Summary
Extracellular stimuli exert their effects on eukaryotic cells via serpentine G-protein-coupled receptors and mediate a vast number of physiological responses. Activated receptors stimulate heterotrimeric G-proteins, consisting of three subunits, α, β and γ. In Dictyostelium discoideum, cAMP binds to the cAMP receptor cAR1, which is coupled to the heterotrimer containing the Gα2 subunit. These studies provide in vivo evidence as to how receptors influence the localization of the G-protein complex prior to and after ligand binding. Previous work has shown that the state of the heterotrimer could be monitored by changes in fluorescence (or Förster) resonance energy transfer (FRET) between the α2- and β-subunits of D. discoideum. We now report the kinetics of G-protein activation as a loss of FRET prior to and after cAMP addition by using total internal reflection fluorescence microscopy (TIRFM). We also performed photobleaching experiments to measure G-protein recovery times. Our data show that inactive and active G-proteins cycle between the cytosol and plasma membrane. These data suggest that cAR1 activation slows the membrane dissociation (`off') rate of the α2 subunit, while simultaneously promoting βγ-subunit dissociation.
Keywords: FRET, G proteins, Chemotaxis
Introduction
G-protein-mediated signal-transduction pathways play an essential role during chemotaxis in the simple eukaryote Dictyostelium discoideum. Genetic analysis and database searches have revealed 11 Gα subunits and a single Gβ and Gγ subunit in this organism (van Es and Devreotes, 1999; Zhang et al., 2001). The Gα2 subunit couples to the cyclic adenosine 3′,5′ monophosphate (cAMP) receptor cAR1 and is essential for development and chemotaxis during aggregation (Janetopoulos et al., 2001; Kumagai et al., 1991). Activated receptors catalyze the exchange of guanosine triphosphate (GTP) for guanosine diphosphate (GDP) and allow both the GTP-bound α-subunit and free βγ complexes to signal to downstream effectors (Gilman, 1987). Over the years, biochemical evidence has accumulated that suggests that membrane-associated Gα and Gβγ subunits dissociate from one another and that this dissociation is necessary for the activation or inhibition of effectors (Northup et al., 1983; Sternweis et al., 1981). However, interpreting earlier in vitro experiments has proven difficult because the use of detergents might have artificially promoted subunit dissociation and because magnesium concentrations in these studies were often much higher than normal physiological levels (Lambert, 2008). Another limitation of in vitro experiments might be the lack of physical influence that normally occurs as G proteins couple to their respective receptor(s) and interact with the plasma membrane. Although it is accepted that the mechanisms underlying the G-protein cycle are well established (Gilman, 1987; Hamm, 1998), there is very little evidence derived from intact cells describing the membrane dynamics of the heterotrimer prior to and after stimulation.
The first successful in vivo attempt to look at G-protein dynamics in response to a stimulus was performed in D. discoideum (Janetopoulos et al., 2001). Fluorescence (or Förster) resonance energy transfer (FRET) between the α2 and β-subunits was used to visualize the conformational change of the heterotrimer. A large loss of FRET in response to chemoattractant suggested dissociation of the subunits. Since then, other groups have examined FRET between the α- and β-subunits in live yeast and mammalian cells, showing decreases and increases in the signal in response to stimulus, respectively (Bunemann et al., 2003; Yi et al., 2003). Studies performed more recently have reported similar findings using bioluminescent resonance energy transfer (BRET) or FRET, and have seen both increases and decreases in the fluorescent signal in response to stimulus (Azpiazu and Gautam, 2004; Frank et al., 2005; Gales et al., 2006; Gibson and Gilman, 2006). These bioluminescent reporters have provided significant clues about the conformational changes that take place in a variety of heterotrimers from various systems.
The heterotrimeric G-proteins play a crucial role during chemoattractant-gradient sensing in eukaryotic organisms (Bagorda and Parent, 2008; Devreotes and Janetopoulos, 2003; Schneider and Haugh, 2006). Cells have a remarkable ability to sense very shallow chemoattractant gradients and respond with sharply localized responses at the leading edge of a cell. Evidence gleaned from many studies suggests that this localization occurs upstream of PI3-kinase activity and downstream of G-protein activation (Charest and Firtel, 2006; Janetopoulos et al., 2001). Single-molecule experiments performed in D. discoideum that examine the interactions between the chemoattractant (cAMP) and the receptor (cAR1) demonstrated that the off-rate of the chemoattractant varied significantly between the front and rear of a cell and also was dependent on the presence of functional G proteins (Ueda et al., 2001). This work suggested that some factor or modification that was linked to the polarized morphology of the cell controlled the binding affinity of chemoattractant to the receptors. This, in turn, might have played a role in amplifying the responses at the leading edge of a cell. It would seem likely that these differences in binding affinities at the front and rear of a cell should influence or might be controlled by the activity of the G proteins. In order to obtain a better understanding of in vivo G-protein activation, total internal reflection microscopy (TIRFM) was combined with a G-protein FRET-based assay while cells were exposed to a variety of conditions. G proteins were monitored directly at the plasma membrane before and after receptor stimulation. We further investigated the interactions of the G proteins with the plasma membrane by performing photobleaching studies. These studies have revealed the dynamic nature of the interactions of the G proteins with the plasma membrane and have helped further elucidate the role that G-protein activation might play in amplifying the response of cells during gradient sensing.
