Abstract
Acute administration of antipsychotic drugs increases dopamine (DA) neuron activity and DA release via D2 receptor blockade. However, it is unclear whether the DA neuron activation produced by antipsychotic drugs is due to feedback from postsynaptic blockade or is due to an action on DA neuron autoreceptors. This was evaluated using two drugs: the first-generation antipsychotic drug haloperidol that has potent D2 blocking properties, and the second generation drug sertindole, which is unique in that it is reported to fail to reverse the apomorphine-induced decrease in firing rate typically associated with DA neuron autoreceptor stimulation. Using single-unit extracellular recordings from ventral tegmental area (VTA) DA neurons in anesthetized rats, both drugs were found to significantly increase the number of spontaneously active DA neurons (population activity). Apomorphine administered within 10 minutes either before or after sertindole reversed the sertindole-induced increase in population activity, but had no effect when administered 1 hour after sertindole. Moreover, both sertindole and haloperidol-induced increase in population activity was prevented when accumbens feedback was interrupted by local infusion of the GABAA antagonist bicuculline into the ventral pallidum. Taken together, these data suggest that antipsychotics increase DA neuron population activity via a common action on the accumbens-ventral pallidal-VTA feedback pathway and thus provide a further elucidation on the mechanism by which antipsychotic drugs affect DA neuron activity. This provides an important insight into the relation between altered DA neuron activity and potential antipsychotic efficacy.
Keywords: antipsychotic, autoreceptor, ventral tegmental area, nucleus accumbens, dopamine, schizophrenia
Introduction
All antipsychotic drugs currently in use are proposed to exert their primary actions via blockade of dopamine (DA) D2 receptors (Carlsson and Lindqvist, 1963; Seeman et al., 1975). Administered acutely, antipsychotic drugs block DA receptors both at postsynaptic sites and at DA autoreceptors (Meltzer and Stahl, 1976; Skirboll et al., 1979; Walters et al., 1973). Furthermore, antipsychotic drugs block the behavioral actions of DA in the limbic system (Kinon and Lieberman, 1996). Owing to their preferential limbic selectivity, likely derived from a low D2 occupancy and/or high relative 5-HT2 receptor affinity (Meltzer et al., 1989), the second-generation antipsychotic drugs are efficacious without producing extrapyramidal side-effects (EPS; Skarsfeldt, 1995). These drugs exhibit limbic system selectivity, in that they block the behavioral actions of low doses of amphetamine (Meng et al., 1998; Natesan et al., 2006), activate c-fos selectively in limbic structures, and increase DA release within the nucleus accumbens (Deutch, 1994). Moreover, upon acute administration, all effective antipsychotic drugs increase the number of DA neurons firing (i.e., DA neuron population activity), whereas their repeated administration causes inactivation of DA neuron firing via induction of depolarization block (Grace et al., 1997). However, whether these effects of antipsychotic drugs on DA neuron activity are due to blockade of DA neuron autoreceptors or feedback from postsynaptic sites, or a combination of actions, is unclear.
Sertindole is a phenylindole derivative second-generation antipsychotic drug that, like most second-generation antipsychotics, exhibits moderate affinity for D2 receptors (Ki= 12nM) and 5-HT2 selectivity (Arnt and Skarsfeldt, 1998; Kane and Tamminga, 1997). Sertindole also exhibits limbic-selective biochemical and behavioral properties (Arnt, 1995; Fink-Jensen and Kristensen, 1994; Hertel, 2006; Sams-Dodd, 1997; Watanabe and Hagino, 1999), it increases DA neuron population activity, and with repeated administration, it induces DA neuron depolarization block (Skarsfeldt and Perregaard, 1990). However, unlike other antipsychotic drugs, upon acute administration, sertindole fails to increase DA neuron firing rate or block the ability of DA neuron autoreceptor agonists to inhibit DA neuron spike firing (Skarsfeldt, 1992); actions that have been associated with DA neuron cell body autoreceptor blockade (Meltzer, 1980; Skirboll et al., 1979). These data suggest that sertindole may exhibit differential actions on DA neuron autoreceptors versus postsynaptic DA receptor targets which, if verified, makes this drug uniquely useful in distinguishing actions on DA neuron autoreceptors from those produced via feedback from postsynaptic sites. By contrasting the effects of a first-generation antipsychotic drug haloperidol, which is known to potently block apomorphine-induced inhibition of DA neurons, with the effects of sertindole we attempted to evaluate the site at which these antipsychotic drugs act to alter DA neuron firing properties. Using in vivo single-unit extracellular recordings from identified DA neurons, we examined the mechanism by which sertindole and haloperidol affect DA neuron activity states. Understanding the mechanism of action of sertindole, as well as other antipsychotic drugs, remains an important issue both in optimizing therapeutic approaches and in guiding drug development.
Materials and Methods
Materials and subjects
Sertindole was generously provided by H. Lundbeck A/S. Glacial acetic acid was obtained from Fisher Scientific (Pittsburgh, PA), and lactic acid from EM Science (Gibbstown, NJ). Haloperidol, 1(S), 9(R) (−) bicuculline methobromide, R(−) apomorphine hydrochloride hemihydrate and all other drugs were obtained from Sigma-Aldrich (St. Louis, MO).
A total of 73 male Sprague–Dawley rats, 280–400g (Hilltop, Scottdale, PA) were used in this study. Handling of all animals, surgery and experiment protocols were in accordance with the guidelines outlined in the United States Public Health Service Guide for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh.
Surgery and electrophysiology recordings from VTA DA neurons
From arrival until the day in which each experiment was performed, rats were housed in pairs in a temperature and humidity controlled facility with lights maintained on a 12h light/dark cycle and food and water available ad libitum. Experiments were typically performed one week following rat arrival to the facility. On the day of the experiment, a rat was transferred to the laboratory, anesthetized with an initial intra-peritoneal injection of 8% chloral hydrate (400mg/kg) and a catheter inserted into the right femoral vein for i.v. administration of drugs. The rat was then mounted on a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA) and burr holes were drilled in the skull overlying the ventral tegmental area (VTA; in mm from bregma, AP: −5.3, ML: −0.6) for electrophysiological recordings, and the ventral pallidum (VP; AP: 0, ML −2.2, DV: −5) for drug microinfusion, according the to the stereotaxic rat atlas of Paxinos and Watson (1998). The rat was placed on a heating pad (FHC, Bowdoinham, ME) to maintain body temperature at ~37° C; anesthesia was monitored periodically and maintained during the duration of the experiment by supplemental injection of chloral hydrate;
Each rat was randomly assigned to control or to a specific pharmacological treatment (see below), and the effects on the activity of VTA DA neurons were investigated using in vivo single-unit extracellular recordings performed as previously described (Lodge and Grace, 2007). Single-barrel glass microelectrodes (in situ impedance, 8–12 MΩ) were constructed from borosilicate glass capillary tubing (2mm outer diameter; World Precision Instruments, Sarasota, FL) using a vertical microelectrode puller (Narishige, model PE-2; Tokyo, Japan), and filled with 2M NaCl in 2% Pontamine sky blue dye. The electrodes were lowered slowly into the VTA (DV: −6 to −9mm) using a hydraulic microdrive (David Kopf Instruments, model 640; Tujunga, CA). Spontaneously firing DA neurons encountered throughout the electrode vertical pass (or track) were distinguished from other VTA neurons and identified using previous well-established criteria (Grace and Bunney, 1983; Grace et al., 2007) and filter settings set to allow accurate distinction of the unique waveform (low pass, 50Hz; high pass, 16 kHz).
Signals from the recording electrode were amplified by a headstage before being fed into a window discriminator/amplifier (1000x gain, 100–4000 Hz band pass; Fintronics Inc., Orange, CT). Signals were also fed into an audio monitor (Grass Instruments, model AM8; Quincy, MA) and displayed on an oscilloscope (BK Precision, model 2120; Yorba Linda, CA) for real-time monitoring. Data were collected using a data acquisition board interface (Microstar laboratory, Bellevue, WA) and monitored on-line using custom-designed computer software (Neuroscope; Brian Lowry).
Effect of pharmacological manipulations on VTA DA neuron activity
Sertindole was chosen based on its reported lack of action on DA neuron autoreceptors in vivo (Skarsfeldt, 1992), and to compare it with another D2 antagonist with known autoreceptor-blocking properties, haloperidol. However, in order to make this determination accurately, it was necessary to demonstrate that the absence of autoreceptor actions of sertindole is indeed present at the time points at which the measurements of DA neuron activity states are performed. Thus, we first examined the effects of acute administration of sertindole on DA neuron activity, and then compared this effect with that produced by autoreceptor-selective dose of apomorphine administered either before or after sertindole.
The effects of sertindole were investigated at 2 time points: 1) effects on a single, identified DA neuron, were measured during drug administration and for 1 hour immediately following drug, and 2) beginning at the 1 hour time point, the persistent effects of sertindole on DA neuron population activity was investigated (elimination half-life of sertindole ~ 2–4 days). Briefly, following isolation of an identified DA neuron, 3 minutes of baseline activity was recorded to calculate average firing rate and the proportion of action potentials occurring in bursts, or percent burst firing (defined as the occurrence of 2 spikes with an interspike interval, ISI of < 80ms indicating the initiation of a burst, with 2 spikes occurring at an ISI >160ms signaling burst termination (Grace and Bunney, 1983). Then, 1.25 mg/kg sertindole was injected i.v through the femoral vein. The firing rate and percent burst firing of the neuron was monitored and recorded during drug application and for the following hour, with firing rate and pattern evaluated at 10 minute intervals (Figure 2A). The effect of sertindole on identified DA neurons was evaluated by comparing baseline activity to the activity during drug application and was further classified as inhibitory or excitatory.
