Abstract
Allogeneic pancreatic islet transplantation theoretically represents a cure for type 1 diabetes. However, current immune suppressive therapies are often associated with undesired side effects. Given this problem, and the shortage of human islet donors, the majority of type 1 diabetes patients cannot currently be offered an islet transplant. However, it has been found that mesenchymal stem cells (MSCs) could exert unique immunosuppressive effects both in vitro and in vivo. Herein we transplanted allogeneic 200 islets alone or in combination with MSCs (3 × 106 cells) under the kidney capsules of diabetic C57LB/6 mouse. We found that the ratios of T helper type 1 (Th1) to Th2 and Tc1 to Tc2 were reduced, and the numbers of naive and memory T cells were down-regulated in peripheral blood after transplantation. In addition, the maturation, endocytosis and interleukin-12 secretion of dendritic cell (DCs)-derived bone marrow cells (BMCs) from receptor mice were suppressed. Rejection reaction was alleviated by MSCs which exerted suppressive effects through T lymphocyte subsets and DCs.
Keywords: dendritic cell, immune modulation, islet transplantation, mesenchymal stem cell, T lymphocyte subsets
Introduction
Islet transplantation is a method to restore β cell mass and achieve glucose homeostasis in type 1 diabetic patients. However, current immune suppressive therapies are often associated with undesired side effects. Given this problem, and the shortage of human islet donors, the majority of type 1 diabetic patients cannot be offered an islet transplant today [1]. Hence, new effective immune suppressive therapies without side effects are clearly warranted. Bone marrow-derived mesenchymal stem cells (MSCs), as non-haematopoietic stem cells derived from the bone marrow stromal system, has demonstrated immunomodulatory activity. Recent in vitro investigations have demonstrated definitively that MSCs may also act as a pleiotropic immune regulator to suppress an ongoing immune process through cytokine secretion and/or direct cell–cell contact to affect nearly all immune cells including T, natural killer (NK), B and dendritic cells (DCs) [2,3]. The effectiveness of MSCs on prevention and treatment of graft-versus-host disease (GVHD) has been reported [4]. We hypothesize that MSCs can prevent rejection of a transplanted islet graft, so how MSCs suppress immune responses in the body needs to be studied. This study aimed to explore the role of MSCs in suppressive effects. In the present study, allogeneic pancreatic islet transplantation was performed in diabetic mice, and the changes of T lymphocyte subsets as well as the maturity and functions of DCs were observed to investigate the immunological mechanism of MSCs in the suppression of host anti-graft rejection.
Methods and materials
Animals
C57BL/6 mice and BALB/c mice were purchased from the Laboratory Animal Centre of Southern Medical University and were maintained in specific pathogen-free conditions (animal house of the Second Clinical Medical College of Jinan University). All animal experiments were approved by the local ethics committee and conducted in accordance with institutional guidelines for animal care and use.
Isolation, culture and identification of MSCs
MSCs from murine compact bone were isolated and culture-expanded as described previously [5]. All MSCs were CD31-, CD34-, CD45-, CD73+, CD105+ and CD106+ analysed flow cytometry (ALTRA' Beckman Coulter, Inc., Brea, CA, USA), and identification was performed by adipogenic and osteogenic induction tests as described previously [5]. Four weeks after osteogenic induction, bone nodules were observed. Alizarin red staining showed that the calcified nodules were red and dense. Four weeks after adipogenic induction, the cells were enlarged, accompanied by fat particles with different sizes. Oil red O staining indicated red in fat particles.
Isolation and purification of pancreatic islets
Pancreatic islets were isolated from adult male BALB/c mice after intraductal collagenase injection (Type V; Sigma, St Louis, MO, USA), followed by automated digestion and purification by centrifugation on Histopaque (1·077 g/ml; Sigma), as described previously [6]. Approximately 154 ± 41 pancreatic islets were obtained from each BALB/c mouse. AO/PI (20 µl; Sigma) staining showed the pancreatic islets were blue, suggesting that the islet cells were viable without injury or apoptosis.
