Abstract
Background
Myocyte contractile dysfunction occurs in pathological remodeling in association with abnormalities in calcium regulation. Mice with cardiac myocyte-specific overexpression of Gαq develop progressive left ventricular (LV) failure associated with myocyte contractile dysfunction and calcium dysregulation. We tested the hypothesis that myocyte contractile dysfunction in the Gαq mouse heart is mediated by reactive oxygen species (ROS), and in particular, oxidative post-translational modifications (OPTM) that impair the function of sarcoplasmic reticulum Ca++-ATPase (SERCA).
Methods and Results
Freshly isolated ventricular myocytes from Gαq mice had marked abnormalities of myocyte contractile function and calcium transients. In Gαq myocardium, SERCA protein was not altered in quantity, but displayed evidence of oxidative cysteine modifications reflected by decreased biotinylated iodoacetamide labeling, and evidence of specific irreversible oxidative modifications consisting of sulfonylation at cysteine 674 and nitration at tyrosines 294/295. Maximal calcium-stimulated SERCA activity was decreased 47% in Gαq myocardium. Cross-breeding Gαq mice with transgenic mice that have cardiac myocyte-specific overexpression of catalase a) decreased SERCA oxidative cysteine modifications, b) decreased SERCA cysteine 674 sulfonylation and tyrosine 294/295 nitration, c) restored SERCA activity, and d) improved myocyte calcium transients and contractile function.
Conclusions
In Gαq-induced cardiomyopathy, myocyte contractile dysfunction is mediated, at least in part, by one or more OPTM of SERCA. Protein OPTM contribute to the pathophysiology of myocardial dysfunction, and thus may provide a target for therapeutic intervention.
Keywords: Cardiac myocytes, sarcoplasmic reticulum ATPase, SERCA, oxidative modification
Introduction
Myocyte contractile dysfunction occurs in several models of pathological remodeling including pressure overload 1–4 and after myocardial infarction 5–8. While myocyte dysfunction appears to be caused, at least in part, by abnormalities in calcium regulation 9, the underlying mechanism remains unclear. There is evidence that reactive oxygen species (ROS) mediate some aspects of pathological myocardial remodeling including myocyte hypertrophy and apoptosis 10–12. Mice with cardiac myocyte-specific overexpression of Gαq develop progressive left ventricular (LV) dilation and failure 13 that is associated with myocyte contractile dysfunction and calcium dysregulation 14. These mice have increased oxidative stress in the myocardium 15, and recently, we demonstrated that concomitant myocyte-specific expression of catalase ameliorated pathological LV remodeling, inhibited myocyte hypertrophy and apoptosis, and preserved LV contractile function 16. Accordingly, we tested the hypothesis that myocyte contractile dysfunction in the Gαq mouse heart is also mediated by ROS, and in particular, involves oxidative post-translational modifications (OPTM) that impair the function of sarcoplasmic reticulum Ca++-ATPase (SERCA) 17.
Methods
Detailed methods are provided in the online supplement. Briefly, transgenic mice with cardiac myocyte-specific overexpression of Gαq (Gαq-40 mice, FVB/N) 13 and WT (FVB/N) mice were cross-bred with transgenic mice having myocyte-specific overexpression of catalase 18, as we previously described 16. Myocytes were isolated, and contraction and intracellular calcium transients were measured as we have described previously 19. SERCA2 activity was measured using calcium-stimulated, thapsigargin-inhibitable calcium45 uptake in an SR membrane preparation by a modification of published methods 14;20, as we have described 21. BIAM-labeling, immunoblotting, and immunohistochemical detection of SERCA OPTM are described in the online supplement. All data are presented as mean ± SEM.
