Summary
Proteolytic activity of separase is required for chiasma resolution during meiosis I in mouse oocytes. Rec8, the meiosis-specific α-kleisin subunit of cohesin, is a key target of separase in yeast. Is the equivalent protein also a target in mammals? We show here that separase cleaves mouse Rec8 at three positions in vitro but only when the latter is hyper-phosphorylated. Expression of a Rec8 variant (Rec8-N) that cannot be cleaved in vitro at these sites causes sterility in male mice. Their seminiferous tubules lack a normal complement of 2 C secondary spermatocytes and 1 C spermatids and contain instead a high proportion of cells with enlarged nuclei. Chromosome spreads reveal that Rec8-N expression has no effect in primary spermatocytes but produces secondary spermatocytes and spermatids with a 4 C DNA content, suggesting that the first and possibly also the second meiotic division is abolished. Expression of Rec8-N in oocytes causes chromosome segregation to be asynchronous and delays its completion by 2-3 hours during anaphase I, probably due to inefficient proteolysis of Rec8-N by separase. Despite this effect, chromosome segregation must be quite accurate as Rec8-N does not greatly reduce female fertility. Our data is consistent with the notion that Rec8 cleavage is important and probably crucial for the resolution of chiasmata in males and females.
Keywords: Chromosome segregation, Cohesin, Meiosis, Oocyte maturation, Separase, Spermatogenesis
Introduction
Sister chromatid cohesion is mediated by a multi-subunit complex called cohesin (Nasmyth, 2005). During mitosis, cohesin resists the tendency of microtubules to pull sister chromatids apart and thereby generates the tension necessary to stabilize connections between the kinetochores and microtubules (Nicklas, 1997; Tanaka et al., 2005). Destruction of sister chromatid cohesion due to proteolytic cleavage of the α-kleisin subunit of cohesin Scc1 (also known as Rad21) by separase triggers the disjunction of sister chromatids (Uhlmann et al., 1999; Uhlmann et al., 2000; Waizenegger et al., 2000). Separase is inhibited through association with securin and cyclin B, which are destroyed by a ubiquitin protein ligase called the anaphase-promoting complex (APC/C) only when all chromosomes have come under tension on the metaphase plate (Gorr et al., 2005; Yanagida, 2005).
In meiosis, reciprocal recombination between homologous non-sister chromatids (crossing-over) creates bivalent chromosomes (bivalents) containing four chromatids that are joined together by sister chromatid cohesion (Petronczki et al., 2003). The cytological manifestation of crossovers is referred to as chiasma. The production of one (or more) chiasma per bivalent ensures that the two centromeres of the maternal chromosome are joined to those of the paternal homolog via cohesin distal to the chiasmata. This has a crucial consequence, namely that it is now possible to generate the tension necessary to stabilize the connections between kinetochores and microtubules by pulling maternal and paternal kinetochores in opposite directions. In mitotic cells, this tension can only be produced by pulling sister kinetochores in opposite directions, known as amphitelic attachment (Tanaka et al., 2005). In yeast, amphitelic attachment is actively prevented during the first meiotic division (meiosis I) by specific `monopolin' proteins (Toth et al., 2000).
In budding yeast, cohesion of bivalents depends on a variant of the cohesin complex that contains a meiosis-specific α-kleisin subunit called Rec8 (Klein et al., 1999). Anaphase I is triggered by activation of separase, which cleaves Rec8 and thereby removes cohesin from chromosome arms (Buonomo et al., 2000). This resolves chiasmata, generating dyad chromosomes (dyads) containing two chromatids whose centromeres remain tied together by cohesin that has resisted separase at meiosis I. Cohesion between sister centromeres left intact facilitates amphitelic attachment of sister kinetochores on the meiosis II spindle, a process that largely resembles that of mitosis, with the key difference that centromeric cohesion in meiosis II had presumably been generated prior to meiosis I and not by a fresh round of DNA replication. Expression of Rec8 variants that cannot be cleaved by separase blocks meiosis I chromosome segregation but has little or no effect on meiotic progression per se (Buonomo et al., 2000; Kitajima et al., 2003).
The discovery that cleavage of the α-kleisin subunits of cohesin by separase triggers meiotic as well as mitotic divisions in yeast suggested that the chemistry of chromosome segregation might be universal in all eukaryotic cells. However, this has been questioned by two findings. The first is the discovery that animal cells possess a second mechanism for removing cohesin from chromosomes during mitosis, one that involves phosphorylation of its Scc3-like subunits (SA1 and SA2) and does not require cleavage of α-kleisin subunits by separase (Hauf et al., 2005). This process precedes separase activation, commencing during prophase. It removes most but not all cohesin from chromosome arms by the time they have aligned on the metaphase plate. Cohesin residing at centromeres is protected from this `prophase pathway' by Mei-S332-like proteins (also known as Shugoshins) (Kitajima et al., 2005; McGuinness et al., 2005). The second finding is the claim that meiosis I in Xenopus oocytes does not require the APC/C-separase pathway (Peter et al., 2001; Taieb et al., 2001). It has been suggested that the first meiotic division in animal cells might be triggered by removal of cohesin from chromosome arms either by the prophase pathway itself or by a process analogous to it. To address rigorously the role of separase during meiosis I, we recently developed a method to deplete separase specifically from mouse oocytes and to replace it by mutated versions (Kudo et al., 2006). This proved that the proteolytic activity of separase is essential for removing cohesin containing Rec8 from bivalents and for resolving chiasmata; a conclusion that is consistent with the finding that a non-degradable version of the separase inhibitor securin blocks chiasmata resolution in oocytes (Herbert et al., 2003; Terret et al., 2003). The chemistry of chiasma resolution might after all be similar in fungi and mammals.
The above studies have left unanswered the issue of whether cohesin containing Scc1 or Rec8 or both mediates cohesion between sister chromatids during meiosis I in animal cells and whether cleavage of either of these α-kleisins is the mechanism by which separase transforms bivalents into dyads. Mouse Rec8, like its fungal namesake, is expressed in meiotic cells, decorates the axial/lateral element (AE/LE) of the synaptonemal complex (SC) during prophase, localizes at the inter-chromatid regions of bivalents, disappears from chromosome arms at the onset of anaphase I in a manner dependent on the protease activity of separase, and persists at centromeres until metaphase II (Eijpe et al., 2003; Lee et al., 2003; Kudo et al., 2006). These features are consistent with the notion that Rec8 confers meiotic sister chromatid cohesion, but do not prove it. The phenotype of mutant mice lacking functional Rec8 has not settled this question because their oocytes or spermatocytes only develop to prophase, and their sister chromatids are at least partially kept in the vicinity of each other until this point (Bannister et al., 2004; Xu et al., 2005). It is therefore still possible that Scc1 or some hitherto-unidentified protein mediates cohesion and its cleavage resolves chiasmata. Such a situation might pertain in Drosophila where the only meiosis-specific α-kleisin-like protein, called C(2)M, does not appear to confer sister chromatid cohesion during alignment of bivalents on meiosis I spindles and might function solely in the double-strand-break repair leading to recombination (Heidmann et al., 2004). In summary, the evidence that Rec8 confers sister chromatid cohesion at the time of the first meiotic division is largely cytological and therefore indirect. There is no direct evidence that Rec8 actually confers sister chromatid cohesion in mammals.
The identity of the key target of separase is not merely of academic interest, as deregulated cleavage could contribute to the chromosome missegregation at meiosis I that causes aneuploidy in humans (Hassold and Hunt, 2001). We therefore set out to identify separase cleavage sites in the mouse meiosis-specific α-kleisin Rec8 and studied in vivo phenotypes caused by the expression of a mutant version (Rec8-N) that cannot be cleaved (at least in vitro).
Results
Identification of separase cleavage sites within Rec8
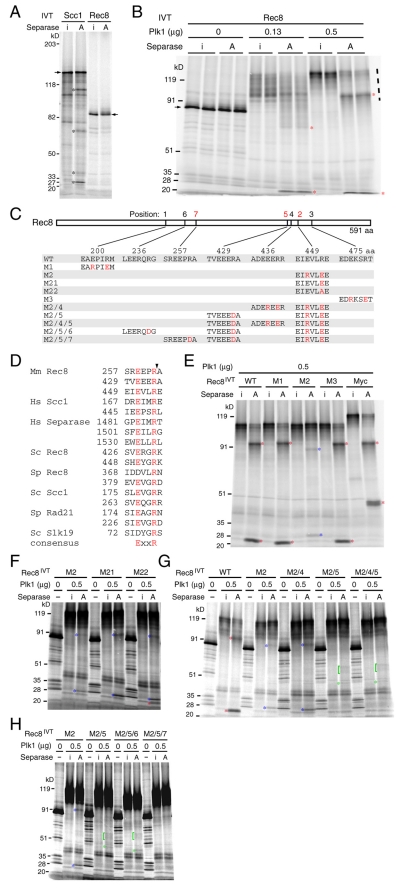
Fragments of α-kleisins cleaved by separase are highly unstable in vivo and are best detected in cells that undergo anaphase synchronously (Uhlmann et al., 1999; Buonomo et al., 2000; Tomonaga et al., 2000; Waizenegger et al., 2000; Kitajima et al., 2003). This largely precludes their detection in mammalian meiotic cells, because it is not possible to obtain homogenous populations of oocytes or spermatocytes at an appropriate stage in sufficient quantities. We therefore used an in vitro system to investigate whether Rec8 possesses separase cleavage sites. [35S]Methionine-labeled human SCC1 tagged at its C-terminus with nine Myc epitopes and mouse Rec8 were synthesized using an in vitro transcription-translation system (IVT) and incubated with active and inactive versions of separase. The former could not be phosphorylated at residues serine 1126 or threonine 1346 whereas the latter contained serine in the place of a crucial catalytic cysteine (Stemmann et al., 2001). Active separase induced Scc1 but not Rec8 cleavage (lanes marked A, Fig. 1A). We detected four of the five SCC1 fragments expected from cleavage at the two known sites (Hauf et al., 2001).