Streptozotocin (STZ) induction of diabetes
C57LB/6 mice were injected intraperitoneally with streptozotocin (STZ; 150 mg/kg; Sigma). Diabetes was monitored by measurement of blood glucose concentration from the tail vein using a blood glucose device (Roche Accu Check III; Roche, Basel, Switzerland). Mice with non-fasting blood glucose >300 mg/dl for 3 consecutive days were considered as onset of diabetes.
Co-transplantation of MSCs and islets
Islets were prepared and 200 islet equivalents (IEQ) transplanted either alone or with MSCs (3 × 106 cells/mouse). One IEQ was defined as an islet mass equivalent to 125 µm in diameter. Thirty-two recipient C57LB/6 diabetic mice were divided randomly into four groups: A group: diabetes mice (n = 8); B group: islets alone (n = 8); C group: islet + MSCs (1 × 106 MSCs via tail vein 3, 2 and 0 days before islet transplantation, n = 8); D group: islet + CD45RB monoclonal antibody (mAb) [CD45RB mAb, 100 µg/mice, intraperitoneally (i.p.), 0, 1, 3, 5, 7 days, MB23G2; Type Culture Collection, Manassas, VA, USA]; and E group: normal C57BL/6 mice (n = 8). Blood was collected by cutting off tails to determine non-fasting blood glucose at 4 p.m. every day for 30 consecutive days. At the same time, the body weights of mice were measured. The left kidney was removed 28 days after transplantation, and blood glucose was monitored for 2 consecutive days. In addition, one mouse left kidney was removed in each group on day 14 and in the remaining mice the left kidney was removed 28 days after transplantation. These left kidneys were fixed with 10% formalin. The tissues were embedded in paraffin and cut into sections, followed by haematoxykin and eosin (H&E) staining for pathological analysis. Under 400× light microscope, 10 fields of visions were chosen randomly to record the number of white blood cells.
Detection of T helper type 1 (Th1), Th2, Tc1 and Tc2 cells, naive and memory T cells
Peripheral blood, 0·5 ml, was obtained on day 30 after pancreatic islet transplantation and mononuclear cells were isolated, followed by stimulation with 50 ng/ml phorbol myristate acetate (PMA), 1 µg/ml ionomycin and 1 µl/ml Brefeldin A (BFA) (Merck, Whitehouse Station, NJ, USA). Then, the mononuclear cells were harvested into tubes (1 × 105 cells in each tube) and incubated with 10 µl of phycoerythrin–Texas-red-X (ECD)-conjugated CD4 and CD8 for 15 min. Cell fixation and perforation were performed, followed by incubation with 10 µl of phycoerythrin (PE)-conjugated interleukin (IL)-4 and interferon (IFN)-γ. Cells were washed and Th1, Th2, Tc1 and Tc2 cells were measured with flow cytometry. Additionally, the mononuclear cells (1 × 105) were incubated with 10 µl of ECD-conjugated CD4 and CD8, fluorescein isothiocyanate (FITC)-conjugated CD44 and PE-conjugated CD62L (BD Pharmingen, San Diego, CA, USA) and the number of naive T cells and memory T cells were detected with flow cytometry.
Detection of maturity and functions of DCs derived from bone marrow cells (BMCs) in recipient mice
Mononuclear cells were isolated from BMCs in recipient mice on day 30 after pancreatic islet transplantation. Purified mononuclear cells (5 × 106/well) were cultured with containing 20 ng/ml granulocyte–macrophage colony-stimulating (GM-CSF) factor and 20 ng/ml IL-4 (PeproTech, London, UK) in six-well plates. Half the media were changed every 2 days. On day 7 of culture, the immature dendritic cells (imDC) were incubated with lipopolysaccharide (LPS) (1 ng/ml; Alexis, Farmingdale, NY, USA) for 2 days, followed by harvesting. Immunophenotyping of dendritic cells was carried out using FITC-conjugated CD11c and CD86, PE-conjugated CD83 and I-Ab (BD Pharmingen). Phenotypes were detected with flow cytometry. Additionally, 5 × 105 mDCs were incubated with FITC-conjugated dextran (1 mg/ml; BD Pharmingen) and mean fluorescence intensity (MFI) was measured to evaluate the ability of mDCs to uptake dextran. Furthermore, the supernatant was obtained and the content of IL-12 was measured with the enzyme-linked immunosorbent assay (ELISA) kit (R&D System, Minneapolis, MN, USA).