Results
Concurrent myocyte-specific catalase overexpression ameliorates contractile dysfunction and calcium dysregulation in myocytes from Gαq mice
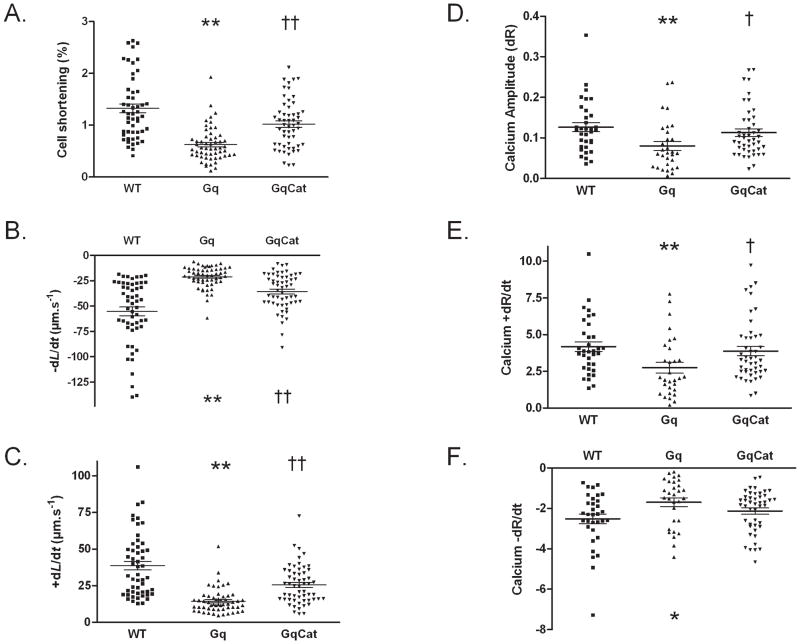
Ventricular myocytes were isolated from mice with myocyte-specific overexpression of Gαq 13–16 and myocyte contractile function and intracellular calcium transients were assessed, as we have described 19;21. In myocytes from Gαq mice (vs. WT), the amplitude of cell shortening was decreased by 53%, and the rates of myocyte shortening and relaxation were reduced by 62% and 63%, respectively (Figure 1, A–C). Likewise, in Gαq myocytes the calcium transient amplitude was decreased by 37%, and the rates of rise and decline were decreased by 34% and 33%, respectively (Figure 1, D–F). Gαq mice were cross-bred with mice that have myocyte-specific overexpression of catalase 18, as we have described 16. In myocytes from Gαq/catalase mice, the amplitude of cell shortening, and the rates of cell shortening and relaxation were improved (Figure 1, A–C); and the abnormalities in calcium transient amplitude and kinetics were ameliorated (Figure 1, D–F).
Figure 1.
Abnormal contractile function and intracellular calcium transients in cardiac myocytes from Gαq overexpressing mice are ameliorated by cross-breeding with mice that overexpress catalase in the myocardium. Ventricular myocytes were isolated from wild-type (WT), Gαq (Gq) or Gαq/catalase (GqCat) mice. Panel A. Cell shortening (% of baseline). Panel B. Velocity of contraction (−dL/dt). Panel C. Velocity of relaxation (+dL/dt). Panel D. Calcium transient amplitude (delta of the ratio (R) of fluorescence 360/380nm). Panel E. Rate of calcium transient rise (+dR/dt). Panel F. Rate of calcium transient decline (−dR/dt). *p<0.05 vs. WT; **p<0.01 vs. WT; †p<0.05 vs. Gαq; ††p<0.01 vs. Gαq; 5–10 cells per heart, 4–5 hearts per group.
Expression of calcium regulating proteins in Gαq myocardium
The protein levels of SERCA, the ryanodine receptor (RyR), phospholamban (PLB) and the sodium/calcium exchanger (NCX) were determined by immunoblotting. RyR protein expression was decreased by 47% in Gαq mice, whereas the expression of SERCA, PLB and NCX was unchanged (Table 1). The decrease in RyR protein was associated with a 42% decrease in mRNA (Online Figure I), suggesting that the decrease in protein was mediated at the transcript level. Concurrent expression of catalase in Gαq/catalase mice had no effect on RyR protein or transcript levels (Table 1; Online Figure I).
Table 1.
