Abstract
Eotaxin-2 is a potent chemoattractant for eosinophils, basophils and T helper type 2 (Th2) lymphocytes. The eotaxin-2/CCL24 receptor CCR3 is expressed in human brain, skin, endothelium and macrophages. The aim of the current study was to evaluate the protective effect of a monoclonal anti-eotaxin-2 antibody on the development of adjuvant-induced arthritis in rats (AIA). Adjuvant arthritis was induced in Lewis rats by intradermal injection of incomplete Freund's adjuvant +Mycobacterium tuberculosis. Rats were treated by intraperitoneal (i.p.) injection with three monoclonal antibodies against eotaxin-2 (G7, G8, D8) three times per week. Controls were treated with total mouse immunoglobulin G (IgG), methotrexate (MTX) or phosphate-buffered saline (PBS). Arthritis severity was evaluated by measuring ankle swelling, arthritic score, whole animal mobility and body weight. Sample joints were obtained for pathological evaluation and postmortem X-ray of ankle joints was performed to document erosions. Significant inhibition of arthritis was observed in rats treated with anti-eotaxin-2 antibodies compared to those treated with immunoglobulin or PBS. Inhibition was manifest in ankle diameter, arthritic score and mobility score. The antibody marked D8 showed the greatest efficacy. The effect was observed both in animals treated before the appearance of arthritis and in those where treatment was begun after development of joint inflammation. Combined treatment with D8 and MTX caused additional protection. Significant reduction of inflammation in D8-treated animals was also demonstrated in pathological and X-ray examinations. Inhibition of eotaxin-2 by monoclonal antibodies has a significant protective effect in adjuvant arthritis. These results may introduce a novel therapeutic target in rheumatoid arthritis and additional inflammatory joint disorders.
Keywords: adjuvant-induced arthritis, eotaxin-2, experimental arthritis, monoclonal antibodies, rheumatology
Introduction
Rheumatoid arthritis (RA) is a common, chronic inflammatory disease, characterized by intense, destructive infiltration of synovial tissue by a broad spectrum of inflammatory cells [1]. Multiple cytokines, derived from macrophages and fibroblasts, are responsible for induction of secretion of both cytokines and chemokines in RA [2]. The accumulation of leucocytes in the joint space leads to secretion of tissue degrading factors, including cytokines and matrix-degrading enzymes.
Chemokines are small cytokines which act as chemoattractants for leucocytes, coordinating both homeostatic trafficking of these cells as well as recruiting specific cell populations to sites of inflammation. Chemokine dysregulation is considered to play a part in a wide spectrum of human disease involving the immune system, including human immunodeficiency virus (HIV) infection [3], malignancy [4] and autoimmunity [5].
The CC chemokine eotaxin-2/CCL11 binds to the eosinophil receptor CCR3, acting as a strong chemoattractant for eosinophils [6], basophils [7] and T helper type 2 (Th2) lymphocytes [8]. However, eotaxin-2 is not the sole ligand for CCR3, which can also be activated by regulated upon activation normal T cell expressed and secreted (RANTES) (CCL5) [9], monocyte chemoattractant protein-3 (MCP-3) (CCL7) and MCP-4 (CCL13) [10].
CCR3, the eotaxin receptor, is a 7-transmembrane G protein-coupled receptor which is expressed by eosinophils, as well as by a wide array of cell types including macrophages and endothelial cells [11]. This chemokine is also expressed on human T helper cells [12]. CCR3 expression was originally studied extensively in the pathogenesis of asthma and allergy, where it continues to pose a therapeutic target [13]. More recently, however, a role for this pathway has emerged in the study of additional inflammatory and autoimmune disorders including inflammatory bowel disease [14], multiple sclerosis [15] and RA. Thus, CCR3 has been shown to play a role in recruitment of leucocytes to synovial tissue in adjuvant-induced arthritis (AIA), a commonly used animal model of RA [16]. In early AIA, CCR3 has been detected in synovial tissue macrophages and lining cells, with a subsequent trend towards declining expression [16]. This has been interpreted as reflecting a role for the eotaxin/CCR3 system in the initial trafficking of leucocytes into the synovial joint. Chemokine inhibition has been tested previously as a therapeutic option in animal models such as AIA [17]. Recently, levels of eotaxin have been shown to be increased in serum of patients with early RA [18] as well as in plasma of patients with juvenile idiopathic arthritis (JIA) [19]. Thus, the eotaxin/CCR3 system appears to be operative both in RA and in the AIA model. In view of these observations, in the current study we have attempted to evaluate the role of eotaxin-2 inhibition in the AIA model.
