Abstract
Background
Interaction of resting platelets with exposed components of the subendothelial matrix is an important early activating event that takes place at sites of vascular injury. Platelet responses to collagen are mediated by the integrin α2β1 and the glycoprotein (GP)VI/Fc receptor (FcR)γ chain complex, while platelet activation by laminin is mediated by the related integrin, α6β1, and similarly requires signaling through GPVI/FcRγ.
Objective
Because the cell adhesion and signaling receptor, PECAM-1, has previously been shown to dampen collagen-induced platelet activation, we sought to determine whether PECAM-1 might similarly regulate platelet activation by laminin.
Methods/Results
We found that PECAM-1 became tyrosine phosphorylated on its cytoplasmic ITIMs following adhesion of either human or murine platelets to immobilized laminin. While the presence or absence of PECAM-1 had no effect on either the rate or extent of platelet adhesion or spreading on laminin, PECAM-1 inhibited laminin-induced phosphorylation of GPVI/FcRγ chain ITAMs, activation of its downstream effector, Syk kinase, and suppressed granule secretion.
Conclusion
Taken together, these data are consistent with previous findings in platelets and other blood and vascular cells that PECAM-1 functions by modulating ITAM-mediated signaling pathways that amplify cellular activation.
Keywords: PECAM-1, laminin, α6β1, GPVI, platelet signaling, ITAM, ITIM
INTRODUCTION
Breach of the vascular endothelium results in rapid adhesion of platelets to the underlying basement membrane – an extracellular matrix sheet composed of glycosaminoglycans, type IV collagen, fibronectin, von Willebrand factor, and one or more members of the laminin family of adhesive glycoproteins [1]. Platelet adhesion to collagen, and to a lesser extent, laminin, initiates a series of biochemical reactions that result in tyrosine phosphorylation of cytoplasmic enzymes and adaptor proteins, calcium mobilization from cytosolic stores, polymerization of cytoskeletal proteins that drive shape change and spreading, rapid conformational activation of cell-surface integrins that enable high-affinity binding to adhesive ligands, and secretion of the contents of platelet alpha and dense granules (for a review, see references [2] and [3]). Together, these events constitute primary hemostasis, and allow formation of a platelet plug designed to prevent blood loss at sites of vascular injury.
The glycoprotein (GP)VI/FcRγ chain complex is a critical mediator of adhesion-induced platelet activation, operating via GPVI-associated Src-family kinases that, upon ligand binding, phosphorylate tyrosine residues located within Immunoreceptor Tyrosine-base Activation Motifs (ITAMs) located within cytoplasmic domain of the FcRγ chain. Phosphorylated FcRγ serves as a docking site for the tyrosine kinase Syk which, with the help of adaptor proteins LAT, SLP76, and Gads, and the tyrosine kinase Btk, activates phospholipase C (PLC)γ2 and/or PLCγ1, which via their lipase activity generate lipid products that support a multitude of cellular activation responses, including calcium mobilization, platelet spreading, integrin activation, and granule secretion [4].
While many of the biochemical events associated with adhesion-initiated platelet activation have been elucidated, the identity and mechanism of action of those receptors that set a physiological threshold for platelet activation and/or return platelets to a resting state is much less well-understood. Of these, the Immunoreceptor Tyrosine-based Inhibitory Motif (ITIM) – bearing inhibitory receptor, PECAM-1 is perhaps the best characterized. PECAM-1 is a 130 kDa member of the Ig-ITIM family that is expressed on platelets, leukocytes and endothelial cells. In leukocytes, PECAM-1 has been shown, upon co-ligation with the TCR, to attenuate calcium release from intracellular stores [5], and can also exert its inhibitory function by suppressing cytokine production [6-9]. In endothelial cells, PECAM-1 has been shown to inhibit apoptotic signals that activate the intrinsic, Bax-mediated pathway of programmed cell death [10-13]. In platelets, numerous laboratories have demonstrated that PECAM-1 inhibits low-dose collagen-induced platelet activation – an effect that has been observed both in vitro and in vivo [14-18]. Interestingly, it appears that PECAM-1 does so by regulating granule secretion – a key amplifier of platelet activation and thrombus formation.
Collagen- and laminin-induced platelet activation have been shown to be mechanistically-linked via their use of the GPVI/FcRγ-chain complex to send activation signals into the cell downstream of ligand binding to integrins α2β1and α6β1, respectively [19]. Because PECAM-1, has previously been shown to dampen collagen-induced platelet activation, we sought to determine whether PECAM-1 might similarly regulate platelet activation by laminin. Our findings provide further support for the notion that PECAM-1 functions to regulate biochemical and cell biological events that amplify platelet activation responses.
MATERIALS AND METHODS
Reagents and Antibodies
Laminin from human placenta, bovine serum albumin, tetramethylrhodamine isothiocyanate (TRITC)-labeled phalloidin, and prostaglandin E1 (PGE1) were purchased from Sigma-Aldrich (St Louis, MO). Soluble calf skin collagen was obtained from BioData (Horsham, PA) and collagen-related peptide (CRP) was synthesized by the Protein Chemistry Core Laboratory of the Blood Research Institute, BloodCenter of Wisconsin. Mouse anti-human PECAM-1 monoclonal antibody (mAb), PECAM-1.3, and rabbit anti-human PECAM-1 polyclonal antibody (pAb), SEW 32-34 have been previously described [5]. Rat anti-mouse PECAM-1 mAb, 390, was generously provided by Dr. Steven Albelda (University of Pennsylvania, Philadelphia, PA) and has also been also previously described [20]. Goat anti-mouse PECAM-1 (clone M-20) pAb and rabbit anti-SHP-2 (clone C-18) pAb were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Unconjugated and fluorescein isothiocyanate (FITC)-conjugated rat anti–integrin α6 (clone GoH3) mAbs and phycoerythrin (PE)-conjugated rat anti-mouse integrin α2 (clone 3H1480) mAbs were obtained from Abcam (Cambridge, MA). Horseradish peroxidase (HRP)-conjugated mouse anti–phospho-tyrosine (PY-20) was obtained from Zymed (San Francisco, CA). Rabbit anti-FcεRI, anti-FcRγ-chain pAbs and mouse anti–phosphotyrosine (clone 4G10) were obtained from Upstate (Lake Placid, NY). PE-conjugated rat anti-mouse PECAM-1 (clone MEC 13.3) mAb was obtained from BD Biosciences (Franklin Lakes, NJ). The rat anti-mouse GPVI (clone JAQ1) mAb and PE-conjugated rat anti-mouse integrin αIIbβ3 (clone JON/A) mAb were purchased from Emfret Analytics (Würzburg, Germany). JAQ1 antibodies were further conjugated with Alexa Fluor 647 using a labeling kit (Invitrogen, Eugene, OR). HRP-conjugated secondary antibodies used for western blot analysis were purchased from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA). For flow cytometry, appropriate conjugated and isotype-matched antibodies were purchased either from Molecular Probes (Invitrogen) or Pharmingen (BD Biosciences). Calcein-AM was purchased from Molecular Probes and (14C) serotonin was obtained from GE Healthcare (Piscataway, NJ).