Results and Discussion
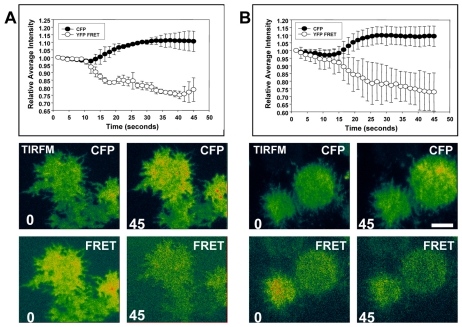
During TIRFM, energy, in the form of an evanescent wave, propagates 100-150 nm into the cell and excites fluorescent molecules at the cell-coverslip interface. This technique provides a very high signal-to-noise ratio and permits the investigation of molecular dynamics at the plasma membrane (Axelrod, 2001). Previously, we monitored G-protein FRET between the α2- and β-subunits in D. discoideum by analyzing the emission profile of large populations of cells in a fluorimeter. We demonstrated that there were rapid changes in the conformation of the G proteins in response to cAMP (Janetopoulos et al., 2001). In this prior study, the FRET fluorescence decreased by approximately 70% upon cAMP application. This large change suggested subunit dissociation between the Gα2 and Gβ subunits. However, it was possible that this loss of FRET was the result of large changes in the conformation of the heterotrimer. In order to analyze only those G proteins that were present at the plasma membrane, cells expressing the same FRET pair were stimulated with a uniform concentration of cAMP and imaged with TIRFM. There was an increase in the Gα2-CFP fluorescence and a simultaneous decrease in the β-YFP FRET fluorescence (Fig. 1A). During continuous stimulation, the membrane G-protein activation did not decline, even though most physiological responses such as PI3K activity or actin polymerization would have subsided. To control for any cell movement and feedback mechanisms from the actin cytoskeleton, which have been shown to play a significant role in signaling cascades, we treated the cells with latrunculin A, an inhibitor of the actin cytoskeleton (Charest and Firtel, 2006). Cells responded with similar kinetics, suggesting no influence of the actin cytoskeleton on G-protein activation (Fig. 1B). Treated and untreated cells both showed a corresponding gain in CFP signal and loss in FRET fluorescence, suggesting activation of the heterotrimer. Control cells exposed to buffer showed no significant changes in fluorescence (supplementary material Fig. S1). The relative changes in membrane FRET fluorescence when compared with the total membrane fluorescent signal were about twofold higher (15-20% vs 7-10%) than those changes seen when comparing signals during whole-cell analysis (Janetopoulos et al., 2001). This is probably due to the high signal-to-noise ratio gained by imaging FRET at the plasma membrane and the avoidance of fluorescent contributions from cytosolic G proteins. There were no changes in the FRET signal in the cytosol when measured using epifluorescence microscopy (not shown). These data suggest that G-protein activation, as measured by loss of G-protein FRET, was local and occurred at or near the plasma membrane. These results also confirm and extend our previous studies on whole cells using fluorimetric analysis that demonstrated that G-protein activation was continuous during prolonged stimulation in spite of most cellular responses having subsided (Janetopoulos et al., 2001). This suggests that adaptation of the response must be downstream or independent of the conformational changes of the heterotrimer.
Fig. 1.
cAMP induces actin-independent heterotrimer disassociation at the plasma membrane. (A) Gα2-null cells expressing the FRET pair (Gα2-CFP and Gβ-YFP) were exposed continuously to ∼1 μM cAMP after 10 seconds of monitoring while CFP and YFP emission at the plasma membrane were simultaneously recorded using TIRF microscopy. As shown in the graph, cells showed a rapid increase in CFP fluorescence and a corresponding decrease in YFP-FRET fluorescence at the plasma membrane after cAMP stimulation. This loss of FRET was maintained in the presence of cAMP. (B) The same experiment was repeated using cells that were treated with latrunculin A, an inhibitor of actin. These cells showed a similar loss in FRET fluorescence, suggesting that cAMP-induced heterotrimer dissociation is actin-independent. Changes in relative cell intensity were normalized to changes in background fluorescence. Graphs are the mean average intensity ± s.e.m. All experiments were done in triplicate with at least 15 cells quantified per day. Representative CFP and FRET images with time stamps (seconds) are shown. Cells were pseudocolored on equal scales to allow for better visualization of changes. Scale bar: 5 μm.
