Abstract
It has been suggested that alveolar and interstitial macrophages play a key role in the pathogenesis of idiopathic pulmonary fibrosis (IPF) by producing proinflammatory and/or fibrogenic cytokines. We showed that inflammatory macrophages expressed folate receptor β (FRβ) while resident macrophages in normal tissues expressed no or low levels of FRβ. In the present study, we examined the distribution of FRβ-expressing macrophages in the lungs of patients with usual idiopathic pulmonary fibrosis (UIP) and mice with bleomycin-induced pulmonary fibrosis (PF) and tested whether the depletion of FRβ-expressing macrophages could suppress bleomycin-induced PF in mice. Immunostaining with anti-human or -mouse FRβ monoclonal antibody (mAb) revealed that FRβ-expressing macrophages were present predominantly in fibrotic areas of the lungs of patients with UIP and mice with bleomycin-induced PF. Intranasal administration of a recombinant immunotoxin, consisting of immunoglobulin heavy and light chain Fv portions of an anti-mouse FRβ mAb and truncated Pseudomonas exotoxin A, increased survival significantly and reduced levels of total hydroxyproline and fibrosis in bleomycin-induced PF. In immunohistochemical analysis, decreased numbers of tumour necrosis factor-α-, chemokines CCL2- and CCL12-producing cells were observed in the immunotoxin-treated group. These findings suggest a pathogenic role of FRβ-expressing macrophages in IPF. Thus, targeting FRβ-expressing macrophages may be a promising treatment of IPF.
Keywords: folate receptor β, immunotoxin, macrophages, pulmonary fibrosis
Introduction
Idiopathic pulmonary fibrosis (IPF) is the most common form of idiopathic interstitial pneumonias, responds poorly to therapy and has a poor prognosis [1,2]. The disease is characterized by alveolar epithelial injury and hyperplasia, infiltration of inflammatory cells, fibroblast hyperplasia, deposition of extracellular matrix and scar formation [3]. The current hypothesis for the aetiopathogenesis of IPF is that repeated insults to the lung lead to abnormal fibrotic response, characterized by an altered mesenchymal phenotype with increased matrix production and reduced matrix mobilization [4]. However, it is still unknown what determines this abnormal fibrotic response to pulmonary injury. Macrophage infiltration is implicated in various types of pulmonary fibrosis (PF). Intriguingly, it has been reported that proinflammatory or profibrotic cytokines produced by alveolar and interstitial macrophages were elevated in the lung of patients with IPF when compared to other lung diseases, suggesting a role of macrophages in the pathogenesis of IPF [5–7].
Bleomycin is used widely to induce pulmonary injury and fibrosis in experimental animals. PF induced by the intratracheal instillation of bleomycin is a well-characterized mouse model and used to study IPF [8]. Using this model, previous studies have demonstrated that macrophages were increased significantly and activated in the lungs [9–11]. These macrophages produce chemokines CCL2 and CCL12 that recruit monocytes into tissues, macrophage colony-stimulating factor (M-CSF) that is important for cell proliferation and survival, and transforming growth factor (TGF-β1) that is involved in the fibrosis [12]. Many preclinical trials that target macrophage-derived molecules were conducted with bleomycin-induced PF [13–15]; however, it remains unknown whether selective depletion of inflammatory macrophages could suppress PF.
Folate receptors (FR) are a family of glycosylphosphatidylinositol-anchored glycoproteins with a high affinity for folic acid and 5-methyltetrahydrofolate [16]. Complementary DNAs for FRα, FRβ and FRγ have been cloned, and their expression is regulated differently in various tissues. Because of overexpression of FRα on cancer cells and FRβ on activated macrophages, significant progress has been made using folate receptor-targeted drugs for the diagnosis and therapy of cancers and inflammatory/autoimmune diseases [17]. Recently, we showed that in contrast to tissue resident macrophages in normal tissues that express no or low levels of FRβ, inflammatory macrophages express FRβ[18]. FRβ-expressing peritoneal macrophages have also been shown to produce TNF-α and reactive oxygen species in mice stimulated with inflammatory stimuli [19]. Furthermore, we demonstrated that an immunotoxin to FRβ depleted synovial macrophages significantly and consequently reduced the numbers of activated fibroblasts and suppressed angiogenesis in rheumatoid arthritis synovium xenografts implanted into severe combined immunodeficiency (SCID) mice [20]. In contrast to FRβ-expressing inflammatory macrophages, tissue resident macrophages with low levels of FRβ were resistant to the immunotoxin. These findings raise the possibility that depletion of FRβ-expressing macrophages could be beneficial for the treatment of human IPF.
In this study, to define the role of FRβ-expressing macrophages in the pathogenesis of IPF, we examined the distribution of FRβ-expressing macrophages in the lungs of patients with usual idiopathic pulmonary fibrosis (UIP) and mice with bleomycin-induced PF, and in mice tested for whether an immunotoxin to FRβ could suppress bleomycin-induced PF.
Materials and methods
Human samples
Surgical biopsy specimens were obtained from the lungs of six patients with UIP at Kagoshima University Hospital, Japan. Control lung tissues were obtained from surgical resections for lung cancers. IPF was diagnosed based on the American Thoracic Society criteria [21]. Informed consent was obtained from all patients and this study was approved by the Human Investigation Committee, Kagoshima University, Japan.