Materials and methods
Monoclonal antibodies (mAbs) against human eotaxin-2
Production of monoclonal antibodies directed against human eotaxin-2
Several clones of mAbs were produced by us according to standard protocols. In short, Balb/C mice were immunized with 20 µg of human eotaxin-2 (Peprotech, Rocky Hill, NJ, USA) followed by four additional boosts. After confirming the presence of polyclonal anti-eotaxin-2 antibodies in the sera, mice were killed and their spleens hybridized with an NS/0 myeloma line, followed by clonal screening for binding to eotaxin-2. The hybridomas were then grown in serum-free media for 2–3 weeks, media collected and concentrated by 100 kDa centricons (Biological Industries, Beit Haemek, Israel).
The cross-reactivity of D8 between human and murine eotaxin-2 [5 µg eotaxin-2 diluted in phosphate-buffered saline (PBS)], with Kd of 0·77 mg and 4 mg, respectively, was determined.
Adhesion assay in the presence of D8
In adhesion assays, rat splenocytes were separated on Ficoll gradient and plated in 10-cm dishes for an overnight incubation. Cells were harvested the next day and pretreated with increasing concentrations of D8 or total mouse immunoglobulin G (IgG) (5–50 µg/ml) for 2 h with rotation. Cells were then centrifuged and plated on 96-well plates precoated with fibronectin. After 1-h incubation, non-adherent cells were washed away and the amount of adherent cells was analysed using the XTT kit (Biological Industries). Similar adhesion assays were performed using splenocytes of C57Bl mice or with peripheral bone marrow cells (PBMCs) collected from healthy donors (Fig. 1a).
Fig. 1.
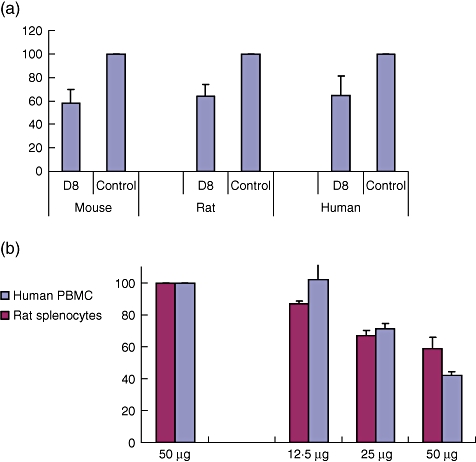
(a) Percentage of adhesion to fibronectin (FN) of D8 (50 mg)-treated compared to immunoglobulin G (IgG)-treated lymphocytes. D8 inhibits adhesion of human, rat and murine lymphocytes to fibronectin. (b) D8 inhibits adhesion of human, rat and murine lymphocytes to fibronectin D8 inhibits migration of lymphocytes towards vascular endothelial growth factor (VEGF).
Migration assays
C57BL/6J-derived splenocytes and human PBMCs pretreated with D8 (30 µg/ml) were plated onto the upper chamber of a transwell system. The lower chamber contained serum-free media supplemented with vascular endothelial growth factor (VEGF) (20 ng/ml). The media in the lower chamber was collected 4 h later and cells counted using flow cytometry (number of cells collected/min) (Fig. 1b).
Induction of AIA model
Six-week-old male Lewis rats were obtained from Harlan Biotech Ltd (Rehovot, Israel). Freund's complete adjuvant was prepared by suspending heat-killed Mycobacterium tuberculosis (Difco, Detroit, MI, USA) in mineral oil at 10 mg/ml. Rats were injected intradermally with 100 µl adjuvant at the base of the tail. Arthritis developed by day 17 post-injection.
Evaluation of effect of anti-eotaxin-2 antibodies compared with non-specific IgG and PBS control on AIA
Rats (eight per group) were treated subsequently by intraperitoneal injection of three monoclonal antibodies directed against eotaxin-2, marked as G7, G8 and D8. Control rats were treated by intraperitoneal injection of non-specific IgG or PBS.
Injections were started on the third day after arthritis induction and were performed three times a week.
Dose–response experiments
In a second set of experiments, D8, the anti-eotaxin-2 antibody showing best protective results, was tested in a dose–response model. Adjuvant arthritis was induced according to the above-described protocol. Animals (six rats per each condition) were treated with D8 intraperitoneally at a dose of 20 µg, 100 µg or 1000 µg, starting on day 3, three times weekly (D8 prevention group). A separate set of animals (six per condition) were treated with identical doses after arthritis onset (D8 treatment group).