Total protein expression of calcium handling proteins in WT and Gαq mice.
| Total protein | |||
|---|---|---|---|
| WT | Gαq | Gαq/Cat | |
| SERCA | 1 ± 0.07 | 1.05 ± 0.07 | 0.99 ± 0.11 |
| RyR | 1 ± 0.09 | 0.53 ± 0.01* | 0.54 ± 0.10* |
| PLB | 1 ± 0.05 | 1.23 ± 0.31 | ND |
| NCX | 1 ± 0.08 | 0.95 ± 0.15 | ND |
Total protein is expressed as the ratio of the protein of interest/GAPDH, normalized to the average value in WT group. Data are the means of 4 hearts in each group (
p<0.05 vs. WT mice).
ND = not done.
OPTM of SERCA in Gαq myocardium
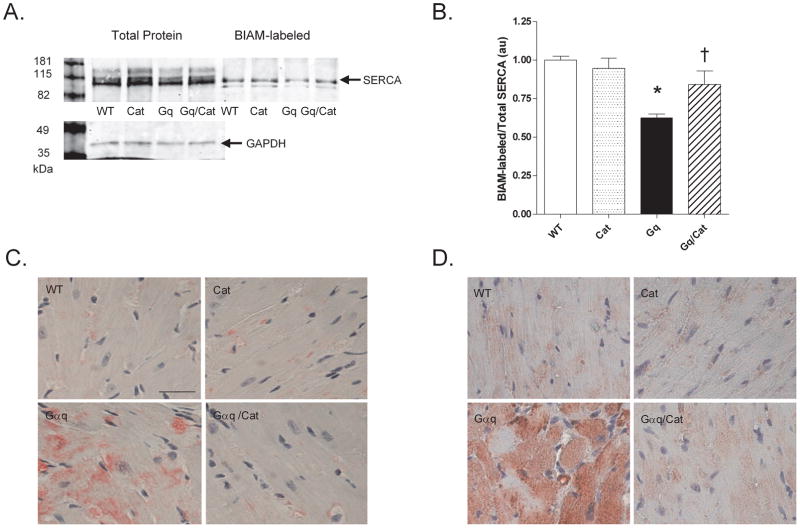
To test whether OPTM may contribute to contractile dysfunction in Gαq myocytes, oxidative thiol modifications of SERCA and RyR were assessed using biotinylated iodoacetamide (BIAM), as we have described 17;22. Compared to WT, the fraction of BIAM-labeled SERCA in Gaq was decreased by 36% (Figure 2, A and B), whereas BIAM-labeling of RyR was unchanged (data not shown). We have developed antibodies directed at SERCA that is sulfonylated at cysteine 674 23 or nitrated at tyrosine 294/295 24. Using these antibodies, immunohistochemistry revealed increased staining for both OPTM diffusely over myocytes in Gαq hearts (Figure 2, C and D). In myocardium from Gαq/catalase mice (compared to Gαq mice), there was a) increased BIAM labeling of SERCA (Figure 2, A and B), indicating a decrease in cysteine oxidation, b) decreased sulfonylation of SERCA cysteine 674 (Figure 2C), and c) decreased nitration of SERCA tyrosine 294/295 (Figure 2D).
Figure 2.
OPTM of SERCA in myocardium from Gαq mice. Panel A. Representative immunoblot for total and BIAM-labeled SERCA in WT, Cat, Gαq and Gαq/Cat mice. Panel B. Ratio of BIAM-labeled to total SERCA. Shown are mean data from 4 hearts in each group (*p<0.001 vs. WT; †p<0.05 vs. Gαq). Panel C and D. Representative micrographs showing increased levels of SERCA sulfonylated at cysteine 674 (Panel C) and nitrated at tyrosine 294/295 (Panel D) distributed diffusely in myocytes from Gαq mice, and the prevention of both OPTM by concurrent expression of catalase in Gαq/catalase mice (bar = 25 μM).
Decreased SERCA activity in Gαq myocardium is restored by catalase
To assess the functional consequence of the observed OPTM, SERCA activity was measured using maximal calcium-stimulated calcium uptake in sarcolemmal membranes, as we have described 21. SERCA-mediated calcium uptake was reduced by 47% in Gαq membranes (Figure 3). In myocytes from Gαq/catalase mice, maximal calcium-stimulated SERCA activity was restored to WT levels (Figure 3).