Reagents
Bleomycin was purchased from Nihon Kayaku Co. (Tokyo, Japan). Mouse anti-human FRβ monoclonal antibody (mAb) (clone 94b) and rat anti-mouse FRβ mAb (clone 5) have been described previously [18,22]. Other antibodies and reagents were antigen retrieval solution (Biocare Medical, LLC, Concord, CA, USA), mouse anti-human CD68 mAb (Dako Japan Co. Kyoto, Japan), rat anti-mouse CD68 mAb (Serotec, Oxford, UK), rabbit anti-mouse TGF-β1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), goat anti-mouse CCL2 antibody (Santa Cruz Biotechnology), goat anti-mouse CCL12 antibody (Santa Cruz Biotechnology) and goat anti-mouse TNF-α antibody (R&D Systems, Minneapolis, MN, USA). The recombinant immunotoxin, which consists of the immunoglobulin (Ig) heavy chain Fv portion of anti-mouse FRβ mAb with PE38 (VH-PE38) and the Ig light chain Fv portion of anti-mouse FRβ mAb (dsFv anti-FRβ-PE38), was prepared as described previously [22]. The half maximal inhibitory concentration 50 (IC50) of the immunotoxin detected as a decrease in propidium iodide staining was 10 ng/ml and 100 ng/ml on FRβ transfected B300-19 cells (a murine pre-B cell line) and thioglycollate-elicited peritoneal macrophages from BALB/c or C57BL/6J mice, respectively [22]. This recombinant immunotoxin contained fewer than five endotoxin units/mg and its purity was confirmed by 4–20% gradient sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) under non-reducing and reducing conditions.
Induction of PF and its intervention with an immunotoxin to FRβ
PF was induced in mice as described previously [23]. Six to 8-week-old male C57BL/6J mice (Charles River Japan, Yokohama, Japan) were anaesthetized with isoflurane, and 100 µl of bleomycin (4 U/kg body weight) in saline was instilled intratracheally to each mouse. On day 3 after bleomycin instillation, mice were treated intranasally with dsFv anti-FRβ-PE38 (immunotoxin) or VH-PE38 (control protein). Each mouse received 30 µl of 0·1 mg/ml of the immunotoxin or control protein every other day until day 19 after bleomycin instillation. Then, survival was monitored for 21 days. On day 21, mice that still survived were killed; the left lungs were used for histological analysis and the right lungs were used for the analysis of hydroxyproline.
All animal procedures were in accordance with the Ethical Guideline for Animal Experiments, Kagoshima University and approved by the University Committee.
Fibrosis scoring in the lungs
The left lungs were frozen, sectioned, fixed in cold acetone and stained with Masson's trichrome. At least four sections from each mouse and 20 randomly selected fields per section were examined by an experienced pathologist in a blinded fashion. Fibrosis was scored according to Ashcroft methods [24].
Analysis of hydroxyproline in lungs
The lungs were air-dried, weighed and subjected to acid hydrolysis in 6 N HCl solution for 16 h at 110°C. The hydrolysate was suspended in an equal volume of 6 N NaOH and filtered. The concentration of hydroxyproline was determined as described previously [25] and expressed as µg/right lung.
Immunohistochemical staining
Lung sections (5 µm) were prepared from formalin-fixed, paraffin-embedded human lungs, deparaffinized and rehydrated. Antigen unmasking was performed using differentiate infected from vaccinated animals (DIVA) reagents according to the manufacturer's instructions. After blocking with 3% skim milk and 10% normal goat serum, sections were stained with mouse anti-human CD68, mouse anti-human FRβ or isotype-matched irrelevant mAb. Staining was developed with a MAX-PO secondary antibody (Nichirei Co. Ltd, Tokyo, Japan) and NovaRED kit (Vector Laboratories, Burlingame, CA, USA). Endogenous peroxidase activity was quenched with 0·3% H2O2. Images were obtained with digital sight CCD camera (DS-Fi1, Nikon, Tokyo, Japan) and computer-aided image analyser (NIS-Elements; Nikon).
Acetone-fixed frozen sections (6 µm) of the lungs from mice with or without bleomycin instillation were stained with anti-mouse CD68, anti-mouse FRβ, anti-TGF-β1, anti-TNF-α, CCL2, CCL12 or isotype-matched irrelevant antibody as described above. Two-colour immunohistochemical staining was performed as described previously [22]. Briefly, sections were stained with primary mAb and visualized with NovaRED (Vector Laboratories). The sections were then incubated with 0·1 M glycine-HCl (pH 2·7) for 1 min to remove mAb and washed with TBST (20 mM Tris–HCl, pH 7·4, 0·15 M NaCl, 0·1% Tween-20). Thereafter, sections were incubated sequentially with another primary mAb for 1 h, biotinylated secondary antibody (Nichirei) for 30 min and alkaline phosphatase-conjugated streptavidin (Nichirei). The second mAb staining was visualized using Vector Blue (Vector Laboratories). Semi-quantitative morphometrical analysis was carried out with a computer-aided image analyser in four sections from each lung. Ten randomly selected fields per section were examined. A colour threshold mask for immunostaining was defined to detect the red and blue colour by sampling, and the same threshold was applied to all samples.
Statistical analysis
A generalized Wilcoxon test of Kaplan–Meier curves was used to evaluate the significance of survival rates. P < 0·05 was accepted as statistically significant. Analysis of variance (anova) was used to test differences for other data between the groups. P < 0·05 was considered to be statistically significant.
