Summary
Background
In the extrinsic pathway, the essential procofactors factor V (fV) and factor VIII (fVIII) are activated to fVIIIa and fVa by thrombin. In the contact pathway and its clinical diagnostic test, the activated partial thromboplastin time assay (aPTT), the sources of procofactor activation are unknown. In the aPTT, factor XII (fXII) is activated on a negatively charged surface and proceeds to activate factor XI (fXI), which activates factor IX (fIX) upon the addition of Ca2+. FIXa feeds thrombin generation through activation of fX. FIXa is an extremely poor catalyst in the absence of its fVIIIa cofactor, which in the intrinsic factor Xase complex increases fXa generation by ~107. One potential aPTT procofactor activator in this setting is fXIa.
Objective
We tested the hypothesis that fXIa can activate fVIII and fV.
Methods
Recombinant fVIII and plasma fV were treated with fXIa and the activities and integrities of each procofactor were measured using commercial clotting assays and SDS-PAGE.
Results
Kinetic analyses of fXIa catalyzed activation and inactivation of fV and fVIII are reported and the timing and sites of cleavage defined.
Conclusions
FXIa activates both procofactors at plasma protein concentrations and computational modeling suggests that procofactor activation during the preincubation phase of the aPTT is critical to the performance of the aPTT. Since the aPTT is the primary tool for the diagnosis and management of Hemophilias A and B as well as in the determination of fVIII inhibitors these findings have potential implications in the clinical setting.
Introduction
Activation of the procofactors fVIII and fV is essential to hemostasis and a deficiency of either is associated with a bleeding phenotype [1]. During tissue factor initiated coagulation, the procofactors are proteolysed to their active forms principally by α-thrombin (thrombin) [2–6]. The initial thrombin is generated by factor Xa (fXa), produced prior to the formation of the prothrombinase complex [7, 8]. The addition of fVIIIa and membrane to fIXa increases fXa generation by 1.7 × 107 over fIXa alone; the addition of fVa and membrane increases thrombin production by 3.5 × 105 over fXa alone [9, 10].
Although most studies of coagulation have focused on the tissue factor pathway, recent studies suggest a role for the contact pathway in thrombosis [11–14]. Analyses of intrinsic pathway function utilizing the activated partial thromboplastin time (aPTT), has been a critical tool for the diagnosis and management of hemophilia A and B [15]. The activation of fVIII and fV is fundamental to the intrinsic pathway [7]. During the preincubation phase of the aPTT, fXII is activated by negatively charged surface and activates fXI to fXIa in the presence of high molecular weight kininogen (HMWK). The result of this initial step is to produce fXIa. The clot measurement phase of the aPTT assay is post recalcification, [22] during which fXIa activates fIX to fIXaβ [16–21] thus continuing the cascade through fXa to thrombin. The steps by which fIXa generation results in sufficient thrombin to yield the aPTT clot time (30–36 seconds) have not been established. FIXa displays a limited catalytic activity in the absence of cofactors and the rate of fX activation by fIXa [10] seems inadequate to provide sufficient fXa and/or thrombin to drive initial procofactor activation within the clot time interval [19]. Since fIXa does not appear capable of producing sufficient initial fXa or subsequent thrombin to activate the procofactors, procofactor activation must occur prior to fXa generation. Potential fVIII and fV activators in the intrinsic pathway include fXIIa, fXIa and kallikrein.
FXIa is a 160 kDa homodimeric serine protease [21–23] that circulates in blood at a concentration of 30 nM in complex with (HMWK) [24]. We tested the hypothesis that fXIa can activate fVIII and fV and the resulting empirical data were integrated into a computational model that describes the dynamics of the aPTT.
Materials and Methods
Materials
Human plasma fV and thrombin were purified as previously described [25, 26]. Excipient-free rfVIII was a gift from Baxter Healthcare (Thousand Oaks, CA). Human fXIa, human von Willibrand factor (VWF) and human activated protein C (APC) were gifts from Haematologic Technologies Inc. (Essex, Vermont). Hirudin was purchased from American Diagnostica (Stamford, CT). Single-chain high molecular weight kininogen (HMWK) was purchased from Enzyme Research Labs Inc. (South Bend, IN). HEPES buffer, polyethylene glycol 8000 (PEG), CaCl2 (Ca2+), ZnCl2 (Zn2+) and NaCl were purchased from Fisher Scientific (Pittsburgh, PA). FXI deficient plasma was purchased from George King Biomedical (Overland Park, KS). Immuno-depleted fVIII and VWF deficient plasma were purchased from Precision Biologic (Dartmouth, Nova Scotia). Immuno-depleted FV deficient plasma, pooled 6-donor normal plasma, corn trypsin inhibitor, plasma-derived fXI, and SN-13a substrate were prepared in-house. 1,2-Dioleolyl-sn-Glycero-3-Phospho-L-Serine (PS) and 1,2-Dioleoyl-sn-Glycero-3-Phosphocholine (PC) were purchased from Avanti Polar Lipids, Inc (Alabaster, AL). Phospholipid vesicles (PCPS) composed of 25% PS and 75% PC were prepared as described [27]. Simplastin Excel S commercial PT reagent and TriniCLOT Automated aPTT reagent were purchased from Trinity Biotech (Bray, Ireland). Immobilon P, polyvinilydene fluoride (PVDF) was from Millipore (Billerica, Massachusetts). Murine anti-human fXI-2 and anti-human FV-17 were produced from the Program Antibody Core Facility (Colchester, Vermont).
FXIa generation in the aPTT
FXI deficient plasma reconstituted with 30 nM plasma derived fXI was incubated with aPTT reagent for 5 minutes at 37°C in a 96 well plate. The reaction was quenched with 0.5 mg/mL of corn typsin inhibitor (CTI) for 5 minutes. The fluorescent substrate specific for FXIa (SN-13a, 100 μM final) [28] was added and hydrolysis was measured in a Biotek (Winooski, VT) Synergy 4 fluorescence plate reader. A calibration curve was developed by the addition of fXIa (5,10,20 and 30 nM) to citrated fXI deficient plasma containing 0.5 mg/mL CTI. SN-13a (100 μM final) was added and the hydrolysis rates were measured.
