Abstract
Objective
Following the end of adjuvant treatment, breast cancer survivors must cope with uncertainty related to the possibility of recurrence and the loss of the “safety net” treatment provides. The present study examined breast cancer survivors' efforts to manage uncertainty by making lifestyle changes, such as improvement in diet and exercise. We further investigated the role of women's common-sense beliefs about their cancer, as described by Leventhal's self-regulation theory, in explaining post-treatment changes.
Method
At 3 weeks and 3 months post-treatment, 79 women who received adjuvant chemotherapy and/or radiation therapy for stages 0-III breast cancer (mean age = 55 years) completed assessments of changes in health practices and other behaviors. Participants also completed measures of beliefs about the causes, course, personal control, and consequences of their cancer.
Results
Survivors reported behavior changes directed toward improving physical, emotional, and spiritual well-being. Results further indicated that women who believed their cancer had more severe consequences and those who attributed the development of cancer or the prevention of recurrence to health behaviors or stress were most likely to report improvement in diet or physical activity and reduction in alcohol use or stress.
Conclusions
Findings suggest that breast cancer survivors are poised to make lifestyle changes after treatment ends, creating an opportune time for health promotion interventions. Understanding women's cancer beliefs could help guide the development of tailored, proactive interventions to improve the health and well-being of breast cancer survivors.
Keywords: breast neoplasms, cancer survivorship, beliefs, attributions, health behavior, oncology
The end of adjuvant chemotherapy and/or radiation therapy is a time of transition and increased distress for women with breast cancer [1-4]. Breast cancer survivors often report feeling as if they have lost a “safety net” when treatment ends [3]. Regularly receiving treatment and attending medical appointments provides an active means for eradicating existing disease and preventing cancer growth. Once treatment is over, this active coping strategy is no longer available. Fear of a cancer recurrence, a common concern among breast cancer patients [5], coupled with the loss of one's primary means for managing cancer, may motivate breast cancer survivors to find new strategies for coping with the uncertainty of their cancer status [4].
Researchers and health care providers are beginning to recognize the need to help survivors develop active, behavioral strategies for coping with uncertainty after treatment ends. Psychosocial interventions are under development with the aim of easing the transition following treatment [6, 7]. The NCI's Facing Forward guide provides suggestions for survivors with respect to health practices, stress management, and coping with physical and psychosocial problems [8]. There are also guidelines for cancer survivors focused on diet, physical activity, alcohol use, and smoking [8-10], and changes in these health practices are common, with 40-50% of cancer survivors reporting positive changes in diet [11-14] and 15-26% reporting increased physical activity [12-14]. While one of these studies [12] used a population-based sample, it is important to note that prevalence rates could reflect more motivated individuals who are willing to respond to surveys.
Whether cancer survivors decide to make lifestyle changes and how they decide which behaviors to change is an important question. Leventhal's self-regulation theory, which is often referred to as the “common-sense model”, posits that individuals' cognitive representations of their illness guide behavioral responses to illness, such as adherence to treatment recommendations and changes in health practices [15, 16]. The key component of this model focuses on “common-sense” or lay beliefs about illness that help individuals to make sense of their illness and, in turn, direct coping efforts. Specifically, the theory proposes that common-sense cognitive models of illness can be characterized along five dimensions: identity (label and symptoms), cause, consequences (short and long-term effects of illness), course (acute, chronic, or recurrent), and control/cure (personal control over the illness and how one effects recovery).
Common-sense illness representations have been useful in predicting health practices among cancer patients. For example, higher levels of perceived control have been associated with use of integrative therapies, lower likelihood of smoking, and utilization of active coping strategies such as problem-solving and seeking support [17-19]. Cancer patients' causal attributions also appear to be important, with findings suggesting that patients who perceive cancer causes to be controllable are more likely to engage in positive health practices [19-21]. For example, breast cancer survivors who attributed their cancer diet, lack of exercise, or alcohol use were more likely to make positive changes in these behaviors [22]. Along similar lines, cancer survivors who believed that good health practices were important in preventing recurrence were more likely to engage in them [21-23]. However, relationships between beliefs about cancer consequences or course and health practices or other coping efforts have not been well-studied.
Most investigations of the prevalence of lifestyle changes or relationships between cancer representations and health practices have utilized cross-sectional designs and have focused on heterogeneous samples on the cancer survivorship continuum, from recently diagnosed to long-term survivors [e.g., 13-14, 17-21]. We argue that patients' common-sense models of cancer may play a salient role in helping patients to “reconstruct a safety net” following the end of treatment. Initially, patients have a relatively uniform active means for coping with cancer: seeking treatment. Following the completion of treatment, a variety of possibilities for active coping efforts exist, and individual differences in beliefs about cancer may play a more significant role in determining behavioral responses. Consistent with this idea, breast cancer survivors have indicated that thinking about causal attributions is more important to them during the recovery period than earlier in the cancer experience [13]. However, this potentially “teachable moment” has not been well studied, and was therefore the focus of the current investigation.
