Abstract
The larval phase of the Drosophila life cycle is characterized by constant food intake, resulting in a two hundred-fold increase in mass over four days. Here we show that the conserved energy sensor AMPK is essential for nutrient intake in Drosophila. Mutants lacking dAMPKα are small, with low triglyceride levels, small fat body cells and early pupal lethality. Using mosaic analysis, we find that dAMPKα functions as a nonautonomous regulator of cell growth. Nutrient absorption is impaired in dAMPKα mutants, and this defect stems not from altered gut epithelial cell polarity but from impaired peristaltic activity. Expression of a wild-type dAMPKα transgene or an activated version of the AMPK target myosin regulatory light chain (MRLC) in the dAMPKα mutant visceral musculature restores gut function and growth. These data suggest strongly that AMPK regulates visceral smooth muscle function through phosphorylation of MRLC. Furthermore, our data show that in Drosophila, AMPK performs an essential cell-nonautonomous function, serving the needs of the organism by promoting activity of the visceral musculature and, consequently, nutrient intake.
Keywords: AMPK, Drosophila, gut, growth, muscle
Introduction
In all organisms, the intake of nutrients is essential for growth, development and the maintenance of cellular function. In single-celled organisms, nutrients enter the cell by active and passive membrane transport. Nutrient intake is more complex in metazoans. Muscles and absorptive epithelial cells in the gastrointestinal tract cooperate to process and digest food. The nervous and endocrine systems regulate the activity of the digestive tract and also signal hunger and satiety. Just as all organisms must ingest nutrients to survive, they must also sense and respond to the availability and quality of nutrients in their environments. For single-celled organisms such as yeast, the composition of the extracellular milieu dictates the nature of intracellular metabolism. These organisms must constantly adapt to the changing external environment. In contrast, metazoans such as mammals can buffer changes in nutrient availability by calling on liver, muscle and fat for storage or release of fuel reserves. Therefore, multicellular organisms not only monitor and respond to the internal energy stores and the external availability of nutrients, but they also must communicate such information among organs to coordinate processes in the whole animal. Although the processes underlying nutrient intake differ in complexity among eukaryotes, some of the same molecular pathways are used to sense and signal nutrient abundance and scarcity.
The yeast sucrose non-fermenting 1 (SNF1) and the mammalian AMP-activated protein kinase (AMPK) pathways exemplify a conserved mechanism that allows single cells and whole animals to respond to depleted energy levels stemming from altered nutrient intake. SNF1 and AMPK are orthologous heterotrimeric serine-threonine kinases, each consisting of a catalytic α subunit and regulatory β and γ subunits, that sense and respond to decreased cellular energy levels (Hardie, 2007). In yeast, SNF1 is essential for adaptation to growth on non-glucose carbon sources. When yeast are grown in non-fermentable sugars such as galactose, glucose metabolism drops, leading to activation of the SNF1 kinase (Woods et al., 1994). SNF1 phosphorylates transcriptional regulators such as the repressor Mig1p, allowing adaptation to nutrient stress through derepression of the genes used to metabolize galactose (Treitel et al., 1998).
Like SNF1 in yeast, AMPK in mammals responds to cellular energy levels. AMPK is activated when the cellular AMP to ATP ratio rises due to a high rate of metabolism or decreased nutrient availability. AMP binds to the regulatory γ subunit of AMPK, leading to both allosteric activation of AMPK and increased phosphorylation of the catalytic α subunit on an activation loop threonine. Once activated, AMPK phosphorylates downstream targets leading to energy conservation and inhibition of ATP consumption (Hardie, 2007). For example, the high rate of ATP use in contracting muscle leads to AMPK activation and inhibition of its target acetyl-CoA carboxylase (ACC), thereby increasing fatty acid oxidation and restoring cellular energy (Hutber et al., 1997).
Over the course of evolution, AMPK has acquired new modes of regulation and new functions to meet the differing circumstances of multicellular life. In mammals, AMPK is regulated not only cell autonomously by energy stress but also cell nonautonomously by circulating factors. For example, the fat hormones leptin and adiponectin rapidly activate AMPK in muscle, leading to accelerated fatty acid oxidation (Minokoshi et al., 2002; Yamauchi et al., 2002). The development of cell-nonautonomous, hormonal regulation of AMPK has been accompanied by additional roles for AMPK that exclusively serve the needs of the organism with no direct profit to the cell. For example, activation of AMPK in hypothalamic neurons by fasting or adiponectin promotes food intake, while inhibition of hypothalamic AMPK by hormones such as leptin and insulin or forced expression of dominant-negative AMPK inhibit feeding inhibits feeding (Kubota et al., 2007; Minokoshi et al., 2004). Together, the cell-nonautonomous regulation and actions of AMPK serve the whole animal by coordinating nutrient intake and energy metabolism across tissues.
Work in the invertebrate Drosophila melanogaster has uncovered a role for AMPK in the regulation of cell polarity during energy stress in the embryonic cuticle, in ovarian follicular cells and in neurons (Lee et al., 2007; Mirouse et al., 2007). To date, metabolic roles for AMPKα in this organism have not been described, although loss of AMPKα in the post-embryonic fly has not been systematically investigated. Here we describe a novel cell-nonautonomous function for AMPK in the regulation of nutrient intake. We show that AMPK is required for gut function and consequently organismal growth in Drosophila. We find that while gut epithelial polarity is maintained in dAMPKα mutants, AMPK acts in the visceral musculature to support peristalsis. Moreover, our data suggest that myosin regulatory light chain (MRLC) acts downstream of or parallel to AMPK to promote visceral muscle function and organismal growth.
Materials and Methods
Fly Stocks
Flies were raised on standard food containing molasses, cornmeal and yeast at 25°C. 4–6 h egg lays were conducted to ensure uncrowded rearing conditions. Drosophila stocks were obtained from: dAMPKα1 (Mirouse et al., 2007); Ubi-GFP, hsFLP, FRT19A (a gift from Thomas Neufeld); lkb14B1–11 (Martin and St Johnston, 2003); dMef2-GAL4 (Ranganayakulu et al., 1995); MHC-GAL4 (Schuster et al., 1996); UAS-sqhEE (Corrigall et al., 2007), byn-GAL4 (Iwaki and Lengyel, 2002); r4-GAL4 (Lee and Park, 2004); TSC1Q87X and UAS-TSC1,TSC2 (Tapon et al., 2001); UAS-FOXO™ (Junger et al., 2003). All other lines were from the Bloomington Stock Center (Bloomington, IN). The dAMPKαΔ39 mutation was generated by imprecise excision of the P element P[SUPor-P]KG09204 (Bloomington), and the dAMPKαΔ39, FRT19A allele was generated by recombination. For UAS-dAMPKα transgenes, the full-length dAMPKα cDNA (clone GH12596, Drosophila Genomics Resource Center, Bloomington, IN) was cloned into pENTR (Invitrogen). The kinase-dead K56R mutation (Mu et al., 2001) was introduced by site-directed mutagenesis (QuikChange, Stratagene) using the following primers: sense: 5’-CAA GGT GGC CGT CAG GAT CCT CAA TCG TCA GAA G-3’ and anti-sense: 5’-CTT CTG ACG ATT GAG GAT CCT GAC GGC CAC CTT G-3’. Gateway cloning (Invitrogen) was used to generate pUAST-dAMPKα and pUAST-dAMPKαK56R. Constructs were injected into Drosophila embryos at Duke University Model Systems Genomics (Durham, NC).
Quantitative RT-PCR
Reverse transcription was performed on 2 µg total RNA isolated from 96 h after egg lay (AEL) larvae using the RETROscript kit (Ambion). Quantitative PCR reactions were performed in triplicate on a Stratagene MX3000P thermocycler using Brilliant SYBR Green Master Mix (Stratagene). Relative amounts of specific transcripts were calculated using the comparative Ct method. The following primers were used: dAMPKα Ex1-F, 5’-GCG TGA GAT CCA GAA CCT AAA G-3’; dAMPKα Ex2-R, 5’-CGT CCA GCA TCA TGT TCG AGA G-3’; dAMPKα Ex2-F, 5’-CCA CCA CAC CAT GGA GTT TTT C-3’; dAMPKα 3’UTR-R, 5’-AAG GGT TTG GGA CGA ATG CAA G-3’; Rp49-F, 5’-GAC GCT TCA AGG GAC AGT ATC TG-3’ and Rp49-R, 5’-AAA CGC GGT TCT GCA TGA G-3’.
Triglyceride Assays
Larvae were sonicated in 140 mM NaCl, 50 mM Tris-HCl, pH 7.4 and 0.1% Triton X-100. Triglyceride concentrations were measured using the Triglyceride Liquicolor Kit (Stanbio Laboratory) and normalized to protein (measured with the Bicinchoninic Acid (BCA) Protein Assay Kit (Pierce)).
