Abstract
The potent anti-tumour activities of γδ T cells have prompted the development of protocols in which γδ-agonists are administered to cancer patients. Encouraging results from small Phase I trials have fuelled efforts to characterize more clearly the application of this approach to unmet clinical needs such as metastatic carcinoma. To examine this approach in breast cancer, a Phase I trial was conducted in which zoledronate, a Vγ9Vδ2 T cell agonist, plus low-dose interleukin (IL)-2 were administered to 10 therapeutically terminal, advanced metastatic breast cancer patients. Treatment was well tolerated and promoted the effector maturation of Vγ9Vδ2 T cells in all patients. However, a statistically significant correlation of clinical outcome with peripheral Vγ9Vδ2 T cell numbers emerged, as seven patients who failed to sustain Vγ9Vδ2 T cells showed progressive clinical deterioration, while three patients who sustained robust peripheral Vγ9Vδ2 cell populations showed declining CA15-3 levels and displayed one instance of partial remission and two of stable disease, respectively. In the context of an earlier trial in prostate cancer, these data emphasize the strong linkage of Vγ9Vδ2 T cell status to reduced carcinoma progression, and suggest that zoledronate plus low-dose IL-2 offers a novel, safe and feasible approach to enhance this in a subset of treatment-refractory patients with advanced breast cancer.
Keywords: cytokines, cytotoxicity, immunotherapy, metastatic breast cancer, Vγ9Vδ2 T cells
Introduction
With more than 1 million new cases diagnosed annually, breast cancer is the most commonly occurring tumour of women. Moreover, with > 370 000 deaths per annum, it is a leading cause of cancer-related mortality. While advances in surgery, radiotherapy, chemotherapy and hormone replacement therapy have irrefutably enhanced early diagnosis and treatment, no effective therapy exists for advanced, invasive, metastatic breast cancer [1].
In such cases, the prospect that immunotherapy may prove effective [2] was supported powerfully by the report that so-called ‘effector-memory’ TEM cells within colorectal cancer provided the best correlate of the absence of early metastases, and of prolonged survival [3]. Similarly, tumour-infiltrating lymphocytes reportedly predict the response to neoadjuvant chemotherapy in breast carcinoma [4]. Most immunotherapeutic modalities seek to induce and boost tumour-specific, adaptive responses via a spectrum of immunization protocols by adoptive transfer of tumour-reactive T cells and by blocking factors, such as cytotoxic T lymphocyte antigen 4 (CTLA4), that suppress antigen-specific T cell activities naturally [5].
In parallel, numerically smaller lymphocyte subsets, including natural killer (NK) cells, and lymphocytes expressing the γδ T cell receptor (TCR) are receiving increasing attention [6–9]. The perceived advantages of these cells is that they respond in high frequency to the increased expression of ‘stress-antigens’ and to the decreased expression of major histocompatibility complex (MHC) antigens, both of which are common signatures of tumour cells. These ‘unconventional’ lymphocytes can home readily to inflammatory or lesional sites, and show immediate effector functions ranging from cytotoxicity, through the chemokine-mediated recruitment of other effector cells, to antigen presentation [8,10,11].
In healthy adults, the major peripheral blood γδ T cell subset expresses the Vγ9Vδ2 TCR, and shows TCR-dependent activation by low molecular weight isoprenoid precursors, including (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMB-PP), derived from many bacteria and protozoa, and isopentenyl pyrophosphate (IPP), that may be over-expressed by virus-infected or tumour cells [12–16]. Tumour cell recognition may be sustained additionally by expression of MHC class I-related chain A (MICA), MHC class I-related chain B (MICA) and/or other ligands for the activating NKG2D receptor, expressed by Vγ9Vδ2+ T cells [17], and by tumour cell surface display of an ‘ecto-F1-ATPase’ that can engage the Vγ9Vδ2 TCR [18]. Vγ9Vδ2+ T cells include those with ‘naive-like’ or ‘central memory-like’ phenotypes (Tnaive, CD45RA+CD27+; TCM, CD45RA-CD27+) that home to secondary lymphoid organs, but that lack immediate effector function, and those with effector/memory-like (TEM, CD45RA-CD27-) and ‘terminally differentiated’ (TEMRA, CD45RA+CD27-) phenotypes that home to sites of inflammation and that display immediate effector function [19].
Aminobisphosphonates are toxic towards osteoclasts and prescribed widely as standard-of-care for osteoporosis, for elderly patients receiving chronic, bone-erosive steroids and for malignant bone metastases. Zoledronate (Zol) is the most potent and efficacious clinically approved aminobisphosphonate [20–23]. Intriguingly, Zol and other aminobisphosphonates provoke accumulation of IPP and related metabolites because they inhibit the downstream enzyme farnesyl pyrophosphate synthase [24,25]. When added to peripheral blood mononuclear cell (PBMC) cultures, Zol + interleukin (IL)-2 induces specifically in Vγ9Vδ2+ T cells activation and effector molecules, including CD25, CD69 and interferon (IFN)-γ, and cytotoxicity markers such as CD107a, perforin and tumour necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) [26] (data not shown). Moreover, Zol-activated Vγ9Vδ2+ T cells kill breast cancer cell lines readily in vitro and in a severe combined immunodeficient (SCID) mouse xenograft model [27] (data not shown).
