Abstract
Traditional homemade brew is believed to represent the highest proportion of alcohol use in sub-Saharan Africa. In Eldoret, Kenya, two types of brew are common: chang’aa, spirits, and busaa, maize beer. Local residents refer to the amount of brew consumed by the amount of money spent, suggesting a culturally relevant estimation method. The purposes of this study were to analyze ethanol content of chang’aa and busaa; and to compare two methods of alcohol estimation: use by cost, and use by volume, the latter the current international standard. Laboratory results showed mean ethanol content was 34% (SD = 14%) for chang’aa and 4% (SD = 1%) for busaa. Standard drink unit equivalents for chang’aa and busaa, respectively, were 2 and 1.3 (US) and 3.5 and 2.3 (Great Britain). Using a computational approach, both methods demonstrated comparable results. We conclude that cost estimation of alcohol content is more culturally relevant and does not differ in accuracy from the international standard.
Keywords: Alcohol, Traditional brew, HIV, Kenya, Cognitive behavioral treatment
Introduction
Of the 33.2 million people in the world estimated to be infected with the HIV virus, 22.5 million of them live in sub-Saharan Africa (UNAIDS/WHO 2007). Because of alcohol’s association with the HIV epidemic (Hargreves 2002; Ayisi et al. 2000) through risky sex (Seage et al. 2002; Apostolopoulos et al. 2002; Jemmot and Brown 2003), U.S. public health agencies, non-governmental organizations and foundations have begun to consistently fund efforts to reduce alcohol use and to stem the HIV epidemic in sub-Saharan Africa. For example, the U.S. National Institute of Alcohol Abuse and Alcoholism has increased funding for Africa-affiliated projects from one study from 1991 to 2000 to 25 studies from 2001 to the present (National Institutes of Health 2008). Because of this trend, international research collaborations are challenged to increase cultural proficiencies and to adapt methods and interventions to local culture, without a loss of precision.
Estimating alcohol use in resource-limited settings is particularly challenging for several reasons: the lack of a “standard drink” where drink type, ethanol content and serving size may vary from region to region within the same country, and the requisite translation and adaption of methods across vastly different cultures and levels of education. In contrast, estimating alcohol use in industrialized countries where the ethanol content and serving sizes are standardized is typically accomplished through the identification of volume in a typical serving size. This approach has been taken in U.S., Canada, Great Britain, Japan and Australia, where standard drinks have been identified that vary from 8 to 19.75 g of ethanol content (Miller et al. 1991; World Health Organization 2001).
Several studies have documented a high rate of alcohol abuse in Kenya (Othieno et al. 2000; Hall et al. 1993; Saunders et al. 1993a, b). In a multi-country study by the World Health Organization of primary care attendees who reported at least one drink in the past year and no past alcohol treatment, Kenyans (78% men) reported the highest alcohol use across the six countries (U.S., Mexico, Norway, Australia, and Bulgaria), including binge drinking (72%), at least six drinks per week (49%), median alcohol consumption in 30 days (799 g) and alcohol dependence (20%). The percentage of drinkers who noted at least one alcohol problem in Kenya (47%) was second only to the U.S. (49%) (Hall et al. 1993; Saunders et al. 1993a, b). In Eldoret in western Kenya, hazardous drinking using the AUDIT questionnaire has been reported by 68% of general medicine outpatients and 53% of HIV-infected outpatients (Shaffer et al. 2004).
Traditional alcoholic brew is often made in homes and villages using seeds, grains, fruit, vegetables or palm sap, and is believed to make up the highest proportion of alcohol use in Africa (Willis 2002; McCall 1996), perhaps because it is typically less expensive than commercially brewed drink (World Health Organization 2004; Willis 2002). One clinical study in the Eldoret area showed that traditional brew is drunk by 54% of rural and 21% of town HIV-infected outpatients (Shaffer et al. 2004). In Kenya, the two most common traditional brews are chang’aa, which refers to distilled spirits, and busaa, a cereal-based fermented beer. Busaa is typically made from the most plentiful source of grain, whether maize, millet or sorghum, while chang’aa is often made from busaa residue as well as sugar, but can also be made from bananas. Although brewing and selling traditional drink is illegal in Kenya (Willis 2002), drinking traditional brew is a common activity during many social and religious ceremonies. For example, drinking busaa is integral to the custom of group genital circumcisions, weddings and funerals (Willis 2002). Once controlled by elder men, drinking of traditional brew has been largely transformed into a commercial enterprise that includes female and young consumers (Willis 2002; Papas et al. 2008) and is believed to be more frequently brewed and sold by women today (Holtzman 2001; McCall 1996; Obot 2007).
