT activity is increased during choline deficiency (53), and this generates more homocysteine as a product; however, the activity of this should be limited by the availability of SAM, which decreases in choline deficiency (54). It is interesting that sarcosine concentrations changed, as sarcosine was recently identified as an intermediate biomarker for the malignant potential of prostate cancers (41). Choline depletion diminished phosphatidylcholine concentrations as expected.
Another grouping of metabolites (myo-inositol, CMPF, hippurate, and 3-indolepropionate) that changed in choline deficiency is related to renal function. In animals, choline deficiency is associated with a defect in reabsorption of water in the renal distal tubule (55), and it is possible that concentrations of these metabolites are lower in choline deficiency because they are being lost with excess water in urine. Myo-inositol, like betaine, is one of the major organic osmolytes used frequently by mammalian cells to regulate volume in response to hypertonic stress (56, 57). Hippurate, 3-indolepropionate and CMPF are uremic toxins that are eliminated from plasma via renal organic anion transporters (OAT) (58). In particular, human OAT1 and OAT3, which are highly expressed in kidney, transport organic anions across the basolateral membrane into the tubular cells of the kidney, recycling out α-ketoglutarate. Hippurate, which also participates with glutaminase in the formation of ammonia in the kidney (59), is decreased during choline depletion, while glutamine is increased (Fig. 4). On the apical side of renal proximal tubule cells, URAT1 is an important OAT for moving anions into the lumen in exchange for urate, which is reabsorbed back into the cell (58). Though changes in organic anion transport could explain the increase in urate and its isomer pseudouridine that we found in plasma of choline-deficient humans, it is possible that these chemicals were released from dying cells secondary to induction of apoptosis. We note that isovaleryl-l-carnitine, which is a caspase-activating proapoptotic factor formed from leucine, and found in blood cells, kidney, liver, and muscle (60, 61), was not elevated in plasma from choline-depleted humans (Fig. 4).
We report that choline deficiency increased plasma concentrations of carnitine and several acylcarnitines (Fig. 4). This is consistent with published studies that report an elevation in plasma carnitine in choline-deficient animals, even while tissue stores are reduced (62, 63). Carnitine is essential for the translocation of acylcarnitines into the mitochondria for β-oxidation of long-chain fatty acids. Transport of carnitine and short-chain acylcarnitines into tissues is facilitated by a recently cloned member of the OCT family, the Na+-driven carnitine organic cation transporter (hOCTN2), which is highly expressed in kidney, skeletal muscle, and heart. This transporter also mediates the transport of betaine (64). Most renal OCTs are expressed on the apical membrane of the tubular cells, and they play an important role in the reabsorption and/or secretion into the lumen of cationic compounds (65). Choline is one such compound that is reabsorbed when plasma concentrations are low, and secreted when plasma levels are high to maintain homeostasis (66). Creatinine and trans-4-hydroxyproline are breakdown products of the skeletal muscle components, creatine phosphate and collagen, respectively. While muscle dysfunction occurs with choline depletion, neither of these metabolites was elevated in the small group of participants with that clinical outcome (Fig. 4). It is possible that creatinine, which is an organic cation like choline and carnitine, was taken up from plasma by human organic cation transporter 2 (hOCT2) and transported across the basolateral membrane in human kidney (67).
We observed that choline depletion was associated with a decrease in pyridoxate, likely from pyridoxal phosphate (PLP), the active form of vitamin B6 (Fig. 4), even though all participants received a vitamin supplement containing 2 mg vitamin B6/d, which is >100% of the RDA. This decrease in vitamin B6 was associated with an increase in erythronate, a keto-acid that, when phosphorylated, participates in vitamin B6 metabolism. PLP acts as a coenzyme for transamination reactions, as well as for other reactions, including the formation of cystathionine from homocysteine; the latter is elevated in choline deficiency (Figs. 4 and 8). Interestingly, B6 is a cofactor in the reactions that produce several molecules that we identified as predictors for increased risk of developing fatty liver when consuming a choline-deficient diet (these include homocysteine, erythronate, 3-methyl-2-oxobutyrate and 3-(4-hydroxyphenyl) lactate). These organic acids are primarily used for energy and fatty acid synthesis in liver. Leucine and valine, and their isomers glutamylvaline and γ-glutamylleucine, are branched-chain amino acids that use the same B6-requiring aminotransferase to form α-keto acids before being oxidized by a common branched-chain α-keto acid dehydrogenase to yield different CoA derivatives. Valine primarily forms propionyl CoA, which is complexed with carnitine to translocate to the inner mitochondria for oxidation. A high basal level of this acylcarnitine in plasma is predictive for humans likely to develop hepatosteatosis when consuming a low-choline diet (Table 1).
