Abstract
In hypertension there is an autonomic imbalance in which sympathetic activity dominates over parasympathetic control. Parasympathetic activity to the heart originates from cardiac vagal neurons located in the nucleus ambiguus. Pre-sympathetic neurons that project to sympathetic neurons in the spinal cord are located in the ventral brainstem in close proximity to cardiac vagal neurons, and many of these pre-sympathetic neurons are catecholaminergic. In addition to their projection to the spinal cord, many of these pre-sympathetic neurons have axon collaterals that arborize into neighboring cardio-respiratory locations and likely release norepinephrine onto nearby neurons. Activation of α2 adrenergic receptors in the central nervous system evokes a diverse range of physiological effects, including reducing blood pressure. This study tests whether clonidine, an α2 adrenergic receptor agonist, alters excitatory glutamatergic, and/or inhibitory GABAergic or glycinergic synaptic neurotransmission to cardiac vagal neurons in the nucleus ambiguus. Cardiac vagal neurons were identified in a in-vitro brainstem slice preparation and synaptic events were recording using whole cell voltage clamp methodologies. Clonidine significantly inhibited GABAergic neurotransmission, but had no effect on glycinergic or glutamatergic pathways to cardiac vagal neurons. This diminished inhibitory GABAergic neurotransmission to cardiac vagal neurons would increase parasympathetic activity to the heart, decreasing heart rate and blood pressure. The results presented here provide a cellular substrate for the clinical use of clonidine as a treatment for hypertension as well as a role in alleviating post-traumatic stress disorder by evoking an increase in parasympathetic cardiac vagal activity, and a decrease in heart rate and blood pressure.
Keywords: ambiguus, clonidine, adrenergic, cardiac, vagal, parasympathetic
1. Introduction
Heart rate is determined by the activity of premotor cardioinhibitory vagal neurons (CVNs) located in the nucleus ambiguus. CVNs are the origin of parasympathetic innervation to the heart and dominate the control of heart rate (Mendelowitz, 1999; Mendelowitz and Kunze, 1991). CVNs are intrinsically silent, and their activity is determined by synaptic activation from GABAergic, glycinergic and glutamatergic neurons, among others(Mendelowitz, 1996; Wang et al., 2001). In many cardiovascular diseases, including hypertension and heart failure, cardiac vagal activity is reduced and unresponsive(Vanoli et al., 1991).
In hypertension there is an autonomic imbalance in which sympathetic activity dominates over parasympathetic control. Pre-sympathetic neurons that project to sympathetic neurons in the spinal cord are located in the ventral brainstem in close proximity to CVNs, and many of these pre-sympathetic neurons are catecholaminergic (Loewy and Spyer, 1990). In addition to their projection to the spinal cord, many of these pre-sympathetic neurons have axon collaterals that arborize into neighboring cardio-respiratory locations. These axon collaterals likely release norepinephrine, as well as glutamate onto nearby neurons (Lipski et al., 1996).
Clonidine, an α2 adrenergic agonist, is used clinically to treat hypertension. Activation of α2 adrenergic receptors in the central nervous system evokes a diverse range of physiological effects, including reducing blood pressure and increasing sinus arrhythmia(Toader et al., 2009). Activation of α2 adrenergic receptors with agonists, such as clonidine, inhibits adenylyl cyclase activity, resulting in a decrease in presynaptic Ca2+ concentrations and inhibition of norepinephrine release. Activation of α2 adrenergic receptors in the ventro-lateral medulla elicits a decrease in brainstem sympathetic activity (Philipp et al., 2002).
Recent studies have shown that activation of α2 receptors with clonidine significantly inhibits GABAergic inhibitory post-synaptic currents (IPSCs) in spinally projecting paraventricular nucleus neurons that can be prevented by the α2 receptor antagonist yohimbine (Li et al., 2005). In contrast, clonidine has been shown to have little effect on glutamatergic excitatory post-synaptic currents (EPSCs) or miniature EPSCs (mEPSCs) in these spinally projecting paraventricular nucleus neurons (Li et al., 2005).