In order to compare the anti-inflammatory effect of D8 with that of a traditional anti-inflammatory agent of known efficacy, one group was treated with intraperitoneal methotrexate (MTX), 0·25 mg/kg, once weekly, starting on day 3 after arthritis induction (MTX prevention group). An additional group was treated with MTX, 0·25 mg/kg once weekly, in combination with D8, 100 µg intraperitoneally given three times a week, starting on day 3 (combined D8–MTX prevention group). A control group was treated with PBS throughout the experiment.
Evaluation of arthritis severity
Body weight in grams was measured every other day as an indicator of systemic inflammation.
For evaluation of paw swelling, ankle and wrist diameter in mm (to one place after the decimal point) were recorded three times a week.
Arthritis score measurement
Each paw was scored on a scale of 0–4 for the degree of swelling, erythema and deformity (maximum score 16 per animal) as follows: 0 = normal, 1 = slight erythema and/or swelling of the ankle or wrist, 2 = moderate erythema and/or swelling of ankle or wrist, 3 = severe erythema and/or swelling of ankle or wrist and 4 = complete erythema and swelling of toes or fingers and ankle or wrist and inability to bend the ankle or wrist. Finger and toe swelling was recorded according to their partial contribution: ankles, each toe scored 0·2; wrist, each finger scored 0·25; the sum of all joints was calculated.
Mobility score
Whole animal mobility was scored between 0 and 4 according to the following definitions: 0 = normal, 1 = slightly impaired, 2 = major impairment, 3 = does not step on paw and 4 = no movement.
Data analysis
Data were analysed using spss software version 16·01. Student's t-test was performed to identify significant differences between experimental groups. Three or more group means were compared by one-way analysis of variance, with an assumed significance level of P < 0·05.
Results
Prevention experiments
In these experiments, treatment was given before the appearance of clinical arthritis (prevention group).
Effect of treatment with anti-eotaxin-2 antibodies on arthritis score
Treatment with anti-eotaxin-2 monoclonal antibodies caused a significant reduction in arthritic score severity, compared to rats treated with PBS. This protective effect was evident in all three antibodies tested (G7, G8 and D8). The protective effect became evident immediately with the appearance of arthritis on day 17 after induction (Fig. 2a). It continued to increase in magnitude until the end of the experiment on day 21. Rats treated with non-specific IgG also showed a reduced arthritic score compared with PBS-treated controls. Treatment with antibodies G7 and D8, however, caused a significant reduction in the arthritic score compared with IgG treatment.
Fig. 2.
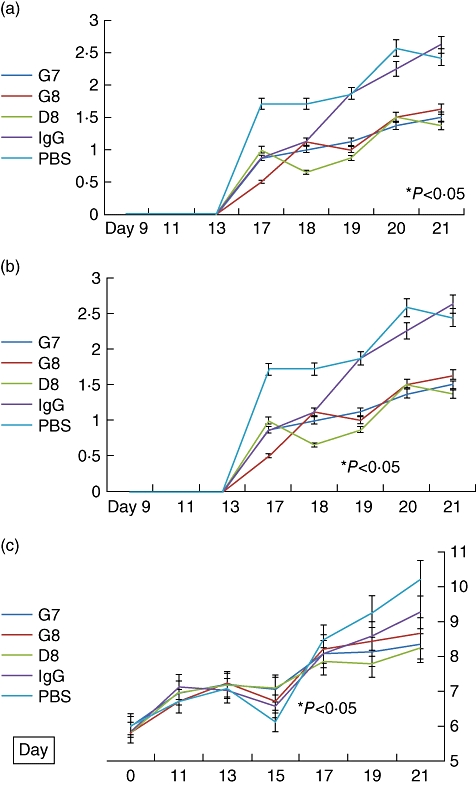
(a) Effect of treatment with anti-eotaxin-2 monoclonal antibodies, immunoglobulin G (IgG) and phosphate-buffered saline (PBS) on the arthritic score (AS) of rats (± standard errors, percentage). Statistically significant differences (P < 0·05) were obtained at every determination, from days 13 to 21, when comparing rats treated with D8 both to rats treated with PBS and to rats treated with IgG. (b) Effect of treatment with anti-eotaxin-2 monoclonal antibodies, IgG and PBS on the mobility score of rats (± standard errors, percentage). Statistically significant differences (P < 0·05) were obtained at every determination, from days 13 to 21, when comparing rats treated with D8 both to rats treated with PBS and to rats treated with IgG. (c) Effect of treatment with anti-eotaxin-2 monoclonal antibodies, IgG and PBS on ankle diameter (± standard errors, percentage). Statistically significant difference (P < 0·05) was obtained between D8 and PBS as of day 19. The difference between D8 and IgG did not reach statistical significance.
