Summary
The apicobasal polarity of epithelia depends on the integrated activity of apical and basolateral proteins, and is essential for tissue integrity and body homeostasis. Yet these tissues are frequently on the move as they are sculpted by active morphogenetic cell rearrangements. How does cell polarity survive these stresses? We analyse this question in the renal tubules of Drosophila, a tissue that undergoes dramatic morphogenetic change as it develops. Here we show that, whereas the Bazooka and Scribble protein groups are required for the establishment of tubule cell polarity, the key apical determinant, Crumbs, is required for cell polarity in the tubules only from the time when morphogenetic movements start. Strikingly, if these movements are stalled, polarity persists in the absence of Crumbs. Similar rescue of the ectodermal phenotype of the crumbs mutant when germ-band extension is reduced suggests that Crumbs has a specific, conserved function in stabilising cell polarity during tissue remodelling rather than in its initial stabilisation. We also identify a requirement for the exocyst component Exo84 during tissue morphogenesis, which suggests that Crumbs-dependent stability of epithelial polarity is correlated with a requirement for membrane recycling and targeted vesicle delivery.
Keywords: Cell polarity, Crumbs, Tissue morphogenesis
Introduction
Polarity and adhesion are hallmark features of epithelial tissues. In the last decade, much has been learnt about the genetic and molecular mechanisms controlling cell polarity in epithelial cells. However, epithelia are extensively remodelled during morphogenesis, when cell rearrangements require the modulation of adhesion and impose a challenge on the stability of cell polarity. In order to unravel the relationship between cell polarity and morphogenesis, we have chosen a simple epithelial system, the renal tubules of the Drosophila embryo. This epithelium has the great advantage that the elaboration of apicobasal polarity and morphogenetic cell rearrangements are clearly separated in time, whereas these two processes occur concurrently in the ectoderm during gastrulation and early germ-band extension.
In the Drosophila embryo, apicobasal polarity is evident in the blastoderm through the apical localisation of Par-6, which depends on the activation of the small GTPase, Cdc42 (Hutterer et al., 2004). At the same time Bazooka (Baz)/Par3 colocalises with the adherens junction component Drosophila E-cadherin, and later acts during gastrulation to stabilise aPKC and Par-6 apically (Harris and Peifer, 2004; Harris and Peifer, 2005). As a consequence, Scribble (Scrib), Lethal giant larvae (Lgl) and Discs large (Dlg) concentrate basolaterally (Bilder et al., 2003; Hutterer et al., 2004; Tanentzapf and Tepass, 2003). Polarity very soon becomes dependent on the activity of Crumbs (Crb) and Stardust (Sdt), which are recruited to the sub-apical domain by Baz. In the epidermis the Crb complex acts to maintain Baz apically and to restrict Scrib, Lgl and Dlg to the basolateral domain (Bilder et al., 2003; Tanentzapf and Tepass, 2003). Embryos mutant for crb or sdt fail to maintain epidermal cell polarity or to establish a zonula adherens (ZA). This results in the loss of epithelial integrity and the epidermis undergoes widespread cell death (Grawe et al., 1996; Tepass et al., 1990; Tepass, 1996). However, despite the expression of Crb and Sdt in all ectodermally derived epithelia, these tissues are differentially affected by the loss of crb or sdt (Tepass et al., 1990), suggesting that distinct features of epithelia underlie differences in their dependence on crb or sdt.
In this paper we show that, whereas the apicobasal polarity of renal tubule cells depends on Baz and Scrib throughout development, Crb and Sdt are required only later as the tubule cells reorganise during extensive morphogenetic movements. If these movements are arrested tubule cell polarity is maintained in the absence of Crb. Furthermore, we provide evidence that the phase when the maintenance of cell polarity depends on Crb coincides with the time when the epithelium shows an enhanced requirement for exocyst function. This result underscores the importance of targeted membrane trafficking for cell polarity during tissue remodelling. Similar observations in other epithelia of the Drosophila embryo indicate a general function for Crb in cell polarity during morphogenesis.
Results
Junctional and polarity proteins are expressed throughout tubule morphogenesis
We first sought to establish the pattern of expression of cell polarity and adhesion proteins in the renal tubules. During stage 10 they bud out from the polarised epithelium of the hindgut primordium, forming short, fat tubes, which elongate dramatically during stages 13-15 by convergent-extension cell rearrangements, to produce the extended mature tubules with just two cells lining the lumen (Fig. 1A,E,I) (Ainsworth et al., 2000). Thus the establishment of cell polarity (before stage 10) and morphogenetic cell rearrangement (stages 13-16) do not overlap in time. This differs from the epidermis where these two processes occur concurrently during gastrulation and early germ-band extension.
Fig. 1.
Renal tubule cells are polarised throughout development. (A-D) Stage 11; (E-H) stage 13; (I-L) stage 16. (A,E,I) Embryos stained for the transcription factor Cut (Ct), to mark renal tubule cells. The renal tubules bud out from the hindgut primordium during stage 11 (A, arrow) and undergo several rounds of cell division. These are completed by stage 13, when the tubules appear as short fat tubes (E, arrow). During stages 13 to 16 they elongate dramatically and narrow to a two-cell circumference (I, arrow). (B,F,J) Crb (red) and Baz (green) are localised to the apical membranes of tubule cells during stages 11-16, with Baz overlapping with, but slightly apical to Crb during stages 11-13. (C,G,K) Dlg (red) localises to the lateral membranes and shows no overlap with Baz (green). (D,H,L) ZAs, marked by Armadillo (Arm, red), are maintained between tubule cells (nuclear Ct, green) throughout cell rearrangements. Dashed white lines outline tubules.
From the time of tubule eversion, during stage 10, the localisation of cell polarity and junctional proteins in the tubules was found to be identical to the epidermal pattern in wild-type embryos (Fig. 1 and data not shown). Significantly, apical and basolateral proteins, as well as ZA-associated proteins, were expressed in defined, non-overlapping domains throughout tubule reorganisation (Fig. 1B-D,F-H,J-L). Thus the process of cell rearrangement does not disrupt apicobasal polarity or cell adhesion during wild-type development.