Antibodies
For the rabbit anti-dAMPKα antibody, a GST-full length dAMPKα fusion protein was partially purified following expression in E. coli, and immunization of rabbits was performed by Quality Control Biochemicals (Hopkinton, MA). Additional antibodies were from the following sources: rabbit anti-phosphoThr172-AMPK (Cell Signaling Technology), mouse anti-tubulin (Developmental Studies Hybridoma Bank (DSHB), Iowa City, IA), sheep anti-Drosophila phosphoSer93-ACC (Pan and Hardie, 2002), goat anti-rabbit HRP (Santa Cruz Biotechnology) and rabbit anti-sheep HRP (Kirkegaard & Perry), rabbit anti-Drosophila myosin heavy chain (Kiehart and Feghali, 1986), mouse anti-Drosophila Fasciclin III and mouse anti-Drosophila Na+/K+-ATPase (DSHB), rabbit anti-GFP (Invitrogen), donkey anti-mouse Cy3 (Jackson ImmunoResearch) and goat anti-rabbit Alexa488 (Molecular Probes). Total levels of the biotin-conjugated enzyme dACC were measured by blotting with streptavidin-HRP (Pierce).
Western Analysis
Larvae were sonicated in 2% SDS and 60 mM Tris-HCl, pH 6.8 with protease inhibitor (Roche Diagnostics) and phosphatase inhibitors 1 and 2 (Sigma-Aldrich). Equal amounts of protein (measured by BCA assay, Pierce) were separated by SDS-PAGE. Western analysis was carried out as described (Gleason et al., 2007).
Immunocytochemistry and Histology
Immunocytochemistry was performed essentially as described (Phillips and Thomas, 2006), and tissues were counterstained with phalloidin (Invitrogen) and DAPI. For histological analysis, third instar guts were fixed in 2.5% glutaraldehyde and 2% formaldehyde in PBS, and 1 µm sections were cut and stained with toluidine blue. Images of sections were collected on a Nikon Eclipse E800 microscope using MetaMorph software. Whole-mount guts and fat bodies with mitotic clones were viewed on a Perkin Elmer Ultraview ERS confocal imaging system attached to an inverted Nikon Eclipse TE300 microscope equipped with 20x (0.45 N.A.) or 40x (0.6 N.A.) objective lenses. Images were collected with Ultraview software and processed in Photoshop CS2.
Mosaic Analysis
dAMPKαΔ39 (or dAMPKα1), FRT19A/UbiGFP, hsFLP, FRT19A embryos (0–6 h AEL) were subjected to a 1 h, 37°C heat shock to induce mitotic clones. 96 h AEL fat bodies were subjected to GFP immunostaining and phalloidin labeling and examined for GFP-negative clones.
Glucose Absorption Assay
72 h AEL larvae were fed food with 5 µCi of D-[1-14C]glucose (0.2 µCi/µL, Amersham) and 2% FD&C No. 1 Blue (McCormick & Co) (Voght et al., 2007). After 4 or 14 h of feeding at 18°C, larvae with blue guts were homogenized in 0.5% Triton X-100, and aliquots of total homogenate were taken for protein measurement (BCA assay, Pierce). Glycogen was extracted from 100 µL of each 0.5% Triton X-100 larval homogenate by boiling for 30 min in 1 M NaOH. Following addition of 0.5 mg unlabeled glycogen, the samples were precipitated with ethanol twice, and the pellets were resuspended in 100 µL H2O and transferred to EcoScint fluid (National Diagnostics, Atlanta, GA) for scintillation counting. Neutral lipids were extracted by adding 2 mL methanol, 2 mL chloroform and 1 mL H2O to 150 µL of each 0.5% Triton X-100 larval homogenate, with vortexing after each addition. Samples were centrifuged for 20 min at 2000 rpm, the organic phase was transferred to a glass vial and allowed to air dry in a hood. The pellet was resuspended in 150 µL chloroform and transferred to EcoScint fluid for scintillation counting. The amount of radiolabeled glucose in the total, glycogen and neutral lipid fractions was measured by scintillation counting and expressed as pg glucose/µg protein. Three experiments were performed in duplicate with 35–45 larvae per group.
Gut Clearance Assay
Larvae were reared on standard food with 1% FD&C No. 1 Blue and transferred to wet Whatman paper in 12-well plates at 96 h AEL. Gut color scores were recorded at 0, 2, 6, 10 and 24 h as follows: 4 = food from anterior midgut to hindgut, 3 = food from central midgut to hindgut, 2 = food in posterior midgut and hindgut, 1 = faint blue color in hindgut, and 0 = clear. For tissue-specific rescues, dAMPKαΔ39/FM7, GFP; UAS-dAMPKα females were mated to FM7, GFP/Y males bearing different GAL4 drivers. For gene-specific rescues, dAMPKαΔ39/FM7, GFP; 24B-GAL4 females were mated to FM7, GFP/Y males bearing various UAS transgenes. Following gut transit assays, larvae were collected for PCR genotyping using the following primers: GAL4-F, 5’-GTC TTC TAT CGA ACA AGC ATG CGA-3’; GAL4-R, 5’-CAT CAT TAG CGT CGG TGA GTG C-3’; UAS-F, 5’-GAT CCA AGC TTG CAT GCC TGC–3’; dAMPKα-R, 5’-CGA ATG GGT ATG GTG CTG C-3’; sqhEE-F, 5’-GCG CCG AGG AGA ATG TGT TCG-3’ and sqhEE-R, 5’-TCC TTG TCC TTG GCA CCG TGC-3’.
Imaging of Peristalsis
Guts were dissected from 96 h AEL larvae into HL3 buffer (Stewart et al., 1994), transferred to coverslips coated with Cell-Tak (BD Biosciences) and adhered to an open-bath chamber filled with HL3. Guts were imaged on an inverted Nikon TE2000U microscope equipped with a 20x (0.75 N.A.) objective lens and differential interference contrast optics (DIC). DIC images were collected every 4 seconds for up to 15 min. At the end of each imaging session, KCl was added to the bath to a final concentration of 50 mM to induce depolarization of the muscle fibers.
Statistics
Data were analyzed by unpaired two-tailed t tests or, in the case of gut transit assays, two-way ANOVA with repeated measures followed by Bonferroni post tests.
Results
Generation of a dAMPKα null allele
To explore the role of AMPK in growth and metabolism in Drosophila melanogaster, we used imprecise P element excision to generate a 2777 bp deletion of genomic DNA that includes the gene CG3719 as well as the 3’ untranslated region (UTR) and last 39 codons of CG3051, the gene encoding the catalytic α subunit of dAMPK (Figure 1A). This mutation, dAMPKαΔ39, is lethal; however, flies with deletions of CG3719 that leave dAMPKα intact are hemizygous and homozygous viable and fertile. The lethality of the dAMPKαΔ39 mutation is completely rescued by ubiquitous expression of a wild-type but not a kinase-dead dAMPKα transgene, indicating that disruption of dAMPKα rather than CG3719 is the cause of lethality in dAMPKαΔ39 mutants.
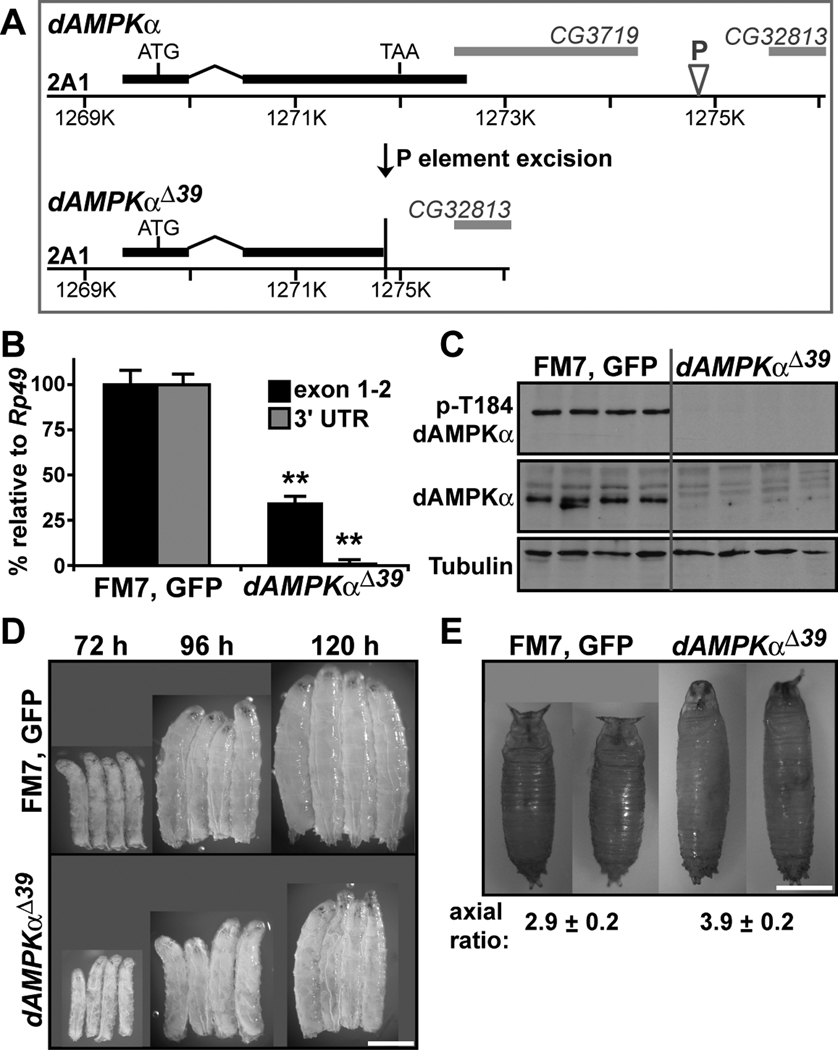
Figure 1. Generation of a dAMPKα mutant allele.