Figure 3.
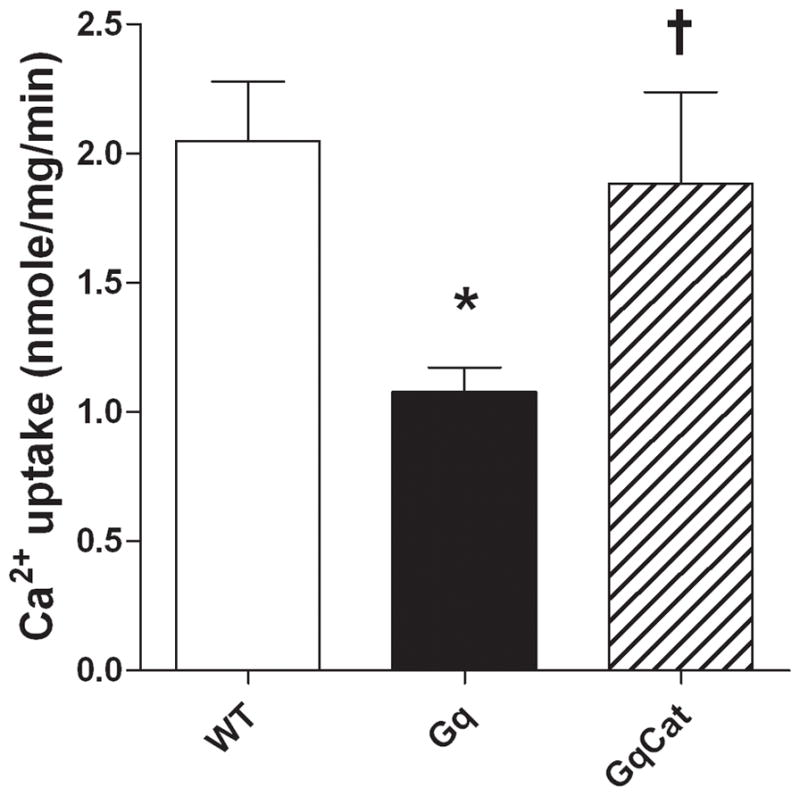
Decreased SERCA activity in Gαq myocardium is corrected by concurrent expression of catalase in Gαq/catalase mice. Maximum SERCA activity was assessed using maximum calcium-stimulated, thapsigargin-inhibited calcium uptake 21. Shown are mean data for 7 – 8 hearts in each group (*p<0.05 vs. WT; †p<0.05 vs. Gαq).
Discussion
We used the Gαq mouse model of dilated cardiomyopathy to examine the role of protein OPTM in mediating myocyte contractile dysfunction. Prior work from Satoh 15 and our group 16 demonstrated increased oxidative stress in the myocardium of these mice. Likewise, our finding of contractile dysfunction and abnormal calcium regulation in Gαq myocytes confirms and extends the prior report by Yatani et al 14. Recently, we demonstrated that concurrent cardiac myocyte-specific overexpression of catalase improved cardiac function in these mice 16. Accordingly, we hypothesized that catalase would improve myocyte function and thereby allow the identification of catalase-sensitive OPTM of myocyte proteins involved in the pathophysiology of contractile dysfunction.