Statistical analysis
spss version 10·0 software was used for statistical analysis and data were presented as mean ± standard deviation. Student's t-test was performed for comparisons of means between two groups and one-way analysis of variance (anova) was used for comparisons among multiple groups. For all tests, P < 0·05 was considered statistically significant.
Results
Effect of co-transplantation islets and MSCs on blood glucose
On day 3 after pancreatic islet transplantation, the blood glucose level (128·7–132·8 mg/dl) reached the minimum in the B (islets alone), C (islet + MSCs) and D (islet + CD45RB mAb) groups. The blood glucose level of the B group was increased gradually 8 days after transplantation, and reached 342·6 mg/dl 16 days after transplantation, the same as the A group (diabetic mice). Blood glucose in the C group was 132·1 mg/dl on day 3 after pancreatic islets transplantation and began to increase gradually on day 12 after transplantation, reaching 216·8 mg/dl on day 28 after transplantation. From days 14 to 28 after transplantation, a significant difference in blood glucose level was observed between the B and C groups (P < 0·05). For mice in the D group, blood glucose reached the minimum on day 3 after transplantation, which was maintained stable thereafter (128·7–139·4 mg/dl) and still higher than that of the E group (normal mice) (99·2–109·9 mg/dl). After removal of the left kidney on day 28 after transplantation, the blood glucose was elevated markedly (Fig. 1).
Fig. 1.
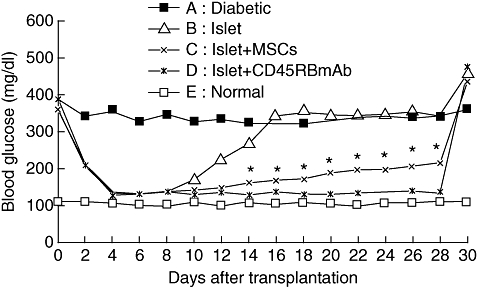
Non-fasting blood glucose levels of streptozotocin-induced diabetic C57LB/6 mice after receiving 200 islets and mesenchymal stem cells (MSCs) transplanted (n = 6 in each group). Nephrectomies were performed 28 days after transplantation.*P < 0·05, compared with the B group (islet alone).
Pathological characteristics of the transplanted kidney
In the pathological analysis on day 14 after transplantation, numerous subcapsular inflammatory cells were observed in the transplant sites of kidneys in B group mice (islet alone) and a few islets were preserved, accompanied by signs of hyperaemia and necrosis (Fig. 2a). There were pancreatic islet cells and a complete cell structure, surrounded by a little lymphocyte infiltration, in the transplantation site of the kidneys of C group mice (Fig. 2b), and no evident inflammatory cells were observed in the kidneys of D group mice (Fig. 2c). On day 30 after transplantation, pathological analysis showed lymphocyte infiltration and fibrous connective tissues in the subcapsular regions, and no islet was observed in the B group (Fig. 2d). The white blood cells were recorded in renal tissue at different times after transplantation under 400× light microscopy. There were a few leucocytes in kidney tissue 14 days and 30 days after transplantation the A group (diabetic mice, 2·24 ± 0·62 and 2·45 ± 0·48) and the D group (islet + CD45Rb mAb, 2·85 ± 0·11 and 2·11 ± 0·21). There was no significant difference compared with the E group (normal mice, 2·01 ± 0·23 and 2·61 ± 0·14) (P > 0·05). In the B group (islet alone, 43·57 ± 3·07 and 18·86 ± 1·89), we found infiltration of numerous white blood cells 14 days and 30 days after transplantation (P < 0·01). The C group (islet + MSCs, 8·48 ± 1·24 and 4·86 ± 0·90) had mild white blood cell infiltration. The number of white blood cells in the C group (islet + MSCs) was significantly lower than that of the B group (islet alone) (P < 0·05).
Fig. 2.

Pathological micrographs of transplanted islets in renal capsules [14 days after transplantation: B group (a), C group (b), D group (c); 30 days after transplantation: B group (d)]. Haematoxylin and eosin staining ×200 magnification.