Results
Distribution of FRβ-expressing macrophages in the lungs of patients with IPF and mice with bleomycin-induced PF
We have demonstrated previously that interstitial macrophages but not alveolar macrophages expressed FRβ in normal human lungs and that macrophages in the lungs of naive mice did not express FRβ[18,22]. When we examined macrophages in the lungs of IPF patients there were more FRβ-expressing interstitial macrophages compared to normal lungs (Fig. 1). In addition, a significant number of alveolar macrophages expressed FRβ. Similar to the findings in human IPF, FRβ-expressing macrophages were observed in alveolar and interstitial macrophages of mouse lungs on day 3 after the intratracheal instillation of bleomycin, and peaked on day 14 (Fig. 2). Interestingly, FRβ-expressing macrophages were observed predominantly in fibrotic areas at every time-point after bleomycin instillation.
Fig. 1.
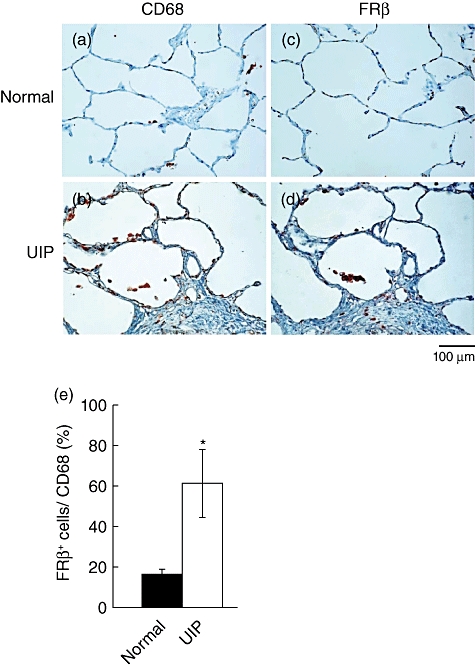
Distribution of folate receptor β(FRβ)-expressing macrophages in the lungs of patients with idiopathic pulmonary fibrosis (IPF). (a, b) CD68- or (c, d) FRβ-expressing macrophages were detected by immunohistochemical staining in the lung tissues of the control (a, c) and usual idiopathic pulmonary fibrosis (UIP) patients (b, d). Photographs are representative of six human normal lung tissues or six UIP lung tissues. Magnification ×200. (e) Ratio of FRβ-expressing macrophages in macrophages of normal lungs ( , n = 6) and IPF lungs (□, n = 6). Values in a graph are the mean ± standard error of the mean of the percentages of FRβ-expressing cells in CD68-expressing cells. *P < 0·05 compared to FRβ-expressing macrophages in normal lung.
, n = 6) and IPF lungs (□, n = 6). Values in a graph are the mean ± standard error of the mean of the percentages of FRβ-expressing cells in CD68-expressing cells. *P < 0·05 compared to FRβ-expressing macrophages in normal lung.
Fig. 2.
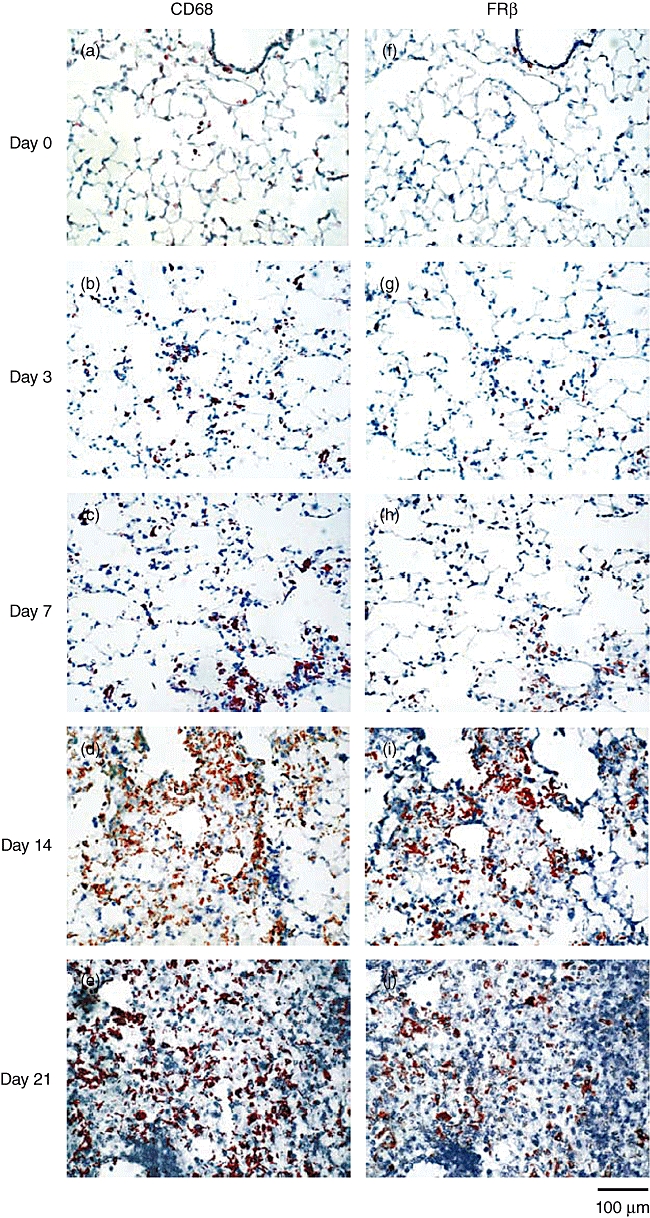
Appearance of folate receptor β(FRβ)-expressing macrophages at different stages in the lungs of mice with bleomycin-induced PF. (a–e) CD68- or (f–j) FRβ-expressing macrophages were detected by immunohistochemical staining in the lungs of mice without bleomycin instillation (n = 3, a, f) and mice on day 3 (n = 3, b, g), 7 (n = 7, c, h), 14 (n = 7, d, i) or 21 (n = 7, e, j) after bleomycin instillation. Photographs are representative of each group. Magnification ×200.