The current substudy was part of an NIH-funded feasibility study (R21AA016884), The Kenya Health Behavior Study, to adapt cognitive behavioral treatment (CBT) to reduce alcohol use among HIV-infected clinical outpatients in Eldoret, Kenya, where there is currently no alcohol treatment except detoxification. The goals of this stage 1 pilot project are to adapt CBT to the cultural context, to train Kenyan lay facilitators in group CBT delivery and psychologists in quality/fidelity monitoring (Phase I), and to evaluate the efficacy of a six-session gender-stratified group CBT to reduce alcohol use among HIV-infected Kenyans recently initiated on ARVs, when compared against a usual care support group (Phase II). Because our alcohol outcome measure is the Timeline Followback (Sobell and Sobell, 1992), we sought to measure and quantify traditional brew within the region so that we could tailor our study methods accordingly. We could find no published reports of systematic chemical analyses of Kenyan traditional brew.
In our experience working with residents of the Eldoret area, local drinkers report the amount of chang’aa and busaa drunk by the amount of Kenyan shillings spent. For example, a local resident will describe drinking “kumi kumi” of chang’aa which means “ten shillings” and “kumi mbili” means “two tens” or “twenty shillings.” Additionally, when we visited traditional breweries and asked the daily quantity of alcohol drunk, drinkers replied to us that they drank, e.g., “500 shillings” per day. Our observations are consistent with the report from a high-volume local brewer in Eldoret that consumers describe alcohol use exclusively in terms of cost in shillings. Based on these observations and in an effort to make our estimation culturally relevant, we sought to determine whether estimating alcohol use by cost would produce as accurate an estimation of ethanol as estimating alcohol use by volume, the current international standard.
Hence, our study had two purposes: to analyze ethanol content of commonly available traditional brew in Eldoret, Kenya; and to compare two different quantitative methods of estimating alcohol use against the gold standard, the volume of ethanol per drink: estimating by drink volume, the current international standard, and estimating by drink cost.
Methods
Data Collection
In November 2007, we collected samples of two types of traditional brew from each of five sites (3 town, 2 rural) that are within 1 hour travel distance from the Moi Teaching and Referral Hospital in Eldoret, Kenya. The 1-hour travel restriction is one of the patient inclusion criteria for our intervention study.
As local residents report that traditional brew is typically drunk as a cheaper alternative to commercial brew and that sites are prevalent throughout Eldoret, brewery sites by definition cater to the poorest individuals. To maximize generalizability, we chose sites representing divergent populations and sizes. The town sites were Huruma, Langas, and Kipkaren estate. The rural sites were Naiberi and Soy. We included breweries located in the two largest, poorest and most concentrated town dwellings in Eldoret: Huruma and Langas, so as to sample locations where most people buy their brew. The Langas site houses a high-volume brewery. In contrast to the town sites, which typically cater to a heterogeneous group of consumers, the rural sites are smaller and typically cater to single tribes. We chose the rural sites based on the two most populous tribes in the area. The Naiberi site caters primarily to the Kalenjin tribe (mainly of Nandi and Keiyo dialects) and the Soy site serves primarily those in the Luhya tribe. In the study catchment area, the Kalenjin tribe is the most populous, while the Luhya tribe is believed to comprise the second largest ethnic group. While four breweries produce and sell alcohol in the same location daily, the Naiberi brewery rotates between different households on different days of the week, so that brewers can engage in other work activities such as farming. The selection of these sites was to insure that our results might generalize across these groups.
Given the regional differences in type of available traditional brew, we intended to limit samples to those accessible and reportable by study participants. From each site, we collected four samples of the commonly available drinks: (1) stronger chang’aa, (2) weaker chang’aa, (3) stronger busaa, and (4) weaker busaa, for a total of 20 samples. All brewers were requested to provide the smallest commonly available serving size for each of the four types of alcoholic drink. Research staff measured the volume, and recorded the cost of each drink. In addition, the time brewed, location of brewing, ingredients and brewing procedures were also documented. Photographs were also taken of the serving receptacles (teacups, glassware and metal cans) for alcoholic drinks.
All samples were to be collected at 12 p.m. and frozen in a −40°C freezer within 1 hour after collection. We chose to collect at 12 p.m. because there was no specific time of day at which most individuals reported frequenting breweries and to control for varying strengths of busaa throughout the day. Because busaaa is a cereal-based fermented drink, the ethanol content may vary after brewing, depending on a variety of factors including rate of oxidation and the amount of sugar and yeast remaining in the brew (Scott and Reed 1975). Frozen samples were then transported to the Kenya Bureau of Standards in Nairobi for analyses of ethanol content. This study was deemed to be “exempt” by the Moi University School of Medicine Institutional Research and Ethics Committee.
Determination of Ethanol Content
Internationally accepted methods for determination of ethanol content were based on the standards of the Association of Official Analytical Chemists (AOAC International 2002). A partial volume of the sample was mixed with distilled water. The mixture was distilled at room temperature then 100 ml of the distillate collected and transferred to a volumetric flask and then filled to the top. Specific gravity of the distillate was then determined at 20°C and the corresponding ethanol content obtained from reference tables (AOAC International 2002). Each of the 20 samples was analyzed in triplicate and results reported as ethanol content, percent volume (i.e., % v/v).
