Abstract
Currently the only treatment for coeliac disease is a lifelong gluten-free diet excluding food products containing wheat, rye and barley. There is, however, only scarce evidence as to harmful effects of rye in coeliac disease. To confirm the assumption that rye should be excluded from the coeliac patient's diet, we now sought to establish whether rye secalin activates toxic reactions in vitro in intestinal epithelial cell models as extensively as wheat gliadin. Further, we investigated the efficacy of germinating cereal enzymes from oat, wheat and barley to hydrolyse secalin into short fragments and whether secalin-induced harmful effects can be reduced by such pretreatment. In the current study, secalin elicited toxic reactions in intestinal Caco-2 epithelial cells similarly to gliadin: it induced epithelial cell layer permeability, tight junctional protein occludin and ZO-1 distortion and actin reorganization. In high-performance liquid chromatography and mass spectroscopy (HPLC-MS), germinating barley enzymes provided the most efficient degradation of secalin and gliadin peptides and was thus selected for further in vitro analysis. After germinating barley enzyme pretreatment, all toxic reactions induced by secalin were ameliorated. We conclude that germinating enzymes from barley are particularly efficient in the degradation of rye secalin. In future, these enzymes might be utilized as a novel medical treatment for coeliac disease or in food processing in order to develop high-quality coeliac-safe food products.
Keywords: Caco-2, coeliac disease, germinating cereal enzymes, innate immunity, permeability
Introduction
The unique composition of cereal prolamins in wheat, barley and rye renders them resistant to gastrointestinal proteolytic enzymes. This is due mainly to a high content of glutamine and proline residues which leads to incomplete degradation of these proteins during normal human digestion [1–3]. Such partial degradation is thought to be one crucial factor in the activation of the immune response in the small-bowel mucosa and the progression of coeliac disease in genetically susceptible people [2,4]. Although coeliac disease has been classified traditionally as a gluten-induced T cell-mediated autoimmune disorder, during the last few years gluten recognition in the mucosal epithelial layer and the role of innate immunity components such as interleukin (IL)-15 [5–9] have claimed increasing attention in discussion of the mechanisms underlying this disease. In particular, modulation of the epithelial barrier is now thought to be a key element in the increased small-intestinal permeability characteristic for coeliac disease [10–13].
Currently the only treatment for coeliac disease is avoidance of gluten prolamins (gliadin, hordein and secalin). Regardless of the fact that oats avenin belongs to the gluten prolamins, there is a large body of evidence for the safety of oat consumption [14–16]. Wheat gliadin has been proved to induce several toxic effects in coeliac disease [6,17–19]. Although rye and barley have also been found to contain several potentially harmful peptides [20,21], which may activate immune reactions in coeliac disease patients, there are only a few studies addressing this issue [22–27]. In practice, rye and barley are excluded from the gluten-free diet mainly in view of their protein homology to wheat.
Because gluten peptides remain fairly intact during gastrointestinal digestion, enzyme supplements have been proposed as novel therapeutic agents for coeliac disease [2,28–32]. Such supplementary proteases might ensure the complete breakdown of gluten epitopes in advance of their absorption in the small bowel. We have proposed previously that the whole mixture of enzymes isolated from germinating wheat – selected evolutionarily for total cleavage of storage proteins during the germination of kernels – are highly efficient in degrading and detoxifying gliadin peptides [33]. In addition to wheat, other cereals also contain a full range of different enzymes with the potential to accelerate gluten degradation. Interestingly, the proteolytic enzyme EP-B2, isolated from germinating barley seeds, has proved recently to be a particularly promising therapeutic tool for gluten detoxification [32,34–36].
To confirm the assumption that rye should be excluded from coeliac disease patients' diet, we investigated whether rye secalin activates toxic reactions in the small-bowel mucosal epithelial cells as extensively as wheat gliadin. Further, we tested the efficacy of germinating enzymes from oat, wheat and barley to hydrolyse secalin peptides and studied whether the harmful effects elicited by secalin can be reduced by such enzymatic pretreatment.
Materials and methods
Preparation of gliadin and secalin
Gliadin was extracted from wheat (Raisio Oy, Raisio, Finland) and secalin from rye flour (Myllyn Paras Oy, Hyvinkää, Finland). For cell culture experiments both were digested using pepsin (P-6887; Sigma-Aldrich, Seelze, Germany) and trypsin (T-7418, Sigma-Aldrich), as described previously [37]. To abolish the harmful effects exerted by pepsin and trypsin enzymes, the samples were heat-inactivated at >95°C for 10 min. Bovine serum albumin (BSA, A8806; Sigma-Aldrich) was treated similarly and served as a negative control for peptic–tryptic (PT) treatment.