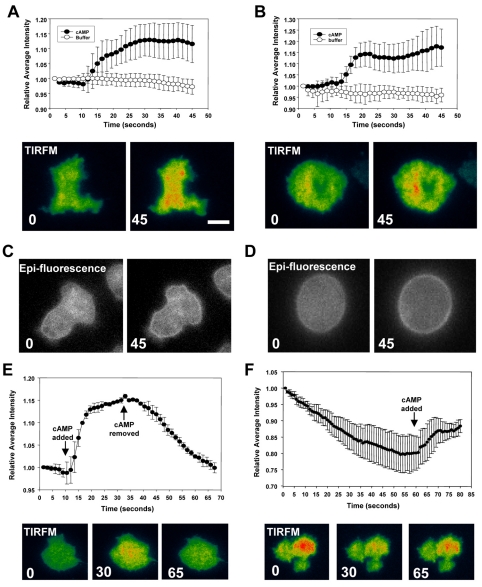
Dictyostelium cells undergo a number of morphological changes, including a cringe response, a rounding-up phase and a return to random movement when exposed to a uniform stimulus of cAMP (Futrelle et al., 1982). To control for any changes in cell shape that might contribute to fluorescent artifacts owing to cell spreading or flattening, cell lines expressing either cAR1-YFP, Gβ-CFP or Gα2-CFP were stimulated with cAMP and imaged with TIRFM. Cells expressing Gβ-CFP or cAR1-YFP alone showed no increase in fluorescence at the membrane surface (supplementary material Fig. S2). Surprisingly, cells expressing Gα2-CFP alone showed a rapid increase in fluorescence at the plasma membrane upon stimulation with cAMP (Fig. 2A; supplementary material Movie 1). Gα2-CFP fluorescence increased rapidly and reached a steady-state maximum within 10 seconds that was about 15% higher than basal levels. This increase in fluorescence mirrored the time course of G-protein activation when G-protein FRET was monitored. As shown above for G-protein activation, the rise in membrane Gα2-CFP signal in response to cAMP stimulation occurred in cells treated with latrunculin A, demonstrating that feedback from the cytoskeleton was also not needed for this response (Fig. 2B, top). This increase in Gα2-CFP fluorescence was also visualized with standard epifluorescence microscopy (Fig. 2C,D, bottom) and confocal microscopy (supplementary material Fig. S3). Using TIRFM, it was found that Gα2-CFP levels returned to basal levels upon removal of cAMP (Fig. 2E; supplementary material Movie 2). To determine whether Gα2-CFP membrane levels remained high after longer periods of exposure to cAMP, cells were stimulated continuously for 5 minutes and then re-examined (Fig. 2F). It has previously been shown that most cellular responses to cAMP would have subsided in cells treated with cAMP for extended periods of time. In addition, cAR1 would be fully phosphorylated (Caterina et al., 1995). After removal of cAMP, there was a decrease in Gα2 signal on the membrane; the loss of Gα2 signal on the membrane had a half-time (approximately 15 seconds) that was similar to that seen when cells were stimulated for only 30 seconds. This showed that the membrane levels of Gα2 remained elevated over the course of minutes and demonstrates that cAR1 phosphorylation had little or no effect on the affinity of Gα2 for the plasma membrane. The recruitment of Gα2-CFP to the plasma membrane is reminiscent of other responses seen during chemotactic stimulation; PI3-kinase and pleckstrin homology (PH) domains are rapidly recruited to the plasma membrane from the cytosol when cells are given a uniform stimulus of cAMP. However, whereas those responses are transient, Gα2-CFP recruitment reflected the presence of cAMP. In a gradient, PI3-kinase and PH domains localize to the leading edge of the cell (Huang et al., 2003). The Gα2-subunit data suggested that the localization of Gα2-CFP should mirror the local concentrations of cAMP, as has been previously suggested for G-protein activation (Janetopoulos et al., 2001; Xu et al., 2005). However, cells imaged in a stable cAMP gradient showed no significant differences between the front and rear when chemotaxing, as assayed by TIRFM. A similar result was found for latrunculin-A-treated cells imaged with epifluorescence (data not shown). Given the small changes in Gα2-CFP localization at the membrane when receptors are saturated, it may not be surprising that small changes in receptor occupancy across the cell were below the level of signal detection. It should be noted that Xu et al. (Xu et al., 2005) showed that G-protein activation mirrored the cAMP gradient by measuring G-protein activation (in cells expressing the same FRET pair: Gα2-CFP and Gβ-YFP) as a ratio of the Gα2-CFP signal in response to an acute stimulus of cAMP (Xu et al., 2005). These data support our hypothesis that changes in both G-protein activation and Gα2-CFP affinity should mirror the cAMP gradient. This result might have significant implications for investigators modeling the gradient-sensing mechanism. A recent model has implicated Gβγ subunits in helping specify the rear of a cell and will be discussed in the section below (Levine et al., 2006). Taken together with the G-protein FRET data, these experiments strongly suggest that the Gα2 subunit, once bound by GTP, is no longer associated with the Gβγ subunit. The Gβγ subunit must diffuse away from the plasma membrane because the membrane β-YFP signal shows no corresponding increase.
Fig. 2.
Gα2-CFP is recruited to the plasma membrane upon cAMP stimulation. (A) Gα2-null cells expressing Gα2-CFP were stimulated with a continual dose of ∼1 μM cAMP or control buffer after 10 seconds of monitoring. (A-D) Cells were imaged with TIRFM (pseudocolor; A,B) or epifluorescence (grayscale; C,D) prior to and after uniform stimulation. After the addition of cAMP, Gα2 was quickly recruited to the plasma membrane. (B) The same experiment as in A was performed in cells treated with 5 μM latrunculin A, an actin inhibitor. Similar results were obtained, suggesting that the recruitment of Gα2 to the plasma membrane does not require F-actin. (E) Cells expressing Gα2-CFP were stimulated with a continual dose of cAMP at 10 seconds and imaged using TIRFM. The cAMP was then withdrawn at 35 seconds. There was a rapid decline in the membrane Gα2-CFP fluorescence after cAMP removal. Images were taken every 1.5 seconds for 67.5 seconds. (F) Cells were stimulated with ∼1 μM cAMP for 5 minutes prior to recording. cAMP was withdrawn at frame 5. There was a rapid decline in the membrane Gα2-CFP fluorescence. Cells were re-stimulated with cAMP after 1 minute and the Gα2-CFP signal on the membrane increased again, demonstrating that the on-rate was also unaffected by prolonged stimulation. Changes in relative cell intensity were normalized to changes in background fluorescence. Graphs are the mean average intensity ± s.e.m. All experiments were done in triplicate with at least 15 cells quantified per day. Representative images with time stamps (seconds) are shown. Cells were pseudocolored on equal scales to allow for better visualization of changes. Scale bar: 5 μm.