Computational Analyses
We categorized chang’aa and busaa as two separate drink categories, due to the two different brewing processes of distillation and fermentation, respectively. The volume of measured ethanol per serving size of brew (in milliliters) was considered the gold standard for all analyses. We calculated the volume of pure ethanol per serving size for each of the 20 samples by multiplying the percent ethanol for each respective drink against the serving size of the drink (milliliters of measured ethanol per serving size). The remaining computations compared two different methods of estimating alcohol use while employing this to be the gold standard.
Estimating by Drink Volume
For the method estimating drink volume, we calculated mean ethanol content for each drink type by averaging the volume of measured ethanol per drink across the ten samples of chang’aa and ten samples of busaa, respectively. This mean ethanol content was considered the estimated ethanol content in a standard drink for each respective drink type (i.e., chang’aa and busaa). We also calculated an error score in milliliters by subtracting the volume of ethanol in the standard drink (per drink type) from the actual volume of ethanol in each drink for each of the 20 samples.
Estimating by Drink Cost
For the method estimating drink cost, we first calculated mean cost of ethanol by dividing the cost for each drink by total ethanol content for each drink at each site. We then averaged this cost for milliliter of ethanol across the ten samples of chang’aa and of busaa, respectively. Finally, we estimated by drink cost by dividing the individual cost of each drink at each site by the mean cost of ethanol (e.g., 40 shillings/0.67). We also calculated an error score in milliliters within each drink type by subtracting the volume of ethanol in each drink when estimated by cost from the measured volume of ethanol in each drink for each of the 20 samples.
International Standard Drink Unit Equivalents
To provide an international comparison for the ethanol content in Kenyan drinks, we converted ethanol content in each of the 20 samples to both U.S. and Great Britain standard drink units, respectively. We first converted milliliters of ethanol in each drink to grams ethanol by multiplying by 0.79 (Miller et al. 1991), and then calculated average grams of ethanol by drink type of chang’aa and busaa, respectively. We divided by the average number of grams ethanol in each drink type by the number of grams identified for each standard drink unit: 14 g in the U.S. and 8 g in Great Britain (World Health Organization, 2001). Finally, we calculated the cost per U.S. standard drink unit of chang’aa and busaa, respectively, by multiplying the volume in milliliters of ethanol in the standard drink by the cost per milliliter of ethanol.
Results
Data Collection
Seventy percent of the samples were collected within 15 min of 12 p.m., while all samples were collected by 12:40 p.m. All samples were frozen in a −40°C freezer within 1 h and 10 min after collection. Frozen samples were then transported to the Kenya Bureau of Standards in Nairobi for analyses of ethanol content.
Brewer Characteristics, Brewing Ingredients and Procedures
All five traditional brewers were female. All brewers reported that busaa was made onsite, while two brewers reporting importing chang’aa from rural areas. The brewing procedures were as follows: Brewers describe making busaa in an average of 6–7 days. Rotting maize flour is mixed with water and fermented for 3–4 days, then heated over a fire on a large pan or iron sheet. Yeast and water is added to the fermented mixture and it is fermented for another 3–4 days. The mixture is then filtered (by squeezing) through a cloth sack to produce the stronger busaa. Adding hot water to the mixture and then filtering again produces the weaker busaa. In order to make chang’aa, brewers start with the mixture left over after the production of busaa. Sugar and water are then added to the mixture, which is fermented for 3–4 days. The mixture is then distilled by boiling over a flame, with a pan of cool water to create condensation (the chang’aa). The first batches of condensation produce the stronger chang’aa, while subsequent batches produce the weaker chang’aa.
Drink Serving Sizes
Drink serving sizes for chang’aa varied from 70 to 260 ml, while drink serving sizes for busaa ranged from 260 to 1,100 ml (Table 1). There was substantial variability in receptacle serving sizes for alcoholic drinks (Fig. 1).
Table 1.