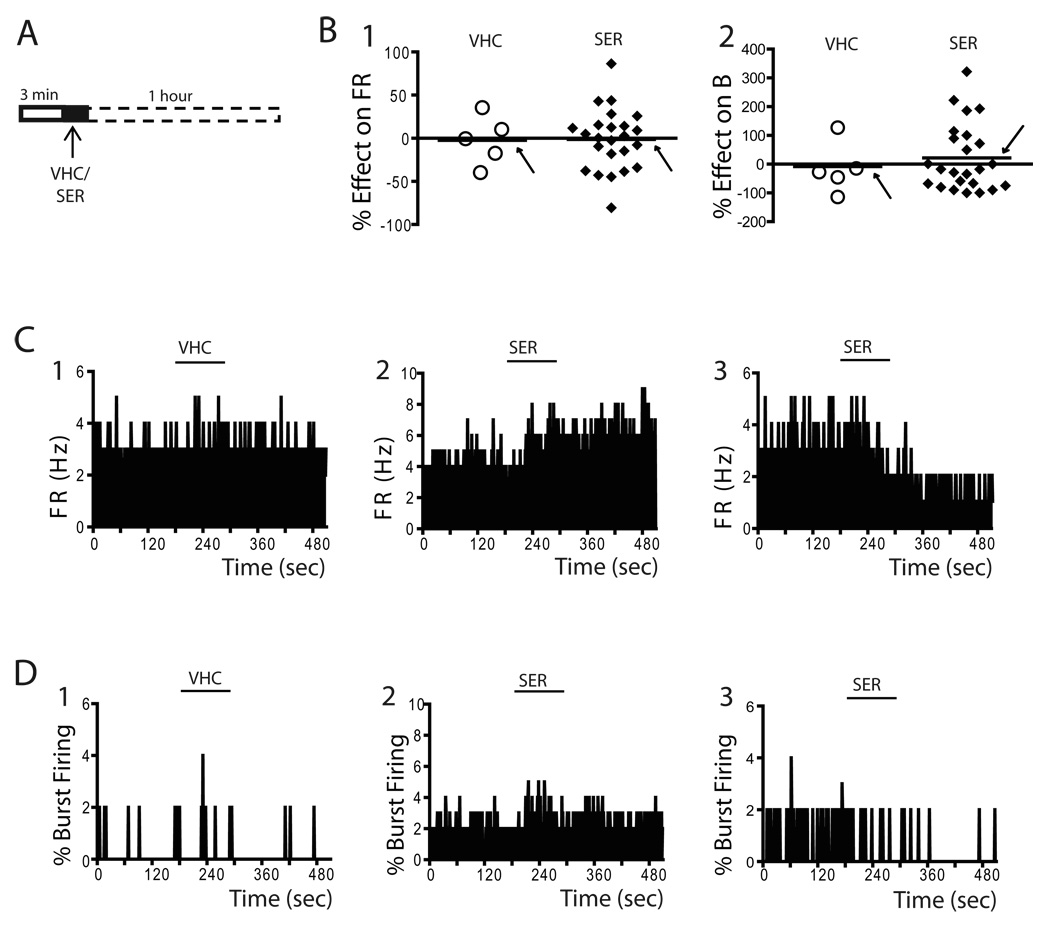
Figure 2. Acute administration of sertindole both activated and inhibited VTA DA neurons.
A) The effects of acute 1.25 mg/kg sertindole on a single DA neuron were examined. Sertindole was administered at arrow after 3 min recording of baseline activity; firing rates and patterns were monitored continuously for one hour following drug administration, with data recorded at 10 minute intervals. B) Plots illustrate the distribution of percent change in firing rate (1) and burst firing (2) for each DA neuron recorded in response to systemic administration of vehicle (open circles) or sertindole (filled diamonds). Horizontal lines illustrate the mean of responses and are indicated by arrows. Sertindole data represent peak effects which usually occurred at cessation of drug administration or immediately after. C) Firing rate histograms of VTA DA neurons following administration of vehicle (1) and sertindole (2, 3). Two types of responses were observed; ~54% of neurons were excited (2), and the remaining inhibited (3). D) Similarly, whereas vehicle administration did not alter the percent burst firing (1), administration of sertindole was found to either increase (2) or decrease (3) burst firing. Horizontal lines indicate duration of vehicle or sertindole injection.
VHC= vehicle; SER= sertindole; FR= firing rate; B= % burst firing
One hour following testing of the effects of sertindole on a single DA neuron, the impact of this sertindole administration on DA neuron population activity was assessed. Between 6 and 9 electrodee tracks separated by 200 µm between adjacent tracks were made by passing the electrode through a predetermined “grid” pattern. For each neuron, firing rate and pattern were recorded. The number of spontaneously active DA neurons encountered in each electrode track was counted and this number represents the “population activity” of the VTA DA neurons. Furthermore, the values of firing rate and percent of burst firing for all neurons encountered were averaged within rats and then between up to 12 rats per condition; the values thus obtained were defined as “average firing rate” and “average percent burst firing”, respectively (Lodge and Grace, 2007).
In the second part of the study the ability of sertindole to prevent or reverse DA neuron inhibition by autoreceptor-selective doses of 30 µg/kg apomorphine was tested Apomorphine was administered either prior to (5–10 minutes; figure 4A) or after (10 minutes or 1 hour; figure 5A1 and 5A2, respectively) sertindole, and the activity of single identified neurons was monitored. Furthermore, for any of the above protocols the effects of apomorphine on the sertindole-induced increase in population activity was examined 1 hour following sertindole administration by recording the activity of all the spontaneously active DA neurons encountered in 6 to 9 electrode track.
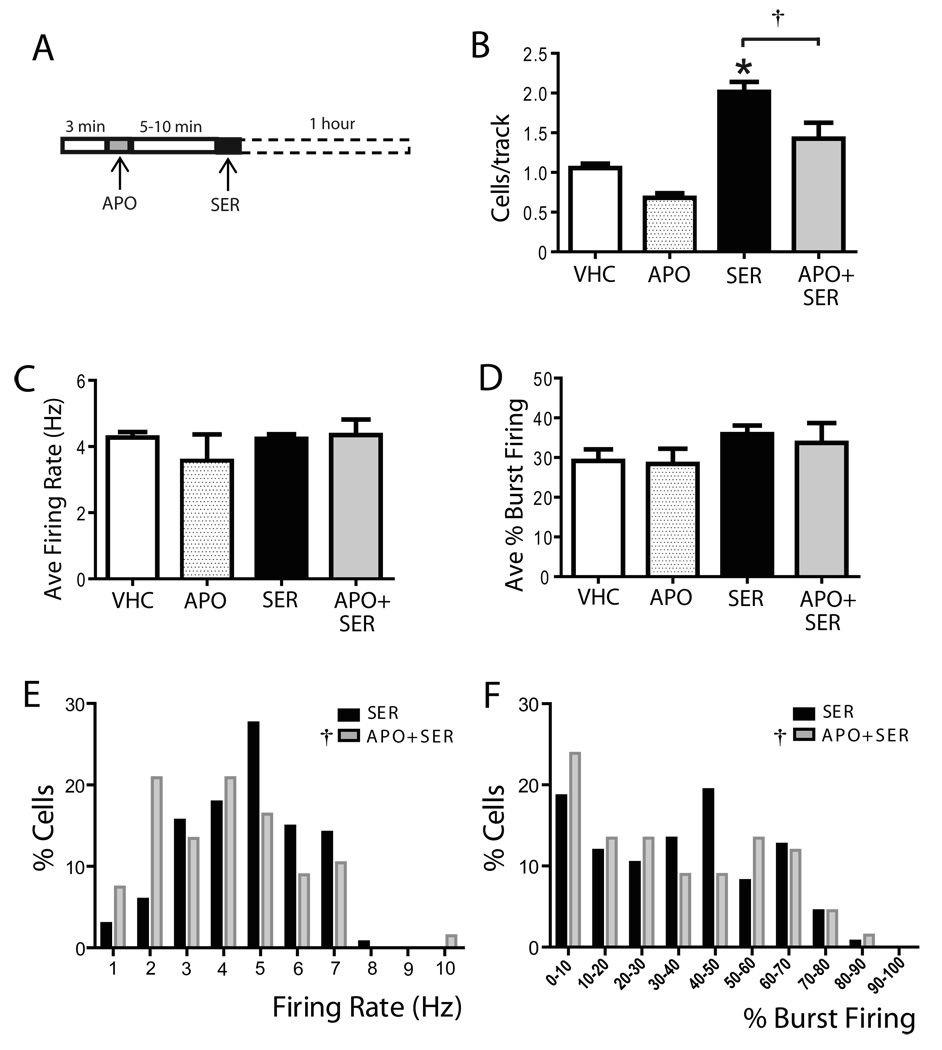
Figure 4. Autoreceptor-selective doses of apomorphine administered shortly before sertindole attenuated the sertindole-induced increase in population activity.
(A) Following 3 min of baseline recording from a single DA neurons, 30 µg/kg apomorphine was administered, followed 5–10 min later by sertindole. One hour following sertindole, DA neuron population activity was assessed. Administration of apomorphine 5–10 minutes before sertindole significantly attenuated the sertindole-induced increase in population activity (B) without altering either average firing rate (C) or the percent burst firing (D). However, apomorphine altered the effects of sertindole on firing rate (E) or burst firing (F) distributions across the population of neurons sampled. VHC= vehicle; SER= sertindole; APO= apomorphine; *represent significant difference of SER from APO (One Way repeated measures ANOVA P≤ 0.001); † represent significant difference of sertindole from APO + SER (One Way repeated measures ANOVA, P≤ 0.001)
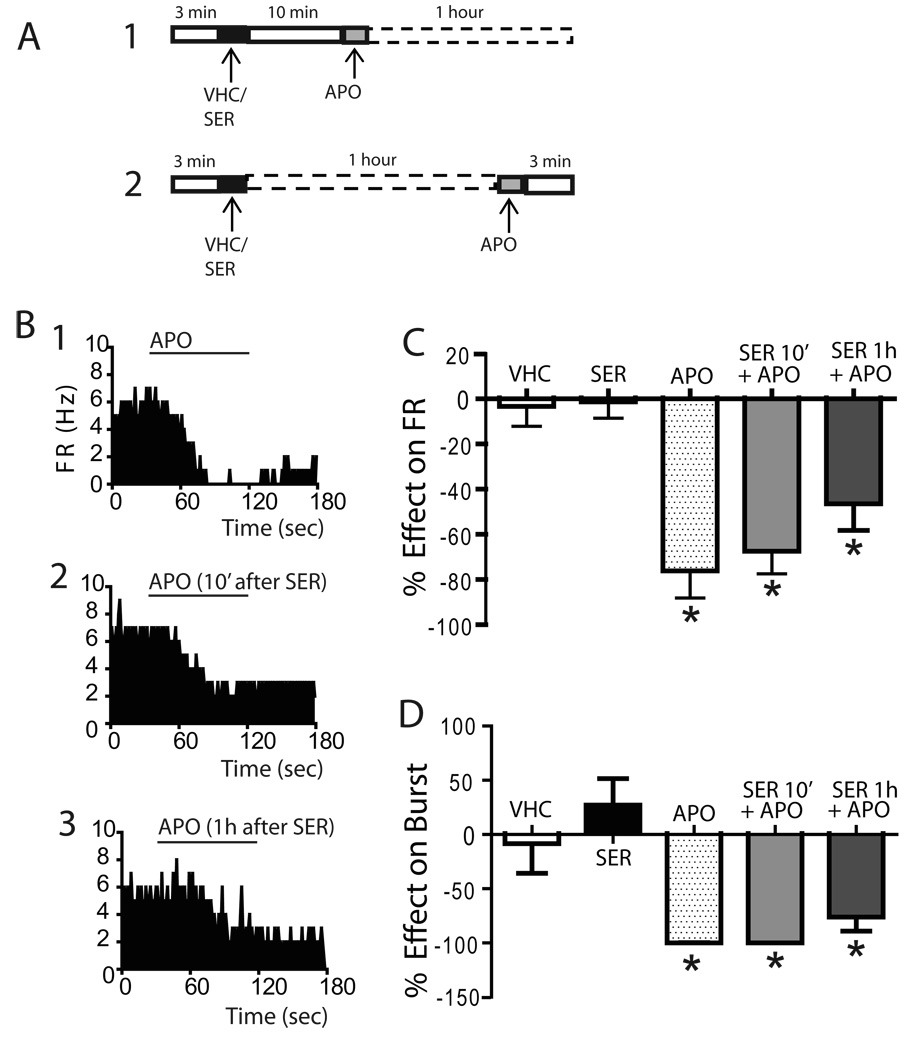
Figure 5. Autoreceptor-selective doses of apomorphine significantly attenuated VTA DA neuron firing rate when administered after sertindole.