Despite its clinical significance, the effect of α2 adrenergic receptor activation on synaptic inputs to parasympathetic cardiac vagal neurons has not yet been studied. The aim of the present study was to test whether activation of α2 adrenergic receptors modulates excitatory glutamatergic, and/or inhibitory GABAergic or glycinergic synaptic neurotransmission to cardiac vagal neurons in the nucleus ambiguus.
2. RESULTS
Application of the α2 adrenergic receptor agonist clonidine (10 μM) did not change holding current in CVNs, but evoked a significant decrease in the frequency of GABAergic neurotransmission to CVNs, see figure 1 (from 5.6±0.9 Hz to 4.7±0.9 Hz, n=9, P<0.05). In addition, clonidine (10 μM) significantly diminished the amplitude of GABAergic events in CVNs (from 61.5±8.1 pA to 49.8±4.0 pA, Fig. 1, n=9, P<0.05). To test if this inhibition of GABAergic neurotransmission evoked by clonidine was due to activating α2 adrenergic receptors, the selective α2 adrenergic receptor antagonist yohimbine (5 μM) was applied prior to clonidine application. Yohimbine by itself evoked a significant decrease in GABAergic IPSC frequency from 5.1±1.0 Hz to 2.4±0.8 Hz, see figure 2, top (n=9, P<0.05). Subsequent bath application of clonidine (10 μM) in the presence of the yohimbine bath did not significantly change the frequency or amplitude of GABAergic events, see figure 2, top (n=9, P>0.05). While yohimbine prevented the responses to clonidine, as expected, it was surprising that yohimbine by itself inhibited GABAergic IPSC frequency. Although the mechanism responsible for the inhibition of GABAergic neurotransmission by yohimbine is unknown, it is likely the result of yohimbine mediated 5-HT1A receptor activation as yohimbine has also been shown to activate 5-HT1A receptors. (Newman-Tancredi et al., 1998). An additional series of experiments were conducted with the more selective α2 adrenergic receptor antagonist atipasmezole (1 μM), see figure 2, bottom. Atipamezole, as did yohimbine, prevented the inhibition of GABAergic neurotransmission to CVNs elicited by the alpha-2 receptor agonist clonidine. Atipamezole also elicited a much smaller, but statistically significant, inhibition of GABA ISPC frequency. This is consistent with atipazmezole having a higher selectivity for alpha-2 receptors over 5-HT1A receptors than yohimbine, but both antagonists have some potency for activation of 5HT1A receptors (Newman-Tancredi et al., 1998).
Figure 1.
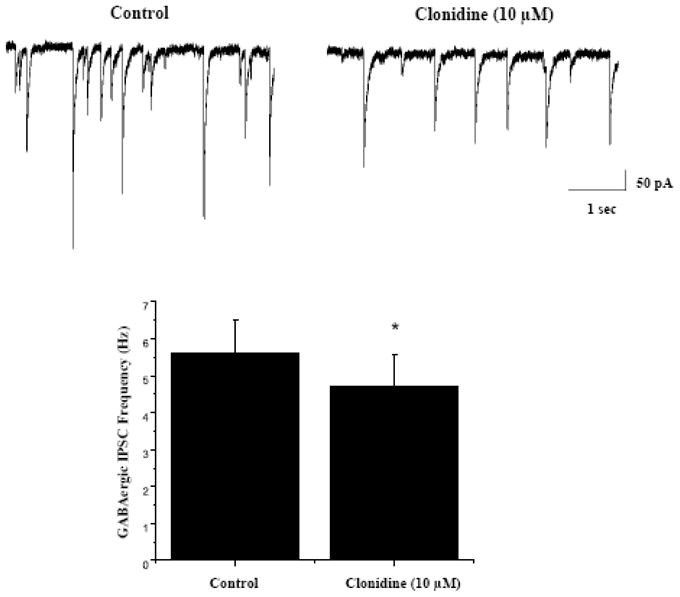
As shown in representative traces, top, and the average results from 9 CVNs, bottom, inclusion of the α2 adrenergic receptor agonist clonidine (10 μM) in the perfusate evoked a significant decrease in the frequency of GABAergic neurotransmission to CVNs (from 5.6±0.9 Hz to 4.7±0.9 Hz, p<0.05).
Figure 2.