Ethanol content, cost and volume of traditional brew in Eldoret, Kenya
| Location purchased (and brewed, if different) | Type of drink | Cost (in kes) | etoh (%) | Serving size in ml | ml of etoh | Cost in kes/ml of etoh | (1) ml etoh estimate using mean % etoh × standard drink | (2) ml etoh estimate using cost ÷ mean cost per ml etoh | Error in ml of etoh/drink for (1) | Error in ml of etoh/drink for (2) |
|---|---|---|---|---|---|---|---|---|---|---|
| Chang’aa | ||||||||||
| Naiberi | Stronger chang’aa | 40 | 0.33 | 260 | 84.50 | 0.47 | 35.17 | 59.97 | −49 | −25 |
| Naiberi | Weaker chang’aa | 35 | 0.22 | 260 | 55.98 | 0.63 | 35.17 | 52.47 | −21 | −4 |
| Langas | Stronger chang’aa | 20 | 0.50 | 70 | 34.67 | 0.58 | 35.17 | 29.98 | 0 | −5 |
| Langas | Weaker chang’aa | 10 | 0.22 | 90 | 19.68 | 0.51 | 35.17 | 14.99 | 15 | −5 |
| Soy | Stronger chang’aa | 10 | 0.18 | 80 | 14.15 | 0.71 | 35.17 | 14.99 | 21 | 1 |
| Soy | Weaker chang’aa | 10 | 0.21 | 80 | 16.71 | 0.60 | 35.17 | 14.99 | 18 | −2 |
| Huruma (Bukembe) | Stronger chang’aa | 20 | 0.51 | 75 | 38.29 | 0.52 | 35.17 | 29.98 | −3 | −8 |
| Huruma (Bukembe) | Weaker chang’aa | 20 | 0.53 | 75 | 40.06 | 0.50 | 35.17 | 29.98 | −5 | −10 |
| Kipkaren estate (Kisii) | Stronger chang’aa | 30 | 0.44 | 70 | 30.77 | 0.98 | 35.17 | 44.97 | 4 | 14 |
| Kipkaren estate (Kisii) | Weaker chang’aa | 20 | 0.24 | 70 | 16.88 | 1.19 | 35.17 | 29.98 | 18 | 13 |
| Sum or mean(SD) | 215 | 0.34 (0.14) | 113 (77.72) | 35.17 (21.80) | 0.67 (0.23) | na | 32.23 (15.82) | |||
| Busaa | ||||||||||
| Naiberi | Stronger busaa | 15 | 0.07 | 260 | 17.50 | 0.86 | 22.94 | 26.16 | 5 | 9 |
| Naiberi | Weaker busaa | 5 | 0.04 | 260 | 11.31 | 0.44 | 22.94 | 8.72 | 12 | −3 |
| Langas | Stronger busaa | 20 | 0.04 | 1100 | 48.18 | 0.42 | 22.94 | 34.88 | −25 | −13 |
| Langas | Weaker busaa | 20 | 0.04 | 1100 | 41.47 | 0.48 | 22.94 | 34.88 | −19 | −7 |
| Soy | Stronger busaa | 10 | 0.05 | 300 | 15.45 | 0.65 | 22.94 | 17.44 | 7 | 2 |
| Soy | Weaker busaa | 5 | 0.05 | 300 | 13.86 | 0.36 | 22.94 | 8.72 | 9 | −5 |
| Huruma | Stronger busaa | 20 | 0.04 | 750 | 31.90 | 0.63 | 22.94 | 34.88 | −9 | 3 |
| Huruma | Weaker busaa | 10 | 0.02 | 550 | 12.85 | 0.78 | 22.94 | 17.44 | 10 | 5 |
| Kipkaren estate | Stronger busaa | 10 | 0.04 | 500 | 21.85 | 0.46 | 22.94 | 17.44 | 1 | −4 |
| Kipkaren estate | Weaker busaa | 10 | 0.03 | 500 | 15.00 | 0.67 | 22.94 | 17.44 | 8 | 2 |
| Sum or mean (SD) | 125 | 0.04 (0.01) | 562 (323.48) | 22.94 (13.04) | 0.57 (0.17) | na | 21.80 (10.28) | |||
ml—milliliter
Etoh—ethanol
Kes—Kenyan shilling
Approximately 65 Kenyan shillings equal 1 U.S. dollar
Fig. 1.

Local serving receptacles of traditional brew
Ethanol Distillation
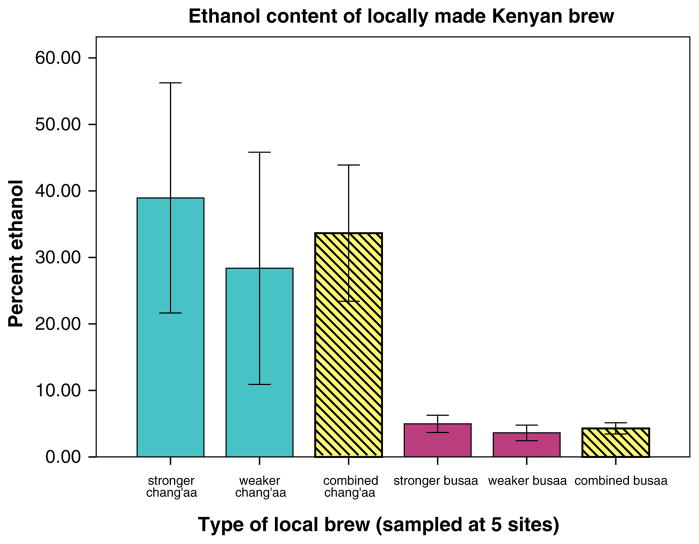
Because 95% confidence intervals of ethanol content among stronger and weaker samples of chang’aa and busaa, respectively, substantially overlapped (see Fig. 2), we collapsed groupings by strength into one combined category of chang’aa and one combined category of busaa for all analyses. Among chang’aa samples, ethanol ranged from 18 to 53% (M = 34%, SD = 14%). Among busaa samples, percent ethanol ranged from 2 to 7% (M = 4%, SD = 1%) (Fig. 3).
Fig. 2.