Tubule cell polarity is not reliant on Crumbs during early development
We assessed the requirement for the activity of cell polarity proteins for tubule development by analysing loss-of-function mutations, where necessary removing both the maternal and zygotic contributions. Tubule cell polarity was lost or severely disrupted already in stage 11 embryos lacking both maternal and zygotic baz or scrib (Fig. 2). By contrast, tubule cells in embryos mutant for crb (or sdt, data not shown) appeared normal at this stage (Fig. 3A-F); Baz and Dlg remained localised (Fig. 3C,D) and electron microscopy revealed that ZAs develop (Fig. 3E,F). Accordingly, in flies carrying the amorphic allele crb8F105, which encodes a truncated protein (Wodarz et al., 1993), Crb protein was still apically localised in tubule cells (Fig. 3B), although it was no longer localised in the epidermis at this stage. Thus the Crb complex is not required for the establishment of polarity and formation of ZAs in the renal tubules or for the maintenance of cell polarity early during their development.
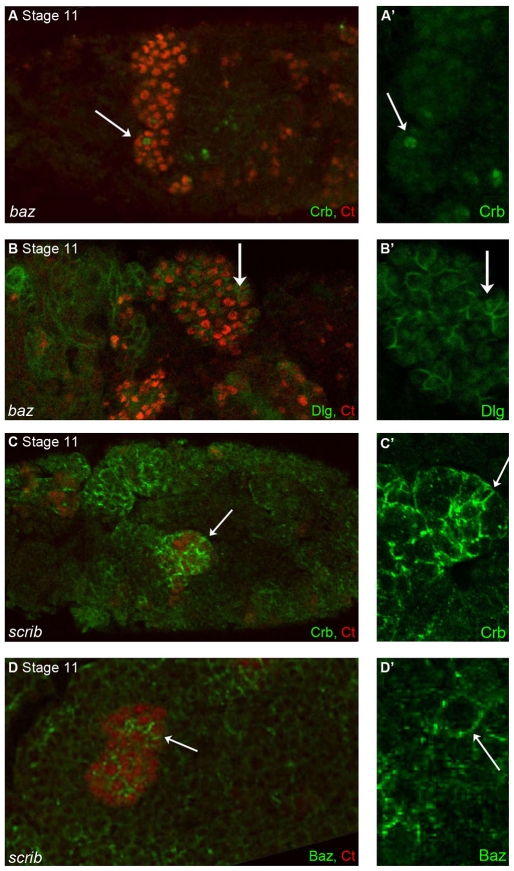
Fig. 2.
Tubule cell polarity is lost in both baz and scrib mutant embryos from the earliest stages of development. Confocal microscopy images of stage 11 baz maternal/zygotic (M/Z) (A,B) and scrib M/Z (C,D) embryos stained for Ct (red) to visualise the renal tubules. (A,B) In baz M/Z mutant tubules Crb levels are strongly reduced and any remaining protein is mis-localised (A,A′, arrow). Dlg has spread around tubule cell membranes (B,B′, arrow). (C,D) In scrib M/Z mutant embryos, both Crb (C,C′, green) and Baz (D,D′, green) are delocalised in tubule cells and can be found all around the cell cortex (arrows).
Fig. 3.
Tubule cells do not require Crb for polarity during early development. (A,B) Stage 11 embryos that are wild type (A) or mutant for the amorphic allele crb8F105 (B) stained for Crb (brown). In wild-type embryos Crb localises apically in both the epidermis (A, arrowhead) and in the renal tubules (A, arrow). In crb mutants the truncated Crb protein is distributed diffusely in the epidermis (B, arrowhead), yet is clearly restricted to the apical membrane of the renal tubules (B, arrow). (C-D″) Lateral view of wild-type (C) and dorsal view of crb (D) stage 11 renal tubules stained for Baz (green) and Dlg (red). crb embryos show a normal localisation of apical and basolateral proteins. (E,F) Transmission electron microscopy images of wild-type (E) and crb (F) tubule cells from stage 11 embryos. ZAs are observed at the apical ends of the lateral membranes of crb mutant tubule cells (F, arrows) as in wild type (E, arrows). Scale bars: 200 nm. (G-I) Overexpressed Crb localises to the apical domain of renal tubules during stages 11-13. (J-K″) Confocal optical sections of stage 13 renal tubules reveal that even when levels of Crb (green) are highly upregulated, Baz (red) localises correctly to the apical domain (K, compare with wild-type embryos in J).
Crb is a potent apical determinant, and strong overexpression in epidermal cells causes expansion of the apical membranes at the expense of the basolateral domain (Wodarz et al., 1995). We overexpressed full-length Crb using a ubiquitous (data not shown) or a tubule-specific driver but found that, despite high levels of Crb (Fig. 3G-K), tubule cell morphology remained unaffected in embryos from stage 11 to 13 (Fig. 3G-K) and the localisation of Baz was unaltered (Fig. 3J,K). Taken together with our loss-of-function data, this shows that the tubules are relatively insensitive to the level of Crb early in their development.
Crumbs becomes essential for tubule cell polarity later in development
We next asked whether there is a requirement for Crb when the tubules are remodelled by cell rearrangements later in development. Tubules of wild-type and crb8F105 mutant embryos at stage 16 show remarkable differences in their morphology. Wild-type tubules have extended, coursing through the body cavity to take up their characteristic positions, whereas the tubules of crb mutant embryos failed to elongate, presenting the appearance of disorganised clusters of cells (Fig. 4A,B). Strikingly, apical and basolateral proteins were delocalised in the tubule cells of stage 16 embryos [Baz, aPKC, Par-6 and Dlg shown in Fig. 4C-F, Lgl, Scrib (as Dlg) not shown]. Ultrastructural analysis revealed that the ZAs have dispersed by this stage (Fig. 4G,H).
Fig. 4.