Fig. 1.
Identification of separase cleavage sites within Rec8 in vitro. [35S]Methionine-labeled mouse wild-type and mutant Rec8 and human SCC1-Myc were prepared in an in vitro transcription-translation system (IVT) and subjected to an in vitro separase cleavage assay using recombinant active separase (lanes marked A) or its inactive mutant (lanes marked i) in the presence or absence of recombinant polo-like kinase 1 (Plk1) as indicated. Autoradiographs of SDS-PAGE gels on which incubated samples were run are shown. Fragments resulting from cleavage at the primary, secondary and tertiary sites are indicated by asterisks and brackets in red, blue and green, respectively. (A) Cleavage assay of mouse Rec8 and C-terminally 9× Myc-tagged human SCC1. Arrows indicate full-length proteins. Cleavage fragments (asterisks) are the bands uniquely seen in A. (B) Cleavage assay in the presence of Plk1. Duplicated samples were run side by side. The amount (μg) of purified Plk1 added to each reaction is indicated. Dashed line (right) shows positions of phosphorylated Rec8. (C) Identities of mouse Rec8 mutants. (D) Alignment of experimentally proven separase target sites (Uhlmann et al., 1999; Buonomo et al., 2000; Tomonaga et al., 2000; Hauf et al., 2001; Sullivan et al., 2001; Waizenegger et al., 2002; Zou et al., 2002; Kitajima et al., 2003). The arrowhead indicates positions of cleavage. Mm, Mus musculus; Hs, Homo sapiens; Sc, Saccharomyces cerevisiae; Sp, Schizosaccharomyces pombe. (E) Cleavage assay of wild-type (WT), M1, M2, M3 and C-terminally 9× Myc-tagged wild-type Rec8 (Myc). (F) Cleavage assay of M2, M21 and M22. (G) Cleavage assay of WT, M2, M2/4, M2/5 and M2/4/5. (H) Cleavage assay of M2, M2/5, M2/5/6 and M2/5/7.
Phosphorylation by the Polo-like kinase Cdc5 has been implicated in regulating Scc1 cleavage in yeast (Alexandru et al., 2001; Hornig and Uhlmann, 2004). Furthermore, yeast Rec8 phosphorylation depends on Cdc5 in vivo (Clyne et al., 2003; Brar et al., 2006). We therefore investigated whether mouse Rec8 can be phosphorylated by human Plk1 and, if so, whether this alters the susceptibility of Rec8 to separase. The electrophoretic mobility of Rec8 in SDS-PAGE was greatly reduced in the presence of Plk1 in a dosage-dependent manner (lanes marked i, Fig. 1B), presumably due to extensive phosphorylation (compare lanes marked i). Remarkably, this was accompanied by the appearance of two prominent cleavage fragments (red asterisks) in the presence of active separase (lanes marked A, Fig. 1B). We obtained a similar result with human Rec8 (data not shown). This suggests that phosphorylation by Plk1 of either Rec8 or separase, or both, promotes Rec8 cleavage, at least in vitro.
Proven separase substrates include Scc1 and separase itself in humans (Hauf et al., 2001; Waizenegger et al., 2002; Zou et al., 2002) and Scc1, Rec8 and Slk19 in yeast (Uhlmann et al., 1999; Buonomo et al., 2000; Tomonaga et al., 2000; Sullivan et al., 2001; Kitajima et al., 2003). The consensus recognition sequence is ExxR (the peptide bond after the Arg being cleaved) (Fig. 1D) and mutations of the conserved Arg alone, or both Arg and Glu, greatly reduce cleavage at that position (Sullivan et al., 2004). To locate the mouse Rec8 cleavage sites, we first tested the effect of mutations M1, M2 and M3 (Fig. 1C,E), which abolished three of its ten ExxR sites. M1 and M3 had no effect on the pattern of cleavage fragments but M2 caused the disappearance of the original fragments and the appearance of two new ones. The simplest explanation for this is that Rec8 has at least two cleavage sites. Separase prefers to cleave at R454, but mutation of this site (M2) revealed a cryptic secondary site elsewhere in the protein. Co-migration of aa 1-454 and aa 455-591 polypeptides produced by IVT and Plk1 treatment with the larger and smaller cleavage fragments produced by separase confirmed that cleavage had occurred close to or at R454 (see supplementary material Fig. S1A). This assignment is consistent with the observation that the mobility of the smaller but not the larger cleavage fragment is reduced by Myc epitopes added to the C-terminus of Rec8 (Fig. 1E). R454E mutation (M21) also abolished Rec8 cleavage at this site, but M22 containing R454A was partially cleaved (Fig. 1F).
We used a similar approach to map the secondary separase cleavage site to R434. Thus, the M2/5 and M2/4/5 mutations abolished the fainter secondary fragments (blue asterisks) produced by M2 Rec8 protein but M2/4 had no effect (Fig. 1G). Co-migration of phosphorylated aa 1-434 and aa 435-591 polypeptides produced by IVT with the larger and smaller secondary cleavage fragments (supplementary material Fig. S1B) confirmed that cleavage had indeed occurred close to or at R434. We noticed that Rec8 protein lacking both primary and secondary sites (M2/5) still gave rise to a cluster of very faint bands (green asterisks and brackets, seen in M2/5 and M2/4/5) when incubated with active but not with inactive separase, which suggests that Rec8 contains a third cleavage site (Fig. 1G). The tertiary cleavage products were still detectable when M2/5/6 but not when M2/5/7 was used as a substrate, suggesting that separase cleaves Rec8 after R262 when R454 and R434 have been mutated (Fig. 1H). We conclude that separase cleaves mouse Rec8 at three positions in vitro (Fig. 1C,D). Rec8 protein with R262D, R434D and R454E mutations will be referred to as Rec8-N.
Generation of transgenic mice expressing Rec8-Myc and Rec8-N-Myc
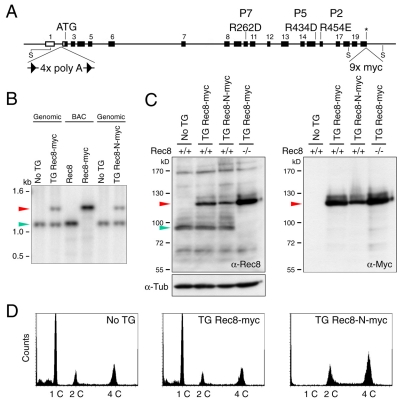
In yeast, non-cleavable Rec8 protein blocks chiasma resolution even when coexpressed with endogenous wild-type Rec8 (Buonomo et al., 2000). We therefore investigated the effect of expressing Rec8-N at physiological levels from a transgene containing the entire Rec8 locus plus neighboring genes on a bacterial artificial chromosome (BAC). Because it was possible that the Rec8-N transgene might cause infertility in both sexes, we also created lines in which expression of Rec8-N from the BAC was prevented by a transcription-terminator cassette flanked by loxP recombination sites (conditional stop cassette) inserted into the first intron. Homologous recombination in Escherichia coli was used first to insert nine Myc epitopes at the C-terminus of Rec8, then to introduce the R262D, R434D and R454E mutations, and finally to insert the conditional stop cassette (Fig. 2A). Transgenes for expression of Rec8-Myc (TG Rec8-Myc) or for silenced Rec8-N-Myc (TG Stop/Rec8-N-Myc) had no effect on the fertility of males or females. The former fully suppressed the infertility of offspring homozygous for a Rec8 deletion (Kudo et al., 2006). Surprisingly, the transgene expressing Rec8-N-Myc (TG Rec8-N-Myc) caused male but not female sterility, as did TG Stop/Rec8-N-Myc when combined with a transgene that expressed Cre recombinase ubiquitously, which caused efficient deletion of the stop cassette (data not shown). Because Rec8-N-Myc females were fertile, all subsequent analyses were performed with mice that expressed Rec8-N unconditionally. Southern blots using a probe that detects a genomic DNA fragment encoding the C-terminus of Rec8 (whose size is altered by the Myc tags) revealed that the TG Rec8-Myc and TG Rec8-N-Myc transgenes were present as single or at most two copies per haploid genome (Fig. 2B).
Fig. 2.