Preparation of murine and human platelets
PECAM-1-deficient mice on a C57BL/6J background were housed and bred in pathogen-free conditions at the Animal Resource Facility of the Medical College of Wisconsin. PECAM-1-negative mice were genotyped by polymerase chain reaction (PCR) and either wild-type littermates or age- and sex-matched wild-type mice used as a controls. Whole blood was obtained from the inferior vena cava using a syringe containing 0.1 volume of 3.8% sodium citrate. Blood from healthy human volunteers was obtained by venipuncture into tubes containing 10% acid-citrate-dextrose (ACD). Blood samples were diluted 1:5 with modified Tyrodes-HEPES buffer (10 mM HEPES [pH 7.4], 12 mM NaHCO3, 137 mM NaCl, 2.7 mM KCl, 5 mM glucose, 0.25% bovine serum albumin [BSA]) and prostaglandin E1 (PGE1) added to a final concentration of 50 ng/mL. Platelet-rich-plasma (PRP) was obtained following centrifugation for 10 minutes at 300g, transferred into a fresh tube, and platelets pelleted at 700g before resuspension in modified Tyrodes-HEPES buffer at 2 × 107 platelets per ml for platelet spreading assays, 1 × 108 platelets per ml for adhesion, flow cytometry, and serotonin release assays, or 6 × 108 platelets per ml for immunoprecipitation/immunoblot analysis.
Quantitative platelet adhesion assays
Adhesion to immobilized substrates was performed as previously described [14,14], with minor modifications. Briefly, 96 well Immulon-2HB microtiter plates (Dynex Technologies, Chantilly, VA) were coated overnight at 4°C with various concentrations of human laminin or calf soluble collagen. After washing and blocking 3% BSA. Calcein-AM-loaded platelets in modified Tyrodes-HEPES buffer containing 2 mM MgCl2 were added to each of triplicate wells and incubated at 37°C for 60 minutes under static conditions. Total fluorescence was determined using a fluorescence plate reader (Cytofluor 4000, PerSeptive Biosystems, Framingham, MA) at 485 nm excitation and 530 nm emission wavelengths. Wells were rinsed with Tyrodes-HEPES buffer 3 times and bound fluorescence measured again. Percent adhesion was calculated as bound fluorescence/total fluorescence × 100.
Immunoprecipitation and immunoblot analysis
700 μl of washed human or murine platelets (6 × 108/mL) suspended in modified Tyrodes-HEPES buffer containing 2 mM Mg2+ were seeded onto laminin or collagen-coated Corning cell culture dishes (Corning Inc., Corning, NY) and incubated at 37 °C for the indicated time period. Reactions were terminated by addition of an equal volume of 2x ice-cold lysis buffer (30 mM HEPES, pH 7.4, 300 mM NaCl, 20 mM EGTA, 0.2 mM MgCl2, 2% Triton X-100 containing 2x protease inhibitor cocktail (CalBiochem, San Diego, CA), 2x phosphatase inhibitor cocktail set II (CalBiochem), and 4 mM sodium orthovanadate. After 30 minutes on ice, lysates were precleared with protein G-Sepharose beads before addition of primary antibody. After overnight incubation at 4°C, immune complexes were captured with protein G-Sepharose beads, eluted off in reducing SDS loading buffer, and subjected to SDS-PAGE/western blot analysis. Binding was detected using enhanced chemiluminescence (Amersham, Piscataway, NJ) and band intensity quantitated using a Kodak 1D imaging system (Scientific Imaging System, New Haven, CT). The degree of phosphorylation was expressed as a ratio of the band intensity for the phosphorylated protein relative to protein antigen.
Platelet spreading assays
Washed platelets derived from either wild-type or PECAM-1-negative mice, or from human blood were resuspended to a concentration of 2 × 107/mL in modified Hepes/Tyrodes buffer containing 2 mM Mg2+, added to laminin-coated coverslips, and incubated for the indicated time-points at 37 °C. After a light rinse, adherent platelets were fixed using 3% paraformaldehyde, permeabilized with 0.1% Triton X-100, and stained for F-actin using tetramethylrhodamine isothiocyanate (TRITC)-labeled phalloidin (0.1 μg/ml). Platelets were visualized using a Nikon TE300 inverted microscopy equipped with a CFI Plan Achromat DL Ph3 100x oil-immersion lens and a Photometrics SenSys charge-coupled device camera (Photometrics, Tucson, AZ). Eight images, encompassing a total of 179 – 264 platelets, were randomly chosen for each time-point and the surface area of each platelet calculated using Metamorph software (Universal Imaging, Downingtown, PA). All analyses were performed under blinded conditions. Statistical significance was determined using the Student t-test for unpaired samples and results expressed at the mean surface area/platelet ± SEM. P values of less than 0.05 were interpreted as statistically significant.
Serotonin release assay
Murine platelets at a concentration of 1 × 108/mL in modified Tyrodes buffer containing 50 ng/ml PGE1 without calcium or magnesium were loaded with 0.2 μCi/ml of [14C] 5-hydroxytryptamine for 1 hour at 37 °C. Labeled platelets were washed by centrifugation and resuspended in modified Tyrodes buffer containing 2 mM MgCl2, added in triplicate to collagen or laminin-coated microtiter wells. Following incubation for 60 minutes at room temperature, non-adherent platelets were removed and the supernatant containing the released serotonin counted in a scintillation counter.