Our initial finding was that cardiac myocyte-specific overexpression of catalase ameliorated the abnormalities in cardiac myocyte contractile function and calcium regulation. This indicates that a catalase-sensitive pathway is involved in mediating myocyte dysfunction, and directed our attention to proteins involved in calcium handling. Of the proteins primarily involved in myocyte calcium regulation, only the expression of RyR was decreased. However, the decreases in RyR protein and its mRNA were not affected by catalase, and thus, are not responsible for the effect of catalase on myocyte function that we observed
SERCA activity was decreased in Gαq myocardium, consistent with prior observations in this 14 and other models of heart failure 9;25. However, the expression of SERCA was not decreased, which is also consistent with prior observations in this mouse 13. Of note, SERCA activity was corrected by concurrent catalase expression, suggesting that OPTM of SERCA might be responsible for decreased SERCA activity. This thesis was further supported by 3 observations. First, in Gαq myocardium there was a decrease in the quantity of BIAM binding to SERCA, which indicates oxidative modification of the most reactive SERCA cysteine, cysteine 674, and potentially other cysteines 17. Importantly, the quantity of BIAM binding to SERCA was restored towards normal in Gαq/catalase mice, confirming that the modification was oxidative in nature. Second, there was immunohistochemical evidence of sulfonylation of SERCA at cysteine-674 23. This OPTM is noteworthy because we have shown that sulfonylation of SERCA cysteine 674 in atherosclerotic aortic smooth muscle is associated with decreased activity 23. Third, there was immunohistochemical evidence of nitration of SERCA tyrosine 294/295 24. Sulfonylation of SERCA at cysteine 674 and nitration of tyrosine at 294/295 provide evidence of irreversible oxidation by elevated oxidants 23. As with BIAM binding, both SERCA sulfonylation and nitration were markedly decreased in Gαq/catalase mice. These findings thus directly identify two specific OPTM of SERCA in the Gαq mouse, and demonstrate that both can be prevented by catalase. This observation implicates H2O2 in the oxidation of SERCA cysteine-674 26 and the nitration of SERCA tyrosines 27. H2O2 is most likely derived via the dismutation of superoxide that is produced by mitochondria and/or oxidases.
While we have identified two specific irreversible OPTM that are associated with decreased SERCA activity, we can not exclude phosphorylation of calcium-regulating proteins due to oxidative regulation of a phosphatase. However, we think it is unlikely that this mechanism could explain our primary observation – correction of maximal calcium-stimulated SERCA activity - since a) SERCA is not known to have regulatory phosphorylation sites, and b) SERCA activity was measured using maximal calcium stimulation, which is not sensitive to phospholamban. On the other hand, oxidative regulation of phosphorylation might contribute to other aspects of contractile dysfunction in this model.
Prior studies in mice have implicated abnormal calcium handling, and in particular, decreased SERCA function in the pathophysiology of myocyte contractile dysfunction 3;4;28;29. Likewise, numerous studies have identified decreased SERCA activity in failing human myocardium 30–32 which, in some cases, has been associated with normal SERCA protein levels 31;32. In preliminary studies we have found immunohistochemical evidence that SERCA is sulfonylated at cysteine 674 in myocardium from patients with heart failure due to dilated cardiomyopathy, but not in myocardium from patients without heart failure (unpublished data), thus suggesting that our findings in the Gαq mouse are relevant to human disease.
Novel findings of this study include the demonstration of a) multiple specific oxidative modifications of SERCA, b) the relationship between SERCA OPTM and reduced SERCA activity and altered cellular calcium handling, and c) the rescue of OPTM, SERCA activity and cellular calcium handling by myocyte-specific overexpression of catalase. Taken together, our data suggest that cardiac myocyte contractile dysfunction in the Gαq mouse is mediated, in part, by catalase-sensitive OPTM of SERCA. These observations suggest that OPTM caused by H2O2 contribute to myocardial dysfunction in pathologic states, such as heart failure, that are associated with increased oxidant levels in the heart.
Novelty and Significance.
What Is Known?
Myocyte dysfunction and calcium dysregulation occur in human heart failure in association with increased reactive oxygen species (ROS) in the myocardium.
Mice with myocyte-specific overexpression of Gαq develop a dilated cardiomyopathy that progresses to heart failure associated with myocyte contractile dysfunction and calcium dysregulation.
ROS are increased in the Gαq overexpressing mouse heart.
What New Information Does This Article Contribute?
Gαq overexpressing mice exhibit specific irreversible oxidative post-translational modifications (OPTM) of sarcoplasmic reticulum Ca2+-ATPase (SERCA) consisting of sulfonylation at cysteine 674 and nitration at tyrosine 294/295.
OPTM of SERCA in Gαq overexpressing mice are associated with reduced SERCA activity in myocardium, myocyte calcium dysregulation and myocyte contractile dysfunction.