Expression of TGF-β1 in FRβ-expressing macrophages
Several cytokines are involved in the fibrosis of bleomycin-induced PF. In particular, it has been shown that interstitial macrophages and fibroblast/myofibroblasts produced TGF-β1 in the lungs of experimental PF [26]. Thus, we used the lung sections of mice on day 7 after bleomycin instillation to examine whether FRβ-expressing macrophages produce TGF-β1. Consistent with our previous findings in an experimental glioma model [22], most FRβ-expressing macrophages were CD68-expressing macrophages (Fig. 3a). Many CD68-expressing macrophages or FRβ-expressing macrophages expressed TGF-β1 (Fig. 3b and c). The percentage of TGF-β1expression in FRβ-expressing macrophages was significantly higher than that in CD68-expressing macrophages. Thus, TGF-β1expression is enriched in FRβ-expressing macrophages in the lungs of mice with bleomycin-induced PF (Fig. 3d).
Fig. 3.
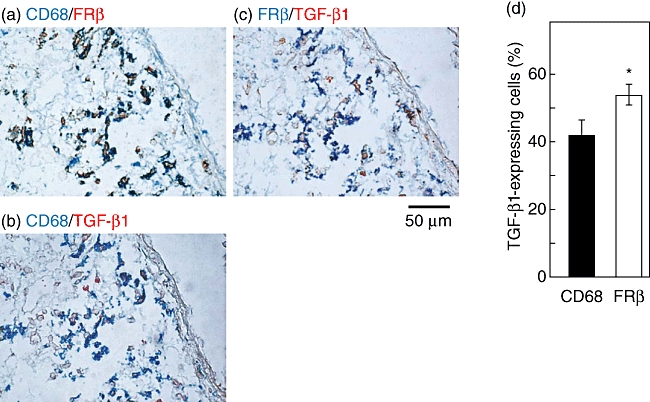
Transforming growth factor (TGF)-β1 expression in folate receptor β(FRβ)-expressing macrophages of lungs of mice with bleomycin-induced pulmonary fibrosis (PF). Co-expression of CD68 and FRβ (a), CD68 and TGF-β1 (b), or FRβ and TGF-β1 (c) were detected by two-colour immunohistochemical staining in the lungs of mice on day 7 after bleomycin instillation. Photographs are representative of seven mice. Magnification ×400. Values in a graph are the mean ± standard error of the mean of percentages of TGF-β1+ cells in CD68- or FRβ-expressing macrophages in the lungs of seven mice (d). *P < 0·05 compared to TGF-β1 expression in CD68-expressing macrophages.
Intervention of bleomycin-induced PF with an immunotoxin to FRβ
To test whether an immunotoxin to FRβ affects the pathogenesis of bleomycin-induced PF in mice, we administered the immunotoxin intranasally to bleomycin-intratracheally instilled mice from days 3 to 19. As shown in Fig. 4, the immunotoxin increased survival significantly compared to the control protein-treated group. Fibrosis in the lungs of the immunotoxin-treated group was significantly less severe compared to the control protein-treated group and the numbers of FRβ-expressing macrophage were decreased (Fig. 5). Total levels of hydroxyproline in the lungs of immunotoxin-treated groups were significantly lower than those in the lungs of the control protein-treated group (Fig. 6).
Fig. 4.
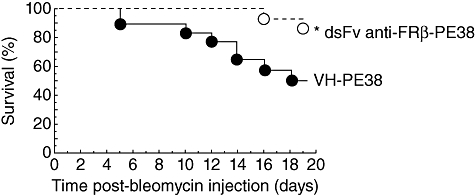
Effect of immunotoxin on the survival of mice with bleomycin-induced pulmonary fibrosis (PF). C57BL/6J mice received intratracheal injection of bleomycin. On day 3 after bleomycin instillation, mice were treated intranasally with the control protein (VH-PE38, n = 30) or an immunotoxin to folate receptor β(FRβ) (dsFv anti-FRβ-PE38, n = 15), every other day from days 3 to 19, and monitored daily for survival. Data are expressed as a Kaplan–Meier plot. *P < 0·05 compared to the control protein-treated group.
Fig. 5.