MSCs enable Th1/Th2 and Tc1 /Tc2 ratios were reduced
Among the peripheral blood mononuclear cells of normal mice, CD3+ T cells consist of CD4+ and CD8+ cells. According to the IFN-γ and IL-4 excreted, CD4+ T cells are subdivided into Th1 cells and Th2 cells, and CD8+ T cells into Tc1 cells and Tc2 cells. The number of Th1 cells was increased and that of Th2 cells was reduced in group A (diabetic mice), resulting in a significantly increased Th1/Th2 ratio compared to the E group (P < 0·05). In the B group, the number of Th1 and Tc1 cells was increased markedly, and that of Th2 and Tc2 cells reduced dramatically. The Th1/Th2 and Tc1/Thc2 ratio was significantly different compared to the E group (P < 0·01). Additionally, in C and D group mice, the numbers of Th1 and Tc1 reduced markedly and those of the Th2 and Tc2 were elevated dramatically. The Th1/Th2 and Tc1/Tc2 ratios were also reduced significantly (P < 0·01) (Figs 3 and 4).
Fig. 3.

The cell percentage of T helper type 1 (Th1)/Th2, Tc1/Tc2 cells in recipient mice in vivo (n = 6–7 in each group). *P < 0·05, **P < 0·01, compared with the E group.
Fig. 4.

The ratio change of T helper type 1 (Th1)/Th2, Tc1/Tc2 cells in recipient mice in vivo (n = 6–7 in each group). *P < 0·05, **P < 0·01, compared with the E group.
MSCs inhibit naive and memory T cells
According to the expression levels of CD62L and CD44, CD44+ T cells can be subdivided into naive D4+ T cells (CD4 CD62Lhigh CD44low) and memory CD4+ T cells (CD4 CD62Llow CD44high) and CD8 into naive CD8+ T cells (CD8 CD62Lhigh CD44low) and memory CD8+ T cells (CD8 CD62Llow CD44high). Results indicated that the number of peripheral naive T cells and memory T cells was increased markedly in the B group mice only (P < 0·05). In the C group mice, the number of peripheral naive T cells and memory T cells was reduced dramatically (P < 0·05), and the reduced number of peripheral naive T cells and memory T cells was more profound in the D group mice (P < 0·01) (Fig. 5).
Fig. 5.

Naive and memory T cells in recipient mice in vivo (n = 6–7 in each group). *P < 0·05, **P < 0·01, compared with the E group.
MSCs suppressed DCs maturation from BMCs
BMCs were obtained on day 30 after transplantation and differentiation of BMCs was induced into mDCs. The expression of CD83, a marker of maturation of mDCs, and CD86, a co-stimulatory molecule, was reduced significantly compared to the E group (normal mice) (P < 0·05). In the B group, the expression of CD83 and CD86 was elevated markedly (P < 0·05). However, in the C and D groups, the expression of CD11c, a marker of cells derived from monocytes, and CD83 and CD86 as well as I-Ab, a major histocompatibility complex (MHC) class II antigen, was reduced dramatically, which was more evident in the C group (P < 0·01). The down-regulated expression of these molecules by MSCs suggested that the DC maturation was reversed, thus inhibiting the T cell function (Fig. 6).
Fig. 6.

Immunophenotypic expression of mature dencdritic cells (mDCs) from bone marrow cells (BMC) in recipient mice in vitro (n = 6–7 in each group). *P < 0·05, **P < 0·01, compared with the E group.
MSCs reduced ability of mDCs to uptake dextran
When compared with the E group (12·9 ± 0·9 MFI), the ability of mDCs to uptake dextran was elevated markedly in the B group (19·2 ± 0·8 MFI) (P < 0·05), and was reduced significantly in the C group (6·3 ± 0·9 MFI) (P < 0·05) or the D group (1·4 ± 0·4 MFI) (P < 0·01).
MSCs reduce the IL-12 level secreted by mDCs
IL-12 was not detected in the imDCs from group E. However, a large amount of IL-12 was measured in mDCs (69·0 ± 12·3 g/ml). IL-12 was secreted mDCs from the A group (61·9 ± 15·4 pg/ml). The amount of IL-12 was increased markedly in mDCs from the B group (89·3 ± 18·2 pg/ml), and was reduced markedly in mDCs from the C group (48·2 ± 7·6 pg/ml) (P < 0. 05) or the D group (27·6 ± 5·4 pg/ml) (P < 0·01).