Role of fXIa in the aPTT
aPTT experiments were performed in normal 6-donor plasma as follows: 100 μL plasma was incubated with 100 μL of aPTT reagent for 5 min at 37°C either in the presence or absence of 0.1 mg/mL of an inhibitory fXIa antibody (αFXI-2). The reagent and plasma mixture were then mixed with: a HEPES buffered saline pH 7.4 (HBS) control buffer, fIXa (0.001–10 nM) or 60 pM rfVIIIa (all additions <5% of plasma volume) immediately prior to recalcification.
FXIa activation of fVIII/fV
Recombinant human fVIII (1.5 nM) and human fV (20 nM) were incubated in HBS, Ca2+ (2 mM), and PEG (0.1%) pH 7.4 at 37°C. Prior to the addition of fXIa (10 nM), the reactions were supplemented with ZnCl2 (10.8 μM), ZnCl2 and HMWK (100 nM) and in the case of fVIII, the reaction was supplemented with ZnCl2, HMWK and VWF (10 μg/mL). Reaction aliquots were removed at specific time points and assayed for fVIIIa and fVa activity using the aPTT and PT clotting assays respectively in a Stago 8 (Diagnostica Stago, Parsippany, New Jersey) clotting apparatus. For the aPTT, fVIII/VWF deficient plasma was pre-incubated with the aPTT reagent for five minutes prior to addition of fVIII and initiation via Ca2+ addition. PT assays were performed in immuno-depleted fV-deficient plasma.
FXIa proteolysis of fVIII/fV/fVa: SDS-Page and NH2-terminal sequencing
Human recombinant fVIII (1.5 μM), human fV (1.5 μM) or thrombin derived human fVa (quenched with the addition of hirudin) were treated with fXIa (100 nM) in HBS, Ca2+ (2 mM), PEG (0.1%) pH 7.4 at 37°C. Aliquots were removed at specific time points for 8–18% SDS-PAGE analysis under reducing conditions and assayed for fVIIIa/fVa activity as described above. Due to the complexity of the cleavage pattern seen in SDS-PAGE, the fV heavy and light chains were separated on a BioCAD perfusion chromatography workstation (Applied Biosystems, Foster City, CA) and individually treated with fXIa as above. The fragments generated when fXIa cleaved the separated chains of fVa, yielded a collection of peptide analogous to that observed when the intact molecule is proteolyzed thus facilitating sequencing. For NH2-terminal sequencing, the proteins were subjected to 8–18% SDS-PAGE under reducing conditions and subsequently transferred to a PVDF membrane and stained with coomassie brilliant blue. NH2-terminal sequencing of rfVIII, and fV HC/LC fragments from PVDF membranes was performed as described [29] in the laboratory of Dr. Alex Kurosky (University of Texas Medical Branch, Galveston, TX)
Computational Model
A mathematical model of the aPTT assay was constructed by integrating a set of reactions describing a fXIa-initiated pathway to fXa formation with a previously described model of tissue factor-initiated thrombin generation [30]. The reaction dynamics represent events that in the empirical assay take place post recalcification, with the concentrations of all modeled proteins set at 30 % of their mean physiologic values to reflect the 3 fold dilution of citrate plasma in the assay. The catalytic sequence leading to the formation of fXIa upon mixing equal volumes of citrate plasma and activator reagent is not modeled. A second order rate constant of 1.9 × 106M−1s−1, the average of several published values [31–33], was used for fXIa activation of fIX; a rate constant of 5700 M−1s−1 [10] was used for fIXa activation of fX; a rate constant of 1 × 106 M−1s−1 [4] was used for fXa activation of fVIII; a rate constant of 0.1 × 106 M−1s−1 [34] was used for fXa activation of fV; and a rate constant of 600 M−1s−1 [35] was used for reaction of fXIa with antithrombin. Second order rate constants for fXIa activation of fV and fVIII were determined in this study. The computational output is αthrombin generation over time with time to 2 nM α-thrombin used as a computational representation of clot time.
Results
The role of fXIa in the intrinsic pathway
The initial rate of fXIa hydrolysis of the SN-13a substrate in CTI citrate plasma was used to construct a standard curve for measuring the development of fXIa activity in plasma (data not shown). From the rate of substrate hydrolysis, the estimated concentration of fXIa generated during the preincubation with the aPTT reagent was 18 nM, which represents 60% activation.
APTT clotting experiments were designed to distinguish the contribution of fXIa to fIX and fVIII activation in the contact pathway. Normal pooled plasma clotted in 30 ± 2 sec. The addition of 0.1 mg/mL of a fXIa inhibitory antibody, αfXI-2, extended the clot time to 74 sec which is consistent with a loss of fXIa activity and comparable to that observed in fXI deficient plasma. In the presence of the fXI-2 inhibitory antibody, the addition of 10 pM fIXa resulted in a 72 sec clot time. When the level of fIXa was raised to 100 pM, the clot time was reduced by only 10 sec to 64 sec which indicates that these levels of fIXa are insufficient to drive normal thrombin generation. In the presence of fXI inhibitory antibody, normalization of the aPTT clot time required the addition of 10 nM fIXa. In contrast to fIXa, addition of thrombin activated rfVIIIa to fVIII deficient plasma showed that the amount of rfVIIIa required to normalize the aPTT clot time is approximately 20 pM, representing activation of only 1.5% of the total fVIII pool. In the presence of the fXI inhibitory antibody, the addition of small amounts of exogenous fVIIIa in addition to exogenous fIXa, reduced the amount of fIXa needed to normalize the aPTT by 20-fold. Due to the high levels of fIXa required to achieve normal aPTT function in the absence of fXIa, these data rule out both fXIIa and prekallikrein as fVIII activators and suggest fXIa as the initial activator of fVIII in the aPTT.
FXIa treatment of rfVIII
FVIII is secreted as a 2-chain glycoprotein (NH2-terminal residues 1–1649; COOH terminal 1650–2332) that circulates at a concentration of approximately 1.5 nM [36]. Thrombin activates fVIII via cleavages at R372, R740 and R1689 yielding a heterotrimeric cofactor (Mr = 50, 43 and 73 kD respectively). The cofactor is unstable due to spontaneous dissociation (T1/2 ~ 100 s) of the 373–740 fragment [37, 38], complicating estimates of fVIIIa cofactor activity. Time course analyses of the activation of fVIII by thrombin showed a maximum increase in fVIII clotting activity of 43-fold achieved within 60 seconds. This level of cofactor activation was defined as maximal and was used to estimate the extent of activation of rfVIII by fXIa.