Fuelled by these attractive properties, recent, albeit small-scale, applications of Vγ9Vδ2+ T cells to haematological and solid-tissue malignancies have yielded promising results [26, 28–33]. Previously, we showed that co-administration of Zol and low-dose IL-2 (i.e. at lower concentration than that required to affect αβ T or NK cells) to late-stage, hormone-resistant prostate cancer patients enhanced Vγ9Vδ2+ T cell responses significantly, with associated clinical benefits [26]. For these reasons, we rationalized that Zol plus low-dose IL-2 may also prove a feasible and safe approach to activate Vγ9Vδ2+ T cells in advanced breast cancer patients in whom all other common treatments have been exhausted.
Materials and methods
Study design
The study included 10 female patients (age 55–70 years; median 63 years, average time since diagnosis: 2037 days) meeting the following eligibility criteria: histopathologically confirmed diagnosis of advanced metastatic breast adenocarcinoma; tumour burden of one to three metastasis sites (brain excluded); adequate organ function [bone marrow: white blood cells (WBC) ≥ 3000/mm3, absolute neutrophil count (ANC) ≥ 1500/mm3, haemoglobin (Hgb) ≥ 9·0g/dl, platelets ≥ 100 000/mm3; liver: bilirubin ≤ 1·5mg/dl and aspartate aminotransferase (AST) ≤ 2× above normal range (30 UI/l); kidney: creatinine ≤ 1·5 mg/dl); performance status ≤ 2 according to the Eastern Cooperative Oncology Group (ECOG) scale [34]; high levels of carcinoembryonic antigen (CEA) and CA15-3 (≥ 2× upper limit of normality); and measurable progressive disease (tumour growth increase > 20%). Eligible patients were also human epidermal growth factor receptor-2 (HER-2)-negative, hormone therapy-resistant, and met strict criteria for acquired chemotherapy resistance: recurrence after primary surgery followed by adjuvant chemotherapy and documented progressive disease after at least one favourable response to treatment, including doxorubicin and taxanes for recurrence. No patient was receiving statins or vitamin D therapy. Patients were excluded if they had chemotherapy, radiation therapy or bisphosphonates up to 4 weeks prior to study entry; severe cardiovascular disease, refractory hypertension, symptomatic coronary artery disease; corrected (for albumin) serum calcium < 8·0 mg/dl; history of autoimmunity; serious intercurrent chronic or acute illnesses; concurrent second malignancy; or concurrent treatment with steroids or other immunosuppressive agents. All patients were recruited and treated over a 1-year period, starting in January 2005. Treatment of patients followed written informed consent from each patient or patient's guardian, according to an institutional review board-approved protocol and in compliance with the Declaration of Helsinki [35]. The ethical rationale was that eligible patients were therapeutically terminal, i.e. all available efficient treatments according to Physician Data Query (PDQ) of the National Cancer Institute had been exhausted. The chosen drugs and doses were considered acceptable, as they showed no relevant toxicity in oncological patients [26]. Immunological parameters were assessed every 3 months (primary end-point); clinical status, according to Response Evaluation Criteria in Solid Tumours (RECIST) definitions [36][progressive disease (PD), stable disease (SD), partial remission (PR), complete remission (CR)] was recorded monthly (secondary end-point). Clinical assessments and analyses were performed by two independent reviewers, using imaging and/or clinical and laboratory measures. Toxicity was evaluated using common terminology criteria for adverse events (CTCAE, version 3·0) of the US National Cancer Institute. Any status was considered evaluable if maintained for ≥ 30 days. Deaths attributable to cancer progression were recorded monthly.
Treatment schedule
Four mg of Zol (Zometa; Novartis, Origgio, Italy) was administered every 21 days by 15-min 100-ml intravenous infusion of saline, followed immediately by subcutaneous delivery of IL-2 (Proleukin, 106 IU; Chiron, Emeryville, CA, USA).
Flow cytometry
Prior to treatment (month 0), and at months 3 (6 days after the fourth Zol + IL-2 administration), 9 (6 days after the 13th Zol + IL-2 administration) and 12 (6 days after the 17th Zol + IL-2 administration), patients' PBMC were obtained by Ficoll-Hypaque centrifugation and stained with fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, PE-Cy5- or allophycocyanin (APC)-conjugated antibodies against CD27, CD45RA, human leucocyte antigen D-related (HLA-DR), CD3, CD56, CD69, TCR pan αβ, TCR pan γδ, TCR Vγ9 or TCR Vδ2 (BD PharMingen, San Diego, CA, USA); 105 cells were analysed on a fluorescence activated cell sorter (FACS)Calibur with CellQuest software (Becton Dickinson, Heidelberg, Germany).