Statistical analysis
Data analysis was performed using SigmaPlot for Windows, version 6.0 (SPSS Inc. Chicago, IL) and/or SAS Learning Edition 4.1 (SAS Institute Inc. Cary, NC). All results were expressed as mean ± SEM. A P-value <0.05 was considered statistically significant. Quantitative data with normal distributions were compared by parametric tests, and data with abnormal distributions were analyzed by non-parametric tests. Multiple groups were analyzed by analysis of variance (ANOVA) or by Kruskall–Wallis tests. In the case of significant main effects, post hoc analysis was performed using Student's t-tests for the ANOVA, or the Wilcoxon test for the Kruskall–Wallis tests. Comparisons between two groups were performed by Student's t-test or Mann–Whitney U-tests.
RESULTS
Platelet adhesion to laminin induces tyrosine phosphorylation of PECAM-1
Platelet adhesion to laminin was first described in 1984 by Ill and Ruoslahti [21], and when performed in the presence of at least 1 mM MgCl2, platelets have consistently been observed to not only adhere, but to undergo cytoskeletally-mediated, laminin-induced cell spreading as well [19,22]. Similar to these studies, we found that human platelets bound and spread on immobilized laminin nearly as well as they did on immobilized collagen (Figure 1B), though the morphology of platelets bound to the two extracellular matrix substrates differed in the degree of activation-induced spreading, especially at early time points (Figure 1A).
Figure 1. Human platelets adhere to immobilized laminin and collagen, but spread with different morphology.
(A) Visualization of platelets bound to immobilized matrix proteins. Washed human platelets resuspended at 1 × 108/mL in 2 mM MgCl2 and 0.5 mM CaCl2 were seeded on surfaces pre-coated with either human placental laminin 511 or soluble calf-skin collagen. After 30 minutes at 37°C, unbound platelets were removed, adherent platelets fixed with 3% paraformaldehyde, and visualized using Hoffman modulation contrast. Results shown are representative of three separate experiments. Note that platelets bind well to immobilized laminin, but do not spread as well as they do on immobilized collagen. (B) Quantitation of platelet adhesion to immobilized matrix proteins. Washed human platelets were loaded with Calcein AM and seeded onto microtiter wells that had been pre-coated with the indicated concentrations of human laminin or collagen. After a 60 minute incubation at 37°C, the percentage of adherent platelets was calculated by taking fluorescence measurements both before and after removal of unbound platelets. Data shown represents mean values ± SEM from three independent experiments performed using triplicate wells.
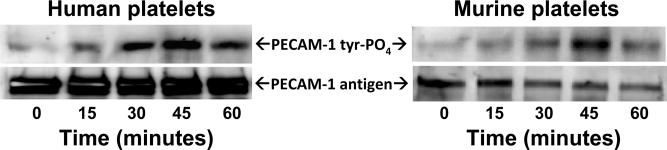
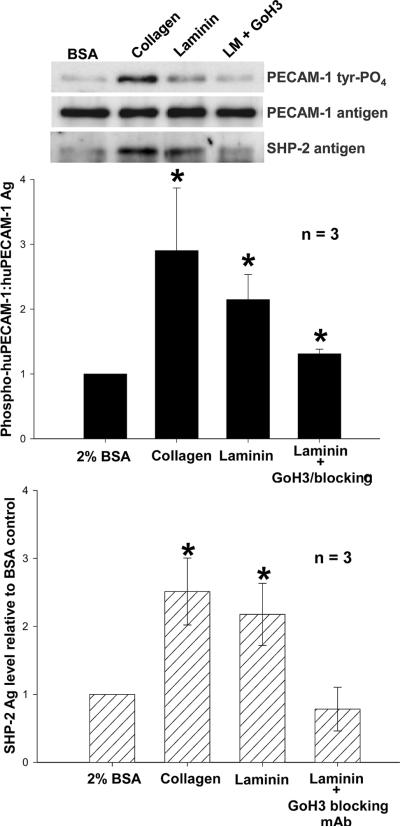
PECAM-1 is thought to function as a negative regulator of collagen-induced platelet activation [14-17] via ligand binding-induced phosphorylation of its cytoplasmic ITIM tyrosine, followed by recruitment of the protein-tyrosine phosphatase, SHP-2 [23]. To determine whether this same negative-feedback pathway might be initiated in response to platelet adhesion to immobilized laminin, we incubated both human and mouse platelets in laminin-coated microtiter wells, allowed them to settle, bind, and spread at 37 °C, and performed immunoblot analysis of cells that were detergent-lysed at various time points up to one hour. As shown in Figure 2, weak PECAM-1 tyrosine phosphorylation could be detected as early as 15 minutes, peaking at 45 minutes, and declining by 60 minutes. Similar to that observed following platelet adhesion to collagen, SHP-2 became associated with the PECAM-1 cytoplasmic domain following platelet adhesion to immobilized laminin (Figure 3). Laminin, and not contaminants in the laminin preparation, was responsible for activation of PECAM-1 and its subsequent recruitment of SHP-2, as addition to platelets of the α6β1-specficic mAb, GoH3 [24], effectively inhibited both responses. Taken together, these data support the notion that the inhibitory function of PECAM-1 is enlisted in response to platelet adhesion to multiple components of the extracellular matrix.
Figure 2. PECAM-1 becomes tyrosine phosphorylated following adhesion of human and mouse platelets to immobilized laminin.
Washed platelets were resuspended at a concentration of 4 × 108/mL in Tyrodes buffer containing 2 mM MgCl2 and 0.5 mM CaCl2 and added to culture plates that had been pre-coated with 50 μg/mL of laminin 511. Platelets were allowed to settle, bind, and spread at 37°C for the indicated times, and then detergent lysed and subjected to immunoprecipitation analysis using mAbs PECAM-1.3 for human platelets and 390 for murine platelets. Western blots were developed using the anti-phosphotyrosine mAb PY20 (top panels) and anti-PECAM-1 polyclonal antibodies SEW32-34 (human) and M-20 (mouse) to visualize antigen loading. Maximal phosphorylation of PECAM-1 at the 45 minute time point reflects the time needed for platelets to settle, make contact with the immobilized laminin, and activate the platelets. The result shown is representative of three separate experiments.