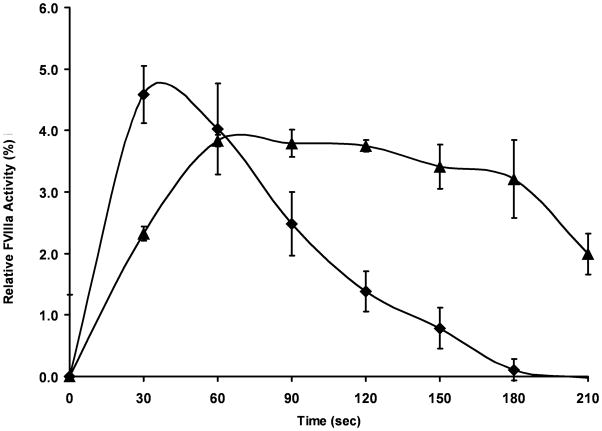
Experiments were performed at plasma concentrations of fVIII (1.5 nM) and fXIa 10 nM (Fig 1) [36]. The functional fVIIIa level reached a maximum of 60 pM in 90 seconds. When the reaction was performed at 10 nM fXIa in the presence of physiologic zinc levels (10.8 μM) the rate and highest level of cofactor activity achieved were approximately doubled. The addition of 100 nM single-chain HMWK with Zn2+ resulted in an additional 3-fold increase in the rate and maximum level of functional fVIIIa. HMWK in the absence of fXIa had no effect on fVIII activity under the same conditions. The fVIII/HMWK/Zn2+ reactions were repeated in the presence of 10 μg/mL VWF. The addition of VWF had little effect on peak fVIIIa activity, however it decreased the rate of activation 2-fold. Once peak activity was achieved, VWF appeared to buffer the activated product leading to a preservation of fVIIIa activity. The addition of the direct thrombin inhibitor hirudin had no effect on the activities of any of these reactions. A second order rate constant (3.9 ×104 M−1s−1) was estimated from the initial rate of fXIa activation of fVIII.
Figure 1. FXIa proteolysis of fVIII.
Recombinant fVIII (1.5 nM) was incubated with fXIa (10 nM) + ZnCl2 (10.8 μM) and HMWK (100 nM) (◆) or + ZnCl2, HMWK and VWF (10 μg/mL) (▲) in HBS/PEG/Ca2+ at 37°C and aliquots were removed every 30 seconds for fVIII activity analysis. Samples were diluted to 75 pM fVIII in 100 μL of fVIII/VWF-deficient plasma and subjected to an aPTT clotting assay. Clot times were transformed into relative fVIII concentrations based on a fVIII standard curve and normalized to percent activation compared to thrombin activated fVIII. Data represent mean +/− SEM for three independent experiments.
FXIa treatment of rfVIII: SDS-PAGE analysis
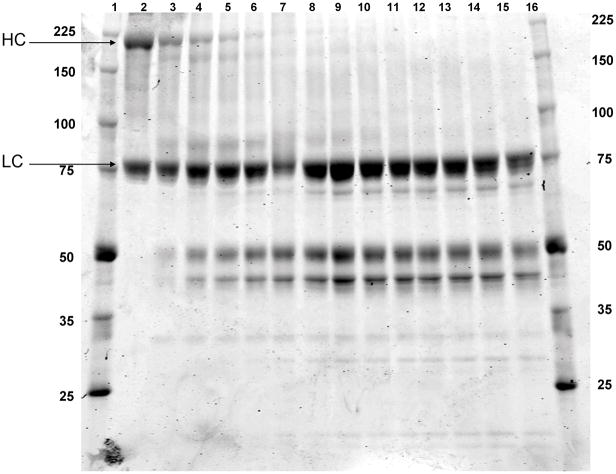
Apparent mass analyses of products were performed using SDS-PAGE (Fig 2). The fVIII procofactor displays products at Mr = 200000 corresponding to the heavy chain and B-domain fragment of fVIII (1–1649), and a lower molecular weight band Mr = 80000 corresponding to the light chain (residues 1650–2332). Within 5 minutes of incubation with fXIa, 60% of the heavy chain is cleaved generating fragments at Mr = 170000, 90000, 50000 and 45000. The heavy chain is completely cleaved within 25 minutes, producing a band pattern similar to that seen with thrombin. However, several low molecular weight bands are also evident: a species at Mr = 28000 and doublet at Mr = 22000/20000 appear concurrent with heavy chain depletion. A single band Mr = 30000 appears throughout the time course; consistent with the fXIa light chain. The fVIII light chain appears to undergo negligible proteolysis with a slight shift in mobility similar to that seen after cleavage at R1689 by thrombin (see figure 3).
Figure 2. FXIa proteolysis of fVIII: SDS-PAGE analysis.
Recombinant fVIII (1.5 μM) was incubated with fXla (100 nM) in HBS/PEG/Ca2+ for 65 min at 37°C. Reaction aliquots were removed every 5 minutes and run out on 8–18% SDS-PAGE followed by coomassie blue staining. Lane 1 and 16: molecular weight standards; Lane 2: rfVIII (T=0); Lanes 3–15: FXla proteolyzed rfVIII (5–65 min).
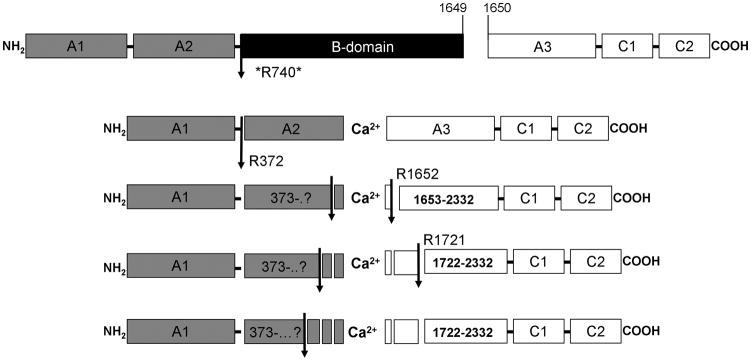
Figure 3. Model of fVIII cleavage by fXIa.
A schematic representation of fVIII cleavages made by fXIa. Arrows indicate cleavages identified by NH2-terminal sequencing. The asterisk at R740 indicates an implied cleavage based on the mobility of thrombin-derived fragments that could not be confirmed by NH2-terminal sequencing.