Discussions
It has been indicated that MSCs exerted an immunosuppressive effect on allogeneic pancreatic islet transplantation [7,8]. MSCs are known to secrete an array of anti-inflammatory and immunoregulatory factors and possess the ability to suppress autoreactive/alloreactive effector Th1 cell activation in the grafts. MSCs secrete or express anti-inflammatory and immunoregulatory factors and possess the ability to inhibit T cell responses by reducing IFN-γ secretion in vitro, and thus suppressing Th1 responses [9–11]. Of note, the bioactive and growth factors secreted by MSCs are thought to be involved in the immunosuppressive/immunoregulatory mechanisms mediated by MSCs [10,12–15]. Using antibodies to human growth factor (HGF), transforming growth factor (TGF)-β1 or IL-6, either alone or in combination, T cell proliferation and/or IFN-γ secretion could not be inhibited when T cells were cultured in the presence of MSCs or conditioned medium prepared from MSCs.
It is suggested that MSCs may exert their effects via a combination of numbers of bioactive and immunoregulatory factors. Importantly, these growth factors have been shown to promote islet survival and enhance beta cell function in several published studies [16–18]. A recent study demonstrated that co-infusion of MSCs with BM enhanced stable mixed chimerism, which was essential for the induction of islet graft survival [19]. In our present study, acute rejection occurred in the islet-alone group; blood sugar rose sharply 4 days after transplantation. We have found the immunoregulatory effect of MSCs on allogeneic islet transplantation, induction of long-term insulin expression and sustained normoglycaemia in diabetic rats. It is suggested that MSCs can promote a microenvironment that limits beta cell damage by providing survival signals to the beta cells, and at the same time MSCs can modulate the local immune responses through the secretion of these immunoregulatory factors in the omentum bearing the grafts. It was surprising that the blood glucose level was elevated slightly on days 12–14 after the C group (islet + MSCs) transplantation. Although, on day 28, the blood glucose level of mice with C group transplantation was different from that of the A group (diabetic mice) without treatment, pathological analysis indicated mild inflammatory cell infiltration in the transplantation sites, suggesting that MSCs conferred immunosuppressive effects on immune rejection reactions, but complete immune tolerance was not observed.
The specific mechanism involved in the immunosuppressive effects conferred by MSCs is poorly understood. It has been postulated that the transplant rejection was related to Th1 effects and immune tolerance was induced mainly by Th2 effects. Graft-versus-host tolerance can be achieved through induction of CD4+ T cell differentiation into Th2 cells. The changes of Tc cells during graft rejection were similar to those of Th1/Th2. Studies found that, in the MSCs–Th1 or MSCs–Th2 co-culture systems, the amount of IFN-γ secreted by Th1 cells was inhibited by MSCs, and that of IL-4 by Th2 cells was elevated [10]. Other studies observed that the T cell subset composition was altered by MSCs and the number of Th2 cells was increased significantly [20]. Our results indicated that the number of Th1 cells and Tc1 cells was increased markedly in mice receiving pancreatic islet transplantation alone (B group) and that of Th2 and Tc2 was reduced, suggesting a Th2 to Th1 shift. However, the number of Th1 cells and Tc1 cells was reduced dramatically in mice with combined (C group) transplantation and that of Th2 cells as well as Tc2 cells increased markedly, suggesting a Th1 to Th2 shift. The present study also showed that the amount of IFN-γ from Th1 cells was reduced and that of IL-4 increased. MSCs act on T cells through the immune-regulating factors, altering T cell subset composition (Th1/Th2 and Tc1/Tc2 ratios). Immune tolerance is induced in recipient mice through Th2 effects and Tc2 effects, thus promoting survival of transplanted islets.