Germination of cereals and comparison of their cleaving capacities
Oat, wheat and barley seeds were germinated in a pilot malting apparatus (Joe White Malting System, Melbourne, Australia) until the most efficient activation of germinating enzymes was achieved at day 8 (data not shown). Germinated grains were isolated and homogenized as described previously [33]. To compare the prolamin degrading capacities of germinating oat, wheat and barley enzymes, crude gliadin and secalin (1 mg/ml) were incubated for 24 h at 37°C in 50 mM Na-acetate buffer, pH 4·0 containing different concentrations of germinated cereal enzymes (0·1–100 µg/ml). The degradation products were analysed by high-performance liquid chromatography and mass spectroscopy (HPLC-MS) and sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE), as described below.
To study toxic in vitro reactions in intestinal epithelial cells, secalin (6 mg/ml) was incubated similarly with the most effective germinated cereal enzyme preparation (0·3 mg/ml) found during the study and digested with pepsin and trypsin as above.
HPLC-MS and SDS-PAGE of PT–secalin and enzymatically pretreated PT–secalin
HPLC-MS was carried out as described previously [33] using a linear trap quadrupole (LTQ) ion trap mass spectrometer connected to a Surveyor HPLC-MS system (Finnigan, San Jose, CA, USA). To compare the prolamin-cleaving capacity of different germinated grains, several representative m/z signals of full-length gliadin and secalin were selected and their disappearance followed after incubation with increasing concentrations (0·1–100 µg/ml) of grain enzymes. The sum of the signals selected was plotted against enzyme concentration and the data fitted in a standard sigmoidal dose–response curve. Half-maximal effective concentration (EC50) values were calculated from these curves, which give an approximation of the enzyme concentration needed to reduce the amount of full-length prolamin by 50%.
The degradation of barley enzyme-pretreated PT–secalin was monitored using similar HPLC-MS conditions. The size of the peptides formed was approximated by retention times of α-gliadin peptides 12-mer (QLQPFPQPQLPY; New England Peptide, Fitchburg, MA, USA) and 33-mer (LQLQPFPQPQLPYPQPQLPYPQPQLPYPQPQPF; New England Peptide), which have been demonstrated to be highly resistant to human digestive enzymes [2,3,38]. Secalin was incubated with or without germinating oat, wheat or barley as described above, whereafter the reaction was stopped by heating (>95°C, 10 min). Subsequently, 2 µl samples were subjected to standard SDS-PAGE analysis on 12% gel.
Cell cultures
Caco-2 cells (passages 19–70, HTB-37; American Type Culture Collection, Rockville, MD, USA) were grown under standard cell culture conditions, maintained in minimum essential medium (Gibco Invitrogen, Paisley, UK) supplemented with 20% fetal bovine serum (FBS; Gibco Invitrogen), 50 U/ml penicillin–streptomycin (Gibco Invitrogen), 1 mM sodium pyruvate (Sigma-Aldrich), 1·5 g/l sodium bicarbonate (Gibco Invitrogen) and 0·1 mM non-essential amino acids (Gibco Invitrogen). The cells were passaged twice a week upon reaching 80% confluence. Before experiments, the culture medium was replaced with starvation media containing 1% fetal bovine serum (FBS), penicillin–streptomycin, sodium pyruvate and non-essential amino acids.
Epithelial cell layer permeability
Caco-2 cells were plated on Millicell Culture (Millipore Corporate, Billerica, MA, USA) and cultured until reaching confluency, measured by Millicell-ERS volt-ohm meter (Millipore Corporate). Once transepithelial resistance (TER) reached 1000 ohms × cm2, cells were starved overnight and challenged with PT–SA, PT–gliadin, PT–secalin or enzymatically pretreated PT–secalin in a concentration of 1 mg/ml. Thereafter, the recovery of TER was measured once an hour until reaching baseline level within 6 h. The experiments were performed independently in duplicate at least six times.
Immunofluorescence stainings
Expression of the tight junction-associated proteins occludin and ZO-1 was investigated in confluent Caco-2 cell layers, cultured on Transwell polyester membrane inserts (Millipore Corporate) for 5–7 days. The cells were then starved overnight and supplemented with PT-digested BSA, gliadin, secalin or enzymatically pretreated secalin and challenged for 24 h. For stainings, samples were fixed with 4% paraformaldehyde, permeabilized and incubated with mouse monoclonal anti-occludin antibody (1:100; Zymed, San Francisco, CA, USA) or mouse anti-ZO-1 antibody (1:100; Zymed) for 60 min. AlexaFluor 568 goat anti-mouse immunoglobulin (Ig)G (1:1000; Invitrogen, Carlsbad, CA, USA) was used as secondary antibody for 30 min. Stainings were visualized with an Olympus BX60 microscope (Olympus, Hamburg, Germany).
