Abstract
Visual-spatial working memory tasks can be decomposed into encoding, and retrieval phases. It was hypothesized that encoding of visual-spatial information is cognitively more challenging than retrieval. This was tested by combining Electroencephalography with a Virtual Reality paradigm to observe the modulation in EEG activity. EEG power analysis results demonstrated an increase in theta activity during encoding in comparison to retrieval whereas alpha activity was significantly higher for retrieval in comparison to encoding. We found that encoding required more cerebral efforts than retrieval. Further, as seen in fMRI studies we observed an encoding/retrieval flip in that encoding and retrieval differentially activated similar neural substrates. Results obtained from sLORETA identified cortical sources in inferior frontal gyrus which is a part of dorsolateral prefrontal cortex (DLPFC) during encoding, whereas the inferior parietal lobe and precuneus cortical sources were identified during retrieval. We further tie our results into studies examining the default network which have shown increased activation in DLPFC occurs in response to increased cerebral challenge while posterior parietal areas show activation during baseline or internal processing tasks. We conclude that encoding of visual-spatial information via VR navigation task is more cerebrally challenging than retrieval.
Keywords: visual-spatial working memory, Electroencephalography, sLORETA, encoding, retrieval, virtual-reality
1. Introduction
Navigating through space requires a variety of cortical networks. These networks are specialized depending on the type of question being asked. For example, in perception a distinction can be made between the spatial component and the non-spatial component resulting in two separate visual processing pathways asking the questions of ‘what is it? (object type) and where is it? (object spatial location) (Smith, 1995, Wilson et al., 1993). Neuro-imaging studies support the formulation that spatial processing chiefly involves dorsal stream – occipito-parietal pathway whereas object processing tend to involve ventral, occipito-temporal pathway (Ungerleider and Mishkin, 1982, Ungerleider and Haxby, 1994). This specificity has been found to extend to the prefrontal cortex, with dorsolateral area responsible for spatial working memory and ventrolateral area responsible for non-spatial working memory (Goldman-Rakic, 1987, Courtney et al., 1996, Levy and Goldman-Rakic, 1999). Thus, there is a consensus that most working memory tasks recruit a network of dorso-lateral prefrontal cortex and parietal and occipital areas (Jonides et al., 1993). It has also been well documented that brain lesions, cerebral ischemia, aging and pharmacological agents can result in disturbance in these visual-spatial networks (Miyamoto et al., 1987, McNamara and Skeleton, 1993) and that deficits in spatial working memory result from dysfunction involving the right posterior parietal and right dorsolateral prefrontal cortex (van Asselen et al., 2006).
Spatial navigation also incorporates a variety of cortical systems. Both behavioral and neuroimaging studies suggest that distinct brain areas become involved according to the nature of the task (Hartley et al., 2003; Janzen & Weststeijn, 2007; Voermans et al., 2004). Utilizing research suggesting two distinct memory systems in navigation, one for route direction and one for relevant object location, Janzen & Weststeijn (2007) suggested that the inferior parietal gyrus, the anterior cingulated gyrus and the right caudate nucleus are involved in coding of route direction whereas the parahippocampal gyri distinguish between relevant and irrelevant landmarks. A similar distinction based on both human and animal data suggest that the hippocampus is involved in acquiring a cognitive map of the environment whereas the caudate nucleus is involved in learning place appropriate responses leading to habitual behavior (Voermans et al. 2004). Another similar distinction has been made between wayfinding in humans which involves adopting a novel route and route following which involves following a previously used route suggests differential involvement of the hippocampus for the first and the caudate for the second (Hartley et al. 2003).
In order to study spatial memory and navigation, we created a virtual reality (VR) environment (virtual corridor) in which individuals encoded spatial information by being shown the navigation route to the target room and later retrieved the internal information necessary to navigate through the corridor and reproduce the exact route to the target room. As noted in a recent fMRI study, few neuroimaging studies have utilized designs which have allowed for a comparison between encoding and retrieval (Kim, Daselaar, & Cabeza, 2010).
One distinction between encoding and retrieval is that encoding emphasizes the processing of external stimuli whereas retrieval involves internal processes. This distinction between externally directed and internally generated processing has produced a large number of studies referred to as baseline or default network as well as referred to as task positive and task negative. As overviewed by Kim et al., the task positive network includes lateral prefrontal cortex (PFC), dorsal parietal cortex, sensory–motor cortices, subcortical areas, and the cerebellum (Cabeza and Nyberg, 2000; Naghavi and Nyberg, 2005; Shulman et al., 1997a), whereas the task-negative network, also known as the default-mode network (Raichle et al., 2001), consists of anterior and medial PFC, the precuneus, and the angular gyrus (Shulman et al., 1997b; Gusnard and Raichle, 2001). A recent fMRI study has shown differential involvement of the default cortical network in terms of encoding and retrieval (Daselaar et al., 2009). Additionally, activity in these areas has also been associated with successful retrieval of information (Wagner et al., 2005). Increased activity in PMR and VPC during successful retrieval and reduced activity during successful encoding has been reported (Daselaar et al., 2009). Given that frontal EEG theta may be viewed as an index of default network activity (Scheeringa et al., 2008), we would expect to find differential encoding/retrieval differences in EEG theta activity.
In the present study, we sought to examine spatial processing during encoding and retrieval of a spatial memory task. Specifically, we designed an EEG study that incorporates spatial processing using a virtual reality (VR) environment. We developed VR based spatial memory tasks including encoding and retrieval conditions enabling: (a) the subjects to experience the sense of presence (Jancke et al., 2009); and (b) to track the changing brain activation patterns during exposure to these VR driven spatial memory task conditions. It was hypothesized that encoding of visual-spatial information in working memory requires more cerebral efforts in comparison to retrieval and that these processes differentially activate the default network.