Naive, i.e. non-sensitized, T cells can exert immunosuppressive effects, but memory can confer adjuvant or inductive effects [21]. In the experiment with T cell receptor (TCR) transgenic mice, results indicated that the responses of naive T cells and memory T cells to specific antigen peptide were suppressed markedly by MSCs. These responses included T cell proliferation, specific cytotoxic response and production of IFN-γ. These findings suggest that MSCs could suppress GVHD through inhibiting the activation of alloreactive T cells, production of cytokines from Th1 cells and the functions of effector T cells. Another study also indicated that the suppressive effects of MSCs could be conferred through direct interaction with target cells, and these effects were reversible. After removal of MSCs or administration of a variety of cytokines, the suppressive effects were discontinued [22]. Our results showed that suppressive effects by MSCs were observed not only in memory CD4+ and memory CD8+ T cells, but also in naive CD4+ T and naive CD8+ T cells. The suppressive effects of memory CD8+ were more profound. MSCs can secret large amounts of CXCL12, a chemokine which promotes the homing of CD8+ memory cells [23]. This may explain why the suppressive effects were more evident on CD8+ T cells.
The immune response is related not only to T cells, but the interaction between DCs and T cells [24]. Therefore, MSCs can confer effects via regulating the production or antigen-presenting ability of DCs which may occur before the activation and proliferation of antigen-specific lymphocytes [25]. Researchers have found that human MSCs could suppress the differentiation and functions of DCs derived from peripheral monocytes or cord blood CD34+ cells [26,27]. Other researchers indicated that MSCs acted on T cells indirectly in which antigen-presenting cells (APCs) were mediators. MSCs could alter the maturity of APCs. Although experiments showed that MSCs could activate highly purified T cells, treatment with APCs suppressed the activation of these cells. Therefore, some authors have postulated that MSCs conferred suppressive effects on DCs directly, which in turn influenced the activation and proliferation of T cells. In addition, inhibition of up-regulation of CD40, CD86 and CD83 suppressed DC maturation. Furthermore, endocytosis of DCs and production of IL-12 and TNF-α were alleviated, resulting in impaired antigen-presenting ability [28,29]. Our results also confirmed the above hypothesis. In mice with combined transplantation of pancreatic islets and MSCs, the expression of CD11c (DCs phenotype derived from monocytes) and CD83 (mature DCs phenotype) was down-regulated markedly, and reduced expression was also observed in MHC class II antigen I-Ab and co-stimulatory molecule CD86, accompanied by an impaired ability to uptake antigens. These findings showed that MSCs could inhibit the maturation of DCs and the semi-maturation phenotype was maintained. Antigen-presenting ability was impaired and the stimulation to T cells was weakened, resulting in survival of transplanted pancreatic islets.
Furthermore, the IL-12 secreted by DCs plays a critical role in the maturation and function of DCs. IL-12 deficiency may upset the balance between Th1 cells and Th2 cells, and differentiation of naive T cells to Th2 can be observed. At the same time, the amount of IFN-γ is reduced and that of IL-10 is increased, leading to ‘non-response’ and tolerance in T cells [30]. In the present study, during DCs maturation IL-12 production was reduced in mice receiving combined transplantation of pancreatic islets and MSCs, suggesting that MSCs conferred suppressive effects on not only the maturation but the function of DCs.
CD45RB antibody, a newly developed tolerance-inducing drug, not only induces immune tolerance but maintains immunosuppressive status, leading to prolonged survival time of grafts [31]. Administration of CD45RB antibody in allogeneic or xenogenic pancreatic islet transplantation can induce immune tolerance and prolong the survival time of transplanted pancreatic islets [32]. In the animals receiving pancreatic islet transplantation and CD45RB antibody, the Th1 to Th2 shift and Tc1 to Tc2 shift. Naive T cells, memory T cells and maturation and functions of monocyte-derived DCs were suppressed. Pathological analysis showed that no evident inflammation was observed in the transplantation sites. In addition, the reduced blood glucose level was maintained stable. These results demonstrate that CD45RB antibody could induce immune tolerance and the immunosuppressive effects conferred by CD45RB antibody were stronger than those by MSCs.