Effect of treatment with anti-eotaxin-2 antibodies on mobility score
In line with the data regarding the arthritic score, treatment with the D8 antibody caused a significant reduction in the mobility score, indicating a protective effect (Fig. 2b). Thus, the average mobility score of animals treated with D8 was 1·37 [standard deviation (s.d.) = 1·06] on day 21 compared with 2·43 (s.d. = 0·76) in animals treated with PBS (P < 0·05).
Effect of treatment with anti-eotaxin-2 antibodies on ankle diameter
On measurement of ankle diameter, which expresses severity of joint swelling, a significant protective effect of anti-eotaxin-2 treatment was demonstrated compared to rats treated with PBS or IgG (Fig. 2c). Similar results were obtained regarding wrist diameter (data not shown).
Histological results
D8-treated rats had lower scores of arthritis, ranging from 2·6 to 3·0 with synovial hyperplasia and scattered inflammatory infiltrates, while most rats treated with PBS (control group) had severe synovitis with panus formation (Fig. 3a).
Fig. 3.
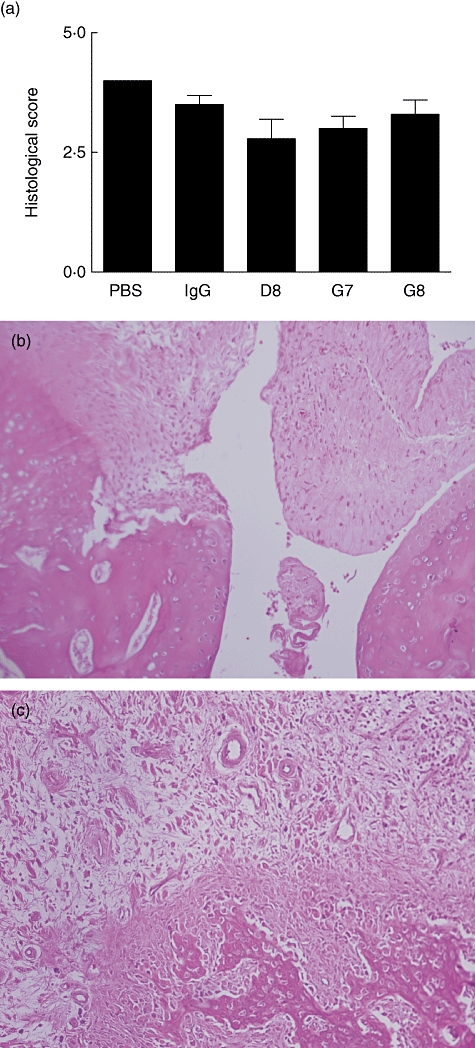
Histological results (a) and representative appearance of joints from rats treated with phosphate-buffered saline (PBS), immunoglobulin G (IgG), D8, G7 and G8 antibodies. (a) Results of histological scoring in rats treated with PBS, IgG, D8, G7 and G8, respectively. An intense inflammatory infiltrate is obvious in the synovial tissue in rats treated with PBS (b) and is absent in rats treated with D8200 µg (c) (haematoxylin and eosin staining).
Figure 3b demonstrates the histological appearance of a joint in a rat treated with D8, while Fig. 3c shows a joint from a control rat treated with PBS.
Effect of treatment with anti-eotaxin-2 antibodies on whole body weight
In order to evaluate the effect of treatment with anti-eotaxin-2 antibodies on the systemic inflammatory response, the average weight of animals was documented. As shown in Fig. 4, anti-eotaxin-2 treatment ameliorated significantly the loss of weight caused by the systemic inflammatory response induced by adjuvant arthritis. Again, the maximal protective effect was observed in animals treated with the D8 antibody, which continued to gain weight throughout the experiment.
Fig. 4.
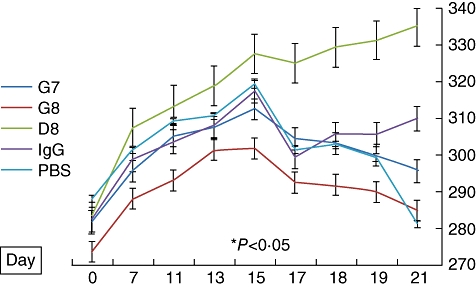
Effect of anti-eotaxin-2 treatment on weight of rats (grams) with adjuvant-induced arthritis (AIA) (± standard errors). A statistically significant difference in weight (P < 0·05) between D8 and phosphate-buffered saline (PBS) was obtained on day 17. The difference between D8 and immunoglobulin G (IgG) did not reach statistical significance.