(A) Protocol illustrating 30 µg/kg apomorphine administration at two time points: (1) 10 min and (2) 1 hour after sertindole. (B) Firing rate histograms of VTA DA neurons illustrate the effect of apomorphine administered alone (1), apomorphine administered 10 minutes after sertindole (2) and 1 hour after sertindole (3). Apomorphine administered both at 10 minutes and at 1 hour following sertindole administration significantly inhibited the firing rate (C) and percent burst firing (D) of identified DA neurons in a manner similar to that observed with apomorphine administered alone.
* represent significant difference post apomorphine versus baseline (Paired t-test, p< 0.05)
The mechanism by which sertindole and haloperidol affected VTA DA neuron population activity was tested by administering the antipsychotic drug following attenuation of the nucleus accumbens (NAc)- VP circuit. Blockade of NAc GABAergic inhibition of the VP was achieved by local microinfusion of 1 ng bicuculline into the VP prior to initiation of recordings. For infusion of bicuculline, stainless steel guide cannulae (Plastics One, 26 gauge; Roanoke, VA) were implanted into the VP and 0.5 µl of bicuculline or vehicle (Dulbecco’s buffer PBS; DB) was infused through a 30 gauge microinfusion injector (Plastic One; Roanoke, VA) extending 2mm from the guide cannula, and connected via a polytetrafluoroethylene tubing (Small Parts Inc, Miami Lakes, FL) to a 5 µl glass syringe (Hamilton Company, model 88011; Reno, NV). The speed of administration was controlled using a BAS syringe pump (Bioanalytical Systems Inc, model MD-1001; W. Lafayette, IN) to administer ~0.2 µl per minute. After isolation of a spontaneously firing DA neuron (approx. 10 minutes following bicuculline infusion), 3 minutes of baseline activity was recorded prior and during administration of either sertindole or haloperidol. The effects of sertindole on DA neurons of rats infused with DB were identical to those of sham rats; therefore, all data were combined. At 1 hour following antipsychotic drug administration, population activity was assessed. The duration of each experiment varied according to the number of DA neurons encountered per electrode track; from 2 hours in rats where ~ 1 cell/track was found, up to 4 hours in rats where ~ 2 cells/track were encountered.
Sertindole (1.25 mg/kg; dissolved in 0.1M acetic acid), apomorphine (30 µg/kg), haloperidol (0.8 mg/kg; dissolved in diluited lactic acid), or vehicles (acetic acid or lactic acid) were prepared in stock and then adjusted with 10M NaOH solution to a pH closer to physiological pH. All drugs and vehicles were then aliquoted and stored at −20° C until i.v. administration.
Histology
At the cessation of each experiment, rats were prepared for histological verification of recording and drug microinfusion sites. The recording sites were marked via electrophoretic ejection of Pontamine sky blue dye from the tip of the recording electrodes (~ −20µA constant current, for 20–30min; Fintronics Inc., bipolar constant current source; Orange, CT). The rats were then deeply anesthetized with an overdose of chloral hydrate, the brains removed and placed in 8% w/v paraformaldehyde in PBS. After a minimum of 48h, brains were cryoprotected with 25% w/v sucrose in PBS until saturated and were then sectioned into 60µm coronal sections with reference to a stereotaxic atlas (Paxinos and Watson, 1998). Brain slices were then mounted onto gelatin-chrom alum-coated slides, and stained with cresyl violet for histochemical verification of electrode and/or cannula sites (Figure 1).
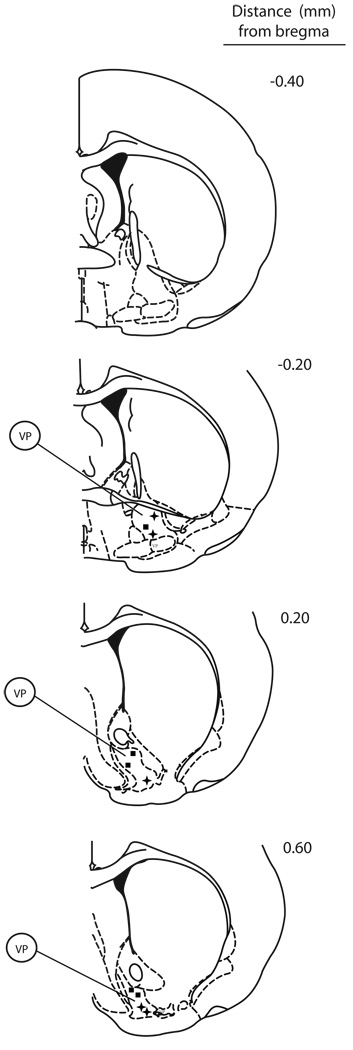
Figure 1. Histology verification of cannulae placements for local infusion of bicuculline in the ventral pallidum.
Coronal sections indicate the location of cannulae tips within the VP in experiments where rats were infused with 1 µg bicuculline prior recording and drug administration. Filled squares refer to cannulae location of rats where sertindole was administered, while plus signs refer to haloperidol administration.
VP= ventral pallidum.
Data Analysis
The electrophysiological activity of VTA DA neuron was analyzed off-line using custom-designed computer software, Neuroscope (Brian Lowry). Statistics were performed using the SigmaStat software program (Systat Software Inc.; San Jose, CA). Paired t-tests were used to examine the acute effects of sertindole and/or apomorphine on identified VTA DA neurons; Pearson Product Moment Correlation analysis was used to test for correlations between the effects of sertindole and baseline activity of DA neurons. One-way ANOVA (Holm-Sidak method, where appropiate), Kruskal-Wallis One Way Analysis of Variance on Ranks (Dunn’s method, where appropiate) were employed to test the effects of sertindole or haloperidol on population activity as well as on the distribution of firing rate or percent of burst firing of the VTA DA neuron. To analyze the effect of apomorphine on sertindole-induced changes in population activity and firing rate/burst firing distributions, a One-way ANOVA with repeated measures followed by Holm-Sidak test was used. The effects of acute sertindole or apomorphine on single, identified neurons are expressed as percent of excitation or inhibition with respect to control/baseline.
All data are reported as mean ± SEM.
Results
Acute sertindole administration increased DA neuron population activity without affecting firing properties
After isolating a spontaneously active DA neuron, 3 minutes of baseline activity was recorded, followed by administration of vehicle (VHC) or the second generation antipsychotic drug sertindole (Figure 2A). Consistent with previous studies (Skarsfeldt, 1992), acute sertindole administration (i.v. injection of 1.25 mg/kg) failed to produce an overall significant change either in the firing rate (n= 24 DA neurons; Paired t-test, p = 0.892) or in the percent burst firing (n= 24 DA neurons; Paired t-test, p= 0.645) when compared to baseline. A high degree of variability in the DA neuron responses was observed both with regard to changes in firing rate (Figure 2B1) and percent of burst firing (Figure 2B2). In order to examine whether this was due to a bimodal action, the responses were divided into excitation and inhibition of firing rate and percent burst firing. Acute sertindole produced a firing rate increase in 13 of the 24 neurons examined (pre SER: 4.1 ± 0.4Hz; post SER: 5.0 ± 0.5Hz; paired t-test, p= 0.002; Figure 2C2); however, only 6 of all neurons examined exhibited a change in firing rate that was > 15%, with an average percent excitation of 40.4 ± 10.2%. (range: 15.4% - 86.4%) The remaining 11 neurons exhibited inhibition to sertindole (pre SER: 3.9 ± 0.4Hz; post SER: 2.9 ± 0.5Hz; paired t-test, p≤ 0.001; Figure 2C3). Of these, 7 showed >15% change in firing rate with a decrease of 42.4 ± 7.2% (range: 18.1% – 80.6%). Similar to the effect on firing rate, sertindole significantly increased the percent burst firing in 9 of the 24 neurons examined (pre SER: 24.5 ± 6.3%; post SER: 44.0 ± 9.3%; paired t-test, p= 0.002; Figure 2D2) with an average of 199.3 ± 61.1% percent increase (range: 49.9% - 630.5%); other 13 neurons showed an attenuation of burst firing (pre SER: 28.8 ± 5.8%; post SER: 14.9 ± 5.6%; paired t-test, p≤ 0.001; Figure 2D3) with an average percent inhibition of burst firing of 63.5 ± 8.4% (range: 17.1% - 100%). In two neurons which displayed no burst activity at baseline, sertindole administration did not alter the pattern of firing, and those neurons continued to fire in a slow, irregular pattern. The responses observed furthermore were not correlated with baseline firing activity. Thus, there was no significant correlation between baseline activity and either the change in firing rate (n= 24 DA neurons; Pearson Product Moment Correlation, r= 0.176, P= 0.411) or the change in percent burst firing (n= 24 DA neurons; Pearson Product Moment Correlation, r = −0.298, P= 0.157). Furthermore, the sertindole-induced alteration in activity was stable for the hour following drug administration.