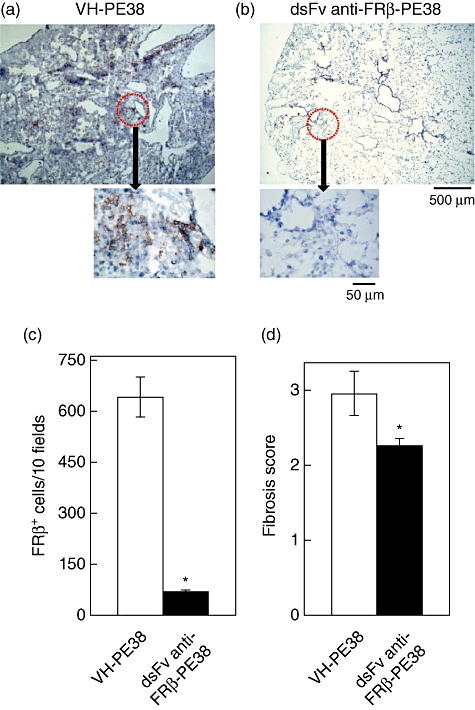
Effect of immunotoxin on histology in the lungs of mice with bleomycin-induced pulmonary fibrosis (PF). Folate receptor β(FRβ)-expressing macrophages were detected by immunohistochemical staining in the lungs on day 21 of control protein- (VH-PE38, a) and immunotoxin-treated (dsFv anti-FRβ-PE38, b) mice with bleomycin-induced PF. Photographs are representative of each group of 13 mice. Magnification ×50, and magnifications (×400) are indicated by arrows in the photographs. (c) Frequency of FRβ-expressing macrophages in the lungs of control protein-treated mice (VH-PE38) and immunotoxin-treated mice (dsFv anti-FRβ-PE38) with bleomycin-induced PF. *P < 0·05 compared to the control protein-treated group, n = 10 mice. (d) Fibrosis scores in control protein- (VH-PE38) and immunotoxin-treated (dsFv anti-FRβ-PE38) groups are presented as the mean ± standard error of the mean of fibrosis scores. *P < 0·05 compared to the control protein-treated group, n = 13 mice.
Fig. 6.
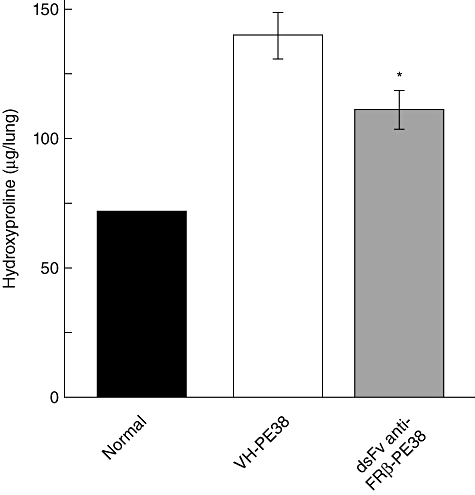
Effect of immunotoxin on hydroxyproline content in the lungs of mice with pulmonary-induced pulmonary fibrosis (PF). Hydroxyproline content was measured in the lungs of mice without PF (control, n = 4), and the control protein- (VH-PE38) or immunotoxin-treated (dsFv anti-FRβ-PE38) mice with bleomycin-induced PF. Data are the mean ± standard error of the mean of hydroxyproline content (µg/right lung) from each group. *P < 0·05 compared to mice without PF or the control protein-treated mice with PF, n = 13 in each group.
Because cytokines such as TNF-α, CCL2 and CCL12 produced by macrophages are known to exhibit profibrotic activity [12,27,28], we compared the numbers of total cells and cells producing each cytokine in the lungs of mice treated with the control protein or immunotoxin. Figure 7 shows that the administration of an immunotoxin to FRβ decreased numbers of total cells, as well as TNF-α-, CCL2- or CCL12-producing cells in the lungs on day 21 compared to the control protein-treated group.
Fig. 7.
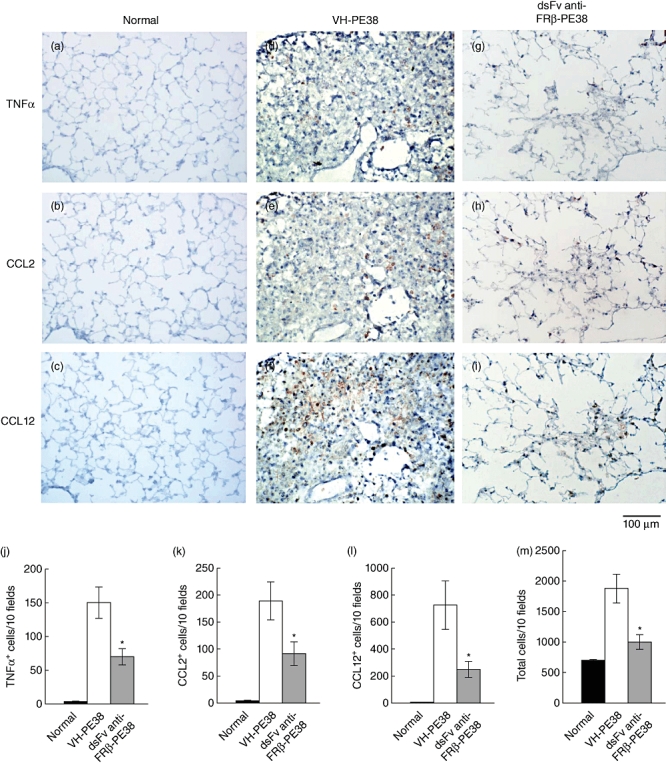
Effect of immunotoxin on the frequency of tumour necrosis factor (TNF)-α-, chemokines CCL2- and CCL12-producing cells in the lungs of mice with bleomycin-induced pulmonary fibrosis (PF). TNF-α- (a, d, g), CCL2- (b, e, h) and CCL12- (c, f, i)-producing cells were detected by immunohistochemical staining in the lungs on day 21 of healthy mice (normal, a–c), and control protein- (VH-PE38, d–f) and immunotoxin-treated (dsFv anti-FRβ-PE38, g–i) mice with bleomycin-induced PF. Photographs are representative of each group of 10 mice. (j–m) Frequency of (j) TNF-α-, (k) CCL2- and (l) CCL12-producing cells, and total cells (m) in the lungs of healthy mice (normal), control protein- (VH-PE38) and immunotoxin-treated mice (dsFv anti-FRβ-PE38) with bleomycin-induced PF. Data are given as the mean ± standard error of the mean. *P < 0·05 compared to the control protein-treated group, n = 10 mice in each group.