The objectives of the present study were as follows. First, we sought to determine to what extent breast cancer patients reported making behavior changes by 3 weeks and 3 months post-treatment, and which behaviors they were most likely to change. While we examined traditional health practices (diet, exercise, and alcohol use), we also extended our investigation to changes in spiritual and religious activities and stress management, among others. A sub-aim was to examine whether women who reported improved health practices were engaging in better health practices than those who did not report improvement (e.g., whether women who reported increasing physical activity were more physically active than others). The second objective was to characterize women's common-sense beliefs about their cancer using Leventhal's model, including beliefs about personal control, consequences, course, causal attributions, and factors believed to prevent recurrence (identity was not examined because participants had already been diagnosed with breast cancer and most identified with this label). The third objective was to determine whether these beliefs, when measured shortly after the end of treatment, were associated with behavior changes both concurrently and prospectively. We hypothesized that women who perceived greater control over their disease, those who perceived cancer to have a more chronic course, and those who perceived more severe consequences of their cancer 3 weeks after treatment ended would be more likely to report making lifestyle changes 3 weeks and 3 months post-treatment. We further anticipated that women would choose to make behavior changes that matched their common-sense ideas regarding what caused their cancer and what may prevent cancer recurrence.
Method
Participants
Participants were women treated with adjuvant chemotherapy and/or radiation therapy for stages 0-III breast cancer. Women with recurrent or metastatic cancer were excluded. Participants were enrolled from five treatment centers in the Midwest during adjuvant treatment as part of a larger study of post-treatment psychological adjustment [24]. Of 113 women approached to participate, 102 subsequently enrolled in the study (an accrual rate of 90%). A total of 79 participants completed measures 3 weeks after the last date of adjuvant chemotherapy or radiation therapy and 71 completed measures 3 months post-treatment. Reasons for nonparticipation or attrition included time constraints and disinterest in participating in research studies. Participants who did not complete the 3-month follow up assessment were not included in prospective analyses examining relationships between predictors at 3 weeks and outcomes at 3 month post-treatment.
Participants ranged in age from 32 to 89 years with a mean age of 55.0 years (SD = 10.8). Additional demographic data and disease characteristics are presented in Table 1. Chi-square analyses indicated that women who were enrolled in, but did not complete the study, did not differ from those who completed all time points on disease or demographic characteristics with the exception of relationship status. Non-completers were more likely to be divorced or separated and less likely to be married or single, χ2 = 10.47, p = .02.
Table 1. Sample Characteristics (N=79).
| Percent of sample | |
|---|---|
| Relationship Status | |
| Married or living with partner | 73.8 |
| Single | 5.7 |
| Divorced or separated | 12.5 |
| Widowed | 7.9 |
| Education | |
| High school graduate or less | 31.8 |
| Some college | 22.7 |
| College graduate | 21.6 |
| Post-graduate degree | 23.9 |
| Income | |
| <$25,000 | 20.6 |
| $25,001-$40,000 | 12.3 |
| $40,001-$55,000 | 13.7 |
| $55,001-$70,000 | 19.2 |
| >$70,000 | 34.2 |
| Ethnicity | |
| Caucasian | 93.2 |
| African American | 2.3 |
| Asian | 1.1 |
| Native American | 1.1 |
| Other | 2.3 |
| Cancer stage | |
| 0 | 5.7 |
| I | 33.3 |
| II | 47.1 |
| III | 13.8 |
| Treatment a | |
| Mastectomy | 27.3 |
| Lumpectomy | 79.5 |
| Chemotherapy | 71.6 |
| Radiation therapy | 86.4 |
| Hormonal therapy | 77.0 |
Treatment categories are not mutually exclusive.
Procedure
Health care providers identified eligible patients and solicited the patients' permission to meet with a researcher at an outpatient clinic appointment (all eligible patients during the study enrollment period were given the opportunity to participate). If the patient gave permission, research staff described the study and subsequently reviewed the informed consent document if the patient expressed interested in participating. If research staff could not be present at the patient's appointment, the patient was contacted by telephone. Participants were mailed self-report assessments 3 weeks and 3 months following their last treatment session and returned questionnaires via postage-paid envelope. All participants signed informed consent documents approved by the University of Iowa Institutional Review Board, and treatment of participants was in accordance with institutional policies and American Psychological Association ethical standards. There were no potential conflicts of interest identified.
Measures
Health Practices
Two aspects of health behaviors were examined: self-reported changes following cancer diagnosis (e.g., had the participant increased her level of physical activity or does she eat more fruits and vegetables?), and the extent to which participants engaged in health practices at the time of the assessments (e.g., the amount and intensity of physical activity or number of servings of fruits and vegetables consumed daily during the prior two-week period). The behavior change measure was the primary outcome of interest, while the current health practices assessment provided a context for behavior change findings.
Behavior changes in a variety of domains were assessed and are presented in Table 2. Women were asked to compare their current behavior with their behavior prior to diagnosis and rate the direction of change as less or have now stopped, about the same, or more or have now started. Similar response options or categories have been used in other studies of cancer survivors' health practices [13, 14, 19]. The behaviors were chosen based on open-ended interviews with breast cancer survivors, behavior changes frequently reported by cancer survivors in the literature [11-14, 25], and ACS and NCI health practices guidelines [8-10].
