Summary
One of the most important processes in fertilization is the fusion of egg and sperm; however, the molecular mechanisms involved in this process are not well understood. So far, using genetic approaches, only two proteins have been demonstrated to be necessary for this process: Izumo in sperm and CD9 in the egg. Here we demonstrate that sperm produced by Tssk6 (Sstk)-null mice present defects that prevent the successful fertilization of eggs in vitro and the fusion to zona-pellucida-free eggs. Tssk6 is a member of the testis-specific serine kinase family of proteins and is expressed postmeiotically in male germ cells. In order for fusion to occur, during the process known as acrosome reaction Izumo needs to relocate from the anterior head to other regions, including the postacrosomal compartment. Tssk6-null sperm fails to relocate Izumo during the acrosome reaction. Agents that interfere with actin dynamics blocked the acrosome-reaction-associated translocation of Izumo that is required for fusion in wild-type sperm. Additionally, actin polymerization was compromised in Tssk6-null sperm. Taken together, our results indicate that Tssk6 is involved in sperm-egg fusion through the regulation of actin polymerization and changes in Izumo localization.
Keywords: Tssk6 (Sstk), Gamete fusion, Izumo, Fertilization, Acrosome reaction
Introduction
Spermatogenesis in the testis is responsible for the production of functional spermatozoa; this process is divided into three stages. Initially, spermatogenesis involves a proliferative phase in which spermatogonia undergo mitotic divisions and generate spermatocytes; second, the meiotic phase is responsible for generating haploid spermatids; finally, the postmeiotic phase, known as spermiogenesis, is characterized by a profound morphological differentiation that starts in the haploid spermatid and culminates in morphologically mature sperm. Spermiogenesis includes the formation of the acrosome, condensation and reorganization of the chromatin, elongation and species-specific reshaping of the cell, and the assembly of the flagellum (Sharpe, 1994). Although the molecular mechanisms regulating these events are poorly understood, it is known that, during spermiogenesis, there are significant changes in both transcription (Hecht, 1988) and translation (Hake et al., 1990). Some of the proteins translated in the haploid spermatid will remain in the morphologically mature sperm after it leaves the testis. Therefore, proteins that are synthesized during spermiogenesis might be necessary for spermatid differentiation and/or for sperm function during fertilization.
Given the importance of phosphorylation events in the regulation of cellular signaling processes and differentiation, it is not surprising that several protein kinases have been shown to be involved in spermatogenesis (Sassone-Corsi, 1997). Some of them are exclusively expressed in germ cells or in the testis (Jinno et al., 1993; Walden and Cowan, 1993; Nayak et al., 1998; Toshima et al., 1998; Tseng et al., 1998; Shalom and Don, 1999; Toshima et al., 1999). Among them, the testis-specific serine kinase (Tssk) gene family is postmeiotically expressed in male germ cells (Bielke et al., 1994; Kueng et al., 1997; Visconti et al., 2001). The conserved testicular expression pattern of Tssk genes as well as the importance of phosphorylation in signaling processes strongly suggest that kinases of the Tssk family have a role(s) in germ-cell differentiation and/or sperm function. The importance of Tssk(s) has been recently demonstrated by phenotypic analysis of Tssk6 (also known as Sstk) knock-out (KO) mice (Mus musculus) (Spiridonov et al., 2005). Male, but not female, Tssk6-null mice are infertile without exhibiting somatic abnormalities. In addition, recently, two groups reported the phenotype of the Tssk1 and Tssk2 double KO. Although one group reported lack of founders due to haploinsufficiency (Xu et al., 2008), the second group was able to obtain Tssk1- and Tssk2-null mice, and showed that these mice were sterile and no other defects were observed (Shang et al., 2007). Taken together, these reports suggest that, similar to Tssk6, Tssk1 and/or Tssk2 are essential for spermiogenesis and/or sperm function.
Tssk6-null mice have a percentage of sperm that are morphologically normal and are able to move progressively.. When in vitro fertilization (IVF) experiments were conducted, none of the eggs were fertilized yet intra-cytoplasmic sperm injection (ICSI) bypassed this defect. When IVF experiments were conducted using eggs in which the zonae pellucidae (ZP) were removed, they were incapable of fertilization owing to lack of sperm-egg fusion. At present, Izumo is the only sperm protein that has been conclusively demonstrated, using genetic approaches, to be necessary for sperm-egg fusion. Antibodies directed against Izumo were used to further explore the Tssk6-null sperm phenotype. In wild-type (WT) sperm, after the acrosome reaction takes place, Izumo changes the immunofluorescence pattern and distributes to the postacrosomal region; this change was not observed in Tssk6-null sperm. Additionally, Tssk6-null mice presented defects in actin polymerization as observed using fluorescent phalloidin staining. Altogether, these data indicate that Tssk6 plays a role in the changes of Izumo localization and that these changes are essential for gamete fusion.
Results
Tssk6-null sperm presents morphological defects
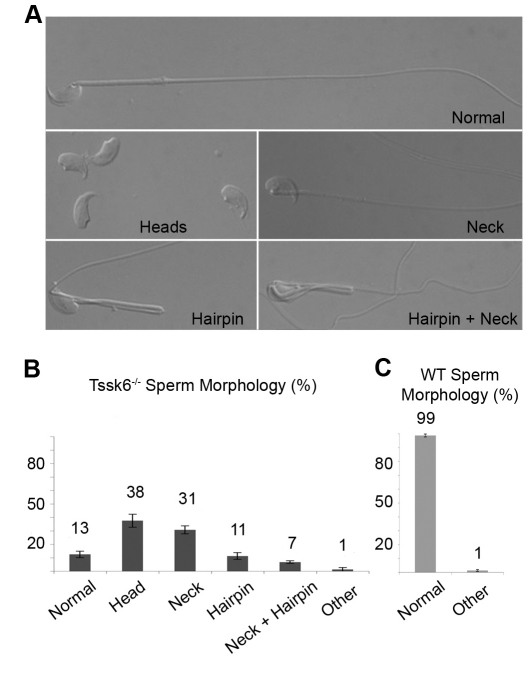
We have recently shown that human and mouse Tssk6 exhibits a very restricted testicular mRNA expression pattern (Hao et al., 2004; Spiridonov et al., 2005). The infertile phenotype of the Tssk6 null raised the issue of whether sperm lacking Tssk6 would present morphological defects. To analyze this possibility, cauda epididymal sperm from WT and Tssk6-null mice were compared using differential interference contrast (DIC) microscopy. The overall head morphology of Tssk6-null sperm did not differ from WT sperm. However, four other major morphological defects were found (Fig. 1). The first characteristic defect was a large number (37.5±4.8%) of detached heads (Fig. 1, Heads). Because a comparable number of detached tails could be found and many of these were still motile in fresh preparations, this defect appeared to be due to breakage of the connection between heads and tails. A second defect, an improper orientation of heads facing backwards that was observed in 30.8±3.0% of the sperm (Fig. 1, Neck), also indicated a defect in the connection between heads and tails. Third, 11.3±2.5% of the spermatozoa presented a hairpin at the level of the annulum (Fig. 1, Hairpin), and 6.8±1.0% presented a combined annular hairpin and the heads-facing-backwards morphology (Fig. 1, Hairpin+Neck). The remainder (12.5±2.4%) of the sperm presented normal morphology.