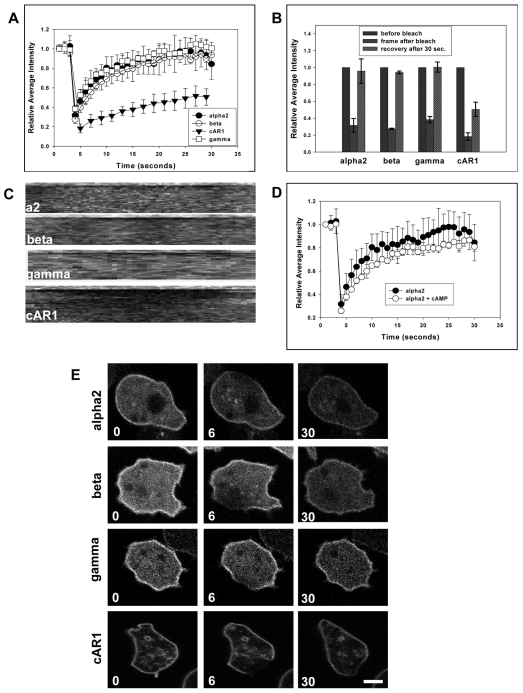
We speculated that the G proteins were cycling between a cytosolic pool and the plasma membrane, with the heterotrimer residing at the plasma membrane for some short time. In the presence of cAMP, activated Gα2 might then be retained at the membrane, while Gβγ would be released via dissociation. Roughly 30% of the heterotrimeric G proteins are in the cytosol and the analysis of cytosolic G-protein FRET has suggested that these G proteins are in the GDP-bound heterotrimeric state (Janetopoulos et al., 2001; Zhang et al., 2001). To investigate this hypothesis, we performed fluorescence recovery after photobleaching (FRAP) experiments using confocal microscopy (Fig. 3E). Interestingly, Gα2-CFP, Gβ-CFP and Gγ-CFP recovered uniformly across the plasma membrane, with a half-time of approximately 5 seconds (Fig. 3A,B; supplementary material Movie 3). cAR1 recovery took much longer and was dependent on the size of the bleached spot, as expected for a transmembrane protein. The recovery rate for the G proteins was independent of the amount of bleached area, further suggesting that the G proteins are cycling between the cytosol and membrane (supplementary material Fig. S4). Kymograph analysis showed that cAR1 diffuses in laterally, whereas the G proteins recovered evenly across the membrane (Fig. 3C). To investigate the Gα2 recovery time when receptors were occupied by chemoattractant, cells expressing Gα2 were continuously stimulated with cAMP and photobleached (Fig. 3D). The recovery times were similar to unstimulated cells. These data show that G-protein heterotrimers are continuously cycling between the cytosol and plasma membrane. Furthermore, the photobleaching data presented here support the argument that there was complete subunit disassociation upon receptor activation. Future experiments should determine the half-time of free cytosolic Gβγ subunits and the roles that locally activated Gα2 subunits play in amplifying the response during chemotaxis. Future studies also need to address whether the Gα2 subunit is in a GDP-bound, empty pocket or GTP-bound state when associated with the plasma membrane. Furthermore, it should be determined whether stably bound Gα2 subunit is interacting with cAR1 or directly with the plasma membrane and/or possibly downstream effector molecules. Interestingly, when Gα2-CFP was expressed in cells lacking cAR1 and cAR3, it still interacted with the plasma membrane, and when FRAP was performed, the Gα2 recovered with kinetics that were similar to those seen in other cell lines examined (supplementary material Fig. S5).
Fig. 3.
G-protein heterotrimers cycle between the cytosol and plasma membrane. FRAP experiments were conducted on Gα2-null cells expressing Gα2-CFP, Gβ-null cells expressing β-CFP, AX2 cells expressing γ-YFP and cAR1/3-null cells expressing cAR1-YFP. Representative cell images before, immediately after bleaching and at the end of 30 seconds are shown. (A) Gα2-CFP, β-CFP and γ-YFP had similar recovery rates, whereas cAR1-YFP recovery was significantly slower. (B) Despite being bleached to comparable levels, cAR1 only recovered 50%, whereas the G proteins reached full recovery after 30 seconds. Fluorescence recovery was graphed as a function of the total intensity of the cell versus the fluorescence of the bleached region and was then normalized to 1. Graphs are the mean average intensity ± s.e.m. All experiments were done in triplicate (n=15). (C) Kymograph analysis showed that Gα2, Gβ and Gγ subunits recovered evenly across the membrane, whereas cAR1 recovery occurred laterally. (D) Gα2-CFP-recovery on rates were independent of the presence of cAMP, because naive cells and cells treated with a saturating dose of cAMP recovered with similar rates. (E) FRAP experiments of Gα2-null cells expressing Gα2-CFP, Gβ-null cells expressing β-CFP and AX2 cells expressing Gγ-CFP show that the G proteins fully recover within 30 seconds, whereas cAR1 does not. Scale bar: 5 μm.