Our analysis did not identify any metabolite as significantly different in early depleters (those who depleted consuming the baseline diet) as compared with other participants at baseline. It would be interesting to know whether similar pathways as described above for the subjects who depleted consuming the choline-deficient diet were also changed in the early depleters, but this would require another study with a much larger sample size of early depleters.
The baseline metabolome used to predict the risk of developing fatty liver during choline deficiency
It is possible that metabolic inefficiencies, perhaps due to genetic polymorphisms, modulate the dietary requirement for choline (17, 18). These inefficiencies could modify the metabolome even when people are consuming normal choline-adequate diets. We found that two different statistical approaches (USC and PLS-DA) identified a common set of metabolites (Table 1) that could predict choline deficiency phenotype (no organ dysfunction vs. developing fatty liver). We did not include the muscle dysfunction group because the group size was small. Both statistical approaches used cross-validation, a technique that estimates how a statistical analysis result can be generalized to an independent data set. We could accurately predict (from cross-validation in USC), at baseline, which participants would develop fatty liver when fed a choline-deficient diet in 80% of Caucasian subjects. When all participants were included, our prediction accuracy fell to 69%. These ethnic differences may reflect differences in prevalence of SNPs in choline metabolism genes. The false-positive rate of prediction during cross-validation for all ethnic groups was 25%, and the false-negative rate was 35% (22% for Caucasians only). Many of the molecules identified to predict the risk of developing fatty liver under choline-deficient dietary conditions were the same as those that changed once the participants were fed a choline-deficient diet, and included choline and methionine pathway metabolites, acylcarnitines, keto-acids, amino acids, and molecules excreted via renal organic anion transporters (Fig. 7). Baseline plasma choline did not predict whether organ dysfunction would develop when consuming a choline-deficient diet, perhaps because plasma choline concentration appears to be maintained above a certain level and does not progressively fall as subjects become depleted of choline.
Compared to those individuals who would develop no organ dysfunction when deprived of choline, the participants who eventually developed fatty liver had higher homocysteine, cysteine, and cystathionine concentrations at baseline, consistent with decreased methylation of homocysteine (Table 1). This occurred despite ingesting the adequate intake for choline, and despite having normal vitamin B12 status. It is interesting that phosphatidylcholine concentrations in plasma were elevated in this group at baseline (Table 1). Phosphatidylcholine in plasma is mostly found in lipoproteins. Propionylcarnitine concentrations were increased, and this group had elevated concentrations of triglyceride and a number of fatty acids in their plasma at baseline. Finally, their liver fat to spleen fat ratio was higher than the no organ dysfunction group at baseline (Table 1). It is possible that this group is already partially choline depleted even when ingesting a purported choline-adequate diet, and therefore had metabolic perturbations similar (though of lesser magnitude) to those that were observed at the end of more severe depletion. It is tempting to suggest that these people had an increase in stearoyl-CoA desaturase (SCD) activity. SCD is the rate-limiting enzyme in monounsaturated fatty acid synthesis. It primarily produces oleate (C18:1,n-9) and palmitoleate (C16:1,n-7) but can form monounsaturated fatty acid species from myristate and odd-numbered long-chain fatty acids that are likely derived from bacterial or plant sources. Recently, plasma palmitoleate was reported as an independent marker of triglyceridemia in healthy men (68). Moreover, mice with a 4-fold increase in SCD activity had significantly more plasma triglycerides and increased VLDL secretion (69). On the other hand, SCD deficiency in mice reduced triglyceride accumulation and VLDL synthesis and secretion from liver, and suppressed endogenous phosphatidylcholine biosynthesis via phosphatidylethanolamine N-methyltransferase, although hepatic phosphatidylcholine synthesis via the Kennedy pathway was enhanced (70).