Fig. 1.
Tssk6-null sperm have morphological defects. (A) DIC-microscopy images of Tssk6-null cauda epididymal sperm. The images show morphological defects that include neck defects that cause sperm heads to point in the wrong direction (Neck), detached heads (Heads), annular hairpins (Hairpin) and a combination of reversed heads and hairpins (Hairpin+Neck). A total of 13% of the sperm have normal morphology (Normal). (B) Quantification of morphological anomalies of Tssk6-null mice sperm. (C) Quantification of control WT sperm morphology, expressed as percentage of the average ± s.d. (200 sperm were counted per experiment; n=6).
Tssk6-null sperm are incapable of fertilization
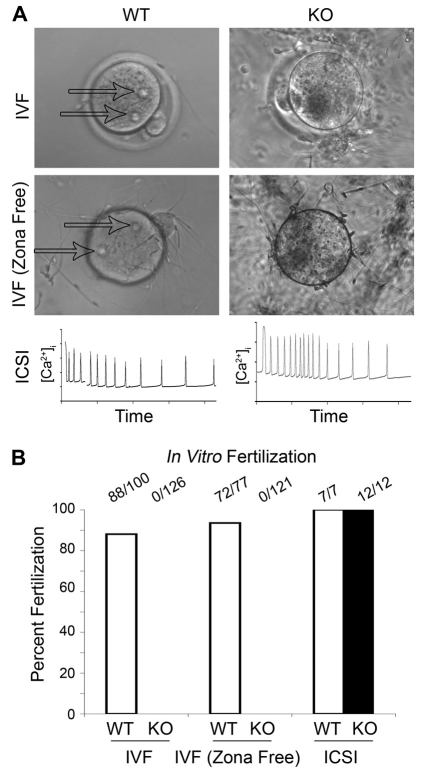
Although only about a tenth of the Tssk6-null sperm population presented normal morphology, such a number of normal sperm should be sufficient under optimal conditions to fertilize eggs in vitro. To test this possibility, IVF experiments were conducted. WT or Tssk6-null sperm were incubated with WT eggs and the efficiency of fertilization was analyzed by counting the percentage of eggs depicting pronuclear formation. In the case of WT sperm, the in vitro fertilization assay was conducted using 2×105 sperm per fertilization drop. Under these conditions, 88% of the eggs were fertilized. When Tssk6-null sperm were analyzed, no fertilization was observed even when 2×106 sperm per drop were used to allow over 2×105 morphologically normal sperm to be present in the fertilization assay (Fig. 2, IVF). By contrast, when egg activation was assessed after ICSI, in which one spermatozoon is physically injected into the eggs cytoplasm, there was no detectable difference between the sperm coming from the mutant mice in comparison with the WT controls. Egg activation was confirmed by measuring the fertilization-induced Ca2+ oscillations (Fig. 2, ICSI). These experiments indicate that Tssk6-null sperm are not capable to fertilize, despite the presence of a sufficient number of morphologically normal sperm.
Fig. 2.
Tssk6 is required for fertilization but not for egg activation. Sperm were obtained from either WT or Tssk6-null mice and used for in vitro fertilization assays as described in the Materials and Methods. (A) Representative images showing WT eggs with (IVF) or without zona pellucida (IVF-Zona Free) after 4 hours of IVF. Note the formation of the two pronuclei in the eggs fertilized with WT sperm (arrows) and the absence of fertilization in the eggs that were exposed to Tssk6-null sperm (KO). The lower panels (ICSI) depict characteristic intracellular Ca2+ transients, indicating equivalent egg activation in the eggs injected with the WT and Tssk6-null sperm. (B) Quantification of the experimental data from A. The data represent a combination of at least four independent experiments and are presented as a percentage of the total eggs used. The total number of eggs used is indicated atop each column.
Tssk6 is required for gamete fusion
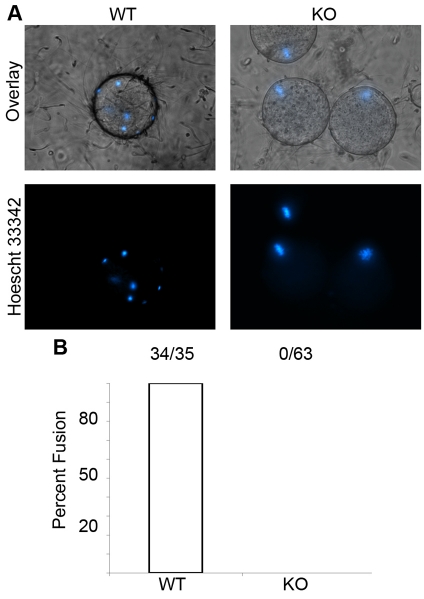
After penetration of ZP, the sperm adheres to the plasma membrane of the egg and fuses with it (Wortzman et al., 2006). When IVF experiments in which the ZP of the eggs were removed, it was noticed that Tssk6-null sperm were still unable to fertilize the egg (Fig. 2, IVF-zona free). To test whether this failure was due to an inability to fuse, ZP-free eggs were loaded with Hoechst 33342 prior to co-incubation with sperm, as described in the Materials and Methods. In this assay, sperm DNA would be labeled only if the sperm are fused to the egg plasma membrane. As expected, WT sperm fused to the eggs in less than 20 minutes (Fig. 3, WT). By contrast, Tssk6-null sperm failed to fuse even after 2 hours of incubation under identical conditions and with a similar number of sperm attached to the membrane, as judged by DIC imaging (Fig. 3, KO). Sperm from Tssk6-null mice were capable of undergoing spontaneous acrosome reaction similar to WT sperm (27±2% and 26±3%, respectively); therefore, the lack of fusion cannot be explained by the absence of acrosome-reacted sperm in the fertilization droplet.
Fig. 3.
Tssk6-null sperm cannot fuse with eggs. Eggs devoid of zona pellucida were loaded with the DNA stain Hoechst 33342 prior to IVF as described in the Materials and Methods. Fusion was assessed by transference of fluorescent Hoechst 33342 from the egg to the fused sperm and observed under DIC and epifluorescence microscopy at 20 and 120 minutes. (A) WT sperm fused to the eggs were stained with Hoechst 33342 after 20 minutes of incubation (WT). Tssk6-null sperm were not stained after 120 minutes incubation, indicating that fusion did not occur (KO). Note that the unfertilized eggs that did not undergo fusion maintain their chromatin aligned at metaphase II as seen in the positive staining (KO). (B) Quantification of the experiments was performed as in Fig. 2. The data represent a combination of four independent experiments and are presented as a percentage of the total eggs used.