How might these dynamic movements of the G proteins influence gradient sensing? A recent model, proposed by Levine et al., suggests that the G proteins themselves make good candidates for the activator and inhibitor molecules that they propose underlie the large gain seen in the responses at the leading edge during gradient sensing (Levine et al., 2006). They suggested that the Gα-subunit might locally activate downstream effectors, whereas diffusible Gβγ subunits could potentially act as inhibitory molecules. This model might be on target because the data presented here suggest that the Gα subunit remains local and the Gβγ subunits diffuse away. Further support for this idea comes from single-molecule analysis of cAR1-YFP, which was shown to have two different receptor populations in the absence of signaling. Some were immobile and some were mobile, suggesting, similar to the finding presented here, that not all of cAR1 is precoupled to the G protein (de Keijzer et al., 2008). The authors further found that the immobile fraction was almost identical in chemotaxing cells at the leading edge to that of cells lacking the Gα2 subunit. They interpreted this to mean that there was uncoupling of the receptor from the G proteins in response to cAMP. Taken together with the data presented here, the single-molecule results suggest that the Gα2 subunits might be binding the membrane or proteins on the membrane (and not the receptor directly) after receptor activation. This local increase of Gα2 subunits at the front of a cell may in turn provide the molecular component for the initial linear amplification that occurs during gradient sensing.
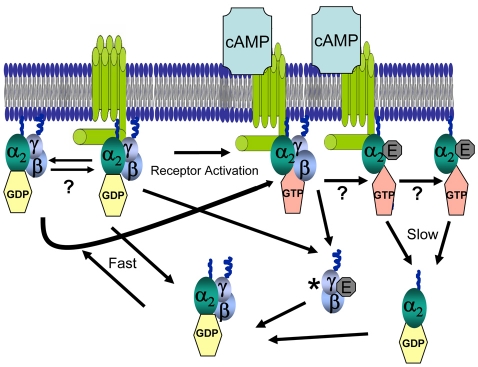
These results show that the receptors and G proteins do not form stable complexes in the absence of signaling. We propose that intact heterotrimers interact transiently with the receptors before receptor activation and are activated by a mechanism similar to that proposed for the classical collision-coupling model (Bourne, 1997; Gilman, 1987) (Fig. 4). However, whereas it is typically thought that the heterotrimeric G proteins move about only within the plasma membrane, our results suggest that they are shuttling back and forth from cytosolic pools. Studies using D. discoideum still must determine whether the shuttling of the G proteins back and forth in the absence of activated receptor occurs between the membrane or receptor, or possibly both. It is also possible that cAMP interacts with receptors that are not `precoupled'. Support for this comes from single-molecule studies imaging the binding of cAMP to the receptors, which demonstrated that cells lacking the Gα and Gβ subunits had an on and off-rate that was faster than wild-type cells (Ueda et al., 2001). The finding that the off-rate of the Gα2 subunit is affected by receptor activation could be consistent with the idea that, once the Gα subunits release GDP, there is a high-affinity state between the receptor and G protein (Oldham and Hamm, 2008). Earlier studies in D. discoideum have also suggested that receptors remain coupled to G proteins during prolonged stimulation (Snaar-Jagalska et al., 1991). Similar findings were found in neurons through a variety of elegant experiments in which the G-protein-coupled receptors (GPCRs) were immobilized and shown to affect the mobility of the Gα subunits (Digby et al., 2006).
Fig. 4.
Model for G-protein cycling in Dictyostelium discoideum. Inactive heterotrimers continuously cycle between the cytosol and cAR1 or the inner leaflet of the plasma membrane. It is likely that the lipid modifications of both Gα2 (palmitoylation and myristoylation) and Gγ (isoprenylation) play a significant role in the heterotrimer interacting with the plasma membrane. Upon binding of cAMP to the receptor, there is a change in receptor conformation that might increase the affinity of the receptor for the Gα2 subunit. The conformational change in cAR1 simultaneously triggers the exchange of GDP for GTP on the Gα2 subunit and the heterotrimer dissociates. Because the Gβγ subunit does not apparently change its residency time on the plasma membrane, time spent in the empty-pocket conformation is probably extremely fast and the Gα2 subunit might remain coupled to the receptor in the GTP-bound state. It is also possible that both GDP- and GTP-bound Gα2 subunits interact with both receptor and the plasma membrane, or effector molecules (E) in the latter case, when activated. This is supported by data showing that the Gα2 subunits still interact with the plasma membrane in cells lacking cAR1 and cAR3. In either situation, this dissociation is complete, and the active Gβγ subunit diffuses away from the membrane. The intrinsic GTPase activity of the Gα2 subunit hydrolyzes the bound GTP and the receptor, membrane or effector molecule and Gα2 subunits dissociate. Because we have been unable to measure changes in FRET in the cytosol, liberated Gβγ subunits probably find free Gα2 subunits and reform the heterotrimer. This model can explain both the loss of G-protein FRET and the lack of a change in membrane Gβ-subunit intensity upon receptor activation.