Ethanol content of local brew with 95% confidence intervals by brew type
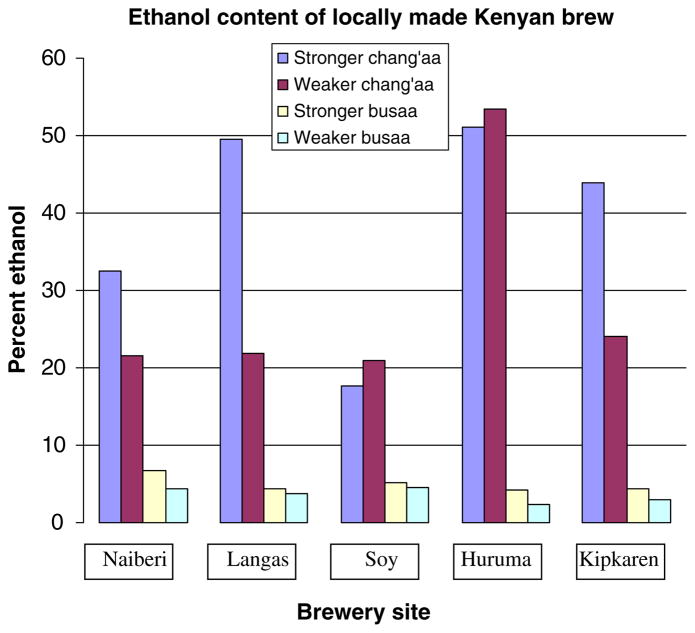
Fig. 3.
Ethanol content of brew sold at each site
Computational Analyses
Volume of measured ethanol per serving size ranged from 14.2 to 84.5 ml for chang’aa and from 11.3 to 48.2 ml for busaa (Table 1).
Estimating by Drink Volume
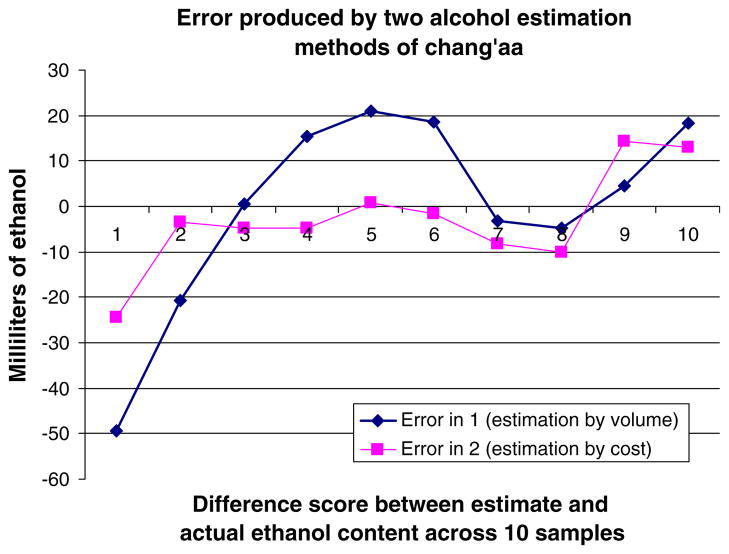
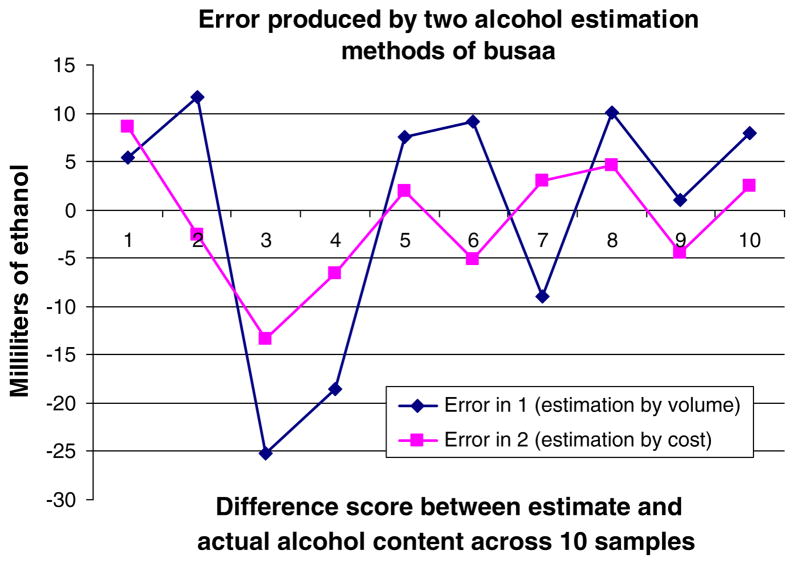
Mean ethanol content for traditional brew was 35.2 and 22.9 ml for chang’aa and busaa, respectively. These were then considered the estimated ethanol content in a standard drink for each respective drink type. After subtracting the volume of ethanol estimated in the standard drink for chang’aa from the measured volume of ethanol for each of the ten chang’aa samples, we found the difference scores to be large overall (Fig. 4). Estimation by volume resulted in substantial under- and over-estimation of actual alcohol content with demonstrated differences of at least (+/) 12.5 ml (equivalent to ½ U.S. standard drink) among 60% of chang’aa samples. Difference scores for busaa were smaller, with 20% of the sample demonstrating differences of at least (+/) 12.5 ml (Fig. 5).
Fig. 4.
Comparison of error across alcohol estimation methods for chang’aa
Fig. 5.