Figure 3. Tyrosine-phosphorylated PECAM-1 recruits SHP-2 following adhesion to immobilized laminin.
Top panel: Washed human platelets were added to laminin-coated tissue culture plates as described in the legend for Figure 2 and incubated for 30-45 minutes at 37°C. Wells coated with 1 mg/ml BSA or 50 μg/mL collagen served as negative and positive controls, respectively. Platelets were then lysed and subjected to immunoprecipitation/western blot analysis as in Figure 2. SHP-2 was detected in the co-immunoprecipitates using polyclonal antibody C-18. The result shown is representative of three individual experiments. Middle and bottom panels: Quantitative analysis of PECAM-1 tyrosine phosphorylation and SHP-2 binding following platelet adhesion to immobilized laminin. Band intensity was determined using a Kodak Molecular Imaging Densitometry System. PECAM-1 tyrosine phosphorylation levels are expressed as the ratio of PY20:PECAM-1 antigen. Data were normalized to values obtained from resting platelets adherent to BSA. Data from three independent experiments were analyzed by the Wilcoxon rank sum test and represented as mean ± SEM. *P < 0.05. Similar results were obtained for murine platelets (not shown).
The rate and extent of platelet adhesion and spreading on immobilized laminin are unaffected by the presence or absence or PECAM-1
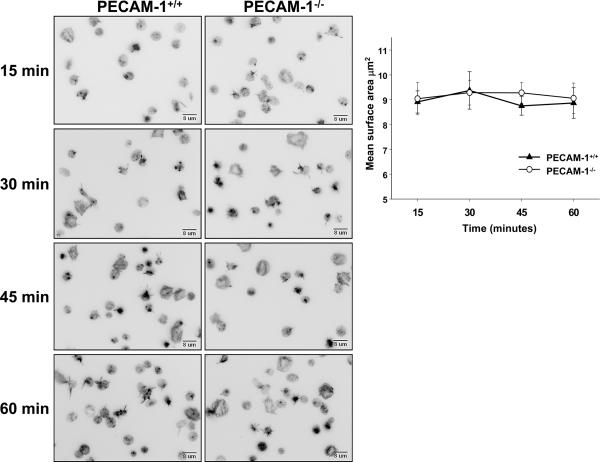
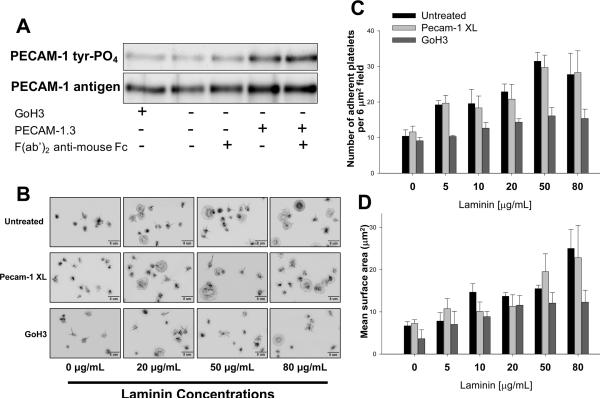
We employed two different approaches to determine whether PECAM-1 might function to inhibit platelet adhesion and spreading following exposure to immobilized laminin. In the first, the rate and extent of spreading of wild-type and PECAM-1-deficient murine platelets on immobilized laminin was compared. As shown in Figure 4, there was no significant difference. The second approach took the tact of Cicmil et al. [16] of adding anti-PECAM-1 mAbs to human platelets, either in the presence or absence of secondary anti-mouse antibodies, to stimulate the inhibitory activity of PECAM-1. As shown in Figure 5, although mAb PECAM-1.3 was able to induce PECAM-1 ITIM tyrosine phosphorylation, its addition had no observable effect on either the number of platelets that became adherent to laminin, or the extent to which they spread following contact.
Figure 4. Rate and extent of spreading of wild-type versus PECAM-1–deficient platelets on immobilized laminin.
Washed platelets isolated from the anticoagulated whole blood of wild-type or PECAM-1–deficient mice were seeded on laminin-coated coverslips in the presence of 2 mM Mg++ and incubated for 15, 30, 45 or 60 minutes at 37°C. Adherent platelets were fixed, permeabilized, stained with TRITC-labeled phalloidin, and visualized by fluorescence microscopy. Left panels – representative images taken over a 60 minute period. Upper right panel - Quantitation of platelet spreading from eight randomly chosen fields (~200 platelets/field) using Metamorph software. Data represent the mean ± SEM from three independent experiments. Statistical significance was determined using the Student t-test for unpaired samples.
Figure 5. PECAM-1 engagement-induced inhibitory signaling does not affect platelet adhesion or spreading on immobilized laminin.
(A) 3 × 108 human platelets/ml were incubated with 5 μg/mL mAb PECAM-1.3 for 10 minutes in the presence or absence of F(ab')2 fragments of anti-mouse IgG Fc and subjected to immunoprecipitation/western blot analysis. GoH3, a specific mAb for the integrin α6 subunit, was used as a negative control. Note that binding and cross-linking of mAb PECAM-1.3 on the platelet surface was able to induce tyrosine phosphorylation of PECAM-1. (B) Effect of antibody-induced inhibitory signaling on platelet spreading on immobilized laminin. Resting human platelets were treated with GoH3 or mAb PECAM-1.3 and cross-linked (Pecam-1 XL) as described in panel A and the platelets added to culture dishes that had been the indicated concentration of laminin. After 30 minutes at 37°C, unbound platelets were removed and the remaining bound platelets were fixed, stained, and analyzed. Panels C and D - The number and mean surface area of adherent platelets from three independent experiments were quantitated from 8 randomly chosen fields of 50 – 300 platelets each, analyzed using Metamorph software, and expressed as the mean ± SEM. Note that although PECAM-1.3 was effective in initiating inhibitory signaling (panel A), this had no effect on platelet adhesion or spreading.