A) Schematic of the dAMPKα locus (top) showing the dAMPKα gene (black), the P element (P) and the genes CG3719 and CG32813 (gray). Imprecise P element excision yielded the dAMPKαΔ39 allele (bottom). B) Q-PCR for exons 1 and 2 (black bars) and the 3’UTR (gray bars) of dAMPKα was performed on RNA isolated from wild type (FM7, GFP/Y) and dAMPKαΔ39/Y third instar larvae. dAMPKα transcript levels were normalized to Rp49 transcript levels. Data are presented as mean ± s.d., **p < 0.01 vs. FM7, GFP/Y. C) Western blot analyses of phosphorylated (p-Thr184, top) and total dAMPKα (middle) and tubulin (bottom) in lysates from FM7, GFP/Y and dAMPKαΔ39/Y third instar larvae. D) FM7, GFP/Y (top) and dAMPKαΔ39/Y larvae (bottom) at 72, 96 and 120 h AEL, scale bar = 1 mm. E) FM7, GFP/Y and dAMPKαΔ39/Y pupae, scale bar = 1 mm. The axial ratio (length/width, mean ± s.d.) is greater in dAMPKαΔ39 mutants compared with WT (p < 0.01).
We performed quantitative RT-PCR to measure dAMPKα transcript levels in wild type and mutant larvae. Primers directed against exon one and the intact portion of exon two showed a 65% reduction in dAMPKα mRNA levels, while primers directed against the 3’UTR failed to amplify a transcript in dAMPKαΔ39 mutants (Figure 1B), suggesting that the loss of the 3’UTR generated an unstable transcript. We were unable to detect dAMPKα protein in dAMPKαΔ39 mutant lysates using either an antibody to phosphorylated AMPKα (Thr184) or an antibody raised against a GST-dAMPKα fusion protein (Figure 1C and Supplementary Figure S1), indicating that the dAMPKαΔ39 mutation acts as a protein null, perhaps due to both transcript and protein instability.
Germline clones that produce dAMPKα-null embryos lead to embryonic lethality due to the essential role of maternally-contributed dAMPK in the regulation of epithelial cell structure (Lee et al., 2007). In contrast, maternal dAMPKα allows dAMPKα-null flies to progress to the larval stage of development. We noted that dAMPKαΔ39 mutant larvae were smaller than wild type larvae from the late second instar; this growth defect became more striking during the third instar (Figure 1D). The molt from second to third instar was delayed by about twelve hours in dAMPKαΔ39 mutants, and the third instar was extended by two days. dAMPKαΔ39 mutants failed to undergo metamorphosis, dying at the end of the third instar or shortly after forming abnormal, elongated pupae (Figure 1E and Table S1, consistent with the lethal phase of dAMPKαD1 mutants (Lee et al., 2007). We also observed decreased larval size and, in uncrowded rearing conditions, early pupal lethality in dAMPKα1 mutants that bear a functionally-null Ser211 to Ala point mutation in the dAMPKα subunit (data not shown) (Mirouse et al., 2007).
Loss of dAMPKα leads to metabolic abnormalities and cell-nonautonomous growth defects
AMPK is a major regulator of metabolism in mammals, affecting nutrient storage and breakdown through multiple targets. For example, AMPK negatively regulates triglyceride synthesis via phosphorylation of the lipogenic enzyme ACC on Ser79 (Ser93 in dACC) (Davies et al., 1990; Pan and Hardie, 2002). While total dACC levels were unchanged in dAMPKαΔ39 larvae, Ser93 phosphorylation was markedly decreased compared with wild type larvae, consistent with dAMPK serving as the primary kinase responsible for phosphorylation of dACC at this site (Figure 2A). Although dACC should be active in dAMPKαΔ39 larvae, whole-body triglyceride levels were significantly decreased in mutant third instars compared with wild type. This difference persisted to the white prepupal stage, demonstrating that impaired triglyceride storage in dAMPKαΔ39 mutants was not simply due to a developmental delay (Figure 2B). We also found significantly decreased triglyceride levels in 96 h AEL dAMPKα1 mutants (µg triglyceride/µg protein: 20.3 ± 7.7 (FM7, GFP/Y, n = 8) vs. 12.0 ± 2.4 (dAMPKα1/Y, n = 10), p < 0.01). In the fat body, increased nutrient storage is accompanied by increased cell size (Britton et al., 2002), and consistent with low triglycerides, dAMPKαΔ39 fat body cells were smaller than wild type fat body cells in third instars and in wandering larvae (Figure 2C).
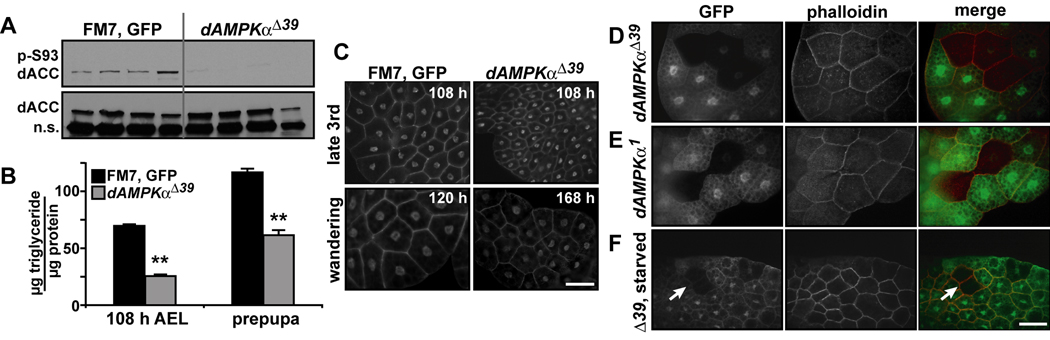
Figure 2. Nutrient storage is decreased in dAMPKα mutant larvae.
A) Western blot analyses of phosphorylated (p-Ser93, top) and total dACC (bottom) in lysates from FM7, GFP/Y and dAMPKαΔ39/Y third instar larvae, n.s., non-specific. B) Whole-body triglyceride levels were measured in FM7, GFP/Y (black bars) and dAMPKαΔ39/Y (gray bars) 108 h AEL larvae (n = 18/group) and white prepupae (n = 13/group) and normalized to protein. Data are presented as mean ± s.e.m., **p < 0.01 vs. FM7, GFP/Y. C) Fat bodies from 108 h AEL and wandering (FM7, GFP/Y: ~120 h AEL, dAMPKαΔ39/Y: ~168 h AEL) larvae were stained with phalloidin and DAPI to label plasma membranes and nuclei. Scale bar = 50 µm. D-F) Heat shock was used to induce clones of fat body cells homozygous for the dAMPKαΔ39 (D) or the dAMPKα1 (E) mutations (GFP negative cells, left panels). Cell membranes were labeled with phalloidin (center panels). Merged images are shown in the right panels, scale bar = 50 µm. F) 24 h starvation of larvae bearing clones had no additional effect on dAMPKαΔ39 homozygous cell size (arrows). Genotypes: D and F) UbiGFP, hsFLP, FRT19A/dAMPKαΔ39, FRT19A. E) UbiGFP, hsFLP, FRT19A/dAMPKα1, FRT19A.
The decreased body and cell size in dAMPKαΔ39 mutants prompted us to ask whether dAMPK serves as an autonomous regulator of growth. Using hsFLP to generate mitotic clones (Golic and Lindquist, 1989), we found that fat body cells homozygous for either the dAMPKαΔ39 or the dAMPKα1 mutation (marked by absence of GFP) were equal in size to surrounding heterozygous cells (Figure 2D and E). Furthermore, starvation of animals bearing clones had no differential effect on cell size within dAMPKαΔ39 clones (Figure 2F). We used the ey-GAL4, UAS-FLP technique to generate homozygous eyes in flies heterozygous for given mutations (Stowers and Schwarz, 1999). In contrast to the altered eye sizes observed when cell-autonomous growth regulators such as dTSC1 or dAkt were mutated (Potter et al., 2001; Verdu et al., 1999), loss of dAMPKα had no obvious effect on eye size (Supplementary Figure S2). Together, these results suggest that AMPK acts as a nonautonomous regulator of cell and organ size and nutrient storage in flies.
Gut function is disrupted in AMPK mutants
In Drosophila larvae, continuous food intake drives a two hundred-fold increase in mass from the beginning of the first instar to the end of the third instar four days later (Church and Robertson, 1966). Once ingested, food is conveyed to the midgut for chemical and mechanical digestion. Two tissues in the gut work in concert to promote nutrient absorption: epithelial cells lining the gut lumen are the initial site of enzymatic breakdown of food and nutrient absorption, and muscle cells on the outside of the gut mix and grind food and propel it through the gut. Absorbed nutrients are used to build larval tissues or are stored as glycogen and triglycerides in the larval fat body, to be released to power metamorphosis. We reasoned that a primary defect in nutrient absorption might underlie decreased body size, impaired triglyceride storage and nonautonomous growth regulation in dAMPKα mutants.
To determine whether nutrient intake might be impaired in dAMPKα mutants, we first measured conversion of ingested glucose into glycogen and triglycerides in wild type and dAMPKαΔ39 mutant larvae. Second instar larvae were fed radiolabeled glucose in food dyed with FD&C Blue No. 1 for four or fourteen hours. At the end of each feeding period, glycogen and neutral lipids were extracted and measured in larvae with blue guts. We found that dAMPKαΔ39 larvae synthesized significantly less glycogen and lipid from the radiolabeled glucose precursor compared with wild type larvae, suggesting that either absorption of nutrients from food or synthesis of glycogen and triglycerides was impaired (Figure 3A).