Izumo fails to relocate after the acrosome reaction in the absence of Tssk6
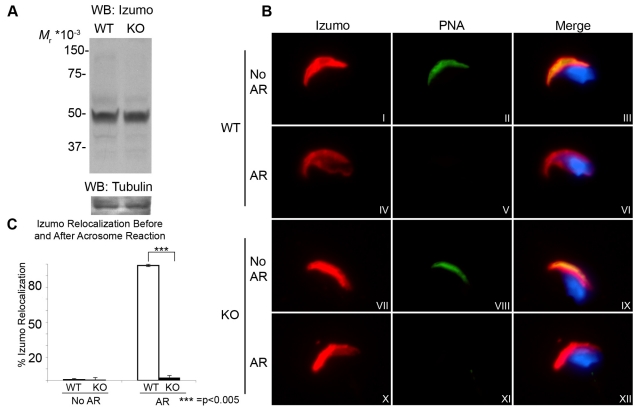
The molecular basis of sperm-egg interaction is not well understood (Primakoff and Myles, 2007). So far, only two molecules have been shown conclusively, using genetic approaches, to be necessary for sperm-egg fusion: the tetraspanin CD9 in the egg (Le Naour et al., 2000) and the immunoglobulin-loop-containing transmembrane protein Izumo in the sperm (Inoue et al., 2005). Taking into consideration that Izumo is the only characterized sperm protein necessary for gamete fusion, its presence in the fusion-deficient Tssk6-null sperm was investigated. Western blot analysis of cauda epididymal sperm from Tssk6-null mice showed no difference in Izumo protein levels when compared to WT mice (Fig. 4A). This experiment indicated that the inability of Tssk6-null sperm to fuse is not due to defects in the expression level of Izumo protein. Anti-Izumo antibodies were then used to localize Izumo in the sperm in conjunction with the peanut lectin PNA, which, in the mouse, stains acrosomal glycoproteins. In intact WT sperm, Izumo was restricted to the dorsal portion of the anterior head (Fig. 4B, panels I to III). After induction of the acrosome reaction with Ca2+ ionophore (A23187), Izumo distributed to a new region opposite to the anterior acrosome including part of the postacrosomal region (Fig. 4B, panels IV to VI). In Tssk6-null mice, similarly to WT, intact sperm also depicted an anterior acrosomal localization of Izumo (Fig. 4B, panels VII to IX). However, contrary to WT sperm, the change in the Izumo staining pattern after the acrosome reaction did not occur in Tssk6-null sperm and Izumo remained in the anterior sperm head (Fig. 4B, panels X to XII). This was not due to a failure of the acrosome reaction because both WT and KO sperm showed the typical loss of PNA staining (Fig. 4B, panels V and XI). Quantification of these results indicate that the change of localization of Izumo occurred in almost all (98.8±1.0%) the acrosome-reacted WT sperm, but essentially did not occur (2.5±1.7%) in Tssk6-null acrosome-reacted sperm (Fig. 4C). Similar values were obtained for spontaneously acrosome-reacted WT and Tssk6-null sperm (data not shown).
Fig. 4.
Izumo fails to relocate after the acrosome reaction in the absence of Tssk6. (A) Cauda epididymal sperm of WT and Tssk6-null (KO) mice was obtained as in the Materials and Methods, and total extracts were analyzed by western blot with anti-Izumo antibodies. The lower panel shows the same membrane re-probed with anti-tubulin mAb as a loading control. (B) Epifluorescence localization analysis of Izumo in WT sperm and Tssk6-null sperm (KO) in either intact sperm (No AR) or after Ca2+-ionophore-induced acrosome reaction (AR). Note that the PNA staining is lost in acrosome-reacted sperm of both WT and Tssk6-null mice. (C) The percentage of sperm presenting posterior head Izumo staining before (No AR) and after (AR) the acrosome reaction. Results represent the mean ± s.d. of four independent experiments in which 200 sperm were counted per condition (***P<0.005).
Tssk6 localizes to the sperm head
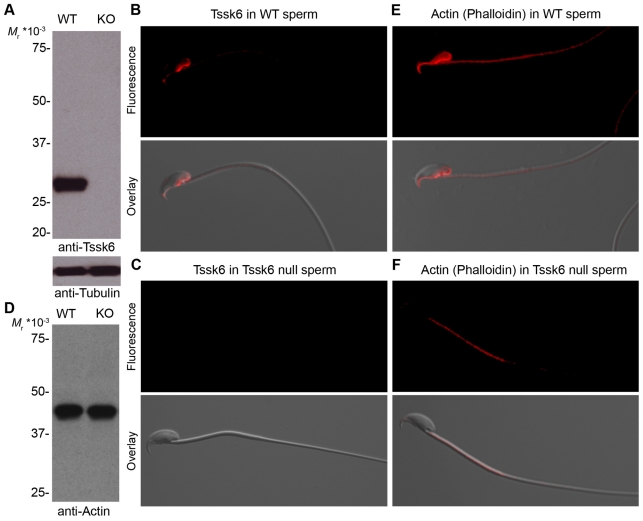
To investigate Tssk6 protein expression in sperm, a monoclonal antibody (mAb) against this molecule was used. Western blotting analysis of cauda epididymal sperm indicated that Tssk6 is maintained throughout sperm maturation and is present in mature sperm (Fig. 5A, lane WT). As a control for the specificity of the anti-Tssk6 mAb, samples from Tssk6-null sperm were also prepared and run in parallel. No signal was detected with this sample (Fig. 5A, lane KO). An epifluorescence microscopy study of WT sperm using the anti-Tssk6 mAb revealed a distribution pattern that included the posterior head and the perforatorium (Fig. 5B). As a control for the specificity of the mAb, identical analysis was done with Tssk6-null sperm and, as expected, no signal was detected (Fig. 5C).
Fig. 5.
Tssk6 and F-actin localize to the posterior head and perforatorium of WT sperm. (A) Cauda epididymal sperm proteins from either WT or Tssk6-null (KO) mice were extracted, separated by polyacrylamide gel electrophoresis, transferred to PVDF and analyzed by western blot with anti-Tssk6 mAb. The signal was observed by incubation with horseradish-peroxidase-labeled anti-mouse IgG antibodies and chemiluminescence reaction. The same membranes were then probed with anti-tubulin antibodies as loading controls (lower panel). (B,C) Sperm from either WT (B) or Tssk6-null (C) mice were fixed and stained with anti-Tssk6 mAbs as described in the Materials and Methods. (D) Western blot reveals comparable levels of total actin of WT and KO sperm. (E,F) Epifluorescence localization of polymerized actin as stained with fluorescently labeled phalloidin in either WT (E) or knock-out (F) sperm incubated for 1 hour in media that support capacitation. In all cases, bottom panels show the overlay with a DIC image for orientation reference. Experiments were repeated at least three times; representative images are shown.