Dose–response experiments
In the series of dose–response experiments, at a dose of 100 µg D8 had a significantly superior protective effect, compared with the low-dose (20 µg) and high-dose (1000 µg) groups (Fig. 5a). Similar results were obtained regarding mobility scores, ankle diameter and animal weight (data not shown).
Fig. 5.
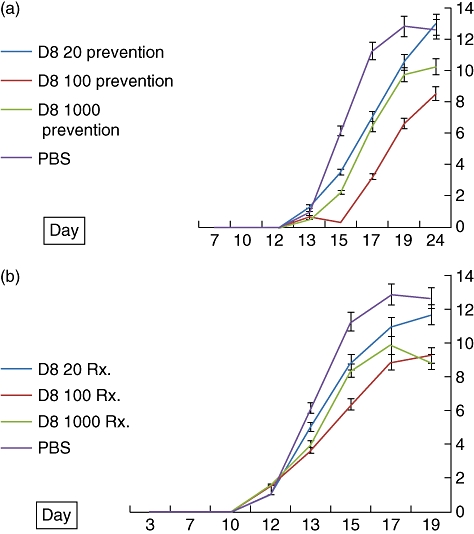
(a) Effect of D8 anti-eotaxin-2 antibody prevention at three doses (20 µg, 100 µg and 1000 µg) on arthtitic score (AS) in adjuvant-induced arthritis (AIA) (prevention group) (± standard error, percentage). Statistically significant differences (P < 0·05) were obtained at every determination from days 17 to 24 when comparing rats treated with D8100 prevention to rats treated with phosphate-buffered saline (PBS). (b) Effect of treatment with D8 antibody at the appearance of arthritis on arthritic score (± standard error, percentage) (treatment group). Statistically significant differences (P < 0·05) were obtained between rats treated with D8 at a dose of 100 µg at the appearance of arthritis on day 12 as of day 13 and to the end of the experiment.
Post-arthritis treatment experiments
In these experiments treatment, was started after the appearance of clinical arthritis (treatment group).
D8 treatment
Treatment with D8 antibody intraperitoneally, beginning at the time of appearance of arthritis, also resulted in a significant reduction in arthritic score severity (Fig. 5b) compared with PBS-treated animals. Similar results were obtained regarding mobility, weight and ankle diameter. As demonstrated in Fig. 5a, in this experiment similar results were obtained at the 100 µg and 1000 µg dose groups.
MTX versus combined D8–MTX prevention
While both MTX and D8100 µg produced significant, comparable protection against development of arthritis, as measured by the arthritic score (compared to PBS-treated controls), the combination of MTX and D8 produced an additional significant protective effect compared to MTX alone, as demonstrated in Fig. 6.
Fig. 6.
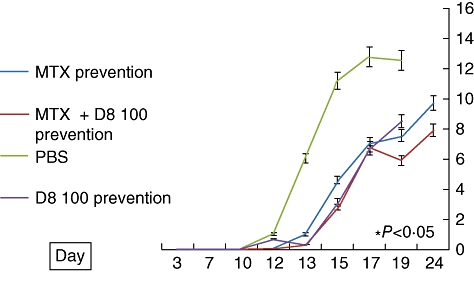
Protective effect of D8 (100 µg), methotrexate (MTX) (0·25 mg/kg) and MTX combined with D8100 µg, compared with phosphate-buffered saline (PBS) controls, on the arthritic score in adjuvant-induced arthritis (AIA) (± standard error, percentage). Both D8100 µg and MTX treatment caused a statistically significant reduction of the arthritis score (P < 0·05) compared with PBS as of day 13. By the end of the experiment on day 24, a statistically significant reduction in the arthritis score was observed in rats treated with MTX + D8100 µg compared with rats treated with MTX alone. A significant difference was also observed on day 24 in the mean ankle width between these groups (not shown).
X-ray results
Post-mortem X-ray demonstrated an intense degree of peri-articular soft tissue swelling in PBS-treated rats, compared with minimal swelling in rats treated with D8 (Fig. 7). In addition, control rats showed signs of decalcification and early erosion, which was not evident in the D8-treated animals.
Fig. 7.