Table 2. Post-Treatment Behavior Changes.
| 3 Weeks Post-Treatment (n = 79) | % | 3 Months Post-Treatment (n = 71) | % |
|---|---|---|---|
| Perform breast self-exam more | 40.0 | Perform breast self-exam more | 36.2 |
| * Eat more fruits and vegetables | 30.8 | * Eat more fruits and vegetables | 30.0 |
| * Drink alcohol less a | 28.9 | * Drink alcohol less a | 27.5 |
| Pray or meditate more | 24.7 | Pray or meditate more | 21.7 |
| * Avoid stressful situations more often | 24.7 | * Avoid stressful situations more often | 15.9 |
| Spend more time with family/friends | 22.1 | Spend more time with family/friends | 26.1 |
| * Do more physical activity | 15.4 | * Do more physical activity | 28.6 |
| Do less household work | 14.3 | Do less household work | 12.9 |
| Do less work outside of the home | 13.3 | Do less work outside of the home | 8.7 |
| Spend more time engaging in hobbies | 12.8 | Spend more time engaging in hobbies | 11.4 |
| Engage in more food safety practices | 11.8 | Engage in more food safety practices | 11.6 |
| Journal/write about experiences more | 11.7 | Journal/write about experiences more | 13.2 |
| Do more relaxing activities | 11.5 | Do more relaxing activities | 10.0 |
| Begin using CAM/use CAM more | 9.2 | Begin using CAM/use CAM more | 10.3 |
| Smoke less b | 9.6 | Smoke less b | 11.6 |
| Get enough sleep more often | 7.9 | Get enough sleep more often | 10.0 |
| * Choose low or non-fat foods more | 5.2 | * Choose low or non-fat foods more | 18.6 |
| Attend religious services more | 2.6 | Attend religious services more | 5.8 |
Note. We report the positive or adaptive behavior change direction (e.g., increased rather than decreased fruit/vegetable consumption). When the adaptiveness of the behavior change was not evident, the change direction that more women reported was used.
36% of participants denied alcohol use; of those who reported consuming alcohol at the time of diagnosis, 44.8% reported drinking less 3 weeks post-treatment and 43.1% reported drinking less 3 months post-treatment.
Of those who reported smoking cigarettes at the time of diagnosis, 58.3% reported they had decreased or quit smoking 3 weeks post-treatment and 88.9% decreased or quit smoking 3 months post-treatment.
Examined as outcomes in logistic regression analyses.
All measures of current health practices were cued to the past two weeks. Participants were asked to indicate number of packs of cigarettes smoked per week and number of alcoholic drinks consumed per week. These questions have been previously used successfully in behavioral health research [26, 27]. We used a modified physical activity measure wherein a physical activity index can be computed by multiplying the number of hours of physical activity by estimates of the relative energy expenditure for activities classified as moderate and vigorous [28]. Diet was assessed with Rapid Food Screeners designed to assess top sources of fat and fruit/vegetable intake for adults in the United States [29].
Common-sense models of cancer
The Illness Perception Questionnaire-Revised (IPQ-R) assessed participants' beliefs about their illness [30]. Three subscales were of interest: acute versus chronic course, personal control, and consequences. An additional section assesses causal attributions and was modified for the current study to make the attributions relevant to breast cancer. Participants were asked to rate the importance of a variety of factors in causing their cancer on a 5-point scale from not at all important to very important. Participants also rated the importance of various factors in preventing recurrence on the same scale. Items were chosen from previous studies of breast cancer survivors' disease attributions [13, 23]. All items are listed in Table 4.
Table 4. Participants' Mean Ratings of Importance of Various Factors in the Development or Recurrence of Cancer.
| Factors Contributing to the Development of Cancer (n = 79) |
Factors Preventing a Cancer Recurrence (n = 79) |
|---|---|
|
|
Note. Values enclosed in parentheses represent the percentage of women listing each factor as moderately to very important. Participants could endorse multiple factors.
Examined as predictors in logistic regression analyses.
Statistical Analyses
Descriptive statistics were used to characterize behavior change measures, health practices, and cancer beliefs (Objectives 1 and 2). Univariate analysis of variance (ANOVA) was employed to compare four key health practices (fat and fruit/vegetable consumption, physical activity, and alcohol use) among women who reported a positive change in the corresponding behavior (e.g., increased physical activity) to those who did not. The comparisons are illustrated in Table 3.
Table 3. Mean Health Practice Scores for Women Reporting a Positive Change in Each Health Practice Versus Those Reporting No Positive Change.
| 3 Weeks Post-Treatment (n= 79) | 3 Months Post-Treatment (n= 71) | |||||
|---|---|---|---|---|---|---|
| Positive Change | No Positive Change | Overall | Positive Change | No Positive Change | Overall | |
| Fat consumption (% daily calories) | 31.3 (2.2) |
33.9 (5.1) |
33.7 (5.0) |
33.8 (3.7) |
33.5 (4.6) |
33.6 (4.4) |
| Fruit/vegetable intake (servings/day) | 4.8 (1.3) |
4.4 (2.2) |
4.5 (2.0) |
5.1 (1.9) |
4.4 (2.0) |
4.6 (52.0) |
| Physical activity score a | 13.8 (9.1) |
10.9 (10.5) |
11.3 (10.3) |
13.6 (13.0) |
11.7 (13.2) |
12.3 (13.1) |
| Alcohol use (drinks/week) | 1.6 (2.6) |
1.9 (2.0) |
1.8 (2.3) |
0.9* (1.5) |
2.6* (2.3) |
1.9 (2.2) |
Note. Standard deviations are enclosed in parentheses.
Scores are based on a formula that multiplies the number of hours of exercise per week by weights based on the intensity of the activity (e.g., the weight for moderate activity is 4). A score of 10 on the physical activity measure would meet the minimum ACS guidelines for cancer prevention (30 minutes of moderate to vigorous activity 5 times weekly) while a score of 15-20 would meet the encouraged duration of 45-60 minutes of activity.