Tubule cells require Crb to maintain polarity during late stages of development. (A,B) Stage 16 wild-type (A) and crb mutant (B) embryos stained for Ct (brown). The renal tubules (arrows) in crb mutant embryos do not elongate fully and lose their normal morphology. (C-F″) Stage 16 renal tubules stained for Dlg (red) and Baz (green) in C and D, Ct (red) and Par-6 (green) in E, and Ct (red) and aPKC (green) in F. The localisation of the apical proteins Baz (D, green), Par-6 (E, green) and aPKC (F, green) is lost in stage 16 crb tubules. Although Dlg (D, red) remains localised to the lateral membranes, cells are irregular in shape (compare D with the wild type in C). (G,H) Transmission electron microscope images of wild-type (G) and crb (H) tubule cells from stage 16 embryos. (G) ZAs are visible in the apical region of the lateral border between all wild-type tubule cells (arrows). (H) ZAs are no longer present between crb mutant tubule cells (arrows indicate the apical region of lateral membranes). Scale bars: 500 nm. (I-N″) Crb overexpression leads to severe polarity defects in stage 16 embryos; Baz (red; I and J) is delocalised (compare J, where two tubules lie next to each other, with the wild type in I), Dlg (red; K and L) spreads around the cell membranes (compare L with the wild type in K) and Arm (red; M and N) is fragmented and reduced in levels (compare N with wild type in M). Dashed white lines outline the tubules.
Since crb is essential for cell polarity and epithelial integrity during remodelling of the tubules, we analysed whether the overexpression of Crb affects this process as well. Crb overexpression resulted in the delocalisation of Baz (Fig. 4I,J) and Dlg (Fig. 4K,L) in stage 16 tubules. This behaviour is in contrast to the insensitivity of Baz localisation to the cutB-Gal4-mediated overexpression of Crb in tubule cells of stage 11-13 embryos (Fig. 3K). Staining for Armadillo, normally localised to ZAs (Peifer, 1993), reveals a loss of junctional integrity in stage 16 embryos (Fig. 4M,N). Consistent with the evidence that tubule cell polarity is compromised, tubules became multilayered cords of cells lacking a clear lumen (Fig. 4K,L). Together, the loss- and gain-of-function phenotypes for crb indicate two stages in the maintenance of renal tubule cell polarity; the first is independent of Crb whereas the second requires its activity.
Crumbs is required during morphogenesis as tubule cells rearrange
In order to discover the underlying cause of this change in the regulation of tubule cell polarity, we sought to pinpoint more precisely the onset of mutant phenotypes in embryos either lacking or overexpressing Crb. The delocalisation of cell polarity markers and changes in tubule morphology that result from the loss of Crb function became apparent only during stage 13 (Fig. 5A-C). This was mirrored by the onset of sensitivity to the overexpression of Crb; Baz remained apically localised at the beginning of stage 13 but became delocalised in late stage 13 tubule cells (Fig. 5D,E). Stage 13 marks the beginning of two major morphogenetic movements in the tubules; elongation by convergent-extension movements and integration of a population of mesodermal cells, which later differentiate as a distinct cell type, the stellate cells (Denholm et al., 2003). These morphogenetic activities occur concurrently and their timing suggests a correlation between Crb-dependent polarity and these events.
Fig. 5.
Crb is required to maintain polarity during late tubule development as they undergo cell rearrangements. (A,A′) In early stage 13 crb mutant embryos, Baz localises normally to the apical membrane of the tubules and the tubule lumen is clearly visible (arrows). (B,B′) In late stage 13 crb mutant embryos there are regions of tubule cells in which Baz has become delocalised and the lumen of the tubule is disrupted (arrow). (C,C′) By stage 14, polarity is disrupted throughout the tubules and there is no apparent lumen (arrow). (D-E″) When Crb is overexpressed in the tubules, there is no effect on polarity at the beginning of stage 13; Baz remains tightly localised to the apical domain (D′). In late stage 13, Crb spreads down the basolateral membranes of the cells and Baz is no longer restricted to the apical domain (E). (F,G) In wild-type tubules Fas II (green) localises along the lateral membranes of tubule cells (arrow) and does not overlap with apical Baz (red) (F). Stellate cells (Tsh, green) integrate into the epithelium during tubule elongation (G). (H-J) In ct mutant embryos the tubules do not elongate but remain as blisters at the junction between the hindgut and midgut (H,I arrows). (I) Tsh-expressing stellate cells are absent from the tubules of ct mutant embryos. (J) Fas II (green) and Baz (red) localisation is not affected in ct mutant tubule cells. (K-L′) ct;crb double mutants have a tubule phenotype similar to single ct mutants; the cells form epithelial blisters of columnar cells, with Baz (red) and Fas II (green) localised to distinct domains.
To test the hypothesis that Crb is required to maintain tubule cell polarity during cell rearrangement, we assessed cell polarity in the absence of Crb function and under conditions in which these morphogenetic movements are arrested. Cut is expressed in tubule cells (Fig. 1) and is required for the development of this tissue. In embryos carrying mutations in cut, tubules bud out from the hindgut primordium but never elongate, remaining as epithelial blisters close to the hindgut-midgut junction (Hatton-Ellis et al., 2007). In addition, stellate cells, marked by Teashirt, did not integrate into the resulting tubule blisters (Fig. 5F,G,H,I). In these embryos tubule cell polarity was unaffected, as revealed by apical Baz and basolateral Fasciclin II (Fas II, Fig. 5J; also known as Fas2). Tubules in cut;crb double-mutant embryos of the same stage resembled those of cut mutants; they formed epithelial blisters in which Baz and Fas II were normally localised (Fig. 5K,L). The delocalisation of Baz and of basolateral proteins, seen in the tubule cells of stage 16 crb mutant embryos (Fig. 4D), did not occur. These data indicate that Crb function is only required for cell polarity during morphogenetic activity.
Crb and Sdt have been ascribed a conserved role in ectodermal epithelia of Drosophila for the maintenance of polarity, namely to restrict the distribution of Baz, aPKC and Par-6 (apical) and Scrib, Dlg and Lgl (basolateral) and, as a consequence, to allow spot adherens junctions to coalesce to form continuous ZA belts (Bachmann et al., 2001; Grawe et al., 1996; Hong et al., 2001; Tepass and Knust, 1993; Wodarz et al., 1993; Tepass, 1996). It has been noted, however, that different epithelia in the embryo show differing sensitivities to the loss of crb (Tepass and Knust, 1993; Wodarz et al., 1993), suggesting that distinct features underlie differences in their dependence on the activity of the Crb complex. Our analysis of the renal tubules now provides an explanation for this apparent phenotypic variation. In early tubules Crb is not required to localise either apical or basolateral cell polarity proteins, ZAs form normally in its absence and tubule cell polarity is maintained. However, as soon as the tubule cells start to reorganise, changing their neighbours and remodelling their junctional contacts, Crb becomes indispensable, both to maintain the polarised distribution of apical and basolateral proteins and for the maintenance of ZAs (Fig. 4H).