PECAM-1 negatively regulates laminin-induced amplification pathways leading to granule secretion
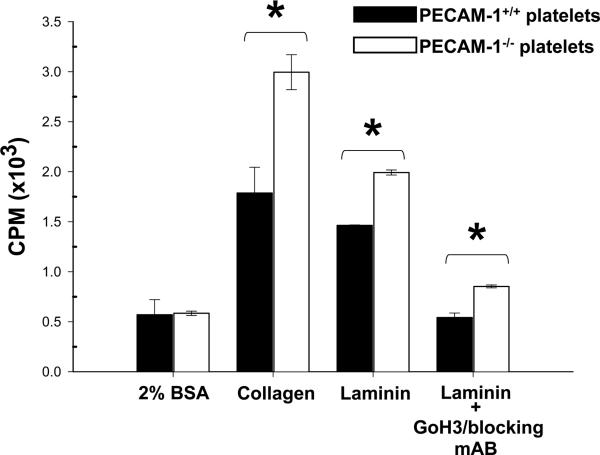
Though PECAM-1 does not appear to control the initial adhesive phenotype of the cell following exposure to adhesive ligands, signaling downstream of PECAM-1 is thought to provide negative feedback regulation of amplification events, in particular secretion of platelet granules. Thus, we [14] and others [15,16] have shown that PECAM-1-deficient murine platelets exhibit enhanced secretion responses to the collagen→α2β1→GPVI/FcRγ→Syk→PLCγ2 platelet activation pathway. To determine whether granule secretion was similarly exaggerated in PECAM-1-deficient platelets exposed to laminin, platelets from wild-type and PECAM-1 knockout mice were isolated, labeled with 14C serotonin, and applied to microtiter wells that have been coated with BSA, collagen, or laminin. As shown in Figure 6, dense granule secretion in response to laminin stimulation was modestly, but significantly, increased in PECAM-1-deficient, relative to wild-type, platelets. This was not due to differential expression of the major cell surface receptors known to regulate platelet responses to collagen, as the expression in PECAM-1-deficient platelets of α6β1 and GPVI was similar to that found in their wild-type counterparts (Table 1).
Figure 6. PECAM-1 regulates laminin-induced granule secretion.
Washed platelets obtained from age- and sex-matched wild-type or PECAM-1-deficient mice were labeled with 14C-serotonin and added in triplicate to wells pre-coated with either 2% BSA, 50 μg/mL of collagen, or 50 μg/mL of laminin. In some cases, platelets were preincubated with 5 μg/mL of GoH3 10 minutes before seeding onto immobilized laminin. After 60 minutes at 37°C, the amount of 14C-serotonin that had been released into the culture media was determined by scintillation counting and expressed as the mean ± SEM. Data shown is representative of two independent experiments. * P < 0.001.
Table 1.
Expression of select membrane glycoproteins on the surface of wild-type and PECAM-1-deficient platelets
| Glycoprotein | PECAM-1+/+ platelets | PECAM-1-/- platelets |
|---|---|---|
| Integrin α6β1 | 32 ± 3 | 28 ± 1 |
| Integrin α2β1 | 60 ± 13 | 61 ± 20 |
| GPVI | 46 ± 2 | 44 ± 2 |
| PECAM-1 | 21 ± 4 | 2 ± 2 |
*Washed platelets at 1 × 108/ml were incubated with 5 μg/ml of the indicated antibody for 15 min at room temperature and then analyzed on a BD LSR II flow cytometer. Values shown are the median fluorescence intensity of triplicate determinations. With the exception of PECAM-1, none of the other differences were significant.
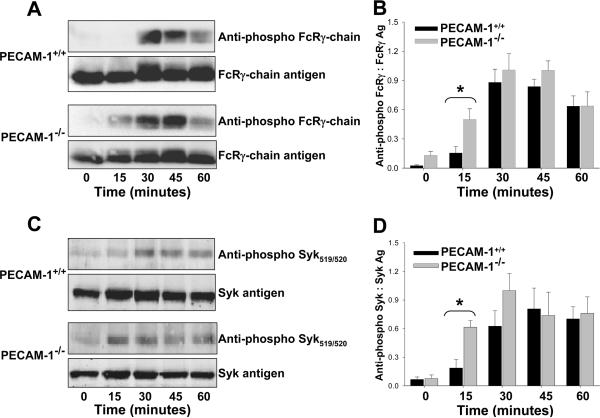
Because granule secretion is downstream of the α6β1→GPVI/FcRγ chain→Syk signaling pathway, we next examined whether the time-course of phosphorylation of these proteins might be influenced by the presence or absence of PECAM-1. As shown in the top panel of Figure 7A, phosphorylation the FcRγ-chain occurred earlier in PECAM-1-/- versus PECAM-1+/+ platelets following exposure to immobilized laminin. The same was found for Syk (panels of Figure 7). Taken together with the results presented in Figure 6, these data suggest that the threshold for laminin-induced platelet activation is normally suppressed by PECAM-1, similar to that previously observed for platelet responses to collagen.
Figure 7. Early activation of the GPVI /FcRγ-chain→Syk platelet activation pathway in PECAM-1-deficient platelets.
Murine platelets were added to laminin-coated microtiter wells for the indicated times, lysed, and subjected to western blot analysis using the indicated antibodies. Panels A and B - PECAM-1 delays laminin-induced FcRγ-chain phosphorylation. A representative immunoblot is shown in (A), and cumulative quantitative data derived from three independent experiments is shown in (B). Panels C and D - PECAM-1 affects the kinetics of laminin-induced Syk phosphorylation. A representative immunoblot is shown in (C), with cumulative quantitative data derived from three independent experiments is shown in (D). Note that PECAM-1 appears to set a higher threshold for platelet activation, as both the FcRγ chain and Syk become phosphorylated earlier in PECAM-1-deficient platelets.