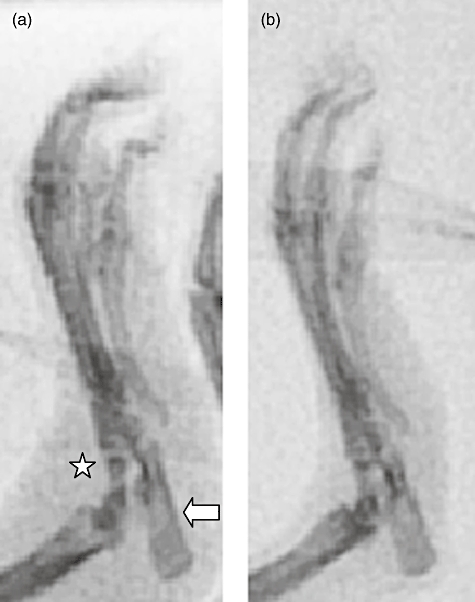
Post-mortem X-ray analysis of joints from rats treated with phosphate-buffered saline (PBS) (a) and D8 (b). (a) Intense periarticular soft tissue swelling is evident in the PBS-treated rats (star), as well as decalcification and early bony erosion at the ankle joint (arrow head); these findings are not evident in the D8-treated rats (b) (representative figures). Similar results were seen in X-rays of the forefeet (not shown).
Discussion
In the current study, we have demonstrated for the first time the efficacy of eotaxin-2 inhibition in the prevention and treatment of AIA.
Eotaxin-2, a CCR3 ligand, has been considered traditionally an important mediator in asthma [13], chronic bronchitis [2] and allergic reactions [6]. While being a major receptor for eotaxin, CCR3 also binds RANTES and MCP-4, thus acting as an important migration regulator for various inflammatory effector cells, including eosinophils, basophils [10] and mast cells [9]. Over recent years it has been become increasingly apparent that chemokines and chemokine receptors play an important role in the pathogenensis of RA [20]. Fibroblast-like synoviocytes have been shown to migrate, proliferate and produce matrix metalloproteinase under regulation of the chemokine system, which may thus play a direct role in the destructive process of RA [21]. This has led to increased interest in animal models of inflammatory arthritis in an attempt to identify potential chemokine therapeutic targets. In the AIA model, CCR2 and CCR3 have been shown to be involved in initial recruitment of leucocytes to synovial tissue [16]. Inhibition of RANTES, a CCR3 agonist, reduced joint inflammation, bone destruction and cell recruitment in the AIA model [22]. Although chemokine inhibition has yet to result in the development of novel effective therapeutics in humans, this strategy is considered currently to be a promising avenue and is the subject of intense investigation [5].
The classical mode of action of eotaxin-2 involves its activity directed towards eosinophil adhesion and chemotaxis [23]. Through downregulation of vascular cell adhesion molecule (VCAM)-1, eotaxin-2 stimulates eosinophils to detach from endothelial cells and migrate into tissue [24]. Acting through MAP-kinase, eotaxin-2 has also been shown to facilitate eosinophil recruitment at sites of allergic inflammation, by shifting their adhesion molecule usage away from VCAM-1 towards an intercellular adhesion molecule (ICAM)-1-dominated pathway [25].
Direct inhibition of the CCR3 receptor has been shown to inhibit eosinophil chemotaxis and is thus a potential therapeutic target [26].
Eotaxin-2 may also have direct inflammatory activity mediated through release of reactive oxygen species [27] and through induction of histamine and leukotriene C-4 (LTC-4) degranulation in basophils. Notably, CCR3 receptors are not limited to eosinophils but are also expressed on monocytes [28], mast cells [9], peripheral memory T cells [29] and Th2 lymphocytes (12). Human dendritic cells (DCs) have been shown to express this receptor in various stages of maturation, and their migration in response to eotaxin can be inhibited by CCR3-specific mAbs [30]. Taken together, these findings indicate that the anti-eotaxin-2/CCR3-directed therapy may have wide therapeutic potential in inflammatory and autoimmune disorders, far exceeding its original role in allergy and atopy.
The results of the current study demonstrate clearly that effective inhibition of eotaxin-2, a CCR3 ligand, has a significantly protective effect in AIA, a well-established model of RA [31]. Our results showed the D8 anti-eotaxin-2 antibody to be effective both as a preventive treatment given before development of arthritis, and more clinically relevant as a therapeutic agent given at the time of the initial manifestation of arthritis. Of note, the central role of eotaxin-2 in inflammatory cell recruitment and adhesion might imply that early inhibition of this chemokine would be particularly effective in amelioration of inflammation. None the less, the results achieved after inflammation was established highlight the multiple roles this chemokine may play, e.g. manipulation of adhesion as well as cell migration, and are encouraging regarding its potential as a therapeutic target.
It is noteworthy that in the dose–response experiments conducted, the maximal effect was observed at an intermediate dose, while treatment with an excess of antibody caused an inferior therapeutic effect. This finding tends to point towards a true physiological effect of the treatment rather than a non-specific toxic effect, which would be expected to intensify with dose escalation. An additional hypothetical explanation could be the induction of neutralizing anti-mouse antibodies by the higher-dosed rats. In the current study, treatment with anti-eotaxin-2 achieved a protective effect which was comparable to that caused by treatment with MTX, an established and effective treatment for RA, which has the capacity to modify joint destruction. The finding that the combination of D8 and MTX achieved an additional improvement compared to MTX alone strengthens the results further and raises the prospect that this strategy may find a role in the management of human inflammatory arthritis, over and above existing therapies. The clinical results are strengthened by the radiological findings, which suggest that anti-eotaxin treatment may prove to be effective in inhibition of erosion.