Deficient actin polymerization in Tssk6-null sperm
Changes in the immunofluorescence pattern of sperm surface proteins during capacitation and acrosome reaction have been described previously (Miranda et al., 2009; Saxena et al., 1986; Primakoff and Myles, 2007; Selvaraj et al., 2007). It has been shown that changes in protein localization are at least in part due to actin-cytoskeleton dynamics (Saxena et al., 1986; Hernandez-Gonzalez et al., 2000). Because of the failure of Izumo to relocate following the acrosome reaction and the defects observed in the head-tail connection in Tssk6-null sperm, the distribution of the actin cytoskeleton in WT and Tssk6-null sperm was investigated. Fluorescent phalloidin was used to localize polymerized actin in capacitated sperm. As previously observed by other authors (Dvorakova et al., 2005; Etkovitz et al., 2007), polymerized actin was observed in the mid-piece and in a region that includes the posterior head and perforatorium of WT sperm (Fig. 5E). Interestingly, the localization of polymerized actin in the head is analogous to the one observed for Tssk6 (Fig. 5B). Furthermore, when phalloidin was used in Tssk6-null sperm, no signal for polymerized actin was observed in the head (Fig. 5F). Nevertheless, western blot analysis indicates that the total level of actin is comparable between WT and Tssk6-null sperm (Fig. 5D)
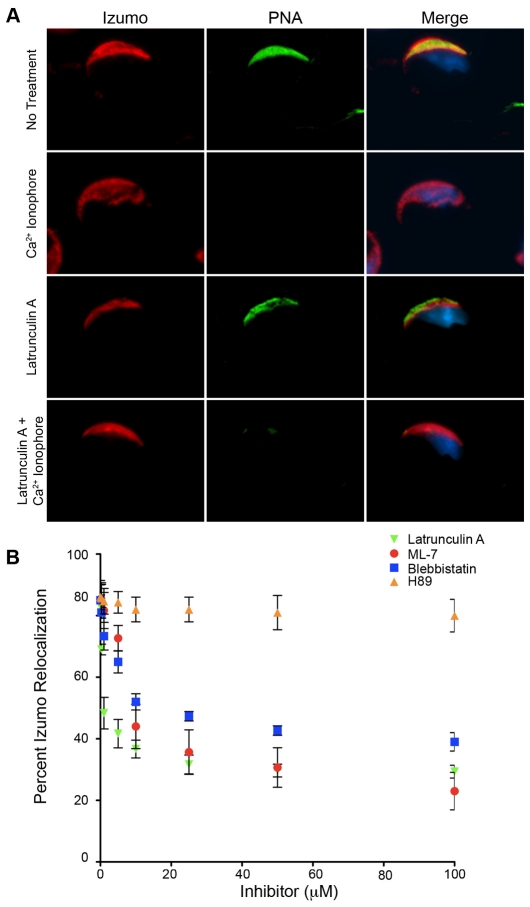
To investigate whether the failure of Izumo to relocate after the acrosome reaction in Tssk6-null sperm was related to the absence of polymerized actin in the sperm head, inhibitors of actin dynamics were used. Latrunculin A, an inhibitor of actin polymerization, caused a significant reduction in the redistribution of Izumo in acrosome-reacted sperm (Fig. 6). As a complementary assay, the myosin-II inhibitor blebbistatin and the myosin-light-chain-kinase inhibitor ML-7 were also used, with similar results (Fig. 6B). The PKA inhibitor H89 was used as a negative control; this inhibitor did not affect the acrosome-reaction-associated changes in Izumo localization (Fig. 6B).
Fig. 6.
Actin and myosin inhibitors block the relocation of Izumo. (A) Anti-Izumo fluorescent staining of WT sperm incubated for 1 hour in media that support capacitation either in the presence or absence of 5 μM latrunculin A. Izumo immunoreactivity examples of intact sperm (no treatment) and Ca2+-ionophore-induced acrosome-reacted sperm are shown. (B) WT sperm were incubated in capacitating medium containing increasing concentrations of latrunculin A, ML-7, blebbistatin or H89 for 1 hour. Then, 5 μM A23187 was added and the sperm incubated for 30 additional minutes. The sperm were then washed, fixed and anti-Izumo immunofluorescence analysis was conducted as described in the Materials and Methods. Points represent the percentage of acrosome-reacted sperm presenting posterior head Izumo staining. Results represent the mean ± s.d. of at least three independent experiments in which 200 sperm were counted per condition.
Discussion
The function of the Tssk kinase family is largely unknown. However, because members of this family are expressed postmeiotically during spermiogenesis, it is hypothesized that they play a role in germ-cell differentiation and/or sperm function. The focus of this study was to further analyze the male-infertility phenotype of Tssk6-null mice. Tssk6 was originally discovered using bioinformatic approaches and different names were given; among them, Tssk4 (Hao et al., 2004) and Sstk (Spiridonov et al., 2005). In mouse and human tissues, Tssk6 is almost exclusively expressed in the testis. Previously, it was demonstrated that Tssk6 interacts with Hsp90-1b, Hsc70 and Hsp70 proteins; this kinase is able to phosphorylate histones H1, H2A, H2AX and H3 but not H2B, H4 or transition protein 1 in vitro (Spiridonov et al., 2005). These results suggest that Tssk6 might have a role in the regulation of postmeiotic chromatin remodeling. Although a fraction of the Tssk6-null sperm population presents a normal morphology and is motile, Tssk6-null male mice are infertile (Spiridonov et al., 2005).
In the present work, we investigated whether sperm lacking Tssk6 are able to fertilize metaphase-II-arrested eggs in vitro. The central observations of this study are that: (1) a significant number of the Tssk6-null sperm present defects in the connection between the head and the flagellum; (2) Tssk6-null sperm are incapable of fusing with eggs; (3) Izumo, a protein known to be necessary for sperm-egg fusion, does not change its localization pattern in Tssk6-null sperm after the acrosome reaction; (4) in WT sperm, Tssk6 localizes to the posterior head, a region in the sperm enriched with polymerized actin; (5) actin polymerization is compromised in Tssk6-null sperm; and (6) inhibitors of actin dynamics abrogate significantly the acrosome-reaction-associated changes in Izumo localization. Taken together, these data indicate that Tssk6 is needed to maintain the sperm structural integrity and suggest that this kinase plays a role in the regulation of actin dynamics. In addition, these data indicate that Tssk6 is involved, either directly or indirectly, in the redistribution of Izumo after the acrosome reaction and that this relocalization might be required for sperm-egg fusion.
The sperm acrosome is a secretory vesicle containing a number of hydrolytic enzymes that help the sperm penetrate the coat of eggs. In response to either physiological or pharmacological stimuli, the sperm undergo a process known as the acrosome reaction, which involves an exocytotic event in which lytic enzymes are released from the acrosome upon binding with the extracellular matrix of an egg (De Blas et al., 2005; Mayorga et al., 2007). Successful completion of the acrosome reaction is an absolute prerequisite for fertilization.
Despite the importance of sperm-egg fusion, little is known about the molecular basis of this event. From the sperm side, the only protein conclusively demonstrated to be necessary in sperm-egg fusion is Izumo, a sperm-specific member of the immunoglobulin superfamily. Using a knockout approach, Inoue et al. showed that females lacking this protein were fertile; by contrast, Izumo-deficient males were sterile (Inoue et al., 2005). Sperm from Izumo-null mice did not seem to have defects in motility or morphology; however, these sperm were not able to fuse with the egg. Immunofluorescence analyses revealed that Izumo localization was restricted to the dorsal portion of the sperm acrosome in intact sperm. However, the sperm region involved in fusion is not the region at which Izumo is found before the acrosomal exocytosis. Although the exact sperm fusogenic region is not fully established, immunoelectromicroscopy experiments strongly suggest that sperm fuses to the egg through a region overlapping either the equatorial segment or the postacrosomal region depending on the species under study (Yanagimachi, 1994).