Difference is significant at p < .05.
Logistic regression models covarying for age and length of treatment (months from surgery or first neoadjuvant treatment to last adjuvant treatment) were used to examine concurrent relationships between domains of cancer beliefs (personal control, acute versus chronic course, consequences, causal attributions, and recurrence prevention beliefs) and selected behavior changes at 3 weeks post-treatment (Objective 3). Logistic regression models adjusting for covariates and behavior changes reported at 3 weeks post-treatment were then employed to examine prospective relationships between cancer beliefs at 3 weeks post-treatment and behavior changes by 3 months post-treatment (Objective 3). Five behavior changes were selected as outcomes based both on the frequency of their endorsement by participants and their theoretical and/or practical relevance (e.g., ACS and NCI recommendations for health practices): fat consumption, fruit and vegetable consumption, physical activity, alcohol use, and stress reduction. Outcome variables were dichotomized as making a positive change in the behavior (e.g., eating more fruits and vegetables) versus not doing so. Because causal attributions and recurrence prevention beliefs were analyzed at the item level, combinations of attributions and outcome variables were specified a priori for testing in logistic regression analyses and are listed in Table 5. Combinations selected were based on our hypothesis that behavior changes would match participants' attributions about factors that may have caused their cancer or can now prevent recurrence.
Table 5. Relationships Tested Between Attributions at 3 weeks Post-Treatment and Behavior Changes by 3 Weeks and 3 Months Post-Treatment.
| Behavior Change(s) Examined | |
|---|---|
| Causal attribution: | |
| Diet or eating habits | Eat more fruits and vegetables Choose low- or non-fat foods more often |
| Lack of exercise | Do more physical activity |
| Use of alcohol | Drink alcohol less |
| Stress or worry | Avoid stressful situations more often |
| Recurrence prevention belief: | |
| Eating a healthy diet | Eat more fruits and vegetables Choose low- or non-fat foods more often |
| Exercise | Do more physical activity |
| Decrease/quit use of alcohol | Drink alcohol less |
| Reducing stress in my life | Avoid stressful situations more often |
Relationships between other potential covariates and outcomes were analyzed, including disease stage, menopausal status, and treatment regimen. However, there were no significant relationships between these variables and behavior changes beyond effects of age and length of treatment (all p values > .10).
Results
Post-Treatment Behavior Changes and Health Practices (Objective 1)
The proportion of participants endorsing behavior changes from pre-diagnosis to post-treatment is reported in Table 2. Participants commonly reported changes in health practices but also in other areas, such as increased prayer/meditation and more frequent avoidance of stress. Frequencies of key health practices are illustrated in Table 3 to provide context for the behavior change findings. The key finding from the data presented in Table 3 is that women who reported positive changes on the behavior change assessment typically reported better health practices than women who did not report positive changes; however, differences were not statistically significant for diet or physical activity measures (p values > .10). Women who reported reducing alcohol use 3 months post-treatment reported less alcohol consumption than those who did not report this change, F(1, 42) = 7.44, p = .009. Data in Table 3 further suggest that women were doing fairly well with respect to most of the health practices examined. Diets were quite close to dietary recommendations for cancer prevention [e.g., 8-10]; scores on fat and fruit/vegetable screeners indicated participants consumed, on average, 32-34% of calories from fat (30% or fewer calories from fat are recommended) and 4 to 5 servings of fruits and vegetables daily (5 or more servings are recommended). Mean scores on the physical activity measure exceeded the minimum ACS guidelines (30 minutes of moderate to vigorous activity 5 times weekly) but fell short of the more aspirational guidelines of 45-60 minutes of activity. Women reported consuming very little alcohol (average of two drinks per week).
Characterization of Common-Sense Models of Breast Cancer (Objective 2)
Means provided below represent scores with a possible range of 1 (low in belief) to 5 (high). At 3 weeks post-treatment, women believed they had moderate control over their cancer (M = 3.48, SD = 0.65). They perceived their cancer to be more acute than chronic, although not strongly so (M = 2.53, SD = 0.57; higher scores indicate belief that cancer is chronic). They perceived their cancer to have moderate consequences for their lives (M = 3.56, SD = 0.61). There were no significant changes in scores on the IPQ-R from 3 weeks to 3 months post-treatment (p values > .10). Table 4 illustrates the rank order of participants' ratings of factors believed to have caused their cancer and factors that may prevent recurrence.
Relationships between Common-Sense Beliefs and Behavior Changes (Objective 3)
Personal control
Personal control was not significantly associated with concurrent (3 weeks post-treatment) or prospective (3 months post-treatment) changes in the five key behaviors after adjusting for age and length of treatment. Women who perceived greater personal control were marginally more likely to report decreasing fat consumption, Wald χ2(1) = 3.55, p = .060, odds ratio = 1.39 and increasing avoidance of stress, Wald χ2(1) = 3.18, p = .075, odds ratio = 1.16, at 3 weeks post-treatment.
Acute versus chronic course
Beliefs about disease course (whether acute or chronic) also was not associated with any of the five behavior changes.