DISCUSSION
The extracellular matrix is a complex, interdigitating network comprised of glycosaminoglycans, structural proteins like collagen and elastin, and multi-adhesive proteins such as fibronectin and laminin. In addition to serving as a repository for cytokines and growth factors that initiate intracellular signaling, and as a structural scaffold that maintains tissue integrity, the matrix also provides a substrate for cell migration and adhesion. As a major constituent of the basement membrane – a specialized sheet-like structure that either surrounds individual cells or is interposed underneath epithelial and endothelial sheets - laminin helps to anchor the matrix to surrounding cells via its association with cell-surface integrins. Of the 24 known integrin pairs, only α3β1, α6β1, α7β1, and α6β4 have been shown to bind laminin [25], and of these, the only laminin receptor expressed on the platelet surface is α6β1 [26], and this integrin has been found to be one of several that are required for efficient platelet adhesion at sites of vascular injury [27].
Laminin was originally isolated in 1979 from a mouse tumor that produced basement membrane proteins [28], however rather than existing as a single entity, laminin is now known to be comprised of a family of proteins consisting of α, β, and γ subunits that assemble into a disulfide-linked, cruciform shaped heterotrimers capable of interacting with other extracellular matrix components such as sulfated lipids, heparan sulfate proteoglycans, and collagen [1]. The 5 known α, 3β, and 3γ chains assemble into at least 15 different, tissue-specific laminin trimers that are named according to their chain composition [29]. Thus, α1β1γ1 is known as laminin 111, α5β1γ1 as 511, and so on. Early studies examining the ability of platelets to bind to and spread on laminin almost universally employed laminin 111 [21,22,26,30], and while Mg++-dependent adhesion to immobilized laminin has been commonly observed, the ability to form filopodia and lamellipodia and spread on immobilized laminin appears to take place only under conditions in which the Mg++ concentration is 1 mM or greater (references[19,22], and this report). In addition, despite the fact that many studies have examined the interaction of platelets with immobilized laminin 111, the expression of this isoform is highly-restricted, and is in fact not present in the walls of most blood vessels [1,31]. Laminins 411 and 511, on the other hand, are much more widely-distributed, produced and secreted by endothelial cells into their basement membranes, and stored in platelet α-granules [1,30-32]. Placenta is also a rich source of laminin 511, highly adhesive to platelets [33], and is the source of laminin used in more recent platelet activation studies [19,33], including this one.
The major findings of the current investigation are that, in addition to the activation events that have been described in many studies to take place following platelet exposure to immobilized laminin [19,21,22,26,30,32,33], a negative feedback inhibitory pathway mediated by PECAM-1 also becomes enlisted, most likely to moderate the effects of laminin-induced platelet activation in the absence of overwhelming exposure to this extracellular matrix protein. Thus, following exposure of platelets to immobilized laminin 511 in the presence of 2 mM MgCl2, PECAM-1 was found to become tyrosine phosphorylated on its cytoplasmic ITIM tyrosines and recruit the protein-tyrosine phosphatase SHP-2 to the inner face of the plasma membrane (Figures 2 and 3), where it presumably downregulates the GPVI/FcRγ chain→Syk activation pathway (Figure 7) leading to dampened granule secretion (Figure 6). These findings, therefore, expand the scope of PECAM-1's regulatory function to include not only platelet activation by collagen [14-17] and VWF [34], but now laminin as well.
All the experiments were performed under static conditions, and therefore the kinetics of platelet activation, and of PECAM-1-regulation of this process differ from those found under in vivo conditions of flow. For example, laminin-induced tyrosine phosphorylation of PECAM-1, Syk, and the FcRγ chain was observed only after 15-30 minutes of incubation in microtiter wells (Figures 2 and 7), while we have previously shown that PECAM-1 exerts its inhibitory effect on thrombus formation in vivo in only 5-10 minutes following vascular injury. This no doubt reflects the time needed for enough platelets to settle onto the immobilized matrix under static conditions to measure their cumulative activation, while in vivo, platelets are continuously forced onto exposed thrombogenic surfaces by the abundance of red cells that force their margination, resulting in a much faster activation response. The reductionist approach taken in the present manuscript to examine regulation of adhesion-initiated signal amplification and control under static conditions, while perhaps not fully able to mimic kinetics that take place in the vasculature, nonetheless reveal the contribution of laminin to platelet granule secretion, and the ability of PECAM-1 to regulate this process.
Given that collagen and laminin each employ GPVI to activate platelets, why are all of the effects of PECAM-1 on collagen-induced platelet activation not also seen with laminin? The answer is likely due to the multivalent nature of collagen, which is able to activate platelets in solution, whereas soluble laminin, a much weaker agonist, is without effect unless first immobilized. Thus, while we and others have shown that PECAM-1 dampens low-dose collagen- or CRP-induced platelet aggregation as well as dense granule secretion [14,35], in the present manuscript we were only able to evaluate its effects on granule secretion and a two key upstream signaling events leading to granule secretion.
A common theme emerging in the field of platelet activation is that large, adhesive ligands present in the extracellular matrix engage platelet receptors that are able to co-opt one or more transmembrane adaptor molecules that contain cytoplasmic ITAM tyrosine residues. These tyrosines become phosphorylated by Src-family kinases shortly after platelets encounter one or more components of the extracellular matrix, resulting in the assembly of kinase-containing protein complexes that mediated feed-forward amplification loops. ITIM-bearing receptors, due to their ability to recruit tyrosine and lipid phosphatases, have been increasingly observed to moderate such activation events [36-38], and PECAM-1 regulation of laminin-induced platelet activation is therefore just the latest example. Future characterization of activatory/inhibitory receptor pairs involved in the regulation of thrombosis, hemostasis and the inflammatory response may yield important new clues leading to improved intervention and management of these clinically important conditions.
ACKNOWLEDGEMENTS
This work was supported by Program Project Grant HL-44612 (to PJN and DKN) from the National Heart, Lung, and Blood Institute of the National Institutes of Health.