Analysis of Vγ9Vδ2+ T cells
RPMI-1640 medium (Gibco, Grand Island, NY, USA) was supplemented with 10% heat-inactivated human pooled AB serum, 2 mM L-glutamine, 20 nM HEPES and 100 U/ml penicillin/streptomycin. PBMC were labelled with 5,6-carboxy-fluorescein-ester (CFSE) (Molecular Probes, Eugene, OR, USA) and cultured with 10 µM IPP (Sigma, St Louis, MO, USA) and 20 U/ml IL-2. After 7 days the number of Vγ9Vδ2+ T cells among CD3+ cells was determined by FACS. IFN-γ and TRAIL levels in 48-h culture supernatants were assessed by enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN, USA). Na-CBZ-L-lysine-thiobenzyl levels in 24-h culture supernatants were determined by N-α-benzyloxycarbonyl-l-lysine thiobenzyl ester (BLT)-esterase assay [19,26,30].
Serum TRAIL, cytokines and chemokines
Serum TRAIL was detected by ELISA; serum granulocyte–macrophage colony-stimulating factor (GM-CSF), IL-2, IL-4, IL-6, IL-8 (CXCL8), IL-12p40, IL-12p70, IL-13, TNF-α, IFN-γ, eotaxin (CCL11), inositol phosphate (IP)-10 (CXCL10), monocyte chemoattractant protein (MCP)-1 (CCL2) and macrophage inflammatory protein (MIP)-1α (CCL3) were determined by xMAP multiplex technology (Luminex, Austin, TX, USA), using Biorad reagents (Bio-Rad Life Sciences, CA, USA). Data were acquired and analysed with the Bioplex Manager Software (Bio-Rad).
Statistical analysis
Differences in survival were assessed using log-rank, Breslow (generalized Wilcoxon) or Tarone–Ware tests. Data from more than two groups were compared using one-way analysis of variance (ANOVA) with Tukey–Kramer multiple comparison test, using Instat software (GraphPad). P-values < 0·05 were considered statistically significant.
Results
Toxicity
One to 3 days after the first Zol + IL-2 administration, five patients developed transient flu-like symptoms that were controlled easily by oral paracetamol. These side effects reprised those reported following pamidronate + IL-2 administration to multiple myeloma and non-Hodgkin's lymphoma patients [28] and Zol + IL-2 injection in advanced prostate cancer patients [26]. Two patients developed local erythema at the site of IL-2 administration. No other haematological, hepatic, renal or neurological toxicity or allergic, autoimmune or fatigue side effect was observed.
Vγ9Vδ2+ T cell responses
Patients displayed pretreatment levels of ∼20–50 × 103 Vγ9Vδ2+ T cells/µl of blood (Fig. 1). Three patients died before month 3, restricting longitudinal analysis to seven patients, three of whom died between months 3 and 11 after precipitous, progressive declines in Vγ9Vδ2+ T cell numbers. Conversely, three of four patients surviving past month 12 showed strikingly robust Vγ9Vδ2+ T cell numbers (Fig. 1), with clear increases in CD69 and/or HLA-DR activation markers detected selectively on total γδ T cells, but not seen on αβ T cells or NK cells (Table 1).
Fig. 1.
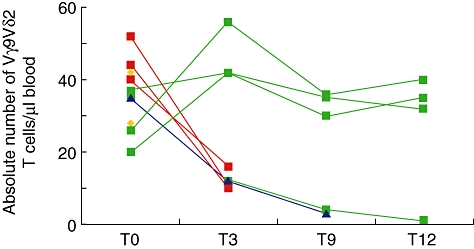
Absolute Vγ9Vδ2 T cell numbers in patients treated with Zoledronate (Zol) + interleukin (IL)-2 prior to treatment (T0), and 3 (T3), 9 (T9) and 12 (T12) months after treatment. Yellow symbols, patients who died before T3; red symbols, patients who died before T9; blue symbols, patients who died before T12; green symbols, patients surviving past T12.
Table 1.
Activation marker expression in γδ, αβ and natural killer (NK) cells from patients treated with Zoledronate and interkeukin (IL)-2.
| Months after treatment |
||||
|---|---|---|---|---|
| 0 | 3 | 9 | 12 | |
| γδ+ CD69+ | 9 | 64 | 47 | 42 |
| γδ+ HLA-DR+ | 10 | 92 | 81 | 87 |
| αβ+ CD69+ | 3 | 8 | 4 | 6 |
| αβ+ HLA-DR+ | 10 | 7 | 9 | 4 |
| NK+ CD69+ | 2 | 0 | 2 | 4 |
| NK+ HLA-DR+ | 4 | 0 | 1 | 3 |
Data indicate the mean percentages of cells expressing CD69 or human leucocyte antigen D-related (HLA-DR) activation antigens. Standard errors (s.e.) were always less than 20% of the means.