Compared to those individuals who did not develop organ dysfunction when deprived of choline, the participants who eventually developed fatty liver had higher keto-acids in plasma, perhaps because of the proposed perturbation in vitamin B6 that we discussed earlier. A number of amino acids were also elevated; these might be altered because of changes in muscle uptake and utilization of amino acids (56, 57) (as noted, there is a link between choline intake and muscle damage). Alternatively, these amino acids could be released from apoptotic cells, as was urate. As noted earlier, choline metabolism and apoptotic mechanisms are linked. It is interesting that the amino acid changes were ranked high in the PLS-DA analysis for their contribution to the prediction of phenotype. This suggests that these changes in amino acid profiles are integral to the mechanisms that influence dietary requirements for choline.
Metabolomic profiling has been successfully used to examine the effects of drugs and toxic compounds on fat accumulation in the liver (71,72,73,74). In addition, the effects of fasting on metabolomic profiles have been described in rodents (75). To our knowledge, this is the first controlled clinical study in which nutrient intake was systematically manipulated and the effects on metabolomic profiles examined in humans. For a small number of molecules that were also assessed using more standard targeted measures, we found that the metabolomic profiling approach was highly correlated. In addition, the metabolomic profiling approach identified changes in metabolic pathways that might not have been examined in a targeted manner. For the first time, we report that there are characteristic changes in amino acid, keto-acid, and carnitine metabolism when humans are made choline deficient. Also, we discovered that a number of molecules cleared by renal transporters are decreased. Finally, we confirmed that choline-deficient humans have elevated urate and pseudouridine concentrations in plasma, consistent with increased apoptosis. Perhaps the choline-specific metabolite markers identified using metabolomic profiling can be used to develop diagnostic test panels that will predict which patients, when treated with total parenteral nutrition, will develop severe liver dysfunction due to choline deficiency (27). It would be interesting to further study the relationship between choline depletion and liver dysfunction in a population in need of total parenteral nutrition. These results show that a baseline metabolic signature can be used to predict the risk of developing fatty liver during choline deficiency. Such an approach to predict how individuals respond to a nutrient intake or deficiency using a baseline metabolome, may have a huge untapped potential in population phenotyping studies, in determining individual nutrient requirements and in designing personalized nutritional interventions.
Supplementary Material
Acknowledgments
The authors thank Yunping Qiu for the PCA and PLS-DA analyses. W.S. performed the statistical computations for data analysis and was responsible for the preparation of the manuscript. K.D. supervised the sample collection and targeted analyses and assisted in the data interpretation and writing of the manuscript. L.M.F. oversaw recruitment of subjects into the study, compliance and safety monitoring. A.B., M.M., and K.L. were responsible for the metabolomic analyses; A.B. also participated in the interpretation of the data. W.J. supervised the PCA and PLS-DA analyses. S.H.Z. was responsible for the conceptualization, funding, implementation and design of the human study, participated in statistical analyses and data interpretation, and provided major input in the writing of the manuscript. There is no financial conflict of interest in relation to this study (S.H.Z. received grant support from Mead Johnson Nutritionals, Balchem, and the Egg Nutrition Research Center for studies other than those described in this paper; S.H.Z. serves on the scientific advisory boards of Metabolon, Inc. and Dupont). This study was funded by grants from the National Institutes of Health to S.H.Z. (DK55865, DK56350, RR00046).