Model of fXIa cleavage of fVIII
A model of fXIa cleavage of rfVIII was constructed (Fig 3). FXIa initially cleaves at R740 and R372 in the heavy chain. Further proteolysis of the A2 domain follows with sequential cleavages occurring at sites upstream from the COOH-terminus, with proteolysis of this region possibly contributing to a reduced fVIIIa cofactor activity. FXIa makes several A3 cleavages most notably at R1652 and R1721. Fragments with the SDS-PAGE mobility of the thrombin derived light chain also suggest a cleavage at R1689; however this could not be verified by NH2-terminal sequencing. The cleavages made by fXIa in the heavy chain at R740 and R372 are identical to those seen with thrombin. Thrombin cleaves the light chain at R1689, which serves to release fVIII from von Willebrand factor [4]. The fXIa cleavage at R1721 may also lead to the release of VWF along with a significant portion of the A3 NH2-terminus.
FXIa treatment of fV/Va
In contrast to fVIII, fV is secreted as a single chain glycoprotein which circulates in plasma at a concentration of 20 nM [39–41]. Thrombin activates fV with cleavages at R709, R1018 and R1545, which release the B-region producing a fVa heavy chain Mr = 105000 (1–709) and a light chain Mr = 74000/72000 (1546–2196) [42]. APC inactivates fVa by proteolysis of the heavy chain at R306, R506 and R679, leading to the dissociation of the A2 domain[43]. When 1.5 μM fV is treated with 10 nM thrombin, complete activation occurs within 15 min, and the cofactor activity is stable for at least an hour. With activation by thrombin, an apparent 20-fold increase in activity is observed when measured in a prothrombin time (PT) assay in fV-deficient plasma. Relative cofactor activity of the fXIa cleaved fV/Va was calculated on the basis of a thrombin-derived fVa standard curve.
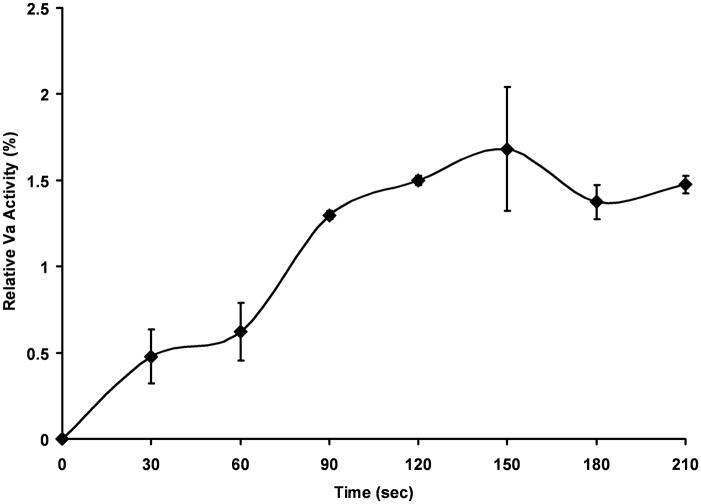
FV activation measurements were performed at physiologically relevant concentrations of fV (20 nM) and fXIa 10 nM (Fig 4). FVa activity reached a maximum of ~0.65 nM (2%) in 150 seconds and remained relatively constant over the 5 minute time course. When the reaction was performed at 10 nM fXIa in the presence of physiologic zinc (10.8 μM) the rate and highest level of cofactor activity achieved were unchanged (data not shown). A second order rate constant (6000 M−1s−1) was estimated from the initial rate of fXIa activation of fV. The addition of 100 nM single-chain HMWK resulted in a decrease in relative fVa activity compared to fV alone. HMWK in the absence of fXIa had no effect on fV activity under the same conditions (data not shown).
Figure 4. FXIa proteolysis of fV: activity analysis.
Human fV (1.5 nM) was incubated with fXIa (10 nM) (◆) in HBS/PEG/Ca2+ at 37°C and aliquots were removed every 30 seconds for fV activity analysis. Samples were diluted to 0.5 nM fV in 100 μL of fV-deficient plasma and subjected to a PT clotting assay. Clot times were transformed into relative fVa concentrations based on a fVa standard curve and normalized to percent activation compared to thrombin activated fVa. Data represent mean +/− SEM for two independent experiments.
FXIa treatment of fV/Va: SDS-PAGE analysis
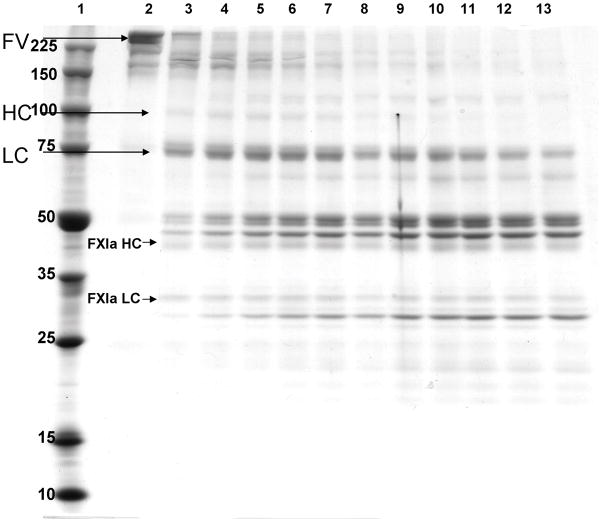
Coomassie blue staining of the fXIa proteolysis time course of fV is presented in figure 5. Intact fV is completely cleaved in 30 minutes with several intermediate fragments evident above Mr = 150000. A trace amount of a species with the mobility of thrombin-derived fVa heavy chain (Mr = 105000) appears after 5 minutes. A species with the mobility of the light chain is evident at 5 minutes and suggests a cleavage at R1545. Three species of Mr = 52000, 50000 and 48000 are observed at 5 minutes and their relative levels remain constant after intact fV has disappeared. A lower molecular weight Mr = 30000 fragment steadily increases over the time course.
Figure 5. FXIa proteolysis of fV: SDS-PAGE analysis.
Plasma derived fV (1 μM) was incubated with fXla (100 nM) in HBS/PEG/Ca2+ for 55 min at 37°C. Reaction aliquots were removed every 5 minutes and run out on 8–18% SDS-PAGE followed by coomassie blue staining. Lane 1: molecular weight standards; Lane 2: fV (T=0); Lanes 3–13: fXIa proteolyzed fV (5–55 min).