The four patients surviving past month 12 showed sharp and progressive decreases in absolute numbers of Vγ9Vδ2+ Tnaive and TCM cells, and corresponding increases in Vγ9Vδ2+ TEM cells, associated with decreased proliferative responses to IPP and reduced cytotoxicity (measured by BLT-esterase and TRAIL), compared to progressive increases in IFN-γ production peaking at 12 months. The pattern of an altered phenotype and function of peripheral Vγ9Vδ2 cells following treatment with zoledronate and IL-2 was strikingly evident when either all the enrolled 10 patients (Figs 2 and 3) or only the four patients surviving past month 12 (data not shown) were compared. Collectively, these changes show that there are patients in whom Zol + IL-2 provokes a specific, striking and durable effector maturation and mobilization of peripheral blood Vγ9Vδ2+ T cells, akin to our and others' observations in different types of cancer [30–33].
Fig. 2.
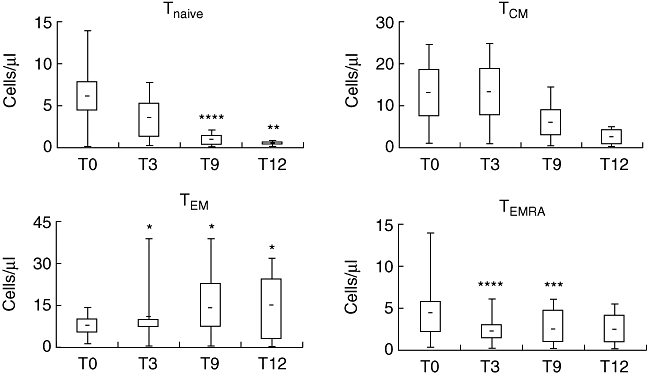
Distribution of Vγ9Vδ2 T cell subsets in patients treated with Zoledronate (Zol) + interleukin (IL)-2 prior to treatment (T0), and 3 (T3), 9 (T9) and 12 (T12) months after treatment. Top, bottom and middle line through boxes, 75th, 25th, and 50th percentiles for Vγ9Vδ2 Tnaive (CD45RA+CD27+), TCM (CD45RA-CD27+), TEM (CD45RA-CD27-), and TEMRA (CD45RA+CD27-) cells. Lines that extend from boxes, highest and lowest values from each subgroup. Lines within boxes, median values. *P < 0·001, **P < 0·005, ***P < 0·01 and ****P < 0·02 compared to T0 values.
Fig. 3.
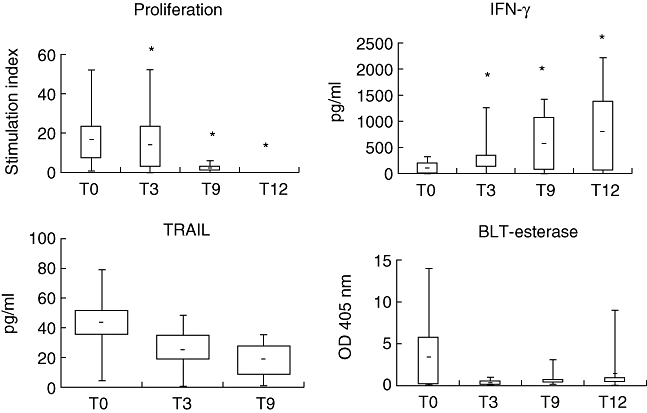
In vitro activity of Vγ9Vδ2 T cells in patients treated with Zoledronate (Zol) + interleukin (IL)-2 prior to treatment (T0), and 3 (T3), 9 (T9) and 12 (T12) months after treatment. Top, bottom and middle line through boxes, 75th, 25th, and 50th percentiles for proliferation of Vγ9Vδ2 T cells (7 days), interferon (IFN)-γ and tumour necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) secretion (48 h), and N-α-benzyloxycarbonyl-l-lysine thiobenzyl ester (BLT)-esterase activity (24 h) in response to isopentenyl pyrophosphate (IPP) and IL-2 stimulation. Optical density (OD), absorbance. Lines that extend from boxes, highest and lowest values from each subgroup. Lines within boxes, median values. *P < 0·001 compared to T0 values.
Clinical responses and correlates
Deterioration in health was invariably preceded by substantial declines in Vγ9Vδ2+ T cell numbers. The three patients sustaining robust Vγ9Vδ2 T cell numbers over 12 months composed one case of PR and two of SD, respectively (Fig. 4a,b). Moreover, a statistically significant correlation was evident between Vγ9Vδ2+ T cell numbers at 12 months and declining CA 15-3 levels (Fig. 4c). Analysis a posteriori of patient sera showed some significant up-regulation in IFN-γ, IL-12p40, TNF-α, GM-CSF, eotaxin, IP-10 and MIP-1α, whereas IL-8 and TRAIL were down-regulated across the treatment period (Table 2). We noted, however, that the three patients whose clinical status improved were identified among others only by their overt differences in numbers and not by the properties and phenotype of their Vγ9Vδ2+ T cells.
Fig. 4.