Consequences
There were no significant concurrent relationships between perceptions of disease consequences and behavior changes at 3 weeks post-treatment. Women who perceived more severe consequences of their cancer at 3 weeks post-treatment were more likely to report increasing the frequency with which they avoided stress by 3 months post-treatment, however, Wald χ2(1) = 4.41, p = .036, odds ratio = 1.37. They were also marginally more likely to have increased the frequency with which they consumed fruits and vegetables, Wald χ2(1) = 3.42, p = .064, odds ratio = 1.28. Perceptions of disease consequences did not significantly predict changes in fat consumption, physical activity, or alcohol use.
Causal attributions
Combinations of causal attributions and behavior changes tested are illustrated in Table 5. Women who attributed the development of their cancer to diet were more likely to report that they had increased consumption of fruits and vegetables, Wald χ2(1) = 5.10, p = .024, odds ratio = 1.90 and decreased fat intake, Wald χ2(1) = 5.84, p = .016, odds ratio = 10.10, at 3 weeks post-treatment. However, this attribution did not predict additional changes in diet at 3 months post-treatment. Women who attributed cancer to a lack of exercise were not more likely to report increased physical activity at 3 weeks post-treatment but were more likely to have increased physical activity by 3 months post-treatment, Wald χ2(1) = 3.90, p = .048, odds ratio = 1.73. Finally, women who attributed their cancer to stress or worry showed a trend toward increasing the frequency with which they avoided stressful situations at both 3 weeks post-treatment, Wald χ2(1) = 3.12, p = .077, odds ratio = 1.50, and 3 months post-treatment, Wald χ2(1) = 3.12, p = .077, odds ratio = 1.75. There were no significant relationships between attributing cancer to alcohol use and decreased alcohol consumption.
Recurrence prevention beliefs
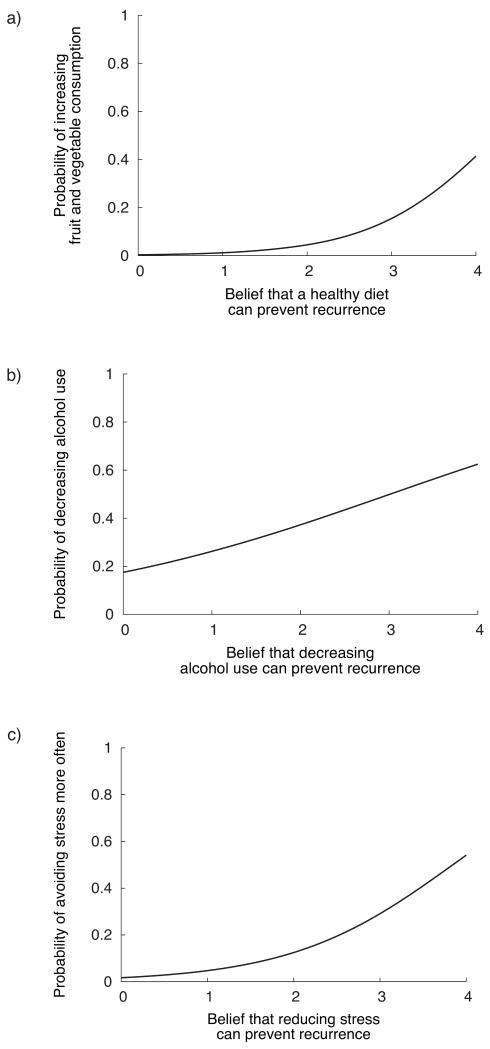
Combinations of recurrence prevention beliefs and behavior changes tested are listed in Table 5. Recurrence prevention beliefs at 3 weeks post-treatment were associated with health practice changes at the same time point, and the relationships are illustrated in Figure 1. Specifically, women who believed that a healthy diet could prevent recurrence were more likely to have increased fruit and vegetable consumption, Wald χ2(1) = 6.20, p = .013, odds ratio = 3.87 (see Figure 1a), but not fat intake. Participants who believed that decreasing or quitting alcohol use could prevent a recurrence were more likely to have reduced their alcohol use, Wald χ2(1) = 4.70, p = .030, odds ratio = 1.67. Similarly, those who believed that decreasing stress could prevent a recurrence were more likely to have increased the frequency with which they avoided stressful situations, Wald χ2(1) = 9.36, p = .002, odds ratio = 2.87. There were no relationships between the belief that exercise could prevent a recurrence and increased physical activity. Moreover, none of the beliefs at 3 weeks post-treatment predicted additional positive behavior changes at 3 months post-treatment.
Figure 1.
Illustration of relationships between participants' beliefs about factors that can prevent cancer recurrence and the likelihood of making corresponding behavior changes by 3 weeks after treatment ended (all models adjusted for age and duration of treatment).
(a) Stronger belief that eating a healthy diet can prevent recurrence was associated with greater probability of increasing fruit and vegetable consumption, Wald χ2(1) = 6.20, p = .013.
(b) Stronger belief that reducing alcohol use can prevent recurrence was associated with greater probability of decreasing alcohol use, Wald χ2(1) = 4.70, p = .030
(c) Stronger belief that reducing stress can prevent a cancer recurrence was associated with greater probability of avoiding stressful situations more often, Wald χ2(1) = 9.36, p = .002
Discussion
Findings from the present study indicate that breast cancer survivors make efforts to make life changes following the end of adjuvant treatment, and changes in health practices are particularly prevalent. Women often reported increasing the frequency of breast self-exam, eating more fruits and vegetables, increasing physical activity, and cutting back on alcohol and tobacco use, with more than one-fourth of participants reporting each of these positive health behavior changes. Moreover, women who reported making these adaptive changes were indeed doing better than their peers on the health practices examined. Breast cancer survivors also made changes that appeared to be directed toward enhancing emotional and spiritual health, with one-fourth of the sample reporting more frequent avoidance of stressful situations or praying or meditating more. Our results suggest that women are poised to make important lifestyle changes after treatment ends, and this may be a sensitive period for health promotion interventions.