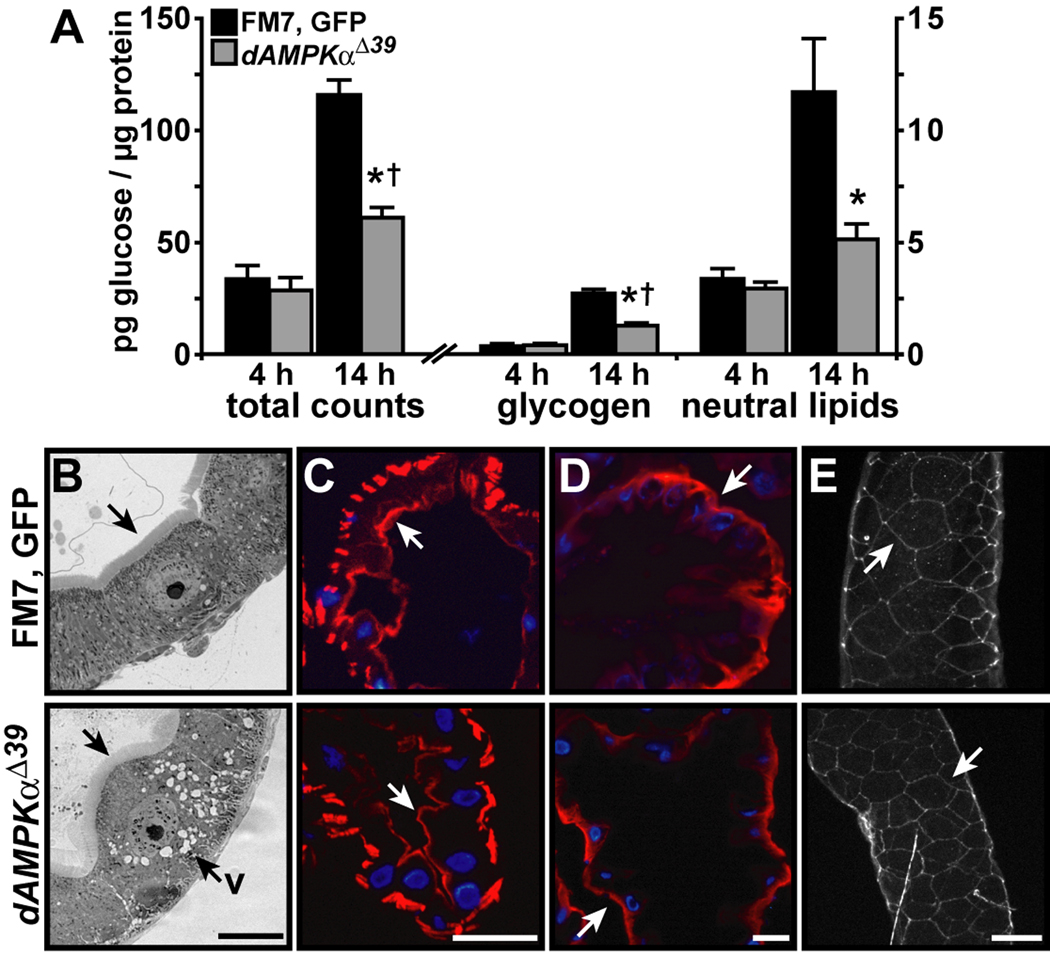
Figure 3. Impaired nutrient absorption despite normal epithelial polarity in dAMPKα mutants.
A) FM7, GFP/Y (black bars) and dAMPKαΔ39/Y larvae (gray bars) were fed radiolabeled glucose for 4 or 14 h, and incorporation of radioactivity into glycogen and neutral lipids was measured. Data are presented as mean ± s.d., *p < 0.05 vs. FM7, GFP/Y at the same time point, †p < 0.05 vs. dAMPKαΔ39/Y at 4 h. For FM7, GFP/Y, all 14 h values differ significantly from 4 h values, p < 0.05. B-E) Representative images of FM7, GFP/Y (top) and dAMPKαΔ39/Y (bottom) midguts. B) Toluidine blue-stained transverse sections of third instar midguts showing the brush border (arrows) in both genotypes and vacuoles (v) in the dAMPKαΔ39 midgut epithelium. C) Phalloidin labeling of the apical layer of filamentous actin (red, arrows) in sections of second instar guts. D, E) Immunocytochemistry for Na+/K+-ATPase (D, red, arrows), and Fasciclin III (E, white, arrows) in sections (D) or whole guts (E) from second instar larvae. Nuclei in C and D are stained with DAPI (blue), scale bars = 50 µm.
Efficient nutrient uptake requires contributions from the epithelial cells that line the gut lumen and absorb nutrients and from the visceral musculature that mechanically digests food and moves it through the gut. In Drosophila, AMPK maintains epithelial polarity during energy stress (Lee et al., 2007; Mirouse et al., 2007); therefore, we assessed the integrity of the epithelial layer in wild type and dAMPKα mutant larval midguts. We observed a pronounced brush border in midgut epithelia from both genotypes, but marked vacuolation in dAMPKαΔ39 mutant epithelial cells (Figure 3B). In both wild type and dAMPKαΔ39 second instar larvae, we observed apical phalloidin labeling of filamentous actin, basolateral localization of the Na+/K+-ATPase in anterior midgut copper cells and lateral distribution of the septate junction marker Fasciclin III (Figure 3C–E) (Baumann, 2001). Additionally, we found that wild type, dAMPKαΔ39 and dAMPKα1 larvae maintained basolateral distribution of the Na+/K+-ATPase and an apical actin layer not only when fed, but also when starved or fed 2-deoxy-glucose (2-DG) to produce energetic stress (Supplementary Figure S3).
We next examined visceral muscle morphology using an antibody against Drosophila myosin heavy chain. In late third instar dAMPKαΔ39 larvae, the midgut musculature displayed a ragged appearance, and both the circular and longitudinal muscles were smaller compared with wild type (Figure 4A). Hindgut muscle fiber thickness was also greatly diminished in dAMPKαΔ39 mutants compared with wild type (Figure 4B). To test visceral muscle function, larvae were grown on blue-dyed food, transferred to wet filter paper at 96 h AEL and scored for the amount of food in the gut over the course of 24 h. At the end of the feeding period, larvae of both genotypes had similar amounts of blue-dyed food in their guts. Most wild type larvae cleared food from their guts within 10 h of removal from food. In contrast, dAMPKαΔ39 and dAMPKα1 mutants retained significant amounts of food in their guts even after 24 h (Figure 4C and D). The same impairment was observed in second instar dAMPKαΔ39 larvae (Supplementary Figure S4A). We noted that larvae bearing imprecise excisions that deleted CG3719 but not dAMPKα exhibited wild type gut function (Supplementary Figure S4B). These data suggest that the blunted glycogen and triglyceride accumulation observed in the glucose absorption experiment were likely to be due to impaired gut motility in dAMPKαΔ39 larvae.
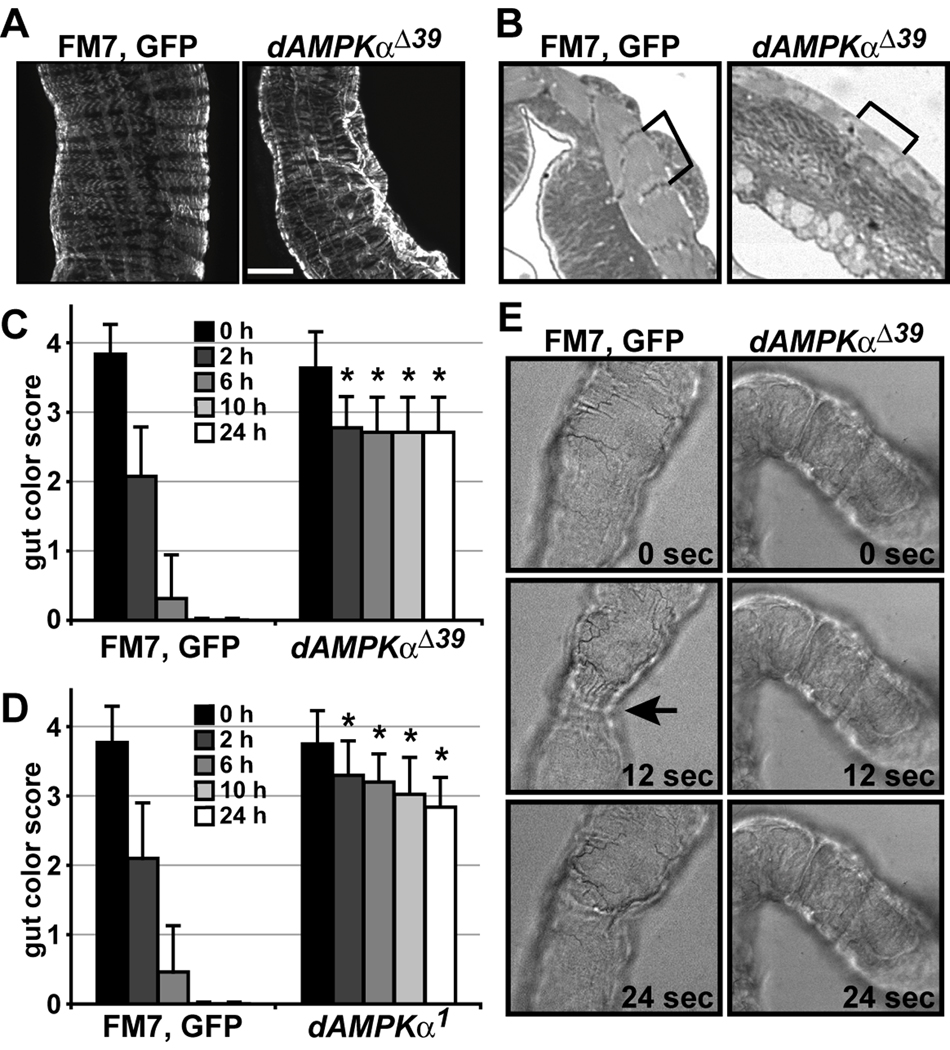
Figure 4. Visceral muscle function is impaired in dAMPKα mutants.