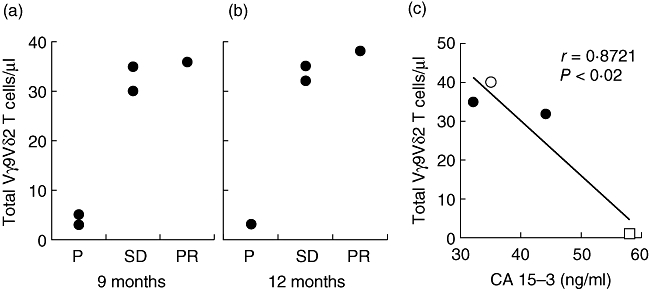
(a, b) Correlation between Vγ9Vδ2 T cell numbers and clinical outcome at T9 (a) and T12 (b). PD: progressive disease; SD: stable disease; PR: partial remission. (c) Inverse correlation between Vγ9Vδ2 T cell numbers and CA15-3 levels, as assessed at T12, in one patient with partial remission (open circles), two patients with stable disease (closed circles) and one patient with progression (open square).
Table 2.
Concentration of cyokines and chemokines in serum from patients treated with Zoledronate and interleukin (IL)-2.
| Months after treatment |
|||
|---|---|---|---|
| 0 | 3 | 9 | |
| IL-8 | 52·1 ± 113·5 | 25·9 ± 36·5* | 28·3 ± 49·9* |
| Eotaxin | 351·5 ± 327·5 | 736·7 ± 708·5* | 455·7 ± 217·3 |
| MIP-1α | 353·7 ± 257·1 | 777·3 ± 668·6* | 524·8 ± 284·9 |
| IP/10 | 670·0 ± 423·0 | 1314·8 ± 1159·1* | 904·8 ± 686·2l |
| MCP-1 | 157·9 ± 90·2 | 148·3 ± 133·5 | 94·1 ± 46·4 |
| IL12p40 | 368·5 ± 157·6 | 474·2 ± 481·1* | 587·0 ± 463·5* |
| IL12p70 | 22·0 ± 26·9 | 33·1 ± 35·3 | 11·5 ± 6·6* |
| IL-2 | 16·8 ± 15·1 | 12·6 ± 8·8 | 10·9 ± 7·7 |
| IL-4 | 5·4 ± 4·6 | 8·2 ± 4·7 | 4·7 ± 4·6 |
| IL-13 | 10·5 ± 10·3 | 16·9 ± 6·5 | 13·8 ± 8·8 |
| TNF-α | 19·2 ± 8·2 | 16·6 ± 7·6 | 18·3 ± 9·2* |
| GM-CSF | 14·6 ± 7·3 | 15·0 ± 8·9 | 18·8 ± 2·1* |
| IL-6 | 9·2 ± 14·0 | 14·9 ± 25·8 | 4·0 ± 3·8 |
| IFN-γ | 22·5 ± 12·2 | 47·9 ± 41·8* | 30·1 ± 14·5 |
| TRAIL | 42·7 ± 13·3 | 22·3 ± 14·4 | 17·2 ± 14·0 |
Data indicate the mean serum concentration (expressed in pg/ml) ± standard deviation (s.d.). GM-CSF, granulocyte–macrophage colony-stimulating factor; IFN, interferon; IP, inositol phosphate; MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; TRAIL, tumour necrosis factor (TNF)-related apoptosis-inducing ligand.
Discussion
The need for innovative treatments of advanced, refractory carcinomas has fuelled investigation of tumour immunology and immunotherapy, with recent studies showing that immunotherapy combined with chemotherapy improves survival benefits relative to chemotherapy alone [37–41]. Indeed, chemotherapeutic agents can sensitize tumours to immune-mediated killing, for instance by up-regulation of death receptors DR5 and Fas, that are bound by TRAIL and FasL, respectively [41].
Pursuing the concept that γδ T cells may compose key components of tumour immunotherapy [7–9], the main aim of this study was to assess whether reproducible phenotypic and functional changes could be induced in the Vγ9Vδ2+ T cell compartment in highly advanced disease. In this regard, there was substantial differentiation of Vγ9Vδ2+ T cells toward an effector/memory-like phenotype in all patients monitored, and a striking and durable maintenance of robust γδ T cell numbers in 30% of patients. This establishes that immunomodulation by Zol + IL-2 can be achieved safely in patients refractory to all other treatments, as was the case in advanced prostate cancer [26]. While highly encouraging, further optimization or modification of the protocol, for example by prescreening patients' cells for responsiveness to Zol + IL-2 in vitro, might benefit additional treatment-refractory patients [28]. Paralleling our data, durable activation of the TEM subset of Vγ9Vδ2+ T cells was reported recently after a single dose of Zol to disease-free breast cancer patients [33].