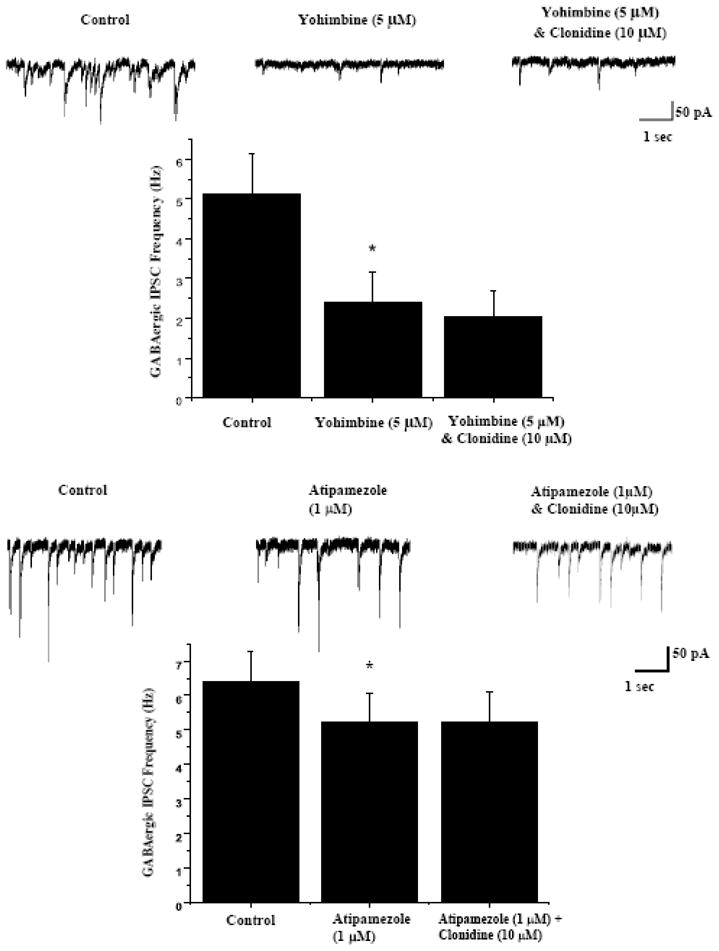
The selective α2 adrenergic receptor antagonist yohimbine (5 μM) was applied prior to clonidine application to examine if there is any endogenous activation of α2 adrenergic receptors and if the responses to clonidine could be blocked by the α2 adrenergic receptor antagonist yohimbine. As shown in representative traces, top, and the average results from 9 CVNs, bottom, yohimbine prevented the inhibition by clonidine, but surprisingly, also evoked a significant decrease in GABAergic IPSC frequency (from 5.1±1.0 Hz to 2.4±0.8 Hz, p<0.05) when applied by itself.
To further study the mechanism by which clonidine and activation of alpha-2 receptors modulates GABAergic neurotransmission to CVNs, the voltage-gated sodium channel blocker tetrodotoxin (TTX, 1μM) was included in the perfusate. Inclusion of TTX abolished action potential dependent synaptic neurotransmission and limited the remaining events to spontaneous miniature inhibitory post-synaptic currents (mIPSCs). There was no significant change in either GABAergic mIPSC frequency or amplitude upon application of clonidine in the presence of TTX (control: 2.6±0.6 Hz, clonidine: 2.4±0.5 Hz; See Fig. 3, n=14, P>0.05).
Figure 3.
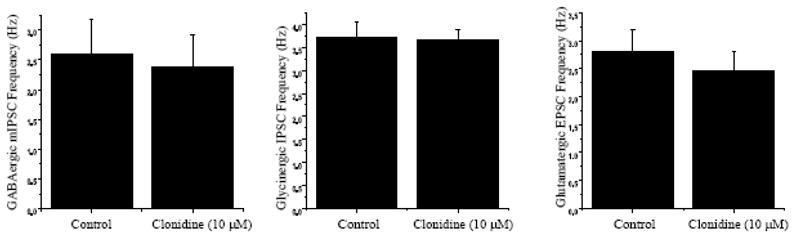
To further study the mechanism by which clonidine modulates GABAergic neurotransmission to CVNs and isolate GABAergic mIPSCs TTX was included in the perfusate. As shown in the left panel, in the presence of TTX there was no significant change in GABAergic mIPSC frequency upon application of clonidine. As shown in the middle panel, clonidine had no significant effect on glycinergic IPSCs in CVNs, and as shown in the right panel, application of clonidine did not produce any significant change in the frequency of glutamatergic EPSC events.