2. Results
Behavioral results
The success rate of the task performance i.e., accurate navigation to the target room was 100%. The average time taken by complete the entire task, from the start room to target room and back to start room was 52.58 +/− 6.23 seconds. The average time taken by subjects to go from start room to target room was 24.83 +/− 3.23 seconds and the average time to come back from target room to start room was found to be 27.75 +/− 2.87 seconds. A paired t-test was used to see if the difference was significant. (T = −0.62, P = 0.55, ns) showed the difference in time taken by the subjects to reach the target room and return to the start room was not significant.
EEG Power analysis results
The EEG power analysis results obtained from Mixed ANOVA Model revealed that three way interaction between Band, ROI, Condition was significant for response variable power F(32,748) = 1.62, P= 0.0177).
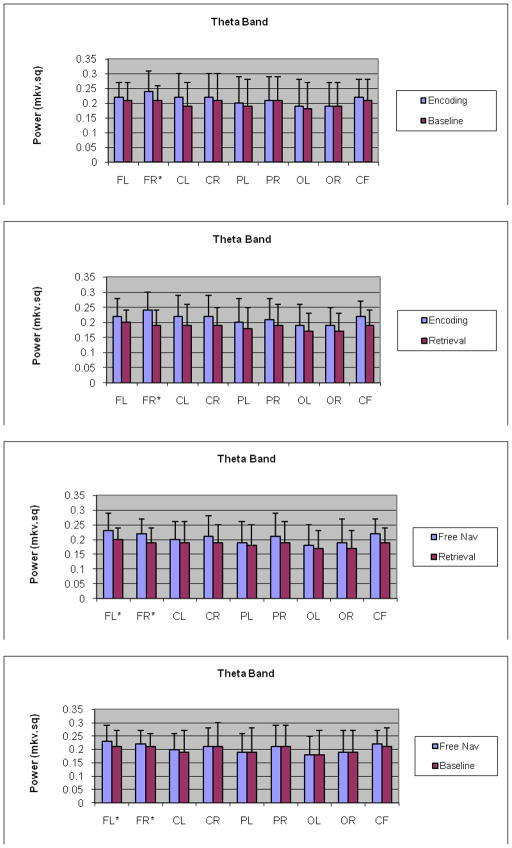
Tukey’s test comparing encoding and baseline at each of the nine ROIs for theta band revealed that for the right frontal region, theta for encoding was significantly higher relative to the baseline t = 2.06, P= 0.0389) (Fig. 1a). No other comparisons were significant. Between encoding and retrieval, encoding was significantly higher for the right frontal region t = 3.4, P= 0.00007) and right central region t = 2.28, P = 0.0275) (Fig. 1b) in comparison to retrieval. Lastly, amongst retrieval and free navigation theta band activity for retrieval was significantly decreased relative to the free navigation at right frontal region (t = −2.11, P= 0.0351) and left frontal region (t = −2.02, P = 0.0439) (Fig. 1c). Interestingly, amongst free navigation and baseline, theta band activity was significantly higher for the free navigation at both right frontal (t = 3.23, P = 0.0038) and left frontal (t = 2.53, P = 0.0149) areas.
Fig 1.
a,b,c,d - Interaction plots for theta band for encoding, retrieval and baseline/free navigation conditions at nine ROIs: a) Graph comparing encoding and baseline for theta band, shows a significant increase in right frontal brain ROI during encoding as compared to baseline; b) Graph comparing encoding and retrieval for theta band, shows a significant increase in right frontal ROI during encoding as compared to retrieval; c) - Graph comparing retrieval and free navigation for theta band, shows a significant increase in right frontal and left frontal ROI during free navigation as compared to retrieval; (d) – graph comparing free navigation and baseline shoes no differences between these conditions; *-significance level, p < .001.
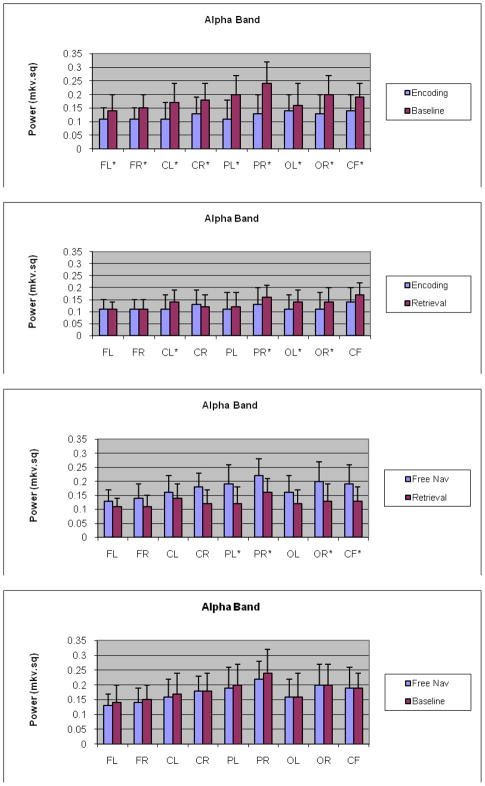
Tukey’s test comparing encoding with baseline and retrieval and baseline at each of the nine ROIs for alpha band revealed that the interaction was statistically significant at all of the nine ROIs with baseline higher relative to encoding and retrieval (Fig. 2a). Amongst encoding and retrieval, alpha band activity for encoding was significantly attenuated relative to the retrieval at left central (t = −2.56, P= 0.0105), right parietal (t = −2.39, P = 0.0221), left occipital (t = −2.04, P = 0.0417) and right occipital ROI (t = −2.02, P = 0.0469) (Fig. 2b). Amongst retrieval and free navigation there was increase in alpha power during free navigation at left parietal, right parietal and right occipital ROI (P < 0.001). Lastly, no significant attenuation of alpha power during free navigation compare to at all ROI under study was observed (P > 0.05).