To study actin reorganization, Caco-2 cells were plated onto eight-chamber glass slides (BD Biosciences, Erembodegem, Belgium) and cultured as described previously [33,37]. The cells were then starved and incubated in the presence of study compounds as above. Intracellular F-actin was stained with phalloidin–fluorescein isothiocyanate (1:300; Sigma-Aldrich). The extent of actin cytoskeleton rearrangement was quantified by measuring the cellular edge covered by membrane ruffles as a percentage of the total length of at least 60 different cell clusters in three independent experiments. Calculations were made with analySIS software (Olympus Soft Imaging System GmbH, Munster, Germany).
Statistical analysis
Results are given as means and standard error of means. The two-tailed Mann–Whitney U-test was used to assess the statistical significance of differences. P-values lower than 0·05 were considered significant.
Results
Comparison of enzymatic degradation of gliadin and secalin
The cleaving capacities of germinating oat, wheat and barley enzymes were compared by incubating gliadin and secalin with several concentrations of enzyme preparations. When calculating the enzyme amounts needed to reduce the level of full-length prolamin by 50% (Table 1), it appeared that the barley enzymes degrade both gliadin and secalin more efficiently than oat and wheat enzymes, even though the differences in EC50 values between the enzyme preparations are small. This is evidenced by Fig. 1, showing the peptide profiles of gliadin (Fig. 1a) and secalin (Fig. 1b) after incubation with germinating grain enzymes. Finally, the SDS-PAGE gel of secalin pretreated with oat, wheat or barley enzymes illustrates clearly the superiority of barley enzymes in secalin degradation (Fig. 1c). Of note, heat-inactivated barley enzymes were not able to cleave secalin (Fig. 1c).
Table 1.
Efficiency of gliadin and secalin degradation by germinated oat, wheat and barley enzyme preparations.
| EC50 (µg/ml) |
||
|---|---|---|
| Enzyme preparation | Gliadin | Secalin |
| Oat | 4·3 (3·1–5·8) | 1·3 (1·2–1·4) |
| Wheat | 2·6 (2·0–3·4) | 1·0 (0·9–1·1) |
| Barley | 2·3 (1·6–3·2) | 0·7 (0·3–1·5) |
Half-maximal effective concentration (EC50) value (µg/ml) represents the mean of enzyme preparation concentrations, needed to reduce the amount of full-length prolamin (1 mg/ml) by 50%. The data are derived from three independent experiments; 95% confidence limits are included in parenthesis.
Fig. 1.
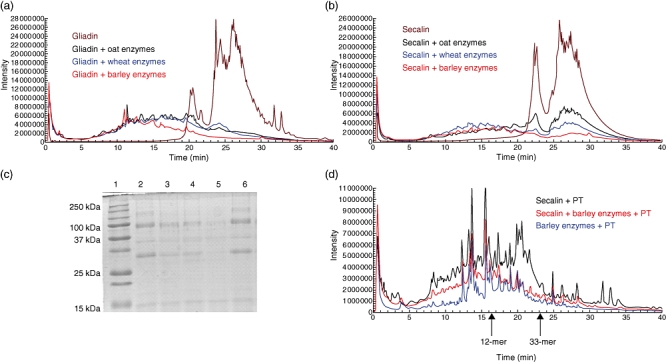
Enzymatic degradation of gliadin and secalin analysed by high-performance liquid chromatography and mass spectroscopy (HPLC-MS) and sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE). HPLC-MS elution profiles of gliadin (a) and secalin (b) (both 6 mg/ml) incubated with 0·3 mg/ml of oat, wheat and barley enzyme preparations. Full range m/z = 480–2000. (c) SDS-PAGE analysis of secalin (1 mg/ml) degraded by germinated grain preparations (1 µg/ml). Lane 1 = size standard, lane 2 = secalin, lane 3 = secalin + germinating oat enzymes, lane 4 = secalin + germinating wheat enzymes, lane 5 = secalin + germinating barley enzymes, lane 6 = secalin + heat inactivated germinating barley enzymes. (d) HPLC-MS elution profiles of pepsin–trypsin (PT)-digested secalin (black line), PT–secalin pretreated with germinating barley enzymes (red line) and germinating barley enzymes + PT (blue line). Full range m/z = 480–2000. Retention times of the standard peptides 12-mer (16·5 min) and 33-mer (23·3 min) are shown by arrows.