To further characterize the role of clonidine on inhibitory neurotransmission to CVNs, clonidine (10 μM) was applied while glycinergic IPSCs were isolated. Clonidine did not elicit any significant change in glycinergic IPSC frequency or amplitude (Control: 3.4±0.3 Hz, Clonidine: 3.5±0.2 Hz; See Fig. 3, n=12, P>0.05).
The effect of clonidine (10 μM) was also tested on excitatory neurotransmission to CVNs. Application of clonidine, while isolating for glutamatergic EPSCs, did not produce any significant change in the frequency or amplitude of EPSC events (Control: 2.8±0.4 Hz, Clonidine: 2.5±0.3 Hz; See Fig. 3, n=17, P>0.05).
3. DISCUSSION
Despite the clinical significance of α2 adrenergic receptors in brainstem cardiovascular control, little is known about the role of α2 adrenergic receptors in modulating synaptic neurotransmission to parasympathetic CVNs that dominate the control of heart rate. Our results demonstrate that activation of α2 adrenergic receptors with clonidine decreases GABAergic neurotransmission to CVNs, but clonidine has no significant effect on CVN holding current, or glycinergic or glutamatergic neurotransmission to CVNs. These results focused on CVNs are similar to the effects of clonidine in other neurons. In Purkinje cells located in the cerebellum as well parvocellular neurons located in hypothalamic paraventricular nuclei, the primary action of clonidine was decreased GABAergic neurotransmission (Han et al., 2002; Hirono and Obata, 2006).
Since clonidine changed GABAergic IPSCs but not GABAergic mIPSCs in CVNs, it is unlikely clonidine directly activates pre-synaptic receptors at GABAergic nerve terminals surrounding CVNs. Rather, the decrease in GABAergic IPSCs is most likely the result of activation of α2 adrenergic receptors located precedent to the GABAergic synapse upon CVNs, perhaps at the GABAergic cell body or prior synapses in the GABAergic brainstem pathway. As there are only a few selective sites of origin of GABAergic neurons that project to CVNs, it is likely α2 adrenergic receptors alters the activity of GABAergic neurons originating in either the immediate vicinity of the nucleus ambiguus and/or in the area of the nucleus tractus solitarius that contain GABAergic neurons that project to CVNs (Frank et al., 2009).
In order to further test if the decrease in GABAergic IPSCs resulted from activation of α α2 adrenergic receptors, the selective α2 adrenergic receptor antagonist yohimbine was applied prior to clonidine application. As expected, yohimbine prevented the clonidine mediated inhibition. However surprisingly there was a significant decrease in GABAergic IPSCs seen upon application of yohimbine by itself. Although the mechanism responsible for the inhibition by yohimbine is unknown, it is likely the result of yohimbine mediated 5-HT1A receptor activation. Although generally considered an α2 adrenergic receptor antagonist, yohimbine has also been shown to have significant 5-HT1A receptor affinity (Newman-Tancredi et al., 1998). Prior work has shown activation of 5-HT1A receptors cause significant decreases in GABAergic transmission to CVNs(Wang et al., 2007). Therefore, the significant decreases in GABAergic events upon application of yohimbine can most likely be attributed to 5-HT1A receptor activation. The more selective α2 adrenergic receptor antagonist atipasmezole also prevented the inhibition of GABAergic neurotransmission to CVNs elicited by the alpha-2 receptor agonist clonidine. Atipamezole also elicited a much smaller, but statistically significant, inhibition of GABA ISPC frequency. This is consistent with atipazmezole having a higher selectivity for alpha-2 receptors over 5-HT1A receptors than yohimbine, but both antagonists have some potency for activation of 5HT1A receptors (Newman-Tancredi et al., 1998).