Male transgenic mice expressing Rec8-N-Myc fail to produce haploid cells. (A) A schematic representation of the mouse Rec8 genomic locus showing the mutagenized loci for generating BAC-mediated transgene constructs. A DNA fragment encoding 9× Myc epitopes (9× Myc) was inserted at the termination codon (asterisk) to make the Rec8-Myc expression construct (for TG Rec8-Myc mice). This construct was further modified to obtain the Rec8-N-Myc expression construct (for TG Rec8-N-Myc mice) by replacing the following residues: 262nd Arg to Asp (R262D), 434th Arg to Asp (R434D) and 454th Arg to Glu (R454E). For a conditional construct (for TG Stop/Rec8-N-Myc mice), a loxP-flanked stop cassette was inserted at the intron 1 (4× polyA). Boxes represent exons. ATG, initiation codon; S, Stu I site; The P represents the position of cleavage sites in Fig. 1C. (B) Southern blot analysis of genomic DNA from transgenic mice and littermates without transgene (No TG) digested by Stu I. The BACs encoding Rec8 or Rec8-Myc were examined as controls (BAC). The 1089 bp Stu I fragment was used as a probe. Expected sizes of fragments encoding the C-termini of Rec8 and Rec8-Myc are 1089 (green arrowhead) and 1478 bp (red arrowhead), respectively. (C) Western blot analysis testing the expression of Rec8-Myc and Rec8-N-Myc in total testicular cell extracts. Genotypes of endogenous Rec8 are indicated. One blot was used for three examinations by anti-Rec8, anti-Myc and anti-tubulin (loading control). Green arrowhead, endogenous Rec8; red arrowhead, Rec8-Myc or Rec8-N-Myc. (D) FACS analysis of testicular cells for the DNA content. Histogram peaks indicated as 1C, 2C and 4C correspond to cells having one, two or four copies of the genome, respectively.
Rec8-N-Myc prevents production of haploid spermatids
Mice heterozygous for TG Rec8-N-Myc developed normally and had unaltered life spans. However, their testes were approximately half as large (in weight) as those of non-transgenic littermates or males heterozygous for TG Rec8-Myc (data not shown). Western blots using antibodies specific for Rec8 or the Myc epitope showed that Rec8-Myc was expressed at a level similar to endogenous Rec8 whereas Rec8-N-Myc was if anything slightly less abundant (Fig. 2C). FACS analysis revealed that the testes of TG Rec8-N-Myc mice lacked any cells with a 1 C DNA content, which constituted the majority of cells in testes of wild-type or TG Rec8-Myc mice (Fig. 2D). About two thirds of the cells in TG Rec8-N-Myc testes had a 4 C DNA content, suggesting that meiotic DNA replication had occurred but was not followed by the nuclear divisions that usually reduce chromatid numbers.
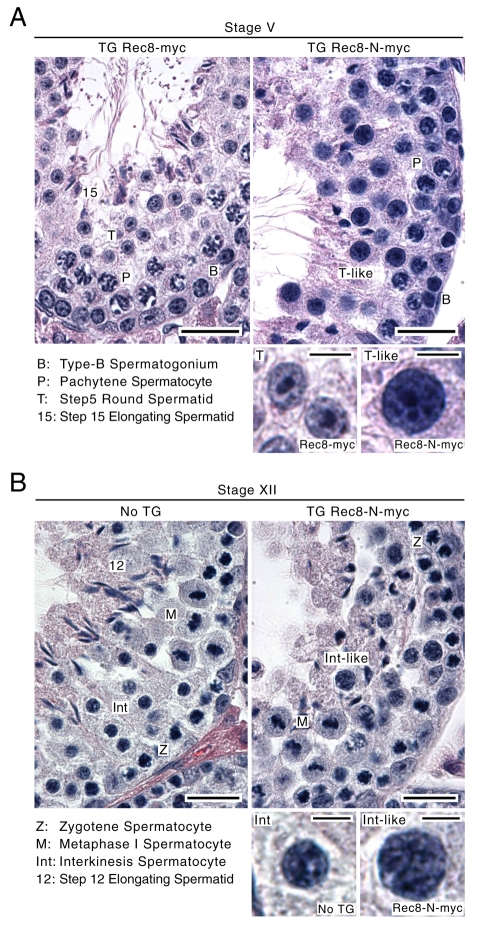
The spermatogenic failure caused by Rec8-N was then histologically studied by comparing cross-sections of seminiferous tubules within the testes from TG Rec8-N-Myc mice, TG Rec8-Myc mice and non-transgenic littermates. Mitotic spermatogonia are situated at the basal lamina of the tubules. As the germ cells differentiate, they migrate toward the lumen. Each cross-section contains a defined combination of cell types at different spermatogenic stages, which facilitates assessment of the developmental process (Russell et al., 1990). Spermatogonia were found at the cortex of TG Rec8-N-Myc tubules at frequencies similar to those found in tubules of non-transgenic littermates or TG Rec8-Myc tubules (Fig. 3A,B), suggesting that mitotic proliferation within spermatogonia is unaffected by Rec8-N expression. Likewise, no abnormalities were detected within populations of primary spermatocytes in meiotic prophase (e.g. pachytene and zygotene in Fig. 3A,B, respectively, and other stages, not shown). Spermatogenic development during meiotic prophase appears therefore to be unaffected by TG Rec8-N-Myc. By contrast, spermatids, either round or elongating types, which are abundant at the luminal region of the tubules at all stages of wild-type and TG Rec8-Myc testes, were absent in TG Rec8-N-Myc tubules. Instead, large numbers of large and densely stained nuclei occupied the luminal part of stage V (Fig. 3A) and other stage tubules (not shown). At the innermost surface of TG Rec8-N-Myc tubules, densely stained irregularly shaped nuclei that resemble elongating spermatids with oversized nuclei and sperm tails were observed (Fig. 3A,B).
Fig. 3.
Spermatogenic failure of TG Rec8-N-Myc males. Hematoxylin and eosin staining of seminiferous tubule sections from transgenic mice (TG) and a non-transgene littermate (No TG). Representative sections of stage V (A) and XII (B) are shown. Representative nuclei for each cell type present in the sections are shown at higher magnification. Scale bars: 20 and 5 μm for low and high magnification images, respectively.
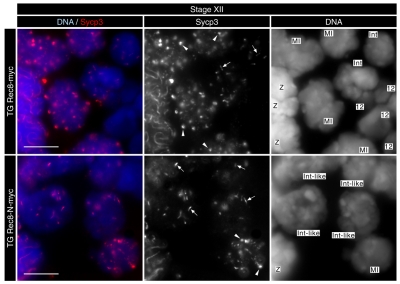
In wild-type mice, spermatocytes undergoing the first and second meiotic divisions are exclusively seen in stage XII tubules (Russell et al., 1990). Such tubules from TG Rec8-N-Myc testes contained metaphase I spermatocytes (Fig. 3B), suggesting that Rec8-N does not block progression from pachytene to metaphase I. Wild-type and TG Rec8-Myc stage XII tubules also contain secondary spermatocytes at interkinesis (interphase between meiosis I and II). TG Rec8-N-Myc tubules at this stage contained spermatocytes whose nuclei were morphologically similar but notably larger (Fig. 3B). To determine the identity of these abnormally large interkinesis-like spermatocytes, we examined stage XII tubule sections using immunofluorescence to detect Sycp3, a major component of the AE/LE. Sycp3 persists in the vicinity of centromeres until the early stages of round spermatid development but there are striking differences in its distribution before and after meiosis I. Sycp3 associated with metaphase I chromosomes is globular and abundant. It is less abundant and adopts the shape of a short bar in post-meiosis I interkinesis nuclei (see Fig. 4). The Sycp3 pattern within the enlarged irregular nuclei of stage XII sections of TG Rec8-N-Myc tubules was similar if not identical to that seen in normal interkinesis nuclei. Because step 1 round spermatids (haploid cells immediately after meiosis II) are not present in stage XII sections (Russell et al., 1990), we suggest that the enlarged irregular nuclei (seen in Fig. 3B) are interkinesis nuclei that are generated by a failure of the first meiotic division but otherwise normal chromosome development. These observations suggest that earlier stages of spermatogenesis up to metaphase I are unaffected in TG Rec8-N-Myc cells and that the first abnormality appears between metaphase I and the interkinesis stages. The large interkinesis and spermatid nuclei and the accumulation of cells with a 4C DNA content suggest that Rec8-N-Myc interferes with nuclear division at the first or possibly both meiotic divisions.
Fig. 4.
Morphological change of Sycp3 after meiosis I is unaffected in TG Rec8-N-Myc spermatocytes. Immunofluorescence images of stage XII tubule sections stained by DAPI (for DNA) and anti-Sycp3 antibody. Arrowheads and arrows indicate typical patterns of Sycp3 signals in metaphase I and post-metaphase I, respectively. Stages are: Z, zygotene; MI, metaphase I; Int, interkinesis; 12, step 12 elongating spermatid. Scale bars: 10 μm.
Normal chromosome development until metaphase I in spermatocytes expressing Rec8-N-Myc
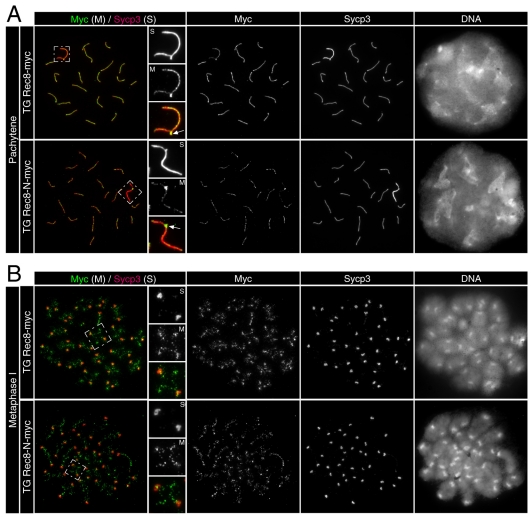
Immunofluorescence staining of Sycp3 and Myc-tagged Rec8 in testicular chromosome spreads revealed that synapsis during pachytene was unaltered in TG Rec8-N-Myc spermatocytes. Typical examples of 19 fully synapsed autosomes together with a pair of sex chromosomes synapsed solely at the pseudo-autosomal region (PAR) from TG Rec8-Myc and TG Rec8-N-Myc testes are shown in Fig. 5A. Importantly, Rec8-N-Myc and Rec8-Myc colocalized with Sycp3 along the AE/LE of pachytene bivalents. The chromosomal distribution of Sycp3 and Myc-tagged Rec8 also appeared normal at preleptotene, leptotene, zygotene and diplotene (data not shown). This contrasts with the phenotype of Rec8- or Smc1β-deficient spermatocytes that enter apoptosis in pachytene (Bannister et al., 2004; Revenkova et al., 2004; Xu et al., 2005). Both Rec8-Myc and Rec8-N-Myc localized to the inter-chromatid axes of metaphase I bivalents, both proximal and distal to chiasmata (Fig. 5B). The presence of chiasmata and the lack of precocious homolog disjunction confirm that crossover formation is normal in TG Rec8-N-Myc spermatocytes. Finally, we observed that metaphase I bivalents contained the normal two sets of doublet foci of centromeres associated with Sycp3 (Fig. 6A). We conclude that Rec8-N has no adverse effect on meiotic chromosome development up to metaphase I.