Comparison of error across alcohol estimation methods for busaa
Estimating by Drink Cost
Mean cost per milliliter of ethanol for traditional brew was 0.67 and 0.57 shillings for chang’aa and busaa, respectively. After subtracting the estimated ethanol content using the cost estimation procedure from the measured ethanol for each of the ten chang’aa samples, we found the difference scores were smaller than those using the standard drink estimation approach. However, 30% of the sample demonstrated differences of at least (+/) 12.5 ml (Fig. 4). The difference scores for busaa were smaller, with 10% of the sample demonstrating differences of at least (+/) 12.5 ml (Fig. 5).
International Standard Drink Unit Equivalents
For chang’aa samples, the U.S. standard drink unit equivalent ranged from 0.8 to 4.8 drinks (Table 2; M = 2.0, SD = 1.2) and the Great Britain standard drink unit equivalent ranged from 1.4 to 8.3 drinks (M = 3.5, SD = 2.2). For busaa samples, the U.S. standard drink unit equivalent ranged from 0.6 to 2.7 drinks (M = 1.3, SD = 0.7) and the Great Britain standard drink unit equivalent ranged from 1.1 to 4.8 drinks (M = 2.3, SD = 1.3). The cost per U.S. standard drink unit for chang’aa was 23.5 shillings and for busaa was 13.2 shillings.
Table 2.
International standard drink unit equivalents of Kenyan traditional brew
| Kenyan drink | Mean ml etoh | Mean grams etoh | U.S. SDU equivalent (14 g) | Great Britain SDU equivalent (8 g) |
|---|---|---|---|---|
| Chang’aa | 35.2 | 27.8 | 2.0 | 3.5 |
| Busaa | 22.9 | 18.1 | 1.3 | 2.3 |
ml—milliliter
Etoh—ethanol
Discussion
This is the first published study reporting systematic chemical analyses of traditional brew in Kenya. We found that the average chang’aa drink was equal to two standard drinks in the U.S. and 3.5 drinks in Great Britain. The average busaa drink was equal to 1.3 drinks in U.S. and 2.3 drinks in Great Britain.
We also sought to determine whether estimating alcohol by cost would produce as accurate an estimation of ethanol content when compared to estimating ethanol content by volume. We found that the accuracy of the two approaches to estimating ethanol content demonstrated at least comparable accuracy. The cost estimation procedure resulted in an 8.4% underestimation overall when applied to chang’aa and a 5% overestimation when applied to busaa.
Given our findings, we conclude that estimating alcohol content of brew by cost, because it is a culturally relevant approach, is preferable. Furthermore, the cost estimation method enables the comparison of data across studies because results using the cost method can be easily converted via simple computation to results using the volume method, and vice-versa.
Estimating drinking by costs may also provide a use tool for intervention studies by increasing awareness of actual costs spent on alcohol and facilitating a “cost-benefit” discussion of excessive drinking costs. Indeed, we already found it useful to incorporate a cost-benefit discussion of money spent on alcohol during the piloting of our CBT intervention. We have also incorporated the cost estimation procedure of alcohol consumption into our alcohol assessment methods. We believe that weekly and monthly feedback to the patients of the amount spent on alcohol will add to the efficacy of CBT, just as providing feedback of unwanted outcomes associated with drinking (e.g., Project Match Research Group 1997), has been shown to be helpful in effecting participant behavior change.
Although our selection of brewing sites was based on location and may not be representative of brewers, we found that all brewers were female, which is consistent with qualitative discussion that brewing and selling alcohol is one of the more salient culturally permitted routes of economic income for women in Kenya and other countries in sub-Saharan Africa (Holtzman 2001; McCall 1996; Ojiji et al. 1993).
A limitation of estimating drinking by cost is that it does not incorporate alcohol made and brewed at home for local or community use, or alcohol offered to clients to taste the quality of the local brew before buying. However, Willis has noted that the evolution of traditional brewing into a commercial enterprise has result in diminished production of alcohol solely for home or communal use (Willis 2002). Another limitation is the potential fluctuation in costs of brew due to rapidly increasing inflation rates in Kenya. However, we conducted a resampling of brew employing 60% of the same sites at a 3-month interval and found that 100% of the costs (i.e., for 12 samples) were duplicated. Both studies were conducted prior to the post-election violence that shook the country from January through March 2008, following a December 27th president election that was declared “biased” by independent observers. In spite of price fluctuations since the violence, consumer cost of brew has not been increased in 1½ years at one high-volume brewery in Eldoret.
Relative stability of crop prices in the Eldoret region is likely precipitated by its location in Rift Valley province, known as the “granary” for the rest of Kenya because of its fertile ground for growing both maize and sugar cane, the two primary ingredients of chang’aa and busaa. Additionally, because rotting maize flour is used in the brew, spoilage is not a concern, extending maize availability even further.
The post-election violence and subsequent upheaval engendered a late planting season in 2008, which may result in a shortage of maize and sugar during the harvest season and possible subsequent price increases. Due to such shifting sociopolitical factors, we recommend that researchers employing cost algorithms to measure alcohol content of brew periodically evaluate them to enhance accuracy.