The origin and location of adrenergic neurons and synaptic terminals that activate the α2 adrenergic receptors and alter GABAergic neurotransmission to CVNs described in this study are unknown. However one intriguing potential source of adrenergic input is the pre-sympathetic neurons that are located in the ventral brainstem in close proximity to CVNs. Many of these pre-sympathetic neurons are catecholaminergic (Loewy and Spyer, 1990) and possess axon collaterals that arborize into other cardiorespiratory medullary regions, including within the nucleus ambiguus, in addition to their projection to sympathetic neurons in the spinal cord (Standish et al., 1995; Ter Horst et al., 1996; Ter Horst et al., 1993). Adrenergic (including α2 and β1 subtypes) receptors have been found within the nucleus ambiguus and shown to play a role in parasympathetic control of cardiovascular function (Gurtu et al., 1983; Paschalis et al., 2009).
Clonidine is clinically useful as an anti-hypertension treatment, and also has been shown to be beneficial in the treatment of post-traumatic stress disorder (Boehnlein and Kinzie, 2007). Our results show the likely site of action of clonidine in the brainstem is via diminishing GABAergic neurotransmission to CVNs. This diminished inhibitory neurotransmission to CVNs would increase parasympathetic activity to the heart, decreasing heart rate and blood pressure. Although the mechanism by which clonidine affects sympathetic control has been previously examined, this is the first evidence suggesting a mechanism by which activation of α2 adrenergic receptors in the brainstem increase parasympathetic activity. The results presented here provide a cellular substrate for the clinical use of clonidine as a treatment for hypertension as well as a role in alleviating post-traumatic stress disorder by evoking an increase in parasympathetic cardiac vagal activity, and a decrease in heart rate and blood pressure.
4. Experimental Procedure
Individual CVNs located in the nucleus ambiguus were identified by the presence of the fluorescent tracer, and differential interference contrast optics along with infrared illumination and infrared-sensitive video detection cameras were used to gain better spatial resolution. The pipettes were filled with a solution consisting of KCl (150 mM), MgCl2 (4 mM), EGTA (10 mM), Na-ATP (2 mM), HEPES (10mM) at a pH of 7.3 for inhibitory GABAergic and glycinergic events, and CsCl (130 mM), HEPES (10 mM), EGTA (10 mM), CaCl2 (1 mM), and MgCl2 (1 mM) at a pH of 7.3 for recording glutamatergic events. CVNs were studied by means of the whole-cell patch-clamp technique and were voltage clamped at a holding potential of −80 mV.
GABAergic IPSCs were isolated by adding strychnine (1μM), a glycinergic receptor antagonist, 6-cyano-7-nitroquinoxaline-2,3-dione ([CNQX] 50μM), a non-NMDA antagonist, and D-2-Amino-5 phosphonovalerate ([AP5] 50μM), a NMDA receptor antagonist, to the perfusate. Gabazine (25μM), a GABAA receptor antagonist, CNQX (50μM) and AP5 (50μM) were included in the perfusate to isolate glycinergic IPSCs. Glutamatergic EPSCs were isolated by adding gabazine (25μM) and strychnine (1μM). In addition to gabazine and strychnine, (TTX) (1μM) was added to the perfusate in order to block action potential dependent events and isolate mEPSCs or mIPSCs. Drugs were applied after a 5–10 min control period and only one experiment was conducted in each slice. Clonidine (10 μM), an α2 agonist, was applied for a period of 5 min by inclusion in the perfusate. In two different sets of experiments yohimbine (5μM), and atipamezole (1 μM), both α2 adrenergic receptor antagonists, were added to the perfusate 5 min before the addition of clonidine (10μM). Concentrations of agonists and antagonists were chosen based on their pharmacological profiles and common dosage in the literature. Preliminary results were conducted with different concentrations of clonidine (ranging from 0.1 to 100 μM) and 10 μM clonidine provided the most consistent and non-saturating responses in our preliminary experiments. MiniAnalysis (Synaptosoft version 4.3.1) was used to analyze all experiments. The threshold for the GABAergic, glycinergic and glutamatergic events was a threshold of 5 times the root mean square of noise. Results are presented as mean ± S.E. and statistically compared with a paired Student’s t-test (for significance of difference *P<0.05), except for the experiments with yohimbine in which an ANOVA with repeated measures was performed.
In an initial surgery, 2–5 day old Sprague-Dawley rats were anesthetized with hypothermia to slow the heart and aid in recovery. A right thoracotomy was performed to expose the heart and the retrograde tracer, rhodamine (XRITC, Molecular Probes, 2% solution, 20–50μL), was then injected into the pericardial sac to retrogradely label CVNs.