Fig 2.
a,b,c,d - Interaction plots for alpha band for encoding, retrieval and baseline/free navigation conditions at nine ROIs: a) Graph comparing encoding and baseline for alpha band, shows a significant increase in all the nine brain ROI during baseline as compared to encoding; b) Graph comparing encoding and retrieval for alpha band, shows a significant increase in left central, right parietal, left occipital and right occipital brain ROI during retrieval as compared to encoding; c) Graph comparing retrieval and free navigation for alpha band, shows an increase in all the nine brain ROI during free navigation as compared to retrieval, reaching significance level at left parietal, right parietal, right occipital and frontal central ROI; (d) graph comparing free navigation and baseline indicating no significant differences between these conditions; *-significance level, p < .001.
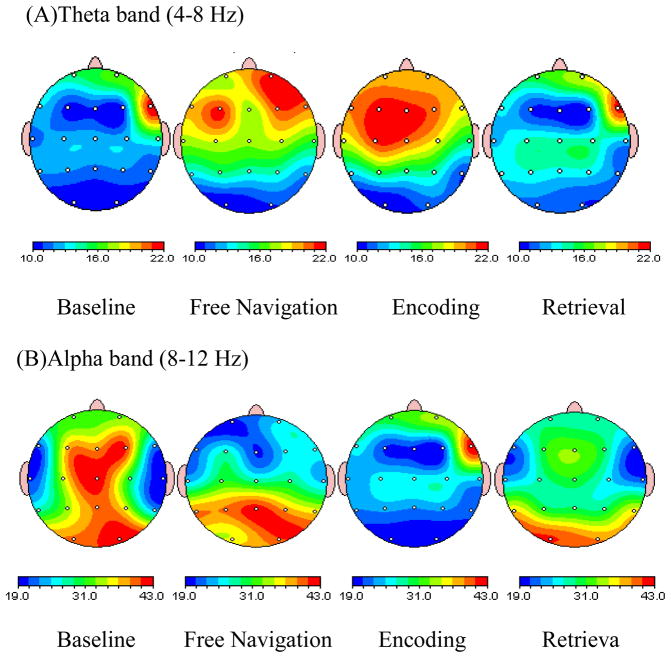
2D brain maps showing topographical distribution of theta and alpha power during all experimental conditions under study are presented in Fig 3a & b. Visually, the topographical distribution of theta activity during free navigation was reminiscent of those during encoding. Specifically, during both encoding and free navigation there was increase in theta power at frontal areas.
Fig. 3.
Brain maps showing topographical distribution of theta and alpha power during free navigation, baseline, encoding and retrieval of visual- spatial working memory task: (top) Brain map showing increase in theta power in right frontal and frontal-central region during encoding and free navigation as compared to retrieval. When encoding is compared with baseline, the increase is again significant in right frontal brain region. Another point of interest is higher theta power at frontal-central areas during free navigation as compared to retrieval; (bottom) Brain map showing a significant increase in alpha power in all the regions during baseline in comparison to encoding and retrieval. When comparing encoding and retrieval, alpha power is slightly higher during retrieval at right parietal, left central and left, right occipital brain regions. When comparing retrieval and free navigation, alpha power reduced at occipital areas. Overall, both encoding and retrieval attenuate alpha power at all ROI.
Tukey’s test comparing encoding and retrieval at each of the nine ROIs for beta band revealed that there was no statistically significant interaction.
Source Localization results
Encoding – Baseline
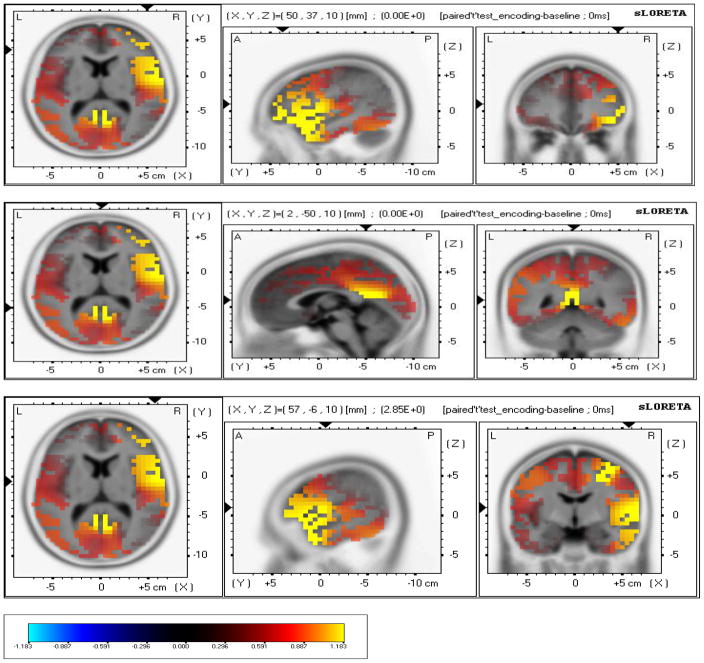
sLORETA localized anatomical sources of electrical neuronal activity recorded using EEG of significant difference between encoding and baseline. There was higher cortical sources solution during encoding in comparison to baseline in Inferior Frontal Gyrus (Broadman area 46); (X= 50, Y= 37, Z= 10) (MNI cords, P < .001); (Fig. 4a,b), Premotor Cortex (Broadman area 6); (X= 57, Y= −6, Z= 10) (MNI cords, P < 0.05), (Fig. 8) and Posterior Cingulate (Broadman area 29); (X= 2, Y= −50, Z= 10) (MNI cords, P < 0.01).