Fig. 5.
Normal chromosome development until metaphase I in TG Rec8-N-Myc spermatocytes. Immunofluorescence images of chromosome spreads stained with anti-Myc and anti-Sycp3 antibodies. (A) Pachytene nuclei and magnified sex chromosomes synapsed at the PAR (arrow) are shown. (B) Metaphase I chromosomes and magnified images of representative bivalents are shown.
Fig. 6.
No nuclear division in spermatocytes expressing Rec8-N-Myc. (A) Immunofluorescence images of chromosome spreads stained with a CREST serum (for centromeres), anti-Sycp3 antibody and DAPI (for DNA). Small panels show the magnified images of boxed areas. (B) Frequencies of nuclei classified by number of centromeres: approximately 40 CREST foci (40× Cen) or approximately 80 CREST foci (80× Cen). Numbers of nuclei examined are indicated (n). (C) Chromosome paintings detecting X (red) and Y (green) chromosomes merged on DAPI images or on anti-Sycp3 images in grayscale. Stages are: Pa, pachytene; Dip, diplotene; Int, interkinesis; RS, round spermatid. (D) Frequencies of nuclei classified by distribution of sex chromosome painting signals: one patch of X chromosome alone (X), one patch of Y chromosome alone (Y) or one or two patches of both (X and Y). Numbers of nuclei examined are indicated (n).
No nuclear division in spermatocytes expressing Rec8-N-Myc
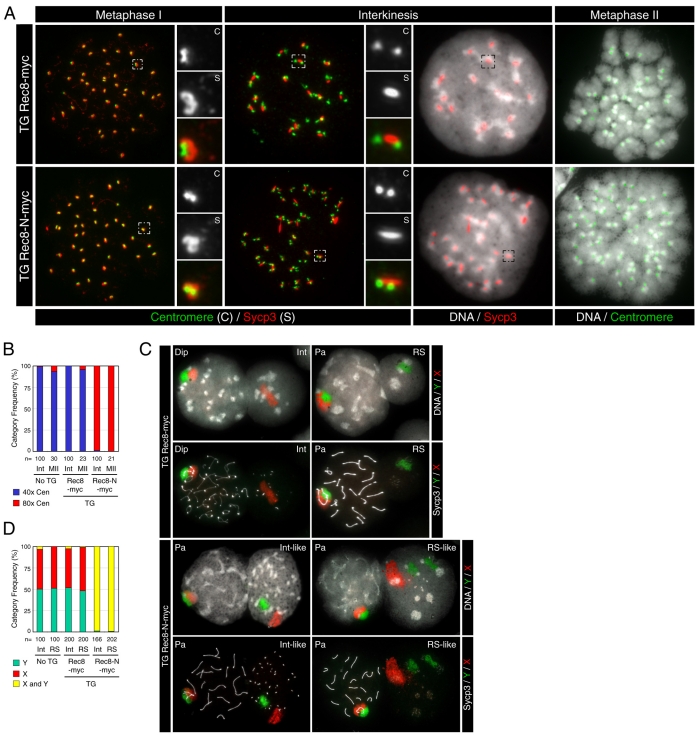
If Rec8-N blocks the first meiotic division without halting meiotic chromosome development, then it should be possible to detect nuclei containing four chromatids of each chromosome with morphologies characteristic of meiosis II chromosomes. To address this, we examined DNA staining (using DAPI), kinetochores (using a CREST serum) and the distribution of Sycp3 in chromosome spreads from TG Rec8-Myc and TG Rec8-N-Myc testes. This revealed three types of abnormal nuclei in TG Rec8-N-Myc testes: those with short bars of Sycp3 and pronounced CREST foci associated with centromeric heterochromatin (Fig. 6A, `Interkinesis'); nuclei with bar-like Sycp3 at chromocenters but lacking CREST foci (not shown); and nuclei without either Sycp3 or pronounced CREST foci (not shown). The CREST and Sycp3 signals (compared with those of wild type) suggest that these three types represent nuclei at interkinesis and at an early and late stage of round spermatid development, respectively. DAPI staining revealed that all three types of nuclei [which would have undergone one (interkinesis) or two (spermatids) meiotic divisions in wild type] were abnormally large, being similar in size or even somewhat larger than those from pachytene or diplotene (see Fig. 6C). Interkinesis nuclei from wild-type or TG Rec8-Myc secondary spermatocytes contained 20 sets of doublet CREST foci associated with Sycp3 signals, which reflects the fact that they have 20 dyads, each containing two chromatids (Fig. 6A,B). By striking contrast, the enlarged interkinesis-like nuclei of TG Rec8-N-Myc spermatocytes contained 40 sets of doublet CREST foci, which implies that they contain 40 dyads. Together, these data suggest that TG Rec8-N-Myc prevents the first meiotic division, and thereby generates 4 C instead of 2 C interkinesis nuclei.
This conclusion was confirmed using chromosome painting to detect the X and Y sex chromosomes. These synapse partially during meiotic prophase (Fig. 5A, Fig. 6C) and segregate at the first meiotic division, which produces secondary spermatocytes with either an X or a Y dyad chromosome. As expected, the interkinesis nuclei from wild-type or TG Rec8-Myc secondary spermatocytes contained either X or Y chromosomes and the ratio of nuclei with X chromosomes to those with Y chromosomes was 1:1 (Fig. 6C,D). By contrast, almost all interkinesis-like nuclei from TG Rec8-N-Myc spermatocytes contained both X and Y chromosomes. We conclude that TG Rec8-N-Myc prevents the first meiotic division at which X and Y chromosomes segregate to `sister' secondary spermatocytes.
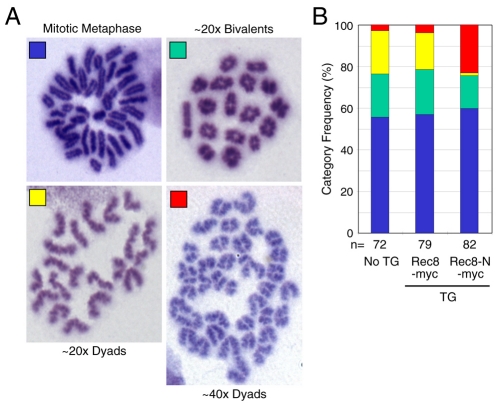
The chromosome spreads from wild-type and TG Rec8-Myc spermatocytes contained three kinds of condensed chromosome sets: those containing 20 bivalents (metaphase I), 20 open arm dyads (metaphase II), and 40 closed arm chromosomes (mitotic metaphase). TG Rec8-N-Myc spermatocytes contained nuclei with mitotic metaphase and metaphase I chromosomes but lacked any containing the 20 open arm dyads characteristic of metaphase II. Notably, they contained nuclei with 40 instead of 20 open arm dyads (Fig. 6A,B). We presume that these abnormal nuclei have entered metaphase II without having undergone the first meiotic division. To confirm this, we prepared chromosome spreads fixed with methanol and acetic acid and measured the fraction of chromosome spreads with four different categories (Fig. 7A) of condensed chromosomes: mitotic metaphase, 20 bivalents (metaphase I), 20 dyads (metaphase II) and 40 dyads (irregular metaphase II). This revealed that TG Rec8-N-Myc testes had few if any chromosome sets containing 20 dyads. However, they contained chromosome sets with 40 dyads with a frequency similar to that of chromosome sets with 20 dyads in wild-type or TG Rec8-Myc testes (Fig. 7B).
Fig. 7.
Double number of dyad chromosomes in metaphase II TG Rec8-N-Myc spermatocytes. (A) Condensed chromosomes found in Giemsa-stained spread preparations: 40 chromosomes with arm closed (Mitotic Metaphase); approximately 20 bivalents; approximately 20 dyads; approximately 40 dyads. (B) Frequencies of different classes of condensed chromosomes shown in A. Numbers of nuclei examined are indicated (n).
Our data are consistent with the notion that Rec8-N blocks the first meiotic division. Does it also block the second? To address this, we examined the distribution of sex chromosomes in enlarged spermatid-like nuclei contained in TG Rec8-N-Myc testes and found that all of them contained both X and Y chromosomes (Fig. 6C,D). Importantly, we frequently observed two separate X or two separate Y chromatids within the same nucleus and sometimes two separate X and two separate Y chromatids within the same nucleus. This suggests that many if not all abnormally large spermatid-like nuclei have a 4 C DNA content and that TG Rec8-N-Myc prevents chromosome segregation during meiosis II as well as meiosis I.