Because germinating barley enzymes proved most efficacious in degrading prolamins, this enzyme mixture was selected for further experiments. Sequential digestion of PT–secalin with germinating barley enzymes was studied by HPLC-MS analysis (Fig. 1d). Pretreatment of PT–secalin with barley enzymes (red line) resulted in much more efficient secalin degradation than PT–digestion alone (black line). After enzymatic pretreatment, most of the long peptides eluting after 15 min had disappeared, leaving only cleavage products of the pepsin, trypsin and barley enzyme preparation (blue line). Examination of the remaining peptide products by comparison to the standard toxic α-gliadin peptides 12-mer (p57–68) and 33-mer (p56–88) eluted at 16·5 and 23·3 min, showed that germinating barley enzymes efficiently hydrolysed coeliac toxic peptides, as only scant short fragments were left (Fig. 1d).
In vitro toxic reactions in intestinal epithelial cells
Culture media exchange caused an immediate drop of TER in all cell cultures, as described elsewhere [33,39]. However, TER began to recover and reached baseline level in control cultures after 6 h (Fig. 2). In contrast, TER in cultures challenged with PT–secalin failed to recover to the baseline levels similarly to those treated with the positive control, PT–gliadin (Fig. 2). When PT–secalin was pretreated with germinating barley enzymes, the TER recovery paralleled that of negative control cultures supplemented with PT–BSA.
Fig. 2.
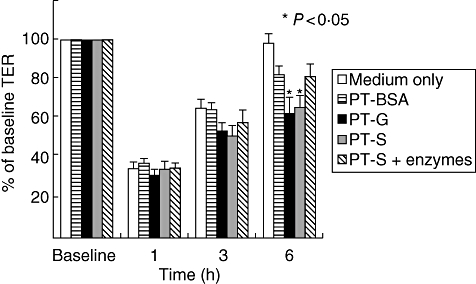
Transepithelial resistance (TER) in Caco-2 cells cultured with medium only or challenged with pepsin–trypsin-digested bovine serum albumin (PT–BSA), pepsin–trypsin-digested gliadin (PT–G), pepsin–trypsin-digested secalin (PT–S) or germinating barley enzyme-pretreated PT–S (PT–S + enzymes) at different time-points. Data are given as mean percentages of baseline TER values ± standard error of the mean derived from six independent experiments performed in duplicate. Statistical analyses by two-tailed Mann–Whitney U-test, where P < 0·05 (*) was considered statistically significant.
This altered barrier function induced by PT–secalin was accompanied by modulation of distinct tight junctional protein expression. In cultures supplemented with either PT–secalin or PT–gliadin the normal curly appearance of occludin and its associated protein ZO-1 expression was altered and the tight junctions were straightened (Fig. 3a and b). In addition, PT–secalin or PT–gliadin-treated cultures showed marked disruption of the occludin network (Fig. 3a). The pretreatment of PT–secalin with germinating barley enzymes was able to circumvent these harmful effects (Fig. 3a and b).
Fig. 3.
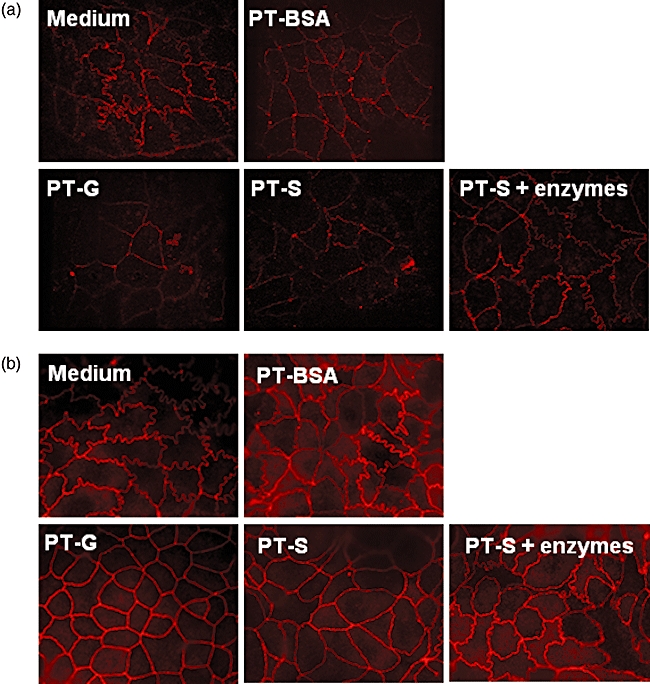
The appearance of tight junctions in Caco-2 cells cultured with medium only, pepsin–trypsin-digested bovine serum albumin (PT–BSA), pepsin–trypsin-digested gliadin (PT–G), pepsin–trypsin-digested secalin (PT–S) or enzymatically pretreated pepsin–trypsin-digested secalin (PT–S + enzymes). Immunofluorescent staining of occludin (a) and ZO-1 (b). Magnification 100×. Pictures are representative images from experiments performed in duplicate three independent times.