In conclusion, the results of the current study shed new light on the functional role of eotaxin-2, heightening its role in the pathogenensis of inflammatory arthritis and underlining it as a promising potential therapeutic target for this spectrum of disease.
Disclosure
None.
References
- 1.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–61. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 2.Arend WP. Physiology of cytokine pathways in rheumatoid arthritis. Arthritis Rheum. 2001;45:101–6. doi: 10.1002/1529-0131(200102)45:1<101::AID-ANR90>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 3.Lusso P. HIV and the chemokine system: 10 years later. EMBO J. 2006;25:447–56. doi: 10.1038/sj.emboj.7600947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zlotnik A. Chemokines and cancer. Int J Cancer. 2006;119:2026–9. doi: 10.1002/ijc.22024. [DOI] [PubMed] [Google Scholar]
- 5.Viola A, Luster AD. Chemokines and their receptors: drug targets in immunity and inflammation. Annu Rev Pharmacol Toxicol. 2008;48:171–97. doi: 10.1146/annurev.pharmtox.48.121806.154841. [DOI] [PubMed] [Google Scholar]
- 6.Kitaura M, Nakajima T, Imai T, et al. Molecular cloning of human eotaxin, an eosinophil-selective CC chemokine, and identification of a specific eosinophil eotaxin receptor, CC chemokine receptor 3. J Biol Chem. 1996;271:7725–30. doi: 10.1074/jbc.271.13.7725. [DOI] [PubMed] [Google Scholar]
- 7.Ponath PD, Qin S, Ringler DJ, et al. Cloning of the human eosinophil chemoattractant, eotaxin. Expression, receptor binding, and functional properties suggest a mechanism for the selective recruitment of eosinophils. J Clin Invest. 1996;97:604–12. doi: 10.1172/JCI118456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bocchino V, Bertorelli G, Bertrand CP, et al. Eotaxin and CCR3 are up-regulated in exacerbations of chronic bronchitis. Allergy. 2002;57:17–22. [PubMed] [Google Scholar]
- 9.Romagnani P, De Paulis A, Beltrame C, et al. Tryptase–chymase double-positive human mast cells express the eotaxin receptor CCR3 and are attracted by CCR3-binding chemokines. Am J Pathol. 1999;155:1195–204. doi: 10.1016/S0002-9440(10)65222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uguccioni M, Mackay CR, Ochensberger B, et al. High expression of the chemokine receptor CCR3 in human blood basophils. Role in activation by eotaxin, MCP-4, and other chemokines. J Clin Invest. 1997;100:1137–43. doi: 10.1172/JCI119624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romagnani P, Annunziato F, Lasagni L, et al. Cell cycle-dependent expression of CXC chemokine receptor 3 by endothelial cells mediates angiostatic activity. J Clin Invest. 2001;107:53–63. doi: 10.1172/JCI9775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sallusto F, Mackay CR, Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997;277:2005–7. doi: 10.1126/science.277.5334.2005. [DOI] [PubMed] [Google Scholar]
- 13.Garcia G, Godot V, Humbert M. New chemokine targets for asthma therapy. Curr Allergy Asthma Rep. 2005;5:155–60. doi: 10.1007/s11882-005-0090-0. [DOI] [PubMed] [Google Scholar]
- 14.Zimmerman NP, Vongsa RA, Wendt MK, Dwinell MB. Chemokines and chemokine receptors in mucosal homeostasis at the intestinal epithelial barrier in inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:1000–11. doi: 10.1002/ibd.20480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simpson J, Rezaie P, Newcombe J, Cuzner ML, Male D, Woodroofe MN. Expression of the beta-chemokine receptors CCR2, CCR3 and CCR5 in multiple sclerosis central nervous system tissue. J Neuroimmunol. 2000;108:192–200. doi: 10.1016/s0165-5728(00)00274-5. [DOI] [PubMed] [Google Scholar]
- 16.Haas CS, Martinez RJ, Attia N, Haines GK, III, Campbell PL, Koch AE. Chemokine receptor expression in rat adjuvant-induced arthritis. Arthritis Rheum. 2005;52:3718–30. doi: 10.1002/art.21476. [DOI] [PubMed] [Google Scholar]