Myocyte-specific overexpression of catalase prevents OPTM of SERCA, restores SERCA activity and improves myocyte calcium dysregulation and contractile dysfunction.
We tested whether myocyte dysfunction in Gαq mice is mediated by OPTM of SERCA. In Gαq myocardium there were specific OPTM of SERCA associated with reduced SERCA activity and impaired calcium-related myocyte function. Myocyte-specific overexpression of catalase prevented SERCA OPTM and rescued SERCA activity and isolated myocyte function. Thus, myocyte contractile dysfunction in Gαq-induced cardiomyopathy is mediated, at least in part, by OPTM of SERCA. More broadly, these observations suggest that protein OPTM may contribute to the pathophysiology of myocardial dysfunction in heart failure and other conditions associated with increased myocardial ROS, and may provide a novel therapeutic target.
Supplementary Material
Acknowledgments
Sources of Funding
Supported by NIH grants HL-061639, HL-064750 (WSC), HL031607 (RAC, XYT), and the NHLBI-sponsored Boston University Cardiovascular Proteomics Center (Contract No. N01-HV-28178, RAC and WSC). SL was supported by a grant from La Fondation pour la Recherche Médicale SPE20051105207.
Non-standard Abbreviations and Acronyms
- BIAM
biotinylated iodoacetamide
- H2O2
hydrogen peroxide
- LV
left ventricular
- OPTM
oxidative post-translational modifications
- PLB
phospholamban
- RyR
ryanodine receptor
- ROS
reactive oxygen species
- SERCA
sarcoplasmic reticulum Ca2+-ATPase
- NCX
sodium/calcium exchanger
Footnotes
Disclosures
None.
References
- 1.Pollack PS, Bailey BA, Budjak R, Fernandez E, Houser SR. Progressive feline pressure-overload: noninvasive assessment correlates with abnormalities in single cells. Am J Physiol. 1993;264:H1307–H1314. doi: 10.1152/ajpheart.1993.264.4.H1307. [DOI] [PubMed] [Google Scholar]
- 2.Dorn GW, Robbins J, Ball N, Walsh RA. Myosin heavy chain regulation and myocyte contractile depression after LV hypertrophy in aortic-banded mice. Am J Physiol. 1994;267:H400–H405. doi: 10.1152/ajpheart.1994.267.1.H400. [DOI] [PubMed] [Google Scholar]
- 3.Ito K, Yan X, Tajima M, Su Z, Barry WH, Lorell BH. Contractile reserve and intracellular calcium regulation in mouse myocytes from normal and hypertrophied failing hearts. Circ Res. 2000;87:588–595. doi: 10.1161/01.res.87.7.588. [DOI] [PubMed] [Google Scholar]
- 4.Ito K, Yan X, Feng X, Manning WJ, Dillmann WH, Lorell BH. Transgenic expression of sarcoplasmic reticulum Ca(2+) atpase modifies the transition from hypertrophy to early heart failure. Circ Res. 2001;89:422–429. doi: 10.1161/hh1701.095522. [DOI] [PubMed] [Google Scholar]
- 5.Capasso JM, Li P, Anversa P. Cytosolic calcium transients in myocytes isolated from rats with ischemic heart failure. Am J Physiol. 1993;265:H1953–64. doi: 10.1152/ajpheart.1993.265.6.H1953. [DOI] [PubMed] [Google Scholar]
- 6.Li P, Park C, Micheletti R, Li B, Cheng W, Sonnenblick EH, Anversa P, Bianchi G. Myocyte performance during evolution of myocardial infarction in rats: effects of propionyl-L-carnitine. Am J Physiol. 1995;268:H1702–H1713. doi: 10.1152/ajpheart.1995.268.4.H1702. [DOI] [PubMed] [Google Scholar]
- 7.Loennechen JP, Wisloff U, Falck G, Ellingsen O. Cardiomyocyte contractility and calcium handling partially recover after early deterioration during post-infarction failure in rat. Acta Physiol Scand. 2002;176:17–26. doi: 10.1046/j.1365-201X.2002.01011.x. [DOI] [PubMed] [Google Scholar]