A) Whole-mount immunocytochemistry for myosin heavy chain showing circular and longitudinal visceral muscle fibers in FM7, GFP/Y (left) and dAMPKαΔ39/Y (right) third instar midguts, scale bar = 50 µm. B) Toluidine blue staining of transverse sections of FM7, GFP/Y (left) and dAMPKαΔ39/Y (right) hindguts. Brackets indicate individual sarcomeres. C, D) Larvae were raised on food containing FD&C No. 1 Blue dye, transferred to Whatman paper at 96 h AEL and scored for the amount of food remaining in the gut over 24 h as described in the Materials and Methods. C) FM7, GFP/Y: n = 16, dAMPKαΔ39/Y: n = 13. D) FM7, GFP/Y: n = 12, dAMPKα1/Y: n = 11. Data are presented as mean ± s.d., **p < 0.01 vs. FM7, GFP/Y at the same time point. E) Still images of FM7, GFP/Y (left) and dAMPKαΔ39/Y (right) third instar guts imaged live in HL3 buffer. Each frame is separated from the next by 12 seconds. The arrow points to constriction of wild type gut muscles.
During peristalsis, the circular muscles of the gut contract behind and relax ahead of a food bolus, and contraction of the longitudinal muscles pushes the food forward. Circular muscle contraction in the absence of longitudinal muscle contraction serves to mix and grind food (Bayliss and Starling, 1899). We assessed peristalsis by imaging the midgut portion of freshly dissected guts in HL3, a buffer that mimics Drosophila hemolymph (Stewart et al., 1994). We observed robust, spontaneous muscle contraction in a majority of wild type guts. However, we failed to see peristaltic contractions of the midgut in any dAMPKαΔ39 mutant guts imaged (Figure 4E and Supplementary movies). In both genotypes, addition of 50 mM KCl to the bath resulted in a rapid contraction followed by a prolonged relaxation, and, in many wild type guts, the resumption of spontaneous contractions.
AMPK activity in muscle is required for normal gut function
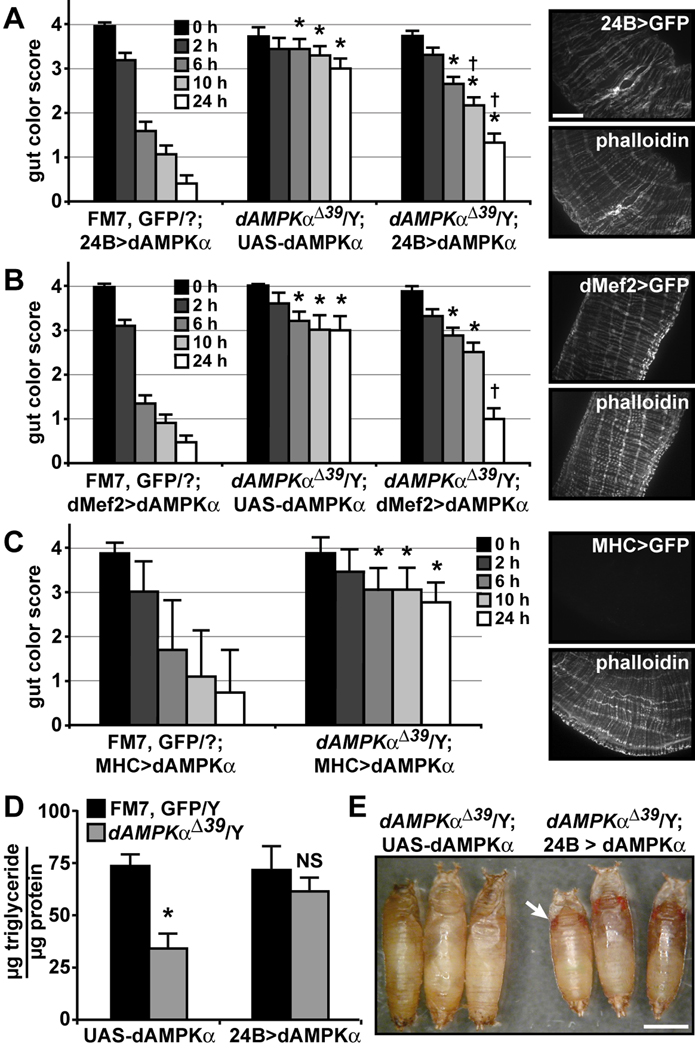
The impaired gut function in dAMPKαΔ39 mutants led to the hypothesis that the growth and metabolic defects observed in these animals stemmed from decreased nutrient uptake rather than cell-autonomous defects in tissues such as the fat body. To test this hypothesis, we attempted to rescue the gut defect in a tissue-specific manner. We measured food transit in larvae with wild type dAMPKα transgene expression driven by different epithelial, fat body, muscle and neuronal GAL4 drivers (Table S2). dAMPKαΔ39 mutant gut function was rescued only when the dAMPKα transgene was expressed in somatic and visceral muscles with either 24B- or dMef2-GAL4 (Figure 5A and B). We confirmed that 24B- and dMef2-GAL4 specifically drove transgenes in visceral muscle and not in the gut epithelium, and we noted that 24B- but not dMef2-GAL4 also drove transgene expression in fat body, as previously described (Supplementary Figure S5) (Kapahi et al., 2004). We were unable to rescue the gut phenotype using another muscle driver, MHC-GAL4; however, MHC-GAL4 drove little if any transgene expression in visceral muscles, despite driving expression in somatic muscles (Figure 5C). Based on these experiments, we conclude that the rescue of dAMPKα mutant gut function is unlikely to be due to expression of UAS-dAMPKα in the somatic musculature.
Figure 5. Muscle-specific expression of dAMPKα rescues dAMPKαΔ39 phenotypes.
A–C) Visceral muscle function was tested using the blue food transit assay. Expression of wild type dAMPKα in muscle using 24B- (A) or dMef2-GAL4 (B) rescued the dAMPKαΔ39 phenotype (compare the center set of bars: dAMPKαΔ39 with UAS-dAMPKα alone, to the right set of bars: dAMPKαΔ39 with GAL4 > dAMPKα). C) MHC-GAL4 was unable to rescue the dAMPKαΔ39 gut transit phenotype. For A–C: FM7, GFP/Y, n = 23–24; dAMPKαΔ39/Y; UAS-dAMPKα, n = 5–7; and dAMPKαΔ39/Y; GAL4 > dAMPKα, n = 18–37. Data are presented as mean ± s.d., *p < 0.01 vs. FM7, GFP/Y, †p < 0.01 vs. dAMPKαΔ39/Y; UAS-dAMPKα. 24B-, dMef2-, and MHC-GAL4-driven GFP expression in visceral muscle (top panels) and phalloidin counter-staining (bottom panels) are shown to the right of each graph. D) Triglyceride levels in 120 h AEL FM7, GFP/Y (black bars) and dAMPKαΔ39/Y larvae (gray bars) with UAS-dAMPKα alone (FM7, GFP, n = 6, dAMPKαΔ39, n = 4) or 24B > dAMPKα (FM7, GFP, n = 8, dAMPKαΔ39, n = 11). Data are presented as mean ± s.d., *p < 0.01 and NS, not significant, vs. FM7, GFP/Y. E) dAMPKαΔ39 mutants (left) died after pupariation, while 24B-GAL4-rescued dAMPKαΔ39 mutants (right) underwent metamorphosis, as indicated by their red eyes (arrow), scale bar = 1 mm.
We further investigated the phenotype of dAMPKαΔ39 mutants rescued with 24B-GAL4 driving UAS-dAMPKα and found that triglyceride content was restored in these animals relative to dAMPKαΔ39 larvae with UAS-dAMPKα alone (Figure 5D). Furthermore, expression of UAS-dAMPKα in muscle and fat body using 24B-GAL4 rescued early pupal lethality and permitted the completion of metamorphosis in 49% of dAMPKαΔ39 mutants (Figure 5E and Supplementary Figure S6). While most 24B>dAMPKα-rescued dAMPKαΔ39 flies were unable to eclose, those that did eclose survived for up to four days as adults. In contrast, expression of UAS-dAMPKα in muscle alone using dMef2-GAL4 permitted rescue of pupal lethality, but not eclosion, in 9% of dAMPKαΔ39 mutants, indicating that fat body expression of dAMPKα is not required for the completion of metamorphosis (Supplementary Figure S6).