- 17.Guglielmotti A, D'Onofrio E, Coletta I, Aquilini L, Milanese C, Pinza M. Amelioration of rat adjuvant arthritis by therapeutic treatment with bindarit, an inhibitor of MCP-1 and TNF-alpha production. Inflamm Res. 2002;51:252–8. doi: 10.1007/pl00000301. [DOI] [PubMed] [Google Scholar]
- 18.Hueber W, Tomooka BH, Zhao X, et al. Proteomic analysis of secreted proteins in early rheumatoid arthritis: anti-citrulline autoreactivity is associated with up regulation of proinflammatory cytokines. Ann Rheum Dis. 2007;66:712–19. doi: 10.1136/ard.2006.054924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Jager W, Hoppenreijs EP, Wulffraat NM, Wedderburn LR, Kuis W, Prakken BJ. Blood and synovial fluid cytokine signatures in patients with juvenile idiopathic arthritis: a cross-sectional study. Ann Rheum Dis. 2007;66:589–98. doi: 10.1136/ard.2006.061853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haringman JJ, Smeets TJ, Reinders-Blankert P, Tak PP. Chemokine and chemokine receptor expression in paired peripheral blood mononuclear cells and synovial tissue of patients with rheumatoid arthritis, osteoarthritis, and reactive arthritis. Ann Rheum Dis. 2006;65:294–300. doi: 10.1136/ard.2005.037176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Vicuna R, Gomez-Gaviro MV, Dominguez-Luis MJ, et al. CC and CXC chemokine receptors mediate migration, proliferation, and matrix metalloproteinase production by fibroblast-like synoviocytes from rheumatoid arthritis patients. Arthritis Rheum. 2004;50:3866–77. doi: 10.1002/art.20615. [DOI] [PubMed] [Google Scholar]
- 22.Shahrara S, Proudfoot AE, Woods JM, et al. Amelioration of rat adjuvant-induced arthritis by Met-RANTES. Arthritis Rheum. 2005;52:1907–19. doi: 10.1002/art.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ying S, Robinson DS, Meng Q, et al. C-C chemokines in allergen-induced late-phase cutaneous responses in atopic subjects: association of eotaxin with early 6-h eosinophils, and of eotaxin-2 and monocyte chemoattractant protein-4 with the later 24-h tissue eosinophilia, and relationship to basophils and other C-C chemokines (monocyte chemoattractant protein-3 and RANTES) J Immunol. 1999;163:3976–84. [PubMed] [Google Scholar]
- 24.Tachimoto H, Burdick MM, Hudson SA, Kikuchi M, Konstantopoulos K, Bochner BS. CCR3-active chemokines promote rapid detachment of eosinophils from VCAM-1 in vitro. J Immunol. 2000;165:2748–54. doi: 10.4049/jimmunol.165.5.2748. [DOI] [PubMed] [Google Scholar]
- 25.Tachimoto H, Kikuchi M, Hudson SA, Bickel CA, Hamilton RG, Bochner BS. Eotaxin-2 alters eosinophil integrin function via mitogen-activated protein kinases. Am J Respir Cell Mol Biol. 2002;26:645–9. doi: 10.1165/ajrcmb.26.6.4741. [DOI] [PubMed] [Google Scholar]
- 26.Heath H, Qin S, Rao P, et al. Chemokine receptor usage by human eosinophils. The importance of CCR3 demonstrated using an antagonistic monoclonal antibody. J Clin Invest. 1997;99:178–84. doi: 10.1172/JCI119145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elsner J, Petering H, Kluthe C, et al. Eotaxin-2 activates chemotaxis-related events and release of reactive oxygen species via pertussis toxin-sensitive G proteins in human eosinophils. Eur J Immunol. 1998;28:2152–8. doi: 10.1002/(SICI)1521-4141(199807)28:07<2152::AID-IMMU2152>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 28.Combadiere C, Ahuja SK, Murphy PM. Cloning and functional expression of a human eosinophil CC chemokine receptor. J Biol Chem. 1995;270:16491–4. doi: 10.1074/jbc.270.28.16491. [DOI] [PubMed] [Google Scholar]
- 29.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–83. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beaulieu S, Robbiani DF, Du X, et al. Expression of a functional eotaxin (CC chemokine ligand 11) receptor CCR3 by human dendritic cells. J Immunol. 2002;169:2925–36. doi: 10.4049/jimmunol.169.6.2925. [DOI] [PubMed] [Google Scholar]
- 31.Williams RO. Rodent models of arthritis: relevance for human disease. Clin Exp Immunol. 1998;114:330–2. doi: 10.1046/j.1365-2249.1998.00785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