Crumbs has a widespread role during tissue morphogenesis
To find out whether the maintenance of epithelial integrity during tissue remodelling is a general function of crb, we analysed epidermal cells, which undergo major rearrangements during germ-band extension. The timing and degree of cell movement varies between trunk and head epidermis. The onset of rearrangement occurs later in the head, where stomodeal invagination starts about an hour after the onset of germ-band elongation in the trunk (Campos-Ortega and Hartenstein, 1997). In the germ-band of crb mutant embryos, loss of cell polarity, as revealed by defects in the localisation of junctional proteins, is first apparent during stage 8 as cells rearrange (Grawe et al., 1996; Tepass, 1996), whereas in the stomodeal region loss of cell polarity, as well as cell movements, was found to occur during stage 10 (Fig. 6A-C). Thus, loss of polarity in crb mutant embryos correlates with the timing of cell rearrangement.
Fig. 6.
Stalling cell movements in the epidermis removes the requirement for Crb to maintain cell polarity. (A-C) As the stomodeum invaginates Baz localisation is progressively lost in the neighbouring epidermal cells (arrows). (D-K) Stage 11 wild-type (D), Kr (E), crb (F,H,J) and Kr;crb (G,I,K) mutant embryos stained for Baz (green) and Neurotactin (Nrt, red; D-G), Sas (green) and Dlg (red; H,I), and E-cad (J,K). Boxed areas in D-G are enlarged in D′-G′ and D″-G″. (D) Baz localises to the apical surfaces of epidermal cells and Nrt to the lateral membranes. (E) The germ band does not extend fully in Kr mutant embryos and cell polarity is unaffected. (F,H,J) In crb mutant embryos, epidermal polarity is lost by stage 11; apical proteins such as Baz (F, green) and Sas (H, green) are delocalised throughout the cell, basolateral proteins such as Nrt (F, red) and Dlg (H, red) spread around the cell membrane, and junctional proteins such as Ecad are fragmented (J). (G,I,K) In Kr;crb double mutants there is a marked rescue of epidermal cell polarity. Baz and Sas localise to the apical membrane (G,I), and Nrt and Dlg are restricted basolaterally (G,I). ZAs are largely rescued, as seen by circumferential labelling of Ecad (compare K with crb mutant J). (L) In crb mutants, most of the cuticle is absent with only fragments of cuticle remaining. (M) Embryos mutant for Kr and crb show a marked rescue of the crb phenotype, as a continuous cuticle is formed. (H, I) Images taken of the epidermis, just posterior to the tip of the germband. (J,K) Enface views of the dorsal epidermis.
We next sought to stall the morphogenetic movements associated with germ-band elongation. In our hands drug-induced stalling (Bertet et al., 2004) is unreliable but we find that germ-band elongation arrests prematurely in Krüppel (Kr) mutant embryos (Fig. 6E) (Bertet et al., 2004; Irvine and Wieschaus, 1994). In these embryos the polarity of epidermal cells was normal (Fig. 6D,E, compare with defects evident in the trunk and tail epidermis of crb mutant embryos in Fig. 6F). In contrast to crb mutant embryos, the epidermis of embryos doubly mutant for crb and Kr exhibited relatively normal distribution of cell polarity and junctional proteins (Fig. 6F-K) and cuticle preparations showed that there is some suppression of the crb phenotype; a continuous, though reduced, cuticle sheet was formed (Fig. 6L,M). We found a similar rescue using a different Kr allele (Kr9, data not shown), confirming the specificity of the effects of Kr loss of function. Together these data show that there is substantial rescue of cell polarity in the epidermis of crb mutant embryos if cell movements are strongly reduced. These data reveal a striking similarity between the behaviour of the epidermis and the tubules; cell polarity in both tissues becomes dependent on Crb when cells rearrange. We therefore suggest that Crb has a conserved role in the maintenance of cell polarity during tissue reorganisation and that the differing sensitivities of tissues to the loss of crb are related to the time and degree of tissue morphogenesis that they undergo.
Cell polarity depends on exocyst-mediated secretion during tissue morphogenesis
The maintenance of cell polarity during cell movement and rearrangement depends on a number of processes, including targeting of transmembrane proteins to specific cell surface domains. This process involves, among others, the function of the exocyst complex to tether secretory vesicles to specific sites (Lipschutz and Mostov, 2002; Whyte and Munro, 2002). The Sec6/8 (exocyst) complex, originally described in S. cerevisiae, is made up of eight subunits. Drosophila homologues of exocyst subunits are required for the targeted delivery of junctional proteins (Langevin et al., 2005), apical proteins, including Crb (Blankenship et al., 2007), Rhodopsin 1 (Beronja et al., 2005), as well as membrane and secreted proteins required for cell growth (Murthy et al., 2003; Murthy et al., 2005; Murthy and Schwarz, 2004). In order to explore further the early resistance of tubule cell polarity to the loss of crb activity, we analysed the effects of the loss of exocyst function in mutants for exo84, by using an allelic combination that removes all zygotic and the majority of maternal Exo84 (Blankenship et al., 2007). Strikingly, loss of epidermal cell polarity at stage 11 was not mirrored in the tubules, where cell polarity and tissue integrity were maintained, manifested by the unaltered distribution of Crb, Baz, Sas and Fas II (Fig. 7A-D). However, stage 16 mutant tubules were abnormally shaped having failed to complete tissue extension (Fig. 7E-H). Apical proteins spread into the lateral membrane domain of the tubule cells (Fig. 7F) or were partially lost from apical domains (Fig. 7H), whereas basolateral proteins remained localised (Fig. 7H).
Fig. 7.