Fig. 4.
a, b, c; - sLORETA localized anatomical sources of electrical neuronal activity for encoding – baseline: a) Images showing identified cortical sources in Inferior Frontal Gyrus (Broadman area 46), Frontal lobe. b) Images showing identified cortical sources in Posterior Cingulate (Broadman area 29), Limbic Lobe. c) Images showing identified cortical sources in Premotor cortex (Broadman area 6), Frontal lobe.
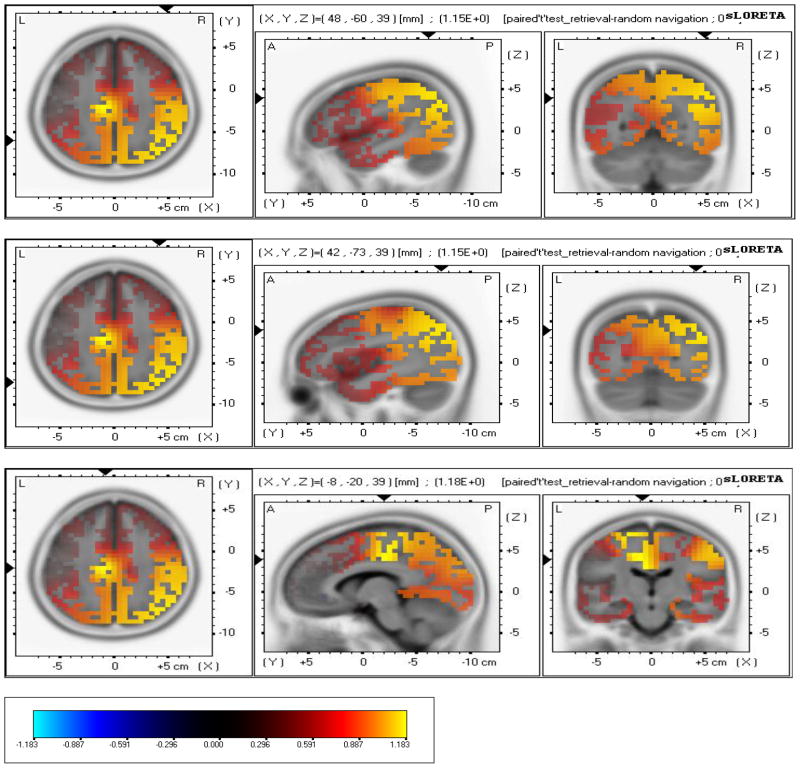
Retrieval – Random Navigation
sLORETA revealed higher cortical sources solution during retrieval in comparison to random navigation in Inferior Parietal Lobule (Broadman area 39, (X= 48, Y= −60, Z= 39) (MNI cords, P < 0.001); (Fig. 5a,b,c), Precuneus (Broadman area 19); (X= 42, Y= −73, Z= 39) (MNI cords, P < 0.001); (Fig. 11), Cingulate Gyrus (Broadman area 24), (X= −8, Y= −20, Z= 39) (MNI cords, P < 0.01).
Fig 5.
a, b, c - sLORETA localized anatomical sources of electrical neuronal activity for retrieval – free navigation: a) Images showing identified cortical sources in Inferior Parietal Lobule (Broadman area 39, 40), Parietal lobe. b) Images showing identified cortical sources in Precuneus (Broadman area 19), Parietal lobe; c) Images showing identified cortical sources Cingulate Gyrus (Broadman area 24), Limbic lobe.
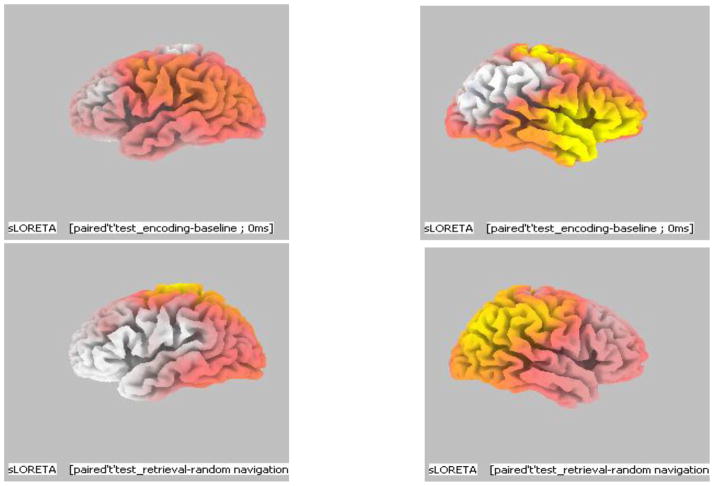
The above results indicated that during a visuo-spatial working memory task the chief brain regions found to be activated were frontal lobe, parietal lobe and limbic lobe. Within the frontal lobe the identified sources were in Inferior Frontal Gyrus (BA 46) and Premotor cortex (BA 6). In the parietal lobe the identified sources were in the Inferior parietal lobule (BA 39, 40) and Precuneus (BA 19) while limbic lobe showed sources in Cingulate gyrus (BA 24) and Posterior Cingulate (BA 29). Overall, during encoding of visuo-spatial information frontal lobe was found to be activated whereas during retrieval of the same information parietal lobe was chiefly activated. Another noticeable factor was that most of the areas activated were located in the right hemisphere of the brain (Fig. 6a & b).
Fig. 6.
a, b - 3D images of identified brain areas during Encoding - Baseline and Retrieval – Free Navigation: a) Brain areas activation during Encoding – baseline: Image of Left brain hemisphere showing no activity during encoding and image of right brain hemisphere showing increased brain activity during encoding in the frontal brain areas. b) Brain areas activated during Retrieval – Free Navigation - Image of Left brain hemisphere showing no signs of activation during retrieval and image of right brain hemisphere showing increased activity during retrieval in the parietal brain areas.