Footnotes
Portions of this work were presented in abstract form at the 49th Annual Meeting of the American Society of Hematology, December 8-11, 2007.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
References
- 1.Scheele S, Nystrom A, Durbeej M, Talts JF, Ekblom M, Ekblom P. Laminin isoforms in development and disease. J Mol Med. 2007;85:825–36. doi: 10.1007/s00109-007-0182-5. [DOI] [PubMed] [Google Scholar]
- 2.Jackson SP. The growing complexity of platelet aggregation. Blood. 2007;109:5087–95. doi: 10.1182/blood-2006-12-027698. [DOI] [PubMed] [Google Scholar]
- 3.Newman PJ, Newman DK. Platelets and the Vessel Wall. 7th Edition 2008. pp. 1378–98. [Google Scholar]
- 4.Watson SP, Auger JM, McCarty OJ, Pearce AC. GPVI and integrin αIIb β3 signaling in platelets. J Thromb Haemost. 2005;3:1752–62. doi: 10.1111/j.1538-7836.2005.01429.x. [DOI] [PubMed] [Google Scholar]
- 5.Newton-Nash DK, Newman PJ. A new role for PECAM-1 (CD31): Inhibition of TCR-mediated signal transduction. J Immunol. 1999;163:682–8. [PubMed] [Google Scholar]
- 6.Tada Y, Koarada S, Morito F, Ushiyama O, Haruta Y, Kanegae F, Ohta A, Ho A, Mak TW, Nagasawa K. Acceleration of the onset of collagen-induced arthritis by a deficiency of platelet endothelial cell adhesion molecule 1. Arthritis Rheum. 2003;48:3280–90. doi: 10.1002/art.11268. [DOI] [PubMed] [Google Scholar]
- 7.Maas M, Stapleton M, Bergom C, Mattson DL, Newman DK, Newman PJ. Endothelial cell PECAM-1 confers protection against endotoxic shock. Am J Physiol Heart Circ Physiol. 2005;288:H159–H164. doi: 10.1152/ajpheart.00500.2004. [DOI] [PubMed] [Google Scholar]
- 8.Carrithers M, Tandon S, Canosa S, Michaud M, Graesser D, Madri JA. Enhanced susceptibility to endotoxic shock and impaired STAT3 signaling in CD31-deficient mice. Am J Pathol. 2005;166:185–96. doi: 10.1016/S0002-9440(10)62243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goel R, Boylan B, Gruman L, Newman PJ, North PE, Newman DK. The proinflammatory phenotype of PECAM-1-deficient mice results in atherogenic diet-induced steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1205–G1214. doi: 10.1152/ajpgi.00157.2007. [DOI] [PubMed] [Google Scholar]
- 10.Bird IN, Taylor V, Newton JP, Spragg JH, Simmons DL, Salmon M, Buckley CD. Homophilic PECAM-1(CD31) interactions prevent endothelial cell apoptosis but do not support cell spreading or migration. J Cell Sci. 1999;112:1989–97. doi: 10.1242/jcs.112.12.1989. [DOI] [PubMed] [Google Scholar]
- 11.Noble KE, Wickremasinghe RG, DeCornet C, Panayiotidis P, Yong KL. Monocytes stimulate expression of the Bcl-2 family member, A1, in endothelial cells and confer protection against apoptosis. J Immunol. 1999;162:1376–83. [PubMed] [Google Scholar]
- 12.Evans PC, Taylor ER, Kilshaw PJ. Signaling through CD31 protects endothelial cells from apoptosis. Transplantation. 2001;71:457–60. doi: 10.1097/00007890-200102150-00020. [DOI] [PubMed] [Google Scholar]
- 13.Gao C, Sun W, Christofidou-Solomidou M, Sawada M, Newman DK, Bergom C, Albelda SM, Matsuyama S, Newman PJ. PECAM-1 functions as a specific and potent inhibitor of mitochondrial-dependent apoptosis. Blood. 2003;102:169–79. doi: 10.1182/blood-2003-01-0003. [DOI] [PubMed] [Google Scholar]
- 14.Patil S, Newman DK, Newman PJ. Platelet endothelial cell adhesion molecule-1 serves as an inhibitory receptor that modulates platelet responses to collagen. Blood. 2001;97:1727–32. doi: 10.1182/blood.v97.6.1727. [DOI] [PubMed] [Google Scholar]
- 15.Jones KL, Hughan SC, Dopheide SM, Farndale RW, Jackson SP, Jackson DE. Platelet endothelial cell adhesion molecule-1 is a negative regulator of platelet-collagen interactions. Blood. 2001;98:1456–63. doi: 10.1182/blood.v98.5.1456. [DOI] [PubMed] [Google Scholar]
- 16.Cicmil M, Thomas JM, Leduc M, Bon C, Gibbins JM. Platelet endothelial cell adhesion molecule-1 signaling inhibits the activation of human platelets. Blood. 2002;99:137–44. doi: 10.1182/blood.v99.1.137. [DOI] [PubMed] [Google Scholar]
- 17.Falati S, Patil S, Gross PL, Stapleton M, Merrill-Skoloff G, Barrett NE, Pixton KL, Weiler H, Cooley B, Newman DK, Newman PJ, Furie BC, Furie B, Gibbins JM. Platelet PECAM-1 inhibits thrombus formation in vivo. Blood. 2006;107:535–41. doi: 10.1182/blood-2005-04-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dhanjal TS, Ross EA, Auger JM, McCarty OJ, Hughes CE, Senis YA, Watson SP. Minimal regulation of platelet activity by PECAM-1. Platelets. 2007;18:56–67. doi: 10.1080/09537100600881396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inoue O, Suzuki-Inoue K, McCarty OJ, Moroi M, Ruggeri ZM, Kunicki TJ, Ozaki Y, Watson SP. Laminin stimulates spreading of platelets through integrin α6β1-dependent activation of GPVI. Blood. 2006;107:1405–12. doi: 10.1182/blood-2005-06-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun J, Williams J, Yan H-C, Amin KM, Albelda SM, DeLisser HM. Platelet Endothelial Cell Adhesion Molecule-1 (PECAM-1) homophilic adhesion is mediated by immunoglobulin-like domains 1 and 2 and depends on the cytoplasmic domain and the level of surface expression. J Biol Chem. 1996;271:18561–70. doi: 10.1074/jbc.271.31.18561. [DOI] [PubMed] [Google Scholar]