Some but not all Rec8-N-Myc persists on chromatin after meiosis I and II
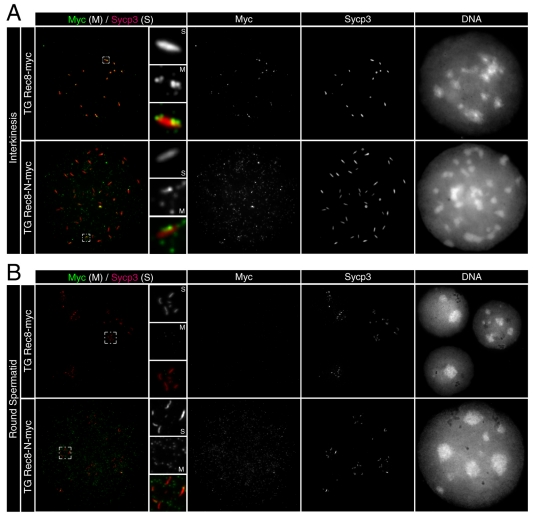
To ascertain whether Rec8-N-Myc persists on chromosomes after anaphase I and II, we compared the distribution of Sycp3 and Myc-tagged Rec8 proteins in chromosome spreads from TG Rec8-Myc and TG Rec8-N-Myc testes. Rec8-Myc was present (along with Sycp3) only at centromeres in interkinesis nuclei (Fig. 8A) but was completely absent from early stage round spermatids that still possessed Sycp3 signals (Fig. 8B). Very faint amounts of Rec8-Myc were detected throughout chromatin, and in particular at chromocenters in later stage round spermatids that lack Sycp3 signals (not shown). By contrast, modest amounts of Rec8-N-Myc protein were present throughout the chromatids of interkinesis-like and early stage of round spermatid nuclei (Fig. 8A,B). These results imply that some but not all Rec8-N-Myc persists on chromatin after the time by which both meiotic divisions should have taken place.
Fig. 8.
Persistence of Rec8-N-Myc on chromatin after meiosis I and II. Immunofluorescence images of chromosome spreads stained with anti-Myc and anti-Sycp3 antibodies. (A) Interkinesis nuclei and representative areas in higher magnification that contain centromeric Rec8 foci are shown. (B) Spermatid nuclei and representative chromocenters in higher magnification that possess Sycp3 signals are shown.
Rec8-N delays but does not block chiasma resolution at meiosis I in oocytes
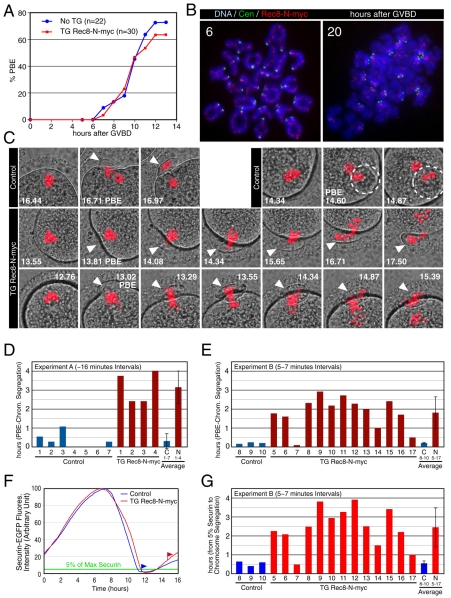
TG Rec8-N-Myc females were fertile with slightly smaller litter size (average 7.8, 11 deliveries of six females) than TG Rec8-Myc females (average 9.6, five deliveries of three females). Oocytes from TG Rec8-N-Myc females extruded the first polar body (PB) with similar timing and efficiency as those from non-transgenic littermates in in vitro culture (Fig. 9A). Importantly, by 20 hours after the germinal vesicle breakdown (GVBD) most oocytes contained 20 dyads, implying that chiasmata had been resolved by metaphase II (Fig. 9B). The lack of any obvious block to meiosis I chromosome segregation or PB formation was not due to a lack of Rec8-N expression, as it was detected along interchromatid axes, both proximal and distal to chiasmata, of metaphase I bivalents. Like Rec8-Myc (Kudo et al., 2006), Rec8-N-Myc was detected in the vicinity of sister centromeres of dyads at metaphase II (Fig. 9B). This contrasts with the situation in oocytes lacking separase activity, in which the persistence of Rec8 along inter-chromatid axes accompanies a complete absence of chiasma resolution (Kudo et al., 2006).
Fig. 9.
Rec8-N delays but does not block chiasma resolution at meiosis I in oocytes. (A) Kinetics of the first polar body extrusion (PBE) of oocytes cultured in vitro. The numbers of oocytes examined are indicated (n). (B) Localization of Rec8-N-Myc on chromosomes in TG Rec8-N-Myc oocytes. Chromosome spreads prepared from oocytes in culture for the indicated time after germinal vesicle breakdown (GVBD) were stained with anti-Myc antibody (red), CREST antiserum (green) and DAPI (blue). (C) Live confocal microscopy of oocytes expressing H2B-mCherry. DIC images (gray) were merged with images of the mCherry channel (red). Frames at indicated time (hours after beginning of filming) were selected from the original time series (supplementary material Movie 1). PBs are indicated by arrowheads or circles (when extruded vertically). (D) Intervals from PBE to the completion of chromosome segregation analyzed by ∼16-minute filming intervals (experiment A, supplementary material Movie 1). Error bar, s,d. (E) As in (D), analyzed by 5-7 minute intervals (experiment B, movies not shown). (F) Representative fluorescence quantifications of securin-EGFP signal in individual oocytes. The maximum value of the signal in each oocyte was set to 100 and relative intensity was plotted. Flags indicate time of the completion of chromosome segregation. Green line indicates the 5% level of the EGFP signal. (G) Intervals from 5% of the EGFP signal to the completion of chromosome segregation analyzed by 5-7 minute filming intervals (experiment B, movies not shown). Error bar, s.d.
These data do not, however, exclude a role for Rec8 cleavage in chiasma resolution. If Rec8-N contained cryptic separase cleavage sites that permitted its eventual removal from the arms of bivalents, then it might merely slow down cleavage and chiasma resolution but not actually block either process. To address this, we used live microscopy to measure the timing of chromosome segregation relative to other anaphase hallmarks, namely PB extrusion (PBE) and securin degradation. Germinal vesicle-stage oocytes injected with mRNAs encoding H2B-mCherry and securin-EGFP were recorded using a confocal microscope either at 16 minutes (experiment A; supplementary material Movie 1) or 5-7 minutes intervals (experiment B, movies not shown). In control oocytes (females with the TG Rec8-Myc or no transgene), chromosome segregation occurred soon after PBE (experiment A, 0.31±0.39 hours, n=7; experiment B, 0.20±0.04 hours, n=3) (Fig. 9C-E; supplementary material Movie 1) (Kudo et al., 2006; McGuinness et al., 2009). By contrast, chromosomes in TG Rec8-N-Myc oocytes linger for about two hours around the oocyte-PB junction before eventually segregating in an asynchronous manner to oocytes and PBs (Fig. 9C; supplementary material Movie 1). The intervals from PBE to the completion of chromosome segregation were 3.15±0.86 hours (experiment A, n=4) and 1.81±0.84 hours (experiment B, n=13) (Fig. 9D,E). The difference between TG Rec8-N-Myc and control oocytes was highly significant (experiment A, P=0.00003; experiment B, P=0.006, Student's t-test). The interval between chromosome segregation and securin destruction, namely the point by which securin sinks to 5% of its maximum, was also significantly greater in TG Rec8-N-Myc oocytes than in controls (experiment B: control, 0.55±0.13 hours, n=3; TG Rec8-N-Myc oocytes, 2.45±1.04 hours, n=13; P=0.011, Student's t-test) (Fig. 9F,G). These results are consistent with the notion that Rec8-N-Myc is cleaved more slowly than wild-type protein in vivo and that this delays chiasma resolution. The fertility of TG Rec8-N-Myc oocytes suggests that abscission between the PB and the oocyte is delayed until chiasmata have been completely resolved.
Discussion
The recent finding that proteolytic activity of separase is essential for the resolution of chiasmata during meiosis I in mouse oocytes (Kudo et al., 2006) is consistent with the notion that they are resolved by cleavage of α-kleisin proteins, as is the case in yeast (Buonomo et al., 2000). To test this, we set out to identify separase cleavage sites in the mouse meiosis-specific α-kleisin Rec8 and then expressed at physiological levels a version (Rec8-N) that is poorly if at all cleaved in vitro. The BAC transgene expressing Rec8-N causes sterility in males, but not in females. Spermatocytes expressing Rec8-N reach metaphase I with bivalents containing a normal complement of chiasmata, but thereafter fail to produce secondary spermatocytes containing the normal complement of 20 dyad chromosomes or to produce haploid gametes. They instead produce cells with 80 chromatids, suggesting that Rec8-N blocks chromosome segregation at the first meiotic division and possibly also at the second. The lack of divisions does not appear to be due to a block of meiotic development because changes in the distribution of Sycp3 and kinetochore proteins normally associated with progression from meiosis I to II appear to take place in the presence of Rec8-N, despite the lack of nuclear division. These observations suggest that cleavage of Rec8 by separase is essential for the first and possibly also for the second meiotic division of mouse spermatocytes. By contrast, Rec8-N has only a modest adverse effect on the fertility of females, blocking neither oocyte divisions. Importantly, chromosome spreads indicate that Rec8-N does not block chiasma resolution in either males or females.