On the day of the experiment, one to three days after the injection of the fluorescent tracer, the animal was anesthetized with isofluorane and sacrificed by cervical dislocation. The brain was rapidly removed and immersed in a cold HEPES buffer (4°C) with the following composition: NaCl (140 mM), KCl (5 mM), CaCl2 (2 mM), glucose (5 mM), HEPES (10 mM). The buffer was continuously oxygenated with 100% O2. Using a dissection microscope, the hindbrain was isolated. The brain was glued to a stage and placed in the slicing chamber of a vibratome filled with the same buffer solution. Coronal slices 500–600 μm in thickness were cut. The slices were then mounted in a perfusion chamber and submerged in a perfusate with the following composition: NaCl (125 mM), KCl (3 mM), CaCl2 (2 mM), NaHCO3 (26 mM), glucose (5 mM), HEPES (5 mM) oxygenated with 95% O2/5% CO2 gas mixture. The osmolarity of all solutions was 285–290 mosM, and the pH was maintained between 7.35 and 7.4. All animal procedures were performed in compliance with the institutional guidelines at The George Washington University and were in accordance with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association and the NIH publication (85–23, revised 1996) ‘Guide for the Care and Use of Laboratory Animals’. The minimal number of animals was used and attention was given to minimize any possible discomfort.
Acknowledgments
Supported by NIH grants HL49965, 59895 and 72006 to D.M.
Abbreviations
- CVNs
cardioinhibitory vagal neurons
- IPSCs
inhibitory post-synaptic currents
- EPSCs
excitatory post-synaptic currents
- mEPSCs
miniature EPSCs
- mIPSCs
miniature IPSCs
- TTX
tetrodotoxin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Boehnlein JK, Kinzie JD. Pharmacologic reduction of CNS noradrenergic activity in PTSD: the case for clonidine and prazosin. J Psychiatr Pract. 2007;13:72–78. doi: 10.1097/01.pra.0000265763.79753.c1. [DOI] [PubMed] [Google Scholar]
- Frank JG, Jameson HS, Gorini C, Mendelowitz D. Mapping and identification of GABAergic neurons in transgenic mice projecting to cardiac vagal neurons in the nucleus ambiguus using photo-uncaging. J Neurophysiol. 2009;101:1755–1760. doi: 10.1152/jn.91134.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurtu S, Sharma DK, Sinha JN, Bhargava KP. Evidence for involvement of alpha 2-adrenoceptors in the nucleus ambiguous in baroreflex-mediated bradycardia. Naunyn Schmiedebergs Arch Pharmacol. 1983;323:199–204. doi: 10.1007/BF00497663. [DOI] [PubMed] [Google Scholar]
- Han SK, Chong W, Li LH, Lee IS, Murase K, Ryu PD. Noradrenaline excites and inhibits GABAergic transmission in parvocellular neurons of rat hypothalamic paraventricular nucleus. J Neurophysiol. 2002;87:2287–2296. doi: 10.1152/jn.2002.87.5.2287. [DOI] [PubMed] [Google Scholar]
- Hirono M, Obata K. Alpha-adrenoceptive dual modulation of inhibitory GABAergic inputs to Purkinje cells in the mouse cerebellum. J Neurophysiol. 2006;95:700–708. doi: 10.1152/jn.00711.2005. [DOI] [PubMed] [Google Scholar]
- Li DP, Atnip LM, Chen SR, Pan HL. Regulation of synaptic inputs to paraventricular-spinal output neurons by alpha2 adrenergic receptors. J Neurophysiol. 2005;93:393–402. doi: 10.1152/jn.00564.2004. [DOI] [PubMed] [Google Scholar]
- Lipski J, Kanjhan R, Kruszewska B, Rong W. Properties of presympathetic neurones in the rostral ventrolateral medulla in the rat: an intracellular study “in vivo’. J Physiol. 1996;490(Pt 3):729–744. doi: 10.1113/jphysiol.1996.sp021181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewy AD, Spyer KM. Central Regulation of Autonomic Functions. Oxford University Press; 1990. [Google Scholar]