- 8.Loennechen JP, Wisloff U, Falck G, Ellingsen O. Effects of cariporide and losartan on hypertrophy, calcium transients, contractility, and gene expression in congestive heart failure. Circulation. 2002;105:1380–1386. doi: 10.1161/hc1102.105258. [DOI] [PubMed] [Google Scholar]
- 9.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 10.Sawyer DB, Siwik DA, Xiao L, Pimentel DR, Singh K, Colucci WS. Role of oxidative stress in myocardial hypertrophy and failure. J Mol Cell Cardiol. 2002;34:379–388. doi: 10.1006/jmcc.2002.1526. [DOI] [PubMed] [Google Scholar]
- 11.Hare JM. Nitroso-redox balance in the cardiovascular system. N Engl J Med. 2004;351:2112–2114. doi: 10.1056/NEJMe048269. [DOI] [PubMed] [Google Scholar]
- 12.Takimoto E, Kass DA. Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension. 2007;49:241–248. doi: 10.1161/01.HYP.0000254415.31362.a7. [DOI] [PubMed] [Google Scholar]
- 13.D’Angelo DD, Sakata Y, Lorenz JN, Boivin GP, Walsh RA, Liggett SB, Dorn GW. Transgenic Galphaq overexpression induces cardiac contractile failure in mice. Proc Natl Acad Sci U S A. 1997;94(15):8121–8126. doi: 10.1073/pnas.94.15.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yatani A, Frank K, Sako H, Kranias EG, Dorn GW. Cardiac-specific overexpression of Galphaq alters excitation- contraction coupling in isolated cardiac myocytes. J Mol Cell Cardiol. 1999;31:1327–1336. doi: 10.1006/jmcc.1999.0966. [DOI] [PubMed] [Google Scholar]
- 15.Satoh M, Matter CM, Ogita H, Takeshita K, Wang CY, Dorn GW, Liao JK. Inhibition of apoptosis-regulated signaling kinase-1 and prevention of congestive heart failure by estrogen. Circulation. 2007;115:3197–3204. doi: 10.1161/CIRCULATIONAHA.106.657981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin F, Lennon-Edwards S, Lancel S, Biolo A, Siwik DA, Pimentel DR, Dorn GW, Kang YJ, Colucci WS. Cardiac-specific overexpression of catalase identifies hydrogen peroxide-dependent and -independent phases of myocardial remodeling and prevents the progression to overt heart failure in G(alpha)q-overexpressing transgenic mice. Circ Heart Fail. 2010;3:306–313. doi: 10.1161/CIRCHEARTFAILURE.109.864785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ying J, Clavreul N, Sethuraman M, Adachi T, Cohen RA. Thiol oxidation in signaling and response to stress: detection and quantification of physiological and pathophysiological thiol modifications. Free Radic Biol Med. 2007;43:1099–1108. doi: 10.1016/j.freeradbiomed.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang YJ, Chen Y, Epstein PN. Suppression of doxorubicin cardiotoxicity by overexpression of catalase in the heart of transgenic mice. J Biol Chem. 1996;271:12610–12616. doi: 10.1074/jbc.271.21.12610. [DOI] [PubMed] [Google Scholar]
- 19.Lim CC, Apstein CS, Colucci WS, Liao R. Impaired cell shortening and relengthening with increased pacing frequency are intrinsic to the senescent mouse cardiomyocyte. J Mol Cell Cardiol. 2000;32:2075–2082. doi: 10.1006/jmcc.2000.1239. [DOI] [PubMed] [Google Scholar]
- 20.Grover AK, Samson SE. Effect of superoxide radical on Ca2+ pumps of coronary artery. Am J Physiol. 1988;255:C297–C303. doi: 10.1152/ajpcell.1988.255.3.C297. [DOI] [PubMed] [Google Scholar]