Renal tubule polarity becomes dependent on the exocyst subunit Exo84 during late stages of development. (A-D) stage 11 wild-type (A,C) and exo84 mutant (B,D) embryos. (A,C) In wild-type embryos Crb (green, A), Baz (red, A) and Sas (red, C) localise apically in the renal tubules (arrows) and epidermis (arrowheads) and Fas II (green, C) localises to the lateral membrane of tubule cells. (B,D) In exo84 mutants the apical localisation of Crb, Baz and Sas is lost in epidermal cells (arrowheads), but is unaffected in the renal tubules and Fas II localises correctly to lateral tubule cell membranes (arrows). (E-H) Stage 16 wild-type (E,G) and exo84 mutant (F,H) tubules. (E,G) In wild-type tubules, Crb (E, green), Baz (E, red) and Sas (G, red) localise to apical cell surfaces and Fas II (G, green) concentrates laterally. (F,H) Stage 16 exo84 mutant tubules remain short and fat and the cells are irregular in shape (compare H with wild type in G). Apical proteins have spread into the lateral membranes (F, arrow) or are partially lost from the apical domains (compare H, with wild type in G, arrowheads). Although basolateral proteins localise as in wild type, the cells appear irregular and more rounded in shape (compare H with wild type in G).
Blankenship et al. (Blankenship et al., 2007) showed that there is a similar phenotype in the epidermis, in that cell polarity, as well as the ZA, is established in exo84 mutants but during stage 9, first Crumbs, and subsequently other apical and junctional proteins mislocalise. The timing of the onset of cell polarity phenotypes in both the epidermis (Blankenship et al., 2007) and the tubules (this study) of exo84 mutant embryos is remarkably similar to the time of appearance of similar defects in these tissues in crb mutant embryos and coincides with the onset of tissue morphogenesis. Thus our data using an allelic combination that strongly reduces exo84 function suggest that early tubule cell polarity is less dependent on the targeted secretion of membrane proteins, through activity of the exocyst, than later in development when exocyst-mediated, targeted membrane delivery underpins tubule cell polarity. These findings support the conclusions of Blankenship et al. (Blankenship et al., 2007), and lead us to suggest further that in the epidermis the initial localisation of Crumbs is largely independent of Exo84 activity but that as cell junctions and cell membranes are remodelled during germ-band extension, there is a dual requirement for both these proteins to maintain cell polarity.
Discussion
Epithelial cell rearrangements are accompanied, and may be driven by, changes in the spatial arrangement of their intercellular contacts (Bertet et al., 2004; Classen et al., 2005; Zallen and Wieschaus, 2004). Contacts with new neighbours are engineered by the reorganisation of intercellular junctions through vesicle recycling; ZAs shrink as the interface disappears between cells that lose contact and expand as borders develop between new neighbours. These changes involve dynamin-dependent endocytosis of ZA components, rab-mediated vesicle trafficking and the targeted recycling of vesicles to specific membrane domains (Classen et al., 2005; Langevin et al., 2005). Here we show that Crb is specifically required to maintain apicobasal polarity and the integrity of ZAs as these cell activities occur. If cell rearrangements are halted or reduced in crb mutants, there is extensive rescue of epithelial integrity. This leads us to propose a model in which the Crb complex is dispensable for the establishment of cell polarity in embryonic epithelia but that as soon as morphogenetic cell rearrangements start, the Crb complex acts both to stabilise apical proteins and to restrict the spread of basolateral proteins (Fig. 8).
Fig. 8.
Model outlining interactions between cell polarity proteins before (left) and during (right) morphogenetic cell rearrangements. The Crb protein group is not required for normal cell polarity in the absence of cell rearrangements. dPATJ, Drosophila PATJ.
The requirement for E-cadherin in different tissues shows a similar dependence on the degree of morphogenetic activity (Tepass et al., 1996; Uemura et al., 1996). It was shown that the zygotic Drosophila E-cadherin mutant phenotype can be rescued in dynamic tissues, for example in the neurectoderm and Malpighian tubules, by suppressing morphogenetic cell movements (Tepass et al., 1996).
It is tempting to speculate that Crb acts by targeting recycling vesicles of ZA components in order to maintain junctional integrity in the elongating renal tubules. Without Crb ZAs are lost and membrane domains no longer remain distinct, leading to the collapse of cell polarity. Alternatively, lack of Crb could result in loss of cell polarity in morphogenetically active tissues and, as a consequence, ZAs cannot be maintained. In this case the primary requirement for Crb during cell movement would be to maintain the apical localisation of Baz/Par-6/aPKC, thereby also ensuring the normal distribution of basolateral proteins (Bilder et al., 2003; Hutterer et al., 2004; Tanentzapf and Tepass, 2003).
The exo84 mutant phenotype in remodelling tissues could be explained by a depletion of apical Crb caused by reduced exocyst-mediated delivery, resulting in a phenotype reminiscent of crb mutant embryos. This view is supported by recent findings showing that expression of dominant-negative Cdc42 in stage 9-11 embryos results in loss of Crb and other proteins from the apical membrane and disruption of cadherin-based adhesion in the morphogenetically active ventral neuroectoderm. Furthermore, mutations in crb enhance the phenotype induced by dominant-negative Cdc42 expression in this tissue (Harris and Tepass, 2008). The authors suggest that Cdc42 normally acts to repress endocytosis of apical proteins including Crb, so that inactivation of Cdc42 results in defects in cell polarity, leading to the loss of ZAs and tissue disruption. Whether Crb ensures ZA plasticity during cell rearrangements by restricting excessive endocytosis of apical proteins indirectly via stabilisation of Baz and/or Par-6 and Cdc42, or in a more direct way by regulating the recycling of junctional proteins remains to be determined. Similar defects in ZA integrity were also observed in the epithelium of the developing adult dorsal thorax upon loss of Cdc42 function. Here, it was shown that Cdc42–Par-6–aPKC control endocytosis by the cytoskeletal regulators Arp2/3 (Georgiou et al., 2008) and Cip4 and WASP (Leibfried et al., 2008). These results complement and support recent data showing that Cdc42, together with PAR-3, PAR-6 and PKC, are required for membrane trafficking in C. elegans coelomocytes and human HeLa cells (Balklava et al., 2007).