Mechanism for impaired food transit in AMPK mutant guts
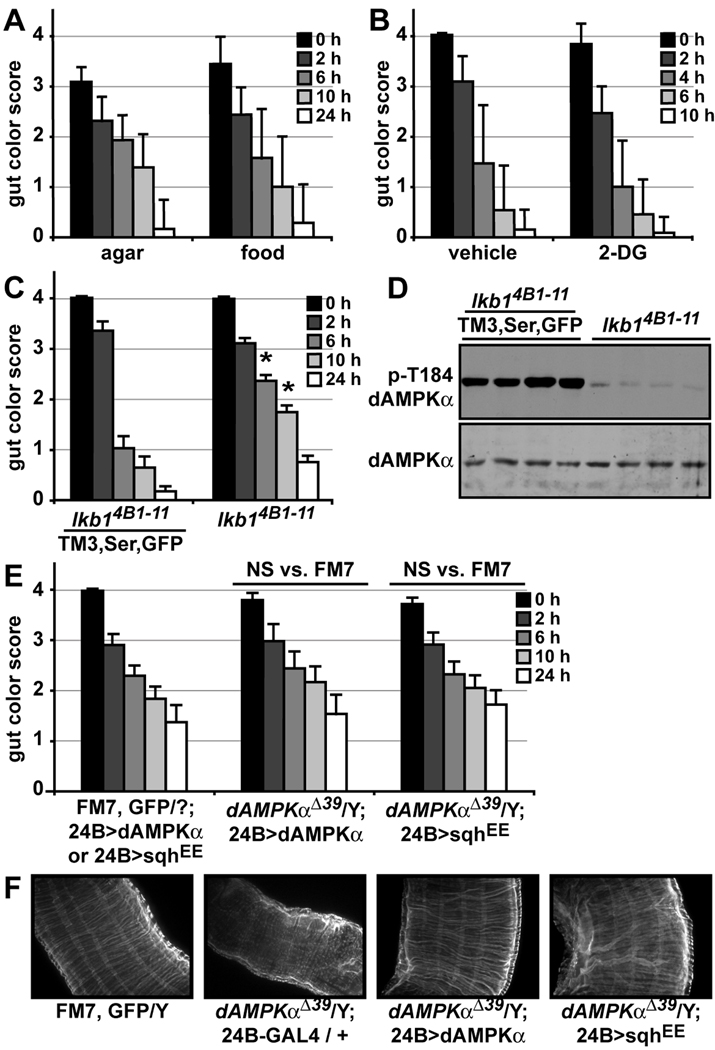
We sought to determine the mechanism by which dAMPK regulates visceral muscle function. We considered the possibility that dAMPK might be sensing the nutrient or energetic content of food and tuning muscle activity to promote maximal absorption of energetically-rich foods. To test this hypothesis, we fed wild type larvae nutrient-rich or nutrient-poor food and measured gut transit. Larvae displayed no differences in movement of either rich, cornmeal/molasses food or nutrient-free agar through their guts (Figure 6A). Furthermore, addition of 2-DG to food also had no effect on gut transit (Figure 6B). In energetically-stressed cells, the serine-threonine kinase LKB1 phosphorylates and activates AMPK (Hawley et al., 2003; Shaw et al., 2004; Woods et al., 2003). Unlike dAMPKα mutants, however, late third instar lkb14B1–11 mutants exhibited normal gut function (Figure 6C) (Martin and St Johnston, 2003). We asked whether AMPK was active in lkb1 mutants by measuring dAMPKα phosphorylation in these animals. We found low but detectable phospho-dAMPKα levels in lysates from late third instar lkb14B1–11 mutants, indicating that AMPK activity may be preserved in these animals (Figure 6D). Together, these results suggest that AMPK may not be playing an energy-sensing role per se in its regulation of gut function, but rather may be transmitting a signal initiated by an unknown upstream regulatory molecule such as a neuropeptide.
Figure 6. Expression of activated MRLC in muscle rescues the dAMPKαΔ39 gut phenotype.
A, B) Visceral muscle function in 96 h AEL wild type larvae was tested using the blue food transit assay. Data are presented as mean ± s.d. A) Larvae were fed 1% agar for 22 h, followed by 4 h of blue-dyed 1% agar (n = 9) or cornmeal/molasses food (n = 14). B) Larvae were fed 10 mM glucose and 1% yeast extract in blue-dyed 1% agar with vehicle (n = 13) or 50 mM 2-DG (n = 11). C) Visceral muscle function in lkb14B1–11/TM3, Ser, GFP (n = 34) and lkb14B1–11 (n = 52) larvae. Data are presented as mean ± s.e.m., *p < 0.05 vs. lkb14B1–11/TM3, Ser, GFP. D) Western blot analysis of phosphorylated (p-Thr184, top) and total dAMPKα (bottom) in lysates from lkb14B1–11/TM3, Ser, GFP and lkb14B1–11 120 h AEL larvae. E) 24B-GAL4 driven expression of an activated MRLC transgene (sqhEE) provided a partial rescue of the dAMPKαΔ39 gut transit phenotype. Compare the middle set of bars: dAMPKαΔ39 with 24B > dAMPKα (n = 13), to the right set of bars: dAMPKαΔ39 with 24B > sqhEE (n = 15). Data are presented as mean ± s.e.m., NS, not significant, vs. FM7, GFP/Y. F) Whole-mount phalloidin labeling showing circular and longitudinal visceral muscle fibers in FM7, GFP/Y, dAMPKαΔ39/Y; 24B-GAL4 / +, dAMPKαΔ39/Y; 24B > dAMPKα and dAMPKαΔ39/Y; 24B > sqhEE third instar midguts.
We next turned to the question of what molecules function downstream of AMPK in visceral muscle. In mammals, AMPK inhibits the Target of Rapamycin (TOR) pathway via phosphorylation of the pathway members TSC2 and raptor, leading to decreased S6K phosphorylation (Gwinn et al., 2008; Inoki et al., 2003). However, we observed equivalent levels of dS6K phosphorylation in wild type and dAMPKαΔ39 mutant guts (Supplementary Figure S7A). Furthermore, genetic manipulation of the TOR pathway by expression of TSC1 and TSC2 or dominant negative TOR in muscle failed to rescue the dAMPKαΔ39 gut transit phenotype (Supplementary Figure S7B and C) (Hennig and Neufeld, 2002; Tapon et al., 2001). Mammalian AMPK phosphorylates and activates the transcription factor FOXO (Greer et al., 2007). However, expression of the constitutively active FOXO™ transgene in muscle also was unable to suppress the impaired food transit in dAMPKαΔ39 flies (Supplementary Figure S7D) (Junger et al., 2003). In Drosophila, AMPK regulates cell polarity in the embryonic epithelium by activating the myosin regulatory light chain (MRLC) orthologue spaghetti squash (sqh) via phosphorylation of two adjacent amino acids (Lee et al., 2007). We asked whether sqh might also play a role in the visceral musculature. Expression of a constitutively active UAS-sqhEE transgene (Corrigall et al., 2007) with the 24B-GAL4 muscle driver produced a rescue of dAMPKαΔ39 gut function that was equivalent to rescue with UAS-dAMPKα (Figure 6E). This transgene expresses a sqh protein containing the two critical sites of phosphorylation mutated to glutamate, thus mimicking the phosphorylated state. We assessed visceral muscle morphology in third instar larvae rescued with 24B-GAL4 driving UAS-dAMPKα or UAS-sqhEE using phalloidin labeling of muscle actin fibers. We found that the ragged, diminished appearance of the unrescued dAMPKαΔ39 visceral musculature was restored to wild type morphology in animals rescued with either transgene (Figure 6F).
Discussion
Nutrients are key determinants of body size. In Drosophila, nutrient-poor diets disrupt endoreplicative cell cycles, inhibit growth and delay metamorphosis (Britton and Edgar, 1998; Colombani et al., 2003). Fed dAMPKα mutants live in a nutrient-rich environment, yet they bear similarities to starved animals. They are small, exhibit delayed pupariation and have low triglyceride levels, despite the role of AMPK as an activator of fatty acid oxidation and a negative regulator of lipogenesis through such targets as ACC. Furthermore, while loss of dAMPKα mutant in the whole animal leads to reduced fat body cell size, loss of dAMPKα in clones of fat body cells or in the whole eye has no effect on cell or organ size, indicating that dAMPK serves as a cell-nonautonomous regulator of growth. We find that dAMPKα mutants are limited in their ability to store nutrients and grow because they fail to move food through their guts efficiently, leading to decreased nutrient absorption through the digestive tract.
Normal gut function requires contributions from polarized epithelial cells that absorb nutrients from food and muscle cells that grind and mix food and move it through the gut. In the mammalian intestine, maintenance of epithelial cell polarity contributes to the establishment of a protective barrier against the environment and to the digestion of nutrients via maintenance of electrochemical gradients for transport and appropriate localization of transporters. Maintenance of epithelial polarity is essential for gut function; flies lacking the gene Gp93 display altered gut epithelial polarity and exhibit a stunted-growth phenotype that resembles effects of starvation (Maynard et al., 2010). Given the established role of dAMPK as a regulator of cell polarity (Lee et al., 2007; Mirouse et al., 2007), we examined the subcellular localization of proteins in normal and energetically-stressed gut epithelia. We find appropriate distribution of basolateral, lateral and apical proteins as well as an apical brush border throughout dAMPKα mutant gut epithelia, indicating that AMPK is not required for maintenance of epithelial polarity in the gut, as is also the case in the Drosophila eye (Amin et al., 2009). We also observe an increased number of vacuoles in the dAMPKα mutant midgut epithelia. This may reflect a compensatory increase in the digestive capacity of these cells. Indeed, microarray analysis shows increased expression of transcripts encoding digestive enzymes such as proteases, lipases and amylases in dAMPKα mutant guts compared with wild type (MLB and MJB, unpublished data).