The SDS-PAGE analysis of fXIa treatment of fVa was performed (data not shown). Significant proteolyses of fVa heavy and light chains were observed, with the heavy chain being completely consumed in 20 minutes. Light chain proteolysis occurs more slowly with approximately 10% intact light chain remaining after 60 minutes. Three species with Mr = 520000/50000/48000 are observed. A Mr = 30,000 band is generated over the time course as seen with fXIa proteolysis of fV.
Model of fXIa cleavage of fV
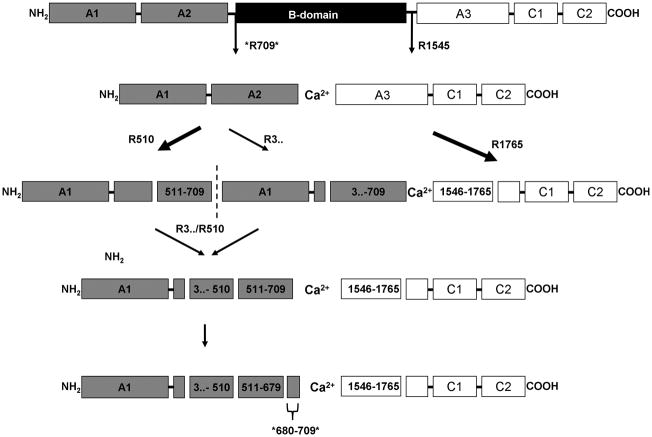
A model of the proteolytic process for fV is presented in figure 6. FVa heavy chain proteolysis occurs via two paths with several intermediates, with initial cleavage either at R510 or at a residue COOH-terminal to R306. Western blotting (data not shown) suggests that the cleavage at R510 is preferred with the alternate cleavage immediately following. The fragment cleaved at the A1–A2 junction runs at Mr = 50000, but does not immuno-react with a fV heavy chain antibody αfV-17 which recognizes an epitope between R307 and R506. This leads to the conclusion that fXIa cleaves COOH-terminal to R306, possibly at the fXa/plasmin cleavage site, R348. However, attempts at trapping this fragment for NH2-terminal sequencing were not successful. Nevertheless, it appears that this fragment is further processed at R510 and R679. FXIa makes a single cleavage in the fVa light chain at R1765.
Figure 6. Model of fV cleavage by fXIa.
A schematic representation of fV cleavages made by fXIa. Arrows indicate cleavages identified by NH2-terminal sequencing. Thick arrows denote the preferred pathway as determined by SDS-PAGE. The asterisks at R709 and R679 indicate an implied cleavage based on an SDS-PAGE-based comparison to thrombin and APC-derived fragments that could not be confirmed by NH2-terminal sequencing.
Numerical Simulation of the aPTT
In order to explore the potential the role of fXIa activation of fVIII and fV in the aPTT, we modified our computational model of tissue factor-initiated thrombin generation [30] to display the dynamics of thrombin generation in the aPTT subsequent to the preincubation phase which provides XIa and (hypothetically) fVIIIa and fVa. Thus, the model reflects the concentrations of proteins and the dynamics seen upon recalcification and describes a pathway initiated by fXIa activation of fIX with limited amounts of fVIIIa and fVa generated during the activation phase.
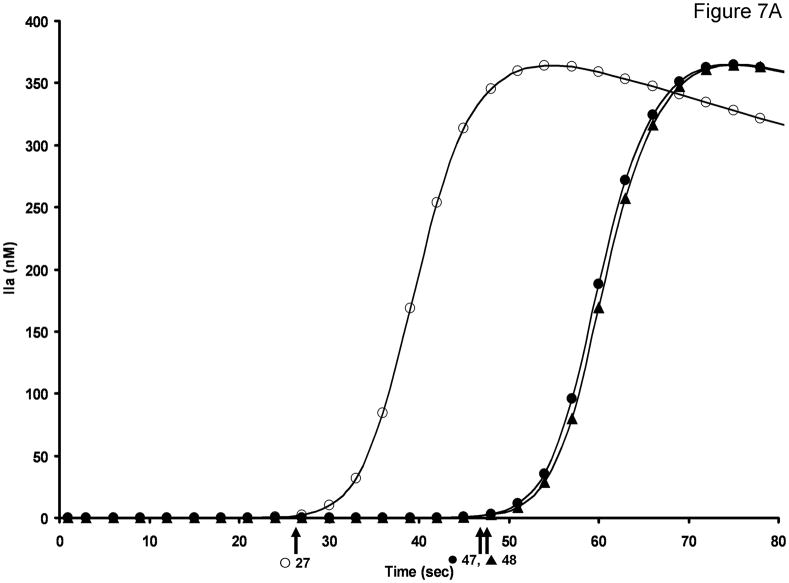
Figure 7a presents a model analysis which compares thrombin formation with and without the preactivation of fVIII and fV. Without procofactor activation prior to recalcification, the time to 2 nM thrombin is 48 seconds, while including the limited preactivation predicted by the empirical study achieves a clot time of 27 secs.
Figure 7. Computational simulation of thrombin generation in the aPTT.
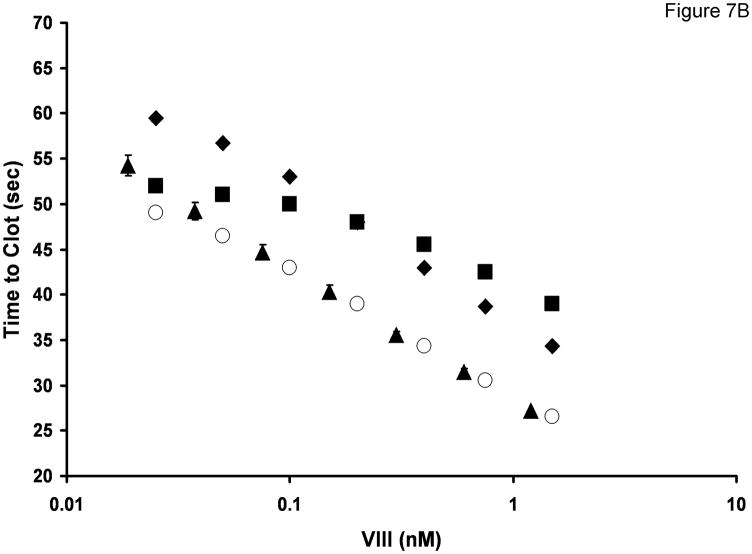
A. Computational simulation of thrombin generation in the aPTT in the presence and absence of procofactor activation by fXa and fXIa. No procofactor activation by either protease (▲) Procofactor activation by fXa post recalcification (●) Procofactor preactivation by fXIa prior to recalcification (○). Time to 2 nM thrombin for each curve is indicated by the arrows. B. Empirical aPTT data (▲) was obtained by performing a fVIII titration in fVIII/VWF deficient plasma reconstituted with 10 μg/mL VWF. Data represent mean +/− SD for two independent experiments (N=2). Computational simulations of thrombin generation in the aPTT in the presence or absence of procofactor preactivation by fXIa: FVIII (4.5%) and fV (1.75%) preactivation by fXIa prior to recalcification (○);fV preactivation only (■); fVIII preactivation only (◆).