Members of the Crb complex also play a critical role in ZA stability and apical membrane delivery or stabilisation during photoreceptor development, when the ZAs enlarge and the apical domain selectively expands as the rhabdomere forms (Berger et al., 2007; Hong et al., 2003; Izaddoost et al., 2002; Pellikka et al., 2002; Richard et al., 2006). These morphogenetic events require the targeted delivery and retention of large amounts of membrane. Here too, it is not yet clear whether Crb acts directly on the stability of ZA components or indirectly, by controlling other polarity proteins. Although Drosophila Par-6 is delocalised in crb mutant photoreceptor cells (Berger et al., 2007), other data suggest that the Crb complex regulates ZA integrity and trafficking of apical membrane via stabilisation of the membrane-associated cytoskeleton, including βH-spectrin (Pellikka et al., 2002) (Mélisande Richard, Nadine Muschalik and E.K., unpublished data).
Vertebrate homologues of members of the fly Crb complex appear to have conserved roles in the control of epithelial integrity. Loss of oko meduzy, one of the five zebrafish crb orthologues, of the sdt orthologue nagie oko or of mosaic eyes, a regulator of Crb, perturbs polarity and morphogenesis of the retinal neuroepithelium and the heart (Malicki and Driever, 1999; Rohr et al., 2006; Wei and Malicki, 2002). In addition, RNAi-mediated knock down of the mammalian Sdt orthologue, Pals1, in Madin Darby canine kidney (MDCK) cells in culture prevents proper delivery of E-cadherin to the cell surface (Wang et al., 2007), a phenotype strikingly similar to that of crb or sdt mutant epithelia in Drosophila.
These observations suggest that, as in the fly, vertebrate epithelia affected by the loss of Crb are those that undergo morphogenetic reorganisation, including cell shape change. Loss of human CRB1 is associated with retinitis pigmentosa and Leber congenital amaurosis, resulting in retinal degeneration and blindness (den Hollander et al., 1999), a phenotype with striking similarity to flies with crb mutant photoreceptor cells, which exhibit light-dependent retinal degeneration (Johnson et al., 2002). In mice mutant for Crb1, photoreceptor cells develop normally but later their ZAs degenerate so that photoreceptors in certain areas of the retina are displaced, followed by cell death (Mehalow et al., 2003; van de Pavert et al., 2004). Further experiments will elucidate whether the defects observed in morphogenetically active epithelia and photoreceptor cells are based on a common cell biological function of the Crb complex in these two cell types.
Materials and Methods
Drosophila stocks and genetics
The mutant strains used were crb11A22, crb8F105, bazxi106, scrib2, onr142-5, cutc145, Kr1 and Kr9. Maternal and zygotic mutants of bazxi106 and scrib2 were made by the FLP-DFS dominant female sterile method as previously described (Chou and Perrimon, 1996). exo84 mutant embryos were generated using onr142-5 [as described by Blankenship et al. (Blankenship et al., 2007)]. For ectopic expression of Crb, UAS-crb30.12e was expressed (Wodarz et al., 1995) using the Gal4 drivers da-GAL4 and cutB-GAL4.
Immunohistochemistry
The following primary antibodies were used: mouse anti-Arm (1:100; Developmental Studies Hybridoma Bank); rabbit anti-Baz (1:1000; a gift from A. Wodarz); rat anti-Crb (1:100); mouse anti-Cut (1:200; a gift from I. Rebay); mouse anti-Dlg (1:500; Developmental Studies Hybridoma Bank); mouse anti-Fas II (1:10; Developmental Studies Hybridoma Bank); mouse anti-Nrt (1:10; Developmental Studies Hybridoma Bank); rabbit anti-SAS (1:500; a gift from D. Cavener); rabbit anti-Tsh (1:300; a gift from S. Cohen). Heat fixation was used for the labelling of Arm, as previously described (Tepass, 1996). For all other staining, embryos were fixed in 4% paraformaldehyde, using standard techniques. Primary incubations were performed overnight, followed by incubation with appropriate biotinylated secondary antibodies and amplification using the Vector Elite ABC Kit (Vector Laboratories). For fluorescent double labelling, the appropriate secondary antibodies were used conjugated with FITC or Cy3 (Jackson Immunoresearch). When required, an additional amplification step using streptavidin-conjugated Cy3 or FITC (Jackson Immunoresearch) was performed.
Image acquisition and manipulation
For transmitted-light microscopy, embryos were mounted in 1,3-diethyl-8-phenylxanthine (DPX) and viewed using a Zeiss Axioplan compound microscope at room temperature with a ×20 (Pan-NeoFluar; NA 0.50; Zeiss) objective, a JVC-KY55B digital camera and Neotech Image Grabber. For confocal microscopy, embryos were mounted in Vectashield fluorescent mounting medium (Vector Laboratories) and viewed at room temperature with a ×40 oil (Leica PL APO; NA 1.25) objective on a Leica SP1 or SP5 scanning laser microscope, and LSM software. Images were processed in Adobe Photoshop 7.0.
Electron microscopy
Transmission electron microscopy was carried out using standard techniques (Grawe et al., 1996). Ultrathin sections were cut on a Reichert OM-U2 ultramicrotome and were analysed using a Philips electron microscope.
We are grateful to Benedicte Sanson and Suzanne Eaton and to members of the Skaer lab for helpful discussions and to Ferdi Grawe, Nan Hu and Xavier Franch-Marro for technical assistance. We thank D. Cavener, S. Cohen, A. Müller, I. Rebay, U. Tepass, A. Wodarz, J. Zallen, the Bloomington stock centre and DSHB for kindly sending us flies and reagents. Our work is supported by the DAAD/ARC. We also acknowledge support from the Wellcome Trust (H.S.), BBSRC, Cambridge European Trust and Balfour Fund (K.C.). Deposited in PMC for release after 6 months.