- 21.Lancel S, Zhang J, Evangelista A, Trucillo MP, Tong X, Siwik DA, Cohen RA, Colucci WS. Nitroxyl activates SERCA in cardiac myocytes via glutathiolation of cysteine 674. Circ Res. 2009;104:720–723. doi: 10.1161/CIRCRESAHA.108.188441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ying J, Tong XY, Pimental DR, Weisbrod RM, Trucillo MP, Adachi T, Cohen RA. Cysteine-674 of the Sarco/Endoplasmic Reticulum Calcium ATPase Is Required for the Inhibition of Cell Migration by Nitric Oxide. Arterioscler Thromb Vasc Biol. 2007 doi: 10.1161/01.ATV.0000258413.72747.23. [DOI] [PubMed] [Google Scholar]
- 23.Ying J, Sharov V, Xu S, Jiang B, Gerrity R, Schoneich C, Cohen RA. Cysteine-674 oxidation and degradation of sarcoplasmic reticulum Ca(2+) ATPase in diabetic pig aorta. Free Radic Biol Med. 2008;45:756–762. doi: 10.1016/j.freeradbiomed.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu S, Ying J, Jiang B, Guo W, Adachi T, Sharov V, Lazar H, Menzoian J, Knyushko TV, Bigelow D, Schoneich C, Cohen RA. Detection of sequence-specific tyrosine nitration of manganese SOD and SERCA in cardiovascular disease and aging. Am J Physiol Heart Circ Physiol. 2006;290:H2220–H2227. doi: 10.1152/ajpheart.01293.2005. [DOI] [PubMed] [Google Scholar]
- 25.Piacentino V, III, Weber CR, Chen X, Weisser-Thomas J, Margulies KB, Bers DM, Houser SR. Cellular basis of abnormal calcium transients of failing human ventricular myocytes. Circ Res. 2003;92:651–658. doi: 10.1161/01.RES.0000062469.83985.9B. [DOI] [PubMed] [Google Scholar]
- 26.Schroder E, Eaton P. Hydrogen peroxide as an endogenous mediator and exogenous tool in cardiovascular research: issues and considerations. Curr Opin Pharmacol. 2008;8:153–159. doi: 10.1016/j.coph.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 27.Oury TD, Tatro L, Ghio AJ, Piantadosi CA. Nitration of tyrosine by hydrogen peroxide and nitrite. Free Radic Res. 1995;23:537–547. doi: 10.3109/10715769509065275. [DOI] [PubMed] [Google Scholar]
- 28.Grieve DJ, Byrne JA, Siva A, Layland J, Johar S, Cave AC, Shah AM. Involvement of the nicotinamide adenosine dinucleotide phosphate oxidase isoform Nox2 in cardiac contractile dysfunction occurring in response to pressure overload. J Am Coll Cardiol. 2006;47:817–826. doi: 10.1016/j.jacc.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 29.Nakayama H, Otsu K, Yamaguchi O, Nishida K, Date MO, Hongo K, Kusakari Y, Toyofuku T, Hikoso S, Kashiwase K, Takeda T, Matsumura Y, Kurihara S, Hori M, Tada M. Cardiac-specific overexpression of a high Ca2+ affinity mutant of SERCA2a attenuates in vivo pressure overload cardiac hypertrophy. FASEB J. 2003;17:61–63. doi: 10.1096/fj.02-0474fje. [DOI] [PubMed] [Google Scholar]
- 30.Periasamy M, Bhupathy P, Babu GJ. Regulation of sarcoplasmic reticulum Ca2+ ATPase pump expression and its relevance to cardiac muscle physiology and pathology. Cardiovasc Res. 2008;77:265–273. doi: 10.1093/cvr/cvm056. [DOI] [PubMed] [Google Scholar]
- 31.Movsesian MA, Karimi M, Green K, Jones LR. Ca(2+)-transporting ATPase, phospholamban, and calsequestrin levels in nonfailing and failing human myocardium. Circulation. 1994;90:653–657. doi: 10.1161/01.cir.90.2.653. [DOI] [PubMed] [Google Scholar]
- 32.Munch G, Bolck B, Brixius K, Reuter H, Mehlhorn U, Bloch W, Schwinger RH. SERCA2a activity correlates with the force-frequency relationship in human myocardium. Am J Physiol Heart Circ Physiol. 2000;278:H1924–H1932. doi: 10.1152/ajpheart.2000.278.6.H1924. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.