3. Discussion
Our results supported the hypothesis that spatial encoding required more effort than retrieval and that these processes differentially involve the default network. This was shown by the increase in theta power from baseline as compared to encoding whereas there was a decrease in theta power during retrieval suggesting that encoding is more demanding or cognitively challenging compared to baseline or retrieval. Frontal-central predominance was seen during encoding when compared with baseline and retrieval. Further, this was differentiated in terms of hemisphere with a greater increase in theta activity in right hemisphere of the brain in comparison to the left during the encoding of our visuo-spatial working memory task. It should also be noted that in comparison to retrieval and baseline, there was an increase in theta during free navigation. Since free navigation and retrieval both require a motor component and encoding did not, this suggests that theta cannot be understood to reflect navigational motor demands per se. It does however suggest that free navigation carries with it a learning component (e.g., possibly formation of the cognitive map) even through our participants were not required to obtain any new knowledge during this task. However, from an evolutionary perspective, it is reasonable to assume that organism would seek to know any route they followed. Thus, both free navigation and encoding contain a learning component which is reflected in theta activity. This is supported in terms of existing literature which has shown working memory encoding is associated by an increase in theta synchronization (Krause et al., 2000, Stam et al., 2002) and that larger theta power during encoding is correlated with a higher retrieval success (Klimesch et al., 2001, Weiss et al., 2000).
In terms of EEG alpha activity, our results are consistent with previous studies which have shown a negative correlation between theta and alpha production. Our study showed an increase in alpha power during baseline compared to encoding and retrieval in all regions. Free navigation also showed greater posterior alpha in comparison to encoding and retrieval. Between encoding and retrieval, alpha activity was significantly higher during retrieval in right parietal, left central, left occipital and right occipital regions. This can be interpreted as encoding of visuo-spatial information being more challenging for the subjects in comparison to retrieval, thereby resulting in a decrease in alpha power during encoding and an increase during retrieval.
We used sLORETA to better articulate the EEG networks involved in encoding and retrieval of the spatial processing during the VR task. Results showed that different neural substrates were involved during encoding and retrieval of visuo-spatial memory. There was a cortical source identified in right DLPFC during encoding of visuo-spatial working memory task. This is consistent with studies showing an increase in activation in DLPFC in response to increasing task demand on a working memory task in healthy individuals (Manoach et al., 1997, D'Esposito et al., 1999) thereby suggesting that activation in DLPFC is associated with increased cerebral challenge allowing for sustained processing of novel sensory stimulus during working memory tasks (Hillary et al., 2006). Thus, tasks requiring greater cognitive control lead to recruitment of DL-PFC which was seen in case of encoding of visuo-spatial working memory. Further, as shown in Figures 4 & 5, our results are consistent with a variety of neuropsychological findings showing the role of the right parietal lobe in spatial memory (e.g., Berryhill & Olson, 2008).
As hypothesized, brain areas activated during retrieval of information were similar to the areas which form part of the default network. Given that Scheeringa et al. (2008) showed a direct link between decreased fMRI default network activity and increased theta activity, our EEG study is consistent with previous fMRI studies showing an increase in activity in the PMR and VPC during successful retrieval and reduced activity during successful encoding (Daselaar et al., 2009). Further, using EEG theta activity we found a similar encoding/retrieval flip in terms of brain areas activated during encoding and retrieval. Our results extend previous fMRI research to show EEG networks reflect the involvement of different networks in encoding and retrieval and may support the idea that retrieval is a partly embedded in the default network of the brain.
4. Conclusion
Overall, it can be concluded from our EEG power analysis and source localization using sLORETA that encoding requires more cerebral efforts from the individual in comparison to retrieval of information during a visual-spatial working memory task. Our results are in support of encoding/retrieval flip, as different neural substrates were employed during encoding and retrieval of information. Also, the pattern of brain areas activated during both, encoding and retrieval support the concept of hemispheric laterality i.e., visual-spatial working memory tasks are localized in the right brain hemisphere.
5. Experimental procedure
Participants and study design
Twelve neurologically normal students were recruited from The Pennsylvania State University for this experiment. All the subjects were right handed according to Edinburgh handedness Inventory (Oldfield, 1971) and had normal or corrected-to-normal vision. The subject’s included 7 male and 5 female subjects with an average age of 22.67 years. They signed an informed consent to a protocol approved by the Institutional Review Board of The Pennsylvania State University. The participants witnessed a Virtual Reality Paradigm, which comprised of four stages: encoding, retrieval, free navigation and baseline stage. EEG was recorded simultaneously and continuously throughout the experiment.
Measures and apparatus
EEG recording
EEG recording was conducted using Ag/AgCl electrodes mounted in a 62-channel Electro-cap (NeuroScan Inc., Eaton, OH). The ground electrode was located 10% anterior to FZ, with linked earlobes served as references. EEG signals were recorded using a programmable DC coupled broadband SynAmps amplifier (NeuroScan, Inc., El Paso, TX.). The EEG signals were amplified (gain 2500, accuracy 0.033/bit) with a recording range set for +/− 55 mV in the DC to 70-Hz frequency range. The EEG signals were digitized at 250 Hz using 16-bit analog-to-digital converters. Impedance was measured at all sites and was kept below 5Kohms.
Virtual Reality Data recording
Virtual corridor was generated by VTK Open GL developing kit (HeadRehab, LLC) providing realistic VR environment and sense of presence to the subjects (see Fig. 7). The subjects were able to freely navigate via virtual corridor using their right thumb in forward, backward and side to side directions. The experimental design to study visual-spatial working memory comprised of four stages:
Fig. 7.