Such changes may be driven by survivors' efforts to alleviate concerns about recurrence by making changes believed to decrease the possibility of recurrence. In fact, most of the changes in health practices match ACS and NCI health guidelines for cancer survivors and breast cancer prevention [8-10]. The changes further appear to reflect individual differences in breast cancer survivors' common-sense representations of their cancer, particularly perceptions of the severity of impact of breast cancer and attributions about factors that may have caused cancer and may prevent a recurrence.
We characterized the typical profile of breast cancer survivors' common-sense beliefs about their disease using the model put forth by Leventhal and colleagues [15, 16]. On average, breast cancer survivors believed they had moderate control over their cancer, they perceived their cancer to have moderate to severe consequences for their lives, and they saw their cancer as more of an acute than chronic condition, although no strongly so.
Moreover, women had fairly strong ideas about what may have caused their cancer and what they could now do to prevent a recurrence. Women rated hormones, environmental toxins or hazards, and genetics or heredity as the most important causes of their cancer, with more than 70% of women citing these factors as important in the development of their cancer, followed by diet, stress and aging. These attributions are at least somewhat consistent with current epidemiological and medical knowledge. Genetics and heredity, along with aging, are known to be strong risk factors for breast cancer, with hormonal factors, obesity (reflected in diet and exercise), and alcohol use conferring more modest risk [31]. Despite controversy regarding links between environmental toxins, such as pesticides, and breast cancer, no definitive evidence for a causal link has yet been found [32]. Women frequently named controllable factors as important in preventing a recurrence. Medical checkups and screenings were seen as most critical in preventing a recurrence, followed by eating a healthy diet, having a positive attitude, exercising, and taking medication. The intriguing finding that 92% of participants believed that a positive attitude was important in preventing a cancer recurrence is consistent with our previous report of gynecologic cancer survivors' recurrence prevention beliefs [21]. Factors such as God's will and chance were seen as less important. This pattern of focusing on controllable factors may be psychologically protective: it may be important for women to believe they can play an instrumental role in preventing recurrence.
Individual differences in common-sense beliefs were modestly successful in explaining behavior changes following treatment, with some domains showing greater predictive utility than others. Contrary to our hypotheses, beliefs about personal control and the course of one's disease were not useful in explaining behavior changes. Beliefs about disease consequences, causal factors, and recurrence prevention were more likely to be related to post-treatment behavior changes, and relationships were in the hypothesized directions. For example, women who perceived more severe consequences of breast cancer were more likely to reduce stress and improve their diet; it may be that these women were more motivated to make changes in their health practices. Post-treatment changes frequently matched participants' causal attributions, with those who attributed the development of their cancer to diet, lack of exercise, or stress making corresponding improvements in their health practices. Changes in health practices similarly corresponded with recurrence prevention beliefs. Women who believed that eating a healthy diet, reducing alcohol use, or reducing stress could prevent a cancer recurrence were more likely to make these changes.
Limitations of this study include the small sample size and relatively homogenous demographics of the sample; almost all participants were Caucasian, and the vast majority were married and well-educated. The sample may be comprised of a more motivated group of individuals who are willing to respond to surveys, although it is important to note that we had a high response rate (90%). Nonetheless, generalizations to other populations of breast cancer survivors may be limited for these reasons. In addition, because it was not feasible to assess health practices prior to diagnosis, we relied on women's self-reports of behavior changes from pre-diagnosis, which may be subject to recall bias. Finally, it is unclear to what extent the behavior changes observed are maintained following the immediate post-treatment period. Maintenance of health behavior may be motivated by different psychosocial factors than those that lead to initial behavior changes [33]. Future work might examine long-term maintenance of changes in diet, exercise, and substance use, and whether women are more likely to maintain changes made following treatment as compared to changes made at other times in their lives.
Nonetheless, results suggest that the immediate post-treatment period is an opportune time to encourage and educate breast cancer survivors regarding positive health practices. We have previously reported that breast cancer survivors experience distress following the end of treatment related to feeling uncertain as to what they should do for their health [2]. Education or other interventions that assist women in identifying appropriate dietary, exercise, or emotional health goals may assist not only in promoting good health practices but also in alleviating distress. Consistent with the suggestion that breast cancer survivors may be particularly receptive to this type of guidance, 79% of breast cancer survivors participating in a recent study expressed interest in a health promotion program [34]. New psychoeducational interventions designed to facilitate post-treatment adjustment by promoting active coping and goal-setting also show promising results [6, 7].
Results of the present study also provide insight into how women decide to make behavior changes and what changes they decide to make. Breast cancer survivors' beliefs that the consequences of breast cancer are severe, that potentially modifiable or controllable factors played a role in the development of their cancer, and that controllable factors can prevent a recurrence appear to motivate positive changes in health practices following cancer treatment. Moreover, women make behavior changes that match their beliefs about what may have caused their cancer and what can prevent a recurrence. Recent work suggests that psychoeducational interventions targeting common-sense beliefs can indeed help patients to develop more accurate or adaptive beliefs about their illness [35-37], and these changes have shown positive behavioral outcomes [35]. Assessing attributions and beliefs about cancer, as well as providing tailored psychoeducational interventions and guidance addressing post-treatment behavior changes, may assist women with breast cancer in navigating this important transition from “patient” to “survivor.”