References
- Zeisel S. H. Choline: critical role during fetal development and dietary requirements in adults. Annu Rev Nutr. 2006;26:229–250. doi: 10.1146/annurev.nutr.26.061505.111156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niculescu M. D., Zeisel S. H. Diet, methyl donors and DNA methylation: interactions between dietary folate, methionine and choline. J Nutr. 2002;132:2333S–2335S. doi: 10.1093/jn/132.8.2333S. [DOI] [PubMed] [Google Scholar]
- Finkelstein J. D. Pathways and regulation of homocysteine metabolism in mammals. Semin Thromb Hemost. 2000;26:219–225. doi: 10.1055/s-2000-8466. [DOI] [PubMed] [Google Scholar]
- Kim Y. I., Miller J. W., da Costa K. A., Nadeau M., Smith D., Selhub J., Zeisel S. H., Mason J. B. Folate deficiency causes secondary depletion of choline and phosphocholine in liver. J Nutr. 1995;124:2197–2203. doi: 10.1093/jn/124.11.2197. [DOI] [PubMed] [Google Scholar]
- Selhub J., Seyoum E., Pomfret E. A., Zeisel S. H. Effects of choline deficiency and methotrexate treatment upon liver folate content and distribution. Cancer Res. 1991;51:16–21. [PubMed] [Google Scholar]
- Varela-Moreiras G., Selhub J., da Costa K., Zeisel S. H. Effect of chronic choline deficiency in rats on liver folate content and distribution. J Nutr Biochem. 1992;3:519–522. [Google Scholar]
- Blusztajn J. K., Zeisel S. H., Wurtman R. J. Developmental changes in the activity of phosphatidylethanolamine N-methyltransferases in rat brain. Biochem J. 1985;232:505–511. doi: 10.1042/bj2320505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine and National Academy of Sciences USA Choline. National Academy Press; Washington, DC: Dietary Reference Intakes for Folate, Thiamin, Riboflavin, Niacin, Vitamin B12, Panthothenic Acid, Biotin, and Choline. 1998;Vol. 1:pp. 390–422. [PubMed] [Google Scholar]
- Busby M. G., Fischer L., Da Costa K. A., Thompson D., Mar M. H., Zeisel S. H. Choline- and betaine-defined diets for use in clinical research and for the management of trimethylaminuria. J Am Diet Assoc. 2004;104:1836–1845. doi: 10.1016/j.jada.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Da Costa K. A., Badea M., Fischer L. M., Zeisel S. H. Elevated serum creatine phosphokinase in choline-deficient humans: mechanistic studies in C2C12 mouse myoblasts. Am J Clin Nutr. 2004;80:163–170. doi: 10.1093/ajcn/80.1.163. [DOI] [PubMed] [Google Scholar]
- Da Costa K. A., Gaffney C. E., Fischer L. M., Zeisel S. H. Choline deficiency in mice and humans is associated with increased plasma homocysteine concentration after a methionine load. Am J Clin Nutr. 2005;81:440–444. doi: 10.1093/ajcn.81.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer L. M., daCosta K., Kwock L., Stewart P., Lu T. S., Stabler S., Allen R., Zeisel S. Sex and menopausal status influence human dietary requirements for the nutrient choline. Am J Clin Nutr. 2007;85:1275–1285. doi: 10.1093/ajcn/85.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z. M., Vance D. E. The active synthesis of phosphatidylcholine is required for very low density lipoprotein secretion from rat hepatocytes. J Biol Chem. 1988;263:2998–3004. [PubMed] [Google Scholar]
- Albright C. D., Salganik R. I., Kaufmann W. K., Vrablic A. S., Zeisel S. H. A p53-dependent G1 checkpoint function is not required for induction of apoptosis by acute choline deficiency in immortalized rat hepatocytes in culture. J Nutr Biochem. 1998;9:476–481. [Google Scholar]