In Drosophila, AMPK acts in visceral muscle to promote peristalsis, thereby enhancing nutrient intake and supporting growth of the whole animal. Our data show that in Drosophila, as in mammals, AMPK positively regulates muscle function (Hutber et al., 1997; Minokoshi et al., 2002; Mu et al., 2001). Furthermore, by acting in visceral muscle to promote nutrient intake, dAMPK fulfills a physiological role that is mechanistically distinct from but functionally analogous to mammalian AMPK in the hypothalamus. In mice, activation of hypothalamic AMPK stimulates food intake (Kubota et al., 2007; Minokoshi et al., 2004). Our results demonstrate that AMPK performs an ancient, conserved role by acting as an important nonautonomous determinant of nutrient supply in flies and mammals.
Expression of wild type dAMPKα in muscle restores gut function and nutrient storage and permits survival through metamorphosis in dAMPKα mutants. Surprisingly, muscle-rescued dAMPKα mutants do not exhibit overgrowth phenotypes consistent with activation of the dTOR pathway, despite the negative regulation of mTOR by AMPK in mammalian cells (Gwinn et al., 2008; Inoki et al., 2003) and the dominant growth-promoting role played by dTOR in flies (reviewed in Edgar, 2006). Therefore, while we cannot exclude that dAMPK inhibits dTOR during energy stress, at this point there is no evidence for a role of AMPK as an important basal regulator of TOR in vivo in flies. Our results indicate that regulation of visceral muscle function is the essential role of dAMPK during the larval stage of development.
Visceral muscle function and morphology are restored in whole-body dAMPKα mutants by expression of a sqh transgene in which Thr21 and Ser22 are replaced by phosphorylation-mimicking glutamate residues. Expression of sqhEE in visceral muscle may improve dAMPKα mutant muscle morphology by promoting muscle contraction and thereby preventing atrophy or by correcting morphology and thereby restoring function; our data do not distinguish between these possibilities. An activated sqh transgene also rescues cell polarity defects in dAMPKα-null embryos (Lee et al., 2007), indicating that sqh acts downstream of or parallel to AMPK in multiple contexts in Drosophila. In mammals, the sqh orthologue MRLC promotes smooth muscle contraction downstream of calcium signaling and myosin light chain kinase (MLCK) activation and G-protein coupled receptor signaling to Rho and Rok (Somlyo and Somlyo, 2003). Additionally, AMPK regulates vascular smooth muscle contraction in mammals (Goirand et al., 2007). The relationship between AMPK and MRLC in smooth muscle may be complex, though most data indicate that AMPK acts upstream of MRLC, either directly or indirectly through phosphorylation of MLCK (Bultot et al., 2009; Horman et al., 2008; Lee et al., 2007). Our data are consistent with AMPK serving as a direct or indirect upstream regulator of MRLC or with AMPK acting in a pathway parallel to MRLC.
The signals that activate AMPK in visceral muscle remain obscure. We do not observe energy-dependent effects of food quality on gut function in Drosophila larvae. Another possibility is that sustained nutrient absorption and visceral muscle contraction in the larval gut lead to high rates of ATP consumption, decreased energy charge and AMPK activation. Indeed, the metabolic activity of the digestive tract is estimated to account for 20% of whole-body oxygen consumption in mammals (Cant et al., 1996). However, the rescue of dAMPKαΔ39 gut function with the activated MRLC transgene suggests that AMPK performs a regulatory role in visceral muscle and not that the gut fails in dAMPKα mutants due to ATP depletion. Furthermore, whole-body lkb14B1–11 mutants do not exhibit the gut transit phenotype, despite low levels of dAMPKα phosphorylation. It may be that basal AMPK activity is sufficient to promote visceral muscle function in lkb14B1–11 mutants. Another possibility is that neuropeptides or neurotransmitters released by the stomatogastric nervous system might activate AMPK through kinases other than LKB1 and promote contraction (Nassel, 2002). Many Drosophila neuropeptides signal through Gq-coupled receptors, and this class of receptors activates AMPK in mammalian cells (Stahmann et al., 2006; Zhang et al., 2008).
The Drosophila AMPK mutant phenotype bears a striking resemblance to the human disease chronic idiopathic intestinal pseudo-obstruction (CIPO) (Stanghellini et al., 2007). CIPO patients exhibit decreased intestinal mobility due to muscular or neural defects that impairs digestion, leading to severe malnutrition and, in many cases, lethality. Although mutations in genes encoding AMPK subunits have not been reported in CIPO patients, it will be of interest to determine whether AMPK or its upstream regulators might play a role in the progression of this disease and other visceral myopathies. If so, drugs that activate AMPK such as the commonly-prescribed, anti-diabetic metformin might be useful in treating these devastating conditions.
We have shown that in Drosophila, AMPK plays an essential role in regulating nutrient intake and supporting growth by acting in the visceral musculature to promote peristalsis. Our results identify a novel cell-nonautonomous function for AMPK in an invertebrate and underscore the importance of gut function in the regulation of organismal growth.
Supplementary Material
Acknowledgements
We thank Graeme Hardie for the phospho-ACC antibody, Daniel Kiehart for the MHC antibody, Jay Brenman, Daniel St Johnston, Franck Pichaud, Eric Olson, Thomas Neufeld and Andrew Arsham for fly stocks, Ed Williamson for gut histology, Melody Esmaeili for technical assistance and members of the Birnbaum laboratory for discussions. Supported by NRSA F32-DK068981 to MLB and NIH Grant R01-DK56886 to MJB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amin N, Khan A, St Johnston D, Tomlinson I, Martin S, Brenman J, McNeill H. LKB1 regulates polarity remodeling and adherens junction formation in the Drosophila eye. Proc Natl Acad Sci U S A. 2009;106:8941–8946. doi: 10.1073/pnas.0812469106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann O. Posterior midgut epithelial cells differ in their organization of the membrane skeleton from other drosophila epithelia. Exp Cell Res. 2001;270:176–187. doi: 10.1006/excr.2001.5343. [DOI] [PubMed] [Google Scholar]
- Bayliss WM, Starling EH. The movements and innervation of the small intestine. J Physiol. 1899;24:99–143. doi: 10.1113/jphysiol.1899.sp000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton JS, Edgar BA. Environmental control of the cell cycle in Drosophila: nutrition activates mitotic and endoreplicative cells by distinct mechanisms. Development. 1998;125:2149–2158. doi: 10.1242/dev.125.11.2149. [DOI] [PubMed] [Google Scholar]
- Britton JS, Lockwood WK, Li L, Cohen SM, Edgar BA. Drosophila's insulin/PI3-kinase pathway coordinates cellular metabolism with nutritional conditions. Dev Cell. 2002;2:239–249. doi: 10.1016/s1534-5807(02)00117-x. [DOI] [PubMed] [Google Scholar]
- Bultot L, Horman S, Neumann D, Walsh MP, Hue L, Rider MH. Myosin light chains are not a physiological substrate of AMPK in the control of cell structure changes. FEBS Lett. 2009;583:25–28. doi: 10.1016/j.febslet.2008.11.022. [DOI] [PubMed] [Google Scholar]
- Cant JP, McBride BW, Croom WJ., Jr The regulation of intestinal metabolism and its impact on whole animal energetics. J Anim Sci. 1996;74:2541–2553. doi: 10.2527/1996.74102541x. [DOI] [PubMed] [Google Scholar]
- Church RB, Robertson FW. Biochemical analysis of genetic differences in the growth of Drosophila. Genet Res. 1966;7:383–407. doi: 10.1017/s0016672300009836. [DOI] [PubMed] [Google Scholar]
- Colombani J, Raisin S, Pantalacci S, Radimerski T, Montagne J, Leopold P. A nutrient sensor mechanism controls Drosophila growth. Cell. 2003;114:739–749. doi: 10.1016/s0092-8674(03)00713-x. [DOI] [PubMed] [Google Scholar]
- Corrigall D, Walther RF, Rodriguez L, Fichelson P, Pichaud F. Hedgehog signaling is a principal inducer of Myosin-II-driven cell ingression in Drosophila epithelia. Dev Cell. 2007;13:730–742. doi: 10.1016/j.devcel.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Davies SP, Sim AT, Hardie DG. Location and function of three sites phosphorylated on rat acetyl-CoA carboxylase by the AMP-activated protein kinase. Eur J Biochem. 1990;187:183–190. doi: 10.1111/j.1432-1033.1990.tb15293.x. [DOI] [PubMed] [Google Scholar]
- Edgar BA. How flies get their size: genetics meets physiology. Nat Rev Genet. 2006;7:907–916. doi: 10.1038/nrg1989. [DOI] [PubMed] [Google Scholar]