FXa, a low efficiency activator of both fVIII [4, 44] and fV [34], is not considered an important activator of the procofactors during tissue factor-initiated thrombin generation [34]. The potential for procofactor activation by fXa (activated by fIXa) was evaluated; however this model only shortened the “clot” time to 47 seconds. When the preactivation phase of the aPTT was modeled producing both fXIa and the low amounts of empirically determined cofactors (e.g. 4.5% fVIIIa (20.3 pM) and 1.8% fVa (105 pM)), these modifications shortened the “clot” time to 27 seconds, consistent with empirical aPTT values for plasma from healthy individuals.
Figure 7b presents a comparison of predicted and measured clotting times in a fVIII aPTT titration. For the case in which the preincubation step generates 12 nM fXIa, along with 4.5% of the fVIII present as fVIIIa and 1.75% of the fV present as fVa, the empirical and model predicted clotting times across a similar range of factor VIII concentrations show excellent agreement. When either preactivation of fVIII (squares) or fV (diamonds) is excluded, the modeled titration does not match the empirical aPTT data. Thus the computational analysis predicts that the performance of the empirical aPTT assay is dependent on not only activation of factor XI during the preactivation step activation, but also on the production of low levels of fVa and fVIIIa prior to recalcification.
Discussion
These studies define the mechanistic order and dynamics of procofactor activation by XIa and support an essential role for fXIa-derived procofactor activation in the aPTT. In purified systems, fXIa activates both fVIII and fV procofactors with cleavages similar to those observed with thrombin activation, however several cleavage sites related to the inactivation of the cofactors appear unique to fXIa (see figures 3 and 6). Empirical and computational aPTT data suggest that the presence of fVIIIa prior to recalcification is necessary for sufficient fXa generation. Factor XIa is the likely source of procofactor activation as aPTT experiments performed in the presence of an inhibitory fXI antibody ruled out fXIIa or kallikrein as potential activators.
The addition of plasma concentrations of zinc and HMWK increase the rate and maximum level of fVIII activation 4-fold. VWF decreased the rate of activation; however it appeared to stabilize the generated fVIIIa product. The level of fVIIIa produced (~60 pM) is equivalent with that required to normalize the aPTT in fVIII deficient plasma. FXIa activation of fV proceeds at a slower rate resulting in half the relative extent of activation (~1.8%) versus (~4.5%).
While thrombin is probably the major activator of both procofactors in the contact pathway, computational modeling of the aPTT is consistent with the need for limited activation of both procofactors prior to recalcification. Both empirical and computational analyses of the dynamics of the aPPT suggest that a small level of thrombin-independent procofactor activation is essential to produce the clot endpoint of the assay.
Potential functions for the contact pathway per-se, remain obscure [13, 14]. FXII, prekallikrein or HMWK deficiencies are not generally associated with bleeding phenotypes and fXI deficiency (hemophilia C) phenotypes vary widely depending on the hemostatic challenge [45–52]. Other than its role in the contact pathway as the activator of fIX and its role in the extrinsic pathway via thrombin feedback activation under certain conditions, additional roles for fXIa remain to be seen [19, 53, 54]. Picomolar levels of fXIa have been detected in the blood of patients with acute and stable coronary artery disease [55]. Recent findings by Cushman et al. suggest that among the procoagulant factors IX, X, XI, XII and XIII, only fXI was independently associated with an increased risk of vascular thromboembolism [56]. Whether or not fXIa procofactor activation plays an important role in these diseases remains to be elucidated.
Acknowledgments
This research was supported by the NIH grant number R01HL034575 Primary Structure of Prothrombin awarded to KG Mann.
References
- 1.Guasch JF, Cannegieter S, Reitsma PH, van’t Veer-Korthof ET, Bertina RM. Severe coagulation factor V deficiency caused by a 4 bp deletion in the factor V gene. Br J Haematol. 1998;101:32–9. doi: 10.1046/j.1365-2141.1998.00664.x. [DOI] [PubMed] [Google Scholar]
- 2.Nesheim ME, Mann KG. Thrombin-catalyzed activation of single chain bovine factor V. J Biol Chem. 1979;254:1326–34. [PubMed] [Google Scholar]
- 3.Suzuki K, Dahlback B, Stenflo J. Thrombin-catalyzed activation of human coagulation factor V. J Biol Chem. 1982;257:6556–64. [PubMed] [Google Scholar]
- 4.Eaton D, Rodriguez H, Vehar GA. Proteolytic processing of human factor VIII. Correlation of specific cleavages by thrombin, factor Xa, and activated protein C with activation and inactivation of factor VIII coagulant activity. Biochemistry. 1986;25:505–12. doi: 10.1021/bi00350a035. [DOI] [PubMed] [Google Scholar]
- 5.Myles T, Yun TH, Leung LL. Structural requirements for the activation of human factor VIII by thrombin. Blood. 2002;100:2820–6. doi: 10.1182/blood-2002-03-0843. [DOI] [PubMed] [Google Scholar]