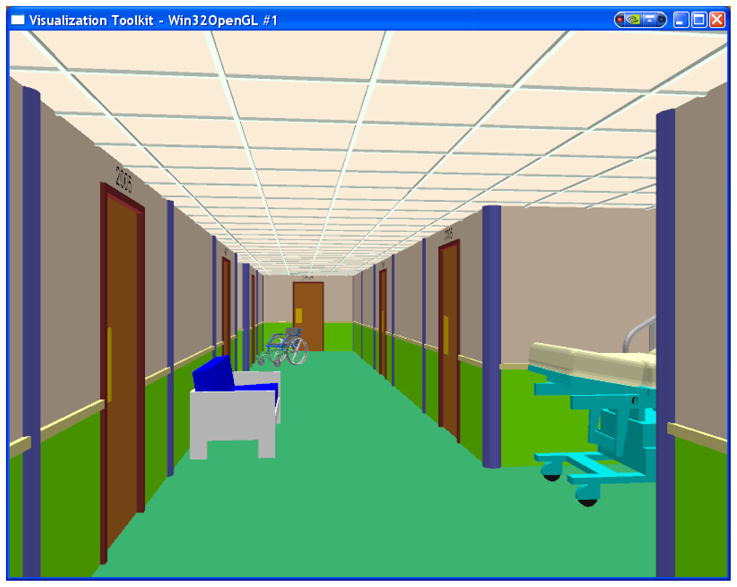
Virtual Reality Corridor used for visual-spatial working memory task – Subjects were shown the designated path through the corridor during encoding and required to: (a) navigate free via VR environment (no goal); and (b) find the designated path shown during encoding using a joystick from start room to the target room and back - retrieval.
Encoding – In this the subject was shown the navigation route to the target room (60s). The subject’s task was to remember the shown route that will be later requested to reproduce;
Retrieval – In which the subject was requested to navigate through the virtual corridor and reproduce exact route to the target room which was shown during encoding stage (60s time limit);
Free navigation; - In which subject was asked to explore the virtual corridor via randomly navigating without any specific goal to reach the destination target (60s);
Baseline – In which subject was requested to look at the screen on which stationary virtual corridor was displayed (60s).
Data Processing
EEG Data processing
In order to reduce the number of independent variables, we collapsed 62 EEG channels into 9 topographical regions of interest (ROIs; Oken and Chiappa, 1986). The 9 ROIs included right frontal (FR: ‘FP2’, ‘AF4’, ‘F6’, ‘F4’, ‘F2’); left frontal (FL: ‘FP1’, ‘AF3’, ‘F5’, ‘F3’, ‘F1’); right central (CR: ‘FC6’, ‘FC4’, ‘FC2’, ‘C6’, ‘C4’, ‘C2’, ‘CP6’, ‘CP4’, ‘CP2’); left central (CL: ‘FC5’, ‘FC3’, ‘FC1’, ‘C5’, ‘C3’, ‘C1’, ‘CP5’, ‘CP3’, ‘CP1’); right parietal (PR: ‘P6’, ‘P4’, ‘P2’, ‘PO6’, ‘PO4’); left parietal (PL: ‘P5’, ‘P3’, ‘P1’, ‘PO5’, ‘PO3’); right occipital (OR: ‘CB2’, ‘O2’); left occipital (OL: ‘CB1’, ‘O1’); and mid frontal central (FC: ‘FZ’, ‘CZ’). The temporal area was excluded in order to reduce the chance of including muscle artifacts. The EEG data was initially processed off-line with EEGLAB 5.03 (Delorme and Makeig, 2004), using Matlab open source toolbox (Mathworks, Natick, USA). Imported data were down sampled to 200 Hz to reduce computing time. After baseline normalization these epochs were automatically screened for unique, non-stereotypic artifacts using a probabilistic function within EEGLAB. This procedure allows the removal of epochs containing signal values exceeding 3 SD and controls for artifacts such as eye blinks, eye movements, and heartbeats. Using Neuroscan’s Scan 4.3 Edit software program, bandpass filtering between 0.5–30 Hz with zero phase-shift was applied to the four virtual reality conditions. Each file was then epoched using a no trigger setting with 256 points, and epochs that overlapped rejected blocks were then rejected. A Fast Fourier Transform in the frequency domain on the artifact free EEG record for the entire 9 region of interests (ROIs) was then performed. Frequency bandwidths were divided according to the following divisions: theta (4–7.5 Hz), alpha (8–12 Hz), beta (12–30 Hz). From the computed FFTs, overall power (uV Sq) averages were computed for each bandwidth using Neuroscan’s analysis program.