On their own, these results imply that Rec8 cleavage might not be necessary for chiasmata resolution. Might the yeast model therefore apply to mammals? Two key findings indicate otherwise. First, it is clear that in oocytes the amount of Rec8-N persisting on chromosomes after separase activation is far less than the amount of Rec8 persisting on chromosomes in oocytes lacking separase activity. The implication is that Rec8-N does not in fact abolish cleavage in vivo. Rec8 must possess additional separase cleavage sites that we have not been able to detect in vitro. Our detection of modest amounts of Rec8-N associated with post-meiosis I chromosomes suggests that mutation of the three sites cleaved by separase in vitro does indeed slow down cleavage in vivo; it does not abolish it. Second, despite the incomplete penetrance of the Rec8-N phenotype, this allele has nevertheless proved a useful reagent for evaluating the role of Rec8 cleavage. In oocytes, where it is possible to film cells undergoing the first meiotic division and thereby obtain kinetic data, we observed that the chiasma resolution occurs asynchronously and is delayed by about two hours following separase activation (as detected by destruction of securin-EGFP). Such data imply that chiasma resolution is normally mediated by Rec8 cleavage. The data do not address directly the mechanism by which chiasmata are eventually resolved in oocytes expressing Rec8-N, but the eventual disappearance of Rec8-N from chromosomes suggests that resolution also arises from cleavage, albeit slower than in wild-type oocytes.
We suggest that the asynchrony of chromosome segregation arises from differences between bivalents in the position of their chiasmata. Bivalents with chiasmata proximal to centromeres will be held together by more cohesin than those with more distal chiasmata and might therefore take longer to be resolved by an inefficient cleavage process. It seems likely that chiasma resolution is also slowed by Rec8-N in spermatocytes (though catching this on film will be a formidable undertaking) and we suggest that this, as opposed to some other unknown role for cohesin cleavage in cell division, is the underlying cause of their meiotic division defect.
According to the above scenario, the differential effects of Rec8-N on male and female gametogenesis (preventing it in males but not females) arises not so much from more rapid cleavage of Rec8-N in oocytes than spermatocytes but rather in the manner in which these two types of cells respond to delayed chiasmata resolution. It is remarkable that Rec8-N delays chromosome segregation by about two hours in oocytes without greatly affecting their inheritance of a complete set of dyads; that is, slower chiasma resolution leads neither to chromosome gain or loss. There are two explanations for this: either abscission of oocytes from the first PB normally does not take place for two or more hours after PBE, providing sufficient time to segregate a complete set of dyads to both oocytes and PB; or the presence of unresolved bivalents in the cleavage furrow actively delays abscission, possibly by delaying inactivation of Aurora B (as recently found in HeLa cells), a process called the abscission checkpoint (Steigemann et al., 2009).
We suggest that a difference in the way that spermatocytes respond to the persistence of bivalents at the cleavage furrow is responsible for the male sterility caused by Rec8-N. It has been suggested that abscission in the presence of chromosome bridges induces furrow regression and formation of tetraploid cells in HeLa cells (Steigemann et al., 2009). Assuming that spermatocytes with delayed chiasma resolution proceed with furrow formation with normal kinetics (as clearly occurs in oocytes), they might then attempt abscission and then regress their furrows in response to the presence of chromatin or regress their furrows without even attempting abscission. Either response could account for the formation of cells with all 80 chromatids and the resulting sterility.
A key aspect of sister chromatid cohesion during meiosis is its persistence at centromeres after meiosis I. Recent work in yeast has shown that recruitment of phosphatase PP2A to centromeres by proteins of the Mei-S332 family is essential for the resistance of centromeric Rec8 to separase at meiosis I (Riedel et al., 2006). To explain this, it has been suggested that phosphorylation of cohesin or even of separase itself might be essential for Rec8 cleavage and that PP2A reverses such phosphorylation in the vicinity of centromeres. The finding that Rec8 but not Scc1 is subject to protection by Mei-S332–PP2A in vivo in yeast is most easily explained if Rec8 were crucial target of PP2A. Hyper-phosphorylation of yeast Rec8 at multiple serine and threonine residues during meiosis I has been reported, but their replacement by alanine appears to have little direct effect on the resolution of chiasmata (Brar et al., 2006). It is therefore still unclear whether Rec8 phosphorylation has a crucial role in promoting its cleavage by separase either in vivo or in vitro. Our finding that cleavage of mouse Rec8 by separase is completely dependent on Plk1 in vitro might be germane to this issue, particularly as Scc1 cleavage is far less dependent on Plk1 under the same conditions (Fig. 1). Plk1 is necessary for hyper-phosphorylation of Rec8 in yeast and has a role in promoting Scc1 cleavage during mitotic divisions (Alexandru et al., 2001; Clyne et al., 2003; Hornig and Uhlmann, 2004; Brar et al., 2006). Our in vitro cleavage data are consistent with the notion that Rec8 phosphorylation might indeed be essential for its cleavage and that PP2A protects sister chromatid cohesion at centromeres by de-phosphorylating Rec8.
In summary, our work suggests that chiasma resolution in mammals is mediated by Rec8 cleavage, as it is in yeast. Rec8 must therefore confer much of the sister chromatid cohesion (though we cannot conclude all) that holds bivalents together. We propose that the chemistry of chiasma resolution is fundamentally the same in fungi and mammals and possibly universal amongst eukaryotes. Given that precocious loss of sister chromatid cohesion could have a role in the chromosome non-disjunction at meiosis I (Hodges et al., 2005), which gives rise to human aneuploidy, it is clearly important to understand better the mechanisms that confer and protect sister chromatid cohesion during meiosis. Further scrutiny of the relative roles of Rec8 and Scc1 in conferring meiotic sister chromatid cohesion as well as the role of their cleavage by separase will be essential for understanding the potential regulation of this process by Mei-S332 and PP2A.
Materials and Methods
In vitro Rec8 cleavage assay
cDNAs encoding mouse Rec8 (GenBank/EMBL/DDBJ accession number AF262055) and human Scc1-Myc (Hauf et al., 2001) were kindly provided by Michael McKay (Peter MacCallum Cancer Research Centre, Melbourne, Australia) and J.-M. Peters (Research Institute of Molecular Pathology, Vienna, Austria), respectively. [35S]Methionine-labeled wild-type or mutant forms of Rec8 and Scc1-Myc proteins were produced using rabbit reticulocyte lysate-based in vitro coupled transcription-translation systems (Promega). The active (PM-2/4 which has S1126A and T1346A) and the inactive (C1129S) separase mutants were coexpressed in 293T cells with human securin, purified and activated as described (Stemmann et al., 2001). GST-human Plk1 was expressed in insect cells and purified as described (Sumara et al., 2002). A pre-incubation mixture composed of 2 μl reticulocyte lysate containing [35S]methionine-labeled protein, 1 μl GST-Plk1 at 0.5 or 0.13 μg/μl and 6 μl cleavage buffer (20 mM HEPES/KOH pH 7.7, 100 mM KCl, 11 mM MgCl2, 0.1 mM CaCl2, 1 mM DTT, 1 mM ATP, 10 mM NaF, 0.5 μM microcystin-LR, 1 mM PMSF) was incubated at 37°C for 30 minutes. One microliter of active or inactive separase was added and the mixture was incubated for an additional 90 minutes at 37°C. The reactions were separated on gradient (8.5-15%) SDS-polyacrylamide gels and the radioactive signal was detected by the PhosphorImager system (Molecular Dynamics).
Mutagenesis and BAC modification
Site-directed mutagenesis of Rec8 cDNA was performed using the QuickChange kit (Stratagene). A BAC clone 546A10 encoding the Rec8 locus from the CT7 library (male CJ7/129SV genomic fragments on pBeloBAC11) was purchased from Invitrogen. Modification of BACs was performed as described (Yang et al., 1997). For each modification, a ∼2 kb genomic fragment that works as ∼1 kb homology arms for both sides was first cloned into pBluescript. For the Myc tagging construct, restriction sites were created at the termination codon and a nucleotide sequence encoding 9×Myc epitope (GEQKLISEEDLN) was inserted. For amino acid replacement, site-directed mutagenesis was performed using the QuickChange kit. For conditional constructs, a loxP flanked transcription silencer cassette consisted of four polyA signal repeats and puroMycin-resistant gene provided by David Tuveson (Cancer Research UK Cambridge Research Institute, Cambridge, UK) (Tuveson et al., 2004), was inserted. After sequencing, the entire insert was cloned into pSV1.RecA and it was electroporated into bacteria harboring the BAC. Induction of homologous recombination and screening of the modified BAC clone was performed according to a previously described method (Yang et al., 1997). Modifications were confirmed by sequencing of the mutagenized regions and the integrity of the BACs was confirmed by restriction enzyme digestions. PCR primer sequences are available upon request.
Mouse strains and generation of transgenic mice
BAC DNA purified by CsCl-EtBr gradient centrifugation and prepared at 1 μg/ml was microinjected into pronuclei of Mus musculus B6CBAF2 zygotes. Transgenic founders confirmed by Southern blot and PCR analysis were bred to B6CBAF1 mice. Offspring that inherited the transgene through the germ line were further bred to B6CBAF1 mice, and 6- to 8-week-old mice were studied. For Southern blots, genomic DNA preparations were digested by Stu I, run on an agarose gel, transferred to a nylon membrane and hybridized with a 32P-labeled 1089 bp Stu I genomic fragment encoding the C-terminus of Rec8.