- 6.Keller FG, Ortel TL, Quinn-Allen MA, Kane WH. Thrombin-catalyzed activation of recombinant human factor V. Biochemistry. 1995;34:4118–24. doi: 10.1021/bi00012a030. [DOI] [PubMed] [Google Scholar]
- 7.Brummel KE, Paradis SG, Butenas S, Mann KG. Thrombin functions during tissue factor-induced blood coagulation. Blood. 2002;100:148–52. doi: 10.1182/blood.v100.1.148. [DOI] [PubMed] [Google Scholar]
- 8.Lawson JH, Kalafatis M, Stram S, Mann KG. A model for the tissue factor pathway to thrombin. I. An empirical study. J Biol Chem. 1994;269:23357–66. [PubMed] [Google Scholar]
- 9.Nesheim ME, Taswell JB, Mann KG. The contribution of bovine Factor V and Factor Va to the activity of prothrombinase. J Biol Chem. 1979;254:10952–62. [PubMed] [Google Scholar]
- 10.Rawala-Sheikh R, Ahmad SS, Ashby B, Walsh PN. Kinetics of coagulation factor X activation by platelet-bound factor IXa. Biochemistry. 1990;29:2606–11. doi: 10.1021/bi00462a025. [DOI] [PubMed] [Google Scholar]
- 11.Renne T, Nieswandt B, Gailani D. The intrinsic pathway of coagulation is essential for thrombus stability in mice. Blood Cells Mol Dis. 2006;36:148–51. doi: 10.1016/j.bcmd.2005.12.014. S1079-9796(06)00014-3 [pii] [DOI] [PubMed] [Google Scholar]
- 12.Tucker EI, Marzec UM, White TC, Hurst S, Rugonyi S, McCarty OJ, Gailani D, Gruber A, Hanson SR. Prevention of vascular graft occlusion and thrombus-associated thrombin generation by inhibition of factor XI. Blood. 2009;113:936–44. doi: 10.1182/blood-2008-06-163675. blood-2008-6-163675 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmaier AH. The elusive physiologic role of Factor XII. J Clin Invest. 2008;118:3006–9. doi: 10.1172/JCI36617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gailani D, Renne T. Intrinsic pathway of coagulation and arterial thrombosis. Arterioscler Thromb Vasc Biol. 2007;27:2507–13. doi: 10.1161/ATVBAHA.107.155952. ATVBAHA.107.155952 [pii] [DOI] [PubMed] [Google Scholar]
- 15.Tripodi A, Mannucci PM. Activated partial thromboplastin time (APTT). New indications for an old test? J Thromb Haemost. 2006;4:750–1. doi: 10.1111/j.1538-7836.2006.01857.x. JTH1857 [pii] [DOI] [PubMed] [Google Scholar]
- 16.Kaplan AP, Silverberg M. The coagulation-kinin pathway of human plasma. Blood. 1987;70:1–15. [PubMed] [Google Scholar]
- 17.Kaplan AP, Joseph K, Shibayama Y, Reddigari S, Ghebrehiwet B, Silverberg M. The intrinsic coagulation/kinin-forming cascade: assembly in plasma and cell surfaces in inflammation. Adv Immunol. 1997;66:225–72. doi: 10.1016/s0065-2776(08)60599-4. [DOI] [PubMed] [Google Scholar]
- 18.Macfarlane RG. An Enzyme Cascade in the Blood Clotting Mechanism, and Its Function as a Biochemical Amplifier. Nature. 1964;202:498–9. doi: 10.1038/202498a0. [DOI] [PubMed] [Google Scholar]
- 19.Osterud B, Bouma BN, Griffin JH. Human blood coagulation factor IX. Purification, properties, and mechanism of activation by activated factor XI. J Biol Chem. 1978;253:5946–51. [PubMed] [Google Scholar]
- 20.Fay PJ. Activation of factor VIII and mechanisms of cofactor action. Blood Rev. 2004;18:1–15. doi: 10.1016/s0268-960x(03)00025-0. S0268960X03000250 [pii] [DOI] [PubMed] [Google Scholar]
- 21.Bouma BN, Griffin JH. Human blood coagulation factor XI. Purification, properties, and mechanism of activation by activated factor XII. J Biol Chem. 1977;252:6432–7. [PubMed] [Google Scholar]
- 22.Fujikawa K, Legaz ME, Kato H, Davie EW. The mechanism of activation of bovine factor IX (Christmas factor) by bovine factor XIa (activated plasma thromboplastin antecedent) Biochemistry. 1974;13:4508–16. doi: 10.1021/bi00719a006. [DOI] [PubMed] [Google Scholar]
- 23.Lindquist PA, Fujikawa K, Davie EW. Activation of bovine factor IX (Christmas factor) by factor XIa (activated plasma thromboplastin antecedent) and a protease from Russell’s viper venom. J Biol Chem. 1978;253:1902–9. [PubMed] [Google Scholar]
- 24.Thompson RE, Mandle R, Jr, Kaplan AP. Association of factor XI and high molecular weight kininogen in human plasma. J Clin Invest. 1977;60:1376–80. doi: 10.1172/JCI108898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katzmann JA, Nesheim ME, Hibbard LS, Mann KG. Isolation of functional human coagulation factor V by using a hybridoma antibody. Proc Natl Acad Sci U S A. 1981;78:162–6. doi: 10.1073/pnas.78.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lundblad RL, Kingdon HS, Mann KG. Thrombin. Methods Enzymol. 1976;45:156–76. doi: 10.1016/s0076-6879(76)45017-6. [DOI] [PubMed] [Google Scholar]
- 27.Higgins DL, Mann KG. The interaction of bovine factor V and factor V-derived peptides with phospholipid vesicles. J Biol Chem. 1983;258:6503–8. [PubMed] [Google Scholar]
- 28.Butenas S, DiLorenzo ME, Mann KG. Ultrasensitive fluorogenic substrates for serine proteases. Thromb Haemost. 1997;78:1193–201. [PubMed] [Google Scholar]
- 29.Edman P. A method for the determination of amino acid sequence in peptides. Arch Biochem. 1949;22:475. [PubMed] [Google Scholar]
- 30.Hockin MF, Jones KC, Everse SJ, Mann KG. A model for the stoichiometric regulation of blood coagulation. J Biol Chem. 2002;277:18322–33. doi: 10.1074/jbc.M201173200. M201173200 [pii] [DOI] [PubMed] [Google Scholar]
- 31.Sun Y, Gailani D. Identification of a factor IX binding site on the third apple domain of activated factor XI. J Biol Chem. 1996;271:29023–8. doi: 10.1074/jbc.271.46.29023. [DOI] [PubMed] [Google Scholar]