Acknowledgments
This work was supported by a Barbara Rosenblum Scholarship for the Study of Women and Cancer from the Sociologists for Women in Society, a graduate research scholarship from the American Psychological Foundation/Council of Graduate Departments of Psychology, a research grant from the University of Iowa, grant T32 MH018931 from the National Institute of Mental Health, and grant KL2 RR0205012 from the National Center for Research Resources.
The authors gratefully acknowledge Lisa Bergman, Heena Maiseri, Mary Mattes, Hetal Pandya, Carolene Robinson, and Sara Sheerer for assistance with participant enrollment and data collection.
References
- 1.Schnipper HH. Life after breast cancer. J Clin Oncol. 2001;19:3581–3584. doi: 10.1200/JCO.2001.19.15.3581. [DOI] [PubMed] [Google Scholar]
- 2.Costanzo ES, Lutgendorf SK, Mattes ML, et al. Adjusting to life after treatment: distress and quality of life following treatment for breast cancer. Br J Cancer. 2007;97:1625–1631. doi: 10.1038/sj.bjc.6604091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ward SE, Viergutz G, Tormey D, deMuth J, Paulen A. Patients' reactions to completion of adjuvant breast cancer therapy. Nurs Res. 1992;41:362–366. [PubMed] [Google Scholar]
- 4.Lethborg CE, Kissane D, Burns WI, Snyder R. “Cast Adrift”: the experience of completing treatment among women with early stage breast cancer. J Psychosoc Oncol. 2000;18:73–90. [Google Scholar]
- 5.Stanton AL, Ganz PA, Rowland JH, Meyerowitz BE, Krupnick JL, Sears SR. Promoting adjustment after treatment for cancer. Cancer. 2005;104:2608–2613. doi: 10.1002/cncr.21246. [DOI] [PubMed] [Google Scholar]
- 6.Cimprich B, Janz NK, Northouse L, Wren PA, Given B, Given CW. Taking CHARGE: A self-management program for women following breast cancer treatment. Psychooncology. 2005;14:704–717. doi: 10.1002/pon.891. [DOI] [PubMed] [Google Scholar]
- 7.Stanton AL, Ganz PA, Kwan L, et al. Outcomes from the Moving Beyond Cancer psychoeducational, randomized, controlled trial with breast cancer patients. J Clin Oncol. 2005;23:6009–6018. doi: 10.1200/JCO.2005.09.101. [DOI] [PubMed] [Google Scholar]
- 8.National Cancer Institute. Facing forward: life after cancer treatment. Bethesda, MD: Author; 2006. [Google Scholar]
- 9.Kushi LH, Byers T, Doyle C, et al. American Cancer Society guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2006;56:254–281. doi: 10.3322/canjclin.56.5.254. [DOI] [PubMed] [Google Scholar]
- 10.Doyle C, Kushi LH, Byers T, et al. Nutrition and physical activity during and after cancer treatment: An American Cancer Society guide for informed choices. CA Cancer J Clin. 2006;56:323–353. doi: 10.3322/canjclin.56.6.323. [DOI] [PubMed] [Google Scholar]
- 11.Maskarinec G, Murphy S, Shumay DM, Kakai H. Dietary changes among cancer survivors. Eur J Cancer Care (Engl) 2001;10:12–20. doi: 10.1046/j.1365-2354.2001.00245.x. [DOI] [PubMed] [Google Scholar]
- 12.Patterson RE, Neuhouser ML, Hedderson MM, Schwartz SM, Standish LJ, Bowen DJ. Changes in diet, physical activity, and supplement use among adults diagnosed with cancer. J Am Diet Assoc. 2003;103:323–328. doi: 10.1053/jada.2003.50045. [DOI] [PubMed] [Google Scholar]
- 13.Taylor SE, Lichtman RR, Wood JV. Attributions, beliefs about control, and adjustment to breast cancer. J Pers Soc Psychol. 1984;46:489–502. doi: 10.1037//0022-3514.46.3.489. [DOI] [PubMed] [Google Scholar]
- 14.Blanchard C, Denniston M, Baker F, et al. Do adults change their lifestyle behaviors after a cancer diagnosis? Am J Health Behav. 2003;27:246–256. doi: 10.5993/ajhb.27.3.6. [DOI] [PubMed] [Google Scholar]
- 15.Leventhal H, Benyamini Y, Brownlee S . Illness representations: theoretical foundations. In: Petrie KJ, Weinman JA, editors. Perceptions of Health and Illness: Current Research and Applications. Routledge; New York: 1997. pp. 19–45. [Google Scholar]
- 16.Leventhal H, Meyer D, Nerenz D. The common-sense representation of illness danger. In: Rachman S, editor. Medical Psychology. Vol. 2. Pergamon; New York: 1980. pp. 7–30. [Google Scholar]
- 17.Henderson JW, Donatelle RJ. The relationship between cancer locus of control and complementary and alternative medicine use by women diagnosed with breast cancer. Psychooncology. 2003;12:59–67. doi: 10.1002/pon.636. [DOI] [PubMed] [Google Scholar]
- 18.Hilton BA. The relationship of uncertainty, control, commitment, and threat of recurrence to coping strategies used by women diagnosed with breast cancer. J Behav Med. 1989;12:39–54. doi: 10.1007/BF00844748. [DOI] [PubMed] [Google Scholar]