Western blot and FACS analyses
For western blot analysis, a testicular cell suspension was made by physical disaggregation of seminiferous tubules with forceps in PBS and precipitated extracellular matrices were removed. Cells were collected by centrifugation, suspended in SDS-PAGE sample buffer and boiled. DNA was sheared by sonication and passed through a 27-gauge needle. Total extracts were run on a SDS-PAGE gel and transferred to a PVDF membrane. For detecting proteins, anti-Rec8 [RαN from rabbit (Eijpe et al., 2003)], anti-human c-Myc epitope [clone 4A6 from mouse (Upstate)] and anti-alpha-tubulin [clone YOL1/34 from rat (Serotec, Oxford, UK)] antibodies were used as primary antibodies, and appropriate secondary antibodies conjugated with HRP (GE Healthcare) were used. FACS analysis for the DNA content of testicular cells was performed by a method previously described (Malkov et al., 1998).
Histological analysis
Histological preparation of testes and staining for hematoxylin and eosine were as described (Peters et al., 2001). Testis cryosections were fixed by 4% PFA in PBS and stained by anti-Sycp3 antibody [clone 10G11 from mouse (Abcam, Cambridge, MA)] and anti-mouse IgG conjugated with Alexa Fluor 488 (Molecular Probes). DNA was counterstained with DAPI. Microscopic examination was performed as described (Kudo et al., 2006).
Preparation and examination of chromosome spreads
Chromosome spreads of testicular cells for immunofluorescence staining were prepared as described (Peters et al., 1997). Anti-human c-Myc epitope antibodies [clone 4A6 and CM-100 from rabbit (Gramsch, Schwabhausen, Germany)], anti-Sycp3 antibodies [clone 10G11 and Knuf from rabbit (Lammers et al., 1994)] and a CREST serum (gift of Arno Kromminga, IPM Biotech, Hamburg, Germany) were used as primary antibodies, and appropriate secondary antibodies conjugated with Alexa Fluor 488 or 568 (Molecular Probes) or Cy5 (Jackson ImmunoResearch) were used. DNA was counterstained with DAPI. Chromosome painting of spermatocyte spreads initially stained for Sycp3 was performed with biotin- and Cy3-labelled mouse X and Y chromosome painting probes (Cambio, Cambridge, UK), respectively. Sycp3 and Y chromosome were visualized by Alexa-Fluor-488 conjugated secondary antibody and Alexa-Fluor-633 conjugated streptavidin, respectively. The experimental procedure was described (Xu et al., 2005). Microscopic examination was performed as described (Kudo et al., 2006). Preparation and Giemsa staining of Metaphase spreads of testicular cells were as described (Peters et al., 2001).
Oocyte studies
Experiments involving oocytes were performed according to our methods described previously (Kudo et al., 2006; McGuinness et al., 2009). Germinal vesicle-stage oocytes were microinjected with mRNAs encoding histone H2B-mCherry and securin-EGFP in M2 media containing IBMX inhibitor. Transferring oocytes to the media without inhibitor after 1-2 hours triggered the meiotic maturation. Live confocal microscopy was performed using a Zeiss LSM510 META microscope equipped with C-Apochromat 40× 1.2 water objective (experiment A, supplementary material Movie 1) and Plan-Neofluor 20× 0.5 dry objective (experiment B). PeCon (Erbach, Germany) environmental microscope incubator was used to maintain 5% CO2 atmosphere, temperature and humidity. Excitation wavelengths were 405, 488, 514 and 561 nm, and LP 420, BP 505-550, BP 530-600 and LP 575 filters were used for detection of Cascade-Blue dextran and EGFP and mCherry fluorescent proteins. During experiment A, AutofocusScreen macro [51] was used to track chromosomes labeled with H2B-mCherry. Five to seven (experiment A) or 20-25 (experiment B) z-stacks were captured every 16 minutes (experiment A) or 5-7 minutes (experiment B) for ∼20 hours. Quantification of the signal was performed with ImageJ software (http://rsb.info.nih.gov/ij/). To measure securin-EGFP signal, individual frames were defined manually for each oocyte and values were normalized to the value measured at the time of GVBD.
Supplementary Material
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/122/15/2686/DC1
We are grateful to Karin Wirth, Alexander Schleiffer, Marina Pasca di Magliano, Pawel Pasierbek and the IMP service and animal house staff for technical assistance, Jan-Michael Peters, Michael McKay, Nathaniel Heintz, David Tuveson and Arno Kromminga for materials, and Marc Kirschner for support. We also thank members of the Nasmyth laboratory for helpful discussion. The Research Institute of Molecular Pathology is funded by Boehringer Ingelheim. This work was partly supported by grants from the Austrian Science Fund (K.N. and N.R.K.), the European Community (contact number: QLG1-CT-2001-02026, shared costs action U2P2) (K.N. and N.R.K.), the Wellcome Trust (K.N.), the Japanese Society for the Promotion of Science and the Institute of Obstetrics & Gynaecology Trust (N.R.K.) and the Deutsche Krebshilfe (O.S.). Deposited in PMC for release after 6 months.
References
- Alexandru, G., Uhlmann, F., Mechtler, K., Poupart, M. A. and Nasmyth, K. (2001). Phosphorylation of the cohesin subunit Scc1 by Polo/Cdc5 kinase regulates sister chromatid separation in yeast. Cell 105, 459-472. [DOI] [PubMed] [Google Scholar]
- Bannister, L. A., Reinholdt, L. G., Munroe, R. J. and Schimenti, J. C. (2004). Positional cloning and characterization of mouse mei8, a disrupted allelle of the meiotic cohesin Rec8. Genesis 40, 184-194. [DOI] [PubMed] [Google Scholar]
- Brar, G. A., Kiburz, B. M., Zhang, Y., Kim, J. E., White, F. and Amon, A. (2006). Rec8 phosphorylation and recombination promote the step-wise loss of cohesins in meiosis. Nature 441, 532-536. [DOI] [PubMed] [Google Scholar]
- Buonomo, S. B., Clyne, R. K., Fuchs, J., Loidl, J., Uhlmann, F. and Nasmyth, K. (2000). Disjunction of homologous chromosomes in meiosis I depends on proteolytic cleavage of the meiotic cohesin Rec8 by separin. Cell 103, 387-398. [DOI] [PubMed] [Google Scholar]
- Clyne, R. K., Katis, V. L., Jessop, L., Benjamin, K. R., Herskowitz, I., Lichten, M. and Nasmyth, K. (2003). Polo-like kinase Cdc5 promotes chiasmata formation and cosegregation of sister centromeres at meiosis I. Nat. Cell Biol. 5, 480-485. [DOI] [PubMed] [Google Scholar]
- Eijpe, M., Offenberg, H., Jessberger, R., Revenkova, E. and Heyting, C. (2003). Meiotic cohesin REC8 marks the axial elements of rat synaptonemal complexes before cohesins SMC1beta and SMC3. J. Cell Biol. 160, 657-670.12615909 [Google Scholar]
- Gorr, I. H., Boos, D. and Stemmann, O. (2005). Mutual inhibition of separase and Cdk1 by two-step complex formation. Mol. Cell 19, 135-141. [DOI] [PubMed] [Google Scholar]
- Hassold, T. and Hunt, P. (2001). To err (meiotically) is human: the genesis of human aneuploidy. Nat. Rev. Genet. 2, 280-291. [DOI] [PubMed] [Google Scholar]
- Hauf, S., Waizenegger, I. C. and Peters, J. M. (2001). Cohesin cleavage by separase required for anaphase and cytokinesis in human cells. Science 293, 1320-1323. [DOI] [PubMed] [Google Scholar]
- Hauf, S., Roitinger, E., Koch, B., Dittrich, C. M., Mechtler, K. and Peters, J. M. (2005). Dissociation of cohesin from chromosome arms and loss of arm cohesion during early mitosis depends on phosphorylation of SA2. PLoS Biol. 3, e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidmann, D., Horn, S., Heidmann, S., Schleiffer, A., Nasmyth, K. and Lehner, C. F. (2004). The Drosophila meiotic kleisin C(2)M functions before the meiotic divisions. Chromosoma 113, 177-187. [DOI] [PubMed] [Google Scholar]
- Herbert, M., Levasseur, M., Homer, H., Yallop, K., Murdoch, A. and McDougall, A. (2003). Homologue disjunction in mouse oocytes requires proteolysis of securin and cyclin B1. Nat. Cell Biol. 5, 1023-1025. [DOI] [PubMed] [Google Scholar]
- Hodges, C. A., Revenkova, E., Jessberger, R., Hassold, T. J. and Hunt, P. A. (2005). SMC1beta-deficient female mice provide evidence that cohesins are a missing link in age-related nondisjunction. Nat. Genet. 37, 1351-1355. [DOI] [PubMed] [Google Scholar]
- Hornig, N. C. and Uhlmann, F. (2004). Preferential cleavage of chromatin-bound cohesin after targeted phosphorylation by Polo-like kinase. EMBO J. 23, 3144-3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima, T. S., Miyazaki, Y., Yamamoto, M. and Watanabe, Y. (2003). Rec8 cleavage by separase is required for meiotic nuclear divisions in fission yeast. EMBO J. 22, 5643-5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima, T. S., Hauf, S., Ohsugi, M., Yamamoto, T. and Watanabe, Y. (2005). Human Bub1 defines the persistent cohesion site along the mitotic chromosome by affecting Shugoshin localization. Curr. Biol. 15, 353-359. [DOI] [PubMed] [Google Scholar]