References
- Ainsworth, C., Wan, S. and Skaer, H. (2000). Coordinating cell fate and morphogenesis in Drosophila renal tubules. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355, 931-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann, A., Schneider, M., Theilenberg, E., Grawe, F. and Knust, E. (2001). Drosophila Stardust is a partner of Crumbs in the control of epithelial cell polarity. Nature 414, 638-643. [DOI] [PubMed] [Google Scholar]
- Balklava, Z., Pant, S., Fares, H. and Grant, B. D. (2007). Genome-wide analysis identifies a general requirement for polarity proteins in endocytic traffic. Nat. Cell Biol. 9, 1066-1073. [DOI] [PubMed] [Google Scholar]
- Berger, S., Bulgakova, N. A., Grawe, F., Johnson, K. and Knust, E. (2007). Unraveling the genetic complexity of Drosophila stardust during photoreceptor morphogenesis and prevention of light-induced degeneration. Genetics 176, 2189-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beronja, S., Laprise, P., Papoulas, O., Pellikka, M., Sisson, J. and Tepass, U. (2005). Essential function of Drosophila Sec6 in apical exocytosis of epithelial photoreceptor cells. J. Cell Biol. 169, 635-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertet, C., Sulak, L. and Lecuit, T. (2004). Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature 429, 667-671. [DOI] [PubMed] [Google Scholar]
- Bilder, D., Schober, M. and Perrimon, N. (2003). Integrated activity of PDZ protein complexes regulates epithelial polarity. Nat. Cell Biol. 5, 53-58. [DOI] [PubMed] [Google Scholar]
- Blankenship, J. T., Fuller, M. T. and Zallen, J. A. (2007). The Drosophila homolog of the Exo84 exocyst subunit promotes apical epithelial identity. J. Cell Sci. 120, 3099-3110. [DOI] [PubMed] [Google Scholar]
- Campos-Ortega, J. A. and Hartenstein, V. (1997). The Embryonic Development of Drosophila Melanogaster. Berlin: Springer-Verlag.
- Chou, T. B. and Perrimon, N. (1996). The autosomal FLP-DFS technique for generating germline mosaics in Drosophila melanogaster. Genetics 144, 1673-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Classen, A. K., Anderson, K. I., Marois, E. and Eaton, S. (2005). Hexagonal packing of Drosophila wing epithelial cells by the planar cell polarity pathway. Dev. Cell 9, 805-817. [DOI] [PubMed] [Google Scholar]
- den Hollander, A. I., ten Brink, J. B., de Kok, Y. J., van Soest, S., van den Born, L. I., van Driel, M. A., van de Pol, D. J., Payne, A. M., Bhattacharya, S. S., Kellner, U. et al. (1999). Mutations in a human homologue of Drosophila crumbs cause retinitis pigmentosa (RP12). Nat. Genet. 23, 217-221. [DOI] [PubMed] [Google Scholar]
- Denholm, B., Sudarsan, V., Pasalodos-Sanchez, S., Artero, R., Lawrence, P., Maddrell, S., Baylies, M. and Skaer, H. (2003). Dual origin of the renal tubules in Drosophila: mesodermal cells integrate and polarize to establish secretory function. Curr. Biol. 13, 1052-1057. [DOI] [PubMed] [Google Scholar]
- Georgiou, M., Marinari, E., Burden, J. and Baum, B. (2008). Cdc42, Par6, and aPKC regulate Arp2/3-mediated endocytosis to control local adherens junction stability. Curr. Biol. 18, 1631-1638. [DOI] [PubMed] [Google Scholar]
- Grawe, F., Wodarz, A., Lee, B., Knust, E. and Skaer, H. (1996). The Drosophila genes crumbs and stardust are involved in the biogenesis of adherens junctions. Development 122, 951-959. [DOI] [PubMed] [Google Scholar]
- Harris, T. J. and Peifer, M. (2004). Adherens junction-dependent and -independent steps in the establishment of epithelial cell polarity in Drosophila. J. Cell Biol. 167, 135-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, T. J. and Peifer, M. (2005). The positioning and segregation of apical cues during epithelial polarity establishment in Drosophila. J. Cell Biol. 170, 813-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, K. P. and Tepass, U. (2008). Cdc42 and Par proteins stabilize dynamic adherens junctions in the Drosophila neuroectoderm through regulation of apical endocytosis. J. Cell Biol. 183, 1129-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton-Ellis, E., Ainsworth, C., Sushama, Y., Wan, S., VijayRaghavan, K. and Skaer, H. (2007). Genetic regulation of patterned tubular branching in Drosophila. Proc. Natl. Acad. Sci. USA 104, 169-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, Y., Stronach, B., Perrimon, N., Jan, L. Y. and Jan, Y. N. (2001). Drosophila Stardust interacts with Crumbs to control polarity of epithelia but not neuroblasts. Nature 414, 634-638. [DOI] [PubMed] [Google Scholar]
- Hong, Y., Ackerman, L., Jan, L. Y. and Jan, Y. N. (2003). Distinct roles of Bazooka and Stardust in the specification of Drosophila photoreceptor membrane architecture. Proc. Natl. Acad. Sci. USA 100, 12712-12717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutterer, A., Betschinger, J., Petronczki, M. and Knoblich, J. A. (2004). Sequential roles of Cdc42, Par-6, aPKC, and Lgl in the establishment of epithelial polarity during Drosophila embryogenesis. Dev. Cell 6, 845-854. [DOI] [PubMed] [Google Scholar]
- Irvine, K. D. and Wieschaus, E. (1994). Cell intercalation during Drosophila germband extension and its regulation by pair-rule segmentation genes. Development 120, 827-841. [DOI] [PubMed] [Google Scholar]
- Izaddoost, S., Nam, S. C., Bhat, M. A., Bellen, H. J. and Choi, K. W. (2002). Drosophila Crumbs is a positional cue in photoreceptor adherens junctions and rhabdomeres. Nature 416, 178-183. [DOI] [PubMed] [Google Scholar]
- Johnson, K., Grawe, F., Grzeschik, N. and Knust, E. (2002). Drosophila crumbs is required to inhibit light-induced photoreceptor degeneration. Curr. Biol. 12, 1675-1680. [DOI] [PubMed] [Google Scholar]