These experiments showing transient interactions between the heterotrimer and plasma membrane are interesting given the predicted electrostatic interactions of the G proteins with the membrane phospholipids (Murray et al., 2001). However, other studies in mammalian cell lines have shown that γ-subunits are capable of translocating from the plasma membrane to the endomembrane in response to receptor activation (Saini et al., 2007). Short-lived interactions have also been shown for the small GTPase Ras in mammalian systems and will probably be found with other lipid-modified signaling enzymes that work at the cytosolic–plasma-membrane interface (Goodwin and Kenworthy, 2005). A recent report using single-molecule imaging suggests that Gαi2 is recruited to CD59 clusters and interacts transiently with the membrane in T24 epithelial cells (Suzuki et al., 2007). Other photobleaching studies have reported similar findings and it is becoming increasingly evident that different heterotrimers probably interact with the receptors and plasma membrane in unique and various ways (Digby et al., 2006; Digby et al., 2008; Lambert, 2008). We speculate that heterotrimers containing other α-subunits in D. discoideum might also show differential dissociation. Such a finding might indicate that different heterotrimeric conformations are capable of activating unique Gβγ effectors or the same Gβγ effectors with different efficiency. Interestingly, the photobleaching data presented in this study suggest that Gβγ subunits coupled to other α-subunits are also transiently interacting with the plasma membrane, because some of these subunits presumably are interacting with receptors other than cAR1. This would further indicate that other heterotrimers have a similar half-time because they interact with very different seven-transmembrane receptors. By contrast, it might be argued that the similar half-times suggest that they are interacting with the plasma membrane and it is the Gβγ subunits that control these transient interactions in the absence of activated receptors. This seems to be the case for some γ-subunits in mammalian cells (Saini et al., 2007). It will be interesting to determine how many different receptors are actually expressed in chemotaxing cells, given that we know from sequence data that there are at least 50 GPCRs, and probably more than 100 (Eichinger et al., 2005).
Materials and Methods
Cell culture
All cell lines were cultured axenically in HL5 medium at 22°C. For development, cells were washed twice with DB buffer (5 mM Na2HPO4, 5 mM KH2PO4,1 mM CaCl2, 2 mM MgCl2) then starved in DB buffer for 6 hours with continuous shaking. Cells were pulsed with 50 nM cAMP every 6 minutes for the last 5 hours of starvation. All transformants were maintained in G418 (20 μg/ml for single transformants, 30 μg/ml for double).
Cell lines
Cells expressing Gα2-CFP in Gα2-null cells, Gα2-CFP and β-YFP in Gα2-null cells and Gβ-YFP in Gβ-null cells were previously described (Janetopoulos et al., 2001). cAR1- and cAR3-nulls and β-null cells were transformed by electroporation with 5.0 μg of plasmid (Insall et al., 1994). G418-resistant clones were selected in 2-3 weeks. The gene encoding the full-length eCFP was fused to the N terminal of Gβ, similar to the Gβ-YFP fusion previously reported. The cloning of the cAR1-YFP was performed as described for cAR1-GFP (Xiao et al., 1997).
Reagents
Latrunculin A and cAMP were obtained from Sigma-Aldrich (St Louis, MO).
Microscopy
Cells were imaged in DB buffer in two-well LabTek chambers (total volume of DB is 1 ml). A micropipette (Eppendorf) was back-loaded with either DB (control) or cAMP (100 μM) and attached to a micropump (Eppendorf Femtojet). The micropipette was attached to a micromanipulator (Eppendorf). Cells were stimulated with cAMP by rapidly bringing in the micropipette to a pre-set position in close proximity to the cells. To remove the cAMP, the micropipette was quickly brought up to a pre-set position approximately 1000 μm above the cells. A 40× PlanNeofluar 1.3 NA wide-field lens for epifluorescence and a 100× PlanFluar 1.45 NA TIRFM lens were used. Images were acquired on a Zeiss Axiovert Marianas Workstation from Intelligent Imaging and Innovations. CFP was imaged using a 40 mW 445-nm laser and a 75 mW 523-nm laser was used for exciting YFP. FRET fluorescence was imaged using a Multispec (Dual View) CFP/YFP Dual EX/EM.
FRAP and confocal-imaging experiments
Cells expressing either Gα2-CFP, Gβ-CFP, Gγ-CFP or cAR1-YFP were imaged on an Olympus FV1000 LSM equipped with an Olympus PlanApoN 60× objective and HeNe lasers at 458 nm (CFP excitation and bleaching) and 515 nm (YFP excitation and bleaching). Regions within CFP-expressing cells were bleached with the 458-nm laser for 100 iterations at 100% laser power. YFP-expressing cells were bleached with the 515-nm laser for eight iterations at 50% laser power. Time lapses were taken every 1 second for the Gα2-CFP, Gβ-CFP and Gγ-CFP cells and every 2 seconds for the cAR1-YFP cells. The resulting bleach spot was between 20-40% of the original intensity. For stimulation experiments with cAMP, cells were given a saturating dose (1 μM) of cAMP and then bleached. Fluorescence recovery was graphed as a function of the total intensity of the cell versus the fluorescence of the bleached region and was then normalized to 1 as previously described (Goodwin and Kenworthy, 2005). Kymograph analyses were performed using the kymograph plug-in from ImageJ, which is available to download for free from the NIH.