- Klein, F., Mahr, P., Galova, M., Buonomo, S. B., Michaelis, C., Nairz, K. and Nasmyth, K. (1999). A central role for cohesins in sister chromatid cohesion, formation of axial elements, and recombination during yeast meiosis. Cell 98, 91-103. [DOI] [PubMed] [Google Scholar]
- Kudo, N. R., Wassmann, K., Anger, M., Schuh, M., Wirth, K. G., Xu, H., Helmhart, W., Kudo, H., McKay, M., Maro, B. et al. (2006). Resolution of chiasmata in oocytes requires separase-mediated proteolysis. Cell 126, 135-146. [DOI] [PubMed] [Google Scholar]
- Lammers, J. H., Offenberg, H. H., van Aalderen, M., Vink, A. C., Dietrich, A. J. and Heyting, C. (1994). The gene encoding a major component of the lateral elements of synaptonemal complexes of the rat is related to X-linked lymphocyte-regulated genes. Mol. Cell. Biol. 14, 1137-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J., Iwai, T., Yokota, T. and Yamashita, M. (2003). Temporally and spatially selective loss of Rec8 protein from meiotic chromosomes during mammalian meiosis. J. Cell Sci. 116, 2781-2790. [DOI] [PubMed] [Google Scholar]
- Malkov, M., Fisher, Y. and Don, J. (1998). Developmental schedule of the postnatal rat testis determined by flow cytometry. Biol. Reprod. 59, 84-92. [DOI] [PubMed] [Google Scholar]
- McGuinness, B. E., Hirota, T., Kudo, N. R., Peters, J. M. and Nasmyth, K. (2005). Shugoshin prevents dissociation of cohesin from centromeres during mitosis in vertebrate cells. PLoS Biol. 3, e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinness, B. E., Anger, M., Kouznetsova, A., Gil-Bernabe, A. M., Helmhart, W., Kudo, N. R., Wuensche, A., Taylor, S., Hoog, C., Novak, B. et al. (2009). Regulation of APC/C activity in oocytes by a Bub1-dependent spindle assembly checkpoint. Curr. Biol. 19, 369-380. [DOI] [PubMed] [Google Scholar]
- Nasmyth, K. (2005). How might cohesin hold sister chromatids together? Philos. Trans. R. Soc. Lond. B Biol. Sci. 360, 483-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklas, R. B. (1997). How cells get the right chromosomes. Science 275, 632-637. [DOI] [PubMed] [Google Scholar]
- Peter, M., Castro, A., Lorca, T., Le Peuch, C., Magnaghi-Jaulin, L., Doree, M. and Labbe, J. C. (2001). The APC is dispensable for first meiotic anaphase in Xenopus oocytes. Nat. Cell Biol. 3, 83-87. [DOI] [PubMed] [Google Scholar]
- Peters, A. H., Plug, A. W., van Vugt, M. J. and de Boer, P. (1997). A drying-down technique for the spreading of mammalian meiocytes from the male and female germline. Chromosome Res. 5, 66-68. [DOI] [PubMed] [Google Scholar]
- Peters, A. H., O'Carroll, D., Scherthan, H., Mechtler, K., Sauer, S., Schofer, C., Weipoltshammer, K., Pagani, M., Lachner, M., Kohlmaier, A. et al. (2001). Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell 107, 323-337. [DOI] [PubMed] [Google Scholar]
- Petronczki, M., Siomos, M. F. and Nasmyth, K. (2003). Un menage a quatre: the molecular biology of chromosome segregation in meiosis. Cell 112, 423-440. [DOI] [PubMed] [Google Scholar]
- Revenkova, E., Eijpe, M., Heyting, C., Hodges, C. A., Hunt, P. A., Liebe, B., Scherthan, H. and Jessberger, R. (2004). Cohesin SMC1 beta is required for meiotic chromosome dynamics, sister chromatid cohesion and DNA recombination. Nat. Cell Biol. 6, 555-562. [DOI] [PubMed] [Google Scholar]
- Riedel, C. G., Katis, V. L., Katou, Y., Mori, S., Itoh, T., Helmhart, W., Galova, M., Petronczki, M., Gregan, J., Cetin, B. et al. (2006). Protein phosphatase 2A protects centromeric sister chromatid cohesion during meiosis I. Nature 441, 53-61. [DOI] [PubMed] [Google Scholar]
- Russell, L. D., Ettlin, R. A., Sinha Hikim, A. P. and Clegg, E. D. (1990). Histological and histopathological evaluation of the testis. Clearwater, FL: Cache River Press.
- Steigemann, P., Wurzenberger, C., Schmitz, M. H., Held, M., Guizetti, J., Maar, S. and Gerlich, D. W. (2009). Aurora B-mediated abscission checkpoint protects against tetraploidization. Cell 136, 473-484. [DOI] [PubMed] [Google Scholar]
- Stemmann, O., Zou, H., Gerber, S. A., Gygi, S. P. and Kirschner, M. W. (2001). Dual inhibition of sister chromatid separation at metaphase. Cell 107, 715-726. [DOI] [PubMed] [Google Scholar]
- Sullivan, M., Lehane, C. and Uhlmann, F. (2001). Orchestrating anaphase and mitotic exit: separase cleavage and localization of Slk19. Nat. Cell Biol. 3, 771-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan, M., Hornig, N. C., Porstmann, T. and Uhlmann, F. (2004). Studies on substrate recognition by the budding yeast separase. J. Biol. Chem. 279, 1191-1196. [DOI] [PubMed] [Google Scholar]
- Sumara, I., Vorlaufer, E., Stukenberg, P. T., Kelm, O., Redemann, N., Nigg, E. A. and Peters, J. M. (2002). The dissociation of cohesin from chromosomes in prophase is regulated by Polo-like kinase. Mol. Cell 9, 515-525. [DOI] [PubMed] [Google Scholar]
- Taieb, F. E., Gross, S. D., Lewellyn, A. L. and Maller, J. L. (2001). Activation of the anaphase-promoting complex and degradation of cyclin B is not required for progression from Meiosis I to II in Xenopus oocytes. Curr. Biol. 11, 508-513. [DOI] [PubMed] [Google Scholar]
- Tanaka, T. U., Stark, M. J. and Tanaka, K. (2005). Kinetochore capture and bi-orientation on the mitotic spindle. Nat. Rev. Mol. Cell Biol. 6, 929-942. [DOI] [PubMed] [Google Scholar]
- Terret, M. E., Wassmann, K., Waizenegger, I., Maro, B., Peters, J. M. and Verlhac, M. H. (2003). The meiosis I-to-meiosis II transition in mouse oocytes requires separase activity. Curr. Biol. 13, 1797-1802. [DOI] [PubMed] [Google Scholar]
- Tomonaga, T., Nagao, K., Kawasaki, Y., Furuya, K., Murakami, A., Morishita, J., Yuasa, T., Sutani, T., Kearsey, S. E., Uhlmann, F. et al. (2000). Characterization of fission yeast cohesin: essential anaphase proteolysis of Rad21 phosphorylated in the S phase. Genes Dev. 14, 2757-2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth, A., Rabitsch, K. P., Galova, M., Schleiffer, A., Buonomo, S. B. and Nasmyth, K. (2000). Functional genomics identifies monopolin: a kinetochore protein required for segregation of homologs during meiosis I. Cell 103, 1155-1168. [DOI] [PubMed] [Google Scholar]
- Tuveson, D. A., Shaw, A. T., Willis, N. A., Silver, D. P., Jackson, E. L., Chang, S., Mercer, K. L., Grochow, R., Hock, H., Crowley, D. et al. (2004). Endogenous oncogenic K-ras(G12D) stimulates proliferation and widespread neoplastic and developmental defects. Cancer Cell 5, 375-387. [DOI] [PubMed] [Google Scholar]
- Uhlmann, F., Lottspeich, F. and Nasmyth, K. (1999). Sister-chromatid separation at anaphase onset is promoted by cleavage of the cohesin subunit Scc1. Nature 400, 37-42. [DOI] [PubMed] [Google Scholar]
- Uhlmann, F., Wernic, D., Poupart, M. A., Koonin, E. V. and Nasmyth, K. (2000). Cleavage of cohesin by the CD clan protease separin triggers anaphase in yeast. Cell 103, 375-386. [DOI] [PubMed] [Google Scholar]
- Waizenegger, I., Gimenez-Abian, J. F., Wernic, D. and Peters, J. M. (2002). Regulation of human separase by securin binding and autocleavage. Curr. Biol. 12, 1368-1378. [DOI] [PubMed] [Google Scholar]
- Waizenegger, I. C., Hauf, S., Meinke, A. and Peters, J. M. (2000). Two distinct pathways remove mammalian cohesin from chromosome arms in prophase and from centromeres in anaphase. Cell 103, 399-410. [DOI] [PubMed] [Google Scholar]
- Xu, H., Beasley, M. D., Warren, W. D., van der Horst, G. T. and McKay, M. J. (2005). Absence of mouse REC8 cohesin promotes synapsis of sister chromatids in meiosis. Dev. Cell 8, 949-961. [DOI] [PubMed] [Google Scholar]
- Yanagida, M. (2005). Basic mechanism of eukaryotic chromosome segregation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 360, 609-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, X. W., Model, P. and Heintz, N. (1997). Homologous recombination based modification in Escherichia coli and germline transmission in transgenic mice of a bacterial artificial chromosome. Nat. Biotechnol. 15, 859-865. [DOI] [PubMed] [Google Scholar]
- Zou, H., Stemman, O., Anderson, J. S., Mann, M. and Kirschner, M. W. (2002). Anaphase specific auto-cleavage of separase. FEBS Lett. 528, 246-250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.