- 32.Sinha D, Seaman FS, Walsh PN. Role of calcium ions and the heavy chain of factor XIa in the activation of human coagulation factor IX. Biochemistry. 1987;26:3768–75. doi: 10.1021/bi00387a005. [DOI] [PubMed] [Google Scholar]
- 33.Pedicord DL, Seiffert D, Blat Y. Substrate-dependent modulation of the mechanism of factor XIa inhibition. Biochemistry. 2004;43:11883–8. doi: 10.1021/bi048964g. [DOI] [PubMed] [Google Scholar]
- 34.Orfeo T, Brufatto N, Nesheim ME, Xu H, Butenas S, Mann KG. The factor V activation paradox. J Biol Chem. 2004;279:19580–91. doi: 10.1074/jbc.M400727200. M400727200 [pii] [DOI] [PubMed] [Google Scholar]
- 35.Soons H, Janssen-Claessen T, Tans G, Hemker HC. Inhibition of factor XIa by antithrombin III. Biochemistry. 1987;26:4624–9. doi: 10.1021/bi00389a005. [DOI] [PubMed] [Google Scholar]
- 36.Butenas S, Parhami-Seren B, Mann KG. The influence of von Willebrand factor on factor VIII activity measurements. J Thromb Haemost. 2009;7:132–7. doi: 10.1111/j.1538-7836.2008.03210.x. JTH3210 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fay PJ, Haidaris PJ, Smudzin TM. Human factor VIIIa subunit structure. Reconstruction of factor VIIIa from the isolated A1/A3-C1-C2 dimer and A2 subunit. J Biol Chem. 1991;266:8957–62. [PubMed] [Google Scholar]
- 38.Lollar P, Parker CG. pH-dependent denaturation of thrombin-activated porcine factor VIII. J Biol Chem. 1990;265:1688–92. [PubMed] [Google Scholar]
- 39.Tracy PB, Eide LL, Bowie EJ, Mann KG. Radioimmunoassay of factor V in human plasma and platelets. Blood. 1982;60:59–63. [PubMed] [Google Scholar]
- 40.Mann KG, Nesheim ME, Tracy PB. Molecular weight of undegraded plasma factor V. Biochemistry. 1981;20:28–33. doi: 10.1021/bi00504a005. [DOI] [PubMed] [Google Scholar]
- 41.Nesheim ME, Foster WB, Hewick R, Mann KG. Characterization of Factor V activation intermediates. J Biol Chem. 1984;259:3187–96. [PubMed] [Google Scholar]
- 42.Krishnaswamy S, Russell GD, Mann KG. The reassociation of factor Va from its isolated subunits. J Biol Chem. 1989;264:3160–8. [PubMed] [Google Scholar]
- 43.Odegaard B, Mann K. Proteolysis of factor Va by factor Xa and activated protein C. J Biol Chem. 1987;262:11233–8. [PubMed] [Google Scholar]
- 44.Lollar P, Knutson GJ, Fass DN. Activation of porcine factor VIII: by thrombin and factor Xa. Biochemistry. 1985;24:8056–64. doi: 10.1021/bi00348a033. [DOI] [PubMed] [Google Scholar]
- 45.Ratnoff OD, Colopy JE. A familial hemorrhagic trait associated with a deficiency of a clot-promoting fraction of plasma. J Clin Invest. 1955;34:602–13. doi: 10.1172/JCI103109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kleinschnitz C, Stoll G, Bendszus M, Schuh K, Pauer HU, Burfeind P, Renne C, Gailani D, Nieswandt B, Renne T. Targeting coagulation factor XII provides protection from pathological thrombosis in cerebral ischemia without interfering with hemostasis. J Exp Med. 2006;203:513–8. doi: 10.1084/jem.20052458. jem.20052458 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Castaman G, Ruggeri M, Rodeghiero F. A new Italian family with severe prekallikrein deficiency. Desmopressin-induced fibrinolysis and coagulation changes in homozygous and heterozygous members. Ric Clin Lab. 1990;20:239–44. doi: 10.1007/BF02900708. [DOI] [PubMed] [Google Scholar]
- 48.Cheung PP, Kunapuli SP, Scott CF, Wachtfogel YT, Colman RW. Genetic basis of total kininogen deficiency in Williams’ trait. J Biol Chem. 1993;268:23361–5. [PubMed] [Google Scholar]
- 49.Rapaport SI, Proctor RR, Patch MJ, Yettra M. The mode of inheritance of PTA deficiency: evidence for the existence of major PTA deficiency and minor PTA deficiency. Blood. 1961;18:149–65. [PubMed] [Google Scholar]
- 50.Leiba H, Ramot B, Many A. Heredity and coagulation studies in ten families with Factor XI (plasma thromboplastin antecedent) deficiency. Br J Haematol. 1965;11:654–65. doi: 10.1111/j.1365-2141.1965.tb00114.x. [DOI] [PubMed] [Google Scholar]
- 51.Asakai R, Chung DW, Davie EW, Seligsohn U. Factor XI deficiency in Ashkenazi Jews in Israel. N Engl J Med. 1991;325:153–8. doi: 10.1056/NEJM199107183250303. [DOI] [PubMed] [Google Scholar]
- 52.Meijers JC, Davie EW, Chung DW. Expression of human blood coagulation factor XI: characterization of the defect in factor XI type III deficiency. Blood. 1992;79:1435–40. [PubMed] [Google Scholar]
- 53.Butenas S, Dee JD, Mann KG. The function of factor XI in tissue factor-initiated thrombin generation. J Thromb Haemost. 2003;1:2103–11. 431. doi: 10.1046/j.1538-7836.2003.00431.x. [pii] [DOI] [PubMed] [Google Scholar]
- 54.Cawthern KM, van’t Veer C, Lock JB, DiLorenzo ME, Branda RF, Mann KG. Blood coagulation in hemophilia A and hemophilia C. Blood. 1998;91:4581–92. [PubMed] [Google Scholar]
- 55.Butenas S, Undas A, Gissel MT, Szuldrzynski K, Zmudka K, Mann KG. Factor XIa and tissue factor activity in patients with coronary artery disease. Thromb Haemost. 2008;99:142–9. doi: 10.1160/TH07-08-0499. 08010142 [pii] [DOI] [PubMed] [Google Scholar]
- 56.Cushman M, O’Meara ES, Folsom AR, Heckbert SR. Coagulation factors IX through XIII and the risk of future venous thrombosis: the Longitudinal Investigation of Thromboembolism Etiology. Blood. 2009;114:2878–83. doi: 10.1182/blood-2009-05-219915. blood-2009-05-219915 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]