After the acrosome reaction, Izumo spreads, entering the post-acrosomal compartment, and permeates the borders of different domains, including a section opposite to the anterior acrosome previously referred as para-acrosomal (Yanagimachi, 1994). Different Izumo staining patterns have been previously observed (Okabe et al., 1987; Inoue et al., 2005) but their connection with antigen relocation caused by the acrosome reaction have not been analyzed in detail. In contrast to Izumo, other proteins reported to change the immunofluorescence pattern after the acrosome reaction seemed to relocalize to the equatorial segment and were not able to pass the post-acrosomal boundary (Myles and Primakoff, 1984; Rochwerger and Cuasnicu, 1992). The differential behavior of proteins during the acrosome reaction suggests that the acrosome-reaction-associated changes in protein redistribution might be an actively regulated process independent of exocytosis.
Although the actin cytoskeleton is widely believed to play an important role in intracellular protein transport, its role in the sperm physiology is poorly understood. The findings presented here indicate that: (1) contrary to WT, Tssk6-null sperm lack phalloidin staining in the head; and (2) Izumo in these sperm failed to appear in the postacrosomal region. These results argue that actin dynamics might be involved in the regulation of Izumo movements during the acrosome reaction. Consistent with this hypothesis is the intricate polymerization and depolymerization movements that have been described in the head of sperm during capacitation and the acrosome reaction (Brener et al., 2003; Dvorakova et al., 2005). In this respect, experimental evidence from different laboratories indicates that actin polymerization occurs during capacitation of boar (Sus scrofa), guinea pig (Cavia cobaya) (Castellani-Ceresa et al., 1993; Cabello-Agueros et al., 2003), mouse, human, bovine (Bos taurus) and ram (Ovis aries) sperm (Brener et al., 2003). These findings are in agreement with results presented here, in which the use of inhibitors of actin dynamics significantly prevented the acrosome-reaction-related changes in Izumo relocalization.
Tssk6-null spermatozoa might prove to be a good model to study the molecular basis of the acrosome reaction. In addition to exocytosis, it is well established that many proteins change their immunofluorescence pattern during the acrosome reaction (Saxena et al., 1986; Kawai et al., 1989). Results in the present work indicate that Tssk6-null sperm are capable of undergoing the acrosome reaction spontaneously or after treatment with Ca2+ ionophore; however, antigen relocation (e.g. Izumo) did not occur. The role of Tssk6 in these events could be due to its activity throughout spermiogenesis and acrosomal biogenesis or might be related to phosphorylation events occurring during capacitation and/or the acrosome reaction. As a working hypothesis, it can be speculated that the Tssk6-null phenotype underscores a role of Tssk6 in the regulation of the actin cytoskeleton. First, most sperm from Tssk6-null mice present structural defects in regions known to be rich in polymerized actin, such as the connective piece and the annulum. Second, in mature sperm, Tssk6 localizes to regions in the head that also stain for polymerized actin; it is noteworthy that a fraction of Izumo also localizes to this region after the acrosome reaction. Third, incubation of WT sperm with latrunculin A (an inhibitor of actin polymerization), blebbistatin (a myosin-II inhibitor) or ML-7 (an inhibitor of myosin light chain kinase) significantly reduced the changes in the immunolocalization pattern of Izumo in acrosome-reacted sperm. As part of this working model, we hypothesize that Tssk6-dependent changes in Izumo localization are essential for the ability of Izumo to regulate sperm-egg fusion.
It is important to stress that protein kinases have become major targets for the development of novel drugs. The observation that Tssk6-null mice are sterile and that Tssk6 is essential for fertilization and gamete fusion highlight the potential use of this kinase as a target for male contraception. Identification of Tssk6-specific inhibitors will be essential to further the understanding of the role of this kinase in reproduction.
Finally, the observations generated by this study demonstrate that, although the acrosome-reaction-associated exocytotic event was unaffected in Tssk6-null sperm, the relocalization of Izumo that normally occurs at this time failed to take place. This finding indicates that, although the initial signaling for both processes involved similar transduction molecules (e.g. Ca2+), the exocytosis process linked with the acrosome reaction can be dissociated from relocalization of Izumo. This work then begins to shed light on the complexity of the acrosome reaction, separating different elements that, because of their simultaneous nature, have been in the past studied as a single process.
Materials and Methods
Reagents, antibodies and media
Reagents and media were obtained from Sigma-Aldrich (St Louis, MO) unless otherwise specified. Antibodies against Izumo were donated by Masaru Okabe (Inoue et al., 2005). Anti-tubulin antibody clone E7 from the Klymkowsky laboratory was obtained from the Developmental Studies Hybridoma Bank, NICHD, NIH. Mouse monoclonal antibody against synthetic peptide corresponding to the C-terminal region (aa 218-273) of mouse Tssk6 was generated and purified with protein G immobilized on agarose from Pierce (Rockford, IL). Monoclonal antibody against actin clone AC-40 was purchased from Sigma-Aldrich (St Louis, MO). Alexa-Fluor-555-conjugated phalloidin and secondary antibodies, Alexa-Fluor-488-conjugated PNA, DAPI and Hoechst 33324 were obtained from Molecular Probes (Eugene, OR). Horseradish-peroxidase-conjugated secondary antibodies were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA). Ca2+ ionophore A23187, latrunculin A, blebbistatin, the myosin-light-chain-kinase inhibitor ML-7 and the PKA inhibitor H89 were obtained from Calbiochem (San Diego, CA).
Animal care and use
All procedures involving animal experimentation were conducted according to approved research animal protocols allowed by the University of Massachusetts Institutional Animal Care and Use Committee. CD1 male mice and B6D2F1 female mice were obtained from Charles River Laboratory, housed and manipulated according to protocol approved by the University of Massachusetts, Amherst's IACUC. Mice null for Tssk6 were bred from an existing colony according to the protocol approved by IACUC of the US FDA, CDER, and genotyped as previously published (Spiridonov et al., 2005).
Sperm sample preparation
Sperm samples were obtained in HEPES-buffered Whitten medium without BSA or NaHCO3 (Whitten and Biggers, 1968) at 37°C from epididymal cauda of Tssk6-null mice or WT littermate control animals. The caudae were squeezed under dissecting microscope in the media above to extract as much sperm as possible. The sperm was left in suspension for 7 minutes before further treatment (Hernandez-Gonzalez et al., 2006).
Western blots
Samples were re-suspended in non-reducing Laemmli buffer (Laemmli, 1970) and boiled for 5 minutes. The samples were then centrifuged at 10,000 g for 5 minutes and the supernatant was reduced for 2 minutes at 95°C with 5% β-mercapto-ethanol. Samples were then separated as described previously (Jha et al., 2006). Briefly, samples were separated in 10% SDS-PAGE at a constant current of 20 mA per gel until the dye front reached the end of the gel. The protein was then blotted to PVDF membranes in tris-glycine-methanol buffer for 60 minutes at a constant potential of 100 V. The membranes were then blocked for at least 1 hour with 5% non-fat dry milk in PBS-0.1% Tween-20. The membranes were then incubated with the appropriate antibody for no less than 1 hour, washed three times for 5 minutes with PBS-0.1% Tween-20, incubated with corresponding peroxidase-conjugated secondary antibody for 30 minutes and washed three times for 5 minutes each with PBS-0.1% Tween-20. The membranes were then developed using chemiluminescence.