In another in vitro model for gliadin toxicity, addition of PT–secalin induced as extensive membrane ruffles at the edge of the Caco-2 cell layer as PT–gliadin treatment (Fig. 4a and b). This toxic effect of PT–secalin was, however, abolished when PT–secalin was pretreated with germinating barley enzymes. These enzymes themselves had no effects on TER, tight junctional protein expression or actin cytoskeleton rearrangement (data not shown).
Fig. 4.
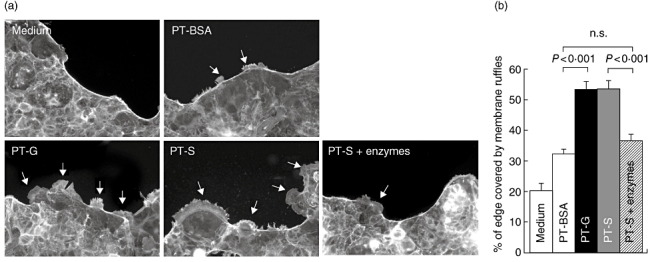
Actin cytoskeleton rearrangement as membrane ruffling in Caco-2 cells. (a) Representative images of actin membrane ruffling (arrows) in Caco-2 cells cultured with medium only, pepsin–trypsin-digested bovine serum albumin (PT–BSA), pepsin–trypsin-digested gliadin (PT–G), pepsin–trypsin-digested secalin (PT–S) or germinating barley enzyme-pretreated PT–S (PT–S + enzymes). Magnification 20×. (b) Quantitation of ruffle formation was measured as percentage of total cell layer edge length. Data are given as mean values ± standard error of the mean derived from three independent experiments performed in duplicate. Statistical analyses by two-tailed Mann–Whitney U-test, where P < 0·05 was considered statistically significant; n.s.: not significant.
Discussion
In this study we have demonstrated that PT–secalin exerts toxic effects similar to those of PT–gliadin in all intestinal epithelial cell culture experiments in vitro: it affected epithelial barrier function by increasing cell monolayer permeability, altered tight junctional protein occludin and ZO-1 expressions and induced extensive actin reorganization at the edges of intestinal epithelial cell clusters. These observations would indicate that secalin contains epitopes which are also able to activate innate immunity pathways in addition to stimulating the T cell-mediated adaptive immunity reactions shown in previous studies [23,27]. These results support the earlier case reports in which it was observed that coeliac disease patients developed symptoms and small-bowel mucosal histological alterations after ingestion of rye [24–26]. Similarly, Kieffer and coworkers [22] demonstrated that coeliac patient serum samples contained antibodies which recognize rye and barley in addition to wheat, suggesting that the immunological response of coeliac disease patients can be activated by all these cereals, including rye.
During recent years, discussion has arisen of alternative therapeutic strategies for coeliac disease. Several external enzymes have been proposed to detoxify gluten epitopes [2,31,34,40]. However, the complexity of the wheat gliadin molecule has made the procedure a very challenging task [28,41]. For the full cleavage of gluten peptides a combination of different enzymes, rather than one specific protease, is probably needed [32,35,42]. To this end, we have introduced previously a natural means of gluten detoxification using a whole mixture of wheat own enzymes, intended evolutionarily for the total digestion of wheat storage proteins during the germination of wheat kernels [33]. In the study in question, germinating wheat proteases cleaved gliadin peptides efficiently into short fragments, whereafter the toxicity of gliadin was diminished markedly in different in vitro models. Similarly, in the current study, germinating cereal enzymes proved particularly powerful in hydrolysing secalin. However, instead of using germinating wheat enzymes, we now compared the efficacy of three different cereal (oat, wheat and barley) enzymes to achieve the maximum cleavage of secalin peptides. Of these, barley enzymes were superior in cleaving gliadin and secalin (Table 1, Fig. 1). Furthermore, when testing the toxic reactions of PT–secalin pretreated with germinating barley enzymes we observed diminished toxicity in all Caco-2 cell culture experiments compared to unprocessed PT–secalin.
In the current study we used the Caco-2 cell line as a model to investigate the toxicity of gluten-containing cereals. As Caco-2 cells originate from the colon, they have some limitations when studying coeliac disease. For example, it has been published that Caco-2 cells do not express a wide variety of different proinflammatory cytokines [43]. IL-15 is a central cytokine in coeliac disease-related innate immune reactions [5,7–9]; however, it is secreted poorly in the cultured intestinal cells as well as organ culture biopsy samples of coeliac disease patients, as shown in previous studies [5,9]. Similarly, we were unable to measure the amount of IL-15 in the Caco-2 culture medium even in the presence of gliadin (data not shown).