Our second aim was to assess whether there was a tumour response to the treatment regimen. In this regard, the study, albeit small, emphasizes strongly a correlation of peripheral blood Vγ9Vδ2+ T cell numbers and status with arrested disease progression, and with a surrogate marker thereof (declining CA 15-3). This evokes a comparable correlation of γδ T cell numbers and activities with arrested disease progression in other scenarios of advanced malignancy [26]. We note, however, that the declining health of seven patients could not be attributed to poor functional conversion of Vγ9Vδ2+ T cells (which was equivalent in all patients). Therefore, there is no obvious need to invoke suppressor γδ T cells, as were described previously in breast cancer [42]. Rather, the critical difference was a failure to sustain robust numbers, implying that high response frequencies compose a key property of lymphoid stress-surveillance [8]. Hence, any future treatment optimizations should respect the need to achieve sufficiently large numbers of relevant γδ T cells.
Given the powerful and diverse immunomodulatory effects of different tumour milieus, the relevant anti-tumour properties of Vγ9Vδ2+ T cells will probably vary in different contexts. In this light, the robust γδ T cell activation reported here showed no direct evidence of enhanced cytotoxic potential, as was observed in the preceding prostate cancer trial [26]. Conversely, the observed increases in cytokines evoke previous demonstrations, including our own, that activated Vγ9Vδ2+ T cells, co-stimulated with IL-2, produce large amounts of IFN-γ, TNF-α, GM-CSF, IP-10 and MIP-1α[43]. A central role of IFN-γ in the anti-tumour activities of γδ T cells was reported in an animal model [44], while IFN-γ can also enhance cytotoxicity indirectly by increasing cells' susceptibility to TRAIL. The central and specific role of Vγ9Vδ2+ T cells in the patients' responses is suggested strongly by the failure to detect significant activation of NK or αβ T cells. Further investigation of the mechanism of action will benefit from longitudinal studies of biopsies that may offer correlates of the status of tumour-infiltrating γδ T cells with outcome.
In summary, this report advances further the case for an expanded application of γδ T cell agonists in solid tumours. Such treatments might be complemented usefully by adoptive transfer of autologous γδ T cells, harvested pretreatment, expanded and reinfused after several months, according to perceived need [9,29,31,32]. Phenotyping of such cells will be critical, given that an expanded population of Vδ1+ T cells producing IL-4 correlated with good prognosis in a 1-year follow-up of lymphoid malignancies [45], whereas Vδ1+ T cells infiltrating breast tumours suppressed T cell responses and blocked dendritic cell function [42]. Although our studies in breast and prostate cancer have focused on advanced disease, the approach may also benefit other patients. Thus, the reported anti-tumour and anti-metastatic properties of Zol underpinned the Austrian Breast and Colorectal Cancer Study Group trial 12, to evaluate the efficacy of 3 years of ovarian suppression plus anastrozole or tamoxifen with or without Zol in premenopausal women with early breast cancer. That study demonstrated that the addition of Zol increased the rate of disease-free survival, compared with endocrine therapy alone [46]. Such results emphasize the need for larger-scale immunological monitoring to gain insight into the full means of efficacy of aminobisphosphonates across a spectrum of disease scenarios.
Acknowledgments
We thank Nicola Gebbia for valuable advice with patient recruiting, Fabio Fulfaro and Alessandra Casuccio for help with statistical analysis, and Elisa Binda and Daniele Santini for helpful suggestion and criticism. The work was supported by PRIN grant 2006068412-002 to F.D.; AIRC grants to M.T and F.D.; ISS Oncoproteomic project 2007-527/B/3A/3 to G.S and F.D.; grants from Cancer Research UK (C28524/A9497) and the Breast Cancer Campaign (2008NovSP08) to M.E.; the Wellcome Trust and the Guy's and St Thomas's Comprehensive Biomedical Research Centre (A.C.H.); a Marie Curie (FP6); and a FEDER (EU/Région Wallone) grant to D.V. Valentina Orlando is a PhD student of the International PhD Programme in Immunopharmacology at the University of Palermo.
Disclosures
F.D. is founding member of a University of Palermo spin-out company (TetraPharm S.r.l.), in which F.D. has a share of equity and to which he acts as non-executive scientific adviser. F.D. is named inventor for patents filed by TetraPharm S.r.l. on products related to those studied in this work. The other authors declare no competing financial interests.