Discussion
In this study, we observed numerous interstitial and alveolar FRβ-expressing macrophages in the lungs of patients with IPF compared to normal lungs.
FRβ-expressing macrophages were not observed in normal mouse lungs, whereas they were present predominantly in fibrotic areas in the lung of bleomycin-induced PF. Increased numbers of FRβ-expressing macrophages in the lungs of bleomycin-instilled mice paralleled the development of PF. This is the first report demonstrating the appearance of FRβ-expressing macrophages in these lungs. Furthermore, the intranasal administration of an immunotoxin to FRβ reduced the hydroxyproline content and fibrosis in the lungs, leading to increased survival in bleomycin-instilled mice.
It has been thought that macrophage-derived TGF-β1 can act on local lung fibroblasts and possibly also recruit fibrocytes [29]. In this study, we showed that FRβ-expressing macrophages produced TGF-β1 at the early stage of bleomycin-induced PF, suggesting that these cells may be involved directly in the pathogenesis of experimental PF. We also noted that the administration of an immunotoxin to FRβ on day 14 did not improve the survival rate of mice with bleomycin-induced PF in which other cell types produce TGF-β1 (data not shown). These results support the notion that FRβ-expressing macrophages may contribute mainly to initiation of bleomycin-induced PF by TGF-β1 production.
Moreover, chemokines CCL2, CCL12 and CCL3, which are also produced by macrophages and epithelial cells, have been identified as profibrotic mediators [30]. Indeed, the lack of CCR2 (the receptor for CCL2 and CCL12) or CCR5 (the receptor for CCL3) suppressed significantly the development of fibrosis [31,32]. In addition, interleukin (IL)-13 has been shown to contribute to the development of PF, presumably by inducing CCL2 and CCL3 [33,34]. The present study demonstrated that the intranasal administration of an immunotoxin to FRβ decreased numbers of cells producing the profibrotic cytokines TNF-α, CCL2 and CCL12. Taken together with previous findings, depletion of FRβ-expressing macrophages in bleomycin-induced PF might reduce various fibrotic cytokines produced by inflammatory macrophages. Thus, targeting FRβ-expressing macrophages will be more effective than targeting individual macrophage-derived fibrogenic molecules.
It has been shown that treatment of mice with mAb against CD11b (pan-monocyte/macrophage marker) or CD11a suppressed PF induced by bleomycin [35]. However, these treatments may suppress innate immunity due to expression of CD11a and CD11b on monocytes and resident tissue macrophages. Indeed, it was reported that treatment with CD11b mAb worsened outcome in rats with Escherichia coli pneumonia [36]. In contrast to CD11b- or CD11a-expressing cells, FRβ-expressing macrophages are very limited in normal tissues. Thus, targeting inflammatory macrophages through FRβ would be an ideal therapy for treating human IPF as the function of monocytes and resident tissue macrophages is not interrupted by this treatment. With the discovery of immunoRNase, in which a toxin moiety and immunoglobulin fragments are replaced by human RNase and a humanized immunoglobulin, respectively [37], the toxicity and immunogenicity of an immunotoxin to FRβ will thus be overcome. Once internalized the RNase moiety in immuneRNase will exert its RNA degrading activity, which will lead to the death of the target cells. Alternatively, folate antagonists can be used because normal tissues express no or low levels of folate receptors, although most have a higher binding affinity for FRα than for FRβ[38,39]. Typically, IPF demonstrates heterogeneically as normal-appearing lung alternating with areas of peripheral fibrosis, interstitial inflammation and honeycomb changes [4]. These inflammatory components appear to consist primarily of macrophages, lymphocytes, plasma cells, neutrophils and eosinophils [40]. At the time of IPF diagnosis it is difficult to determine the distribution of activated macrophages without a lung biopsy. Folate-targeted diagnostic radiopharmaceuticals bind to folate receptors and are taken up predominantly by activated macrophages [17,40]. Thus, FolateScan, which images FR-expressing cells [41], will help to predict optimal therapeutic timing and monitor the therapeutic efficacy of targeting FRβ-expressing macrophages in IPF patients. It has been suggested that abnormalities in multiple pathways involved in wound-healing and inflammation lead to the development of IPF [42]. Thus, the most effective approach would be to target multiple profibrotic pathways, including the simultaneous activation of macrophages.
In conclusion, this study showed that inflammatory macrophages in the lungs from human and experimental PF expressed FRβ. Selective depletion of FRβ-expressing macrophages suppressed the development of bleomycin-induced PF significantly, thus implying that targeting FRβ-expressing macrophages may be promising for the treatment of IPF.
Acknowledgments
This work was supported by KAKENHI (20790377), a Grant-in-Aid for Young Scientists (B) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan, and the Kodama Memorial Fund Medical Research.
Disclosure
The authors have no financial conflict of interest.