Of note, it was observed that PT–BSA had minor toxic effects in all cell culture experiments when compared to cells cultured without any supplementation, as also seen in earlier studies [33,37]. This is due probably to remaining amounts of pepsin and trypsin in the samples in spite of heat inactivation. In earlier studies it has been demonstrated that contaminating trypsin might alter gliadin peptide-binding characteristics by direct binding to intestinal epithelial cell brush border membranes [44]. Therefore, the inclusion of PT-treated control in the experiments is important to reveal the true effect exerted by toxic compounds. In spite of these limitations, the Caco-2 cell line has been used widely in coeliac disease research, especially when studying the toxicity of gliadin [11,12,37,45,46].
We conclude that rye secalin activates toxic reactions in vitro similarly to wheat gliadin and should thus be excluded from the diet of coeliac disease patients, as suggested previously [23,25–27]. However, the taste and nutritional value of rye is highly appreciated in some countries and therefore coeliac-safe processed rye-based products are called for. Our present results indicate that the whole mixture of germinating barley enzymes is highly efficient in the elimination of residual toxic peptides of rye secalin and could therefore be of value in the medical treatment of coeliac disease or in the food industry in developing novel coeliac-safe food products in the future.
Acknowledgments
The authors thank Mrs Jaana Leskinen and Mr Jorma Kulmala for technical assistance. The Celiac Disease Study Group and the current work have been financially supported by the Tampere Graduate School in Biomedicine and Biotechnology, the Academy of Finland, the Pediatric Research Foundation, the Competitive Research Funding of the Tampere Hospital District, the Finnish Celiac Society, the Finnish Cultural Foundation, the Sigrid Juselius Foundation and the European Comission (contract number MRTN-CT-2006-036032).
Disclosure
None.
References
- 1.Bruce G, Woodley JF, Swan CH. Breakdown of gliadin peptides by intestinal brush borders from coeliac patients. Gut. 1984;25:919–24. doi: 10.1136/gut.25.9.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shan L, Molberg O, Parrot I, et al. Structural basis for gluten intolerance in celiac sprue. Science. 2002;297:2275–9. doi: 10.1126/science.1074129. [DOI] [PubMed] [Google Scholar]
- 3.Hausch F, Shan L, Santiago NA, Gray GM, Khosla C. Intestinal digestive resistance of immunodominant gliadin peptides. Am J Physiol Gastrointest Liver Physiol. 2002;283:G996–G1003. doi: 10.1152/ajpgi.00136.2002. [DOI] [PubMed] [Google Scholar]
- 4.Sollid LM. Coeliac disease: dissecting a complex inflammatory disorder. Nat Rev Immunol. 2002;2:647–55. doi: 10.1038/nri885. [DOI] [PubMed] [Google Scholar]
- 5.Maiuri L, Ciacci C, Auricchio S, Brown V, Quaratino S, Londei M. Interleukin 15 mediates epithelial changes in celiac disease. Gastroenterology. 2000;119:996–1006. doi: 10.1053/gast.2000.18149. [DOI] [PubMed] [Google Scholar]
- 6.Maiuri L, Ciacci C, Ricciardelli I, et al. Association between innate response to gliadin and activation of pathogenic T cells in coeliac disease. Lancet. 2003;362:30–7. doi: 10.1016/S0140-6736(03)13803-2. [DOI] [PubMed] [Google Scholar]
- 7.Di Sabatino A, Ciccocioppo R, Cupelli F, et al. Epithelium derived interleukin 15 regulates intraepithelial lymphocyte Th1 cytokine production, cytotoxicity, and survival in coeliac disease. Gut. 2006;55:469–77. doi: 10.1136/gut.2005.068684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yokoyama S, Watanabe N, Sato N, et al. Antibody-mediated blockade of IL-15 reverses the autoimmune intestinal damage in transgenic mice that overexpress IL-15 in enterocytes. Proc Natl Acad Sci USA. 2009;106:15849–54. doi: 10.1073/pnas.0908834106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mention JJ, Ben Ahmed M, Begue B, et al. Interleukin 15: a key to disrupted intraepithelial lymphocyte homeostasis and lymphomagenesis in celiac disease. Gastroenterology. 2003;125:730–45. doi: 10.1016/s0016-5085(03)01047-3. [DOI] [PubMed] [Google Scholar]