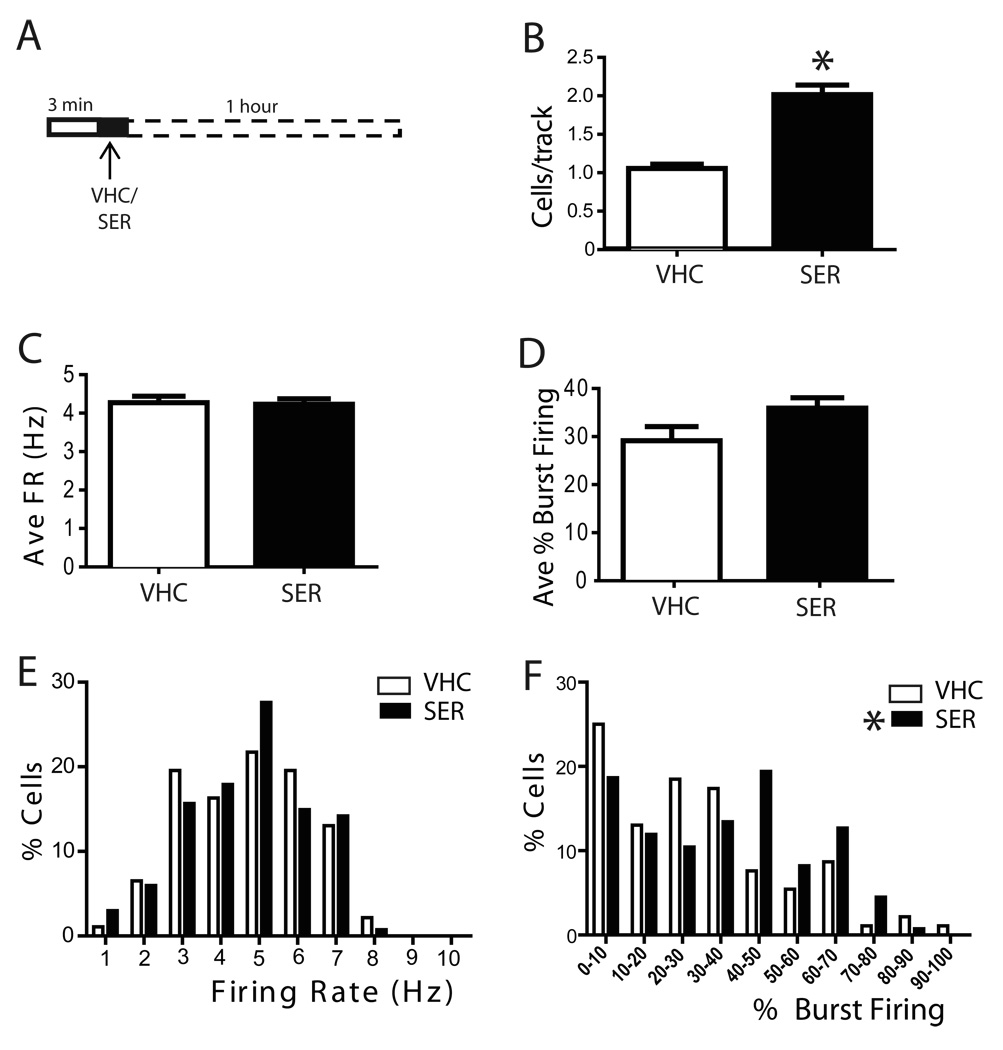
The effect of sertindole on DA neuron population activity (i.e., the number of DA neurons firing per electrode track) was evaluated 1 hour following antipsychotic drug infusion. Rats injected with the sertindole VHC exhibited an average of 1.1 ± 0.1 spontaneous active DA neurons per electrode track, fired at an average firing rate of 4.3 ± 0.2Hz and showed an average percent of burst firing of 29.1 ± 2.9% (n= 12 rats, n= 92 DA neurons). Administration of sertindole induced a prominent increase in DA neuron population activity, producing nearly a doubling in number of active neurons, which was significantly different from vehicle (VHC: 1.1 ± 0.1, n= 12 rats, n= 92 DA neurons; SER: 2 ± 0.1, n= 10 rats, n= 134 DA neurons; One Way ANOVA, Holm-Sidak Method F(1,20)= 55.86, P≤ 0.001; Figure 3B). The sertindole-induced increase in population activity occurred without a significant change in average firing rate (n= 10 rats; One Way ANOVA F(1,20)= 0.0354, P= 0.853; Figure 3C), the average percent burst firing (n= 10 rats; One Way ANOVA F(1,20)= 3.199, P= 0.089; Figure 3D) or the firing rate distribution (n= 10 rats; One Way ANOVA, F(1,224)= 0.008, P= 0.928; Figure 3E) of the DA neurons recorded. However, when the distribution in percent burst firing was plotted, an overall significant effect was revealed, in that there was a decrease in the number of DA neurons with low burst firing and an increase in the number of DA neurons exhibiting high percent burst firing (n= 10 rats; Kruskal-Wallis One Way ANOVA on Ranks, H= 4.374, P= 0.036; Figure 3F).
Figure 3. Acute sertindole administration significantly activated VTA DA neuron population activity and altered burst firing distribution.
A) Schematic of the pharmacological protocol employed in this set of experiments, sertindole was applied while recording the activity of single DA neurons. At one hour following sertindole administration, DA neuron population activity was assessed. Sertindole (1.25 mg/kg) increased the population activity of DA neurons (B) without affecting the average firing rate (C) or average percent burst firing (D). Furthermore, sertindole did not change the distribution of firing rates (E), although it did significantly alter the distribution of burst firing across the neurons recorded (F). VHC= vehicle; SER= sertindole; Cells/track= number of spontaneously firing DA neurons encountered in each electrode tracks. FR= firing rate; B= % burst firing, percent of spikes fired in bursts. *represent significant difference of SER effect from VHC (One Way ANOVA, P≤ 0.001).
Taken together, these data indicate that, although sertindole did not affect the activity of individual DA neurons, acute administration of sertindole produced an overall significant activation of VTA DA neurons within the first hour following administration, which was reflected as an increase in population activity and in the number of neurons exhibiting elevated burst firing.
Effects of low doses of apomorphine on the sertindole-induced increase in VTA DA neuron population activity
In contrast with other D2-blocking drugs, sertindole is reported to fail to reverse the attenuation of DA neuron firing produced by low, putative autoreceptor-selective, doses of DA agonists, such as apomorphine (Skarsfeldt, 1992). However, it is not clear whether this failure is due to lack of blockade, or instead due to a delayed time course of D2 autoreceptor antagonism. In this set of experiments, the time course of sertindole on autoreceptor-mediated inhibition of DA neuron firing was tested (see methods). Thus, 30µg/kg of the DA agonist apomorphine was administered 5 to 10 minutes prior to sertindole (Figure 4A) and, as shown previously by Skarsfeldt (1992), sertindole had no effect on the apomorphine-induced inhibition of firing rate. Administration of apomorphine shortly before sertindole administration significantly reduced the ability of sertindole to increase DA neuron population activity as measured one hour later (SER: 2.0 ± 0.1, n= 10 rats, n= 134 DA neurons; APO + SER: 1.4 ± 0.2, n= 6 rats, n = 67 DA neurons; One Way repeated measure ANOVA, F(9,5)= 12.08, P= 0.018; Fig 4B). Apomorphine administered prior to sertindole did not change the average firing rate (One Way repeated measures ANOVA, F(9,5)= 0.367, P= 0.571; Fig 4C) or the percent burst firing (One Way repeated measures ANOVA, F(9,5)= 1.028, P= 0.357; Fig 4D) when compared to sertindole alone. However, apomorphine administered prior to sertindole altered the firing rate distribution (One Way repeated measures ANOVA, F(133,66)= 139.048, P<0.001; Fig 4E) and the effects of sertindole on the distribution of percent burst firing (One Way repeated measures ANOVA, F(133,66)= 122.508, P< 0.001; Fig 4F).
The ability of apomorphine to reverse the effects of sertindole was also examined by testing the response to apomorphine administered after sertindole. A previous in vivo 3H-raclopride binding study indicated that sertindole reached significant DA D2 receptor occupancy within 5 to 15 min after i.v. injection (L. Tøttrup Brennum, unpublished observation). To determine if there were any delayed actions following steady-state occupancy, 2 time points were tested: i) apomorphine administered 10 minutes after sertindole (Figure 5A1) and ii) apomorphine administered 1 hour after sertindole (Figure 5A2). Consistent with previous reports, administration of apomorphine induced an immediate and pronounced inhibition of both firing rate (pre APO: 4.9 ± 0.8Hz; post APO: 1.3 ± 0.8Hz; % inhibition: 76.1 ± 12.0; n= 5 DA neurons; paired t-test, t4= 6.721, p= 0.002; Figure 5B1 and Figure 5C) and percent burst firing (pre APO: 38.4 ± 12.6%; post APO: 0%; 100% inhibition; n= 5 DA neurons; paired t-test, p= 0.039; Figure 5D). When administered 10 min after sertindole, apomorphine still induced a significant decrease in DA neuron firing rate with a 67.4 ± 10.0% inhibition (pre APOafter10min: 4.4 ± 0.4Hz, post APOafter10min: 1.5 ± 0.5Hz; n= 6 DA neurons; paired t-test, p= 0.0008; Figure 5B2, 5C), and a complete suppression of burst firing (pre APOafter10min: 33.6 ± 14.3%, post APOafter10min: 0; n= 5 DA neurons; paired t-test, p= 0.039; Figure 5D). Similarly, apomorphine continued to significantly attenuate the decrease in DA neuron firing rate even when apomorphine was administered 1 hour after sertindole (pre APOafter1h: 4.2 ± 0.6Hz, post APOafter1h: 2.2 ± 0.6Hz; % inhibition: 46.5 ± 11.7; n= 6 DA neurons; paired t-test, p= 0.006; Figure 5B3, 5C) and percent burst firing (pre APOafter10min: 51.1 ± 12.1%, post APOafter10min: 16.2 ± 10.1; % inhibition: 76.1 ± 13, n= 6 DA neurons; paired t-test, p= 0.007; Figure 5D). Therefore, even when examined at different time points, sertindole still demonstrated an inability to block apomorphine action on assessments normally associated with DA autoreceptor stimulation.