Immunocytochemistry
Cells were obtained as previously described and allowed to adhere to clean glass slides in drops at room temperature for 10 minutes. The excess liquid was removed and the sperm fixed with either methanol-free 4% paraformaldehyde in PBS (for phalloidin staining) or 3.7% formaldehyde in PBS (containing up to 1.5% methanol, for antibody staining) for 5 minutes at room temperature. The fixing solution was then removed and the cells were permeabilized with 0.5% Triton X-100 in PBS for 5 minutes at room temperature. The permeabilizing solution was removed and the slides were blocked for not less than 2 hours with PBS 0.1% Tween-20 0.5% BSA. After blocking, the slides were incubated overnight at 4°C with the corresponding antibody in blocking buffer. The following day, the slides were washed ten times with PBS-0.1% Tween-20 and then incubated with the appropriate secondary antibody and desired counterstains diluted in blocking buffer for no less than 2 hours at room temperature. The slides were then washed ten times in PBS-0.1% Tween-20, mounted with SlowFade Light (Molecular Probes, Eugene, OR) mounting media, and left at 4°C until imaged. Imaging was performed with a Nikon Eclipse 200 inverted microscope equipped with DIC optics, epifluorescence, a CCD camera and Open Lab imaging software.
Mouse egg preparation
Metaphase-II eggs were obtained from B6D2F1 (C57BL/6J × DBA/2J) female mice (6- to 10-weeks old) superovulated by injection of 5 IU of serum gonadotropin from a pregnant mare (PMSG; Sigma, St Louis, MO) followed 48 hours later by 5 IU of human chorionic gonadotropin (hCG; Sigma). Eggs were collected from the oviduct 14 hours post-hCG, and were washed with HEPES-buffered Tyrode-lactate solution (Parrish et al., 1988) with 5% heat-inactivated fetal calf serum (FCS; Gibco, BRL, Grand Island, NY). Cumulus cells were removed with 0.1% bovine testes hyaluronidase (Sigma, St Louis, MO). In experiments requiring eggs without zona pellucida, the eggs were treated with Tyrode's Acidic Solution and zona-free eggs were then washed in Tyrode-lactate solution and left to recover in fertilization medium (see bellow M16 medium) for at least 30 minutes at 37°C in a 5% CO2 incubator.
IVF
IVF assays were performed following published protocols (Hogan et al., 1986) with slight modifications. Briefly, sperm were obtained as described above and left to capacitate in CO2-equilibrated M16 media (M7292; Sigma, St Louis, MO) overlaid with light mineral oil in a 37°C incubator with 5% CO2 for 90 minutes. Eggs were obtained as described above and separated in CO2-equilibrated M16 media drops overlaid with light mineral oil in a 37°C incubator with 5% CO2. Sperm were then added to the egg drops at final concentrations ranging from 105 sperm/ml to 2×106 sperm/ml and incubated for 4 hours. Eggs were then washed off sperm by passing them through consecutive pre-equilibrated M16 media drops and left at 37°C until scored for fertilization.
ICSI
The ICSI procedure was carried out as previously described (Yoon and Fissore, 2007) using Narishige manipulators (Medical Systems Corp., NY) mounted on a Nikon diaphot microscope (Nikon, Tokyo, Japan). All manipulations were carried out in a 50 μl drop of HEPES-buffered CZB medium (Chatot et al., 1990) containing 0.1% polyvinyl alcohol (PVA, Mr=30-70×103, Sigma) under light mineral oil at room temperature. Sperm were diluted 1:1 in medium containing 12% w/v polyvinylpyrrolidone (PVP, Mr=360×103, Sigma). A single sperm was aspirated into a blunt-ended pipette driven by a PiezoDrill (Burleigh, Rochester, NY), and several Piezo pulses were applied to separate the head from the tail. The zona pellucida and plasma membrane of the egg were penetrated by applying Piezo pulses. The sperm head was then released into the cytoplasm of the egg from the pipette. Different intensity pulses were used to penetrate the zona pellucida and plasma membrane.
[Ca2+]i monitoring
Monitoring of intracellular Ca2+ concentration ([Ca2+]i) was performed as described before (Yoon and Fissore, 2007). Briefly, eggs were incubated in TL-HEPES supplemented with 1 μM Fura-2-acetoxymethyl ester (Fura-2-AM; Molecular Probes, Eugene, OR) and 0.02% pluronic acid (Molecular Probes) for 20 minutes at room temperature, and were then transferred into 300 μl of TL-HEPES on a glass-bottom culture dish (MatTek Corporation, Ashland, MA) covered with light mineral oil for fluorescence imaging. [Ca2+]i changes were monitored simultaneously using a 20× objective lens on a Nikon Diaphot inverted microscope (Nikon) equipped with a temperature-controlled stage (20/20 Technology, Wilmington, NC) and a xenon lamp (Osram, Germany). The excitation wavelength was alternated between 340 nm and 380 nm by a computer-controlled filter wheel (Ludl Electronic Products, Hawthorne, NY) and the emission of light was passed through a 510-nm barrier filter and collected with cool SNAP ES digital camera (Roper Scientific, Tucsan, AZ). Captured images were taken every 20 seconds and analyzed by SimplePCI software (Compix Imaging, Cranberry, PA). [Ca2+]i values are reported as the ratio of 340:380-nm fluorescence in the whole egg.
Fusion assays
Sperm-egg fusion assay was optimized using CD1 mice owing to their cost and availability. Previously published protocols with slight modifications were used (Conover and Gwatkin, 1988; Wortzman et al., 2006). Briefly, sperm were obtained as above and left to capacitate as described for the IVF assays. Eggs were obtained as above and, after zona removal, left in M16 media to equilibrate and recuperate for 30 minutes, after which the eggs were moved to a new drop of M16 media containing 1 μg/ml of Hoechst 33342 stain and incubated for 30 minutes, after which period the eggs were washed in three consecutive M16 media drops. The main modification to the published protocols was the addition of a de-stain step of 45 minutes in a fresh pre-equilibrated M16 media drop and a new three-drop wash of the eggs before transfer to the assay drop. Sperm was then added to the assay drop in concentrations ranging from 2×104 sperm/ml to 2×105 sperm/ml. The eggs were then scored for fusion at 20 minutes and 120 minutes using an epifluorescence microscope.
Inhibitor and ionophore treatments
For the analysis of Izumo localization, sperm were incubated in HEPES-buffered Whitten medium containing 5 mg/ml of BSA and 10 mM NaHCO3 for 60 minutes at 37°C. The acrosome reaction was then induced with Ca2+ ionophore A23187 for 30 minutes at 37°C. When inhibitors were used, the appropriate inhibitor was included in the media from the beginning of the incubation. The different inhibitors were used in the range of 0 to 100 μM (Boyle et al., 2001; Valderrama et al., 2001; Nolan et al., 2004; Matson et al., 2006).
Data expression and statistical analysis
IVF, ICSI and fusion experiments were pooled and data expressed as a percentage of the total number of eggs used. The number of eggs used can be found atop each column. Because these experiments are expressed on a percent basis, no error bars are presented. In experiments in which error bars are presented, the bars represent one standard deviation (s.d.). In order to assess significance, the Student's t-test was used.