- 10.Schulzke JD, Schulzke I, Fromm M, Riecken EO. Epithelial barrier and ion transport in coeliac sprue: electrical measurements on intestinal aspiration biopsy specimens. Gut. 1995;37:777–82. doi: 10.1136/gut.37.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sander GR, Cummins AG, Henshall T, Powell BC. Rapid disruption of intestinal barrier function by gliadin involves altered expression of apical junctional proteins. FEBS Lett. 2005;579:4851–5. doi: 10.1016/j.febslet.2005.07.066. [DOI] [PubMed] [Google Scholar]
- 12.Lammers KM, Lu R, Brownley J, et al. Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology. 2008;135:194–204. doi: 10.1053/j.gastro.2008.03.023. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tripathi A, Lammers KM, Goldblum S, et al. Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proc Natl Acad Sci USA. 2009;106:16799–804. doi: 10.1073/pnas.0906773106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janatuinen EK, Pikkarainen PH, Kemppainen TA, et al. A comparison of diets with and without oats in adults with celiac disease. N Engl J Med. 1995;333:1033–7. doi: 10.1056/NEJM199510193331602. [DOI] [PubMed] [Google Scholar]
- 15.Hogberg L, Laurin P, Falth-Magnusson K, et al. Oats to children with newly diagnosed coeliac disease: a randomised double blind study. Gut. 2004;53:649–54. doi: 10.1136/gut.2003.026948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koskinen O, Villanen M, Korponay-Szabo I, Lindfors K, Maki M, Kaukinen K. Oats do not induce systemic or mucosal autoantibody response in children with coeliac disease. J Pediatr Gastroenterol Nutr. 2009;48:559–65. doi: 10.1097/MPG.0b013e3181668635. [DOI] [PubMed] [Google Scholar]
- 17.Sturgess R, Day P, Ellis HJ, et al. Wheat peptide challenge in coeliac disease. Lancet. 1994;343:758–61. doi: 10.1016/s0140-6736(94)91837-6. [DOI] [PubMed] [Google Scholar]
- 18.Anderson RP, Degano P, Godkin AJ, Jewell DP, Hill AV. In vivo antigen challenge in celiac disease identifies a single transglutaminase-modified peptide as the dominant A-gliadin T-cell epitope. Nat Med. 2000;6:337–42. doi: 10.1038/73200. [DOI] [PubMed] [Google Scholar]
- 19.Stenman SM, Lindfors K, Korponay-Szabo IR, et al. Secretion of celiac disease autoantibodies after in vitro gliadin challenge is dependent on small-bowel mucosal transglutaminase 2-specific IgA deposits. BMC Immunol. 2008;9:6. doi: 10.1186/1471-2172-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wieser H. The precipitating factor in coeliac disease. Baillières Clin Gastroenterol. 1995;9:191–207. doi: 10.1016/0950-3528(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 21.Vader LW, Stepniak DT, Bunnik EM, et al. Characterization of cereal toxicity for celiac disease patients based on protein homology in grains. Gastroenterology. 2003;125:1105–13. doi: 10.1016/s0016-5085(03)01204-6. [DOI] [PubMed] [Google Scholar]
- 22.Kieffer M, Frazier PJ, Daniels NW, Coombs RR. Wheat gliadin fractions and other cereal antigens reactive with antibodies in the sera of coeliac patients. Clin Exp Immunol. 1982;50:651–60. [PMC free article] [PubMed] [Google Scholar]
- 23.Kilmartin C, Wieser H, Abuzakouk M, Kelly J, Jackson J, Feighery C. Intestinal T cell responses to cereal proteins in celiac disease. Dig Dis Sci. 2006;51:202–9. doi: 10.1007/s10620-006-3108-0. [DOI] [PubMed] [Google Scholar]
- 24.Baker PG, Read AE. Oats and barley toxicity in coeliac patients. Postgrad Med J. 1976;52:264–8. doi: 10.1136/pgmj.52.607.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anand BS, Piris J, Truelove SC. The role of various cereals in coeliac disease. Q J Med. 1978;47:101–10. [PubMed] [Google Scholar]
- 26.Dicke WK, Weijers HA, Van De Kamer JH. Coeliac disease. II. The presence in wheat of a factor having a deleterious effect in cases of coeliac disease. Acta Paediatr. 1953;42:34–42. doi: 10.1111/j.1651-2227.1953.tb05563.x. [DOI] [PubMed] [Google Scholar]
- 27.Bracken SC, Kilmartin C, Wieser H, Jackson J, Feighery C. Barley and rye prolamins induce an mRNA interferon-gamma response in coeliac mucosa. Aliment Pharmacol Ther. 2006;23:1307–14. doi: 10.1111/j.1365-2036.2006.02876.x. [DOI] [PubMed] [Google Scholar]
- 28.Shan L, Marti T, Sollid LM, Gray GM, Khosla C. Comparative biochemical analysis of three bacterial prolyl endopeptidases: implications for coeliac sprue. Biochem J. 2004;383:311–18. doi: 10.1042/BJ20040907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gass J, Ehren J, Strohmeier G, Isaacs I, Khosla C. Fermentation, purification, formulation, and pharmacological evaluation of a prolyl endopeptidase from Myxococcus xanthus: implications for celiac sprue therapy. Biotechnol Bioeng. 2005;92:674–84. doi: 10.1002/bit.20643. [DOI] [PubMed] [Google Scholar]