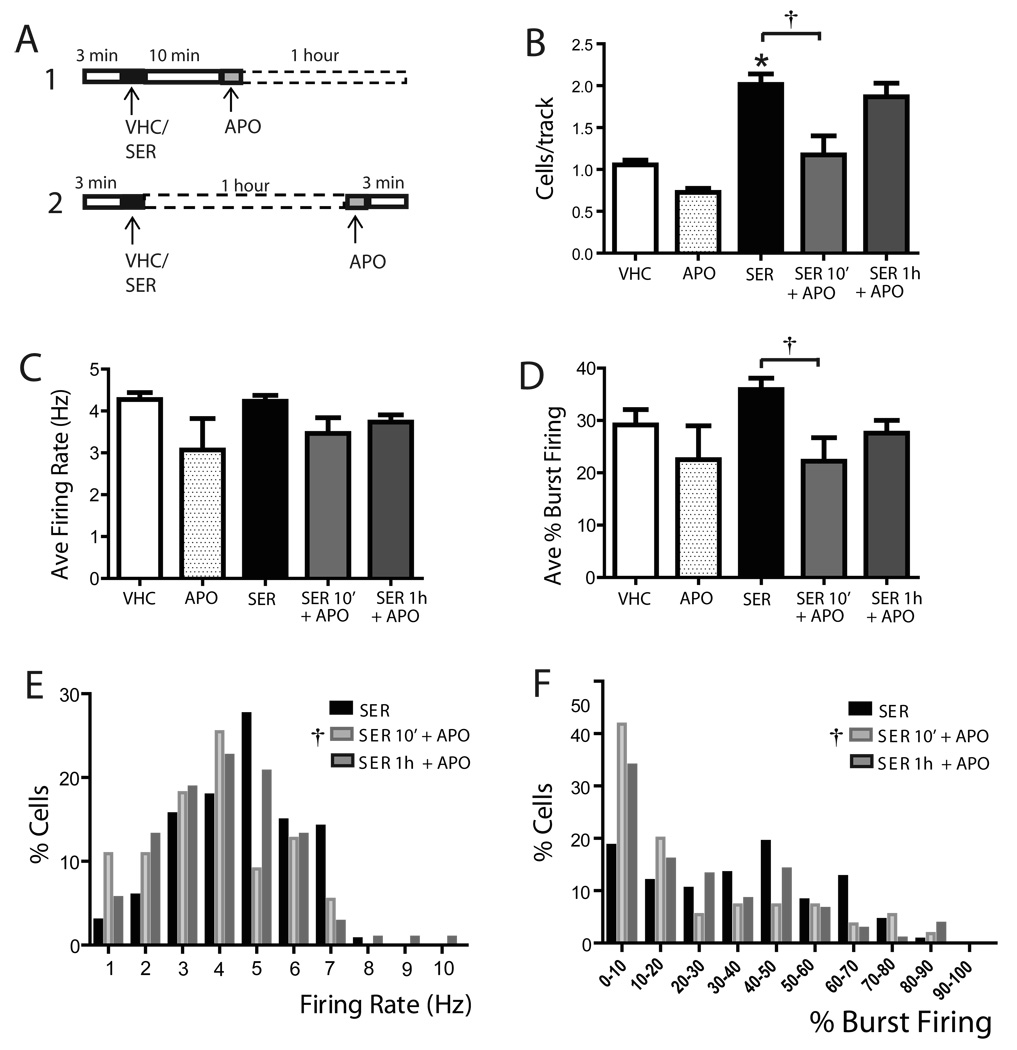
With respect to the effects on VTA DA neuron population activity, apomorphine administered 10 min after sertindole completely reversed the sertindole-induced increase in the number of spontaneously active DA neurons encountered per electrode track (SER: 2.0 ± 0.1, n= 10 rats, n= 134 DA neurons; SER10min +APO: 1.2 ± 0.2, n= 7 rats, n=55 DA neurons; One Way repeated measures ANOVA, F(9,13)= 6.916, P= 0.009; Figure 6B). In contrast, apomorphine failed to reverse the sertindole-induced increase in population activity when administered 1 hour following sertindole (SER1h +APO: n= 8 rats, n=106 DA neurons; One Way repeated measures ANOVA, P> 0.05; Figure 6B). When examined in terms of average firing rate of the population of neurons sampled, apomorphine did not affect firing rate in any of the protocols examined (One Way repeated measures ANOVA, F(9,13)= 1.907, P= 0.188; Figure 6C). Interestingly, apomorphine administered 10 min after sertindole significantly decreased the percent burst firing (One Way repeated measures ANOVA, F(9,13)= 5.377, P= 0.02; Figure 6D), whereas it did not change percent burst firing when administered 1 hour after sertindole (One Way repeated measures ANOVA, P> 0.05; Figure 6D). In addition, apomorphine induced a pronounced leftward shift in the firing rate distribution curves by increasing the percent of neurons firing at low frequencies when administered at 10 minutes (One Way repeated measures ANOVA, F= 56.031, P< 0.001; Figure 6E) but not at 1 hour following sertindole (One Way repeated measures ANOVA, P> 0.05; Figure 6E). Furthermore, apomorphine significantly reversed the effects of sertindole on the distribution of burst firing when administered shortly after (One Way repeated measures ANOVA, F(133,159)= 34.488, P< 0.001; Figure 6F) but not when administer 1 hour following sertindole (One Way repeated measures ANOVA, P≥ 0.05; Figure 6F).
Figure 6. Autoreceptor-selective dose of apomorphine administered after sertindole produced a time-dependent attenuation of the sertindole-induced increase in population activity.
A) Protocol showing administration of 30 µg/kg apomorphine either 10 min (1) or 1 hour (2) after sertindole; in both cases, after ~1 hour following sertindole administration, DA neuron population activity was assessed. (B) Apomorphine reversed the sertindole-induced increase in population activity when administered shortly after sertindole, but not when it was administered 1 hour after sertindole. (C) Apomorphine administered either 10 min or 1 hour after sertindole did not alter the average firing rate. (D) Apomorphine administered shortly after sertindole significantly decreased the percent burst firing, but did not affect burst firing when administered 1 hour after sertindole. (E) Apomorphine significantly altered the firing rate distribution only when administered at 10 minutes following sertindole, producing a leftward shift in firing rate distribution. However, no significant changes were observed when apomorphine is administered 1 hour after sertindole. (F) Apomorphine significantly affected the burst firing distribution when administered at 10 min following sertindole, but not when administered 1 hour after sertindole. VHC= vehicle; SER= sertindole; APO= apomorphine; SER 10’ + APO= sertindole injected 10 minutes prior to apomorphine; SER 1h + APO= sertindole injected 1 hour prior to apomorphine; *represents significant difference between SER and APO (One Way repeated measures ANOVA P≤ 0.001); † representd significant difference between sertindole and SER 10’ + APO (One Way repeated measures ANOVA, P≤ 0.001)
Blockade of accumbens-pallidal transmission prevented the sertindole-induced increase in DA neuron population activity
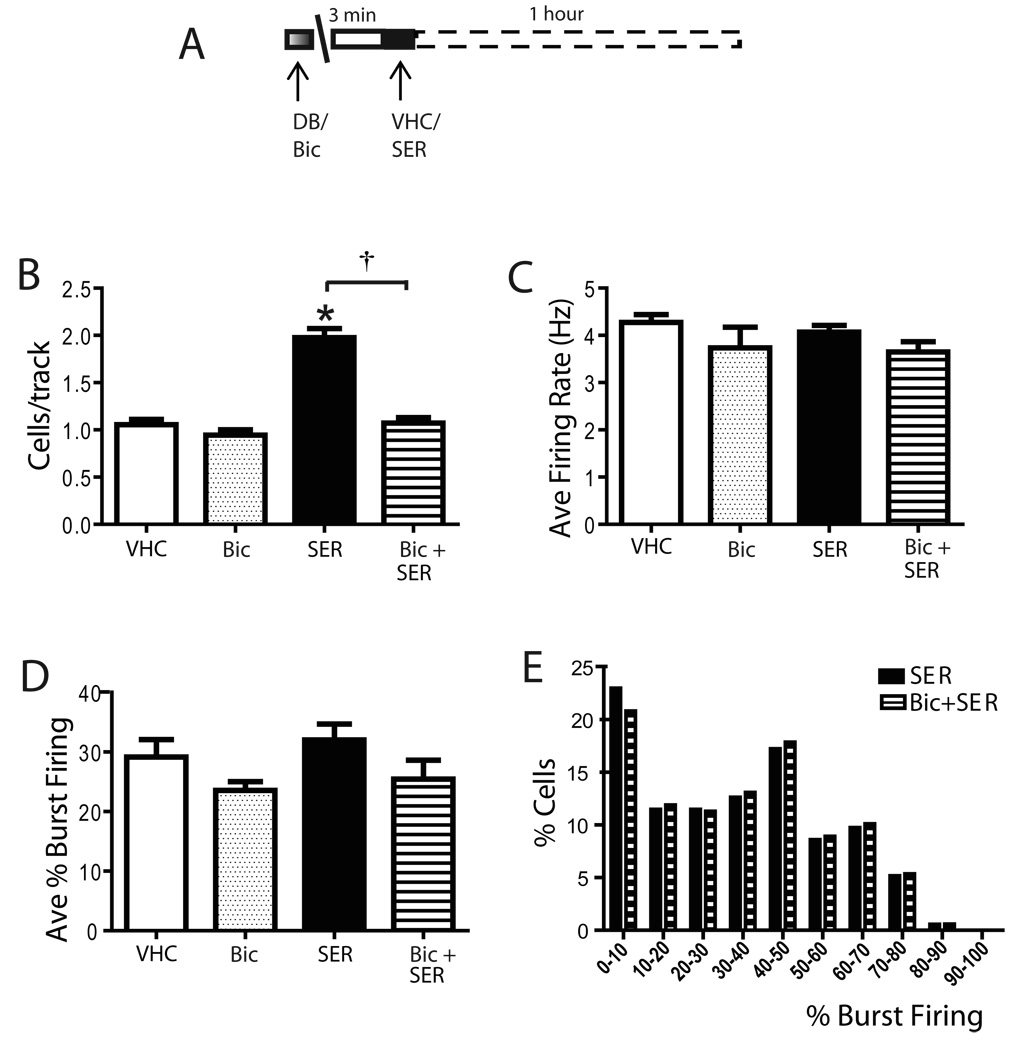
Previous studies have shown that the population activity of VTA DA neurons is dependent on a circuit that includes NAc-VP-VTA pathway (Floresco and Grace, 2003; Floresco et al., 2001). Thus, activation of the NAc would produce an inhibition of the VP which, in turn, disinhibits DA neurons of the VTA. Antipsychotic drugs are known to activate the NAc (Hertel, 2006; Hetey and Drescher, 1986; Marcus et al., 1996), presumably via blockade of DA receptors. Therefore, it is possible that the observed increase in DA neuron population activity may be mediated via the NAc-VP-VTA pathway. To test this hypothesis, the NAc-mediated inhibition was blocked by local injection into the VP of the GABAA antagonist bicuculline prior to antipsychotic drug administration (Figure 7A). In control rats sertindole induced a significant increase in VTA DA neuron population activity similar to that observed in unoperated rats (Figure 7B). Consistent with our previous study (Floresco and Grace, 2003) local infusion of 1 ng (0.5 µl) bicuculline into the VP did not significantly affect any of the parameters of VTA DA neuron population activity when compared to vehicle. Thus, following bicuculline injection, an average of 1 ± 0.1 DA neurons were encountered per electrode track (n= 4 rats, n= 34 DA neurons; One Way ANOVA, F(1,14)= 1.167, P= 0.298; Figure 7B), the average firing rate for DA neurons was of 3.7 ± 0.4Hz (One Way ANOVA, F(1,14)= 2.075, P= 0.172; Fig 7C) and the average percent of burst firing was 24.5 ± 2% (Kruskal-Wallis One Way ANOVA on Ranks, H= 0.941, P= 0.332; Figure 7D). Infusion of bicuculline into the VP prior to recording prevented the sertindole-induced increase in DA neuron population activity (SER: 2.0 ± 0.1, n= 14 rats, n=174 DA neurons; Bic+SER: 1.1 ± 0.1, n= 8 rats, n=75 DA neurons; Kruskal-Wallis One Way ANOVA on Ranks, H= 13.947, P≤ 0.001; Figure 7B) without significantly affecting the average firing rate (One Way ANOVA, F(1,20)= 2.888, P= 0.105; Figure 7C) or the average percent burst firing (One Way ANOVA, F(1,20)= 2.368, P= 0.139; Figure 7D). Furthermore, infusion of bicuculline into the VP did not alter the effects of sertindole on the burst firing distribution (Kruskal-Wallis One Way ANOVA on Ranks, H= 0.156, P= 0.693; Figure 6D). Taken together with the results from the previous experiment with apomorphine, these data suggested that sertindole-induced increase in population activity is mediated through activation of the NAc-VP-VTA pathway.