- Langevin, J., Morgan, M. J., Sibarita, J. B., Aresta, S., Murthy, M., Schwarz, T., Camonis, J. and Bellaiche, Y. (2005). Drosophila exocyst components Sec5, Sec6, and Sec15 regulate DE-Cadherin trafficking from recycling endosomes to the plasma membrane. Dev. Cell 9, 365-376. [DOI] [PubMed] [Google Scholar]
- Leibfried, A., Fricke, R., Morgan, M. J., Bogdan, S. and Bellaiche, Y. (2008). Drosophila Cip4 and WASp define a branch of the Cdc42-Par6-aPKC pathway regulating E-cadherin endocytosis. Curr. Biol. 18, 1639-1648. [DOI] [PubMed] [Google Scholar]
- Lipschutz, J. H. and Mostov, K. E. (2002). Exocytosis: the many masters of the exocyst. Curr. Biol. 12, R212-R214. [DOI] [PubMed] [Google Scholar]
- Malicki, J. and Driever, W. (1999). oko meduzy mutations affect neuronal patterning in the zebrafish retina and reveal cell-cell interactions of the retinal neuroepithelial sheet. Development 126, 1235-1246. [DOI] [PubMed] [Google Scholar]
- Mehalow, A. K., Kameya, S., Smith, R. S., Hawes, N. L., Denegre, J. M., Young, J. A., Bechtold, L., Haider, N. B., Tepass, U., Heckenlively, J. R. et al. (2003). CRB1 is essential for external limiting membrane integrity and photoreceptor morphogenesis in the mammalian retina. Hum. Mol. Genet. 12, 2179-2189. [DOI] [PubMed] [Google Scholar]
- Murthy, M. and Schwarz, T. L. (2004). The exocyst component Sec5 is required for membrane traffic and polarity in the Drosophila ovary. Development 131, 377-388. [DOI] [PubMed] [Google Scholar]
- Murthy, M., Garza, D., Scheller, R. H. and Schwarz, T. L. (2003). Mutations in the exocyst component Sec5 disrupt neuronal membrane traffic, but neurotransmitter release persists. Neuron 37, 433-447. [DOI] [PubMed] [Google Scholar]
- Murthy, M., Ranjan, R., Denef, N., Higashi, M. E., Schupbach, T. and Schwarz, T. L. (2005). Sec6 mutations and the Drosophila exocyst complex. J. Cell Sci. 118, 1139-1150. [DOI] [PubMed] [Google Scholar]
- Peifer, M. (1993). The product of the Drosophila segment polarity gene armadillo is part of a multi-protein complex resembling the vertebrate adherens junction. J. Cell Sci. 105, 993-1000. [DOI] [PubMed] [Google Scholar]
- Pellikka, M., Tanentzapf, G., Pinto, M., Smith, C., McGlade, C. J., Ready, D. F. and Tepass, U. (2002). Crumbs, the Drosophila homologue of human CRB1/RP12, is essential for photoreceptor morphogenesis. Nature 416, 143-149. [DOI] [PubMed] [Google Scholar]
- Richard, M., Grawe, F. and Knust, E. (2006). DPATJ plays a role in retinal morphogenesis and protects against light-dependent degeneration of photoreceptor cells in the Drosophila eye. Dev. Dyn. 235, 895-907. [DOI] [PubMed] [Google Scholar]
- Rohr, S., Bit-Avragim, N. and Abdelilah-Seyfried, S. (2006). Heart and soul/PRKCi and nagie oko/Mpp5 regulate myocardial coherence and remodeling during cardiac morphogenesis. Development 133, 107-115. [DOI] [PubMed] [Google Scholar]
- Tanentzapf, G. and Tepass, U. (2003). Interactions between the crumbs, lethal giant larvae and bazooka pathways in epithelial polarization. Nat. Cell Biol. 5, 46-52. [DOI] [PubMed] [Google Scholar]
- Tepass, U. (1996). Crumbs, a component of the apical membrane, is required for zonula adherens formation in primary epithelia of Drosophila. Dev. Biol. 177, 217-225. [DOI] [PubMed] [Google Scholar]
- Tepass, U. and Knust, E. (1993). Crumbs and stardust act in a genetic pathway that controls the organization of epithelia in Drosophila melanogaster. Dev. Biol. 159, 311-326. [DOI] [PubMed] [Google Scholar]
- Tepass, U., Theres, C. and Knust, E. (1990). crumbs encodes an EGF-like protein expressed on apical membranes of Drosophila epithelial cells and required for organization of epithelia. Cell 61, 787-799. [DOI] [PubMed] [Google Scholar]
- Tepass, U., Gruszynski-DeFeo, E., Haag, T. A., Omatyar, L., Torok, T. and Hartenstein, V. (1996). shotgun encodes Drosophila E-cadherin and is preferentially required during cell rearrangement in the neurectoderm and other morphogenetically active epithelia. Genes Dev. 10, 672-685. [DOI] [PubMed] [Google Scholar]
- Uemura, T., Oda, H., Kraut, R., Hayashi, S., Kotaoka, Y. and Takeichi, M. (1996). Zygotic Drosophila E-cadherin expression is required for processes of dynamic epithelial cell rearrangement in the Drosophila embryo. Genes Dev. 10, 659-671. [DOI] [PubMed] [Google Scholar]
- van de Pavert, S. A., Kantardzhieva, A., Malysheva, A., Meuleman, J., Versteeg, I., Levelt, C., Klooster, J., Geiger, S., Seeliger, M. W., Rashbass, P. et al. (2004). Crumbs homologue 1 is required for maintenance of photoreceptor cell polarization and adhesion during light exposure. J. Cell Sci. 117, 4169-4177. [DOI] [PubMed] [Google Scholar]
- Wang, Q., Chen, X. W. and Margolis, B. (2007). PALS1 regulates E-cadherin trafficking in mammalian epithelial cells. Mol. Biol. Cell 18, 874-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, X. and Malicki, J. (2002). nagie oko, encoding a MAGUK-family protein, is essential for cellular patterning of the retina. Nat. Genet. 31, 150-157. [DOI] [PubMed] [Google Scholar]
- Whyte, J. R. and Munro, S. (2002). Vesicle tethering complexes in membrane traffic. J. Cell Sci. 115, 2627-2637. [DOI] [PubMed] [Google Scholar]
- Wodarz, A., Grawe, F. and Knust, E. (1993). CRUMBS is involved in the control of apical protein targeting during Drosophila epithelial development. Mech. Dev. 44, 175-187. [DOI] [PubMed] [Google Scholar]
- Wodarz, A., Hinz, U., Engelbert, M. and Knust, E. (1995). Expression of crumbs confers apical character on plasma membrane domains of ectodermal epithelia of Drosophila. Cell 82, 67-76. [DOI] [PubMed] [Google Scholar]
- Zallen, J. A. and Wieschaus, E. (2004). Patterned gene expression directs bipolar planar polarity in Drosophila. Dev. Cell 6, 343-355. [DOI] [PubMed] [Google Scholar]