Because the association between cost and alcohol content were similar across town and rural breweries in our study, we are reasonably confident that our findings are generalizable to this region in Kenya. However, given the regional variability in types of traditional brews in Kenya and other African countries, we echo the recommendation by Obot that research collaborations perform local alcohol research to increase understanding and relevance of research prior to conducting alcohol prevalence and outcome studies in resource-limited settings (Obot 2001). Based on reports of agricultural experts, brewers and residents of Kenya, brew types in Kenya vary depending on regional availability of crops. For example, in the Coast province, brew is made from coconut, while in the Central province, muratina, a honey wine, is prevalent. In other parts of western Kenya, busaa is made from sorghum and chang’aa is made from bananas. Not only does brew type vary within Kenya, but it varies across other African countries including Tanzania, Uganda, and Nigeria, where brew is made from bananas, bamboo, coconut, millet, rice, sorghum, and maize (Mosha et al. 1996; Ojiji et al. 1993; Shayo et al. 1998; World Health Organization 2004). Furthermore, Kenyan residents have told us that reporting brew consumed by amount of money spent is common in other parts of the country as well. It is unknown, however, whether costs per milliliter of ethanol is consistent across Kenya. Together, these observations suggest a need for identifying traditional brews, analyzing respective ethanol content and conducting related cost estimation analyses within each respective “catchment” area targeted by a study.
Although this study did not examine these hypotheses, those of limited financial means may find the exercise of remembering money spent on alcohol to be particularly salient in the face of limited resources, and this could generalize to poor strata of the population in other countries as well. Indeed, our clinical observations in the U.S. suggest that drugs of abuse are often referred to by the amount spent (e.g., a “dime bag” (US $10) of marijuana or cocaine). Future inquiries are needed to determine whether the cost estimation methods can be extended to impoverished populations in industrialized countries and to other categories of substances (e.g., commercial alcohol, illicit drugs).
Traditional brew is believed to represent the highest proportion of alcohol use in Kenya. Despite its prevalence and potential impact on HIV risk behaviors in sub-Saharan Africa, traditional brew has been largely understudied. Our finding that a typical Kenyan chang’aa drink in Eldoret is equal to two U.S. standard drinks underscores the need for such research in order to accurately estimate alcohol exposure.
Acknowledgments
This work was supported by NIAAA-funded R21AA016884. We acknowledge Sammy Kimani for his input about Kenyan cultural customs, Lucien Butuba for his assistance with data collection and Naman Nyabanda for his expertise with Kenyan farming and agriculture.
Contributor Information
Rebecca K. Papas, Email: Rebecca.papas@yale.edu, Department of Medicine, Yale University School of Medicine, New Haven, CT, USA
John E. Sidle, Department of Medicine, Moi University Faculty of Health Sciences, P.O. Box 5550, Eldoret, Kenya
Emmanuel S. Wamalwa, P.O. Box 5550, Eldoret, Kenya
Thomas O. Okumu, Kenya Bureau of Standards, Nairobi, Kenya
Kendall L. Bryant, National Institute of Alcohol Abuse and Alcoholism, Rockville, MD, USA
Joseph L. Goulet, Department of Medicine, Yale University School of Medicine, New Haven, CT, USA
Stephen A. Maisto, Department of Psychology, Syracuse University, Syracuse, NY, USA
R. Scott Braithwaite, Department of Medicine, Yale University School of Medicine, New Haven, CT, USA.
Amy C. Justice, Department of Medicine, Yale University School of Medicine, New Haven, CT, USA
References
- AOAC International. Official method 920.56. 17. Gaithersburg, M. D: Association of Analytical Communities; 2002. Official Methods of Analysis of AOAC International. [ii], 1.02. [Google Scholar]
- Apostolopoulos Y, Sonmez S, Yu CH. HIV-risk behaviors of American spring break vacationers: a case of situational disinhibition? International Journal of STD and AIDS. 2002;13:733–743. doi: 10.1258/095646202320753673. [DOI] [PubMed] [Google Scholar]
- Ayisi JG, van Eijk AM, ter Kuil OF, Kolczak MS, Otieno JA, Misore AO, et al. Risk factors for HIV infection among asymptomatic pregnant women attending an antenatal clinic in western Kenya. International Journal of STD and AIDS. 2000;11:393–401. doi: 10.1258/0956462001916119. [DOI] [PubMed] [Google Scholar]
- Hall W, Saunders JB, Babor TF, Aasland OG, Amundsen A, Hodgson R, et al. The structure and correlates of alcohol dependence: WHO collaborative project on the early detection of persons with harmful consumption-III. Addiction (Abingdon, England) 1993;88:1627–1636. doi: 10.1111/j.1360-0443.1993.tb02037.x. [DOI] [PubMed] [Google Scholar]