Data analysis and statistics
Images were analyzed using Slidebook from 3I. CFP and YFP intensities were calculated by first subtracting any changes in background fluorescence and then normalizing to the first frame of the movie. FRET fluorescence was further normalized by subtracting the bleed-through of the CFP fluorescence from the YFP channel. CFP bleed-through into the YFP channel was calculated by reading the CFP and YFP emission of CFP-expressing cells. Based on those numbers, the bleed-through percentage was determined to be 26%. Bleed-through of YFP into the CFP was determined to be insignificant (0.07%). After subtracting any changes in background fluorescence, the FRET YFP channel was further normalized by subtracting the changes in CFP emission multiplied by 0.26. This corrected for any changes in bleed-through from the CFP channel. All data shown represent the average of three independent experiments plus the standard error of the mean.
Supplementary Material
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/122/15/2597/DC1
We thank the Devreotes lab for providing the cAR1 and cAR3 nulls. This research was supported in part by the Vanderbilt Institute for Integrative Biosystems Research and Education. Support for J.C. was provided by the Systems Biology Bioengineering Undergraduate Research Experience. Program support for C.A.E. was provided by the Vanderbilt Biomedical Research and Education Training Office, an Institutional Research and Academic Career Developmental Award from the NIGMS/NIH 5K12 GM068543-04, and NIHGM080370 to C.J. Deposited in PMC for release after 12 months.
References
- Axelrod, D. (2001). Total internal reflection fluorescence microscopy in cell biology. Traffic 2, 764-774. [DOI] [PubMed] [Google Scholar]
- Azpiazu, I. and Gautam, N. (2004). A fluorescence resonance energy transfer-based sensor indicates that receptor access to a G protein is unrestricted in a living mammalian cell. J. Biol. Chem. 279, 27709-27718. [DOI] [PubMed] [Google Scholar]
- Bagorda, A. and Parent, C. A. (2008). Eukaryotic chemotaxis at a glance. J. Cell Sci. 121, 2621-2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne, H. R. (1997). How receptors talk to trimeric G proteins. Curr. Opin. Cell Biol. 9, 134-142. [DOI] [PubMed] [Google Scholar]
- Bunemann, M., Frank, M. and Lohse, M. J. (2003). Gi protein activation in intact cells involves subunit rearrangement rather than dissociation. Proc. Natl. Acad. Sci. USA 100, 16077-16082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina, M. J., Hereld, D. and Devreotes, P. N. (1995). Occupancy of the Dictyostelium cAMP receptor, cAR1, induces a reduction in affinity which depends upon COOH-terminal serine residues. J. Biol. Chem. 270, 4418-4423. [DOI] [PubMed] [Google Scholar]
- Charest, P. G. and Firtel, R. A. (2006). Feedback signaling controls leading-edge formation during chemotaxis. Curr. Opin. Genet. Dev. 16, 339-347. [DOI] [PubMed] [Google Scholar]
- de Keijzer, S., Serge, A., van Hemert, F., Lommerse, P. H., Lamers, G. E., Spaink, H. P., Schmidt, T. and Snaar-Jagalska, B. E. (2008). A spatially restricted increase in receptor mobility is involved in directional sensing during Dictyostelium discoideum chemotaxis. J. Cell Sci. 121, 1750-1757. [DOI] [PubMed] [Google Scholar]
- Devreotes, P. and Janetopoulos, C. (2003). Eukaryotic chemotaxis: distinctions between directional sensing and polarization. J. Biol. Chem. 278, 20445-20448. [DOI] [PubMed] [Google Scholar]
- Digby, G. J., Lober, R. M., Sethi, P. R. and Lambert, N. A. (2006). Some G protein heterotrimers physically dissociate in living cells. Proc. Natl. Acad. Sci. USA 103, 17789-17794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digby, G. J., Sethi, P. R. and Lambert, N. A. (2008). Differential dissociation of G protein heterotrimers. J. Physiol. 586, 3325-3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichinger, L., Pachebat, J. A., Glockner, G., Rajandream, M. A., Sucgang, R., Berriman, M., Song, J., Olsen, R., Szafranski, K., Xu, Q. et al. (2005). The genome of the social amoeba Dictyostelium discoideum. Nature 435, 43-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank, M., Thumer, L., Lohse, M. J. and Bunemann, M. (2005). G Protein activation without subunit dissociation depends on a G{alpha}(i)-specific region. J. Biol. Chem. 280, 24584-24590. [DOI] [PubMed] [Google Scholar]
- Futrelle, R. P., Traut, J. and McKee, W. G. (1982). Cell behavior in Dictyostelium discoideum: preaggregation response to localized cyclic AMP pulses. J. Cell Biol. 92, 807-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gales, C., Van Durm, J. J., Schaak, S., Pontier, S., Percherancier, Y., Audet, M., Paris, H. and Bouvier, M. (2006). Probing the activation-promoted structural rearrangements in preassembled receptor-G protein complexes. Nat. Struct. Mol. Biol. 13, 778-786. [DOI] [PubMed] [Google Scholar]
- Gibson, S. K. and Gilman, A. G. (2006). Gialpha and Gbeta subunits both define selectivity of G protein activation by alpha2-adrenergic receptors. Proc. Natl. Acad. Sci. USA 103, 212-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman, A. G. (1987). G proteins: transducers of receptor-generated signals. Annu. Rev. Biochem. 56, 615-649. [DOI] [PubMed] [Google Scholar]