References
- 1.Thomeer MJ, Vansteenkiste J, Verbeken EK, Demedts M. Interstitial lung diseases: characteristics at diagnosis and mortality risk assessment. Respir Med. 2004;98:567–73. doi: 10.1016/j.rmed.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 2.Noble PW. Idiopathic pulmonary fibrosis: natural history and prognosis. Clin Chest Med. 2006;27:S11–16. doi: 10.1016/j.ccm.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Wilson MS, Wynn TA. Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol. 2009;2:103–21. doi: 10.1038/mi.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White ES, Lazar MH, Thannickal VJ. Pathogenetic mechanisms in usual interstitial pneumonia/idiopathic pulmonary fibrosis. J Pathol. 2003;201:343–54. doi: 10.1002/path.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Homolka J, Ziegenhagen MW, Gaede KI, Entzian P, Zissel G, Müller-Quernheim J. Systemic immune cell activation in a subgroup of patients with idiopathic pulmonary fibrosis. J Respir. 2003;70:262–9. doi: 10.1159/000072007. [DOI] [PubMed] [Google Scholar]
- 6.Kadota J, Mizunoe S, Mito K, et al. High plasma concentrations of osteopontin in patients with interstitial pneumonia. Respir Med. 2005;99:111–17. doi: 10.1016/j.rmed.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 7.Ide M, Ishii H, Mukae H, et al. High serum levels of thrombospondin-1 in patients with idiopathic interstitial pneumonia. Respir Med. 2008;102:1625–30. doi: 10.1016/j.rmed.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Moore BB, Hogaboam CM. Murine models of pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008;294:L152–60. doi: 10.1152/ajplung.00313.2007. [DOI] [PubMed] [Google Scholar]
- 9.Okuma T, Terasaki Y, Kaikita K, et al. C-C chemokine receptor 2 (CCR2) deficiency improves bleomycin-induced pulmonary fibrosis by attenuation of both macrophage infiltration and production of macrophage-derived matrix metalloproteinases. J Pathol. 2004;204:594–604. doi: 10.1002/path.1667. [DOI] [PubMed] [Google Scholar]
- 10.Moore BB, Murray L, Das A, Wilke CA, Herrygers AB, Toews GB. The role of CCL12 in the recruitment of fibrocytes and lung fibrosis. Am J Respir Cell Mol Biol. 2006;35:175–81. doi: 10.1165/rcmb.2005-0239OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishida Y, Kimura A, Kondo T, et al. Essential roles of the CC chemokine ligand 3-CC chemokine receptor 5 axis in bleomycin-induced pulmonary fibrosis through regulation of macrophage and fibrocyte infiltration. Am J Pathol. 2007;170:843–54. doi: 10.2353/ajpath.2007.051213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baran CP, Opalek JM, McMaken S, et al. Important roles for macrophage colony-stimulating factor, CC chemokine ligand 2, and mononuclear phagocytes in the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med. 2007;176:78–89. doi: 10.1164/rccm.200609-1279OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piguet PF, Vesin C. Treatment by human recombinant soluble TNF receptor of pulmonary fibrosis induced by bleomycin or silica in mice. Eur Respir J. 1994;7:515–18. doi: 10.1183/09031936.94.07030515. [DOI] [PubMed] [Google Scholar]
- 14.Tanino Y, Makita H, Miyamoto K, et al. Role of macrophage migration inhibitory factor in bleomycin-induced lung injury and fibrosis in mice. Am J Physiol Lung Cell Mol Physiol. 2002;283:L156–62. doi: 10.1152/ajplung.00155.2001. [DOI] [PubMed] [Google Scholar]
- 15.Inoshima I, Kuwano K, Hamada N, et al. Anti-monocyte chemoattractant protein-1 gene therapy attenuates pulmonary fibrosis in mice. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1038–44. doi: 10.1152/ajplung.00167.2003. [DOI] [PubMed] [Google Scholar]
- 16.Elnakat H, Ratnam M. Distribution, functionality and gene regulation of folate receptor isoforms: implications in targeted therapy. Adv Drug Deliv Rev. 2004;56:1067–84. doi: 10.1016/j.addr.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Low PS, Henne WA, Doorneweerd DD. Discovery and development of folic-acid-based receptor targeting for imaging and therapy of cancer and inflammatory diseases. Acc Chem Res. 2008;41:120–9. doi: 10.1021/ar7000815. [DOI] [PubMed] [Google Scholar]
- 18.Nagayoshi R, Nagai T, Matsushita K, et al. Effectiveness of anti-folate receptor beta antibody conjugated with truncated Pseudomonas exotoxin in the targeting of rheumatoid arthritis synovial macrophages. Arthritis Rheum. 2005;52:2666–75. doi: 10.1002/art.21228. [DOI] [PubMed] [Google Scholar]
- 19.Xia W, Hilgenbrink AR, Matteson EL, Lockwood MB, Cheng JX, Low PS. A functional folate receptor is induced during macrophage activation and can be used to target drugs to activated macrophages. Blood. 2009;113:438–46. doi: 10.1182/blood-2008-04-150789. [DOI] [PubMed] [Google Scholar]
- 20.Nagai T, Tanaka M, Tsuneyoshi Y, et al. In vitro and in vivo efficacy of a recombinant immunotoxin against folate receptor beta on the activation and proliferationof rheumatoid arthritis synovial cells. Arthritis Rheum. 2006;54:3126–34. doi: 10.1002/art.22082. [DOI] [PubMed] [Google Scholar]