- 30.Sollid LM, Khosla C. Future therapeutic options for celiac disease. Nat Clin Pract Gastroenterol Hepatol. 2005;2:140–7. doi: 10.1038/ncpgasthep0111. [DOI] [PubMed] [Google Scholar]
- 31.Stepniak D, Spaenij-Dekking L, Mitea C, et al. Highly efficient gluten degradation with a newly identified prolyl endoprotease: implications for celiac disease. Am J Physiol Gastrointest Liver Physiol. 2006;291:G621–9. doi: 10.1152/ajpgi.00034.2006. [DOI] [PubMed] [Google Scholar]
- 32.Siegel M, Bethune MT, Gass J, et al. Rational design of combination enzyme therapy for celiac sprue. Chem Biol. 2006;13:649–58. doi: 10.1016/j.chembiol.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 33.Stenman SM, Venalainen JI, Lindfors K, et al. Enzymatic detoxification of gluten by germinating wheat proteases: implications for new treatment of celiac disease. Ann Med. 2009;41:390–400. doi: 10.1080/07853890902878138. [DOI] [PubMed] [Google Scholar]
- 34.Gass J, Vora H, Bethune MT, Gray GM, Khosla C. Effect of barley endoprotease EP-B2 on gluten digestion in the intact rat. J Pharmacol Exp Ther. 2006;318:1178–86. doi: 10.1124/jpet.106.104315. [DOI] [PubMed] [Google Scholar]
- 35.Gass J, Bethune MT, Siegel M, Spencer A, Khosla C. Combination enzyme therapy for gastric digestion of dietary gluten in patients with celiac sprue. Gastroenterology. 2007;133:472–80. doi: 10.1053/j.gastro.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 36.Tye-Din JA, Anderson RP, Ffrench RA, et al. The effects of ALV003 pre-digestion of gluten on immune response and symptoms in celiac disease in vivo. Clin Immunol. 2010;134:289–95. doi: 10.1016/j.clim.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Lindfors K, Blomqvist T, Juuti-Uusitalo K, et al. Live probiotic Bifidobacterium lactis bacteria inhibit the toxic effects induced by wheat gliadin in epithelial cell culture. Clin Exp Immunol. 2008;152:552–8. doi: 10.1111/j.1365-2249.2008.03635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arentz-Hansen H, Korner R, Molberg O, et al. The intestinal T cell response to alpha-gliadin in adult celiac disease is focused on a single deamidated glutamine targeted by tissue transglutaminase. J Exp Med. 2000;191:603–12. doi: 10.1084/jem.191.4.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li N, DeMarco VG, West CM, Neu J. Glutamine supports recovery from loss of transepithelial resistance and increase of permeability induced by media change in Caco-2 cells. J Nutr Biochem. 2003;14:401–8. doi: 10.1016/s0955-2863(03)00071-8. [DOI] [PubMed] [Google Scholar]
- 40.Mitea C, Havenaar R, Drijfhout JW, Edens L, Dekking L, Koning F. Efficient degradation of gluten by a prolyl endoprotease in a gastrointestinal model: implications for coeliac disease. Gut. 2008;57:25–32. doi: 10.1136/gut.2006.111609. [DOI] [PubMed] [Google Scholar]
- 41.Matysiak-Budnik T, Candalh C, Cellier C, et al. Limited efficiency of prolyl-endopeptidase in the detoxification of gliadin peptides in celiac disease. Gastroenterology. 2005;129:786–96. doi: 10.1053/j.gastro.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 42.Ehren J, Moron B, Martin MT, Bethune E, Gray GM, Khosla C. A food-grade enzyme preparation with modest gluten detoxification properties. PLoS ONE. 2009;4:e6313. doi: 10.1371/journal.pone.0006313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jung HC, Eckmann L, Yang SK, et al. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bolte G, Osman A, Mothes T, Stern M. Peptic–tryptic digests of gliadin: contaminating trypsin but not pepsin interferes with gastrointestinal protein binding characteristics. Clin Chim Acta. 1996;247:59–70. doi: 10.1016/0009-8981(95)06220-3. [DOI] [PubMed] [Google Scholar]
- 45.Ciccocioppo R, Finamore A, Ara C, Di Sabatino A, Mengheri E, Corazza GR. Altered expression, localization, and phosphorylation of epithelial junctional proteins in celiac disease. Am J Clin Pathol. 2006;125:502–11. doi: 10.1309/DTYR-A91G-8R0K-TM8M. [DOI] [PubMed] [Google Scholar]
- 46.Barone MV, Gimigliano A, Castoria G, et al. Growth factor-like activity of gliadin, an alimentary protein: implications for coeliac disease. Gut. 2007;56:480–8. doi: 10.1136/gut.2005.086637. [DOI] [PMC free article] [PubMed] [Google Scholar]