- Goodwin, J. S. and Kenworthy, A. K. (2005). Photobleaching approaches to investigate diffusional mobility and trafficking of Ras in living cells. Methods 37, 154-164. [DOI] [PubMed] [Google Scholar]
- Hamm, H. E. (1998). The many faces of G protein signaling. J. Biol. Chem. 273, 669-672. [DOI] [PubMed] [Google Scholar]
- Huang, Y. E., Iijima, M., Parent, C. A., Funamoto, S., Firtel, R. A. and Devreotes, P. (2003). Receptor-mediated regulation of PI3Ks confines PI(3,4,5)P3 to the leading edge of chemotaxing cells. Mol. Biol. Cell 14, 1913-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insall, R. H., Soede, R. D., Schaap, P. and Devreotes, P. N. (1994). Two cAMP receptors activate common signaling pathways in Dictyostelium. Mol. Biol. Cell 5, 703-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janetopoulos, C., Jin, T. and Devreotes, P. (2001). Receptor-mediated activation of heterotrimeric G-proteins in living cells. Science 291, 2408-2411. [DOI] [PubMed] [Google Scholar]
- Kumagai, A., Hadwiger, J. A., Pupillo, M. and Firtel, R. A. (1991). Molecular genetic analysis of two G alpha protein subunits in Dictyostelium. J. Biol. Chem. 266, 1220-1228. [PubMed] [Google Scholar]
- Lambert, N. A. (2008). Dissociation of heterotrimeric g proteins in cells. Sci. Signal. 1, re5. [DOI] [PubMed] [Google Scholar]
- Levine, H., Kessler, D. A. and Rappel, W. J. (2006). Directional sensing in eukaryotic chemotaxis: a balanced inactivation model. Proc. Natl. Acad. Sci. USA 103, 9761-9766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, D., McLaughlin, S. and Honig, B. (2001). The role of electrostatic interactions in the regulation of the membrane association of G protein beta gamma heterodimers. J. Biol. Chem. 276, 45153-45159. [DOI] [PubMed] [Google Scholar]
- Northup, J. K., Smigel, M. D., Sternweis, P. C. and Gilman, A. G. (1983). The subunits of the stimulatory regulatory component of adenylate cyclase. Resolution of the activated 45,000-dalton (alpha) subunit. J. Biol. Chem. 258, 11369-11376. [PubMed] [Google Scholar]
- Oldham, W. M. and Hamm, H. E. (2008). Heterotrimeric G protein activation by G-protein-coupled receptors. Nat. Rev. Mol. Cell Biol. 9, 60-71. [DOI] [PubMed] [Google Scholar]
- Saini, D. K., Kalyanaraman, V., Chisari, M. and Gautam, N. (2007). A family of G protein betagamma subunits translocate reversibly from the plasma membrane to endomembranes on receptor activation. J. Biol. Chem. 282, 24099-24108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, I. C. and Haugh, J. M. (2006). Mechanisms of gradient sensing and chemotaxis: conserved pathways, diverse regulation. Cell Cycle 5, 1130-1134. [DOI] [PubMed] [Google Scholar]
- Snaar-Jagalska, B. E., Van Es, S., Kesbeke, F. and Van Haastert, P. J. (1991). Activation of a pertussis-toxin-sensitive guanine-nucleotide-binding regulatory protein during desensitization of Dictyostelium discoideum cells to chemotactic signals. Eur. J. Biochem. 195, 715-721. [DOI] [PubMed] [Google Scholar]
- Sternweis, P. C., Northup, J. K., Smigel, M. D. and Gilman, A. G. (1981). The regulatory component of adenylate cyclase: purification and properties. J. Biol. Chem. 256, 11517-11526. [PubMed] [Google Scholar]
- Suzuki, K. G., Fujiwara, T. K., Sanematsu, F., Iino, R., Edidin, M. and Kusumi, A. (2007). GPI-anchored receptor clusters transiently recruit Lyn and G alpha for temporary cluster immobilization and Lyn activation: single-molecule tracking study 1. J. Cell Biol. 177, 717-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda, M., Sako, Y., Tanaka, T., Devreotes, P. and Yanagida, T. (2001). Single-molecule analysis of chemotactic signaling in Dictyostelium cells. Science 294, 864-867. [DOI] [PubMed] [Google Scholar]
- van Es, S. and Devreotes, P. N. (1999). Molecular basis of localized responses during chemotaxis in amoebae and leukocytes. Cell Mol. Life Sci. 55, 1341-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, Z., Zhang, N., Murphy, D. B. and Devreotes, P. N. (1997). Dynamic distribution of chemoattractant receptors in living cells during chemotaxis and persistent stimulation. J. Cell Biol. 139, 365-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, X., Meier-Schellersheim, M., Jiao, X., Nelson, L. E. and Jin, T. (2005). Quantitative imaging of single live cells reveals spatiotemporal dynamics of multistep signaling events of chemoattractant gradient sensing in Dictyostelium. Mol. Biol. Cell 16, 676-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi, T. M., Kitano, H. and Simon, M. I. (2003). A quantitative characterization of the yeast heterotrimeric G protein cycle. Proc. Natl. Acad. Sci. USA 100, 10764-10769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, N., Long, Y. and Devreotes, P. N. (2001). Ggamma in dictyostelium: its role in localization of gbetagamma to the membrane is required for chemotaxis in shallow gradients. Mol. Biol. Cell 12, 3204-3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.