Cortical Sources of EEG as Revealed by s LORETA
It should be noted that LORETA has been successfully used in a number of recent EEG studies (Babiloni et al., 2006, 2010). In this study, the EEG data were initially processed off-line using EEGLAB 5.03 45 using Matlab open source toolbox (Mathworks, Natick, USA) after which it was input into Key Institute’s sLORETA – Standardized Low Resolution Brain Electromagnetic Tomography software program (Pasqual-Marqui, 2002). Each of the four artifact free EEG files: for encoding, retrieval, random navigation and baseline for each subject were transformed into a sLORETA individual analysis file. The transformed individual files were then averaged together to form sLORETA group analysis files. Nineteen ROIs were constructed with the “all nearest voxels” method using sLORETA software (Pascual-Marqui, 2002). The sLORETA/eLORETA software package was then used to perform the statistical analyses. The methodology used is non-parametric. It is based on estimating, via randomization, the empirical probability distribution for the max-statistic (e.g. the maximum of a t or an F statistic), under the null hypothesis. The brain areas activated during the following conditions were compared: encoding with baseline and retrieval with free navigation. Based on the scalp-recorded electric potential distribution, the standardized low resolution brain electromagnetic tomography (sLORETA) software (publicly available free academic software at (http://www.uzh.ch/keyinst/loreta.htm) was used to compute the cortical three-dimensional distribution of current density. Computations were made in a realistic head model (Fuschs et al., 2002), using the MNI152 template (Mazziotta et al., 2001), with the three-dimensional solution space restricted to cortical gray matter, as determined by the probabilistic Talairach atlas (Lancaster et al., 2000). The intracerebral volume was partitioned in 6239 voxels at 5 mm spatial resolution. Thus, sLORETA images represent the standardized electric activity at each voxel in neuroanatomic Montreal Neurological Institute (MNI) space as the exact magnitude of the estimated current density (Musso et al., in press). Anatomical labels as Broadman areas are also reported using MNI space, with correction to Talairach space, similar to Babiloni et la., 2010. Only voxels significant at p = 0.01 or p=005 after correction for multiple comparisons were retained, i.e. the significance of changes in activity compared to baseline was assessed using non-parametric analysis adapted for sources comparisons and implemented in sLORETA which correct for multiple comparisons (Holmes et al., 1996).
EEG Data Analysis
A mixed-effects analysis of variance (Mixed ANOVA) model to analyze the data was used. The model included the 3 fixed-effects factors: frequency bands, ROIs and subject and all with-in subject factors were considered as random effects factor. Power was taken as the response variable. The statistical question we wanted to answer was if the effects of the 3 fixed-effects factors and their interactions, in the presence of the random-effects factor i.e. subject was significant on the response variable Power. SAS 9.2 software package was used to conduct Mixed ANOVA. Before fitting the model exploratory data analysis (EDA) was carried out to identify any potential problems of the data. Following which assumptions of normality and equal variance were checked. The Mixed ANOVA model was fitted using Proc Mixed in SAS. Finally the following interactions were considered for significance: Band*ROI, Band*Condition, ROI*Condition, Band*ROI*Condition.
Acknowledgments
This study was supported by NIH Grant RO1 NS056227-01A2 “Identification of Athletes at Risk for Traumatic Brain Injury” awarded to Dr. Slobounov, PI. We would like to thank Elena Slobounov for VR programming.
Abbreviations
- EEG
Electroencephalography
- sLORETA
standardized low resolution brain electromagnetic tomography
- DLPFC
dorsolateral prefrontal cortex
- ROI
region of interest
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Babiloni C, Binetti E, Casetta G Dal Fonto, et al. Sources of cortical rhythms in adults during physiological aging: a multi-center EEG study. Clin Neurophysiol. 2006;117(2):252–268. [Google Scholar]
- Babiloni C, Marzano N, Iacoboni M, et al. Resting state cortical rhythms in athletes: A high-resolution EEG study. Brain Res Bull. 2010;81:149–156. doi: 10.1016/j.brainresbull.2009.10.014. [DOI] [PubMed] [Google Scholar]
- Berryhill M, Olson I. The right parietal lobe is critical for visual working memory. Neuropsychologia. 2008;46:1767–1774. doi: 10.1016/j.neuropsychologia.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Courtney SM, Ungerleider LG, Keil LG, Haxby JV. Object and spatial visual working memory activate separate neural systems in human cortex. Cereb Cortex. 1996;6:39–49. doi: 10.1093/cercor/6.1.39. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR, Ballard D, Lease J. Maintenance versus manipulation of information held in working memory: an event-related fMRI study. Brain Cogn. 1999;41:66–86. doi: 10.1006/brcg.1999.1096. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Prince SE, Dennis NA, Hayes SM, Kim H, Cabeza R. Posterior midline and ventral parietal activity is associated with retrieval success and encoding failure. Frontiers in Human Neuroscience. 2009;3:1–10. doi: 10.3389/neuro.09.013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Circuitry of primate prefrontal cortex and regulation of behaviour by representational memory. In: Plum F, Mountcastle U, editors. Handbook of physiology. Vol. 5. Washington, DC: American Physiological Society; 1987. pp. 373–417. [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Hartley T, Maguire E, Spiers H, Burgess N. The well-worn route and the path less traveled: distinct neural bases of route following and wayfinding in humans. Neuron. 2003;37:877–888. doi: 10.1016/s0896-6273(03)00095-3. [DOI] [PubMed] [Google Scholar]
- Hillary FG, Genova HM, Chiaravalloti ND, Rypma B, DeLuca J. Prefrontal Modulation of Working Memory Performance in Brain Injury and Disease. Human Brain Mapping. 2006;27:837–847. doi: 10.1002/hbm.20226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancke L, Cheetham M, Baumgartner T. Virtual reality on the role or prefrontal cortex in adults and children. Front Neurosci. 2009;3:52–59. doi: 10.3389/neuro.01.006.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzen G, Weststeijn C. Neural representation of object location and route direction: an evernt-related fMRI study. Brain Res. 2007;1165:116–125. doi: 10.1016/j.brainres.2007.05.074. [DOI] [PubMed] [Google Scholar]