- Da Costa K. A., Niculescu M. D., Craciunescu C. N., Fischer L. M., Zeisel S. H. Choline deficiency increases lymphocyte apoptosis and DNA damage in humans. Am J Clin Nutr. 2006;84:88–94. doi: 10.1093/ajcn/84.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resseguie M., Song J., Niculescu M. D., da Costa K. A., Randall T. A., Zeisel S. H. Phosphatidylethanolamine N-methyltransferase (PEMT) gene expression is induced by estrogen in human and mouse primary hepatocytes. FASEB J. 2007;21:2622–2632. doi: 10.1096/fj.07-8227com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Costa K. A., Kozyreva O. G., Song J., Galanko J. A., Fischer L. M., Zeisel S. H. Common genetic polymorphisms affect the human requirement for the nutrient choline. FASEB J. 2006;20:1336–1344. doi: 10.1096/fj.06-5734com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlmeier M., da Costa K. A., Fischer L. M., Zeisel S. H. Genetic variation of folate-mediated one-carbon transfer pathway predicts susceptibility to choline deficiency in humans. Proc Natl Acad Sci U S A. 2005;102:16025–16030. doi: 10.1073/pnas.0504285102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savendahl L., Mar M. H., Underwood L., Zeisel S. Prolonged fasting results in diminished plasma choline concentration but does not cause liver dysfunction. Am J Clin Nutr. 1997;66:622–625. doi: 10.1093/ajcn/66.3.622. [DOI] [PubMed] [Google Scholar]
- Zeisel S. H., da Costa K. A., Franklin P. D., Alexander E. A., Lamont J. T., Sheard N. F., Beiser A. Choline, an essential nutrient for humans. FASEB J. 1991;5:2093–2098. [PubMed] [Google Scholar]
- Jensen H. H., Batres-Marquez S. P., Carriquiry A., Schalinske K. L. Choline in the diets of the US population: NHANES, 2003–2004. FASEB J. 2007;21:lb219. [Google Scholar]
- Cho E., Zeisel S. H., Jacques P., Selhub J., Dougherty L., Colditz G. A., Willett W. C. Dietary choline and betaine assessed by food-frequency questionnaire in relation to plasma total homocysteine concentration in the Framingham Offspring Study. Am J Clin Nutr. 2006;83:905–911. doi: 10.1093/ajcn/83.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G. M., Carmichael S. L., Laurent C., Rasmussen S. A. Maternal nutrient intakes and risk of orofacial clefts. Epidemiology. 2006;17:285–291. doi: 10.1097/01.ede.0000208348.30012.35. [DOI] [PubMed] [Google Scholar]
- Shaw G. M., Carmichael S. L., Yang W., Selvin S., Schaffer D. M. Periconceptional dietary intake of choline and betaine and neural tube defects in offspring. Am J Epidemiol. 2004;160:102–109. doi: 10.1093/aje/kwh187. [DOI] [PubMed] [Google Scholar]
- Xu X., Gammon M. D., Zeisel S. H., Bradshaw P. T., Wetmur J. G., Teitelbaum S. L., Neugut A. I., Santella R. M., Chen J. High intakes of choline and betaine reduce breast cancer mortality in a population-based study. FASEB J. 2009;23:4022–4028. doi: 10.1096/fj.09-136507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman A., Dubin M., Moukarzel A., Jenden D., Roch M., Rice K., Gornbein J., Ament M. Choline deficiency: a cause of hepatic steatosis during parenteral nutrition that can be reversed with intravenous choline supplementation. Hepatology. 1995;22:1399–1403. [PubMed] [Google Scholar]
- Buchman A. L., Ament M. E., Sohel M., Dubin M., Jenden D. J., Roch M., Pownall H., Farley W., Awal M., Ahn C. Choline deficiency causes reversible hepatic abnormalities in patients receiving parenteral nutrition: proof of a human choline requirement: a placebo-controlled trial. J Parenter Enteral Nutr. 2001;25:260–268. doi: 10.1177/0148607101025005260. [DOI] [PubMed] [Google Scholar]
- Sheard N. F., Tayek J. A., Bistrian B. R., Blackburn G. L., Zeisel S. H. Plasma choline concentration in humans fed parenterally. Am J Clin Nutr. 1986;43:219–224. doi: 10.1093/ajcn/43.2.219. [DOI] [PubMed] [Google Scholar]
- Nehra V., Angulo P., Buchman A. L., Lindor K. D. Nutritional and metabolic considerations in the etiology of nonalcoholic steatohepatitis. Digest Dis Sci. 2001;46:2347–2352. doi: 10.1023/a:1012338828418. [DOI] [PubMed] [Google Scholar]