- Hargreves JR. Socioeconomic status and risk of HIV infection in an urban population in Kenya. Tropical Medicine & International Health. 2002;7:793–802. doi: 10.1046/j.1365-3156.2002.00943.x. [DOI] [PubMed] [Google Scholar]
- Holtzman J. The food of elders, the “ration” of women: brewing, gender and domestic processes among the Samburu of northern Kenya. American Anthropologist. 2001;103:1041–1058. doi: 10.1525/aa.2001.103.4.1041. [DOI] [Google Scholar]
- Jemmot LS, Brown EJ. Reducing HIV sexual risk among African women who use drugs: hearing their voices. The Journal of the Association of Nurses in AIDS Care. 2003;14:19–26. doi: 10.1177/1055329002239187. [DOI] [PubMed] [Google Scholar]
- McCall M. Rural brewing, exclusion and development policy-making. Focus on Gender. 1996;4:29–38. doi: 10.1080/741922167. [DOI] [PubMed] [Google Scholar]
- Miller WR, Heather N, Hall W. Calculating standard drink units: international comparisons. British Journal of Addiction. 1991;86:43–47. doi: 10.1111/j.1360-0443.1991.tb02627.x. [DOI] [PubMed] [Google Scholar]
- Mosha D, Wangabo J, Mhinzi G. African traditional brews: how safe are they? Food Chemistry. 1996;57:205–209. doi: 10.1016/0308-8146(95)00192-1. [DOI] [Google Scholar]
- National Institutes of Health. [Accessed 15 May 2008];ERA commons computer retrieval of information on scientific projects. 2008 http://crisp.cit.nih.gov/
- Obot IS. The measurement of drinking patterns and alcohol problems in Nigeria. Journal of Substance Abuse. 2001;12:169–181. doi: 10.1016/S0899-3289(00)00047-X. [DOI] [PubMed] [Google Scholar]
- Obot IS. Nigeria: alcohol and society today. Addiction (Abingdon, England) 2007;102:519–522. doi: 10.1111/j.1360-0443.2006.01619.x. [DOI] [PubMed] [Google Scholar]
- Ojiji OO, Dagona ZK, Tamen FI. Women, children and alcohol: a study of local brewers in Jos, Nigeria. In: Obot IS, editor. Epidemiology and Control of Substance Abuse in Nigeria. Jos, Nigeria: Centre for Research and Information on Substance Abuse; 1993. pp. 95–102. [Google Scholar]
- Othieno CJ, Kathuku DM, Ndetei DM. Substance abuse in outpatients attending rural and urban health centres in Kenya. East African Medical Journal. 2000;77:592–595. doi: 10.4314/eamj.v77i11.46728. [DOI] [PubMed] [Google Scholar]
- Papas RK, Ayuku DO, Sidle JE, Omolo OE, Ballidawa JB, Songole R, et al. A stage 1 cognitive behavioral trial to reduce alcohol use among HIV-infected Kenyans. (Oral) Research society on alcoholism annual meeting; July 2–July 27, 2008.2008. [Google Scholar]
- Project Match Research Group. Matching alcoholism treatments to client heterogeneity: project MATCH posttreatment drinking outcomes. Journal of Studies on Alcohol. 1997;58:7–29. [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Amundsen A, Grant M. Alcohol consumption and related problems among primary health care patients: WHO collaborative project on early detection of persons with harmful alcohol consumption-I, 15. Addiction (Abingdon, England) 1993a;88:349–362. doi: 10.1111/j.1360-0443.1993.tb00822. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, DeLaFuente JR, Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction (Abingdon, England) 1993b;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Scott D, Reed G. Enzymes in food processing. New York: Academic Press; 1975. [Google Scholar]
- Seage GR, Holte S, Gross M, Koblin B, Marmor M, Mayer KH, et al. Case-crossover study of partner and situational factors for unprotected sex. Journal of Acquired Immune Deficiency Syndrome. 2002;31:429–432. doi: 10.1097/00126334-200212010-00010. [DOI] [PubMed] [Google Scholar]
- Shaffer DN, Njeri R, Justice AC, Odero WW, Tierney WM. Alcohol abuse among patients with and without HIV infection attending public clinics in western Kenya. East African Medical Journal. 2004;81:594–598. [PubMed] [Google Scholar]
- Shayo NB, Nnko SAM, Gidamis AB, Dillon VM. Assessment of cyanogenic glucoside (cyanide) residues in Mbege: an opaque traditional Tanzanian beer. International Journal of Food Sciences and Nutrition. 1998;49:333–338. doi: 10.3109/09637489809089407. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline followback: a technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen J, editors. Measuring alcohol consumption. 1. New Jersey: Humana; 1992. pp. 41–72. [Google Scholar]
- UNAIDS/WHO. AIDS epidemic update: December 2007. Geneva: UNAIDS/WHO; 2007. [Google Scholar]
- Willis J. Potent brew: a social history of alcohol in East Africa 1850–1999. London and Nairobi: The British Institute of East Africa; 2002. Serial (Book, Monograph) [Google Scholar]
- World Health Organization. The alcohol use disorders identification test: guidelines for primary care geneva. WHO; 2001. [Google Scholar]
- World Health Organization. Global status report on alcohol 2004. Geneva: WHO; 2004. [Google Scholar]