- Gleason CE, Lu D, Witters LA, Newgard CB, Birnbaum MJ. The role of AMPK and mTOR in nutrient sensing in pancreatic beta-cells. J Biol Chem. 2007;282:10341–10351. doi: 10.1074/jbc.M610631200. [DOI] [PubMed] [Google Scholar]
- Goirand F, Solar M, Athea Y, Viollet B, Mateo P, Fortin D, Leclerc J, Hoerter J, Ventura-Clapier R, Garnier A. Activation of AMP kinase alpha1 subunit induces aortic vasorelaxation in mice. J Physiol. 2007;581:1163–1171. doi: 10.1113/jphysiol.2007.132589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golic KG, Lindquist S. The FLP recombinase of yeast catalyzes site-specific recombination in the Drosophila genome. Cell. 1989;59:499–509. doi: 10.1016/0092-8674(89)90033-0. [DOI] [PubMed] [Google Scholar]
- Greer EL, Oskoui PR, Banko MR, Maniar JM, Gygi MP, Gygi SP, Brunet A. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem. 2007;282:30107–30119. doi: 10.1074/jbc.M705325200. [DOI] [PubMed] [Google Scholar]
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, Hardie DG. Complexes between the LKB1 tumor suppressor, STRADalpha/beta and MO25alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig KM, Neufeld TP. Inhibition of cellular growth and proliferation by dTOR overexpression in Drosophila. Genesis. 2002;34:107–110. doi: 10.1002/gene.10139. [DOI] [PubMed] [Google Scholar]
- Horman S, Morel N, Vertommen D, Hussain N, Neumann D, Beauloye C, El Najjar N, Forcet C, Viollet B, Walsh MP, Hue L, Rider MH. AMP-activated protein kinase phosphorylates and desensitizes smooth muscle myosin light chain kinase. J Biol Chem. 2008;283:18505–18512. doi: 10.1074/jbc.M802053200. [DOI] [PubMed] [Google Scholar]
- Hutber CA, Hardie DG, Winder WW. Electrical stimulation inactivates muscle acetyl-CoA carboxylase and increases AMP-activated protein kinase. Am J Physiol. 1997;272:E262–E266. doi: 10.1152/ajpendo.1997.272.2.E262. [DOI] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- Iwaki DD, Lengyel JA. A Delta-Notch signaling border regulated by Engrailed/Invected repression specifies boundary cells in the Drosophila hindgut. Mech Dev. 2002;114:71–84. doi: 10.1016/s0925-4773(02)00061-8. [DOI] [PubMed] [Google Scholar]
- Junger MA, Rintelen F, Stocker H, Wasserman JD, Vegh M, Radimerski T, Greenberg ME, Hafen E. The Drosophila forkhead transcription factor FOXO mediates the reduction in cell number associated with reduced insulin signaling. J Biol. 2003;2:20. doi: 10.1186/1475-4924-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehart DP, Feghali R. Cytoplasmic myosin from Drosophila melanogaster. J Cell Biol. 1986;103:1517–1525. doi: 10.1083/jcb.103.4.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota N, Yano W, Kubota T, Yamauchi T, Itoh S, Kumagai H, Kozono H, Takamoto I, Okamoto S, Shiuchi T, Suzuki R, Satoh H, Tsuchida A, Moroi M, Sugi K, Noda T, Ebinuma H, Ueta Y, Kondo T, Araki E, Ezaki O, Nagai R, Tobe K, Terauchi Y, Ueki K, Minokoshi Y, Kadowaki T. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab. 2007;6:55–68. doi: 10.1016/j.cmet.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Lee G, Park JH. Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics. 2004;167:311–323. doi: 10.1534/genetics.167.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Koh H, Kim M, Kim Y, Lee SY, Karess RE, Lee SH, Shong M, Kim JM, Kim J, Chung J. Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature. 2007;447:1017–1020. doi: 10.1038/nature05828. [DOI] [PubMed] [Google Scholar]
- Martin SG, St Johnston D. A role for Drosophila LKB1 in anterior-posterior axis formation and epithelial polarity. Nature. 2003;421:379–384. doi: 10.1038/nature01296. [DOI] [PubMed] [Google Scholar]
- Maynard JC, Pham T, Zheng T, Jockheck-Clark A, Rankin HB, Newgard CB, Spana EP, Nicchitta CV. Gp93, the Drosophila GRP94 ortholog, is required for gut epithelial homeostasis and nutrient assimilation-coupled growth control. Dev Biol. 2010;339:295–306. doi: 10.1016/j.ydbio.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- Mirouse V, Swick LL, Kazgan N, St Johnston D, Brenman JE. LKB1 and AMPK maintain epithelial cell polarity under energetic stress. J Cell Biol. 2007;177:387–392. doi: 10.1083/jcb.200702053. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mu J, Brozinick JT, Jr, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell. 2001;7:1085–1094. doi: 10.1016/s1097-2765(01)00251-9. [DOI] [PubMed] [Google Scholar]
- Nassel DR. Neuropeptides in the nervous system of Drosophila and other insects: multiple roles as neuromodulators and neurohormones. Prog Neurobiol. 2002;68:1–84. doi: 10.1016/s0301-0082(02)00057-6. [DOI] [PubMed] [Google Scholar]
- Pan DA, Hardie DG. A homologue of AMP-activated protein kinase in Drosophila melanogaster is sensitive to AMP and is activated by ATP depletion. Biochem J. 2002;367:179–186. doi: 10.1042/BJ20020703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips MD, Thomas GH. Brush border spectrin is required for early endosome recycling in Drosophila. J Cell Sci. 2006;119:1361–1370. doi: 10.1242/jcs.02839. [DOI] [PubMed] [Google Scholar]
- Potter CJ, Huang H, Xu T. Drosophila Tsc1 functions with Tsc2 to antagonize insulin signaling in regulating cell growth, cell proliferation, and organ size. Cell. 2001;105:357–368. doi: 10.1016/s0092-8674(01)00333-6. [DOI] [PubMed] [Google Scholar]
- Ranganayakulu G, Zhao B, Dokidis A, Molkentin JD, Olson EN, Schulz RA. A series of mutations in the D-MEF2 transcription factor reveal multiple functions in larval and adult myogenesis in Drosophila. Dev Biol. 1995;171:169–181. doi: 10.1006/dbio.1995.1269. [DOI] [PubMed] [Google Scholar]
- Schuster CM, Davis GW, Fetter RD, Goodman CS. Genetic dissection of structural and functional components of synaptic plasticity. II. Fasciclin II controls presynaptic structural plasticity. Neuron. 1996;17:655–667. doi: 10.1016/s0896-6273(00)80198-1. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- Stahmann N, Woods A, Carling D, Heller R. Thrombin activates AMP-activated protein kinase in endothelial cells via a pathway involving Ca2+/calmodulin-dependent protein kinase kinase beta. Mol Cell Biol. 2006;26:5933–5945. doi: 10.1128/MCB.00383-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanghellini V, Cogliandro RF, de Giorgio R, Barbara G, Salvioli B, Corinaldesi R. Chronic intestinal pseudo-obstruction: manifestations, natural history and management. Neurogastroenterol Motil. 2007;19:440–452. doi: 10.1111/j.1365-2982.2007.00902.x. [DOI] [PubMed] [Google Scholar]
- Stewart BA, Atwood HL, Renger JJ, Wang J, Wu CF. Improved stability of Drosophila larval neuromuscular preparations in haemolymph-like physiological solutions. J Comp Physiol [A] 1994;175:179–191. doi: 10.1007/BF00215114. [DOI] [PubMed] [Google Scholar]
- Stowers RS, Schwarz TL. A genetic method for generating Drosophila eyes composed exclusively of mitotic clones of a single genotype. Genetics. 1999;152:1631–1639. doi: 10.1093/genetics/152.4.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapon N, Ito N, Dickson BJ, Treisman JE, Hariharan IK. The Drosophila tuberous sclerosis complex gene homologs restrict cell growth and cell proliferation. Cell. 2001;105:345–355. doi: 10.1016/s0092-8674(01)00332-4. [DOI] [PubMed] [Google Scholar]
- Treitel MA, Kuchin S, Carlson M. Snf1 protein kinase regulates phosphorylation of the Mig1 repressor in Saccharomyces cerevisiae. Mol Cell Biol. 1998;18:6273–6280. doi: 10.1128/mcb.18.11.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdu J, Buratovich MA, Wilder EL, Birnbaum MJ. Cell-autonomous regulation of cell and organ growth in Drosophila by Akt/PKB. Nat Cell Biol. 1999;1:500–506. doi: 10.1038/70293. [DOI] [PubMed] [Google Scholar]
- Voght SP, Fluegel ML, Andrews LA, Pallanck LJ. Drosophila NPC1b promotes an early step in sterol absorption from the midgut epithelium. Cell Metab. 2007;5:195–205. doi: 10.1016/j.cmet.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D. LKB1 Is the Upstream Kinase in the AMP-Activated Protein Kinase Cascade. Curr Biol. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Woods A, Munday MR, Scott J, Yang X, Carlson M, Carling D. Yeast SNF1 is functionally related to mammalian AMP-activated protein kinase and regulates acetyl-CoA carboxylase in vivo. J Biol Chem. 1994;269:19509–19515. [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- Zhang J, Xie Z, Dong Y, Wang S, Liu C, Zou MH. Identification of nitric oxide as an endogenous activator of the AMP-activated protein kinase in vascular endothelial cells. J Biol Chem. 2008;283:27452–27461. doi: 10.1074/jbc.M802578200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.