- Song J., da Costa K. A., Fischer L. M., Kohlmeier M., Kwock L., Wang S., Zeisel S. H. Polymorphism of the PEMT gene and susceptibility to nonalcoholic fatty liver disease (NAFLD) FASEB J. 2005;19:1266–1271. doi: 10.1096/fj.04-3580com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H., Wang J., Li C., Hirose A., Nozaki Y., Takahashi M., Ono M., Akisawa N., Iwasaki S., Saibara T., Onishi S. The phosphatidylethanolamine N-methyltransferase gene V175M single nucleotide polymorphism confers the susceptibility to NASH in Japanese population. J Hepatol. 2007;46:915–920. doi: 10.1016/j.jhep.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Jun D. W., Han J. H., Jang E. C., Kim S. H., Jo Y. J., Park Y. S., Chae J. D. Polymorphisms of microsomal triglyceride transfer protein gene and phosphatidylethanolamine N-methyltransferase gene in alcoholic and nonalcoholic fatty liver disease in Koreans. Eur J Gastroenterol Hepatol. 2009;21:667–672. doi: 10.1097/MEG.0b013e3283196adc. [DOI] [PubMed] [Google Scholar]
- Romeo S., Cohen J. C., Hobbs H. H. No association between polymorphism in PEMT (V175M) and hepatic triglyceride content in the Dallas Heart Study. FASEB J. 2006;20:2180. doi: 10.1096/fj.06-1004ufm. author reply 2181–2182. [DOI] [PubMed] [Google Scholar]
- Zeisel S. People with fatty liver are more likely to have the PEMT rs7946 SNP, yet populations with the mutant allele do not have fatty liver. FASEB J. 2006;20:2181–2182. [Google Scholar]
- Da Costa K., Cochary E. F., Blusztajn J. K., Garner S. C., Zeisel S. H. Accumulation of 1,2-sn-diradylglycerol with increased membrane-associated protein kinase C may be the mechanism for spontaneous hepatocarcinogenesis in choline-deficient rats. J Biol Chem. 1993;268:2100–2105. [PubMed] [Google Scholar]
- Zeisel S. H. Nutrigenomics and metabolomics will change clinical nutrition and public health practice: insights from studies on dietary requirements for choline. Am J Clin Nutr. 2007;86:542–548. doi: 10.1093/ajcn/86.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keun H. C., Athersuch T. J. Application of metabonomics in drug development. Pharmacogenomics. 2007;8:731–741. doi: 10.2217/14622416.8.7.731. [DOI] [PubMed] [Google Scholar]
- Jacobs A. An FDA perspective on the nonclinical use of the X-Omics technologies and the safety of new drugs. Toxicol Lett. 2009;186:32–35. doi: 10.1016/j.toxlet.2008.08.013. [DOI] [PubMed] [Google Scholar]
- Rubino F. M., Pitton M., Di Fabio D., Colombi A. Toward an “omic” physiopathology of reactive chemicals: thirty years of mass spectrometric study of the protein adducts with endogenous and xenobiotic compounds. Mass Spectrom Rev. 2009;28:725–784. doi: 10.1002/mas.20207. [DOI] [PubMed] [Google Scholar]
- Beger R. D., Sun J., Schnackenberg L. K. Metabolomics approaches for discovering biomarkers of drug-induced hepatotoxicity and nephrotoxicity. Toxicol Appl Pharmacol. 2009;243:154–166. doi: 10.1016/j.taap.2009.11.019. [DOI] [PubMed] [Google Scholar]
- Sreekumar A., Poisson L. M., Rajendiran T. M., Khan A. P., Cao Q., Yu J., Laxman B., Mehra R., Lonigro R. J., Li Y., Nyati M. K., Ahsan A., Kalyana-Sundaram S., Han B., Cao X., Byun J., Omenn G. S., Ghosh D., Pennathur S., Alexander D. C., Berger A., Shuster J. R., Wei J. T., Varambally S., Beecher C., Chinnaiyan A. M. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009;457:910–914. doi: 10.1038/nature07762. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Koc H., Mar M. H., Ranasinghe A., Swenberg J. A., Zeisel S. H. Quantitation of choline and its metabolites in tissues and foods by liquid chromatography/electrospray ionization-isotope dilution mass spectrometry. Anal Chem. 2002;74:4734–4740. doi: 10.1021/ac025624x. [DOI] [PubMed] [Google Scholar]
- Fishbein M., Gardner K., Potter C., Schmalbrock P., Smith M. Introduction of fast MR imaging in the assessment of hepatic steatosis. Magn Reson Imaging. 1997;15:287–293. doi: 10.1016/s0730-725x(96)00224-x. [DOI] [PubMed] [Google Scholar]
- Saadeh S., Younossi Z. M., Remer E. M., Gramlich T., Ong J. P., Hurley M., Mullen K. D., Cooper J. N., Sheridan M. J. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745–750. doi: 10.1053/gast.2002.35354. [DOI] [PubMed] [Google Scholar]
- Ma X., Holalkere N. S., Kambadakone R. A., Mino-Kenudson M., Hahn P. F., Sahani D. V. Imaging-based quantification of hepatic fat: methods and clinical applications. Radiographics. 2009;29:1253–1277. doi: 10.1148/rg.295085186. [DOI] [PubMed] [Google Scholar]