References
- 1.Reeder JG, Vogel VG. Breast cancer risk management. Clin Breast Cancer. 2007;7:833–40. doi: 10.3816/CBC.2007.n.047. [DOI] [PubMed] [Google Scholar]
- 2.Tan PH, Lota AS. Interaction of current cancer treatments and the immune system: implications for breast cancer therapeutics. Exp Opin Pharmacother. 2008;9:2639–60. doi: 10.1517/14656566.9.15.2639. [DOI] [PubMed] [Google Scholar]
- 3.Pagès F, Berger A, Camus M, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–66. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 4.Hornychova H, Melichar B, Tomsova M, et al. Tumor-infiltrating lymphocytes predict response to neoadjuvant chemotherapy in patients with breast carcinoma. Cancer Invest. 2009;26:1024–31. doi: 10.1080/07357900802098165. [DOI] [PubMed] [Google Scholar]
- 5.Gattinoni L, Powell DJ, Rosenberg SA, et al. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383–93. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol. 2006;90:1–50. doi: 10.1016/S0065-2776(06)90001-7. [DOI] [PubMed] [Google Scholar]
- 7.Kabelitz D, Wesch D, He W. Perspectives of γδ T cells in tumor immunology. Cancer Res. 2007;67:5–8. doi: 10.1158/0008-5472.CAN-06-3069. [DOI] [PubMed] [Google Scholar]
- 8.Hayday AC. γδ T cells and the lymphoid stress-surveillance response. Immunity. 2009;31:184–96. doi: 10.1016/j.immuni.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 9.June CH, Blazar BR, Riley JL. Engineering lymphocyte subsets: tools, trials and tribulations. Nat Rev Immunol. 2009;9:704–16. doi: 10.1038/nri2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brandes M, Willimann K, Bioley G, et al. Cross-presenting human γδ T cells induce robust CD8+ alphabeta T cell responses. Proc Natl Acad Sci USA. 2009;106:2307–12. doi: 10.1073/pnas.0810059106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landmeier S, Altvater B, Pscherer S, et al. Activated human γδ T cells as stimulators of specific CD8+ T-cell responses to subdominant Epstein Barr virus epitopes: potential for immunotherapy of cancer. J Immunother. 2009;32:310–21. doi: 10.1097/CJI.0b013e31819b7c30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Constant P, Davodeau F, Peyrat MA, et al. Stimulation of human γδ T cells by nonpeptidic mycobacterial ligands. Science. 1994;264:267–70. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka Y, Morita CT, Nieves E, et al. Natural and synthetic non-peptide antigens recognized by human γδ T cells. Nature. 1995;375:155–8. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 14.Morita CT, Mariuzza R, Brenner MB. Antigen recognition by human γδ T cells: pattern recognition by the adaptive immune system. Springer Semin Immunopathol. 2000;22:191–217. doi: 10.1007/s002810000042. [DOI] [PubMed] [Google Scholar]
- 15.Eberl M, Hintz M, Reichenberg A, et al. Microbial isoprenoid biosynthesis and human γδ T cell activation. FEBS Lett. 2003;544:4–10. doi: 10.1016/s0014-5793(03)00483-6. [DOI] [PubMed] [Google Scholar]
- 16.Gober HJ, Kistowska M, Angman L, et al. Human γδ T cell receptor cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197:163–8. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das H, Groh V, Kuijl C, et al. MICA engagement by human Vγ9Vδ2 T cells enhances their antigen-dependent effector function. Immunity. 2003;15:83–93. doi: 10.1016/s1074-7613(01)00168-6. [DOI] [PubMed] [Google Scholar]
- 18.Scotet E, Martinez LO, Grant E, et al. Tumor recognition following Vγ9Vδ2 T cell receptor interactions with a surface F1-ATPase-related structure and apolipoprotein A-I. Immunity. 2005;22:71–80. doi: 10.1016/j.immuni.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 19.Dieli F, Poccia F, Lipp M, et al. Differentiation of effector/memory Vd2 T cells and migratory routes in lymph nodes or inflammatory sites. J Exp Med. 2003;198:391–7. doi: 10.1084/jem.20030235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Major P. The use of zoledronic acid, a novel, highly potent bisphosphonate, for the treatment of hypercalcemia of malignancy. Oncologist. 2002;7:481–91. doi: 10.1634/theoncologist.7-6-481. [DOI] [PubMed] [Google Scholar]
- 21.Major P, Cook R. Efficacy of bisphosphonates in the management of skeletal complications of bone metastases and selection of clinical endpoints. Am J Clin Oncol. 2002;25:S10–S18. doi: 10.1097/00000421-200212001-00003. [DOI] [PubMed] [Google Scholar]
- 22.Brahim A, Scher N, Williams G, et al. Approval summary for zoledronic acid for treatment of multiple myeloma and cancer bone metastases. Clin Cancer Res. 2003;9:2394–9. [PubMed] [Google Scholar]
- 23.FDA approves ZOMETA for treatment of cancer-related bone complication. Expert Rev Anticancer Ther. 2002;2:137–8. doi: 10.1586/14737140.2.2.137. [DOI] [PubMed] [Google Scholar]