In summary, in combined transplantation of allogeneic pancreatic islets and MSCs, T cell proliferation was suppressed by MSCs and the Th1/Th2 and Tc1/Tc2 ratios were altered with the Th1 to Th2 shift and Tc1 to Tc2 shift. In addition, the naive T cells and memory T cells were also suppressed and the maturation and function of mononuclear cell-derived DCs was inhibited. These effects resulted finally in alleviating immune rejection. However, the immunosuppressive effect of MSCs was lower than CD45Rb mAb in vivo. MSCs did not produce full immune suppression in recipient mice because there are no autologous MSCs, or MSCs lack concentration; further in-depth studies are needed.
Acknowledgments
This work was supported by the National Natural Science Foundation (no. 30772042), the Natural Science Foundation of Guangdong (no. 6027540) and The Science and Technology Project of Shenzhen (no. 200601017) and the Medical Science and Technology Foundation of Guangdong (no. B2008158). We would like to thank Mr Qianglin Duan from Tongji University for critical reading of the manuscript.
Disclosure
No competing financial interests exist.
References
- 1.Ichii H, Ricordi C. Current status of islet cell transplantation. J Hepatobiliary Pancreat Surg. 2009;16:101–12. doi: 10.1007/s00534-008-0021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keating A. How do mesenchymal stromal cells suppress T cells? Cell Stem Cell. 2008;2:106–8. doi: 10.1016/j.stem.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Jones S, Horwood N, Cope A, Dazzi F. The antiproliferative effect of mesenchymal stem cells is a fundamental property shared by all stromal cells. J Immunol. 2007;179:2824–31. doi: 10.4049/jimmunol.179.5.2824. [DOI] [PubMed] [Google Scholar]
- 4.Li H, Guo Z, Jiang X, Zhu H, Li X, Mao N. Mesenchymal stem cells alter migratory property of T and dendritic cells to delay the development of murine lethal acute graft-versus-host disease. Stem Cells. 2008;26:2531–41. doi: 10.1634/stemcells.2008-0146. [DOI] [PubMed] [Google Scholar]
- 5.Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–8. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- 6.Li FR, Qiu L, Yang XF, Qi H, Ren LL, Zhou HX. The protective effects of ulinastatin and adenosine triphosphate on islet function during islet isolation and after islet transplantation. Pancreas. 2009;38:227–30. doi: 10.1097/MPA.0b013e3181788e3d. [DOI] [PubMed] [Google Scholar]
- 7.Figliuzzi M, Cornolti R, Perico N, et al. Bone marrow-derived mesenchymal stem cells improve islet graft function in diabetic rats. Transplant Proc. 2009;41:1797–800. doi: 10.1016/j.transproceed.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 8.Solari MG, Srinivasan S, Boumaza I, et al. Marginal mass islet transplantation with autologous mesenchymal stem cells promotes long-term islet allograft survival and sustained normoglycemia. J Autoimmun. 2009;32:116–24. doi: 10.1016/j.jaut.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Aksu AE, Horibe E, Sacks J, et al. Co-infusion of donor bone marrow with host mesenchymal stem cells treats GVHD and promotes vascularized skin allograft survival in rats. Clin Immunol. 2008;127:348–58. doi: 10.1016/j.clim.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–22. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 11.Boumaza I, Srinivasan S, Witt WT, et al. Autologous bone marrow-derived rat mesenchymal stem cells promote PDX-1 and insulin expression in the islets, alter T cell cytokine pattern and preserve regulatory T cells in the periphery and induce sustained normoglycemia. J Autoimmun. 2009;32:33–42. doi: 10.1016/j.jaut.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–84. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 13.Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–43. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 14.Meisel R, Zibert A, Laryea M, Gobel U, Daubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619–21. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 15.Zhang B, Liu R, Shi D, et al. Mesenchymal stem cells induce mature dendritic cells into a novel Jagged-2-dependent regulatory dendritic cell population. Blood. 2009;113:46–57. doi: 10.1182/blood-2008-04-154138. [DOI] [PubMed] [Google Scholar]