Figure 7. Sertindole increased VTA DA neuron population activity by activating the accumbens-VP pathway.
A) Protocol illustrating sertindole administration following local infusion of bicuculline; 1 hour after sertindole administration DA population activity was assessed. (B) Infusion of bicuculline into the VP prevented the sertindole-induced increase in population activity. In contrast, bicuculline did not alter the average firing rate (C) or the percent burst firing (D) of VTA DA neurons, nor did it alter the distribution in percent burst firing (E). VHC= vehicle; DB= Dulbecco’s Buffer PBS; Bic = bicuculline infused into the VP; SER= sertindole; Bic + SER= bicuculline infused in VP, sertindole injected i.v. *represent significant difference between sertindole and VHC (One Way ANOVA, P≤ 0.001); † represents significant difference between sertindole and Bic + SER (Kruskal-Wallis One Way ANOVA on Ranks, P≤ 0.001).
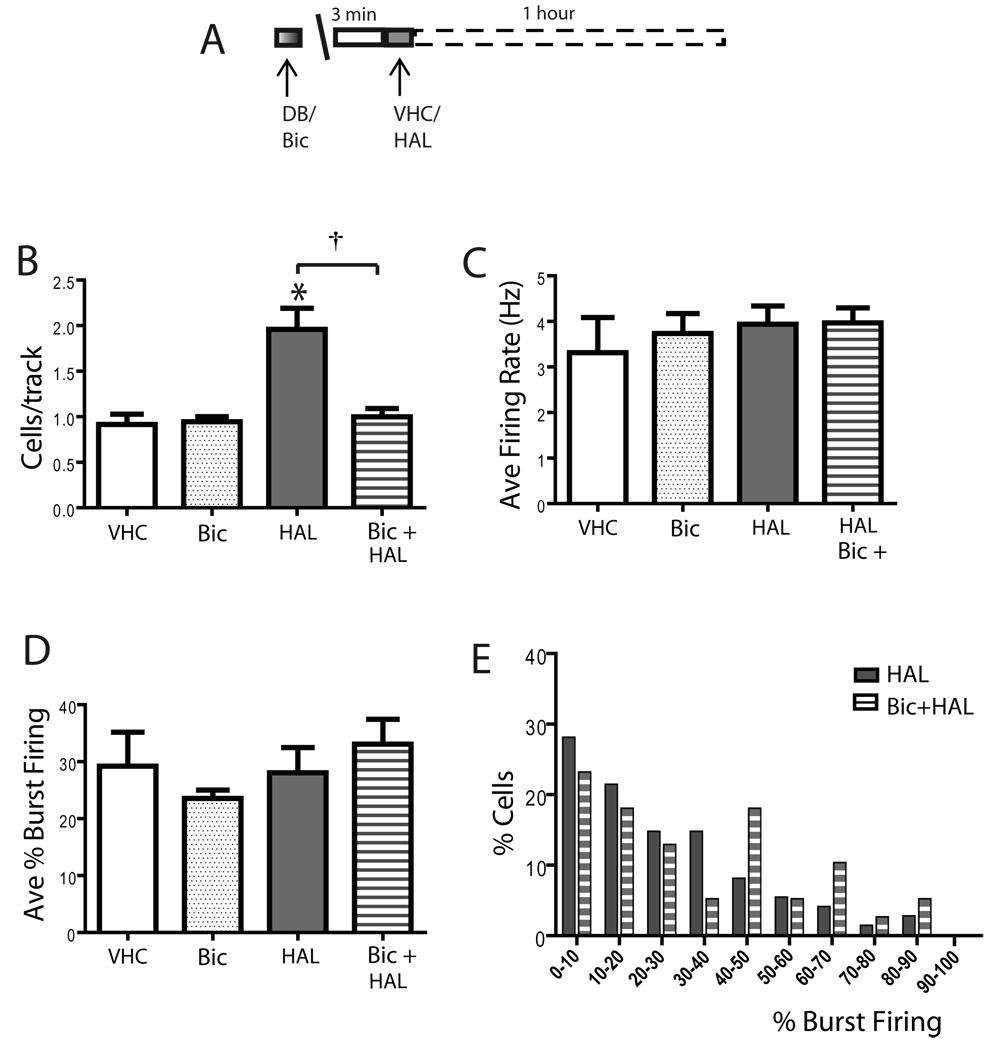
To examine whether the activation of DA neuron population activity via the NAc-VP-VTA pathway is a common mechanism of antipsychotic drug effects on DA neuron population activity, the dependence of this pathway on the actions of a first generation, classical antipsychotic drug with known autoreceptor-blocking properties was tested (Figure 8A). Consistent with previous reports, in unoperated rats 0.8 mg/kg haloperidol potently increased DA neuron population activity, yielding a pronounced increase in the number of spontaneously active DA neurons (VHC: 1.0 ± 0.1, n= 4 rats, n=35 DA neurons; HAL: 2.0 ± 0.2, n= 6 rats, n=75 DA neurons; One Way ANOVA, F(1,8)= 9.040, P= 0.0017; Figure 8B), without affecting the average firing rate (One Way ANOVA, F(1,8)= 3.557, P= 0.096; Figure 8C) or average percent burst firing (One Way ANOVA, F(1,8)= 0.0175, P= 0.898; Figure 8D). As with sertindole, infusion of bicuculline into the VP completely prevented the haloperidol-induced increase in population activity (HAL: 2.0 ± 0.2, n= 6 rats, n=75 DA neurons; Bic+HAL: 1.0 ± 0.1, n= 6 rats, n= 39 DA neurons; One Way ANOVA, F(1,10)= 14.856, P= 0.003; Figure 8B). In addition, bicuculline infusion failed to affect the average firing rate (One Way ANOVA, F(1,10)= 0.002, P= 0.965; Figure 8C) or the percent burst firing (One Way ANOVA, F(1,10)= 0.591, P= 0.46; Figure 8D) observed in rats injected with haloperidol alone. Furthermore, there was no change in the distribution in percent burst firing (Kruskal-Wallis One Way ANOVA on Ranks, H= 1.350, P= 0.245; Figure 8E).
Figure 8. Haloperidol increased VTA DA population activity through activation of the NAc-VP pathway.
A) Protocol illustrating haloperidol administration following local infusion of bicuculline; 1 hour after haloperidol administration DA population activity was assessed. (B) Similar to sertindole, blockade of the nucleus NAc-VP pathway by infusion of bicuculline into the VP also prevented the increase in population activity following haloperidol (0.8 mg/kg) administration, without affecting the average firing rate (C), the percent of burst firing (D), or the distribution in percent burst firing (E). VHC= vehicle; DB= Dulbecco’s Buffer PBS; Bic = bicuculline infused into the VP; HAL= haloperidol; Bic + HAL= bicuculline infused in VP, haloperidol injected i.v. *represents significant difference between haloperidol and vehicle (One Way ANOVA, P< 0.05); † represents significant difference between haloperidol and Bic + HAL (One Way ANOVA, P< 0.05).
Therefore, both the first-generation antipsychotic drug haloperidol, which exhibits DA autoreceptor blocking properties, and the second-generation drug sertindole, which fails to block DA neuron autoreceptors, appear to increase DA neuron population activity via common mechanisms within the NAc-VP-VTA pathway.
Discussion
The present study evaluated the mechanism by which acute administration of a first- (haloperidol) and second- (sertindole) generation antipsychotic drug affected DA neuron firing in the rat VTA. Intravenous administration of sertindole induced a prominent and significant increase in DA neuron population activity 1 hour following administration. Furthermore, selective blockade of GABAergic transmission in the NAc-VP pathway reversed the increase in population activity produced by either sertindole or haloperidol administration. Thus, these data demonstrate that antipsychotic drugs affect DA neuron population activity via an action within the NAc rather than by direct blockade of DA neuron cell body autoreceptors.