- 24.Roelofs AJ, Jauhiainen M, Mönkkönen H, et al. Peripheral blood monocytes are responsible for γδ T cell activation induced by zoledronic acid through accumulation of IPP/DMAPP. Br J Haematol. 2009;144:245–50. doi: 10.1111/j.1365-2141.2008.07435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitrofan LM, Pelkonen J, Mönkkönen J. The level of ATP analog and isopentenyl pyrophosphate correlates with zoledronic acid-induced apoptosis in cancer cells in vitro. Bone. 2009;45:1153–60. doi: 10.1016/j.bone.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 26.Dieli F, Vermijlen D, Fulfaro F, et al. Targeting human gamma-delta T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 2007;67:7450–7. doi: 10.1158/0008-5472.CAN-07-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simoni D, Gebbia N, Invidiata FP, et al. Design, synthesis, and biological evaluation of novel aminobisphosphonates possessing an in vivo antitumor activity through a γδ-T lymphocytes-mediated activation mechanism. J Med Chem. 2008;51:6800–7. doi: 10.1021/jm801003y. [DOI] [PubMed] [Google Scholar]
- 28.Wilhelm M, Kunzmann V, Eckstein S, et al. γδ T cells for immune therapy of patients with lymphoid malignancies. Blood. 2003;102:200–6. doi: 10.1182/blood-2002-12-3665. [DOI] [PubMed] [Google Scholar]
- 29.Abe Y, Muto M, Nieda M, et al. Clinical and immunological evaluation of zoledronate-activated Vg9 γδ T-cell-based immunotherapy for patients with multiple myeloma. Exp Hematol. 2009;37:956–68. doi: 10.1016/j.exphem.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Dieli F, Gebbia N, Poccia F, et al. Induction of γδ T-lymphocyte effector functions by bisphosphonate zoledronic acid in cancer patients in vivo. Blood. 2003;102:2310–11. doi: 10.1182/blood-2003-05-1655. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi H, Tanaka Y, Yagi J, et al. Safety profile and anti-tumour effects of adoptive immunotherapy using γδ T cells against advanced renal cell carcinoma: a pilot study. Cancer Immunol Immunother. 2007;56:469–76. doi: 10.1007/s00262-006-0199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bennouna J, Bompas E, Neidhardt EM, et al. Phase-I study of Innacell γδ, an autologous cell-therapy product highly enriched in g9d2 T lymphocytes, in combination with IL-2, in patients with metastatic renal cell carcinoma. Cancer Immunol Immunother. 2008;57:1599–609. doi: 10.1007/s00262-008-0491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santini D, Martini F, Fratto ME, et al. In vivo effects of zoledronic acid on peripheral γδ T lymphocytes in early breast cancer patients. Cancer Immunol Immunother. 2009;58:31–8. doi: 10.1007/s00262-008-0521-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55. [PubMed] [Google Scholar]
- 35.World Medical Association Declaration of Helsinki: recommendations guiding medical doctors in biomedical research involving human subjects. Adopted by the 18th World Medical Assembly, Helsinki, Finland, 1964 and as revised in Tokyo, 1975, in Venice, 1983, in Hong Kong, 1989. Version with changes of 1997.
- 36.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 37.Lake RA, Robinson BW. Immunotherapy and chemotherapy – a practical partnership. Nat Rev Cancer. 2005;5:397–405. doi: 10.1038/nrc1613. [DOI] [PubMed] [Google Scholar]
- 38.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 39.Feldman EJ, Brandwein J, Stone R, et al. Phase III randomized multicenter study of a humanized anti-CD33 monoclonal antibody, lintuzumab, in combination with chemotherapy, versus chemotherapy alone in patients with refractory or first-relapsed acute myeloid leukemia. J Clin Oncol. 2005;23:4110–16. doi: 10.1200/JCO.2005.09.133. [DOI] [PubMed] [Google Scholar]
- 40.Linck D, Lentini G, Tiemann M, et al. Sequential application of chemotherapy and monoclonal CD20 antibody: successful treatment of advanced composite-lymphoma. Leuk Lymph. 2005;46:285–8. doi: 10.1080/10428190400015535. [DOI] [PubMed] [Google Scholar]
- 41.Mattarollo SR, Kenna T, Nieda M, et al. Chemotherapy pretreatment sensitizes solid tumor-derived cell lines to Va24+ NKT cell-mediated cytotoxicity. Int J Cancer. 2006;119:1630–7. doi: 10.1002/ijc.22019. [DOI] [PubMed] [Google Scholar]
- 42.Peng G, Wang HY, Peng W, et al. Tumor-infiltrating γδ T cells suppress T and dendritic cell function via mechanisms controlled by a unique toll-like receptor signaling pathway. Immunity. 2007;27:334–48. doi: 10.1016/j.immuni.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 43.Vermijlen D, Ellis P, Langford C, et al. Distinct cytokine-driven responses of activated blood γδ T cells: insights into unconventional T cell pleiotropy. J Immunol. 2007;178:4304–14. doi: 10.4049/jimmunol.178.7.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao Y, Yang W, Pan M, et al. γδ T cells provide an early source of interferon gamma in tumor immunity. J Exp Med. 2003;198:433–42. doi: 10.1084/jem.20030584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Catellani S, Poggi A, Buzzone A, et al. Expansion of Vd1 T lymphocytes producing IL-4 in low-grade non-Hodgkin lymphomas expressing Ul-16-binding proteins. Blood. 2007;109:2078–85. doi: 10.1182/blood-2006-06-028985. [DOI] [PubMed] [Google Scholar]
- 46.Gnant M, Mlineritsch B, Schippinger W, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360:679–91. doi: 10.1056/NEJMoa0806285. [DOI] [PubMed] [Google Scholar]