- Boudonck K. J., Mitchell M. W., Nemet L., Keresztes L., Nyska A., Shinar D., Rosenstock M. Discovery of metabolomics biomarkers for early detection of nephrotoxicity. Toxicol Pathol. 2009;37:280–292. doi: 10.1177/0192623309332992. [DOI] [PubMed] [Google Scholar]
- Lawton K. A., Berger A., Mitchell M., Milgram K. E., Evans A. M., Guo L., Hanson R. W., Kalhan S. C., Ryals J. A., Milburn M. V. Analysis of the adult human plasma metabolome. Pharmacogenomics. 2008;9:383–397. doi: 10.2217/14622416.9.4.383. [DOI] [PubMed] [Google Scholar]
- Evans A. M., DeHaven C. D., Barrett T., Mitchell M., Milgram M. Integrated, nontargeted ultrahigh performance liquid chromatography/elctrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal Chem. 2009;81:6656–6667. doi: 10.1021/ac901536h. [DOI] [PubMed] [Google Scholar]
- Barrett T., Dehaven C. D., Alexander D. C. U.S. Patent and Trademark Office; Washington, DC: System, method, and computer program product using a database in a computing system to compile and compare metabolomic data obtained from a plurality of samples US Patent 7,433,787; issued October 27, 2008. 2008 [Google Scholar]
- Storey J. D., Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci U S A. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung K. Y., Bumgarner R. E. Multiclass classification of microarray data with repeated measurements: application to cancer. Genome Biol. 2003;4:R83. doi: 10.1186/gb-2003-4-12-r83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson W., Downer P., Lever M., Chambers S. T., George P. M. Effects of orange juice and proline betaine on glycine betaine and homocysteine in healthy male subjects. Eur J Nutr. 2007;46:446–452. doi: 10.1007/s00394-007-0684-5. [DOI] [PubMed] [Google Scholar]
- Cui Z., Vance D. E. Expression of phosphatidylethanolamine N-methyltransferase-2 is markedly enhanced in long term choline-deficient rats. J Biol Chem. 1996;271:2839–2843. doi: 10.1074/jbc.271.5.2839. [DOI] [PubMed] [Google Scholar]
- Zeisel S. H., Zola T., daCosta K., Pomfret E. A. Effect of choline deficiency on S-adenosylmethionine and methionine concentrations in rat liver. Biochem J. 1989;259:725–729. doi: 10.1042/bj2590725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael U. F., Cookson S. L., Chavez R., Pardo V. Renal function in the choline-deficient rat. Proc Soc Exp Biol Med. 1975;150:672–676. doi: 10.3181/00379727-150-39103. [DOI] [PubMed] [Google Scholar]
- Horio M., Yamauchi A., Moriyama T., Imai E., Orita Y. Osmotic regulation of amino acids and system A transport in Madin-Darby canine kidney cells. Am J Physiol Cell Physiol. 1997;272:C804–C809. doi: 10.1152/ajpcell.1997.272.3.C804. [DOI] [PubMed] [Google Scholar]
- Kempson S. A., Montrose M. H. Osmotic regulation of renal betaine transport: transcription and beyond. Pflügers Arch. 2004;449:227–234. doi: 10.1007/s00424-004-1338-6. [DOI] [PubMed] [Google Scholar]
- Srimaroeng C., Perry J. L., Pritchard J. B. Physiology, structure, and regulation of the cloned organic anion transporters. Xenobiotica. 2008;38:889–935. doi: 10.1080/00498250801927435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzurik R., Spustova V., Krivosikova Z., Gazdikova K. Hippurate participates in the correction of metabolic acidosis. Kidney Intl. 2001;78:S278–S281. doi: 10.1046/j.1523-1755.2001.59780278.x. [DOI] [PubMed] [Google Scholar]
- Ferrara F., Bertelli A., Falchi M. Evaluation of carnitine, acetylcarnitine and isovalerylcarnitine on immune function and apoptosis. Drugs Exp Clin Res. 2005;31:109–114. [PubMed] [Google Scholar]
- Pontremoli S., Melloni E., Viotti P. L., Michetti M., Di Lisa F., Siliprandi N. Isovalerylcarnitine is a specific activator of the high calcium requiring calpain forms. Biochem Biophys Res Commun. 1990;167:373–380. doi: 10.1016/0006-291x(90)91775-n. [DOI] [PubMed] [Google Scholar]
- Carter A. L., Frenkel R. The relationship of choline and carnitine in the choline deficient rat. J Nutr. 1978;108:1748–1754. doi: 10.1093/jn/108.11.1748. [DOI] [PubMed] [Google Scholar]