Blockade of postsynaptic dopamine receptors in the nucleus accumbens mediates the increase in VTA DA neuron population activity
Numerous studies have documented the importance of D2 receptor blockade in the therapeutic efficacy of antipsychotic drugs (Abi-Dargham, 2004; Arnt and Skarsfeldt, 1998; Kapur and Remington, 2001; O'Donnell and Grace, 1998). Moreover, it is suggested that at least a part of this therapeutic action is mediated via an effect on DA neuron activity states (Grace et al., 1997). Acute administration of both first-generation and second-generation antipsychotic drugs is known to activate DA neuron firing; in particular, they cause an increase in DA neuron population activity (Bunney and Grace, 1978; Chiodo and Bunney, 1983; Grace and Bunney, 1986; Boye and Rompre, 2000), and augment burst firing and firing rate when tested on individual neurons (Bunney, 1988; Bunney et al., 1991; Bunney et al., 1973; Chiodo and Bunney, 1983). However, which of these effects are mediated via an action on the highly sensitive, firing rate-modulating autoreceptors on DA neuron somata and which are due to activation of feedback pathways via blockade of DA receptors on dopaminoceptive neurons is not well understood.
Sertindole was found to replicate the ability of other antipsychotic drugs to increase the number of DA neurons firing spontaneously. However, sertindole failed reverse the inhibition of DA neuron firing produced by low, autoreceptor-selective doses of apomorphine (Skarsfeldt, 1992). Blockade of D2 autoreceptors localized on the somatodendritic region of substantia nigra pars compacta DA neurons by haloperidol was shown to cause a modest increase in the firing rate of spontaneously active DA neurons, accompanied by an increase in input resistance and depolarization of membrane potentials in some of these neurons (Pucak and Grace, 1996). Although acute sertindole did not produce an alteration in burst firing or firing rate when evaluated over the population of DA neurons recorded, acute sertindole administration was found to have a bimodal effect, with almost half of the DA neurons exhibiting a significant level of excitation and half exhibiting inhibition. Furthermore, as previously shown (Skarsfeldt, 1992), sertindole failed to reverse the apomorphine-induced inhibition of DA neuron firing, in contrast to the actions of haloperidol and other D2-blocking drugs. Therefore, it is reasonable to assume that the ability of sertindole to increase DA neuron population activity is not due to an effect on DA neuron autoreceptors, but instead is mediated via blockade of DA receptors within postsynaptic targets. This model is borne out by the current study. Thus, data indicate that D2 antagonists will increase neuronal firing in the NAc (Hu et al., 2005; Hu and White, 1997; Perez et al., 2006). Moreover, activation of the NAc will produce a GABAergic inhibition of firing of the VP, relieving DA neurons from inhibition and increasing DA neuron population activity (Floresco and Grace, 2003; Floresco et al., 2001). Consistent with this model, injection of the GABA antagonist bicuculline into the VP, which should block the effects of NAc activation on this structure, also blocks the ability of sertindole to increase DA neuron population activity. Thus, both sertindole and haloperidol, and likely other DA D2 antagonist antipsychotic drugs, appear to exert their effects on DA neuron population activity via their effects on this feedback pathway.
Although most of these data are consistent with an inability of sertindole to block DA autoreceptors, it is unclear why low doses of apomorphine administered shortly after sertindole can nonetheless attenuate the ability of sertindole to increase DA neuron population activity. It is possible that this effect of apomorphine may be due to sites of action other than on neuronal somatodendritic regions. Thus, although low doses of apomorphine may not exert postsynaptic actions on neurons in the striatum at low doses (Skirboll et al., 1979), it has been shown that very low extracellular concentrations of DA are capable of stimulating presynaptic DA receptors, such as those on prefrontal cortical afferents to the accumbens (O'Donnell and Grace, 1994). Indeed, we have shown that attenuation of medial prefrontal cortex (mPFC) activity will attenuate ventral hippocampal (vHPC) drive of NAc neurons (Belujon and Grace, 2008). Given that vHPC drive of the NAc determines DA neuron population activity (Floresco et al., 2001), interruption of this drive may prevent sertindole-induced activation of the NAc, thereby circumventing the effect on DA neuron population activity. On the other hand, it is unclear why apomorphine administered one hour after sertindole failed to reverse the increase in DA neuron population activity. It may be that, following an extended period of DA receptor blockade, other compensatory mechanisms may be induced that segregate the effects of NAc DA D2 blockade from VTA neuron activity. One possibility is an induction of ventral subiculum (vSub)-NAc long-term potentiation (Goto and Grace, 2005). Indeed, following induction of LTP in this pathway, our studies show that the mPFC is no longer required for vSub drive of NAc neurons (Belujon and Grace, 2008).
Conclusions
These data highlight the importance of striatofugal pathways in the effects of antipsychotic drugs on DA neuron population activity. Both first- and second-generation antipsychotic drugs are known to activate c-fos in the NAc (Deutch, 1994; Fink-Jensen and Kristensen, 1994), which is consistent with the role of NAc activation in driving DA neuron population activity. We have proposed previously that the ability of antipsychotic drugs to activate DA neuron population firing will serve to blunt the actions of these drugs on blocking DA transmission in the NAc; i.e., by increasing DA neuron activity, acute administration of antipsychotic drugs would increase DA release that would work against antipsychotic drug-induced D2 receptor blockade (Grace et al., 1997). However, our recent studies show that, in a developmental disruption model of schizophrenia based on prenatal administration of metholazoxymethanol acetate (Moore et al., 2006), population activity within the VTA is already in an elevated state (Lodge and Grace, 2007), and moreover, this appears to underlie the DA system hyper-responsivity in this model (Lodge and Grace, 2008). Under these conditions, one would predict that antipsychotic drugs would exert greater levels of DA D2 blockade due to the absence of this compensatory process. Such a model could account for the rapid onset of antipsychotic action reported to occur in schizophrenia patients (Agid et al., 2008; Breier et al., 2002; Kapur et al., 2005).
Acknowledgement
The authors would like to thank Niki Macmurdo and Emily Mahar for technical assistance, Brian Lowry for the custom-design software Neuroscope, and Dr. Daniel Lodge for helpful discussions. This work was supported by USPHS MH57440 and a research grant from H. Lundbeck H/S to A. A. Grace.
The authors declare that this work was supported in part by a research grant from H. Lundbeck H/S to A. A. Grace.
Footnotes
Disclosure/conflict of interest
Dr. O. Valenti has received partial support from Lundbeck
Dr. A. A. Grace has the following conflicts to disclose:
Consultant: Johnson & Johnson, Taisho; research grants from Lundbeck, Abbott, pharmaceutical company talks (Abbott, Roche, Lundbeck); advisory boards (as non-member invited speaker, including Astra-Zeneca, Novartis, Boehringer Ingelheim); legal consulting: Phillips/Lyttel representing Galaxo Smith Klein; Schiff-Harden representing Sandoz Pharmaceutical.
References
- Abi-Dargham A. Do we still believe in the dopamine hypothesis? New data bring new evidence. International Journal of Neuropsychopharmacology. 2004;7 Suppl 1:S1–S5. doi: 10.1017/S1461145704004110. [DOI] [PubMed] [Google Scholar]
- Agid O, Kapur S, Warrington L, Loebel A, Siu C. Early onset of antipsychotic response in the treatment of acutely agitated patients with psychotic disorders. Schizophrenia Research. 2008;102(1–3):241–248. doi: 10.1016/j.schres.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Arnt J. Differential effects of classical and newer antipsychotics on the hypermotility induced by two dose levels of D-amphetamine. European Journal of Pharmacology. 1995;283(1–3):55–62. doi: 10.1016/0014-2999(95)00292-s. [DOI] [PubMed] [Google Scholar]
- Arnt J, Skarsfeldt T. Do novel antipsychotics have similar pharmacological characteristics? A review of the evidence. Neuropsychopharmacology. 1998;18(2):63–101. doi: 10.1016/S0893-133X(97)00112-7. [DOI] [PubMed] [Google Scholar]
- Belujon P, Grace AA. Critical role of the prefrontal cortex in the regulation of hippocampus-accumbens information flow. Journal of Neuroscience. 2008;28(39):9797–9805. doi: 10.1523/JNEUROSCI.2200-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boye SM, Rompre PP. Behavioral evidence of depolarization block of dopamine neurons after chronic treatment with haloperidol and clozapine. Journal of Neuroscience. 2000;20(3):1229–1239. doi: 10.1523/JNEUROSCI.20-03-01229.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier A, Meehan K, Birkett M, David S, Ferchland I, Sutton V, Taylor CC, Palmer R, Dossenbach M, Kiesler G, Brook S, Wright P. A double-blind, placebo-controlled dose-response comparison of intramuscular olanzapine and haloperidol in the treatment of acute agitation in schizophrenia. Archives of General Psychiatry. 2002;59(5):441–448. doi: 10.1001/archpsyc.59.5.441. [DOI] [PubMed] [Google Scholar]
- Bunney BS. Effects of acute and chronic neuroleptic treatment on the activity of midbrain dopamine neurons. Annals of the New York Academy of Sciences. 1988;537:77–85. doi: 10.1111/j.1749-6632.1988.tb42097.x. [DOI] [PubMed] [Google Scholar]
- Bunney BS, Chiodo LA, Grace AA. Midbrain dopamine system electrophysiological functioning: a review and new hypothesis. Synapse. 1991;9(2):79–94. doi: 10.1002/syn.890090202. [DOI] [PubMed] [Google Scholar]
- Bunney BS, Grace AA. Acute and chronic haloperidol treatment: comparison of effects on nigral dopaminergic cell activity. Life Science. 1978;23(16):1715–1727. doi: 10.1016/0024-3205(78)90471-x. [DOI] [PubMed] [Google Scholar]