We thank Masaru Okabe (Osaka University, Japan) for his generous contribution of anti-Izumo antibodies. We also thank Dominique Alfandari and Anna Maria Salicioni (University of Massachusetts, Amherst) for their input. This study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development through grants NIH HD038082, NIH HD044044 and NIH HD050341 (to P.E.V.) and NIH F31HD049324 (to J.S.). Deposited in PMC for release after 12 months.
References
- Bielke, W., Blaschke, R. J., Miescher, G. C., Zurcher, G., Andres, A. C. and Ziemiecki, A. (1994). Characterization of a novel murine testis-specific serine/threonine kinase. Gene 139, 235-239. [DOI] [PubMed] [Google Scholar]
- Boyle, J. A., Chen, H. and Bamburg, J. R. (2001). Sperm incorporation in Xenopus laevis: characterisation of morphological events and the role of microfilaments. Zygote 9, 167-181. [DOI] [PubMed] [Google Scholar]
- Brener, E., Rubinstein, S., Cohen, G., Shternall, K., Rivlin, J. and Breitbart, H. (2003). Remodeling of the actin cytoskeleton during mammalian sperm capacitation and acrosome reaction. Biol. Reprod. 68, 837-845. [DOI] [PubMed] [Google Scholar]
- Cabello-Agueros, J. F., Hernandez-Gonzalez, E. O. and Mujica, A. (2003). The role of F-actin cytoskeleton-associated gelsolin in the guinea pig capacitation and acrosome reaction. Cell Motil. Cytoskeleton 56, 94-108. [DOI] [PubMed] [Google Scholar]
- Castellani-Ceresa, L., Mattioli, M., Radaelli, G., Barboni, B. and Brivio, M. F. (1993). Actin polymerization in boar spermatozoa: fertilization is reduced with use of cytochalasin D. Mol. Reprod. Dev. 36, 203-211. [DOI] [PubMed] [Google Scholar]
- Chatot, C. L., Lewis, J. L., Torres, I. and Ziomek, C. A. (1990). Development of 1-cell embryos from different strains of mice in CZB medium. Biol. Reprod. 42, 432-440. [DOI] [PubMed] [Google Scholar]
- Conover, J. C. and Gwatkin, R. B. (1988). Pre-loading of mouse oocytes with DNA-specific fluorochrome (Hoechst 33342) permits rapid detection of sperm-oocyte fusion. J. Reprod. Fertil. 82, 681-690. [DOI] [PubMed] [Google Scholar]
- De Blas, G. A., Roggero, C. M., Tomes, C. N. and Mayorga, L. S. (2005). Dynamics of SNARE assembly and disassembly during sperm acrosomal exocytosis. PLoS Biol. 3, e323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorakova, K., Moore, H. D., Sebkova, N. and Palecek, J. (2005). Cytoskeleton localization in the sperm head prior to fertilization. Reproduction 130, 61-69. [DOI] [PubMed] [Google Scholar]
- Etkovitz, N., Rubinstein, S., Daniel, L. and Breitbart, H. (2007). Role of PI3-kinase and PI4-kinase in actin polymerization during bovine sperm capacitation. Biol. Reprod. 77, 263-273. [DOI] [PubMed] [Google Scholar]
- Hake, L. E., Alcivar, A. A. and Hecht, N. B. (1990). Changes in mRNA length accompany translational regulation of the somatic and testis-specific cytochrome c genes during spermatogenesis in the mouse. Development 110, 249-257. [DOI] [PubMed] [Google Scholar]
- Hao, Z., Jha, K. N., Kim, Y. H., Vemuganti, S., Westbrook, V. A., Chertihin, O., Markgraf, K., Flickinger, C. J., Coppola, M., Herr, J. C. et al. (2004). Expression analysis of the human testis-specific serine/threonine kinase (TSSK) homologues. A TSSK member is present in the equatorial segment of human sperm. Mol. Hum. Reprod. 10, 433-444. [DOI] [PubMed] [Google Scholar]
- Hecht, N. B. (1988). Post-meiotic gene expression during spermatogenesis. Prog. Clin. Biol. Res. 267, 291-313. [PubMed] [Google Scholar]
- Hernandez-Gonzalez, E. O., Lecona-Valera, A. N., Escobar-Herrera, J. and Mujica, A. (2000). Involvement of an F-actin skeleton on the acrosome reaction in guinea pig spermatozoa. Cell Motil. Cytoskeleton 46, 43-58. [DOI] [PubMed] [Google Scholar]
- Hernandez-Gonzalez, E. O., Sosnik, J., Edwards, J., Acevedo, J. J., Mendoza-Lujambio, I., Lopez-Gonzalez, I., Demarco, I., Wertheimer, E., Darszon, A. and Visconti, P. E. (2006). Sodium and epithelial sodium channels participate in the regulation of the capacitation-associated hyperpolarization in mouse sperm. J. Biol. Chem. 281, 5623-5633. [DOI] [PubMed] [Google Scholar]
- Hogan, B., Costantini, F. and Lacy, E. (1986). Manipulating The Mouse Embryo: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
- Inoue, N., Ikawa, M., Isotani, A. and Okabe, M. (2005). The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature 434, 234-238. [DOI] [PubMed] [Google Scholar]
- Jha, K. N., Salicioni, A. M., Arcelay, E., Chertihin, O., Kumari, S., Herr, J. C. and Visconti, P. E. (2006). Evidence for the involvement of proline-directed serine/threonine phosphorylation in sperm capacitation. Mol. Hum. Reprod. 12, 781-789. [DOI] [PubMed] [Google Scholar]
- Jinno, A., Tanaka, K., Matsushime, H., Haneji, T. and Shibuya, M. (1993). Testis-specific mak protein kinase is expressed specifically in the meiotic phase in spermatogenesis and is associated with a 210-kilodalton cellular phosphoprotein. Mol. Cell. Biol. 13, 4146-4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai, Y., Hama, T., Mayumi, T., Okabe, M., Matzno, S., Kohama, Y. and Mimura, T. (1989). Flow cytometric analysis of mouse sperm using monoclonal anti-sperm antibody OBF13. J. Reprod. Immunol. 16, 71-82. [DOI] [PubMed] [Google Scholar]
- Kueng, P., Nikolova, Z., Djonov, V., Hemphill, A., Rohrbach, V., Boehlen, D., Zuercher, G., Andres, A. C. and Ziemiecki, A. (1997). A novel family of serine/threonine kinases participating in spermiogenesis. J. Cell Biol. 139, 1851-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680-685. [DOI] [PubMed] [Google Scholar]
- Le Naour, F., Rubinstein, E., Jasmin, C., Prenant, M. and Boucheix, C. (2000). Severely reduced female fertility in CD9-deficient mice. Science 287, 319-321. [DOI] [PubMed] [Google Scholar]
- Matson, S., Markoulaki, S. and Ducibella, T. (2006). Antagonists of myosin light chain kinase and of myosin II inhibit specific events of egg activation in fertilized mouse eggs. Biol. Reprod. 74, 169-176. [DOI] [PubMed] [Google Scholar]
- Mayorga, L. S., Tomes, C. N. and Belmonte, S. A. (2007). Acrosomal exocytosis, a special type of regulated secretion. IUBMB Life 59, 286-292. [DOI] [PubMed] [Google Scholar]