- Sheard N. F., Krasin B. Restricting food intake does not exacerbate the effects of a choline-deficient diet on tissue carnitine concentrations in rats. J Nutr. 1994;124:738–743. doi: 10.1093/jn/124.5.738. [DOI] [PubMed] [Google Scholar]
- Wagner C. A., Lukewille U., Kaltenbach S., Moschen I., Broer A., Risler T., Broer S., Lang F. Functional and pharmacological characterization of human Na(+)-carnitine cotransporter hOCTN2. Am J Physiol Renal Physiol. 2000;279:F584–F591. doi: 10.1152/ajprenal.2000.279.3.F584. [DOI] [PubMed] [Google Scholar]
- Launay-Vacher V., Izzedine H., Karie S., Hulot J. S., Baumelou A., Deray G. Renal tubular drug transporters. Nephron. 2006;103:97–106. doi: 10.1159/000092212. [DOI] [PubMed] [Google Scholar]
- Acara M., Roch R. F., Rennick B. Bidirectional renal tubular transport of free choline: a micropuncture study. Am J Physiol. 1979;236:F112–F118. doi: 10.1152/ajprenal.1979.236.2.F112. [DOI] [PubMed] [Google Scholar]
- Urakami Y., Kimura N., Okuda M., Inui K. Creatinine transport by basolateral organic cation transporter hOCT2 in the human kidney. Pharm Res. 2004;21:976–981. doi: 10.1023/b:pham.0000029286.45788.ad. [DOI] [PubMed] [Google Scholar]
- Paillard F., Catheline D., Duff F. L., Bouriel M., Deugnier Y., Pouchard M., Daubert J. C., Legrand P. Plasma palmitoleic acid, a product of stearoyl-coA desaturase activity, is an independent marker of triglyceridemia and abdominal adiposity. Nutr Metab Cardiovasc Dis. 2008;18:436–440. doi: 10.1016/j.numecd.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Attie A. D., Krauss R. M., Gray-Keller M. P., Brownlie A., Miyazaki M., Kastelein J. J., Lusis A. J., Stalenhoef A. F., Stoehr J. P., Hayden M. R., Ntambi J. M. Relationship between stearoyl-CoA desaturase activity and plasma triglycerides in human and mouse hypertriglyceridemia. J Lipid Res. 2002;43:1899–1907. doi: 10.1194/jlr.m200189-jlr200. [DOI] [PubMed] [Google Scholar]
- Dobrzyn A., Dobrzyn P., Miyazaki M., Sampath H., Chu K., Ntambi J. M. Stearoyl-CoA desaturase 1 deficiency increases CTP:choline cytidylyltransferase translocation into the membrane and enhances phosphatidylcholine synthesis in liver. J Biol Chem. 2005;280:23356–23362. doi: 10.1074/jbc.M502436200. [DOI] [PubMed] [Google Scholar]
- Kovacic P. Unifying mechanism for addiction and toxicity of abused drugs with application to dopamine and glutamate mediators: electron transfer and reactive oxygen species. Med Hypoth. 2005;65:90–96. doi: 10.1016/j.mehy.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Maddox J. F., Luyendyk J. P., Cosma G. N., Breau A. P., Bible R. H., Jr, Harrigan G. G., Goodacre R., Ganey P. E., Cantor G. H., Cockerell G. L., Roth R. A. Metabonomic evaluation of idiosyncrasy-like liver injury in rats cotreated with ranitidine and lipopolysaccharide. Toxicol Appl Pharmacol. 2006;212:35–44. doi: 10.1016/j.taap.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Duarte I. F., Stanley E. G., Holmes E., Lindon J. C., Gil A. M., Tang H., Ferdinand R., McKee C. G., Nicholson J. K., Vilca-Melendez H., Heaton N., Murphy G. M. Metabolic assessment of human liver transplants from biopsy samples at the donor and recipient stages using high-resolution magic angle spinning 1H NMR spectroscopy. Anal Chem. 2005;77:5570–5578. doi: 10.1021/ac050455c. [DOI] [PubMed] [Google Scholar]
- Griffin J. L., Bonney S. A., Mann C., Hebbachi A. M., Gibbons G. F., Nicholson J. K., Shoulders C. C., Scott J. An integrated reverse functional genomic and metabolic approach to understanding orotic acid-induced fatty liver. Physiol Genomics. 2004;17:140–149. doi: 10.1152/physiolgenomics.00158.2003. [DOI] [PubMed] [Google Scholar]
- van Ginneken V., Verhey E., Poelmann R., Ramakers R., van Dijk K. W., Ham L., Voshol P., Havekes L., Van Eck M., van der Greef J. Metabolomics (liver and blood profiling) in a mouse model in response to fasting: a study of hepatic steatosis. Biochim Biophys Acta. 2007;1771:1263–1270. doi: 10.1016/j.bbalip.2007.07.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.