- Bunney BS, Walters JR, Roth RH, Aghajanian GK. Dopaminergic neurons: effect of antipsychotic drugs and amphetamine on single cell activity. Journal of Pharmacology and Experimental Therapeutics. 1973;185(3):560–571. [PubMed] [Google Scholar]
- Carlsson A, Lindqvist M. Effect of Chlorpromazine or Haloperidol on Formation of 3methoxytyramine and Normetanephrine in Mouse Brain. Acta Pharmacologica et Toxicologica. 1963;20:140–144. doi: 10.1111/j.1600-0773.1963.tb01730.x. [DOI] [PubMed] [Google Scholar]
- Chiodo LA, Bunney BS. Typical and atypical neuroleptics: differential effects of chronic administration on the activity of A9 and A10 midbrain dopaminergic neurons. Journal of Neuroscience. 1983;3(8):1607–1619. doi: 10.1523/JNEUROSCI.03-08-01607.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutch AY. Identification of the neural systems subserving the actions of clozapine: clues from immediate-early gene expression. Journal of Clinical Psychiatry. 1994;55 Suppl B:37–42. [PubMed] [Google Scholar]
- Fink-Jensen A, Kristensen P. Effects of typical and atypical neuroleptics on Fos protein expression in the rat forebrain. Neuroscience Letters. 1994;182(1):115–118. doi: 10.1016/0304-3940(94)90220-8. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Grace AA. Gating of hippocampal-evoked activity in prefrontal cortical neurons by inputs from the mediodorsal thalamus and ventral tegmental area. Journal of Neuroscience. 2003;23(9):3930–3943. doi: 10.1523/JNEUROSCI.23-09-03930.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Todd CL, Grace AA. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. Journal of Neuroscience. 2001;21(13):4915–4922. doi: 10.1523/JNEUROSCI.21-13-04915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Dopamine-dependent interactions between limbic and prefrontal cortical plasticity in the nucleus accumbens: disruption by cocaine sensitization. Neuron. 2005;47(2):255–266. doi: 10.1016/j.neuron.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons--1. Identification and characterization. Neuroscience. 1983;10(2):301–315. doi: 10.1016/0306-4522(83)90135-5. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. Induction of depolarization block in midbrain dopamine neurons by repeated administration of haloperidol: analysis using in vivo intracellular recording. Journal of Pharmacology & Experimental Therapeutics. 1986;238(3):1092–1100. [PubMed] [Google Scholar]
- Grace AA, Bunney BS, Moore H, Todd CL. Dopamine-cell depolarization block as a model for the therapeutic actions of antipsychotic drugs. Trends in Neuroscience. 1997;20(1):31–37. doi: 10.1016/S0166-2236(96)10064-3. [DOI] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends in Neurosciences. 2007;30(5):220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Hertel P. Comparing sertindole to other new generation antipsychotics on preferential dopamine output in limbic versus striatal projection regions: mechanism of action. Synapse. 2006;60(7):543–552. doi: 10.1002/syn.20322. [DOI] [PubMed] [Google Scholar]
- Hetey L, Drescher K. Influence of antipsychotics on presynaptic receptors modulating the release of dopamine in synaptosomes of the nucleus accumbens of rats. Neuropharmacology. 1986;25(10):1103–1109. doi: 10.1016/0028-3908(86)90157-7. [DOI] [PubMed] [Google Scholar]
- Hu XT, Dong Y, Zhang XF, White FJ. Dopamine D2 receptor-activated Ca2+ signaling modulates voltage-sensitive sodium currents in rat nucleus accumbens neurons. Journal of Neurophysiology. 2005;93(3):1406–1417. doi: 10.1152/jn.00771.2004. [DOI] [PubMed] [Google Scholar]
- Hu XT, White FJ. Dopamine enhances glutamate-induced excitation of rat striatal neurons by cooperative activation of D1 and D2 class receptors. Neuroscience Letters. 1997;224(1):61–65. doi: 10.1016/s0304-3940(97)13443-7. [DOI] [PubMed] [Google Scholar]
- Kane JM, Tamminga CA. Sertindole (Serdolect): preclinical and clinical findings of a new atypical antipsychotic. Expert Opinion on Investigational Drugs. 1997;6(11):1729–1741. doi: 10.1517/13543784.6.11.1729. [DOI] [PubMed] [Google Scholar]
- Kapur S, Arenovich T, Agid O, Zipursky R, Lindborg S, Jones B. Evidence for onset of antipsychotic effects within the first 24 hours of treatment. American Journal of Psychiatry. 2005;162(5):939–946. doi: 10.1176/appi.ajp.162.5.939. [DOI] [PubMed] [Google Scholar]
- Kapur S, Remington G. Dopamine D(2) receptors and their role in atypical antipsychotic action: still necessary and may even be sufficient. Bioogical Psychiatry. 2001;50(11):873–883. doi: 10.1016/s0006-3223(01)01251-3. [DOI] [PubMed] [Google Scholar]
- Kinon BJ, Lieberman JA. Mechanisms of action of atypical antipsychotic drugs: a critical analysis. Psychopharmacology. 1996;124(1–2):2–34. doi: 10.1007/BF02245602. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. Journal of Neuroscience. 2007;27(42):11424–11430. doi: 10.1523/JNEUROSCI.2847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Amphetamine activation of hippocampal drive of mesolimbic dopamine neurons: a mechanism of behavioral sensitization. Journal of Neuroscience. 2008;28(31):7876–7882. doi: 10.1523/JNEUROSCI.1582-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus MM, Nomikos GG, Svensson TH. Differential actions of typical and atypical antipsychotic drugs on dopamine release in the core and shell of the nucleus accumbens. European Neuropsychopharmacology. 1996;6(1):29–38. doi: 10.1016/0924-977x(95)00056-u. [DOI] [PubMed] [Google Scholar]
- Meltzer HY. Relevance of dopamine autoreceptors for psychiatry: preclinical and clinical studies. Schizophrenia Bulletin. 1980;6(3):456–475. doi: 10.1093/schbul/6.3.456. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Matsubara S, Lee JC. The ratios of serotonin2 and dopamine2 affinities differentiate atypical and typical antipsychotic drugs. Psychopharmacology Bulletin. 1989;25(3):390–392. [PubMed] [Google Scholar]
- Meltzer HY, Stahl SM. The dopamine hypothesis of schizophrenia: a review. Schizophrenia Bulletin. 1976;2(1):19–76. doi: 10.1093/schbul/2.1.19. [DOI] [PubMed] [Google Scholar]
- Meng ZH, Feldpaush DL, Merchant KM. Clozapine and haloperidol block the induction of behavioral sensitization to amphetamine and associated genomic responses in rats. Brain Research Molecular Brain Research. 1998;61(1–2):39–50. doi: 10.1016/s0169-328x(98)00196-x. [DOI] [PubMed] [Google Scholar]
- Moore H, Jentsch JD, Ghajarnia M, Geyer MA, Grace AA. A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: implications for the neuropathology of schizophrenia. Bioogicall Psychiatry. 2006;60(3):253–264. doi: 10.1016/j.biopsych.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natesan S, Reckless GE, Nobrega JN, Fletcher PJ, Kapur S. Dissociation between in vivo occupancy and functional antagonism of dopamine D2 receptors: comparing aripiprazole to other antipsychotics in animal models. Neuropsychopharmacology. 2006;31(9):1854–1863. doi: 10.1038/sj.npp.1300983. [DOI] [PubMed] [Google Scholar]
- O'Donnell P, Grace AA. Tonic D2-mediated attenuation of cortical excitation in nucleus accumbens neurons recorded in vitro. Brain Research. 1994;634(1):105–112. doi: 10.1016/0006-8993(94)90263-1. [DOI] [PubMed] [Google Scholar]
- O'Donnell P, Grace AA. Dysfunctions in multiple interrelated systems as the neurobiological bases of schizophrenic symptom clusters. Schizophrenia Bulletin. 1998;24(2):267–283. doi: 10.1093/oxfordjournals.schbul.a033325. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th Edition. Academic Press; 1998. [Google Scholar]
- Perez MF, White FJ, Hu XT. Dopamine D(2) receptor modulation of K(+) channel activity regulates excitability of nucleus accumbens neurons at different membrane potentials. Journal of Neurophysiology. 2006;96(5):2217–2228. doi: 10.1152/jn.00254.2006. [DOI] [PubMed] [Google Scholar]
- Pucak ML, Grace AA. Effects of haloperidol on the activity and membrane physiology of substantia nigra dopamine neurons recorded in vitro. Brain Research Molecular Brain Research. 1996;713(1–2):44–52. doi: 10.1016/0006-8993(95)01460-8. [DOI] [PubMed] [Google Scholar]
- Sams-Dodd F. Effect of novel antipsychotic drugs on phencyclidine-induced stereotyped behaviour and social isolation in the rat social interaction test. Behavioural Pharmacology. 1997;8(2–3):196–215. [PubMed] [Google Scholar]
- Seeman P, Chau-Wong M, Tedesco J, Wong K. Brain receptors for antipsychotic drugs and dopamine: direct binding assays. Proceedings of the National Academy of Sciences of the United States of America. 1975;72(11):4376–4380. doi: 10.1073/pnas.72.11.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarsfeldt T. Electrophysiological profile of the new atypical neuroleptic, sertindole, on midbrain dopamine neurones in rats: acute and repeated treatment. Synapse. 1992;10(1):25–33. doi: 10.1002/syn.890100105. [DOI] [PubMed] [Google Scholar]
- Skarsfeldt T. Differential effects of repeated administration of novel antipsychotic drugs on the activity of midbrain dopamine neurons in the rat. European Journal of Pharmacology. 1995;281(3):289–294. doi: 10.1016/0014-2999(95)00260-r. [DOI] [PubMed] [Google Scholar]
- Skarsfeldt T, Perregaard J. Sertindole, a new neuroleptic with extreme selectivity on A10 versus A9 dopamine neurones in the rat. European Journal of Pharmacology. 1990;182(3):613–614. doi: 10.1016/0014-2999(90)90067-g. [DOI] [PubMed] [Google Scholar]
- Skirboll LR, Grace AA, Bunney BS. Dopamine auto- and postsynaptic receptors: electrophysiological evidence for differential sensitivity to dopamine agonists. Science. 1979;206(4414):80–82. doi: 10.1126/science.482929. [DOI] [PubMed] [Google Scholar]
- Walters JR, Roth RH, Aghajanian GK. Dopaminergic neurons: similar biochemical and histochemical effects of gamma-hydroxybutyrate and acute lesions of the nigro-neostriatal pathway. Journal of Pharmacology & Experimental Therapeutics. 1973;186(3):630–639. [PubMed] [Google Scholar]
- Watanabe M, Hagino Y. The atypical antipsychotic sertindole enhances efflux of dopamine and its metabolites in the rat cortex and striatum. European Journal of Pharmacology. 1999;367(1):19–23. doi: 10.1016/s0014-2999(98)00936-4. [DOI] [PubMed] [Google Scholar]