- 21.Ill CR, Engvall E, Ruoslahti E. Adhesion of platelets to laminin in the absence of activation. J Cell Biol. 1984;99:2140–5. doi: 10.1083/jcb.99.6.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hindriks G, Ijsseldijk MJ, Sonnenberg A, Sixma JJ, de Groot PG. Platelet adhesion to laminin: role of Ca2+ and Mg2+ ions, shear rate, and platelet membrane glycoproteins. Blood. 1992;79:928–35. [PubMed] [Google Scholar]
- 23.Jackson DE, Ward CM, Wang R, Newman PJ. The protein-tyrosine phosphatase SHP-2 binds PECAM-1 and forms a distinct signaling complex during platelet aggregation. Evidence for a mechanistic link between PECAM-1 and integrin-mediated cellular signaling. J Biol Chem. 1997;272:6986–93. doi: 10.1074/jbc.272.11.6986. [DOI] [PubMed] [Google Scholar]
- 24.Sonnenberg A, Janssen H, Hogervorst F, Calafat J, Hilgers J. A complex of platelet glycoproteins Ic and IIa identified by a rat monoclonal antibody. J Biol Chem. 1987;262:10376–83. [PubMed] [Google Scholar]
- 25.Nishiuchi R, Takagi J, Hayashi M, Ido H, Yagi Y, Sanzen N, Tsuji T, Yamada M, Sekiguchi K. Ligand-binding specificities of laminin-binding integrins: a comprehensive survey of laminin-integrin interactions using recombinant α3β1, α6β1, α7β1 and α6β4 integrins. Matrix Biol. 2006;25:189–97. doi: 10.1016/j.matbio.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Sonnenberg A, Modderman PW, Hogervorst F. Laminin receptor on platelets is the integrin VLA-6. Nature. 1988;336:487–9. doi: 10.1038/336487a0. [DOI] [PubMed] [Google Scholar]
- 27.Gruner S, Prostredna M, Schulte V, Krieg T, Eckes B, Brakebusch C, Nieswandt B. Multiple integrin-ligand interactions synergize in shear-resistant platelet adhesion at sites of arterial injury in vivo. Blood. 2003 doi: 10.1182/blood-2003-05-1391. [DOI] [PubMed] [Google Scholar]
- 28.Timpl R, Rohde H, Robey PG, Rennard SI, Foidart JM, Martin GR. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979;254:9933–7. [PubMed] [Google Scholar]
- 29.Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, Engel J, Engvall E, Hohenester E, Jones JC, Kleinman HK, Marinkovich MP, Martin GR, Mayer U, Meneguzzi G, Miner JH, Miyazaki K, Patarroyo M, Paulsson M, Quaranta V, Sanes JR, Sasaki T, Sekiguchi K, Sorokin LM, Talts JF, Tryggvason K, Uitto J, Virtanen I, von der MK, Wewer UM, Yamada Y, Yurchenco PD. A simplified laminin nomenclature. Matrix Biol. 2005;24:326–32. doi: 10.1016/j.matbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Geberhiwot T, Ingerpuu S, Pedraza C, Neira M, Lehto U, Virtanen I, Kortesmaa J, Tryggvason K, Engvall E, Patarroyo M. Blood platelets contain and secrete laminin-8 (α4β1γ1) and adhere to laminin-8 via α6β1 integrin. Exp Cell Res. 1999;253:723–32. doi: 10.1006/excr.1999.4653. [DOI] [PubMed] [Google Scholar]
- 31.Miner JH, Patton BL, Lentz SI, Gilbert DJ, Snider WD, Jenkins NA, Copeland NG, Sanes JR. The laminin α chains: expression, developmental transitions, and chromosomal locations of α1-5, identification of heterotrimeric laminins 8-11, and cloning of a novel α3 isoform. J Cell Biol. 1997;137:685–701. doi: 10.1083/jcb.137.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang JC, Chang HH, Lin CT, Lo SJ. The integrin α6β1 modulation of PI3K and Cdc42 activities induces dynamic filopodium formation in human platelets. J Biomed Sci. 2005;12:881–98. doi: 10.1007/s11373-005-9021-2. [DOI] [PubMed] [Google Scholar]
- 33.Nigatu A, Sime W, Gorfu G, Geberhiwot T, Anduren I, Ingerpuu S, Doi M, Tryggvason K, Hjemdahl P, Patarroyo M. Megakaryocytic cells synthesize and platelets secrete α5-laminins, and the endothelial laminin isoform laminin 10 (α5β1β1) strongly promotes adhesion but not activation of platelets. Thromb Haemost. 2006;95:85–93. [PubMed] [Google Scholar]
- 34.Rathore V, Stapleton MA, Hillery CA, Montgomery RR, Nichols TC, Merricks EP, Newman DK, Newman PJ. PECAM-1 negatively regulates GPIb/V/IX signaling in murine platelets. Blood. 2003;102:3658–64. doi: 10.1182/blood-2003-06-1888. [DOI] [PubMed] [Google Scholar]
- 35.Wilkinson R, Lyons AB, Roberts D, Wong MX, Bartley PA, Jackson DE. Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31) acts as a regulator of B-cell development, B-cell antigen receptor (BCR)-mediated activation, and autoimmune disease. Blood. 2002;100:184–93. doi: 10.1182/blood-2002-01-0027. [DOI] [PubMed] [Google Scholar]
- 36.Unkeless JC, Jin J. Inhibitory receptors, ITIM sequences and phosphatases. Curr Opin Immunol. 1997;9:338–43. doi: 10.1016/s0952-7915(97)80079-9. [DOI] [PubMed] [Google Scholar]
- 37.Bolland S, Ravetch JV. Inhibitory pathways triggered by ITIM-containing receptors. Adv Immunol. 1999;72:149–77. doi: 10.1016/s0065-2776(08)60019-x. [DOI] [PubMed] [Google Scholar]
- 38.Gibbins JM. The negative regulation of platelet function: extending the role of the ITIM. Trends Cardiovasc Med. 2002;12:213–9. doi: 10.1016/s1050-1738(02)00164-0. [DOI] [PubMed] [Google Scholar]