- 16.Lim JY, Min BH, Kim BG, et al. Combinations of growth factors enhance the potency of islets in vitro. Pancreas. 2009;38:447–53. doi: 10.1097/MPA.0b013e318197a62e. [DOI] [PubMed] [Google Scholar]
- 17.Vasavada RC, Gonzalez-Pertusa JA, Fujinaka Y, Fiaschi-Taesch N, Cozar-Castellano I, Garcia-Ocana A. Growth factors and beta cell replication. Int J Biochem Cell Biol. 2006;38:931–50. doi: 10.1016/j.biocel.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Suarez-Pinzon WL, Lakey JR, Brand SJ, Rabinovitch A. Combination therapy with epidermal growth factor and gastrin induces neogenesis of human islet {beta}-cells from pancreatic duct cells and an increase in functional {beta}-cell mass. J Clin Endocrinol Metab. 2005;90:3401–9. doi: 10.1210/jc.2004-0761. [DOI] [PubMed] [Google Scholar]
- 19.Itakura S, Asari S, Rawson J, et al. Mesenchymal stem cells facilitate the induction of mixed hematopoietic chimerism and islet allograft tolerance without GVHD in the rat. Am J Transplant. 2007;7:336–46. doi: 10.1111/j.1600-6143.2006.01643.x. [DOI] [PubMed] [Google Scholar]
- 20.Rainsford E, Reen DJ. Interleukin 10, produced in abundance by human newborn T cells, may be the regulator of increased tolerance associated with cord blood stem cell transplantation. Br J Haematol. 2002;116:702–9. doi: 10.1046/j.0007-1048.2001.03321.x. [DOI] [PubMed] [Google Scholar]
- 21.Dutt S, Tseng D, Ermann J, et al. Naive and memory T cells induce different types of graft-versus-host disease. J Immunol. 2007;179:6547–54. doi: 10.4049/jimmunol.179.10.6547. [DOI] [PubMed] [Google Scholar]
- 22.Krampera M, Glennie S, Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–9. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 23.Mazo IB, Honczarenko M, Leung H, et al. Bone marrow is a major reservoir and site of recruitment for central memory CD8+ T cells. Immunity. 2005;22:259–70. doi: 10.1016/j.immuni.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Lutz MB, Kurts C. Induction of peripheral CD4+ T-cell tolerance and CD8+ T-cell cross-tolerance by dendritic cells. Eur J Immunol. 2009;39:2325–30. doi: 10.1002/eji.200939548. [DOI] [PubMed] [Google Scholar]
- 25.Jung YJ, Ju SY, Yoo ES, et al. MSC-DC interactions: MSC inhibit maturation and migration of BM-derived DC. Cytotherapy. 2007;9:451–8. doi: 10.1080/14653240701452057. [DOI] [PubMed] [Google Scholar]
- 26.Ramasamy R, Fazekasova H, Lam EW, Soeiro I, Lombardi G, Dazzi F. Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation. 2007;83:71–6. doi: 10.1097/01.tp.0000244572.24780.54. [DOI] [PubMed] [Google Scholar]
- 27.Chen L, Zhang W, Yue H, et al. Effects of human mesenchymal stem cells on the differentiation of dendritic cells from CD34+ cells. Stem Cells Dev. 2007;16:719–31. doi: 10.1089/scd.2007.0065. [DOI] [PubMed] [Google Scholar]
- 28.Beyth S, Borovsky Z, Mevorach D, et al. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105:2214–19. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- 29.English K, Barry FP, Mahon BP. Murine mesenchymal stem cells suppress dendritic cell migration, maturation and antigen presentation. Immunol Lett. 2008;115:50–8. doi: 10.1016/j.imlet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Van Parijs L, Perez VL, Biuckians A, Maki RG, London CA, Abbas AK. Role of interleukin 12 and costimulators in T cell anergy in vivo. J Exp Med. 1997;86:1119–28. doi: 10.1084/jem.186.7.1119. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Deng S, Moore DJ, Huang X, et al. Cutting edge: transplant tolerance induced by anti-CD45RB requires B lymphocytes. J Immunol. 2007;178:6028–32. doi: 10.4049/jimmunol.178.10.6028. [DOI] [PubMed] [Google Scholar]
- 32.Visser L, Poppema S, de Haan B, et al. Prolonged survival of rat islet xenografts in mice after CD45RB monotherapy. Transplantation. 2004;77:386–91. doi: 10.1097/01.TP.0000111741.85249.EC. [DOI] [PubMed] [Google Scholar]