- 21.American Thoracic Society and the European Respiratory Society (ERS) Idiopathic pulmonary fibrosis: diagnosis and treatment:international consensus statement. Am J Respir Crit Care Med. 2000;161:646–64. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 22.Nagai T, Tanaka M, Tsuneyoshi Y, et al. Targeting tumor-associated macrophages in an experimental glioma model with a recombinant immunotoxin to folate receptor beta. Cancer Immunol Immunother. 2009;58:1577–86. doi: 10.1007/s00262-009-0667-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gharaee-Kermani M, Ullenbruch M, Phan SH. Animal models of pulmonary fibrosis. Methods Mol Med. 2005;117:251–9. doi: 10.1385/1-59259-940-0:251. [DOI] [PubMed] [Google Scholar]
- 24.Ashcroft T, Simpson JM, Timbrell VJ. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. Clin Pathol. 1988;41:467–70. doi: 10.1136/jcp.41.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reddy GK, Enwemeka CS. A simplified method for the analysis of hydroxyproline in biological tissues. Clin Biochem. 1996;29:225–9. doi: 10.1016/0009-9120(96)00003-6. [DOI] [PubMed] [Google Scholar]
- 26.Higashiyama H, Yoshimoto D, Okamoto Y, Kikkawa H, Asano S, Kinoshita M. Receptor-activated Smad localisation in bleomycin-induced pulmonary fibrosis. J Clin Pathol. 2007;60:283–9. doi: 10.1136/jcp.2006.037606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith RE, Strieter RM, Phan SH, Lukacs N, Kunkel SL. TNF and IL-6 mediate MIP-1alpha expression in bleomycin-induced lung injury. J Leukoc Biol. 1998;64:528–36. [PubMed] [Google Scholar]
- 28.McMillan TR, Moore BB, Weinberg JB, et al. Exacerbation of established pulmonary fibrosis in a murine model by gammaherpesvirus. Am J Respir Crit Care Med. 2008;177:771–80. doi: 10.1164/rccm.200708-1184OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lloyd CM, Minto AW, Dorf ME, et al. RANTES and monocyte chemoattractant protein-1 (MCP-1) play an important role in the inflammatory phase of crescentic nephritis, but only MCP-1 is involved in crescent formation and interstitial fibrosis. J Exp Med. 1997;185:1371–80. doi: 10.1084/jem.185.7.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore BB, Kolodsick JE, Thannickal VJ, et al. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am J Pathol. 2005;166:675–84. doi: 10.1016/S0002-9440(10)62289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishida Y, Kimura A, Kondo T, et al. Essential roles of the CC chemokine ligand 3-CC chemokine receptor 5 axis in bleomycin-induced pulmonary fibrosis through regulation of macrophage and fibrocyte infiltration. Am J Pathol. 2007;170:843–54. doi: 10.2353/ajpath.2007.051213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belperio JA, Dy M, Burdick MD, et al. Interaction of IL-13 and C10 in the pathogenesis of bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2002;27:419–27. doi: 10.1165/rcmb.2002-0009OC. [DOI] [PubMed] [Google Scholar]
- 34.Ma B, Zhu Z, Homer RJ, Gerard C, Strieter R, Elias JA. The C10/CCL6 chemokine and CCR1 play critical roles in the pathogenesis of IL-13-induced inflammation and remodeling. J Immunol. 2004;172:1872–81. doi: 10.4049/jimmunol.172.3.1872. [DOI] [PubMed] [Google Scholar]
- 35.Piguet PF, Rosen H, Vesin C, Grau GE. Effective treatment of the pulmonary fibrosis elicited in mice by bleomycin or silica with anti-CD-11 antibodies. Am Rev Respir Dis. 1993;147:435–41. doi: 10.1164/ajrccm/147.2.435. [DOI] [PubMed] [Google Scholar]
- 36.Zeni F, Parent C, Correa R, et al. ICAM-1 and CD11b inhibition worsen outcome in rats with E. coli pneumonia. J Appl Physiol. 1999;87:299–307. doi: 10.1152/jappl.1999.87.1.299. [DOI] [PubMed] [Google Scholar]
- 37.De Lorenzo C, D'Alessio G. From immunotoxins to immunoRNases. Pharm Biotechnol. 2008;9:210–14. doi: 10.2174/138920108784567254. [DOI] [PubMed] [Google Scholar]
- 38.Jackman AL, Theti DS, Gibbs DD. Antifolates targeted specifically to the folate receptor. Adv Drug Deliv Rev. 2004;56:1111–25. doi: 10.1016/j.addr.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 39.van der Heijden JW, Oerlemans R, Dijkmans BA, et al. Folate receptor beta as a potential delivery route for novel folate antagonists to macrophages in the synovial tissue of rheumatoid arthritis patients. Arthritis Rheum. 2009;60:12–21. doi: 10.1002/art.24219. [DOI] [PubMed] [Google Scholar]
- 40.Parra ER, Kairalla RA, Ribeiro de Carvalho CR, Eher E, Capelozzi VL. Inflammatory cell phenotyping of the pulmonary interstitium in idiopathic interstitial pneumonia. Respiration. 2007;74:159–69. doi: 10.1159/000097133. [DOI] [PubMed] [Google Scholar]
- 41.Matteson EL, Lowe VJ, Prendergast FG, et al. Assessment of disease activity in rheumatoid arthritis using a novel folate targeted radiopharmaceutical FolatescanTM. Clin Exp Rheumatol. 2009;27:253–9. [PMC free article] [PubMed] [Google Scholar]
- 42.Maher TM, Wells AU, Laurent GJ. Idiopathic pulmonary fibrosis: multiple causes and multiple mechanisms? Eur Respir J. 2007;30:835–9. doi: 10.1183/09031936.00069307. [DOI] [PubMed] [Google Scholar]