- Miranda, P. V., Allaire, A., Sosnik, J. and Visconti, P. E. (2009). Localization of low-density detergent-resistant membrane proteins in intact and acrosome-reacted mouse sperm. Biol. Reprod. 80, 897-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myles, D. G. and Primakoff, P. (1984). Localized surface antigens of guinea pig sperm migrate to new regions prior to fertilization. J. Cell Biol. 99, 1634-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak, S., Galili, N. and Buck, C. A. (1998). Immunohistochemical analysis of the expression of two serine-threonine kinases in the maturing mouse testis. Mech. Dev. 74, 171-174. [DOI] [PubMed] [Google Scholar]
- Nolan, M. A., Babcock, D. F., Wennemuth, G., Brown, W., Burton, K. A. and McKnight, G. S. (2004). Sperm-specific protein kinase A catalytic subunit Calpha2 orchestrates cAMP signaling for male fertility. Proc. Natl. Acad. Sci. USA 101, 13483-13488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe, M., Adachi, T., Takada, K., Oda, H., Yagasaki, M., Kohama, Y. and Mimura, T. (1987). Capacitation-related changes in antigen distribution on mouse sperm heads and its relation to fertilization rate in vitro. J. Reprod. Immunol. 11, 91-100. [DOI] [PubMed] [Google Scholar]
- Parrish, J. J., Susko-Parrish, J., Winer, M. A. and First, N. L. (1988). Capacitation of bovine sperm by heparin. Biol. Reprod. 38, 1171-1180. [DOI] [PubMed] [Google Scholar]
- Primakoff, P. and Myles, D. G. (2007). Cell-cell membrane fusion during mammalian fertilization. FEBS Lett. 581, 2174-2180. [DOI] [PubMed] [Google Scholar]
- Rochwerger, L. and Cuasnicu, P. S. (1992). Redistribution of a rat sperm epididymal glycoprotein after in vitro and in vivo capacitation. Mol. Reprod. Dev. 31, 34-41. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi, P. (1997). Transcriptional checkpoints determining the fate of male germ cells. Cell 88, 163-166. [DOI] [PubMed] [Google Scholar]
- Saxena, N., Peterson, R. N., Sharif, S., Saxena, N. K. and Russell, L. D. (1986). Changes in the organization of surface antigens during in-vitro capacitation of boar spermatozoa as detected by monoclonal antibodies. J. Reprod. Fertil. 78, 601-614. [DOI] [PubMed] [Google Scholar]
- Selvaraj, V., Buttke, D. E., Asano, A., McElwee, J. L., Wolff, C. A., Nelson, J. L., Klaus, A. V., Hunnicutt, G. R. and Travis, A. J. (2007). GM1 dynamics as a marker for membrane changes associated with the process of capacitation in murine and bovine spermatozoa. J. Androl. 28, 588-599. [DOI] [PubMed] [Google Scholar]
- Shalom, S. and Don, J. (1999). Tlk, a novel evolutionarily conserved murine serine threonine kinase, encodes multiple testis transcripts. Mol. Reprod. Dev. 52, 392-405. [DOI] [PubMed] [Google Scholar]
- Shang, P., Baarends, W. M., Dooijes, D., Elfferich, P., Wijgerde, M., Ooms, M. P., Looijenga, L. H., Dohle, G. R., Van Eenennaam, H., Gossen, J. A. et al. (2007). Testis-specific serine/threonine kinases encoded by the retrogenes Tssk1 and Tssk2 are essential for spermatogenesis in mouse. In XIX North American Testis Workshop: “Chromosome Structure and Gene Expression”, p. 115. Tampa, FL: Hyatt Regency.
- Sharpe, R. M. (1994). Regulation of spermatogenesis. In The Physiology of Reproduction (ed. E. Knobil and J. D. Neill), pp. 1363-1434. New York: Raven Press.
- Spiridonov, N. A., Wong, L., Zerfas, P. M., Starost, M. F., Pack, S. D., Paweletz, C. P. and Johnson, G. R. (2005). Identification and characterization of SSTK, a serine/threonine protein kinase essential for male fertility. Mol. Cell. Biol. 25, 4250-4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toshima, J., Koji, T. and Mizuno, K. (1998). Stage-specific expression of testis-specific protein kinase 1 (TESK1) in rat spermatogenic cells. Biochem. Biophys. Res. Commun. 249, 107-112. [DOI] [PubMed] [Google Scholar]
- Toshima, J., Tanaka, T. and Mizuno, K. (1999). Dual specificity protein kinase activity of testis-specific protein kinase 1 and its regulation by autophosphorylation of serine-215 within the activation loop. J. Biol. Chem. 274, 12171-12176. [DOI] [PubMed] [Google Scholar]
- Tseng, T. C., Chen, S. H., Hsu, Y. P. and Tang, T. K. (1998). Protein kinase profile of sperm and eggs: cloning and characterization of two novel testis-specific protein kinases (AIE1, AIE2) related to yeast and fly chromosome segregation regulators. DNA Cell Biol. 17, 823-833. [DOI] [PubMed] [Google Scholar]
- Valderrama, F., Duran, J. M., Babia, T., Barth, H., Renau-Piqueras, J. and Egea, G. (2001). Actin microfilaments facilitate the retrograde transport from the Golgi complex to the endoplasmic reticulum in mammalian cells. Traffic 2, 717-726. [DOI] [PubMed] [Google Scholar]
- Visconti, P. E., Hao, Z., Purdon, M. A., Stein, P., Balsara, B. R., Testa, J. R., Herr, J. C., Moss, S. B. and Kopf, G. S. (2001). Cloning and chromosomal localization of a gene encoding a novel serine/threonine kinase belonging to the subfamily of testis-specific kinases. Genomics 77, 163-170. [DOI] [PubMed] [Google Scholar]
- Walden, P. D. and Cowan, N. J. (1993). A novel 205-kilodalton testis-specific serine/threonine protein kinase associated with microtubules of the spermatid manchette. Mol. Cell. Biol. 13, 7625-7635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitten, W. K. and Biggers, J. D. (1968). Complete development in vitro of the pre-implantation stages of the mouse in a simple chemically defined medium. J. Reprod. Fertil. 17, 399-401. [DOI] [PubMed] [Google Scholar]
- Wortzman, G. B., Gardner, A. J. and Evans, J. P. (2006). Analysis of Mammalian sperm-egg membrane interactions during in vitro fertilization. Methods Mol. Biol. 341, 89-101. [DOI] [PubMed] [Google Scholar]
- Xu, B., Hao, Z., Jha, K. N., Zhang, Z., Urekar, C., Digilio, L., Pulido, S., Strauss, J. F., 3rd, Flickinger, C. J. and Herr, J. C. (2008). Targeted deletion of Tssk1 and 2 causes male infertility due to haploinsufficiency. Dev. Biol. 319, 211-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagimachi, R. (1994). Fertility of mammalian spermatozoa: its development and relativity. Zygote 2, 371-372. [DOI] [PubMed] [Google Scholar]
- Yoon, S. Y. and Fissore, R. A. (2007). Release of phospholipase C zeta and [Ca2+]i oscillation-inducing activity during mammalian fertilization. Reproduction 134, 695-704. [DOI] [PubMed] [Google Scholar]