- 19.Christensen AJ, Moran PJ, Ehlers SL, Raichle K, Karnell L, Funk G. Smoking and drinking behavior in patients with head and neck cancer: effects of behavioral self-blame and perceived control. J Behav Med. 1999;22:407–418. doi: 10.1023/a:1018669222706. [DOI] [PubMed] [Google Scholar]
- 20.Buick DL. Illness representations and breast cancer: coping with radiation and chemotherapy. In: Petrie KJ, Weinman JA, editors. Perceptions of Health and Illness: Current Research and Applications. Routledge; New York: 1997. pp. 379–409. [Google Scholar]
- 21.Costanzo ES, Lutgendorf SK, Bradley SL, Rose S, Anderson B. Cancer attributions, distress, and health practices among gynecologic cancer survivors. Psychosom Med. 2005;67:972–980. doi: 10.1097/01.psy.0000188402.95398.c0. [DOI] [PubMed] [Google Scholar]
- 22.Rabin C, Pinto B. Cancer-related beliefs and health behavior change among breast cancer survivors and their first-degree relatives. Psychooncology. 2006;15:701–712. doi: 10.1002/pon.1000. [DOI] [PubMed] [Google Scholar]
- 23.Stewart DE, Cheung AM, Duff S, et al. Attributions of cause and recurrence in long-term breast cancer survivors. Psychooncology. 2001;10:179–183. doi: 10.1002/pon.497. [DOI] [PubMed] [Google Scholar]
- 24.Costanzo ES, Lutgendorf SK, Mattes ML, Trehan S, Robinson CB, Tewfik F, Roman SL. Adjusting to life after treatment: distress and quality of life following treatment for breast cancer. Br J Cancer. 2007;97:1625–1631. doi: 10.1038/sj.bjc.6604091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tatsumura Y, Maskarinec G, Shumay DM, Kakai H. Religious and spiritual resources, CAM, and conventional treatment in the lives of cancer patients. Altern Ther Health Med. 2003;9:64–71. [PubMed] [Google Scholar]
- 26.Cohen S, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM. Social ties and susceptibility to the common cold. JAMA. 1997;277:1940–1944. [PubMed] [Google Scholar]
- 27.Miller GE, Cohen S, Herbert TB. Pathways linking major depression and immunity in ambulatory female patients. Psychosom Med. 1999;61:850–860. doi: 10.1097/00006842-199911000-00021. [DOI] [PubMed] [Google Scholar]
- 28.Kohl HW, Blair SN, Paffenbarger RS, Macera CA, Kronnenfeld JJ. A mail survey of physical activity habits as related to measured physical fitness. Am J Epidemiol. 1988;127:1228–1239. doi: 10.1093/oxfordjournals.aje.a114915. [DOI] [PubMed] [Google Scholar]
- 29.Block G, Gillespie C, Rosenbaum EH, Jensen C. A rapid food screener to assess fat and fruit and vegetable intake. Am J Prev Med. 2000;18:284–288. doi: 10.1016/s0749-3797(00)00119-7. [DOI] [PubMed] [Google Scholar]
- 30.Moss-Morris R, Weinman J, Petrie KJ, Horne R, Cameron LD, Buick D. The Revised Illness Perception Questionnaire (IPQ-R) Psychol Health. 2002;17:1–16. [Google Scholar]
- 31.American Cancer Society. Breast cancer facts & figures 2009-2010. Atlanta, GA: [Google Scholar]
- 32.Calle EE, Frumkin H, Henley SJ, Savitz DA, Thun MJ. Organochlorines and breast cancer risk. CA Cancer J Clin. 2002;52:301–309. doi: 10.3322/canjclin.52.5.301. [DOI] [PubMed] [Google Scholar]
- 33.Rothman AJ. Toward a theory-based analysis of behavioral maintenance. Health Psychol. 2000;19(1S):64–69. doi: 10.1037/0278-6133.19.suppl1.64. [DOI] [PubMed] [Google Scholar]
- 34.Demark-Wahnefried W, Peterson B, McBride C, Lipkus I, Clipp E. Current health behaviors and readiness to pursue lifestyle changes among men and women diagnosed with early stage prostate and breast carcinomas. Cancer. 2000;88:674–684. [PubMed] [Google Scholar]
- 35.Petrie KJ, Cameron LD, Ellis CJ, Buick D, Weinman J. Changing illness perceptions after myocardial infarction: An early intervention randomized controlled trial. Psychosom Med. 2002;64:580–586. doi: 10.1097/00006842-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Karamanidou C, Weinman J, Horne R. Improving hemodialysis patients' understanding of phosphate-binding medication: A pilot study of a psycho-educational intervention designed to change patients' perceptions of the problem and treatment. Br J Health Psychol. 2008;13:205–214. doi: 10.1348/135910708X288792. [DOI] [PubMed] [Google Scholar]
- 37.Fortune DG, Richard HL, Griffiths CEM, Main CJ. Targeting cognitive-behaviour therapy to patients' implicit model of psoriasis: Results from a patient preference controlled trial. Br J Clin Psychol. 2004;43:65–82. doi: 10.1348/014466504772812977. [DOI] [PubMed] [Google Scholar]