- Jonides J, Smith E, Koeppe R, Awh E, Minoshima S, Mintun S. Spatial working memory in humans as revealed by PET. Nature. 1993;363:583–584. doi: 10.1038/363623a0. [DOI] [PubMed] [Google Scholar]
- Kim H, Daselaar S, Cabeza R. Overlapping brain activity between episodic memory encoding and retrieval: Roles of the task-positive and task-negative networks. NeuroImage. 2010;49:1045–1054. doi: 10.1016/j.neuroimage.2009.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Yonelinas A, Kroll NE, Lazzara M, Rohm D, Gruber W. Theta synchronization during episodic retrieval: neural correlates of conscious awareness. Brain Res Cogn Brain Res. 2001;12:33–38. doi: 10.1016/s0926-6410(01)00024-6. [DOI] [PubMed] [Google Scholar]
- Krause CM, Sillanmaki L, Koivisto M, et al. The effects of memory load on event-related EEG desynchronization and synchronization. Clin Neurophysiol. 2000;111:2071–2078. doi: 10.1016/s1388-2457(00)00429-6. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Kochunov PV, Mikiten SA. Automated Talairach Atlas labels for functional brain mapping. Human Brain Mapping. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R, Goldman-Rakic PS. Association of storage and processing functions in the dorsolateral prefrontal cortex of the nonhuman primate. J Neurosci. 1999;19:5149–5158. doi: 10.1523/JNEUROSCI.19-12-05149.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS, Schlaug G, Siewert B, Darby DG, Bly BM, Benfield A, Edelman RR, Warach S. Prefrontal cortex fMRI signal changes are correlated with working memory load. Neuroreport. 1997;8:545–549. doi: 10.1097/00001756-199701200-00033. [DOI] [PubMed] [Google Scholar]
- Mazziotta J, Toga AE, Fox A, Lancaster P, Zilles J, Woods KR, Paus T. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM. Philos Trans R Soc Lond Biol Sci. 2001;29:1293–1322. doi: 10.1098/rstb.2001.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Skelton RW. The neuropharmacological and neurochemical basis of place learning in the Morris water maze. Brain Res Rev. 1993;18:33–49. doi: 10.1016/0165-0173(93)90006-l. [DOI] [PubMed] [Google Scholar]
- Musso F, Brinkmyer J, Mobascher A, Warbrick T, Winterer G. Spontaneous brain activity and EEG microstates. A novel EEG/fMRI analysis approach to explore resting-state networks. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2010.01.093. in press. YNIMG-07017: No of Pages: 14; 4C. [DOI] [PubMed] [Google Scholar]
- Miyamoto M, Kato J, Narumi S, Nagaoka A. Characteristics of memory impairment following lesioning of the basal forebrain and medial septal nucleus in rats. Brain Res. 1987;419:19–31. doi: 10.1016/0006-8993(87)90564-6. [DOI] [PubMed] [Google Scholar]
- Naghavi HR, Nyberg L. Common fronto-parietal activity in attention, memory, and consciousness: shared demands on integration? Conscious Cogn. 2005;14:390–425. doi: 10.1016/j.concog.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Oken BS, Chiappa KH. Statistical issues concerning computerized analysis of brainwave topography. Ann Neurol. 1986;19:493–494. doi: 10.1002/ana.410190511. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui RD. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details, Methods Find. Exp Clin Pharmacol. 2002;24:5–12. [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheeringa R, Nastiaansen M, Peterson K, Oostenveld R, Norris D, Hagoort P. Frontal theta EEG activity correlates negatively with the default mode network in resting state. Int J Psychophysiology. 2008;67:242–251. doi: 10.1016/j.ijpsycho.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Corbetta M, Buckner RL, Fiez JA, Miezin FM, Raichle ME, Petersen SE. Common blood flow changes across visual tasks: I. Increases in subcortical structures and cerebellum but not in nonvisual cortex. J Cogn Neurosci. 1997a;9:624–647. doi: 10.1162/jocn.1997.9.5.624. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin F, Raichle ME, Petersen SE. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cogn Neurosci. 1997b;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Smith EE. Spatial versus object working memory: PET investigations. J Cogn Neurosci. 1995;7:337–358. doi: 10.1162/jocn.1995.7.3.337. [DOI] [PubMed] [Google Scholar]
- Stam CJ, Anne-Marie van Cappellen van Walsum, Micheloyannis S. Variability of EEG synchronization during a working memory task in healthy subjects. International Journal of Psychophysiology. 2002;46:53–66. doi: 10.1016/s0167-8760(02)00041-7. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Haxby JV. ‘What ‘and ‘where ‘in the human brain. Current Opinion Neurobiol. 1994;4:157–165. doi: 10.1016/0959-4388(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M. Two cortical visual systems. In: Ingle DJ, Goodale MA, Mansfield RJW, editors. The analysis of visual behavior. Cambridge: 1982. pp. 549–586. [Google Scholar]
- van Asselen M, Kessels R, Neggers S, Kappelle L, Frijins C, Poastma A. Brain areas involved in spatial working memory. Neuropsychologia. 2006;44:1185–1194. doi: 10.1016/j.neuropsychologia.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Voermans N, Petersson K, Daudey L, Weber B, van Spaendonck K, Kremer H, Fernández G. Interaction between the human hippocampus and the caudae nucleus during route recognition. Neuron. 2004;43:427–435. doi: 10.1016/j.neuron.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Weiss S, Muller HM, Rappelsberger P. Theta synchronization predicts efficient memory encoding of concrete and abstract nouns. NeuroReport. 2000;11:2357–2361. doi: 10.1097/00001756-200008030-00005. [DOI] [PubMed] [Google Scholar]
- Weiss S, Rappelsberger P. EEG coherence within the 13–18 Hz band as a correlate of a distinct lexical organization of concrete and abstract nouns in humans. Neurosci Lett. 1996;209:17–20. doi: 10.1016/0304-3940(96)12581-7. [DOI] [PubMed] [Google Scholar]
- Wilson FA, O’Scalaidhe SP, Goldman-Rakic PS. Dissociation of object and spatial processing domains in primate prefrontal cortex. Science. 1993;260:1955–1958. doi: 10.1126/science.8316836. [DOI] [PubMed] [Google Scholar]