- Mendelowitz D. Firing properties of identified parasympathetic cardiac neurons in nucleus ambiguus. Am J Physiol. 1996;271:H2609–2614. doi: 10.1152/ajpheart.1996.271.6.H2609. [DOI] [PubMed] [Google Scholar]
- Mendelowitz D. Advances in Parasympathetic Control of Heart Rate and Cardiac Function. News Physiol Sci. 1999;14:155–161. doi: 10.1152/physiologyonline.1999.14.4.155. [DOI] [PubMed] [Google Scholar]
- Mendelowitz D, Kunze DL. Identification and dissociation of cardiovascular neurons from the medulla for patch clamp analysis. Neurosci Lett. 1991;132:217–221. doi: 10.1016/0304-3940(91)90305-d. [DOI] [PubMed] [Google Scholar]
- Newman-Tancredi A, Nicolas JP, Audinot V, Gavaudan S, Verriele L, Touzard M, Chaput C, Richard N, Millan MJ. Actions of alpha2 adrenoceptor ligands at alpha2A and 5-HT1A receptors: the antagonist, atipamezole, and the agonist, dexmedetomidine, are highly selective for alpha2A adrenoceptors. Naunyn Schmiedebergs Arch Pharmacol. 1998;358:197–206. doi: 10.1007/pl00005243. [DOI] [PubMed] [Google Scholar]
- Paschalis A, Churchill L, Marina N, Kasymov V, Gourine A, Ackland G. beta1-Adrenoceptor distribution in the rat brain: an immunohistochemical study. Neurosci Lett. 2009;458:84–88. doi: 10.1016/j.neulet.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Philipp M, Brede M, Hein L. Physiological significance of alpha(2)-adrenergic receptor subtype diversity: one receptor is not enough. Am J Physiol Regul Integr Comp Physiol. 2002;283:R287–295. doi: 10.1152/ajpregu.00123.2002. [DOI] [PubMed] [Google Scholar]
- Standish A, Enquist LW, Escardo JA, Schwaber JS. Central neuronal circuit innervating the rat heart defined by transneuronal transport of pseudorabies virus. J Neurosci. 1995;15:1998–2012. doi: 10.1523/JNEUROSCI.15-03-01998.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ter Horst GJ, Hautvast RW, De Jongste MJ, Korf J. Neuroanatomy of cardiac activity-regulating circuitry: a transneuronal retrograde viral labelling study in the rat. Eur J Neurosci. 1996;8:2029–2041. doi: 10.1111/j.1460-9568.1996.tb00723.x. [DOI] [PubMed] [Google Scholar]
- Ter Horst GJ, Van den Brink A, Homminga SA, Hautvast RW, Rakhorst G, Mettenleiter TC, De Jongste MJ, Lie KI, Korf J. Transneuronal viral labelling of rat heart left ventricle controlling pathways. Neuroreport. 1993;4:1307–1310. doi: 10.1097/00001756-199309150-00005. [DOI] [PubMed] [Google Scholar]
- Toader E, Cividjian A, Quintin L. Recruitment of cardiac parasympathetic activity: effects of clonidine on cardiac vagal motoneurones, pressure lability, and cardiac baroreflex slope in rats. Br J Anaesth. 2009;102:322–330. doi: 10.1093/bja/aen390. [DOI] [PubMed] [Google Scholar]
- Vanoli E, De Ferrari GM, Stramba-Badiale M, Hull SS, Jr, Foreman RD, Schwartz PJ. Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ Res. 1991;68:1471–1481. doi: 10.1161/01.res.68.5.1471. [DOI] [PubMed] [Google Scholar]
- Wang J, Irnaten M, Mendelowitz D. Characteristics of spontaneous and evoked GABAergic synaptic currents in cardiac vagal neurons in rats. Brain Res. 2001;889:78–83. doi: 10.1016/s0006-8993(00)03112-7. [DOI] [PubMed] [Google Scholar]
- Wang X, Dergacheva O, Kamendi H, Gorini C, Mendelowitz D. 5-Hydroxytryptamine 1A/7 and 4alpha receptors differentially prevent opioid-induced inhibition of brain stem cardiorespiratory function. Hypertension. 2007;50:368–376. doi: 10.1161/HYPERTENSIONAHA.107.091033. [DOI] [PubMed] [Google Scholar]


